Abstract
It is well established that cerebellar granule cell precursors (GCPs) initially derive from progenitors in the rhombic lip of the embryonic cerebellar primordium. GCPs proliferate and migrate tangentially across the cerebellum to form the external granule cell layer (EGL) in late embryogenesis and early postnatal development. It is unclear whether GCPs are specified exclusively in the embryonic rhombic lip or whether their precursor persists in the neonate. Using transgenic mice expressing DsRed under the human glial fibrillary acidic protein (hGFAP) promoter, we found 2 populations of DsRed+ cells in the EGL in the first postnatal week defined by bright and faint DsRed fluorescent signal. Bright DsRed+ cells have a protein expression profile and electrophysiological characteristics typical of astrocytes, but faint DsRed+ cells in the EGL and internal granule cell layer (IGL) express markers and physiological properties of immature neurons. To determine if these astroglial cells gave rise to GCPs, we genetically tagged them with EGFP or βgal reporter genes at postnatal day (P)3-P5 using a hGFAP promoter driven inducible Cre recombinase. We found that GFAP promoter+ cells in the EGL are proliferative and express glial and neural stem cell markers. In addition, immature granule cells (GCs) en route to the IGL at P12 as well as GCs in the mature cerebellum, 30 days after recombination, express the reporter protein, suggesting that GFAP promoter+ cells in the EGL generate a subset of granule cells. The identification of glial cells which function as neuronal progenitor cells profoundly impacts our understanding of cellular plasticity in the developing cerebellum.
Keywords: cerebellum, GFAP, astrocytes, neural stem cells, granule cells, external granule cell layer, Bergmann glial cells, Math1, GABA interneurons
INTRODUCTION
The earliest cerebellar precursor cells are segregated into three distinct regions, the neuroepithelium of the 4th ventricle, the cerebellar white matter, and the anterior rhombic lip. The neuroepithelium of the 4th ventricle gives rise to the neurons of the cerebellar nuclei, the Purkinje cells, and the cerebellar interneurons for the internal granular layer (IGL). Cerebellar interneurons for the molecular layer (ML) arise from precursors with glial characteristics in the interlobular and deep white matter (Silbereis et al., 2009). By contrast, the precursors of the granule cells (GCs) migrate from the anterior rhombic lip to the cerebellar primordium, spreading tangentially across its surface to form the external granule layer (EGL); from birth and extending until P20 in the mouse, they exit the cell cycle and migrate down the fibers of the Bergmann glia to populate the IGL (Alder et al., 1996; Hallonet et al., 1990; Komuro et al., 2001; Wingate and Hatten, 1999). The rhombic lip precursors express Math1, a neurogenic bHLH transcription factor, which is required in these cells for the genesis of hindbrain nuclei before E12 and for cerebellar GCs from E12 until E17 (Ben-Arie et al., 1997; Hatten and Heintz, 1995; Lin et al., 2001; Machold and Fishell, 2005; Wang and Zoghbi, 2001).
In the rhombic lip and EGL, fate mapping studies using a tamoxifen inducible Math1-CreERT2 transgenic mouse line revealed that distinct pools of Math1-expressing progenitor cells are generated de novo from a Math1-negative precursor (Machold and Fishell, 2005). These studies suggest that a class of non-committed neural stem/precursor cells (NSC/NPC) gives rise to the committed, highly proliferative Math1-positive cells that eventually differentiate into cerebellar GCs. In Math1-LacZ knock-in animals where the Math1 gene is replaced with a LacZ reporter the EGL does not form. However, a small population of Math1-LacZ positive cells are generated from the rhombic lip and migrate tangentially over the surface of the cerebellum. These cells are retained postnatally and express the astroglial marker GFAP (Jensen et al., 2004) Reinforcing these findings and the notion that not all cells of the EGL are Math1-expressing committed neuronal progenitors, cells isolated from the outer edge of the EGL can differentiate into mature astrocytes when exposed to Sonic Hedgehog and Bone Morphogenetic Proteins in vitro (Okano-Uchida et al., 2004). However, the generation of mature cerebellar GCs from postnatal Math1-negative NSC/NPCs in the intact animal has not been demonstrated.
In embryogenesis, neurogenic progenitors arise from earlier precursors that exhibit glial properties, such as the expression of the astrocytic glutamate-aspartate transporter (Glast), the brain lipid binding protein (Blbp), and the intermediate filaments nestin and vimentin (Malatesta et al., 2000). These neural precursors, also called radial glia, express factors that activate the human GFAP promoter, and give rise to juvenile and adult NSC/NPCs of the subventricular zone and dentate gyrus, in which both the mouse and human GFAP promoters are expressed (Casper and McCarthy, 2006; Doetsch et al., 1999; Ganat et al., 2006; Garcia et al., 2004; Seri et al., 2001). In this paper, we examine whether the EGL contains neural progenitor cells with glial characteristics that generate GCs. We used several lines of transgenic mice expressing reporter genes under the human GFAP promoter (hGFAP) to characterize astroglial cells in the EGL of neonatal mice. The GCE mice, carrying a tamoxifen-inducible Cre recombinase (CreErT2), were then used to permanently mark cells with GFAP promoter activity with reporter genes and study their fate at specific time points in cerebellar development. Our results indicate that GFAP-expressing cells in the EGL generate GCs in vivo in a temporally restricted window, limited to the first two postnatal weeks of cerebellar development.
RESULTS
The EGL contains GFAP-expressing cells with characteristics of neural progenitors
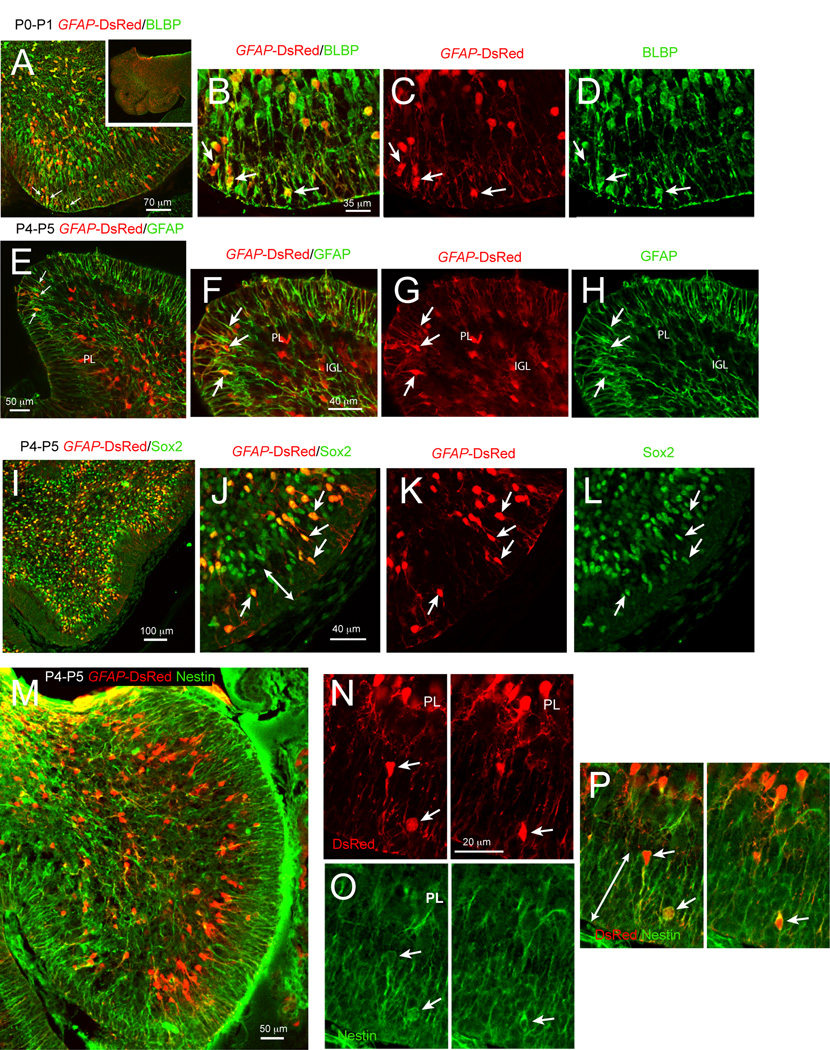
In our experiments, we used transgenic lines driven by the hGFAP to identify, characterize and fate map astroglial cells in the cerebellum of neonatal mice. The hGFAP promoter is expressed in radial glia, earlier than the mouse GFAP promoter, which is not highly expressed at embryonic stages (see GENSAT database at: http://www.gensat.org/imagenavigatorfileselection.jsp?gene.id=507&mouseage.id=8). However, after birth, both promoters largely identify the same cell types. The mouse GFAP promoter is highly expressed both by Bergmann glia and by other glial cells throughout the cerebellum (http://www.gensat.org/imagenavigatorfileselection.jsp?gene.id=507&mouseage.id=9). Consistently, in hGFAP-DsRed mice, the DsRed reporter colocalizes with endogenous GFAP protein expression at neonatal stages (Fig. 1 E–H). In the EGL of neonates, DsRed-fluorescent cells were small, bipolar or unipolar in shape, and observed at P0-P5, but not in the EGL of P8 mice (data not shown). This finding was confirmed in hGFAP-GFP mice (data not shown). At P0-P2, DsRed-fluorescent cells were found throughout the EGL (Fig 1 A–D). By P4 the majority of bright DsRed-fluorescent cells were in the inner EGL and at both time points contacted the pial surface with a single process (Fig. 1 E–H and see Supplementary Fig. 1). We also found DsRed-fluorescent cells in other regions of the cerebellum, including the IGL and PL (Bergmann glia), as expected (Fig. 1). To characterize the DsRed-fluorescent cells in the EGL, we stained for several astroglial markers. Blbp, a marker of radial glia and immature astrocytes (Ganat et al., 2002; Malatesta et al., 2003), stained the majority of DsRed-fluorescent cells in the EGL at P0-P2 (Fig. 1 A–D). As expected, the classical astrocytic marker GFAP was not expressed in P0-P2 cerebellum (data not shown). By P4, DsRed-fluorescent cells in the EGL stained positive for GFAP (Fig. 1 E–H).
