Abstract
Aims
Clinical studies demonstrate attenuation of trigeminal-related pain states such as migraine by intranasal CO2 application. This study investigated the underlying mechanisms of this observation and its potential use to reverse trigeminal pain and hypersensitivity.
Main methods
We used a behavioral rat model of capsaicin-induced trigeminal thermal hyperalgesia, intranasal CO2 application and several pharmacologic agents such as carbonic anhydrase, acid-sensing ion channels (ASICs), and TRPV1 blocker as well as acidic buffer solutions to investigate and mimick the underlying mechanism.
Key findings
Intranasal CO2 application produced a robust dose-dependent antihyperalgesic effect in rats that lasted at least one hour. Blockade of nasal carbonic anhydrase with a dorzolamide solution (Trusopt® ophthalmic solution) showed only a non-significant decrease of the antihyperalgesic effect of intranasal CO2 application. Pharmacologic blockade of ASICs or TRPV1 receptor significantly attenuated the antihyperalgesic effect of CO2 application. The effect of intranasal CO2 application could be mimicked by application of pH4, but not pH 5, buffer solution to the nasal mucosa. As with CO2 application, the antihyperalgesic effect of intranasal pH4 buffer was blocked by nasal application of antagonists to ASICs and TRPV1 receptors.
Significance
Our results indicate that intranasal CO2 application results in a subsequent attenuation of trigeminal nociception, mediated by protonic activation of TRPV1 and ASIC channels. A potential central mechanism for this attenuation is discussed. The antihyperalgesic effects of intranasal CO2 application might be useful for the treatment of trigeminal pain states.
Keywords: trigeminal, pain, hyperalgesia, hypersensitivity, capsaicin, TRPV1, ASIC, carbon dioxide
Introduction
Trigeminal nerve-mediated pain has a major impact on human health, and significantly debilitates the functioning of the afflicted (Tölle T et al. 2006; Bigal M et al. 2007). Conditions such as migraine headache, trigeminal neuralgia, non-migraine headache and dental pain reduce well-being and negatively affect productivity and the economy. Unfortunately, many pharmacological approaches to alleviate trigeminal pain are rife with harmful side effects and/or risks of addiction, necessitating a continued search for other methods of pain relief.
One treatment for trigeminal pain under development is the nasal application of carbon dioxide (CO2). There is evidence that CO2 can decrease the release of pain-related neurotransmitters from cultured sensory neurons (Vause C et al. 2007). In addition, clinical observations have found intranasal CO2 to alleviate headache by an unknown mechanism (Spierings EL 2005). On the other hand, intranasal CO2 is known to produce discharges in rat trigeminal afferent (Thuerauf N et al. 1991) as well as medullary neurons (Anton F et al. 1991) and a stinging nasal pain sensation in human subjects (Hummel T et al. 2000; Hummel T et al. 2003), which has led to its use as a noxious stimulus in psychophysical pain studies (Hummel T et al. 2003).
Previous studies have observed the production of a widespread analgesic effect by activation of nociceptive afferents (Cui JG et al. 1997; Sandkuhler J 2000). For example, in a recent study using a herpes-mediated GABAb receptor knockdown, we found that repeated activation of Aδ-thermonociceptors in the sciatic nerve of rats produced hypoalgesia for responses mediated by activation of saphenous nociceptors, an effect mediated by activation of inhibitory spinal interneurons (Jones TL et al. 2005). Thus, it is possible that the apparent trigeminal antinociceptive effect of nasal CO2 on migraine headache and other trigeminal pain might be mediated through a similar mechanism of widespread nociceptive inhibition evoked by afferent activation.
Therefore, we investigated whether nasal CO2 would attenuate nociceptive behavioral responses to stimuli applied to the trigeminally-innervated facial skin of rats.
Methods
Animals
Male Sprague-Dawley rats (200 to 300 g, Charles River) were housed in a 12-h light: 12-h dark environment and provided food and water ad libitum. Effort was made to minimize discomfort and to reduce the number of animals used. At the end of each experiment, animals were euthanized by CO2 asphyxiation. The protocol of this study was approved by the Stanford University Institutional Animal Care and Use Committee.
Heat withdrawal test
Animals were lightly anesthetized with urethane (1000 mg/kg ip). It has been shown in previous studies that light urethane anesthesia (800–1000 mg/kg ip) does not alter withdrawal latencies to noxious thermal stimuli administered to paws (Yeomans DC et al. 1996; Zachariou V et al. 1997) or areas innervated by the trigeminal nerve (Tzabazis A et al. 2004). The experimenter performing the behavioral tests was blinded to the rat treatment group assignment. Thirty minutes after urethane administration, the animals’ faces were shaved closely with a hair clipper without causing damage to the skin and blackened with India ink applied with a single-use cotton swab. All animals were tested for baseline withdrawal latency using a heat lamp set at a C thermonociceptor selective intensity of 190 mW/cm2 (Yeomans DC et al. 1996; Yeomans DC and Proudfit HK 1996). The mean latency to response to noxious heat (facial withdrawal or flinching) was calculated as the average of four trials’ response time in seconds. Maximum (cut-off) latency was set at 20 s to avoid tissue damage to the face.
