Abstract
Hyperglycemia is one of the major factors for hemorrhagic transformation after ischemic stroke. In this study, we tested hydrogen gas on hemorrhagic transformation in a rat focal cerebral ischemia model. Sprague–Dawley rats (n=72) were divided into the following groups: sham; sham treated with hydrogen gas (H2); Middle Cerebral Artery Occlusion (MCAO); and MCAO treated with H2 (MCAO+H2). All the rats received an injection of 50% dextrose (6ml/kg intraperitoneally) and underwent MCAO 15 min later. Following a 90 min ischemic period, hydrogen was inhaled for 2 hr during reperfusion. We measured the level of blood glucose at 0 hr, 0.5 hr, 4 hr, and 6 hr after dextrose injection. Infarct and hemorrhagic volumes, neurologic score, oxidative stress (evaluating by the level of 8OHG, HNE and nitrotyrosine), MMP-2/MMP-9 activity were measured at 24 hr after ischemia. We found that hydrogen inhalation for 2 hr reduced infarct and hemorrhagic volumes and improved neurological functions. This effect of hydrogen is accompanied by a reduction of the expressions of 8OHG, HNE, nitrotyrosine and the activity of MMP-9. Furthermore, a reduction of the blood glucose level from 500±32.51 to 366±68.22 mg/dl at 4 hr after dextrose injection was observed in hydrogen treated animals. However, the treatment had no significant effect on the expression of ZO-1, occluding, collagen IV or AQP4. In conclusion, hydrogen gas reduced the infarction, hemorrhagic transformation, and improved neurological functions in rat. The potential mechanisms of decreased oxidative stress and glucose levels after hydrogen treatment warrant further investigation.
Keywords: Hydrogen, Hyperglycemia, MCAO, Neuroprotection
Hyperglycemia is a major risk factor of mortality and morbidity after ischemic stroke (Bruno et al., 1999; Capes et al., 2001). Experimental studies have shown that preischemic hyperglycemia aggravated reperfusion brain injury (Pulsinelli et al., 1982; Yip et al., 1991) and has a major impact on postischemic hemorrhagic transformation, possibly by increasing the production of reactive oxygen species or reactive nitrogen species including hydroxyl radical (• OH), superoxide anion (O2−), hydrogen peroxide (H2O2), nitric oxide (NO), and peroxynitrite (ONOO−) (Li et al., 1999; Bonnefont-Rousselot, 2002). Although there are endogenous defenses in brain tissues against oxidative injury, excessive production of reactive oxygen species after ischemic stroke results in DNA fragmentation, lipid peroxidation, inactivation of proteins, and cell death (Szabo, 2007). There is currently no effective treatment to limit the occurrence or effect of hemorrhagic transformation after stroke which is a major factor limiting the use of tissue plasminogen activator (tPA) therapy, the only effective and approved stroke treatment to date (Orso et al., 2008).
Hydrogen gas has been demonstrated to neutralize free radicals and reduce oxidative stress in heart and liver ischemia/reperfusion injuries (Fukuda et al., 2007; Hayashida et al., 2008). Hydrogen gas seemed selectively reduce hydroxyl radical and peroxynitrite in vitro and exerted antioxidant effect by decreasing 4-hydroxynonenal (4-HNE) (the specific marker for lipid peroxidation) and 8OHG (nucleic acid oxidation marker) in a rat MCAO model (Ohsawa et al., 2007). In this study, we examined whether hydrogen inhalation provided neuroprotective effects for acute hyperglycemia-induced hemorrhage after focal ischemia in rats.
EXPERIMENTAL PROCEDURES
This protocol was evaluated and approved by the Institutional Animal Care and Use Committee at Loma Linda University, Loma Linda, California, USA.
MCAO model
A total of 72 male Sprague-Dawley rats weighing 250 to 300 g were divided into the following groups: Sham surgery (Sham), MCAO, Sham treated with hydrogen for 2 hr (Sham+H2), and MCAO treated with hydrogen for 1 hr or 2 hr (MCAO +H21hr/MCAO +H 22hr). Rats were allowed free access to food and water before and after surgery. Anesthesia was induced with ketamine and xylazine (80 mg/kg +10mg/kg i.p.) followed by atropine at a dose of 0.1 mg/kg s.c. During surgery and postoperative period rectal temperature was maintained at 37.5°C by means of a feedback-controlled heating pad. Rats were injected with 50% dextrose (6 ml/kg) intraperitoneally 15 min before MCAO to induce acute hyperglycemia.
MCAO was induced as reported previously (Longa et al., 1989) and modified (Kawamura et al., 1991). Briefly, the right common carotid artery, internal carotid artery, and external carotid artery were surgically exposed. The external carotid artery was then isolated and coagulated. A 4-0 nylon suture with the tip rounded by a flame was inserted into the internal carotid artery through the external carotid artery stump and gently advanced to occlude the MCA. After 90 min of occlusion, the suture was carefully removed to restore blood flow, the neck incision was closed, and the rats were allowed to recover. Blood was obtained from the tail artery for analysis of glucose level before, during, and after the operation. For the treatment group, H2 was administered for 1 or 2 hr immediately after ischemia via a tank premixed with 2.9% hydrogen, 21% oxygen and 76.1% nitrogen (2.9%, Praxair, CA, USA). The concentration of hydrogen was checked prior to the procedure using a Hydrogen-meter (H2 Scan, CA, USA).
Neurological scores
The neurological deficit was evaluated in a blinded fashion at 24 hr after MCAO using the scoring system reported by Garcia et al. with several modifications (Garcia et al., 1995). The score given to each rat at the completion of the evaluation was the summation of all six individual test scores (Spontaneous Activity; Symmetry in the Movement of Four Limbs; Forepaw Outstretching; Climbing; Body Proprioception and Response to Vibrissae Touch). The minimum neurological score was 3 and the maximum was 18.
2, 3, 5-Triphenyltetrazolium Chloride staining and evaluation of infarction volume
Rats were deeply anesthetized with ketamine at 24 hr after MCAO and perfused transcardially with 0.1 mol/L phosphate buffered solution (PBS) to remove intravascular blood until the outflow fluid from the right atrium was colorless. The brains were rapidly removed and sliced into 2-mm-thick coronal sections with a matrix (Kent Scientific Corporation). We first took pictures of the whole and sliced brain to observe if hemorrhagic transformation was present. Then brain slices were immersed in 2% 2, 3, 5-triphenyltetrazolium chloride (TTC; Sigma) for 30 min at 37°C in the dark (Yin et al., 2003). The infarction (unstained) area and hemisphere area of each section were traced and measured using an Image J analysis system. The possible interference of brain edema with infarct volume was corrected by standard methods (whole contralateral hemisphere volume-non ischemic ipsilateral hemisphere volume) and the infracted volume was expressed as a percentage of the whole contralateral hemisphere (Gao et al., 2006).
Spectrophotometric assay of intracerebral hemorrhage
Cerebral hemorrhage was quantified with a previously described spectrophotometric assay (Choudhri et al., 1997) with some modification. Initially, a standard curve was obtained using a “virtual” model of hemorrhage. Hemispheric brain tissue was obtained from naive rats subjected to complete transcardial perfusion to remove intravascular blood. Incremental volumes of homologous blood (0, 2, 4, 8, 16, 32 μl) were added to each brain tissue sample with phosphate buffered saline (PBS) to reach a total volume of 3 ml, followed by homogenization for 30 sec, sonication on ice for 1 min, and centrifugation at 13,000 rpm for 30 min. Drabkin’s reagent (1.6 ml, Sigma) was added to 0.4 ml supernatant aliquots and allowed to stand for 15 min at room temperature. Optical density was measured and recorded at 540 nm with a spectrophotometer (Spectronix 3000, Milton-Roy, Rochester NY, USA). These procedures yielded a linear relationship between measured hemoglobin concentrations in perfused brain and the volume of added blood. Hemorrhage measurements were performed on brains already stained with TTC for infarct quantitation. It has been shown that TTC staining did not alter the spectrophotometric hemoglobin assay (Asahi et al., 2000).