Figure 1. Cells expressing immature glial markers and GFAP promoter activity are present in the cerebellar EGL.
In hGFAP-DsRed mice, red fluorescence is evident in bipolar cells within the EGL co-expressing Blbp (A–D), GFAP (E–H), Sox2 (I–L), and Nestin (M–P) immunoreactivities. Note that at P0–P1 these cells occupy the external portion of the EGL, whereas by P4–P5 they can be seen throughout the EGL, although preferentially in the inner EGL. (B–D), (F–H) and (J–L) are magnifications of (A), (E) and (I), respectively. All images were acquired with a laser-scanning confocal microscope and are 1 µm thick optical sections.
Based on recent findings showing that hGFAP promoter-expressing radial glial cells in the embryonic rhombic lip are progenitors for GCs (Schuller et al., 2008; Spassky et al., 2008), we stained for known markers of NSCs such as nestin, Sox2 and Mushahi1 (Ellis et al., 2004; Imura et al., 2006; Kaneko et al., 2000; Zappone et al., 2000). We found that DsRed-fluorescent cells stained for Sox2 (Fig. 1 I–L) and nestin (Fig. 1 M–O). In addition, about 60% of DsRed-fluorescent cells expressed Mushashi1 (data not shown) and 55% (± 13.7) were proliferative cells expressing the cell cycle protein Ki67 (Supplementary Fig. 1). Thus, a subpopulation of EGL cells in the neonatal cerebellum displays antigenic properties of astrocytes and proliferating neural progenitor cells.
Upon closer examination of cerebellar sections, we found that DsRed-fluorescent cells exhibited different degrees of fluorescence intensity; quantification of the intensity of fluorescence revealed that DsRed cells could be categorized in two distinct subtypes with either faint or bright fluorescence (Fig. 2 A–C for fluorimetry and examples of bright and faint DsRed cells). Some cells with faint DsRed-fluorescence were observed both in the EGL and in the IGL. Considering that DsRed half-life is 4 days, faint DsRed-fluorescent cells may display DsRed fluorescence without an active GFAP promoter and could be the daughters of GFAP+ cells in the EGL. To explore the phenotype of the faint DsRed-fluorescent cells, we immunostained for markers of granule cell precursors (GCPs) such as Zic1/2 and Reelin (Aruga et al., 2002; Jensen et al., 2004). In the EGL, incipient ML and IGL, faint DsRed-fluorescent cells stained positive for Reelin (Fig. 2 D–I) and Zic1/2 (Fig. 2 J–O). Importantly, bright DsRed-fluorescent cells in the EGL did not stain for Reelin (Fig. 2 D–I, arrowheads) or Zic1/2 (Fig. 2 J–M, arrowhead), suggesting that they were not yet committed to a neuronal lineage.
Figure 2. Faint DsRed-fluorescent cells express markers of granule cells.
(A), fluorimetric quantification of intensity of red fluorescence reveals two subpopulations of DsRed cells in hGFAP-DsRed mice. (B,C) Confocal Z-stacks and projections illustrating bright (arrowheads) and faint (arrows) DsRed fluorescence in the EGL and IGL. (D–I) faint red fluorescence is visible in cells displaying Reelin immunoreactivity at both P2 and P4 (D–I, arrows), whereas bright DsRed fluorescent cells do not display Reelin immunostaining (arrowheads). (J–O) faint red fluorescence is also visible in Zic 1/2 positive cells (J–M, arrows). In contrast, cells with bright red fluorescence are negative for Zic1/2 (J–O, arrowhead). All images are 1 µm thick single confocal optical section; side panels show Z-stack projections.
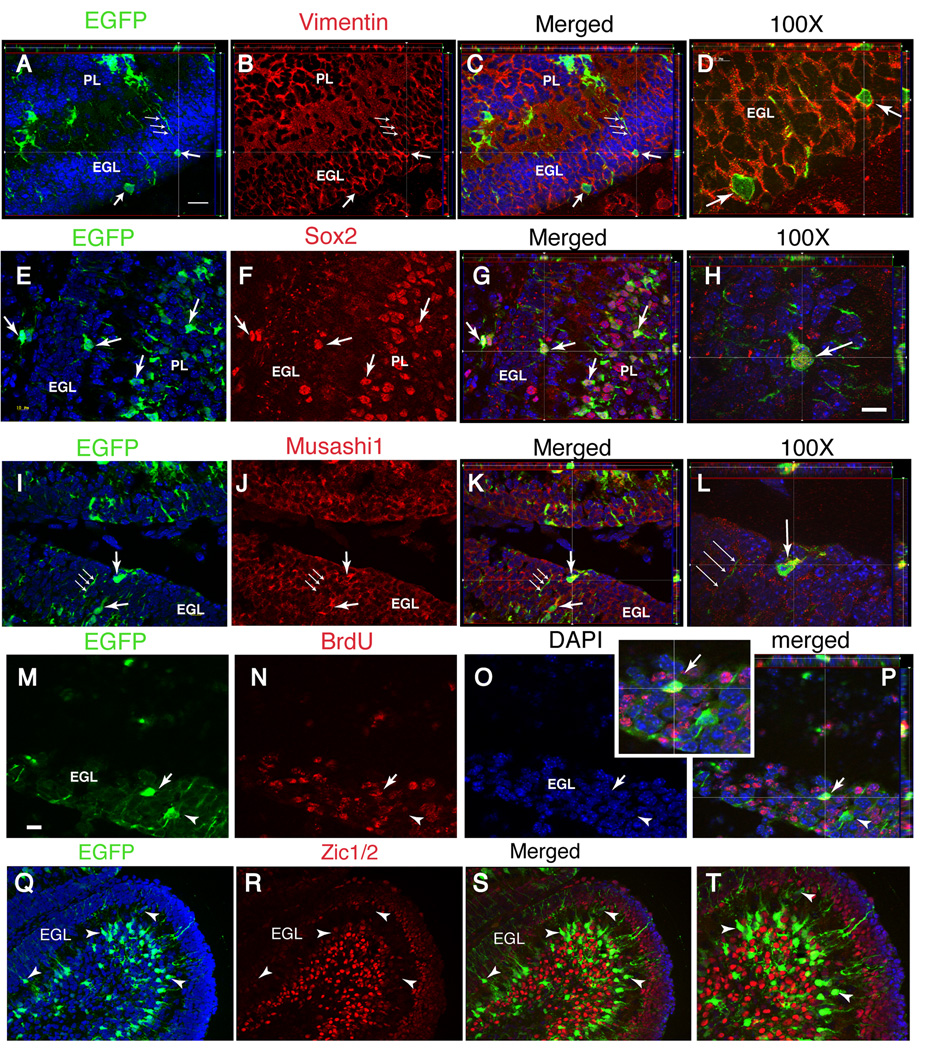
To further examine the lineage of, hGFAP promoter+ cells, we used hGFAP-CreERT2 (GCE) transgenic mice. In this mouse model, tamoxifen treatment induces Cre recombinase activity and permanently activates the expression of the β-galactosidase (βgal) or EGFP reporter genes in hGFAP promoter+ cells. Previous reports have shown that in GCE mice reporter gene expression is activated exclusively in cells of the glial lineage (Ganat et al., 2006; Silbereis et al., 2009). Specifically, reporter+ cells in the cerebellum were NeuN-negative at short time points after recombination, and conversely, NeuN+ GCs and Purkinje cells were devoid of reporter immunoreactivity (Silbereis et al., 2009) (see also Fig. 6 J–M). We confirmed these results by characterizing the phenotype of reporter+ cells in the cerebellar cortex 2 days after treating GCE mice with tamoxifen in the neonatal period. Neonatally tagged reporter+ cells in the EGL were bipolar or unipolar, contacted the pial surface with a single process, and could be found at all levels within the EGL, including its outer half (Fig. 3A–D; Supplementary Fig. 2 D – F). Bergmann glia and astrocyte-like cells in the IGL and white matter were also reporter+ (Supplementary Fig. 2 A – F) (Silbereis et al., 2009). Furthermore, the molecular characteristics of reporter+ cells in the EGL of GCE mice 2 days after recombination were similar to the bright DsRed+ cells described above. Cre-tagged cells expressed the immature glial filament vimentin (Fig. 3 A–D), the transcription factor Sox2 (Fig. 3E–H), and the radial glial marker Musashi-1 (Fig. 3 I–L). To investigate whether the reporter+ cells in the cerebellar cortex were proliferative, animals received an injection of the S-phase marker bromodeoxyuridine (BrdU) at P5 and the sections were immunostained for BrdU at P7. Approximately 30–40% of the EGFP+ cells in the EGL were BrdU-positive (Fig. 3 M–P) and about half expressed Math1 immunoreactivity (Supplementary Fig. 2 G – I). Moreover, two days after recombination, reporter+ cells did not express markers for early postmitotic GCs in the inner EGL, ML, and IGL, including Zic1/2 (Fig. 3 Q–T, arrowheads) and Tag-1 (Supplementary Fig. 2 J – L).