Capsaicin sensitization and CO2 administration
After baseline latencies were obtained, one cheek of the animals was sensitized with two 20 μl applications, administered 20 minutes apart, of 15 mM synthetic capsaicin (Sigma-Aldrich, St. Louis, MO, USA) applied and spread with a pipette tip. Twenty minutes after the second application, the animals were tested again for response latencies to heat. CO2 delivered from a cylinder containing 100% CO2 and was administered using an adjustable pressure reducer via a single-use 14G intravenous catheter (BD Insyte™ Autoguard™, Becton, Dickinson Infusion Therapy systems Inc., Sandy, USA) inserted approximately 1 mm into one nare. The rats were then tested immediately, and at 30 and 60 minutes after CO2 administration (n = 9). Three groups of animals were treated with CO2 at a flow rate of 0.8 l/min for 20 s, 0.8l/min for 40 s, and 0.4 l/min for 40 s (n = 9 per group), respectively. For control groups, some rats received compressed air at a flow rate of 0.8 l/min for 40 s (n = 9); another group was treated with saline applied to the skin and spread using a pipette tip and received CO2 at a flow rate of 0.8 l/min for 40 s (n = 9). As a third and fourth control group animals treated with saline but no CO2 or sensitized with capsaicin without intranasal CO2 (n=9), respectively, were tested at time points to match time points of the groups mentioned above. Hind paw withdrawal latencies were tested in two additional groups after capsaicin sensitization of the cheek and with or without intranasal administration of CO2 at a flow rate of 0.8l/min for 40 s.
Pharmacological experiments
To investigate potential underlying mechanisms of CO2 antihyperalgesia, capsaicin sensitized animals were pretreated with 50 μl of a 55.4 mM solution of the clinically available carbonic anhydrase blocker dorzolamide (Trusopt®, MSD Inc, White House Station, NJ, USA). Fifteen minutes later, rats were administered CO2 at a flow rate of 0.8 l/min for 40 seconds (n = 6). Dorzolamide has been used to block carbonic anhydrase in cultured retinal neural cells (Kniep EM et al. 2006) as well as in mice after topical application on the retina (Akaishi T et al. 2009).
A second group (n = 6) was nasally pretreated with 50 μl of 2 mM solution of the non-specific acid sensing channel (ASICs) blocker amiloride (Sigma-Aldrich, St. Louis, MO, USA) in normal saline applied topically to the nostril. Amiloride has been shown to dose-dependently block ASICs in neurons (Leffler A et al. 2006, Zhang M et al. 2008). Fifteen minutes after applying amiloride, rats were administered CO2 nasally at a flow rate of 0.8 l/min for 40 seconds (n = 6).
A third group (n = 6) was nasally pretreated with 50 μl of a 5 mM solution of the TRPV1 channel blocker capsazepine (Sigma-Aldrich, St. Louis, MO, USA) in 10% tween 20 (Bio-Rad, Hercules, CA, USA), 10% ethanol (EMD chemicals, USA) and 80% normal saline. Capsazepine has been shown to be highly selective in blocking TRPV1 with low affinity for other channels (Seabrook GR et al. 2002, Leffler A et. al. 2006). Fifteen minutes after applying capsazepine, rats were nasally administered CO2 at a flow rate of 0.8 l/min for 40 seconds (n = 6). Appropriate control groups (n=6), i. e. treated with the amiloride and capsazepine vehicle, respectively, were also tested. Since we used a commercially available formulation of dorzolamide in an a proprietary aqueous solution with inactive ingredients such as hydroxyethyl cellulose, mannitol, sodium citrate dihydrate, and sodium hydroxide in unknown amounts (Trusopt®), we could not include a group treated with the appropriate dorzolamide vehicle. A fourth group (n = 6) was nasally pretreated with 50 μl of amiloride and 50 μl of capsazepine, then administered CO2 at a flow rate of 0.8 l/min for 40 seconds (n = 6). To investigate the possibility that CO2 induced antihyperalgesia was mediated by acidification of the nasal mucosa, additional groups (n = 6 each) were administered four 6 μl applications of pH 4 and pH 5 buffer, respectively, per nostril, 2 minutes apart. This application method was found to be the most effective application method in preliminary studies. Effects of cheek withdrawal latencies were then tested immediately, and at 30 and 60 minutes after the fourth buffer administration. As a control, other groups (n = 6 each) were pretreated with amiloride or capsazepine, respectively, as described above before intranasal administration of pH 4 buffer and tested in identical manner.
Statistics
Data is presented as mean ± SEM. Behavioral data was analyzed for significance by two-way ANOVA with repeated measures followed by a post-hoc Bonferroni analysis. P-values lower than 0.05 were considered significant. All statistical analysis were performed using GraphPad Prism version 5.0 for Mac OS X (GraphPad Software, San Diego California USA, http://www.graphpad.com).
