Abstract
Schizophrenia patients suffer from cognitive impairments that are not satisfactorily treated by currently available medications. Cognitive dysfunction in schizophrenia encompasses deficits in several cognitive modalities that can be differentially responsive to different medications and are likely to be mediated by different neurobiological substrates. Translational animal models of cognitive deficits with relevance to schizophrenia are critical for gaining insights into the mechanisms underlying these impairments and developing more effective treatments. The 5-choice serial reaction time task (5-CSRTT) is a cognitive task used in rodents that allows simultaneous assessment of several cognitive modalities, including attention, response inhibition, cognitive flexibility, and processing speed. Administration of N-methyl-D-aspartate (NMDA) glutamate receptor antagonists disrupts multiple 5-CSRTT performance measures in a way that mirrors various cognitive deficits exhibited by schizophrenia patients. Some of these disruptions are partially attenuated by antipsychotic medications that exhibit partial effectiveness on cognitive dysfunction in schizophrenia, suggesting that the model has predictive validity. Examination of the effects of pharmacological manipulations on 5-CSRTT performance disruptions induced by NMDA antagonists have implicated a range of brain regions, neurotransmitter systems, and specific receptor subtypes in schizophrenia-like impairment of different cognitive modalities. Thus, disruption of 5-CSRTT performance by NMDA antagonists represents a valuable tool for exploring the neurobiological bases of cognitive dysfunction in schizophrenia.
Introduction
This article reviews the various measures provided by the 5-choice serial reaction time task (5-CSRTT) and discusses the cognitive constructs to which these measures correspond, their relevance to cognitive dysfunction in schizophrenia, and the effects of N-methyl-D-aspartate (NMDA) receptor antagonists on these measures. This review further addresses convergent and divergent findings from other cognitive tests assessing the same cognitive constructs. An overview of the evidence for the involvement of various neurotransmitters and brain circuits in NMDA antagonist-induced deficits in the 5-CSRTT is provided in Supplement 1. The focus of this review is on experimental findings from animal studies, with some discussion of relevant human studies to clarify the significance of the findings in animals to schizophrenia. Where not otherwise specified, the text refers to studies using rats as the experimental subject. A glossary of experimental procedures is provided in Table S1 (see Supplement 1); these terms appear in italics in the text.
Cognitive deficits in schizophrenia
Cognitive impairment is recognized as a core deficit of schizophrenia [1] and is highly correlated with functional impairment [2–4]. Currently available treatments often do not improve cognitive deficits in schizophrenia patients and may even aggravate them [5–7]. The newer atypical antipsychotic medications are widely considered to show greater promise for the improvement of cognition in schizophrenia than the older typical antipsychotics [6,8–10]. However, recent clinical trials suggest that both typical and atypical antipsychotics may confer limited cognitive benefits [11]. In any case, it is clear that all currently available medications produce at best a partial amelioration of symptoms that falls short of restoring normal functioning [3,11,12]. Improved understanding of the etiology of cognitive dysfunction in schizophrenia is needed to allow the development of more effective treatments for these deficits. For this purpose, the development and validation of translational animal models of cognitive deficits in schizophrenia, a shortage of which still exists [13], are crucially important.
Importantly, accrued evidence suggests that “cognition” cannot be treated as a unitary concept when investigating cognitive dysfunction in schizophrenia [14]. Schizophrenia patients exhibit deficits in a wide range of cognitive modalities, including attention [15], response inhibition and impulse control [16], cognitive flexibility [17], and processing speed [18]. Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS), an interdisciplinary initiative by the National Institute of Mental Health aimed at developing new interventions for cognitive deficits in schizophrenia, compiled a list of cognitive performance dimensions affected in schizophrenia that includes at least eight separable cognitive domains [14]. Clinical studies indicate that antipsychotic medications differ in their effects on the various aspects of cognition in schizophrenia patients. For example, the atypical antipsychotic clozapine improves attention but has fewer or no effects on working memory [8,19]. The atypical antipsychotic risperidone, in contrast, improves working memory function in schizophrenia patients [4,8,19,20], as well as enhancing attention [21–23]. Moreover, animal studies assessing schizophrenia-like cognitive deficits revealed that novel compounds with potential procognitive effects show efficacy in tests of one cognitive modality but may be ineffective in tests assessing different cognitive functions. For instance, acute administration of agonists at Group II metabotropic glutamate receptors ameliorated working memory deficits induced by the psychotomimetic phencyclidine (PCP) [24] but did not improve or even exacerbated PCP-induced attentional deficits [25,26], preattentional gating [27–31], and verbal memory [32]. These observations emphasize the need for assessing a broad range of cognitive domains when examining potential treatments for cognitive dysfunction in schizophrenia.