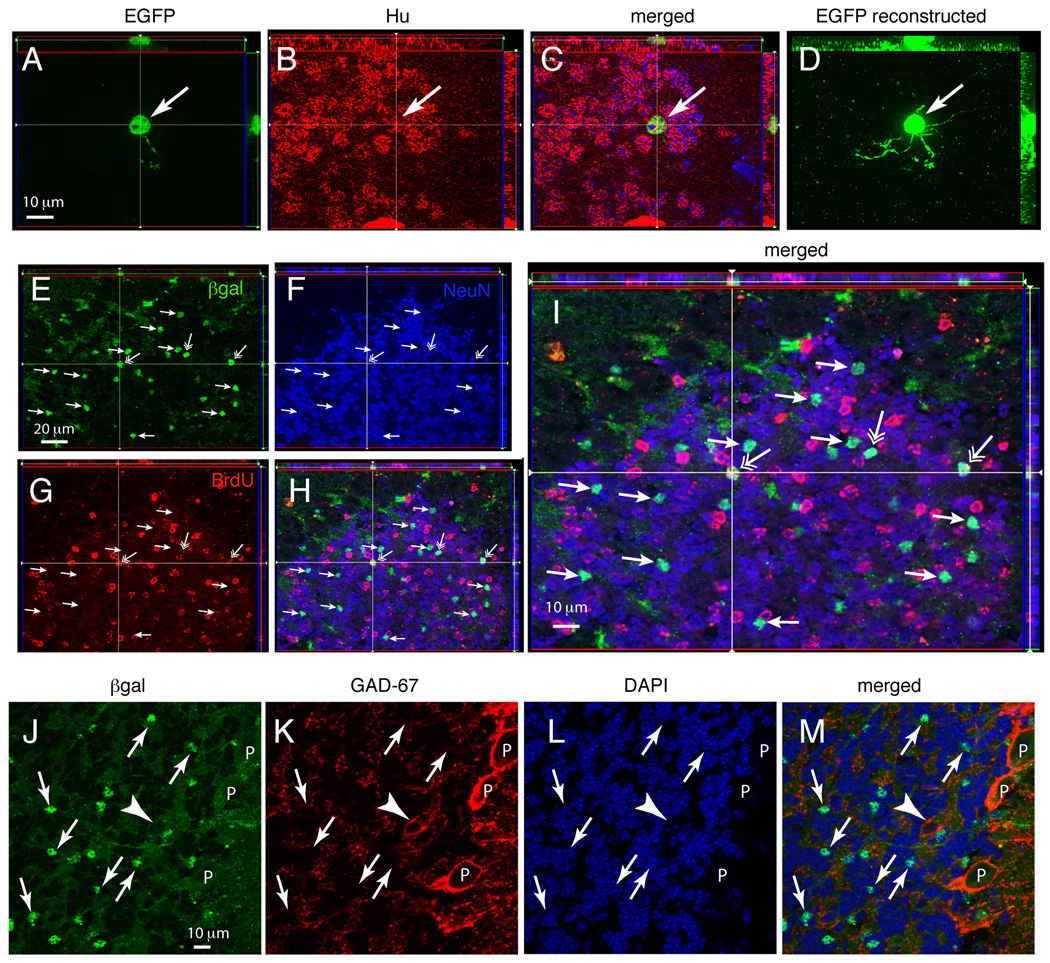
Figure 6. GFAP promoter-positive cells generate granule cells in the perinatal period.
Double-immunostaining for reporter gene expression and cell specific markers in the GC layer of CGE; CAG-EGFP mice (EGFP, A–D) or GCE;R26R mice (βgal, E–M) analyzed at P35 after two tamoxifen injections at P5. (A–C), apotome acquired 1 µm-thick Z-plane projections of tissue sections double immunostained for the EGFP reporter (A), Hu (B) and merged images (C) in the GC layer. (D), reconstructed image of the EGFP staining obtained from a 6 µm-thick Z-stack of 1 µm-thick slices from the same view as in A–C. (E–H), 1 µm-thick Z-plane projections of sections triple immunostained for βgal (E), NeuN (F), BrdU (G) and merged images (H). Panel (I) is a 2X magnification of (H). Many reporter-positive cells are NeuN-positive (small arrows) some of which also incorporate the mitotic marker BrdU (open arrows). (J–M), 1 µm-thick Z-plane projections of sections double immunostained for βgal (J) and GAD-67 (K); DAPI nuclear staining (L) and merged images (M). The images demonstrate that βgal staining is in small cells in the IGL with 5–6-µm diameter cell body (arrows), whereas reporter expression is absent in Purkinje cells (P) and large GAD-67 positive neurons of the IGL (likely Golgi cells, arrowhead). All images are Apotome acquired 1 µm-thick Z-stack projections. Scale bars, 10 µm for (A–D) and 20 µm for (E–H); scale bar in (J) 10 µm for (J–M).
Figure 3. Glial cells with GFAP promoter activity rapidly transition to a GCP phenotype in the EGL.
Double-immunostaining for reporter gene expression and cell specific markers in CGE;CAG-EGFP mice analyzed at P7 after two tamoxifen injections at P5. The antibody markers are indicated above each image. (D,H,L,P,T), higher magnification of (C,G,K,O,S), respectively. The reporter-positive cells in the EGL express vimentin (A–D), Sox2 (E–H) and Musashi1 (I–L). After BrdU incorporation at P5, about 50% of reporter+ cells are BrdU+ (arrows in M–P). In contrast, reporter+ cells do not express the postmitotic GC marker Zic1/2 (Q–T). All panels illustrate apotome 1 µm-thick Z-plane projections. Scale bar in (H) 10 µm for (D,H,L) and 25 µm for (P,T); scale bar in (M) 20 µm for all other panels.