Results
Effect of CO2 and air on capsaicin sensitized cheek
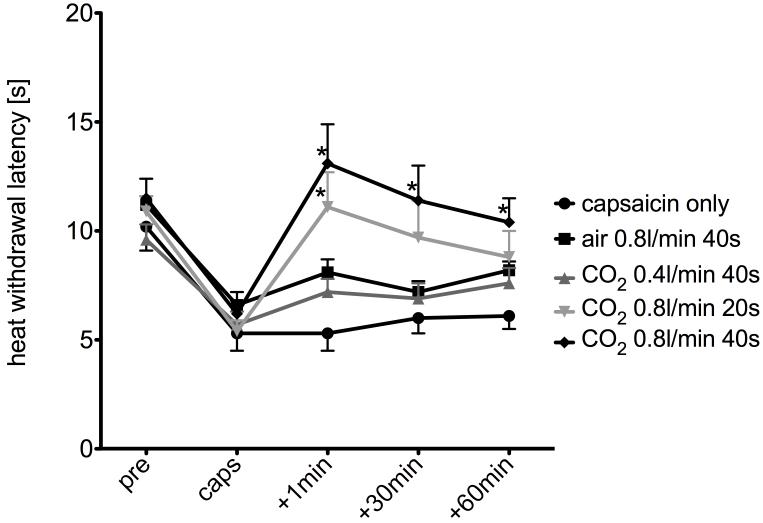
After sensitization of facial skin of rats with topical capsaicin, facial withdrawal response latencies evoked by C fiber selective radiant heat dropped from 10.2±1.1 s to 5.3±0.8 s (Fig. 1). The analysis of variance indicated that withdrawal latency was significantly affected by treatment (F0.05,4,16=10.96, Fcrit=3.01) and by timepoint (F0.05,4,16=14.09, Fcrit=3.01). Lightly anesthetized rats tolerated nasal CO2 administration well. Slight moving of the head was observed when placing the intravenous catheter into the nare, but usually stopped within a few seconds. CO2 administration was only started when the animals were lying still.
Fig. 1.
Effect of intranasal CO2 on capsaicin-induced heat sensitization: After topical capsaicin application, there was a robust decrease in withdrawal latencies to a heat stimulus applied to the cheek (circles). Air applied with a flowrate of 0.8 l/min for 40 s (squares) and CO2 with a flowrate of 0.4 l/min for 40 s (dark grey triangles), respectively, did not show significant antihyperalgesic effects. CO2 with a flowrate of 0.8 l/min for 20 s (light grey triangles) exhibited a significant (p < 0.05, ANOVA) antihyperalgesic effect immediately after application, which decreased with time, whereas CO2 with a flowrate of 0.8 l/min for 40 s (rhombs) demonstrated a significant (p < 0.05, ANOVA) antihyperalgesic effect for every observed time point after application. * indicates statistical significance as compared to “capsaicin only” (two way-ANOVA, p<0.05).
The group that received CO2 at 0.8 l/min for 40 s exhibited a withdrawal latency of 13.1±1.8 s immediately following treatment indicating an antihyperalgesic effect when compared to the post-capsaicin baseline. Similarly, a group that received CO2 at 0.8 l/min for the shorter period of 20 s exhibited a withdrawal latency of 11.1±1.6 s immediately following treatment. Post hoc Bonferroni analysis revealed that both of these groups’ average latency responses were significantly higher than that of the control group that received no CO2 treatment and not significantly different from one another. The group of animals that received CO2 at the lower flow rate of 0.4 l/min for 40 s exhibited a latency withdrawal of 7.2±0.6 s immediately following treatment, which was not significantly different from capsaicin-treated control. Control animals that received air at a flow rate of 0.8 l/min for 40 s exhibited a heat withdrawal latency of 8.1±0.6 s for the sensitized cheek immediately following treatment, which was also not significantly different from the “capsaicin only” group.
Duration of CO2 effect on sensitized cheek
Thirty and 60 minutes after CO2 application, the group that received CO2 at 0.8 l/min for 40 s exhibited average response latencies of 11.4±1.6 s and 10.4±1.1 s, respectively (Fig. 1), which were both significantly higher than the control capsaicin-sensitized group that did not receive CO2 treatment. Thirty minutes after CO2 application, the group that received CO2 at 0.8 l/min for 20 s exhibited an average response latency of 9.7±1.7 seconds, which was significantly higher than control. Sixty minutes after treatment this effect (8.8±1.2 s) was no longer significant, there was however a trend towards an increased withdrawal latency (Fig. 1). At no point were the response latencies of the group that received CO2 at 0.4 l/min for 40 s or air at 0.8l/min for 40 s significantly higher as compared to the capsaicin-sensitized control group (Fig. 1).
Effect of CO2 on nonsensitized cheek skin
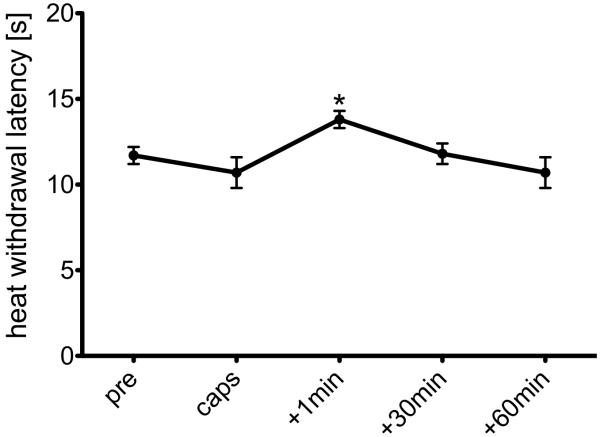
Withdrawal latencies of the group that received CO2 (0.8 l/min for 40 s) after topical application of saline to the cheek instead of capsaicin exhibited a small increase in withdrawal latency to 13.8±0.5 s immediately after treatment, which indicated a significant antinociceptive effect when compared to non-sensitized control animals that did not receive CO2 (p<0.05; Fig. 2). The control group that received no CO2 exhibited no statistically significant latency changes over the same time period.
Fig. 2.