ELISA for the nitrotyrosine
The amount of nitrotyrosine was measured at 24 hr after MCAO by nitrotyrosine Chemiluminescence Detection Assay Kit following the protocol from the manufacturer (Chemicon 30577). Briefly, 96-well plates were coated with 5 μg/ml nitrated BSA overnight, and blocked with blocking buffer for 2 hr. 50 μl of test or standard samples and 50 μl of 2X Anti-Nitrotyrosine were added to each well, then the plates were incubated at 37°C for 60 min. 100 μl per well of 1X Anti-Rabbit IgG, HRP-conjugate was added and incubated at 37°C for 60 min. After incubating with 75 μl of the freshly prepared LumiGLO® Chemiluminescent Substrate for 10 min, samples were measured with the luminescence as relative light units (RLU). The amount of nitrotyrosine was calculated with the standard curve and the plates were washed between each step.
Western blot
Equal amounts (30 μg) of protein in tissue extracts (same as used for zymography) mixed with loading buffer, were separated by Tris–glycine SDS–PAGE. After separation, proteins were transferred onto a nitrocellulose membrane and blocked with 5% nonfat dry milk in TBS, pH 7.4, containing 0.1% Tween 20 (TBS-T) at 4°C overnight. Then, the membrane was incubated with the primary antibodies: (1) mouse anti-4-Hydroxy-2-Nonenal (HNE) 1:100 (ab48506), (2) mouse anti-nitrotyrosine 1:500 (sc-32757), (3) ZO-1(sc-10804), (4) collagen IV (MAB 1910) (5) Occludin (sc-8145) and (6) AQP4 (sc-28623) diluted in the blocking buffer. After washing with TBS-T, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody at room temperature for 60 min. As for the internal control, the same membrane was probed with an antibody against β-actin (Santa Cruz, 1:1000) after being stripped. Immunoblots were probed with ECL kit reagents (Amersham) and then exposed to x-ray film. The x-ray films were scanned and the optical density was determined with Bio-Rad Image analysis software.
Zymography
Animals were euthanized at 24 hr after MCAO for zymography. Rats were deeply anesthetized and transcardially perfused with PBS through the left ventricle. The ipsilateral brain hemisphere was used to analyze the activity of MMP-2/MMP-9. Briefly, samples were homogenized in lysis buffer (5% 1M Tris-HCl, pH7.4, 15% 1M NaCl, 1% Nonidet P-40, 0.1% SDS and 0.1% deoxycholic acid) including protease inhibitors. After centrifugation, the supernatant was collected and the total protein concentration was determined using the Bradford assay (Bio-Rad). Samples were loaded and separated by 10% Tris-tricine gel with 0.1% gelatin as a substrate. After separation by electrophoresis, the gel was renatured and then incubated with development buffer at 37°C for 48 hr. After development, the gel was stained with 0.5% Coomassie blue R-250 for 30 min and then destained appropriately. The activity of MMP-9/MMP-2 was quantified with Image J software.
Morphological assessment
Rats from each group were used for morphological study at the end of the experiment (24 hr after MCAO). Rats were anesthetized and transcardiacally perfused with PBS and 10% paraformaldehyde as described previously (Yin et al., 2003). Brains were quickly removed and postfixed in 4% paraformaldehyde overnight then immersed in 30% sucrose in PBS until they reached the bottom of the tubes. Coronal tissue sections (10μm) were cut with a cryostat (Leica LM3050S). Sections from each group were divided into several subsets for Nissl staining and immunofluorescence, respectively. For Nissl staining, the sections were hydrated in 1% toluidine blue at 50°C for 20 min. After rinsing with distilled water, they were dehydrated and mounted with Permount. For immunofluorescence staining, series of sections were used for incubation with the following primary polyclonal antibodies: (1) 4-Hydroxy-2-Nonenal (HNE) antibody 1:100 (ab48506) and (2) 8 Hydroxyguanosine (8OHG) antibody 1:200 (ab10802) overnight. The sections were then treated with donkey anti-mouse/goat IgG-fluorescein isothiocyanate (FITC) (green) and observed under OLYMPUS BX51 microscopy. For triple fluorescence labeling, sections were used for triple labeling with mouse anti-HNE 1:100 and goat anti-mouse IgG-fluorescein isothiocyanate (FITC) (green); goat anti-8OHG 1:200 and donkey anti-goat IgG-FITC; TUNEL fluorescence kit (green, fluorescein dUTP and dNTP Kit, Roche Inc.); mouse anti-NeuN (MAB377), mouse anti-nitrotyrosine (sc-32757), rabbit anti-GFAP (AB5804), goat anti-CD34 (sc-7045), rabbit anti-VWF (sc-14014), rabbit anti-Iba-1 (Ionized calcium binding adaptor molecule-1, Iba-1) (Millipore) 1:200 and corresponding IgG-tetramethylrhodamine isothiocyanate (TRITC) (red fluorescein). The nuclei were stained with DAPI (blue fluorescein). Sections were observed under an OLYMPUS BX51 microscope with fluorescence light. The TRITC was excited by dye laser at 557 nm and emitted at 576 nm. The FITC was exited by an argon laser at 490 nm and emitted at 525 nm. DAPI was excited by dye laser at 358 nm and emitted at 461 nm. Merged images were generated by means of Image-Pro-Plus software.
Statistical analysis
Data are presented as means ± SEM. Statistical differences between the various groups were assessed by one-way ANOVA followed by the Tukey or Mann–Whitney rank sum test. Comparisons between the 2 groups for the infarction volume and the neurological scores were assessed by the unpaired t test. A value of P<0.05 was considered statistically significant.
RESULTS
Physiological data
All systemic parameters were within normal range but the glucose levels in all animal groups during surgery were significantly higher than the level at the time point of dextrose injection. Hydrogen gas significantly reduced the blood glucose level 500±32.51 to 366±68.22 mg/dl at 4 hr after dextrose injection that is about 1hr after the hydrogen inhalation (Fig. 2C).