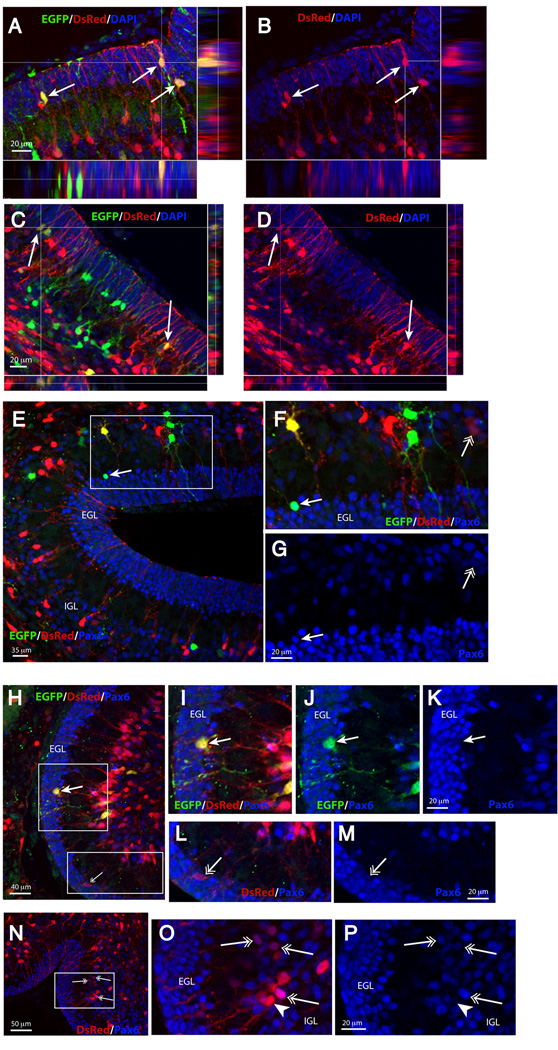
To demonstrate that DsRed-fluorescent cells belong to the GFAP lineage and thus that faint DsRed-fluorescent cells may derive from bright DsRed-fluorescent cells, we crossed the hGFAP-DsRed mice to the GCE mice carrying the EGFP reporter gene. Pups received three injections of tamoxifen over three consecutive days (P1 to P3). The pups were sacrificed 3 days after the first injection (P4). DsRed-fluorescent cells, both the bright (Fig. 4 A–D) as well as the faintly labeled (Fig. 4 C , D and H–L) co-localized the EGFP reporter protein in the EGL, demonstrating that the cells marked by the DsRed transgene and those marked by Cre recombination are in the same lineage. Not all the DsRed-fluorescent cells were EGFP+, because the recombination efficiency of the GCE mice is only 30% (Silbereis et al., 2009) and the GFAP-DsRed reporter mice similarly do not target 100% of GFAP+ cells. Furthermore, 52 ± 5.9% of the faint DsRed fluorescent cells in the EGL and IGL co-localized Pax6 immunoreactivity (Fig. 4 H–P; Supplementary Fig. 3). Some bright red Bergmann glial cells were Pax6+, as some Bergamnn glia display persisting Pax6 immunoreactivity. As expected, some reporter+ cells also colocalized Pax6 three days after the first tamoxifen injection in GCE mice (Fig. 4E–G, closed arrow), and more extensive colocalization with Pax6 was observed seven days after recombination (Fig. 7). Thus, genetic fate mapping suggested a rapid transition from an astroglial phenotype, exemplified by the bright DsRed-fluorescent cells, to a neuronal GCP phenotype in the inner EGL and IGL of neonatal mice, exemplified by the faint DsRed-fluorescent cells.
Figure 4. Characterization of EGFP reporter expression and DsRed fluorescence in CGE;CAG-EGFP; hGFAP-DsRed mice.
Triple transgenic mice were analyzed at P4 after tamoxifen injections at P1–P3. (A–D), arrows: EGFP+ cells in the EGL express strong (A–B) or weak (C–D) DsRed fluorescence. Blue is DAPI. (E–G), closed arrow: an EGFP+ cell in the EGL that expresses Pax6 immunoreactivity (blue); open arrow: faint DsRed fluorescent cells that expresses weak Pax6 immunoreactivity; (H–M), closed arrow: an EGFP+/DsRed fluorescent cell in the EGL that is Pax6-negative; open arrow: a faint DsRed fluorescent cell in the EGL that expresses weak Pax6 immunoreactivity; (N–P), open arrows: DsRed fluorescent cells in the IGL/Purkinje cell layer that express weak or strong Pax6 immunoreactivity; closed arrowhead: strong DsRed fluorescent cell in the Purkinje cell layer (presumably a Bergmann glial cell) that is Pax6-negative. All images are 1 µm thick single confocal optical sections; side panels show Z-stack analyses.
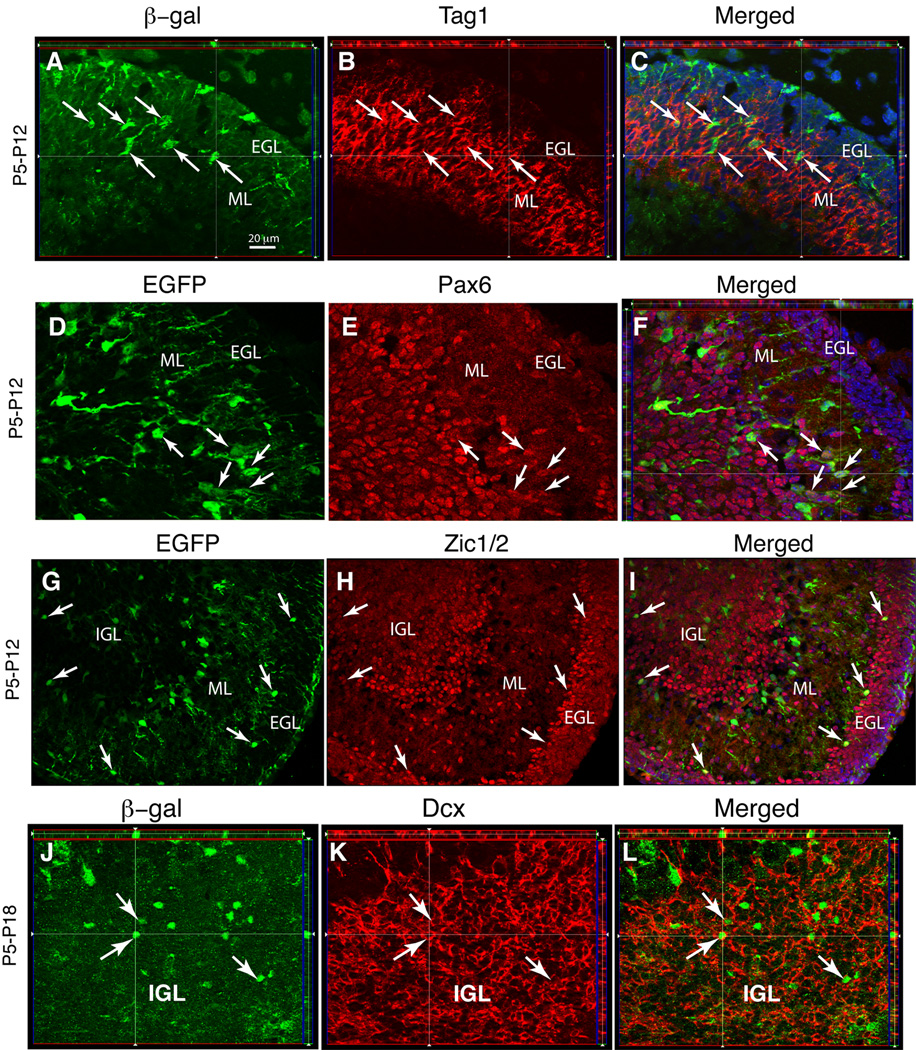
Figure 7. GFAP-expressing ancestors give rise to granule neuron precursors that migrate from the EGL to the IGL.
Single 1 µm-thick Z-plane projections of staining for reporter gene expression and cell specific markers in GCE;R26R mice (A–C and J–L) and CGE;CAG-EGFP mice (D–I) analyzed at P12 or P18 after two tamoxifen injections at P5, as indicated. (A–F) Tag1+ and Pax6+ GCPs co-expressing reporter immunoreactivity emerge in the inner aspect of the EGL one week after tamoxifen injection (arrows) and migrate to the IGL. (G–L) EGFP/Zic1/2 and βgal/Dcx double-positive young neurons appear in the inner aspect of the EGL and in the IGL (arrows) 7 and 13 days after tamoxifen treatment, respectively. All images are Apotome acquired 1 µm-thick Z-stack projections. Scale bar, 20 µm.
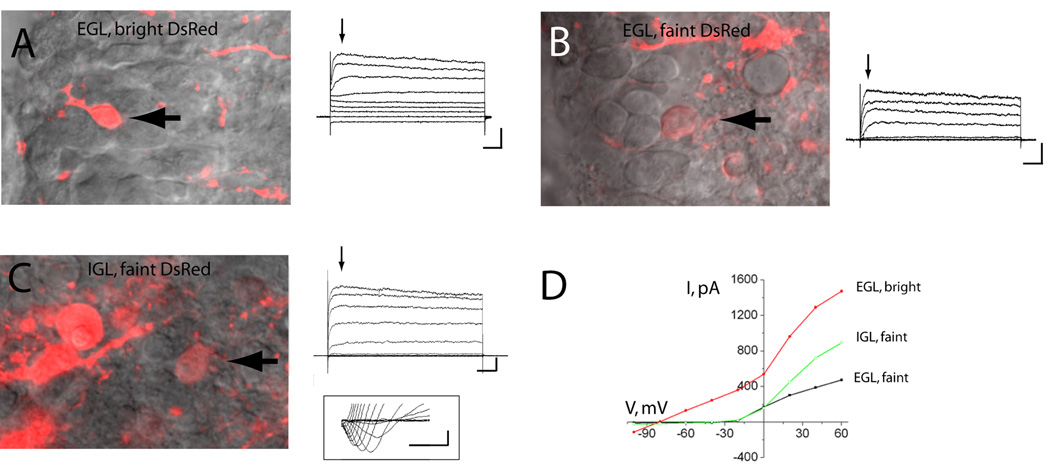
We thus hypothesized that bright DsRed+ cells represent a newly identified glial population and faint DsRed+ cells are GCPs or immature granule neurons. To more thoroughly characterize these two populations, we obtained whole-cell patch clamp recordings of DsRed-fluorescent cells. In acute sagittal slices from the P2-P4 cerebellum, bright DsRed-fluorescent cells in the EGL displayed biophysical characteristics of astrocytes such as hyperpolarized resting potentials (VR, mean of −81 mV, n=13, Table 2) and low input resistances (mean of 236 MΩ, n=13, Table 2 and Fig. 5). Faint DsRed-fluorescent cells in the EGL displayed characteristics of immature neurons with high mean input resistances of 4 GΩ and mean resting potentials of −51 mV (n=17, Table 2 and Fig. 5). Similarly, faint DsRed-fluorescent cells in the IGL displayed characteristics of GCPs but with slightly lower input resistance (n=17, Table 2, Fig. 5).