NMDA receptor antagonists as an inducing condition in animal models of cognitive deficits with relevance to schizophrenia
Dysfunction of NMDA glutamate receptors has been suggested to be a likely contributor to schizophrenia pathophysiology, specifically to cognitive dysfunction in schizophrenia [33,34]. Blockade of NMDA receptors by noncompetitive NMDA antagonists produces a schizophrenia-like state in healthy humans [35–46], and exacerbates the symptoms of schizophrenia patients [38,47–50]. Exposure to NMDA antagonists has emerged as a well-accepted inducing condition in tests of various aspects of schizophrenia-like deficits. Importantly, and most relevant to this review, administration of NMDA antagonists, such as PCP or ketamine, to healthy humans also induces profound disruptions of cognition [51], including attentional deficits [52–57], cognitive inflexibility [56,58], and slowed processing speed [59]. Similarly, the exacerbation of schizophrenia symptoms after NMDA antagonist administration includes worsening of cognitive symptoms [50]. NMDA antagonist administration also disrupts cognitive performance in experimental animals, including rats, mice, and monkeys [25,60–108,109–137].
The best exposure regimen for inducing cognitive deficits with NMDA antagonists in experimental animal models has been a subject of some debate. Acute administration of NMDA antagonists has been successfully employed to induce schizophrenia-like cognitive deficits [25,60,61,64–67,69,83,84,96,100,109,111]. However, acute administration can also result in profound general behavioral disruption, such as ataxia and sedation, and motivational deficits that may confound cognitive test results [101,117,138,139].
Some studies avoid these problems by exposing animals to a subchronic regimen of NMDA antagonists, followed by a period of drug washout and measurement of cognitive effects in the drug-free state. (For the purposes of this review, the term “subchronic administration” is defined as a limited number, usually 5–15, of discrete administrations of a drug, given 1–2 times daily, while “chronic administration” is reserved for continuous delivery of a drug for several days, e.g. via an osmotic minipump.) Cognitive deficits have been observed after such NMDA antagonist treatments [62,63,68,70,78,86,87,89–93,105,107,108,110,118], though not in all cases [66,71,78,101,117,119,120,140]. There are reports of lasting cognitive deficits in humans chronically using phencyclidine or ketamine even after cessation of drug-taking [38,43,141–145; but see 146,147]. However, the results of such studies are confounded by several factors, such as potentially pre-existing cognitive deficits in persons likely to abuse NMDA antagonists, as well as concurrent abuse of other psychoactive drugs by virtually all users of NMDA antagonists. For example, while Cosgrove and Newell observed cognitive deficits in chronic PCP users after 12 hours of abstinence from PCP, the PCP user group also reported significantly higher levels of alcohol drinking than the control group [145]. In studies where NMDA antagonists were administered under controlled conditions, both the schizophrenia-like state evoked in healthy subjects and symptom exacerbation induced in schizophrenia patients by NMDA antagonists were typically observed only during acute intoxication, not during withdrawal or post-drug [35,36,40,50–53,56–59,148–151]. Nevertheless, the possibility remains that long-term frequent administration of high doses of NMDA antagonists to humans (which cannot ethically be performed in controlled experimental settings) may result in enduring neuronal changes that lead to lasting cognitive impairments.