Effect of intranasal CO2 insufflation (0.8l/min for 40 s) on heat withdrawal latencies on capsaicin vehicle treated (non sensitized) cheeks. Heat withdrawal latency one minute after CO2 insufflation was significantly different from baseline indicating a short-termed antinociceptive effect of CO2 in non-sensitized skin (*: p<0.05, t-test)
Effect of CO2 on paw withdrawal latencies
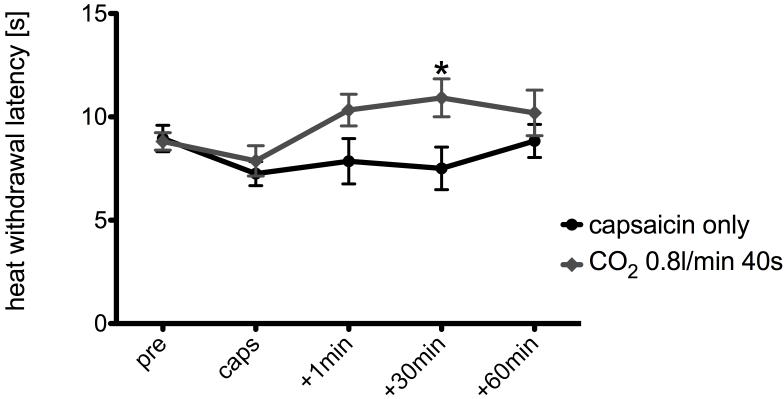
Right paw withdrawal latencies to noxious heat stimulation after capsaicin sensitization of the left cheek were decreased to 7.8±0.7 and 7.2±0.6 s in the group compared to the pre-sensitization baseline (8.8±0.4 and 8.9±0.6 s). In the group that received intranasal administration of CO2 at a flow rate of 0.8l/min for 40 s paw withdrawal latencies increased to 10.3±0.8 s immediately after, 10.9±0.9 s 30 minutes after, and 10.2±1.1 s 60 minutes after CO2 treatment compared to 7.8±1.1 s, 7.5±1.0 s, and 8.8±0.8 s for the animals that did not receive CO2 (Fig. 3). The analysis of variance indicated that withdrawal latency was significantly affected by treatment (F0.05,4,16=8.51, Fcrit=3.01).
Fig. 3.
Effect of intranasal CO2 insufflation (0.8l/min for 40 s) on right hind paw withdrawal latencies after capsaicin sensitization of the left cheek. * indicates statistical significance as compared to “capsaicin only” (two way-ANOVA, p<0.05)
Pharmacology
All animals tested for pharmacology were administered CO2 at 0.8 l/min for 40 s based on the hypothesis that CO2 would acidify the nasal mucosa and that protons thus released would activate nasal nociceptors. The analysis of variance indicated that cheek withdrawal latencies were significantly increased by treatment (F0.05,5,20=11.26, Fcrit=2.71) and by timepoint (F0.05,4,16=8.97, Fcrit=3.01). Post-hoc Bonferroni analysis revealed that the average withdrawal response latency of 10.8±0.9 s for animals that received CO2 treatment following nasal application of the carbonic-anhydrase inhibitor dorzolamide (Trusopt® ophthalmic solution) indicated a non-significant decrease from the antihyperalgesia evidenced in the group treated only with CO2 (Fig. 3A). Similarly, at 30 and 60 minutes after CO2 exposure, animals pretreated with dorzolamide showed a non-significant attenuation of the antihyperalgesic effect of CO2 as indicated by a decrease in post-CO2 withdrawal latencies compared to animals exposed to CO2 without dorzolamide pretreatment (Fig. 4A).
Fig. 4.
Effects of drugs on CO2 induced antihyperalgesic effect: A) Application of the carbonic anhydrase blocker dorzolamide before intranasal administration of CO2 with a flowrate of 0.8 l/min for 40 s non-significantly (p > 0.05, ANOVA) blocked the CO2 induced antihyperalgesia as compared to CO2 application without pretreatment. B) Application of an the acid-sensing ion channel blocker amiloride before intranasal administration of CO2 with a flow rate of 0.8 l/min for 40 s significantly (p < 0.05, ANOVA) blocked the CO2 induced antihyperalgesic effect immediately after application. C) Application of the TRPV1 blocker capsazepine before intranasal administration of CO2 with a flowrate of 0.8 l/min for 40 s also significantly (p < 0.05, ANOVA) blocked the CO2 induced antihyperalgesic effect 30 and 60 minutes after application of CO2. # indicates statistical significance as compared to “CO2 0.8 l/min 40s” (two way-ANOVA, p<0.05).
Animals that received CO2 treatment following nasal pretreatment with the mixed ASIC channel blocker amiloride exhibited a response latency of 7.3±0.7 s in the sensitized cheek immediately after CO2 treatment, which was significantly lower than response latencies of the group that received only CO2, indicating an attenuation of the antihyperalgesic effect and possible mediation of that effect by ASICs. Thirty and 60 minutes after CO2 exposure withdrawal latencies were 7.5±1.3 s and 6.8±1.0 s, respectively which were also significantly shorter than those of animals treated with CO2 without amiloride pretreatment (Fig. 4B). Animals intranasally treated with the appropriate vehicle for amiloride prior to intranasal CO2 application did not show a significant attenuation of the antihyperalgesic effect (p>0.05, data not shown).