Table 2.
Electrophysiological characteristics of DsRed-fluorescent cells recorded in the EGL and IGL from P1-P4 mice.
| DsRed fluorescence/properties |
Location | Resting potential, VR (mean ± SEM, mV) |
Input Resistance, RIN (MΩ) |
|---|---|---|---|
| Bright Red | EGL | −81.5 ± 0.6, n=13 | 235.7 ± 22.1, n=13 |
| Faint Red | EGL | −48.8 ± 1.6, n=16 * | 4,119 ± 472, n=16 * |
| Faint Red | IGL | −51.3 ± 0.9, n=17 * | 3,100 ± 170, n=17 * |
* and ** each compared to bright red cells: p<0.001.
Figure 5. Bright DsRed-fluorescent cells display biophysical properties of astrocytes.
(A,B), left panels: photographs of a bright (A) and faint DsRed-fluorescent cells in the EGL (B) and IGL (C). The arrows point to the recorded cells. Right panels: Representative traces of whole cell currents. The cell membrane was stepped from −100 to +60 mV by 20 mV from a holding potential −60 mV. Scale bars: 500 pA (A), 200 pA (B and C), 50 ms. Inset in (C): Sodium currents activated by 10 mV-depolarizing voltage pulse following a prepulse to −110 mV (protocol with leak-subtraction). Scale bar: 100 pA/1 ms. (D) Current-voltage (I/V) relationships from traces shown in (C to D).
Collectively, these data suggest that GFAP promoter+ cells in the EGL generate GCPs in the EGL that differentiate into GCs in the IGL.
GFAP promoter+ cells generate granule neurons and glia in the IGL
We then assessed the long-term fate of the Cre-tagged cells. For these experiments, GCE mice carrying R26R or CAG-EGFP reporter lines were administered two tamoxifen injections at P3 or P5 to permanently activate βgal or EGFP expression in GFAP promoter+ cells. Reporter+ cells (i.e., βgal+ or EGFP+ cells) were phenotyped 35 days following recombination.
We found that 35 days following either P2 or P5 tamoxifen treatment, a large number of reporter+ cells in the IGL were Hu- and NeuN-positive (Fig. 6A–H). These βgal- or GFP-positive neurons were negative for GAD67 (Fig. 6J–M) and had a morphology resembling that of GCs, including a small round soma of 5–6 µm, emanating 4 to 5 thin dendrites (Fig. 6A and D). Unbiased stereological analyses confirmed that 73.6 ± 6.6% of βgal+ cells in the IGL were NeuN+ granule cells, 20.0 ± 5.1% were GFAP+ astrocytes and 2.6% were labeled by the Rip antibody, a marker for immature and myelinating oligodendrocytes.
To examine whether reporter-tagged GCs were indeed born at the time of tamoxifen injection, animals received a single BrdU injection at P5 following tamoxifen injection and the sections were immunostained for BrdU 35 days later. Indeed, we found that some βgal/NeuN-double positive granule cells in the IGL colocalized BrdU (Fig. 6E–I).
Collectively, these data suggest that glial cells with GFAP promoter activity in the early postnatal cerebellar EGL change their identity, from precursors with the molecular and physiological phenotype of astroglia and neural stem cells to Pax6+ proliferating GCPs in the EGL and eventually GCs, and only a minority remains in the glial lineage.
GFAP promoter+ cells in the EGL generate immature granule cells en route to the IGL
We next performed a series of experiments to show that GCs were generated from GFAP promoter+ cells in the EGL. If astroglial cells in the EGL generate GCs that migrate to the IGL, we should find intermediate cell types, i.e., reporter+ immature GCs en route from the EGL to the IGL. Indeed, at P12 (i.e. 7 days following tamoxifen administration at P5), some of the βgal+ cells had taken up residence in deeper levels of the EGL and the ML and had acquired Tag1, Pax6 and Zic1/2 immunoreactivity (Fig. 7A–I ). Many of these cells had an elongated soma and leading lagging processes characteristic of cells migrating from the inner EGL and ML (arrows in Fig. 7A–C and Fig. 7D–E) to the granule layer. Some reporter+/Zic1/2+ cells were already situated in the IGL (Fig. 7G–I). By P15 (i.e. 10-days after tamoxifen injection), all βgal+ cells in the EGL cells were Tag1 immunoreactive and βgal/Tag1-double positive were found throughout the entire extent of the ML (data not shown). At P18, very few Dcx+ or Tag1+ cells remained in the ML. However, by this age, βgal/Dcx double immunoreactive cells could be observed in the IGL (Fig. 7J–L). These data suggest that cells with an active GFAP promoter located in the EGL generate GCPs that migrate through the ML to reach the IGL.
GFAP promoter+ cells acting as progenitors are transient in the EGL
Considering that GFAP promoter+ cells using hGFAP-DsRed were not found in the EGL by P12, we hypothesized that induction of Cre reporter activity at P12 in the GCE mice should not lead to GC labeling. To test this hypothesis, we injected tamoxifen to induce reporter gene expression in the GFAP promoter+ precursors at P12-P14 and harvested tissue at P35 or P90. Neither time point revealed genesis of GCs in the cerebellum from the GFAP lineage, as revealed by Hu, GAD-67 and NeuN double staining in either the molecular or granule cell layers (data not shown).
DISCUSSION
Using transgenic mouse models that drive either transient or permanent reporter gene expression in GFAP promoter+ cells, we show in this article that GFAP+ cells located in the EGL of neonatal mice give rise to proliferative GCPs in the EGL, which migrate through the ML and differentiate into postmitotic GCs in the IGL. These data add to the growing consensus that the EGL is not composed of a homogeneous population of committed Math1+ GCPs, but encompasses cells of multiple lineages and/or stages of development (Machold and Fishell, 2005; Okano-Uchida et al., 2004).
The transformation of GFAP promoter+ cells into GCs is a gradual process, which is apparent in both mouse models used in this paper. In the hGFAP-DsRed mice, strongly fluorescent cells were proliferative, invariably expressed markers of astroglia and neural stem cells (e.g., GFAP, nestin, Blbp, Sox2, Musashi1), and exhibited electrophysiological properties of astroglia. In contrast, weakly fluorescent cells, which presumably had already downregulated the GFAP promoter but still expressed red fluorescence due to the long half-life of the DsRed protein, expressed molecular markers of GCPs (e.g., reelin, Pax6 and Zic1/2) and exhibited electrophysiological characteristics of immature GCs. Similarly, short-term fate mapping from hGFAP promoter+ cells using the GCE mice in the first week of murine life was almost exclusively restricted to astroglial and NSC lineages (e.g., GFAP-, vimentin-, Sox2-, Musashi1-positive cells). A P12, one week after tamoxifen injection at P5, fate mapped hGFAP+ cells expressed the GCP markers Zic1/2, Pax6, Dcx, and Tag1 in the inner EGL, ML and IGL. By P18, the reporter/Tag1 double-positive cells disappeared from the ML, concomitantly with the appearance of reporter/Dcx double-positive cells in the IGL. To confirm this migratory population gave rise to mature GCs we analyzed the mature cerebellum 30 days after targeting. This analysis revealed that in the adult IGL, the majority (74%) of reporter+ cells were mature GCs. Double labeling of reporter+ cells in the IGL with BrdU and NeuN confirmed that these reporter+ GCs derived from neonatal GFAP+ precursors that proliferated at the time of targeting or shortly thereafter. Because we also mark Bergmann glia with our transgenics we cannot completely exclude that these cells represent a source of GCPs. However, the spatiotemporal dynamics of the GCP development in targeted cells coupled with the numerous studies that have established that the vast majority GCPs likely arise in the EGL render this possibility unlikely (Ben-Arie et al., 1997; Hatten and Heintz, 1995; Lin et al., 2001; Machold and Fishell, 2005).
Thus, we suggest that GFAP+/ Nestin+/Sox2+/Musahi1+ glial cells residing in the neonatal EGL generate granule neurons via GCPs, which express Pax6, Tag1, Zic1/2 (Alder et al., 1996). This is consistent with an earlier report suggesting that multipotential Math1/Tag1 negative cells isolated from the postnatal cerebellum can generate Tag1+/Math1+ GCs in vitro and, after transplantation, in vivo (Klein et al., 2005). However, it is possible that the cerebellar GFAP-lineage progenitors are separate from the Math1+ progenitors, and that they differentiate directly into Pax6+ GCPs that rapidly migrate to the IGL. The genesis of granule neurons from GFAP+ precursors is a transient event of postnatal development, as GCE targeting at after P12 revealed that the in vivo fate of GFAP+ cells is restricted to Bergmann glia and parenchymal astrocytes.