An alternative approach involves repeated treatment with an NMDA antagonist, followed by a drug-free period and then assessment of cognitive performance upon repeated re-exposure to the NMDA antagonist, with the drug on board (for the purposes of this review, this regimen will be referred to as “repeated administration”). Repeated PCP administration reduces or even eliminates ataxia and other behaviorally disruptive effects that are seen after the first PCP administration [101,117,138], and may also allow the development of a degree of tolerance to initial profound cognitive deficits induced by acute PCP, such that the animal can perform the cognitive task, thereby permitting the quantification of deficits. Upon re-challenge with additional PCP injections, selective cognitive impairments are observed [71,101,117,138]. This administration regimen therefore permits investigation of the schizophrenia-like cognitive deficits induced by the acute actions of NMDA antagonists, while avoiding the confounding effects of nonspecific behavioral disruption and excessive cognitive disruption induced by the first NMDA antagonist administration.
Different NMDA antagonist administration regimens may be better suited for modeling deficits in certain cognitive domains and less suited for other cognitive deficits. For example, subchronic NMDA antagonist administration followed by washout and testing in the drug-free state appears to produce robust impairment of cognitive flexibility and disinhibition of impulsive responding, while its effects on attentional performance seem less consistent (see below). These differential effects are likely due to distinct neurochemical effects induced by the various NMDA antagonist administration regimens.
Of note, while numerous studies report the effects of single acute administrations of NMDA antagonists, many of these studies administer several doses of NMDA antagonists using a within-subjects design. While the experimental design of these studies generally includes several days of drug washout between drug administrations, profound carryover effects on cognitive performance after only 1–2 exposures even after washout periods of 10 days or more have been observed at least in the case of PCP [26,71,121,122]. Thus, the cognitive effects reported in studies nominally using a “single acute administration” design may be most reflective of the effect of repeated, though discontinuous, administrations of NMDA antagonists.
Finally, different species and strains may differ substantially in their sensitivity to NMDA antagonists. Therefore, treatment doses may vary significantly between studies using different experimental animals. While 5-CSRTT research has been conducted predominantly in rats, this review includes corroborating evidence from other species when such data are available, and discusses instances of strain differences in NMDA antagonist effects.
5-choice serial reaction time task
The 5-CSRTT was originally developed as a test of attention [152,153] based on Leonard’s choice reaction time task [154]. Some researchers have suggested that it constitutes a rodent analog of the continuous performance task (CPT) that is used to quantify attention in humans [155]; however, certain key differences to the CPT exist (see below). Numerous studies have demonstrated the construct validity of the 5-CSRTT as a test of attention [13]. Additionally, the 5-CSRTT provides measures of a number of other cognitive domains with relevance to schizophrenia, including response disinhibition/impulsivity, cognitive flexibility/compulsivity, and processing speed. Originally developed for rats, a mouse version of the 5-CSRTT has existed for some time [156,157].
The task requires the experimental animal to monitor five equidistant locations for the presentation of a visual stimulus (see Figure 1). The animal indicates detection of the stimulus by performing a nosepoke in the location where the light flash was presented. Trials are usually initiated by the animal via head entry into the reinforcer magazine. After the intertrial interval (ITI) elapses, a brief light is presented pseudorandomly in one of the apertures. Nosepokes performed in the location of the light stimulus during a fixed limited hold period (“correct responses”) are rewarded with a reinforcer, most commonly a food pellet for rats and liquid sweetened milk for mice. Nosepokes in a different aperture (“incorrect responses”) result in a brief timeout period, usually signaled by a change in lighting in the operant chamber (e.g., extinguishing the house light if the task is performed with lights on, or illuminating the house light if the task is performed with lights off), and no reinforcer delivery. Failures to make any nosepoke during the limited hold periods (“omissions”), nosepokes in any aperture made before presentation of the target stimulus (“premature responses”), continued nosepokes after a correct response but before collection of the reward (“perseverative responses”), or nosepokes during the timeout period (“timeout responses”) are also recorded and usually result in initiating or resetting of a timeout period. Finally, the latency of the animal to make a correct or incorrect response (“correct latency” and “incorrect latency”) and the time it takes the animal to retrieve the reinforcer (“reward latency”) are also recorded.
Figure 1.

Schematic of a rat’s performance in the 5-choice serial reaction time task.