Similarly, rats nasally pretreated with the TRPV1 blocker capsazepine exhibited response latencies of 8.0±0.6 s in the sensitized cheek after CO2 treatment, which was significantly lower than the group treated only with CO2, and remained so at 30 (6.7±0.4 s) and 60 minutes (6.9±0.5 s) after treatment indicating a robust blockade of the antihyperalgesic effect of CO2 (Fig. 4C). Again, pretreatment with the appropriate vehicle did not lead to an attenuation of the antihyperalgesic effect observed after intranasal CO2 at 0.8 l/min for 40 s (p>0.05, data not shown).
The group that was pretreated with both amiloride and capsazepine showed significantly shorter withdrawal latencies, when compared to capsaicin-sensitized animals treated with CO2 alone, immediately, 30, and 60 minutes after CO2 treatment as well (p<0.05, data not shown).
Effect of acidic buffer on withdrawal responses of capsaicin-sensitized cheeks
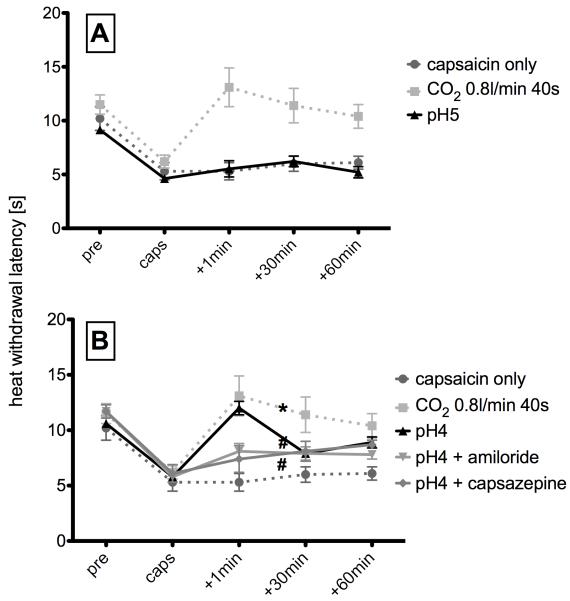
In an effort to further examine the influence of the acidification of the nasal mucosa by CO2, a group of animals that received intranasal pH 4 buffer but not those that received pH 5 buffer treatment exhibited a significantly elevated average cheek withdrawal latency (12.0±0.6 s) in sensitized sensitized rats immediately after treatment when compared to controls (Fig. 4). Analysis of variance indicated that withdrawal latency was significantly affected by treatment (F0.05,5,20=11.85, Fcrit=2.71) and by timepoint (F0.05,4,16=21.28, Fcrit=3.01). Post-hoc Bonferroni analysis demonstrated a significant attenuation of the capsaicin-induced hyperalgesia by intranasal pH 4 buffer, but no significant difference from the average response latency of the group that received CO2 (0.8 l/min, 40 s). At thirty and 60 minutes after intranasal application of pH 4 buffer, no antihyperalgesic effect observed, indicating a relatively short duration of effect.
Animals that received pretreatment with amiloride or capsazepine prior to buffer application exhibited significantly lower withdrawal latencies immediately after intranasal pH4 buffer administration as compared to animals that received pH4 buffer alone. Thirty and 60 minutes after buffer administration, there was no significant difference between groups (Fig. 5).
Fig. 5.
Effects of pH buffer: A) Intranasal administration of pH5 buffer did not change the decrease in withdrawal latency to a nociceptive heat stimuli after capsaicin sensitization. B) Intranasal administration of pH4 buffer produced a significant (p<0.05, ANOVA) antihyperalgesic effect immediately after application (+1min). This effect was significantly (p < 0.05, ANOVA) blocked by pretreatment with the acid-sensing ion channel blocker amiloride (black triangles), as well as by the TRPV1 blocker capsazepine (rhombs). * indicates statistical significance as compared to “capsaicin only” (two way-ANOVA, p<0.05). # indicates statistical significance as compared to “pH4” (two way-ANOVA, p<0.05).
Discussion
Our results indicate that intranasal administration of CO2 produces a dose-dependent antinociceptive/antihyperalgesic effect on trigeminal nociception. Forty seconds of CO2 at a flow rate of 0.8l/min produced a substantial attenuation of trigeminal nociception in capsaicin-sensitized rats for at least 60 minutes. In addition, animals that were not sensitized with capsaicin prior to nasal application of CO2 showed a significant increase in withdrawal latencies to a heat stimulus indicating, a slight antinociceptive effect. We hypothesized that the mechanism underlying these effects involves nasal mucosal carbonic anhydrase catalyis of CO2 to form carbonate and protons. Hypothetically, the resulting free protons would activate acid sensing ion channels (ASICs) as well as TRPV1 channels on mucosal trigeminal nociceptor terminals, resulting in afferent activation. This afferent activity, upon reaching the CNS, would then induce a wide-spread inhibition of further incoming trigeminal nociceptive activity, probably through activation of inhibitory interneurons (Hummel T et al. 2000; Jones TL et al. 2005) or by activation of trans-segmental inhibitory control circuitry (Bouhassira D et al. 1995). Some components of this hypothesis were tested using behavioral pharmacological methods.