The genetic and molecular programs that restrict the potential of GFAP+ cells in the cerebellum is an interesting topic for further study. We were unable to determine the extent of the granule population generated from postnatal GFAP+ cells with our GCE mouse model, as we target only a subset of astroglial cells in the EGL at P3-P5. The embryonic origin of GFAP+ cells in the EGL is also presently unclear. These cells may represent a portion of the early migratory population from the anterior rhombic lip, as are the early Math1+ GCPs, although we cannot exclude that they are derived from an alternative source such as the subventricular zone radial glia.
This study provides further insight into the progenitor populations present in the neonatal cerebellum in which there is widespread genesis of multiple cells type. Numerous studies report that multipotential, neurosphere-generating NSCs derived from the postnatal developing cerebellum can also generate granules cells, interneurons and glia in vitro (Klein et al., 2005; Lee et al., 2005; Sottile et al., 2006). The antigenic profile of the GFAP+ precursors that we identify in the neonatal cerebellum is similar to that of embryonic radial glia and neurogenic astrocytes that populate postnatal SVZ niches (Bani-Yaghoub et al., 2006; Episkopou, 2005; Kamei et al., 1998; Kaneko et al., 2000; Pixley and de Vellis, 1984).
In a previous paper we reported that hGFAP+ cells similar to those reported here populate the cerebellar white matter at these neonatal stages of development and progressively migrate outward into the cerebellar cortex, developing into parvalbumin+ basket and stellate cerebellar GABAergic interneurons of the ML (Silbereis et al., 2009). Together, these results highlight the heterogeneity of GFAP+ cells in the neonatal brain and the likely possibility that instructive cues present in the local microenvironments of these cell populations are critical to drive their fate into particular cell lineages. It remains to be determined whether the neurons, astrocytes and oligodendrocytes generated from GFAP+ cells at this time period arise from subpopulations of these cells or whether each GFAP+ precursor maintains both neurogenic and gliogenic potential.
In conclusion, we have identified GFAP+ precursor cells that generate cerebellar GCs. Our data underscore the surprising plasticity of glial cells in the immature mammalian brain. We suggest that neurogenic GFAP+ cells similar to embryonic radial glia and to the neural stem cells of the dentate gyrus (DG) and the SVZ exist in the neonatal cerebellum. Whereas the DG and SVZ niches allow the maintenance of these neural stem cells throughout life, the cerebellum supports their neurogenic potential only for the first two weeks of rodent postnatal development, after which GFAP+ cells restrict their fate exclusively to astrocytes. We cannot, however, exclude that under special conditions, such as in vivo alterations of their normal environment, or intrinsic changes in their genetic makeup, such glial cells might revert into a neural stem cell-like state and support aberrant cerebellar neurogenesis or tumor formation.
EXPERIMENTAL METHODS
Mice and Genotyping
The hGFAP-DsRed mice expressing DsRed under the human GFAP promoter (hGFAP-DsRed mice) were produced and kindly provided by N.A. Jensen and J.V. Nielsen (Noraberg et al., 2007).
The hGFAP-GFP mice were obtained from the Jackson Lab (FVB/N Tg(GFAPGFP)14Mes/J, donating investigator Dr. Albee Messing, Jackson Labs, USA).
The GFAPCreERT2 (GCE) mice were generated as previously described and back-crossed to C57/B6 mice to the F6 generation (Ganat et al., 2006). GCE transgenic mice carry a Cre recombinase-estrogen receptor type 2 fusion protein (CreErT2) placed under control of the human GFAP promoter, which is active in radial glia, astrocytes and adult neural stem cells (Brenner et al., 1994; Ganat et al., 2006). Genotyping was done by PCR using primers to the Cre gene (5'-GCAACGAGTGATGAGGTTCGCAAG-3') (forward) and the ERT2 5'- TCCGCCGCATAACCAGTGAAACAG-3') (reverse) to generate a band of 307 bp (Ganat et al., 2006). GCE mice were crossed with either the R26R LacZ (R26R) reporter mice (Soriano, 1999) or the CAG-EGFP reporter mice (Nakamura et al., 2006) in order to conduct fate mapping experiments. All animal experiments comply with Institutional and National policies and guidelines.
Control experiments were performed to validate these mice as previously reported (Ganat et al., 2006): no detectable βgalacrosidase (βgal) activity (i.e. X-gal staining) was present in GCE;R26R mice without tamoxifen treatment, and postnatal tamoxifen injections did not result in reporter labeling of Purkinje neurons, which are exclusively generated during embryogenesis (Fig. 6 J–M) (Silbereis et al., 2009).
Induction of Cre Recombination via Tamoxifen Treatment
In order to induce Cre recombination in GFAP promoter expressing cells, GCE mice crossed with reporter lines were administered either tamoxifen or its metabolite 4-hydroxy-tamoxifen at a dosage of 33 mg/kg (two i.p. administration separated by 4 hr) for mice P5 and younger or 60 mg/kg (i.p., single administration) for older animals from a 2 mg/ml stock solution prepared in autoclaved sunflower seed oil and stored at −20 °C. For the analyses of cell proliferation, 2-bromodeoxyuridine (BrdU) was injected (50 µg/g, i.p.) after the last tamoxifen injection.
Tissue Preparation and Immunohistochemistry
Mice were deeply anesthetized and sacrificed via transcardial perfusion with 4% paraformaldehyde (PFA) and the brains were then post-fixed for one to three hours depending on the age. Samples were transferred to 20% sucrose overnight for cryoprotection and then stored at −80 °C. Serial 10 µm-cryosections were obtained as previously described (Ganat et al., 2006). For immunohistochemistry, sections were blocked in phosphate buffer saline (PBS) containing 0.1% Tween-20/0.2% Triton (PBS-T) containing 10% goat serum (10% GS/PBS-T) or 10% donkey serum (10% DS/PBS-T), and then incubated in primary antibody in 5% GS/PBS-T or DS/PBS-T overnight at 4°C. For a list of antibodies used and their sources, see Table 1. Sections were washed thoroughly and then reacted to the secondary antibody of the appropriate species. The secondary antibodies used were Alexa Fluor series (1:1000; Invitrogen) RRX or Cyanine series (1:500, Jackson Labs). BrdU staining was done sequentially. First immunohistochemistry for all antigens other than BrdU was performed as described above. Section were post-fixed for 15 minutes at room temperature in 4% PFA and then incubated for 45 minutes in 1.5 N HCl, followed by immunohistochemistry for BrdU.
Table 1.
List of antibodies with their concentration
| Antibody | Species | Concentration | Source | Catalog # |
|---|---|---|---|---|
| βgal | Rabbit | 1/10,000 | Cappel | S5975 |
| BLBP | Rabbit | 1/500 | Chemicon | AB9558 |
| BrdU | Rat | 1/500 | Accurate Chemical | OBT0030 |
| DCX | Rabbit | 1/100 | Santa Cruz | SC28939 |
| DCX | Goat | 1/100 | Santa Cruz | SC 8066 |
| Gad-67 | Chemicon | |||
| GFAP | Rabbit | 1/1000 | Dako | Z0334 |
| GFAP-Cy3 | Mouse | 1/500 | Sigma | C9205 |
| GFP | chicken | 1/250–1/2000 | Abcam | 13970-100 |
| Hu | mouse | 1/500 | Molecular Probes/Invitrogen |
|
| Ki67 | Rabbit | 1/200 | Vector laboratories | T0423 |
| LeX/CD15 | Mouse | 1/ | BD Bioscience | 559045 |
| Math1 | Rabbit | 1/ | Jane Johnson | |
| Musashi1 | Rabbit | 1/1000 | Chemicon | ABS977 |
| Nestin | Mouse | 1/2000 | Chemicon | MAB353 |
| NeuN | Mouse | 1/500 | Chemicon | MAB377-18111840 |
| Pax6 | Rabbit | 1/250 | Covance | PRB-278P |
| PCNA | Mouse | 1/100 | Upstate | 5-347-14115 |
| PSA-NCAM | Rabbit | 1/1000 | U. Rutishauser, Sloan- Kettering |
|
| Reelin | Mouse | 1/500 | Chemicon | MAB5364 |
| Sox2 | Rabbit | 1/1000 | Chemicon | AB5603 |
| Tag1 | Mouse | 1/200 | Iowa Hybridoma Bank | |
| Vimentin | Mouse | 1/500 | Sigma | V5255 |
| Zic1/2 | Rabbit | 1/500 | Rosalind Segal, Dana Farber Institute |
For the analyses of hGFAP-DsRed mice, brains were quickly removed from deeply anesthetized animals and placed in 4% paraformaldehyde overnight at 4°C. The next day, 100 µm-thick slices were prepared using a vibratome (Leica VTS 1000, Germany). Free-floating sections were blocked in Tris Buffered Saline (TBS) containing 0.1% Triton X100 + 0.1% Tween-20 + 2% bovine serum albumin (BSA), incubated in primary antibodies (Table 1) and processed as described above. Immunostaining for EGFP was carried out with an antibody against GFP (Abcam, 1:250) and Cy5 dye (Jackson, 1:25) as secondary antibody. Staining was replicated at least in 4–5 slices from three different mice.