By allowing the simultaneous examination of multiple aspects of cognition, the 5-CSRTT offers a valuable tool for efficiently assessing the effects of various manipulations thought to induce schizophrenia-like disruptions in different cognitive domains, as well as the effectiveness of various treatments on these disruptions. While the predictive validity of the 5-CSRTT for potential therapeutics for cognitive deficits in schizophrenia remains difficult to assess in the absence of well-established positive controls that treat cognitive symptoms, numerous studies have documented robust predictive validity of the 5-CSRTT for manipulations impairing or improving attentional performance across rodents and humans [13]. Moreover, a variety of manipulations can further extend the versatility of this task. For example, in addition to divided spatial attention, assessed by the requirement for the subject to simultaneously monitor five different potential stimulus locations, researchers can gauge sustained attention by increasing the number of trials in a challenge test session and comparing performance on earlier vs. later trials. Additionally, attentional load can be increased by using a variable ITI. This modification prevents the use of mediating strategies based on time estimation, which is possible with a fixed ITI duration, because the animal must attend to the apertures throughout the ITI instead of only during the short time window when the stimulus is expected to occur.
A recently developed modification of the 5-CSRTT addresses a major difference between the 5-CSRTT and the human CPT, namely, the absence in the 5-CSRTT of “non-signal” stimuli after whose presentation the subject needs to withhold responding to receive a reward. The newly developed 5C-CPT adds a condition in which all apertures are simultaneously illuminated; in these trials, the animal is required to withhold responding in any aperture to receive the reward [158]. The 5C-CPT therefore allows the assessment of inhibition of responding to irrelevant stimuli, a key component of the human CPT.
Attention: accuracy, correct and incorrect responses, and omissions
Attentional function is severely disrupted in schizophrenia [15]. Schizophrenia patients perform well below healthy controls in the CPT, a human attention task with some analogies to the 5-CSRTT [159–163]. The main measure of attentional performance in the 5-CSRTT is accuracy (sometimes also referred to as “percent correct responses”), defined as the total number of correct responses divided by the sum of correct and incorrect responses. Accuracy is a conservative measure of attention because it is independent of omissions. Consequently, this measure is relatively impervious to potential confounds such as sedation, locomotor impairment, or motivational changes.
In some cases, the stringency of the accuracy measure may preclude detection of attentional changes resulting from pharmacological or other manipulations. Animals with greater entrainment to respond to the light stimulus are more likely to withhold responding after failure to attend to the visual stimulus instead of ‘guessing’ which hole was lit, thus making an omission error instead of an incorrect response. Such omissions are particularly likely to occur when using mice as the experimental subjects, because mice appear to have better entrainment to the light stimulus compared to rats [13,164]. An increase in omissions, typically accompanied by a decrease in total correct responses, can therefore reflect a deficit in attention, even if accuracy is unaltered. Of course, such a pattern can also result from non-cognitive disruptions, such as sedation, locomotor impairment, or reduced motivation. However, inspection of other 5-CSRTT measures can often rule out these confounds. Reduced motivation for the food reward (e.g., because of feeding prior to testing in the task) reduces the total number of trials completed by the animal and the number of head entries into the collection magazine [165], as well as the latency to retrieve the reward [166]. Sedation or locomotor impairment would tend to increase response latencies and reward collection latencies in the task. Therefore, if 5-CSRTT omissions are increased and/or total correct responses are decreased without concomitant decreases in head entries into the magazine, decreases in completed trials, or slowed latencies, it is likely that the disruptions reflect a true attentional deficit instead of motivational deficits, sedation, or locomotor impairment.
The above considerations illustrate a crucial point about the 5-CSRTT. It is critically important to consider the various parameters measured by the task in combination. While this review addresses different 5-CSRTT measures separately in the interest of clarity, the final interpretation of the effects of any manipulation on the 5-CSRTT should only be made after taking into account how all of the task measures are affected.