Forty seconds of CO2 exposure of the nasal mucosa at a flow rate of 0.8 l/min was found to produce a clear facial antihyperalgesic effect. In evidence that this effect was mediated by catalysis of CO2 to protons and bicarbonate, amiloride, an ASIC inhibitor, was found to significantly decrease the antihyperalgesic effect of CO2. Amiloride is a diuretic that has been shown to block ASIC channels (Ferreira J et al. 1999), and to suppress ASIC mediated responses in patch clamp experiments (Dube GR et al. 2005) as well as in vivo rodent pain models (Ferreira J et al. 1999; Dube GR et al. 2005).
Pretreatment of the nasal mucosa with the TRPV1 antagonist capsazepine blocked the antihyperalgesia produced by CO2 application indicating the involvement of TRPV1 channels. Dorzolamide solution, a clinically available carbonic anhydrase inhibitor formulated for ophthalmic mucosa (Trusopt®), demonstrated only a non-significant trend in attenuating the CO2 mediated antihyperalgesia at the tested concentration despite the fact that carbonic anhydrase is expressed in 79% of TRPV1-positive trigeminal neurons (Tanimoto T et al. 2005), The non-significance of this finding may be due to a lack of alternative concentrations of the Trusopt® product or an attenuation of the anti-hyperalgesic effect by one of the numerous excipients of the dorzolamide formulation such as sodium hydroxide, which is a strong base. However, since other carbonic anhydrase inhibitors such as acetazolamide are insoluble in aequous solution, we were limited to the Trusopt® preparation.
Lastly, to provide additional evidence that it was, in fact mucosal acidification that produced the antihyperalgesia, pH 4 buffer was administered to the nose of animals sensitized with capsaicin and was found to have a significant antihyperalgesic effect of short duration. Notably, in animals not sensitized with capsaicin, pH 4 buffer was not found to have an effect, again suggesting proton signaling in the induction of CO2 antihyperalgesia. One possible explanation for the shorter-termed effect of the nasal pH 4 buffer solution as compared to the intranasal CO2 administration is the different temporal and spatial distribution of an aqueous solution when compared to CO2 gas. In humans, it has been shown that low-viscosity solutions were swallowed rapidly after nasal administration (Frasnelli J et al. 2005) thus decreasing the time of interaction with the nasal mucosa. On the other hand, CO2 gas molecules would readily pass through the mucosa. One might also argue that an intranasally administered fluid will not cover the same surface area of the nasal mucosa as compared to an intranasal gas insufflation, thus interacting with fewer afferent receptors. This is consistent with our our observation of a shorter-termed antinociceptive effect after administrating 0.8l/min CO2 for 20s as compared to the rats that received 0.8l/min CO2 for 40 s.
Vause et al. (Vause C et al. 2007) have recently shown that treatment of cultured trigeminal neurons with CO2 under isohydric conditions inhibits sensory nerve activation as induced by capsaicin or KCl, and leads to a subsequent decrease in neuropeptide, i. e. CGRP, release. They proposed an involvement of inhibition of calcium channels. However, Vause et al. also observed a significant increase in CGRP release when trigeminal ganglion neurons were cultured in acidic media and after CO2 exposure of cell cultures, which also leads to a drop in culture medium pH. These findings support our observation that intranasal CO2 leads to activation of mucosal primary afferents by protons liberated by a CO2 induced drop in nasal pH.
Consistent with our rodent findings, Spierings E. L. (Spierings EL 2005; Spierings ELH 2005) presented a randomized, double blind, placebo-controlled, parallel-group study where he investigated the effects of non-inhaled, intranasal CO2 on migraine headache. In the CO2 group 29.8% of patients were pain-free at 2 hours having treated migraine headaches of mild, moderate, or severe intensity, whereas only 9.2% of patients in the placebo group were pain-free. Since there were no clinically significant systemic and only minor and short-lived adverse effects observed, it was concluded that non-inhaled, intranasal CO2 is a well-tolerated and safe alternative for the abortive treatment of migraine headaches.
To our knowledge, there are no previous studies that demonstrated antinociceptive/antihyperalgesic effects of intranasal CO2 application in a behavioral animal model. Thuerauf et al. investigated mucosal potentials elicited by CO2 stimuli in rats (Thuerauf N et al. 1991) observing a reduction in amplitudes of negative mucosal potentials after repetitive application of high-concentration (>90% v/v CO2) stimuli. Hummel et al. (Hummel T et al. 2003) reported that a continuous stimulus of CO2 applied to human subjects results in a decrease in pain ratings, sometimes even to non-painful levels as opposed to CO2 stimuli of a length of up to 1600 ms, which induce robust pain sensations. These observations support the present findings of an antinociceptive/antihyperalgesic effect of nasal CO2 application, since the 0.8 l/min flow rate applied in this study should lead to a high CO2 concentration in the nasal cavity. In addition, exposure length for the antinociceptive/antihyperalgesic effect in the present study was 40 s compared to 1.2 s and 0.2 to 1.6 s in the studies of Thuerauf et al. and Hummel et al., respectively.