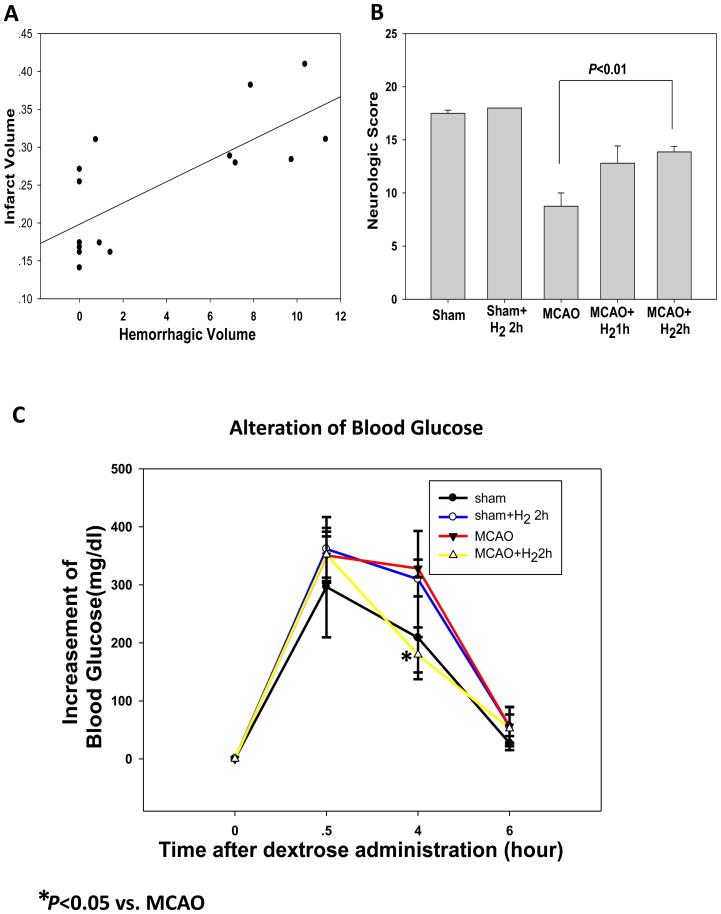
Fig. 2.
(A) The severity of hemorrhage correlated positively with infarct volumes (P<0.05). MCAO: n=8; other groups: n=6. (B) Neurological scores. Grades of 3 to 18 are used. The treatment of hydrogen inhalation for 2hr but not 1hr significantly improved the neurological deficit as compared to MCAO (P<0.05). (C) Alteration of blood glucose in the rats of different groups at different time points after dextrose administration (n=4). The glucose levels in all animal groups during surgery (0.5hr after dextrose administration) were significantly higher than the level at the time point of dextrose injection. Meanwhile the treatment with hydrogen significantly reduced the blood glucose level at 4hr that is about 1hr after the hydrogen inhalation.
Effect of hydrogen on brain infarction, hemorrhagic volume and neurological score
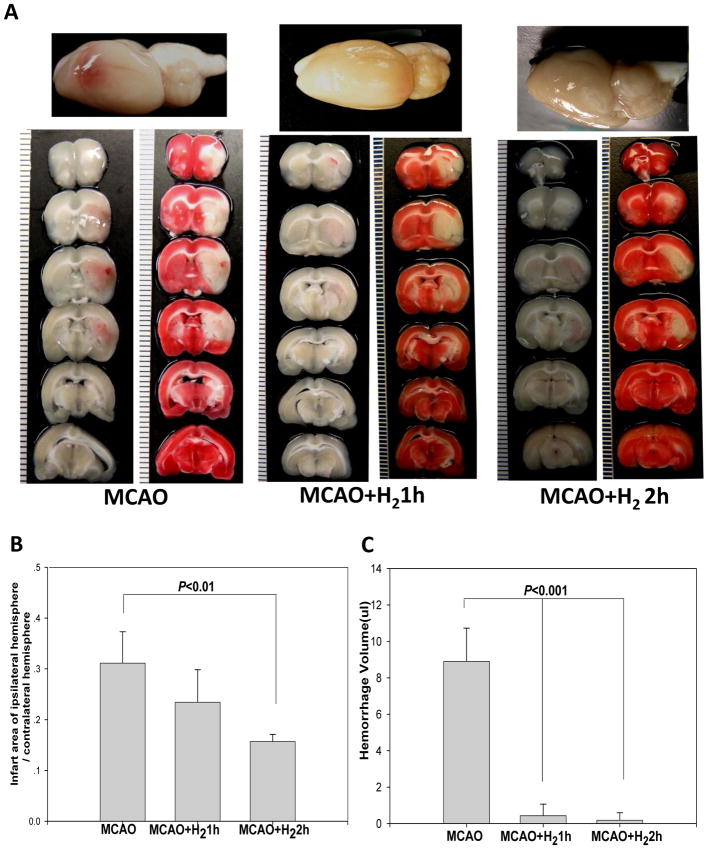
Hyperglycemia induced hemorrhagic transformation in ischemic territories in MCAO rats. Hydrogen inhalation for 2 hr significantly reduced infarct and hemorrhage volumes (from 8.89±1.83 to 0.18±0.41 ul, P<0.001) at 24 hr after reperfusion when compared with MCAO. In addition, 1 hr treatment reduced hemorrhagic volume (from 8.89±1.83 to 0.43±0.63 ul, P<0.001) but failed to reduce infarct volume significantly (Fig. 1). Concomitantly, the severity of hemorrhage seemed to correlate positively with infarct volume (Fig. 2A). Neurological deficits were worsened in the hyperglycemic group when compared with the sham rats. Statistical analysis of neurological scores showed that hydrogen inhalation for 2 hr but not 1 hr significantly ameliorated neurological deficits as compared with MCAO (P<0.05) (Fig. 2B).
Fig. 1.
(A) Whole brain and brain slices with and without TTC staining showed that the hyperglycemia with MCAO induced extensive hemorrhagic transformation and severe infarction at 24hr after the reperfusion, while the treatment with H2 for 1hr or 2hr resulted in only moderate infarction and minor hemorrhagic transformation. (B) Statistic analysis of infarct ratio. Hydrogen inhalation for 2hr significantly reduced infarct volumes at 24hr after reperfusion compared with MCAO (P<0.05). Meanwhile 1hr treatment failed to reduce infarct volume significantly. (C) Statistic analysis of hemorrhagic volumes. Hydrogen inhalation for both 1hr and 2hr significantly reduced hemorrhage volumes at 24hr after reperfusion compared with MCAO (P<0.05). Data represent the mean±SEM. MCAO: n=8; other groups: n=6.
Nitrotyrosine and HNE expression
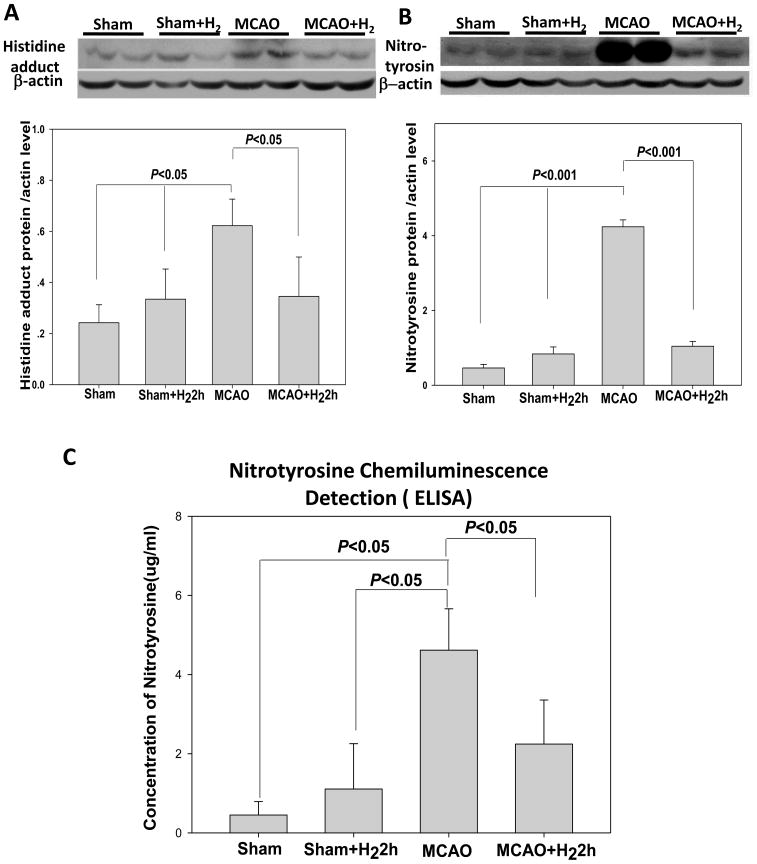
Western Blot analysis of brain tissues from the infarct area showed a significant increase of nitrotyrosine level in the MCAO group at 24 hr after reperfusion when compared to the sham-operated group (P<0.05); this high level of nitrotyrosine was significantly attenuated by hydrogen inhalation (P<0.05 vs. MCAO) although they were still higher than that in the sham group (Fig. 3B). The expression pattern of HNE shown as histidine adduct was similar to that of nitrotyrosine (Fig. 3A).