Cell Counting and Confocal analysis
Unbiased estimates of cell number were obtained via a Zeiss Axioskope 2 Mot Plus (Carl Zeiss, Thornwood, NY, USA) attached to a motorized stage and connected to a computer running the Stereoinvestigator Software (MicroBrightfield, Colchester, VT, USA). Serial parasagittal sections (one every 400 µm) were used for all counts and a minimum number of 3 animals was used for all analyses. Contours of the EGL, ML, IGL, interlobule WM, deep cerebellar nuclei and SVZ of the fourth ventricle were drawn. Grid sizes of 300 µm × 300 µm were assigned and then sampled with a counting box of 100 µm × 100 µm × 6 µm. Cells were assessed and counted for double immunostaining using a dual 594 and 488 exposure filter.
Images and Z-stacks containing 5–10 Z-sections spaced by 1 to 2 µm over 5 to 20 µm were acquired on a laser-scanning confocal microscope (Olympus FluoView 1000) with a 40x dry objective (N.A. 0.75) or a 60x oil objective (N.A. 1.42), or on an ApoTome Axiovert 200M using a 40x oil objective equipped and analyzed with Axiovision 4.5 software (Carl Zeiss, Thornwood, NY, USA).
Flourimetry
To define the relative intensity of dsRed bright and dsRed faint cells in the EGL, we used 5 sections from 3 different hGFAP-dsRed mouse brains. Using a Leica LSM 510 confocal microscope, 8 µm Z stack images comprised of 1 µm z stack projections were acquired at identical settings for laser intensity and detector gain (Leica, Wetzlar, Germany). 3D reconstructions of the cells were made using the Imaris imaging software (Bitplane, St. Paul, MN, USA). Cell bodies were then traced and the fluorescence intensity quantified using the Leica Application Suite (LAS) imaging software. The data were clustered according to their intensity units at intervals of 30 and plotted as a histogram. 47% of cells had values of less than 90 intensity units, 47% had values of greater than 120, while less than 7% had values between 90 and 120. Those cells which had values less than 90 were counted as dsRed faint cells, while those cells that had values greater than 120 were classified as dsRed bright; cells between 90 and 120 were excluded. Counts were made manually using a single 1 µm Z-stack projection using the LAS cell counting application.
Patch clamp recording in acute slices
Sagittal cerebellar slices were prepared as described previously (Huang et al., 2004). Briefly, 2- to 3-day old mice were anesthetized using pentobarbital (50 mg/kg) and decapitated. A rapid craniotomy that removed the occipital bone and mastoid processes allowed the cerebellum to be quickly detached, removed, and chilled (0–4°C) in 95% O2-5% CO2-saturated artificial cerebrospinal fluid (aCSF). The standard aCSF contained (in mM): 125 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 25 NaHCO3, and 10 glucose, pH 7.4 (when equilibrated with a mixture of 95% O2 and 5% CO2). Next, the cerebellum was glued (using cyanoacrylate glue) to the stage of a vibratome, and sagittal slices (200 µm-thick) were cut in cold oxygenated aCSF. After a recovery period of at least 1 hr in aCSF, slices were placed in a flow-through chamber, held in position by a nylon mesh that was glued to a U-shaped platinum wire, and continuously superfused with oxygenated ACSF. The chamber was mounted on the stage of an upright microscope (BX61; Olympus Optical, Melville, NY) equipped with a 60x water immersion objective, infrared optics, and an Olympus Fluoview 1000 confocal microscope. Prior to recording, the tissue was scanned in the Z axis to find DsRed-fluorescent cells. We could visually find faint and bright DsRed in the same region for recordings. Recordings were made using an Axopatch-200B amplifier (Axon Instruments, Foster City, CA). Cells were recorded at a holding potential of −60 mV in the voltage clamp configuration. Current signals were low-pass filtered at 1–5 kHz and digitized on-line at 5–20 kHz using a Digidata 1320 digitizing board (Axon Instruments) interfaced with an IBM-compatible computer. Settings were determined by compensating the transients of a small (−5 mV) 10 ms-voltage step. Capacitive and leak currents were not subtracted. Data acquisition and analysis were performed using PClamp Version 8 (Axon Instruments, Foster City, CA). Holding potentials were adjusted offline for the junction potential (4 mV). All values in the text are given as mean ± standard error of the mean. For comparison of two groups, two-tailed unpaired Student’s t-tests were used.
Supplementary Material
Representatives examples of Ki67 immunoreactivity in the EGL of hGFAP-DsRed mice at P0 (green in A–D and blue in I–L) and at P4 (green in E–H and blue in M–O). All images were acquired with a laser-scanning confocal microscope and are 1 µm thick single optical sections.
Double-immunostaining for reporter gene expression and cell specific markers in CGE;CAG-EGFP mice analyzed at P7 after two tamoxifen injections at P5. The reporter-positive cells in the cerebellar cortex express GFAP (A–C), Nestin (D–F), and occasionally Math1 (G–I, arrows), while the majority are Mash1 negative (G–I, arrowheads). (J–O): Reporter+ cells (arrowheads) do not express postmitotic neuron markers, including Tag-1 (J–L). All images are Apotome acquired 1 µm-thick Z-stack projections. Scale bar in (A) 20 µm.
(A–C) reporter+ cells (green) that are pax6+ (red, arrows) and pax6-negative (arrowheads) in the EGL. Blue is DAPI. (D–E), open arrows: faint DsRed fluorescent cells in the IGL that express weak Pax6 immunoreactivity (blue); closed arrowheads: bright DsRed fluorescent cells in the IGL that are Pax6-negative. (F–G), closed arrows: DsRed fluorescent cells in the EGL that expresses no Pax6 immunoreactivity. All images are 1 µm thick single confocal optical sections.