We have previously demonstrated that tonic activation of sciatic Aδ afferents attenuates nociceptive responses mediated by the activation of saphenous C-afferents (Jones TL et al. 2005), implying activation of a trans-segmental inihibitory process. This effect could be counteracted by intrathecal administration of a GABAb receptor antagonist or by selective silencing of the GABAb receptor gene in sensory neurons using an HSV vector, demonstrating at least partial mediation by presynaptic GABAb receptors on nociceptors. Similarly, Cui et al. (Cui JG et al. 1997) and Sandkuhler (Sandkuhler J 2000) have shown that low frequency tonic activation of Aδ afferents leads to a centrally mediated “long term depression” of subsequent nociceptive input, i.e. activity of one type of afferents modulates the nociceptive input of other afferents. More recently, Lapirot et al. has shown that facial grooming time after formalin injection into the vibrissa of rats can be suppressed by a conditioning noxious stimulus (formalin injection into the hindpaw, Lapirot O et al 2009). This phenomenon is known as diffuse noxious inhibitory controls (DNIC) and comprises a pathway that is activated when two concomitant painful stimuli are applied at the same time (van Wijk G et al. 2010). Our observation of increased paw withdrawal latencies after capsaicin-sensitization and intranasal CO2 insufflation suggest that similar central mechanisms are critical in mediating the antinociceptive/antihyperalgesic effect of nasal CO2. Such potential mechanisms are currently under study in our laboratory.
In summary, the present study suggests a possible mechanism underlying the results of the clinical observations of analgesic effects of nasal CO2 in patients with migraine. Our results provide evidence for a centrally-mediated antinociceptive/antihyperalgesic effect of intranasal CO2 application mediated by activation of afferents in the nasal mucosa by protons via ASICs and TRPV1 channels (Spierings EL 2005). Furthermore, these studies suggest that nasal CO2 may be useful for the treatment of somatic trigeminal pain states. Considering this and taking into account the clinical effects reported by Spierings (Spierings EL 2005; Spierings ELH 2005), it seems likely that the observed antihyperalgesic effect after intranasal CO2 administration affects all types of pain mediated by the trigeminal system, and possibly non-trigeminal pain. However, additional investigations are necessary to further elucidate the underlying mechanisms and the potential of this therapeutic approach in humans.
Conclusions
Our results indicate that intranasal CO2 application might be a useful treatment option for trigeminal pain states. These effects are likely mediated by protonic activation of TRPV1 and ASIC channels on afferent neurons innervating the nasal mucosa.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
Capnia, Inc., provided funding for this project. Also, Dr. Yeomans is on the Scientific Advisory Board of Capnia.
References
- Akaishi T, Odani-Kawabata N, Ishida N, Nakamura M. Ocular hypotensive effects of anti-glaucoma agents in mice. Journal of Ocular Pharmacology and Therapeutics. 2009;25(5):401–408. doi: 10.1089/jop.2009.0006. [DOI] [PubMed] [Google Scholar]
- Anton F, Peppel P, Euchner I, Handwerker HO. Controlled noxious chemical stimulation: responses of rat trigeminal brainstem neurons to CO2 pulses applied to the nasal mucosa. Neuroscience Letters. 1991;123(2):208–211. doi: 10.1016/0304-3940(91)90932-j. [DOI] [PubMed] [Google Scholar]
- Bigal M, Rapoport A, Aurora S, Sheftell F, Tepper S, Dahlof C. Satisfaction with current migraine therapy: experience from 3 centers in US and Sweden. Headache. 2007;47(4):475–479. doi: 10.1111/j.1526-4610.2007.00752.x. [DOI] [PubMed] [Google Scholar]
- Bouhassira D, Chitour D, Villaneuva L, Le Bars D. The spinal transmission of nociceptive information: modulation by the caudal medulla. Neuroscience. 1995 Dec;69(3):931–8. doi: 10.1016/0306-4522(95)00269-o. [DOI] [PubMed] [Google Scholar]
- Cui JG, O’Connor WT, Ungerstedt U, Linderoth B, Meyerson BA. Spinal cord stimulation attenuates augmented dorsal horn release of excitatory amino acids in mononeuropathy via a GABAergic mechanism. Pain. 1997;73(1):87–95. doi: 10.1016/s0304-3959(97)00077-8. [DOI] [PubMed] [Google Scholar]
- Dube GR, Lehto SG, Breese NM, Baker SJ, Wang X, Matulenko MA, et al. Electrophysiological and in vivo characterization of A-317567, a novel blocker of acid sensing ion channels. Pain. 2005;117(1-2):88–96. doi: 10.1016/j.pain.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Ferreira J, Santos ARS, Calixto JB. Antinociception produced by systemic, spinal and supraspinal administration of amiloride in mice. Life Sciences. 1999;65:1059–1066. doi: 10.1016/s0024-3205(99)00336-7. [DOI] [PubMed] [Google Scholar]
- Frasnelli J, van Ruth S, Kriukova I, Hummel T. Intranasal concentrations of orally administered flavors. Chemical Senses. 2005;30(7):575–582. doi: 10.1093/chemse/bji051. [DOI] [PubMed] [Google Scholar]
- Hummel T, Mohammadian P, Marchl R, Kobal G, Lötsch J. Pain in the trigeminal system: irritation of the nasal mucosa using short- and long-lasting stimuli. International Journal Psychophysiology. 2003;47(2):147–158. doi: 10.1016/s0167-8760(02)00150-2. [DOI] [PubMed] [Google Scholar]