Fig. 3.
(A) Representative immunoblots of HNE (Histidine adduct) at 24 hr in the ischemic hemisphere (with β-actin as a loading control). Quantification of the western blot analysis showed increased Histidine adduct levels in the MCAO group. Meanwhile hydrogen treatment reduced the protein level (P<0.05, versus MCAO). (B) Representative immunoblots of nitrotyrosine at 24 hr in the ischemic hemisphere (with β-actin as a loading control). Following MCAO, a significant increase of nitrotyrosine protein was detected in MCAO group when compared to sham-operated group. This high level of expression was significantly attenuated by hydrogen treatment. (C) Chemiluminescence detection of nitrotyrosine with ELISA assay (n=6). In MCAO groups, the protein level significantly increased when compared with sham and sham+H2 (P<0.05). However, the elevated expression of nitrotyrosine protein was significantly decreased by hydrogen treatment (P<0.05, vs. MCAO). There were no statistical differences between sham and sham+H2 groups (P>0.05).
ELISA for the nitrotyrosine
The nitrotyrosine assayed by ELISA was shown in Fig. 3C. In the MCAO group, the level (4.62±1.04ug/ml) was significantly increased when compared with the sham (0.45±0.34ug/ml)and sham+H2 (1.11±1.15ug/ml) (P<0.05). However, the elevated expression of nitrotyrosine was significantly decreased by hydrogen treatment (2.24±1.11ug/ml, P<0.05, vs. MCAO, ANOVA). There were no statistical differences between sham and sham+H2 groups (P>0.05).
MMP-2/9 activity
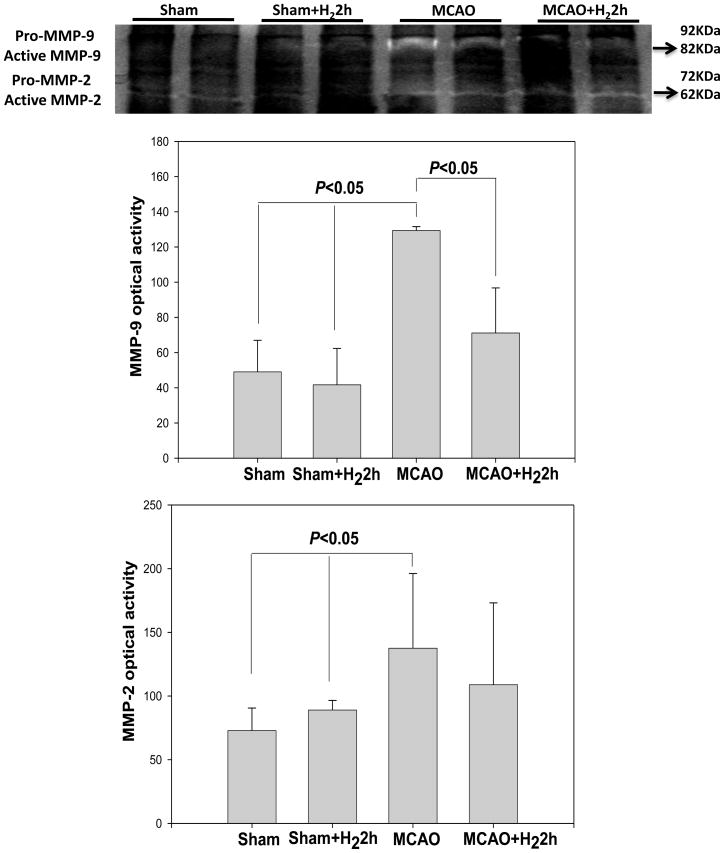
Total MMP-2 and MMP-9 levels were quantified at 24 hr after MCAO (Fig. 4). Densitometric analysis showed an increased expression of activated MMP-2/MMP-9 in the MCAO group. Compared with the MCAO, MMP-9 activity was significantly reduced by hydrogen gas (P<0.05), even though MMP-2 activity was not changed (p>0.05).
Fig. 4.
Gelatin zymography from cerebral cortex in all study groups. Zymograms showed intense of active MMP-9 (82kD) and MMP-2 (62kD) on the ischemic side at 24hr after reperfusion in MCAO group, and only mild expression in the hydrogen treated groups. Densitometric analysis of these bands showed that MMP-9 activity was significantly reduced following the treatment with hydrogen (P<0.05), however the MMP-2 activity was not changed by the treatment significantly (P>0.05). Values are expressed as mean ± SEM of 6 rats per group.
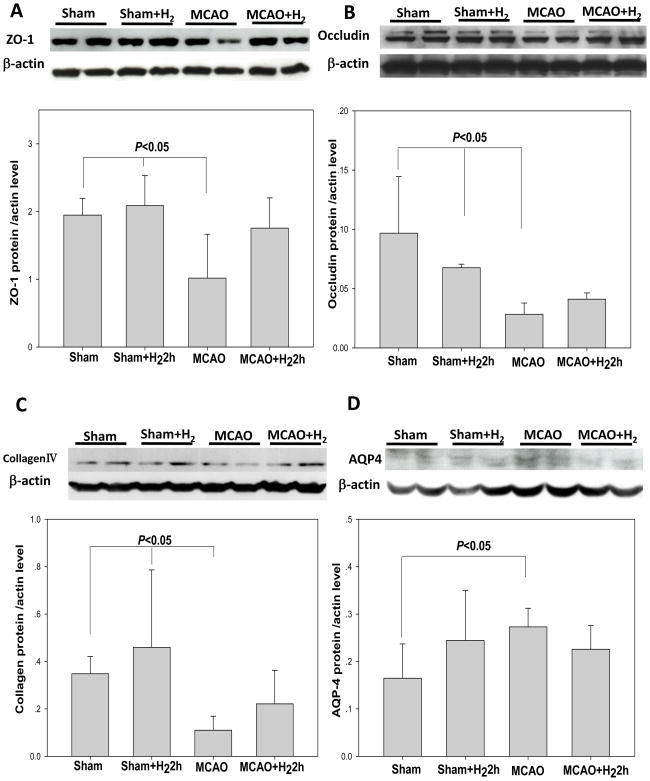
Extracellular matrix molecules and AQP4 expression
Western Blot analysis of brain tissues from the infarct area showed a significant decrease of extracellular matrix molecules such as ZO-1, occluding and collagen IV protein level in the MCAO group at 24 hr after reperfusion when compared to the sham-operated group (P<0.05); however, this level of protein was not significantly elevated by hydrogen inhalation (P>0.05 vs. MCAO) (Fig. 5A, B, C). Following MCAO, a significant increase of AQP4 protein was detected in MCAO group when compared to sham-operated group (P<0.05). This high level of protein expression was not significantly attenuated by hydrogen treatment (P>0.05 vs. MCAO).