Acknowledgments
We acknowledge the expert technical support of Elise Cheng. This work was supported by grants from the National Institute of Health P01 NS 35476, R01 NS060750 (F.V.) and R01 NS048256, DC007681 (A.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alder J, Cho NK, Hatten ME. Embryonic precursor cells from the rhombic lip are specified to a cerebellar granule neuron identity. Neuron. 1996;17:389–399. doi: 10.1016/s0896-6273(00)80172-5. [DOI] [PubMed] [Google Scholar]
- Aruga J, Inoue T, Hoshino J, Mikoshiba K. Zic2 controls cerebellar development in cooperation with Zic1. J Neurosci. 2002;22:218–225. doi: 10.1523/JNEUROSCI.22-01-00218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bani-Yaghoub M, Tremblay RG, Lei JX, Zhang D, Zurakowski B, Sandhu JK, Smith B, Ribecco-Lutkiewicz M, Kennedy J, Walker PR, Sikorska M. Role of Sox2 in the development of the mouse neocortex. Dev Biol. 2006;295:52–66. doi: 10.1016/j.ydbio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Ben-Arie N, Bellen HJ, Armstrong DL, McCall AE, Gordadze PR, Guo Q, Matzuk MM, Zoghbi HY. Math1 is essential for genesis of cerebellar granule neurons. Nature. 1997;390:169–172. doi: 10.1038/36579. [DOI] [PubMed] [Google Scholar]
- Brenner M, Kisseberth WC, Su Y, Besnard F, Messing A. GFAP promoter directs astrocyte-specific expression in transgenic mice. J Neurosci. 1994;14:1030–1037. doi: 10.1523/JNEUROSCI.14-03-01030.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper KB, McCarthy KD. GFAP-positive progenitor cells produce neurons and oligodendrocytes throughout the CNS. Mol Cell Neurosci. 2006;31:676–684. doi: 10.1016/j.mcn.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci U S A. 1999;96:11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis P, Fagan BM, Magness ST, Hutton S, Taranova O, Hayashi S, McMahon A, Rao M, Pevny L. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci. 2004;26:148–165. doi: 10.1159/000082134. [DOI] [PubMed] [Google Scholar]
- Episkopou V. SOX2 functions in adult neural stem cells. Trends Neurosci. 2005;28:219–221. doi: 10.1016/j.tins.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Ganat Y, Soni S, Chacon M, Schwartz ML, Vaccarino FM. Chronic hypoxia up-regulates Fibroblast Growth Factor ligands in the perinatal brain and induces Fibroblast Growth Factor -responsive radial glial cells in the sub-ependymal zone. Neuroscience. 2002;112:977–991. doi: 10.1016/s0306-4522(02)00060-x. [DOI] [PubMed] [Google Scholar]
- Ganat YM, Silbereis J, Cave C, Ngu H, Anderson GM, Ohkubo Y, Ment LR, Vaccarino FM. Early postnatal astroglial cells produce multilineage precursors and neural stem cells in vivo. J Neurosci. 2006;26:8609–8621. doi: 10.1523/JNEUROSCI.2532-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- Hallonet ME, Teillet MA, Le Douarin NM. A new approach to the development of the cerebellum provided by the quail-chick marker system. Development. 1990;108:19–31. doi: 10.1242/dev.108.1.19. [DOI] [PubMed] [Google Scholar]
- Hatten ME, Heintz N. Mechanisms of neural patterning and specification in the developing cerebellum. Annu Rev Neurosci. 1995;18:385–408. doi: 10.1146/annurev.ne.18.030195.002125. [DOI] [PubMed] [Google Scholar]
- Huang H, Barakat L, Wang D, Bordey A. Bergmann glial GlyT1 mediates glycine uptake and release in mouse cerebellar slices. J Physiol. 2004;560:721–736. doi: 10.1113/jphysiol.2004.067801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imura T, Nakano I, Kornblum HI, Sofroniew MV. Phenotypic and functional heterogeneity of GFAP-expressing cells in vitro: differential expression of LeX/CD15 by GFAP-expressing multipotent neural stem cells and non-neurogenic astrocytes. Glia. 2006;53:277–293. doi: 10.1002/glia.20281. [DOI] [PubMed] [Google Scholar]
- Jensen P, Smeyne R, Goldowitz D. Analysis of cerebellar development in math1 null embryos and chimeras. J Neurosci. 2004;24:2202–2211. doi: 10.1523/JNEUROSCI.3427-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei Y, Inagaki N, Nishizawa M, Tsutsumi O, Taketani Y, Inagaki M. Visualization of mitotic radial glial lineage cells in the developing rat brain by Cdc2 kinase-phosphorylated vimentin. Glia. 1998;23:191–199. doi: 10.1002/(sici)1098-1136(199807)23:3<191::aid-glia2>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Sakakibara S, Imai T, Suzuki A, Nakamura Y, Sawamoto K, Ogawa Y, Toyama Y, Miyata T, Okano H. Musashi1: an evolutionally conserved marker for CNS progenitor cells including neural stem cells. Dev Neurosci. 2000;22:139–153. doi: 10.1159/000017435. [DOI] [PubMed] [Google Scholar]
- Klein C, Butt SJ, Machold RP, Johnson JE, Fishell G. Cerebellum- and forebrain-derived stem cells possess intrinsic regional character. Development. 2005;132:4497–4508. doi: 10.1242/dev.02037. [DOI] [PubMed] [Google Scholar]
- Komuro H, Yacubova E, Rakic P. Mode and tempo of tangential cell migration in the cerebellar external granular layer. J Neurosci. 2001;21:527–540. doi: 10.1523/JNEUROSCI.21-02-00527.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Kessler JD, Read TA, Kaiser C, Corbeil D, Huttner WB, Johnson JE, Wechsler-Reya RJ. Isolation of neural stem cells from the postnatal cerebellum. Nat Neurosci. 2005;8:723–729. doi: 10.1038/nn1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JC, Cai L, Cepko CL. The external granule layer of the developing chick cerebellum generates granule cells and cells of the isthmus and rostral hindbrain. J Neurosci. 2001;21:159–168. doi: 10.1523/JNEUROSCI.21-01-00159.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machold R, Fishell G. Math1 is expressed in temporally discrete pools of cerebellar rhombic-lip neural progenitors. Neuron. 2005;48:17–24. doi: 10.1016/j.neuron.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hack MA, Hartfuss E, Kettenmann H, Klinkert W, Kirchnoff F, Gotz M. Neuronal or Glial Progeny: Regional Differences in Radial Glia Fate. Neuron. 2003;37:751–764. doi: 10.1016/s0896-6273(03)00116-8. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Harfuss E, Gotz M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development. 2000;127:5253–5263. doi: 10.1242/dev.127.24.5253. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Colbert MC, Robbins J. Neural crest cells retain multipotential characteristics in the developing valves and label the cardiac conduction system. Circ Res. 2006;98:1547–1554. doi: 10.1161/01.RES.0000227505.19472.69. [DOI] [PubMed] [Google Scholar]
- Noraberg J, Jensen CV, Bonde C, Montero M, Nielsen JV, Jensen NA, Zimmer J. The developmental expression of fluorescent proteins in organotypic hippocampal slice cultures from transgenic mice and its use in the determination of excitotoxic neurodegeneration. Altern Lab Anim. 2007;35:61–70. doi: 10.1177/026119290703500121. [DOI] [PubMed] [Google Scholar]
- Okano-Uchida T, Himi T, Komiya Y, Ishizaki Y. Cerebellar granule cell precursors can differentiate into astroglial cells. Proc Natl Acad Sci U S A. 2004;101:1211–1216. doi: 10.1073/pnas.0307972100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pixley SKR, de Vellis J. Transition Between Immature Glia and Mature Astrocytes Studied with a Monoclonal Antibody to Vimentin. Developmental Brain Research. 1984;15:201–209. doi: 10.1016/0165-3806(84)90097-x. [DOI] [PubMed] [Google Scholar]
- Schuller U, Heine VM, Mao J, Kho AT, Dillon AK, Han YG, Huillard E, Sun T, Ligon AH, Qian Y, Ma Q, Alvarez-Buylla A, McMahon AP, Rowitch DH, Ligon KL. Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma. Cancer Cell. 2008;14:123–134. doi: 10.1016/j.ccr.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbereis J, Cheng E, Ganat YM, Ment LR, Vaccarino FM. Precursors with glial fibrillary acidic protein promoter activity transiently generate GABA interneurons in the postnatal cerebellum. Stem Cells. 2009;27:1152–1163. doi: 10.1002/stem.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature Gen. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Sottile V, Li M, Scotting PJ. Stem cell marker expression in the Bergmann glia population of the adult mouse brain. Brain Res. 2006;1099:8–17. doi: 10.1016/j.brainres.2006.04.127. [DOI] [PubMed] [Google Scholar]
- Spassky N, Han YG, Aguilar A, Strehl L, Besse L, Laclef C, Ros MR, Garcia-Verdugo JM, Alvarez-Buylla A. Primary cilia are required for cerebellar development and Shh-dependent expansion of progenitor pool. Dev Biol. 2008;317:246–259. doi: 10.1016/j.ydbio.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang VY, Zoghbi HY. Genetic regulation of cerebellar development. Nat Rev Neurosci. 2001;2:484–491. doi: 10.1038/35081558. [DOI] [PubMed] [Google Scholar]
- Wingate RJ, Hatten ME. The role of the rhombic lip in avian cerebellum development. Development. 1999;126:4395–4404. doi: 10.1242/dev.126.20.4395. [DOI] [PubMed] [Google Scholar]
- Zappone MV, Galli R, Catena R, Meani N, De Biasi S, Mattei E, Tiveron C, Vescovi AL, Lovell-Badge R, Ottolenghi S, Nicolis SK. Sox2 regulatory sequences direct expression of a (beta)-geo transgene to telencephalic neural stem cells and precursors of the mouse embryo, revealing regionalization of gene expression in CNS stem cells. Development. 2000;127:2367–2382. doi: 10.1242/dev.127.11.2367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representatives examples of Ki67 immunoreactivity in the EGL of hGFAP-DsRed mice at P0 (green in A–D and blue in I–L) and at P4 (green in E–H and blue in M–O). All images were acquired with a laser-scanning confocal microscope and are 1 µm thick single optical sections.
Double-immunostaining for reporter gene expression and cell specific markers in CGE;CAG-EGFP mice analyzed at P7 after two tamoxifen injections at P5. The reporter-positive cells in the cerebellar cortex express GFAP (A–C), Nestin (D–F), and occasionally Math1 (G–I, arrows), while the majority are Mash1 negative (G–I, arrowheads). (J–O): Reporter+ cells (arrowheads) do not express postmitotic neuron markers, including Tag-1 (J–L). All images are Apotome acquired 1 µm-thick Z-stack projections. Scale bar in (A) 20 µm.
(A–C) reporter+ cells (green) that are pax6+ (red, arrows) and pax6-negative (arrowheads) in the EGL. Blue is DAPI. (D–E), open arrows: faint DsRed fluorescent cells in the IGL that express weak Pax6 immunoreactivity (blue); closed arrowheads: bright DsRed fluorescent cells in the IGL that are Pax6-negative. (F–G), closed arrows: DsRed fluorescent cells in the EGL that expresses no Pax6 immunoreactivity. All images are 1 µm thick single confocal optical sections.