- Hummel T, Schiessl C, Wendler J, Kobal G. Peripheral and central nervous changes in patients with rheumatoid arthritis in response to repetitive painful stimulation. International Journal Psychophysiology. 2000;37(2):177–183. doi: 10.1016/s0167-8760(00)00087-8. [DOI] [PubMed] [Google Scholar]
- Jones TL, Sweitzer SM, Peters MC, Wilson SP, Yeomans DC. GABAB receptors on central terminals of C-afferents mediate intersegmental Adelta-afferent evoked hypoalgesia. European Journal of Pain. 2005;9(3):233–242. doi: 10.1016/j.ejpain.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Kniep EM, Roehlecke C, Ozkucur N, Steinberg A, Reber F, Knels L, Funk RH. Inhibition of apoptosis and reduction of intracellular pH decrease in retinal neural cell cultures by a blocker of carbonic anhydrase. Investigative Ophthalmology and Visual Science. 2006;47(3):1185–1192. doi: 10.1167/iovs.05-0555. [DOI] [PubMed] [Google Scholar]
- Lapirot O, Chebbi R, Monconduit L, Artola A, Dallel R, Luccarini P. NK1 receptor-expressing spinoparabrachial neurons trigger diffuse noxious inhibitory controls through lateral parabrachial activation in the male rat. Pain. 2009;142(3):245–254. doi: 10.1016/j.pain.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Leffler A, Mönter B, Koltzenburg M. The role of the capsaicin receptor TRPV1 and acid-sensing ion channels (ASICS) in proton sensitivity of subpopulations of primary nociceptive neurons in rats and mice. Neuroscience. 2006;139(2):699–709. doi: 10.1016/j.neuroscience.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J. Long-lasting analgesia following TENS and acupuncture: spinal mechanisms beyond gate control; Proceedings of the 9th World Congress of Pain; Seattle, IASP press. 2000.p. 1154. [Google Scholar]
- Seabrook GR, Sutton KG, Jarolimek W, Hollingworth GJ, Teague S, Webb J, Clark N, Boyce S, Kerby J, Ali Z, Chou M, Middleton R, Kaczorowski G, Jones AB. Functional properties of the high-affinity TRPV1 (VR1) vanilloid receptor antagonist (4-hydroxy-5iodo-3methoxyphenylacetate ester) iodo-resiniferatoxin. Journal of Pharmacology and Experimental Pharmaceutics. 2002;303(3):1052–1060. doi: 10.1124/jpet.102.040394. [DOI] [PubMed] [Google Scholar]
- Spierings EL. Abortive treatment of migraine headache with non-inhaled, intranasal carbon dioxide: A randomized, double-blind, placebo-controlled, parallel-group study. Headache. 2005;45:809. [Google Scholar]
- Spierings ELH. Non-Inhaled, Intranasal Carbon Dioxide for the Abortive Treatment of Migraine Headache: Efficacy, Tolerability, and Safety. Annals of Neurology. 2005;58:S17. [Google Scholar]
- Tanimoto T, Takeda M, Nasu M, Kadoi J, Matsumoto S. Immunohistochemical co-expression of carbonic anhydrase II with Kv1.4 and TRPV1 in rat small-diameter trigeminal ganglion neurons. Brain Research. 2005;1044(2):262–265. doi: 10.1016/j.brainres.2005.02.082. [DOI] [PubMed] [Google Scholar]
- Thuerauf N, Friedel I, Hummel C, Kobal G. The mucosal potential elicited by noxious chemical stimuli with CO2 in rats: is it a peripheral nociceptive event? Neuroscience Letters. 1991;128:297–300. doi: 10.1016/0304-3940(91)90283-y. [DOI] [PubMed] [Google Scholar]
- Tölle T, Dukes E, Sadosky A. Patient burden of trigeminal neuralgia: results from a cross-sectional survey of health state impairment and treatment patterns in six European countries. Pain Practice. 2006;6(3):153–160. doi: 10.1111/j.1533-2500.2006.00079.x. [DOI] [PubMed] [Google Scholar]
- Tzabazis A, Klyukinov M, Manering N, Nemenov M, Shafer SL, Yeomans DC. Differential activation of trigeminal C or Aδ nociceptors by infrared diode laser in rats: Behavioral evidence. Brain Research. 2004;1037:148–156. doi: 10.1016/j.brainres.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Van Wijk G, Veldhuijzen DS. Perspective on Diffuse Noxious Inhibitory Controls as a Model of Endogenous Pain Modulation in Clinical Pain Syndromes. Journal of Pain. doi: 10.1016/j.jpain.2009.10.009. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Vause C, Bowen E, Spierings E, Durham P. Effect of carbon dioxide on calcitonin gene-related peptide secretion from trigeminal neurons. Headache. 2007;47(10):1385–1397. doi: 10.1111/j.1526-4610.2007.00850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans DC, Pirec V, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: behavioral evidence. Pain. 1996;68(1):133–140. doi: 10.1016/S0304-3959(96)03176-4. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: electrophysiological evidence. Pain. 1996;68(1):141–150. doi: 10.1016/S0304-3959(96)03177-6. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Goldstein BD, Yeomans DC. Low but not high rate noxious radiant skin heating evokes a capsaicin-sensitive increase in spinal cord dorsal horn release of substance P. Brain Research. 1997;752:143–150. doi: 10.1016/s0006-8993(96)01466-7. [DOI] [PubMed] [Google Scholar]
- Zhang M, Gong N, Lu YG, Jia NL, Xu TL, Chen L. Functional characterization of acid-sensing ion channels in cultured neurons of rat inferior colliculus. Neuroscience. 2008;154(2):461–472. doi: 10.1016/j.neuroscience.2008.03.040. [DOI] [PubMed] [Google Scholar]