Fig. 5.
(A) Representative immunoblots of ZO-1 at 24 hr in the ischemic hemisphere (with β-actin as a loading control). Following MCAO, a significant decrease of ZO-1 protein was detected in MCAO group when compared to sham-operated group. However, the protein expression was not significantly increased by hydrogen treatment. (B) and (C) Representative immunoblots of Occludin and collagen IV at 24 hr in the ischemic hemisphere (with β-actin as a loading control). Quantification of the western blot analysis showed hydrogen treatment did not increase both of the two proteins level (P>0.05, versus MCAO). (D) Representative immunoblots of AQP4 at 24 hr in the ischemic hemisphere (with β-actin as a loading control). Following MCAO, a significant increase of AQP4 protein was detected in MCAO group when compared to sham-operated group. This high level of protein expression was not significantly attenuated by hydrogen treatment (P>0.05). Values are expressed as mean ± SEM of 6 rats per group.
Morphological analysis
Nissl staining
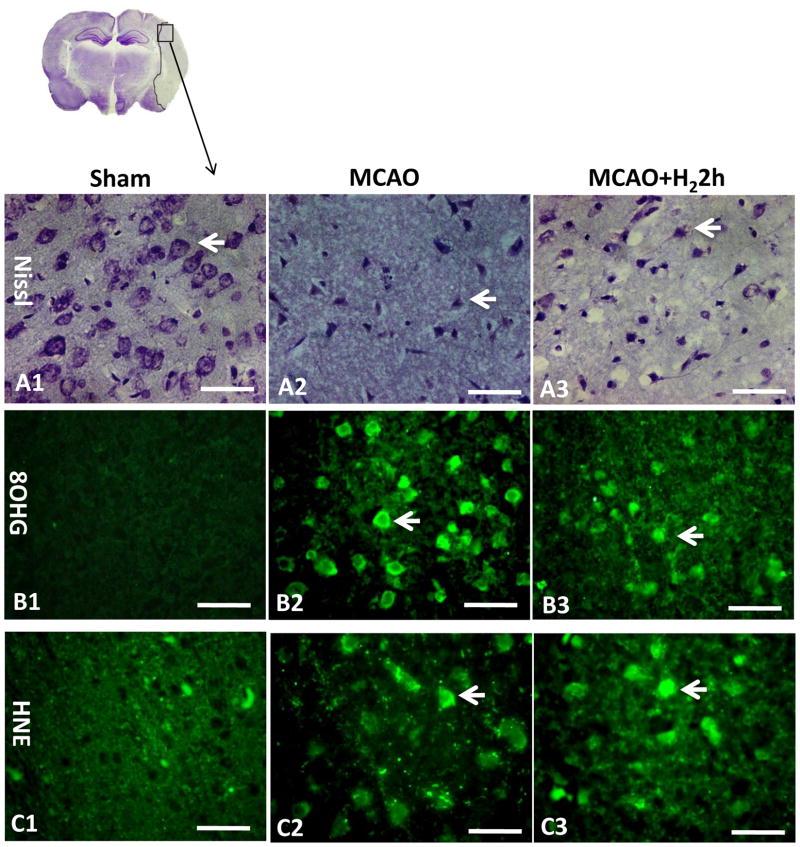
Intact cells (Fig. 6A1) were found in sham operated animals as well as in all non-ischemic regions as evidenced by Nissl staining. After MCAO, cells with aberrant morphology (triangular in shape and dark stained due to condensation of cytoplasm and karyoplasms) were visible in the penumbral and ischemic core area (Fig. 6A2). Hydrogen gas increased the number of surviving cells in the penumbral area (Fig. 6A3).
Fig. 6.
Nissl staining and immunohistochemistry (n=3). Intact neuronal cells could be found in sham operated animals (A1). After severe ischemia, cells with aberrant morphology (triangular in shape exhibiting a dark staining due to condensation of cytoplasm and karyoplasms) were seen in the penumbral region and arrows indicate deformed neurons (A2). Hydrogen treatment group seemed to have more surviving cells in the penumbral area (A3). No immunoreactivities of 8OHG and HNE were observed in the sham-operated rats (B1, C1). Massive immunoreactivity of 8OHG in cells was found in the infarct region at 24 hr after MCAO (B2). However, the extent of 8OHG immunostaining decreased in treated rats (B3). HNE was found robustly expressed in the cells in the MCAO group but only mildly expressed in the treatment group (C2, C3). Arrows indicate cells that are positive for 8OHG and HNE. Scale bars indicated 50μm.
8OHG and HNE immunofluorescence
Immunostaining of 8OHG and HNE at 24 hr after MCAO were shown in Fig. 6B, 6C. Sections from sham-operated rats were negative for 8OHG and HNE staining (Fig. 6B1, C1). Massive immunoreactivity of 8OHG in cells was found in the infarction region at 24 hr after MCAO (Fig. 6B2). The level of 8OHG immunostaining decreased in hydrogen treated rats (Fig. 6B3). HNE in the neurons was highly expressed in the MCAO group and mildly expressed in hydrogen treated group (Fig. 6C2, C3).
Triple immunofluorescence staining
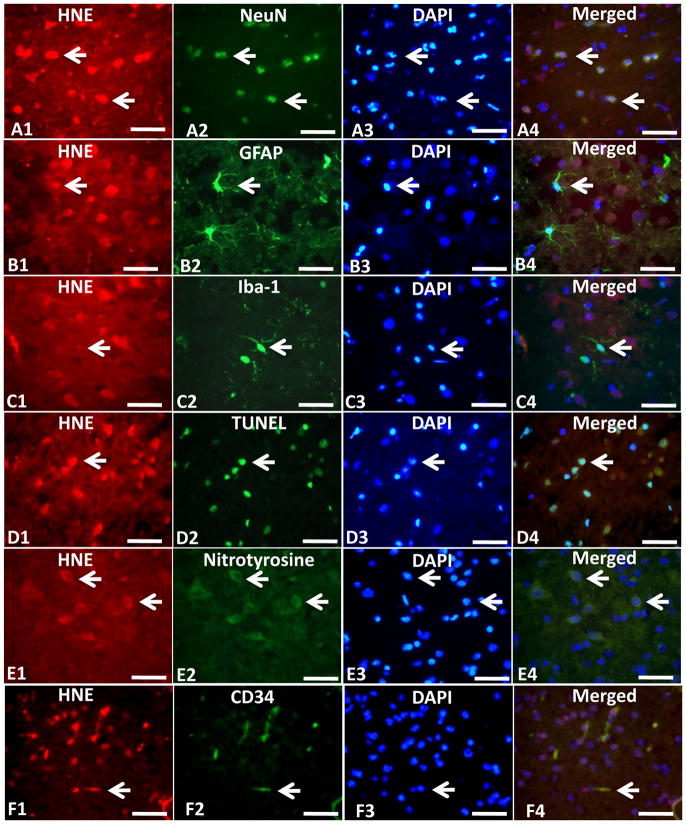
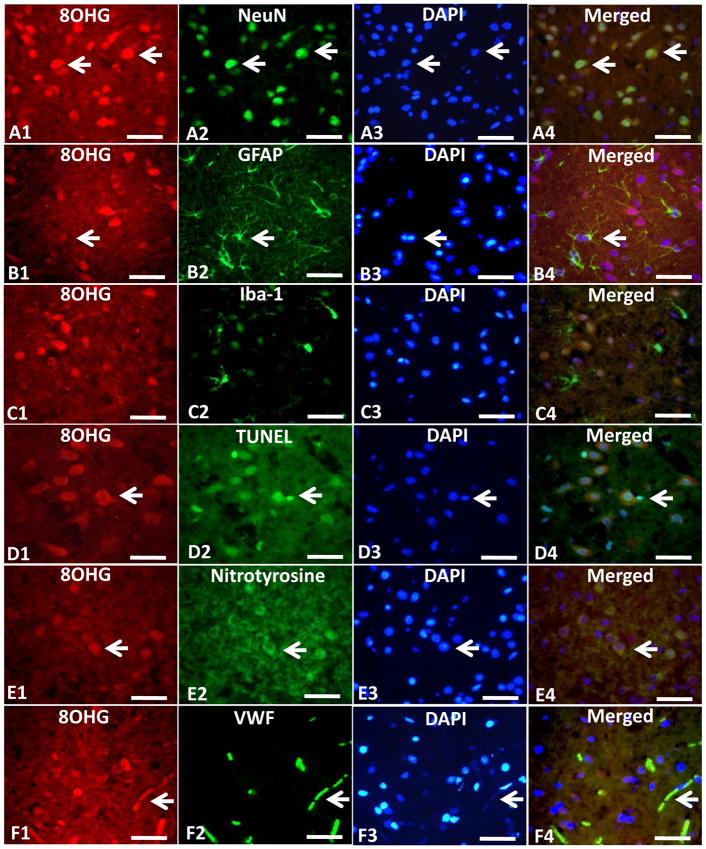
The HNE and 8OHG positive cells in ipsilateral ischemic hemisphere in MCAO group were expressed by TRITC (Fig. 7A1, B1, C1, Fig. 8A1, B1, C1). The NeuN, GFAP, and Iba-1 positive cells showing the positive neurons, astrocytes and microglia were expressed by green fluorescence (Fig. 7A2, B2, C2, Fig. 8A2, B2, C2), and the Nuclei of cells in the same section were expressed by blue fluorescence from DAPI staining (Fig. 7A3, B3, C3, Fig. 8A3, B3, C3). Color merging showed that HNE and 8OHG positive cells were co-localized mostly with neurons but rarely with astrocytes or microglia (Fig. 7A4, B4, C4, Fig. 8A4, B4, C4). The TUNEL positive cells were in the ischemic hemisphere (Fig. 7D2, Fig. 8D2) which also showed the positive expression of HNE and 8OHG when merged together (Fig. 7D4, Fig. 8D4). The HNE and 8OHG positive neurons also expressed the protein of nitrotyrosine which showed co-localization (Fig. 7E4, Fig. 8E4). CD34 and VWF (Fig. 7F2, Fig. 8F2) showed endothelium in the ischemic zone and those CD34 and VWF positive endothelial cells co-expressed HNE and 8OHG (Fig. 7F4, Fig. 8F4).
Fig. 7.
Triple immunofluorescence staining (n=3). Triple immunofluorescence staining showed the co-localization of HNE (A1, B1 and C1) with NeuN (A2) but rarely with astrocytes shown by GFAP (B2) or microglia shown by Iba-1(C2). HNE (D1, E1 and F1) also co-localized with TUNEL (D2) nitrotyrosine (E2) or vascular endothelial shown by CD34 (F2) in the cerebral cortex at 24hr after middle cerebral artery occlusion (MCAO). HNE (red) was expressed by tetramethylrhodamine isothiocyanate(TRITC). NeuN, GFAP, Iba-1, Nitrotyrosine and CD34 (green) were expressed by fluorescein isothiocyanate(FITC). The apoptosis is marked by the terminal deoxynucleotidyl transferase (TdT)-mediated biotinylated UTP nick end-labeling (TUNEL) assay (green). Scale bars: 50um. Small white arrows indicated examples of the positive cells.
Fig. 8.
Triple immunofluorescence staining (n=3). Triple immunofluorescence staining showed the co-localization of 8OHG (A1, B1 and C1) with NeuN (A2) but rarely with astrocytes shown by GFAP (B2) or microglia shown by Iba-1(C2). 8OHG (D1, E1 and F1) also co-localized with TUNEL (D2), nitrotyrosine (E2) or vascular endothelial shown by VWF (F2) in the cerebral cortex at 24 hr after middle cerebral artery occlusion (MCAO). 8OHG (red) was expressed by tetramethylrhodamine isothiocyanate (TRITC). NeuN, GFAP, Iba-1, Nitrotyrosine and VWF (green) were expressed by fluorescein isothiocyanate (FITC). The apoptosis was marked by the terminal deoxynucleotidyl transferase (TdT)-mediated biotinylated UTP nick end-labeling (TUNEL) assay (green). Scale bars: 50um. Small white arrows indicated examples of the positive cells.
DISCUSSION
This study expanded the previous observation that hydrogen not only reduced brain infarction after MCAO in rats (Ohsawa et al., 2007) but also decreased hyperglycemia-enhanced hemorrhagic transformation after MCAO. In addition, we used two treatment protocols and we observed an interesting result that hydrogen gas applied for 1 hr after MCAO reduced only hemorrhagic transformation but not brain infarction, indicating hydrogen gas might directly influence hemorrhagic transformation, independent of reduction of infarction. Since brain infarction is correlated to neurological function, it was within expectation that hydrogen gas reduced brain infarction and improved neurological function. One unexpected result from this study is that hydrogen reduced, even though only transiently at 4 hr after ischemia, glucose levels and this observation may indicate other mechanisms may be involved in the neuroprotection by hydrogen in addition to the existing mechanisms that hydrogen reduced DNA damage and lipid peroxidation which were also shown by this study from 8OHG and HNE experiments. We studied hyperglycemia-induced hemorrhagic conversion in this ischemic model for two main reasons (Qin et al., 2007). First, because hyperglycemia is strongly associated with hemorrhagic transformation and tPA-associated cerebral hemorrhage, therefore this model is clinically relevant. The second reason is the model produces a consistent hemorrhagic transformation after MCAO with reperfusion. The alternative embolic model with tPA-induced reperfusion is less consistent.
There are a couple of gases that are used as therapies such as nitric oxide (NO), carbon monoxide (CO), and hydrogen sulfate (H2S) (Elrod et al., 2007; Kobayashi et al., 2007; Foresti et al., 2008). Hydrogen reacts with oxidizing elements such as chlorine, fluorine, and hydroxyl radical (Labiche and Grotta, 2004), which may explain its anti-oxidative effect after ischemic brain injury (Ohsawa et al., 2007). Inhalation of hydrogen gas has been reported to reduce infarct size in the rat models of myocardial, intestinal and hepatic ischemia/reperfusion injuries (Fukuda et al., 2007; Hayashida et al., 2008; Zheng et al., 2009). In all of these injuries, hydroxyl radicals and peroxynitrite are dominant reactive oxygen species which indiscriminately interact with nucleic acids, lipids and proteins to cause DNA fragmentation, lipid oxidation, and protein nitrification (Valko et al., 2007). Unfortunately, there is no endogenous detoxification system for hydroxyl radicals and peroxynitrite in human body (Ohsawa et al., 2007). Hydrogen gas selectively scavenges hydroxyl radical may offer a unique cytoprotective pathway after ischemic and reperfusion injuries (Wood and Gladwin, 2007).
Hemorrhagic transformation in this study may be broadly related to deleterious events during reperfusion injury with hyperglycemia. Historically, reperfusion injury has been associated with the generation of oxygen-derived free radicals that damage proteins and lipids in cell membranes, thus amplifying cell death. Also, hyperglycemia may increase reactive oxygen species (ROS), elicit ROS-dependent signal transduction events (Li et al., 1999; Bonnefont-Rousselot, 2002) and exaggerate acidosis occurring shortly after the ischemic insult, which may increase neuronal and glial damage (Hoxworth et al., 1999). The effect of hydrogen gas after ischemic and reperfusion injuries may be related to the scavenging of hydroxyl radicals. In this study, we examined hydrogen-mediated molecular changes at 24 hr after ischemia, by staining brain sections with antibodies to 8OHG in order to assess the extent of nucleic acid oxidation, and with antibodies to HNE to assess lipid peroxidation. For both of these oxidative markers, staining was substantially reduced in hydrogen treated rats as compared to MCAO rats (Fig. 6B, C). We also detect the amount of HNE and nitrotyrosine, which are markers of lipid peroxidation and protein nitrification respectively (Fig. 3) and found a distinct hydrogen-dependent decrease of expression. Taken together, we observed similar results that hydrogen gas reduced oxidative injuries and that may be responsible at least partially for the reduction of hemorrhagic transformation. What’s more, from the observation of triple staining, we found the co-localization of 8OHG and HNE with the endothelia in the ischemic zone which suggested the DNA damage and lipid peroxidation from the ischemia with acute hyperglycemia. So we presumed that the reduction of hemorrhagic transformation may come from the protection of endothelia which is the component of the Blood Brain Barrier and important for formation of brain edema. And the reduction of these oxidative agents might contribute to the endothelia as well as the neurons and glia.
Matrix metalloproteinases (MMPs), especially gelatinases (MMP-2 and 9), are up-regulated in cerebral ischemia and closely associated with blood-brain barrier (BBB) disruption (Rosenberg et al., 1998), edema formation (Pfefferkorn and Rosenberg, 2003), and hemorrhagic transformation (Sumii and Lo, 2002). In an acute cerebral ischemia, BBB disruption, associated with upregulated expression of MMP-2 and MMP-9, may exacerbate neuronal damage (Copin et al., 2005; Yang et al., 2007) and hemorrhagic transformation following treatment with recombinant tissue plasminogen activator (Wang and Lo, 2003). In this study, we found that hydrogen treatment decreased the upregulation of active MMP-9 which may be related to the protection of BBB and the reduction of hemorrhagic conversion in the present study. The decrease in blood brain barrier breakdown in turn results in decreased cerebral infarction, hemorrhagic transformation, as well as improved functional outcome. Although activated MMP-9 could directly damage neurovascular substrates such as the tight junction proteins (ZO-1, claudin-5, occcludin), and the basal lamina (collagen IV, laminin, and fibronectin) and led to a breakdown of the BBB with ultimate hemorrhage and edema (Asahi et al., 2001; Rosenberg and Yang, 2007). However, in this study we did not find a significant increase of the expression of ZO-1, occluding or collagen IV compared with MCAO after hydrogen treatment even though there was a tendency of elevation of these proteins. This may suggest that the inhibition of MMP-9 activity is not sufficient for the persistent increase of some of the extracellular matrix molecules. The important role that MMP-9 plays in the development of brain hemorrhage may be related to the hyper-stimulation of other target genes such as VEGF as reported by others (Hu et al., 2009). The data we obtained in this study suggested that the effect of hydrogen on extracellular matrix molecules may act synergistically with other agent to play a disruptive role in BBB integrity, although the molecules alone do not achieve statistical significance after treatment. Therefore, the potent effect of hydrogen against hemorrhagic transformation may be via different molecular pathways besides the MMP-9 inhibition that needs further investigation.
Aquaporin4 (AQP4) is the predominant form of AQP enriched in the CNS (Jung et al., 1994). It is constitutively expressed in astrocyte foot processes near capillaries and in ependymal cells where it regulates brain water homeostasis by acting as a key constituent of the blood–brain barrier (BBB) and the blood–CSF barrier (Nielsen et al., 1997). In hypoxia and ischemia, a previous study has shown that disruption of BBB and increased permeability of blood vessels was linked to up-regulation of AQP4 in brain (Kaur et al., 2006). However, in this study, the decreased hemorrhage transformation seemed not come from the adjustment of AQP4 expression through the observation of western blot which showed no significant decline of AQP4 after the treatment of hydrogen. So we speculated that the increased permeability or rupture of brain vessels may be the result of different injuries via multiple pathways.
As mentioned above, we have observed a unique phenomenon that hydrogen gas inhalation reduced blood glucose levels at 4 hr after MCAO (about 1 hr after the hydrogen inhalation). We do not know the mechanisms for this observation. In literature, there was a report that scavenging of reactive oxygen species exerted beneficial effects in diabetic mice by preservation of β-cell function (Kaneto et al., 1999). In addition, intake of hydrogen-rich water normalized oral glucose tolerance test in some patients with impaired glucose tolerance (Kajiyama et al., 2008). However, more evidences are needed to support the acute mechanistic action of hydrogen gas on blood glucose levels.
In conclusion, hydrogen gas appeared to be a promising therapy to reduce hemorrhagic transformation after ischemia-reperfusion injury in rats. However, the action of hydrogen may be multifold involving anti-oxidative stress, matrix proteins, and glucose levels. The exact mechanisms and signaling pathways involved in the protective effect of hydrogen in cerebral ischemia/reperfusion injury accompanied with acute hyperglycemia need to be elucidated in the future.
Acknowledgments
The authors thank Mr. Matt Peterson for the excellent English editorial assistance.
This study was partially supported by grants from NIH NS43338 and NS53407 to J.H.Z
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asahi M, Asahi K, Wang X, Lo EH. Reduction of tissue plasminogen activator-induced hemorrhage and brain injury by free radical spin trapping after embolic focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2000;20:452–457. doi: 10.1097/00004647-200003000-00002. [DOI] [PubMed] [Google Scholar]
- Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefont-Rousselot D. Glucose and reactive oxygen species. Curr Opin Clin Nutr Metab Care. 2002;5:561–568. doi: 10.1097/00075197-200209000-00016. [DOI] [PubMed] [Google Scholar]
- Bruno A, Biller J, Adams HP, Jr, Clarke WR, Woolson RF, Williams LS, Hansen MD. Acute blood glucose level and outcome from ischemic stroke. Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators. Neurology. 1999;52:280–284. doi: 10.1212/wnl.52.2.280. [DOI] [PubMed] [Google Scholar]
- Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32:2426–2432. doi: 10.1161/hs1001.096194. [DOI] [PubMed] [Google Scholar]
- Choudhri TF, Hoh BL, Solomon RA, Connolly ES, Jr, Pinsky DJ. Use of a spectrophotometric hemoglobin assay to objectively quantify intracerebral hemorrhage in mice. Stroke. 1997;28:2296–2302. doi: 10.1161/01.str.28.11.2296. [DOI] [PubMed] [Google Scholar]
- Copin JC, Goodyear MC, Gidday JM, Shah AR, Gascon E, Dayer A, Morel DM, Gasche Y. Role of matrix metalloproteinases in apoptosis after transient focal cerebral ischemia in rats and mice. Eur J Neurosci. 2005;22:1597–1608. doi: 10.1111/j.1460-9568.2005.04367.x. [DOI] [PubMed] [Google Scholar]
- Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci U S A. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresti R, Bani-Hani MG, Motterlini R. Use of carbon monoxide as a therapeutic agent: promises and challenges. Intensive Care Med. 2008;34:649–658. doi: 10.1007/s00134-008-1011-1. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Asoh S, Ishikawa M, Yamamoto Y, Ohsawa I, Ohta S. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem Biophys Res Commun. 2007;361:670–674. doi: 10.1016/j.bbrc.2007.07.088. [DOI] [PubMed] [Google Scholar]
- Gao D, Zhang X, Jiang X, Peng Y, Huang W, Cheng G, Song L. Resveratrol reduces the elevated level of MMP-9 induced by cerebral ischemia-reperfusion in mice. Life Sci. 2006;78:2564–2570. doi: 10.1016/j.lfs.2005.10.030. [DOI] [PubMed] [Google Scholar]
- Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation Stroke. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- Hayashida K, Sano M, Ohsawa I, Shinmura K, Tamaki K, Kimura K, Endo J, Katayama T, Kawamura A, Kohsaka S, Makino S, Ohta S, Ogawa S, Fukuda K. Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochem Biophys Res Commun. 2008;373:30–35. doi: 10.1016/j.bbrc.2008.05.165. [DOI] [PubMed] [Google Scholar]
- Hoxworth JM, Xu K, Zhou Y, Lust WD, LaManna JC. Cerebral metabolic profile, selective neuron loss, and survival of acute and chronic hyperglycemic rats following cardiac arrest and resuscitation. Brain Res. 1999;821:467–479. doi: 10.1016/s0006-8993(98)01332-8. [DOI] [PubMed] [Google Scholar]
- Hu Q, Chen C, Yan J, Yang X, Shi X, Zhao J, Lei J, Yang L, Wang K, Chen L, Huang H, Han J, Zhang JH, Zhou C. Therapeutic application of gene silencing MMP-9 in a middle cerebral artery occlusion-induced focal ischemia rat model. Exp Neurol. 2009;216:35–46. doi: 10.1016/j.expneurol.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Jung JS, Bhat RV, Preston GM, Guggino WB, Baraban JM, Agre P. Molecular characterization of an aquaporin cDNA from brain: candidate osmoreceptor and regulator of water balance. Proc Natl Acad Sci U S A. 1994;91:13052–13056. doi: 10.1073/pnas.91.26.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiyama S, Hasegawa G, Asano M, Hosoda H, Fukui M, Nakamura N, Kitawaki J, Imai S, Nakano K, Ohta M, Adachi T, Obayashi H, Yoshikawa T. Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr Res. 2008;28:137–143. doi: 10.1016/j.nutres.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Kaneto H, Kajimoto Y, Miyagawa J, Matsuoka T, Fujitani Y, Umayahara Y, Hanafusa T, Matsuzawa Y, Yamasaki Y, Hori M. Beneficial effects of antioxidants in diabetes: possible protection of pancreatic beta-cells against glucose toxicity. Diabetes. 1999;48:2398–2406. doi: 10.2337/diabetes.48.12.2398. [DOI] [PubMed] [Google Scholar]
- Kaur C, Sivakumar V, Zhang Y, Ling EA. Hypoxia-induced astrocytic reaction and increased vascular permeability in the rat cerebellum. Glia. 2006;54:826–839. doi: 10.1002/glia.20420. [DOI] [PubMed] [Google Scholar]
- Kawamura S, Yasui N, Shirasawa M, Fukasawa H. Rat middle cerebral artery occlusion using an intraluminal thread technique. Acta Neurochir (Wien) 1991;109:126–132. doi: 10.1007/BF01403007. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Ishikawa K, Matsumoto H, Kimura S, Kamiyama Y, Maruyama Y. Synergetic antioxidant and vasodilatory action of carbon monoxide in angiotensin II - induced cardiac hypertrophy. Hypertension. 2007;50:1040–1048. doi: 10.1161/HYPERTENSIONAHA.107.097006. [DOI] [PubMed] [Google Scholar]
- Labiche LA, Grotta JC. Clinical trials for cytoprotection in stroke. NeuroRx. 2004;1:46–70. doi: 10.1602/neurorx.1.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li PA, Liu GJ, He QP, Floyd RA, Siesjo BK. Production of hydroxyl free radical by brain tissues in hyperglycemic rats subjected to transient forebrain ischemia. Free Radic Biol Med. 1999;27:1033–1040. doi: 10.1016/s0891-5849(99)00152-5. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Nagelhus EA, miry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17:171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- Orso F, Baldasseroni S, Maggioni AP. The role of thrombolysis in acute ischemic stroke. Herz. 2008;33:498–506. doi: 10.1007/s00059-008-3154-9. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn T, Rosenberg GA. Closure of the blood-brain barrier by matrix metalloproteinase inhibition reduces rtPA-mediated mortality in cerebral ischemia with delayed reperfusion. Stroke. 2003;34:2025–2030. doi: 10.1161/01.STR.0000083051.93319.28. [DOI] [PubMed] [Google Scholar]
- Pulsinelli WA, Waldman S, Rawlinson D, Plum F. Moderate hyperglycemia augments ischemic brain damage: a neuropathologic study in the rat. Neurology. 1982;32:1239–1246. doi: 10.1212/wnl.32.11.1239. [DOI] [PubMed] [Google Scholar]
- Qin Z, Karabiyikoglu M, Hua Y, Silbergleit R, He Y, Keep RF, Xi G. Hyperbaric oxygen-induced attenuation of hemorrhagic transformation after experimental focal transient cerebral ischemia. Stroke. 2007;38:1362–1367. doi: 10.1161/01.STR.0000259660.62865.eb. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Estrada EY, Dencoff JE. Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke. 1998;29:2189–2195. doi: 10.1161/01.str.29.10.2189. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Yang Y. Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia. Neurosurg Focus. 2007;22:E4. doi: 10.3171/foc.2007.22.5.5. [DOI] [PubMed] [Google Scholar]
- Sumii T, Lo EH. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke. 2002;33:831–836. doi: 10.1161/hs0302.104542. [DOI] [PubMed] [Google Scholar]
- Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Wang X, Lo EH. Triggers and mediators of hemorrhagic transformation in cerebral ischemia. Mol Neurobiol. 2003;28:229–244. doi: 10.1385/MN:28:3:229. [DOI] [PubMed] [Google Scholar]
- Wood KC, Gladwin MT. The hydrogen highway to reperfusion therapy. Nat Med. 2007;13:673–674. doi: 10.1038/nm0607-673. [DOI] [PubMed] [Google Scholar]
- Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- Yin D, Zhou C, Kusaka I, Calvert JW, Parent AD, Nanda A, Zhang JH. Inhibition of apoptosis by hyperbaric oxygen in a rat focal cerebral ischemic model. J Cereb Blood Flow Metab. 2003;23:855–864. doi: 10.1097/01.WCB.0000073946.29308.55. [DOI] [PubMed] [Google Scholar]
- Yip PK, He YY, Hsu CY, Garg N, Marangos P, Hogan EL. Effect of plasma glucose on infarct size in focal cerebral ischemia-reperfusion. Neurology. 1991;41:899–905. doi: 10.1212/wnl.41.6.899. [DOI] [PubMed] [Google Scholar]
- Zheng X, Mao Y, Cai J, Li Y, Liu W, Sun P, Zhang JH, Sun X, Yuan H. Hydrogen-rich saline protects against intestinal ischemia/reperfusion injury in rats. Free Radic Res. 2009;43:478–484. doi: 10.1080/10715760902870603. [DOI] [PubMed] [Google Scholar]