Administration of NMDA antagonists induces attentional deficits in the 5-CSRTT. Accuracy in the 5-CSRTT was decreased in rats after acute [67,96] or repeated administrations [71] of PCP (see Figure 2A), or acute administration of dizocilpine, another NMDA antagonist also referred to as MK-801 [83,167], at moderate doses (0.05–0.06 mg/kg). Acute low doses of dizocilpine (≤0.03 mg/kg) were without effect [84,168], and acute higher doses led to a significant increase in omissions without affecting accuracy, indicating a general, nonspecific behavioral suppression that affected correct and incorrect responses equally [123,168; but see 66,100]. Repeated administration of high doses (0.25 mg/kg) of dizocilpine did not produce attentional deficits in the 5-CSRTT when testing was conducted 24 h after drug administration [66]. This negative finding may indicate that attentional performance is less sensitive than other cognitive modalities to disruption by NMDA antagonist pretreatment in the absence of acute drug exposure, and is more robustly disrupted while the drug is on board. DBA, but not C57/BL6, mice also exhibited decreases in 5-CSRTT accuracy after acute PCP administration (see Figure 3A, D) [25]. However, 5-CSRTT accuracy was unaffected by acute ketamine administration in mice [124], mirroring the observation that ketamine does not reliably disrupt attention in humans [51,58,148,149,151], possibly because it has lower affinity and less selectivity for the NMDA receptor than PCP [169]. Chronic clozapine, an atypical antipsychotic with some effectiveness against attentional deficits in schizophrenia [8,19], attenuated disruptions in 5-CSRTT accuracy induced by repeated PCP [71] (see Figure 2A), indicating that this model may have predictive validity [170]. Further supporting this possibility, acute administration of haloperidol, a typical antipsychotic that is less effective against attentional deficits in schizophrenia [171–173], did not affect 5-CSRTT accuracy disruption induced by acute dizocilpine, while acute clozapine attenuated the deficit [66]. In contrast, chronic treatment with a different atypical antipsychotic, quetiapine, did not attenuate 5-CSRTT deficits induced by repeated PCP [122]. As discussed above, cognitive impairments respond differentially to different antipsychotic medications [8,19]. NMDA antagonist-induced disruptions in 5-CSRTT performance may be similarly differentially sensitive to different therapeutics. However, recent reports suggest that quetiapine may in fact improve attentional deficits in schizophrenia patients, although relatively few studies are available so far [174]. As stated above, conclusive assessment of the predictive validity of a model for cognitive schizophrenia deficits is complicated by the fact that documented ameliorative effects of any medication on these deficits remain limited and controversial.
Figure 2.
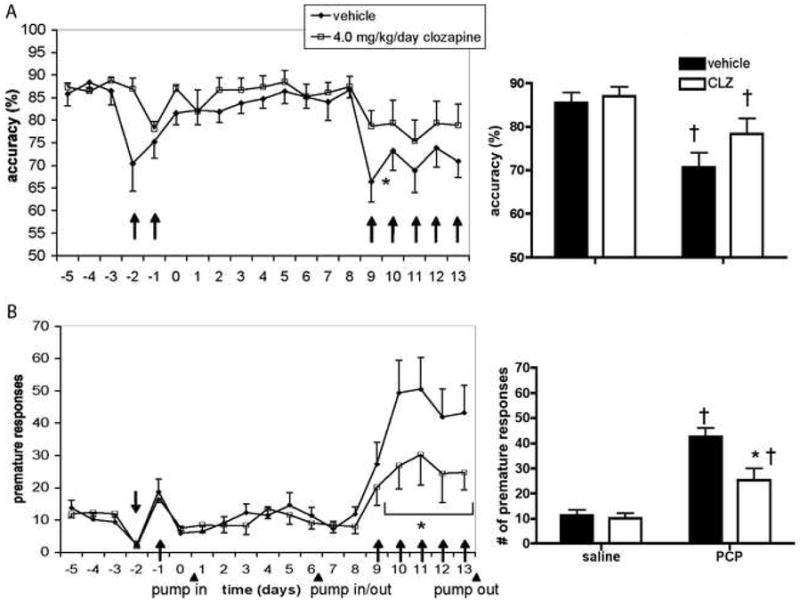
Effects of chronic clozapine treatment on 5-CSRTT performance disruption induced by repeated PCP. Accuracy (A) and premature responses (B) are shown as mean±SEM. *P<0.05 vs. vehicle group; †P<0.05 vs. performance after saline injections; ↑ or ↓ indicates a PCP injection. CLZ = clozapine; pump in/out = beginning/end of chronic clozapine treatment via subcutaneous minipumps. Adapted from [71], © Springer-Verlag 2007, with kind permission from Springer Science+Business Media.
Figure 3.
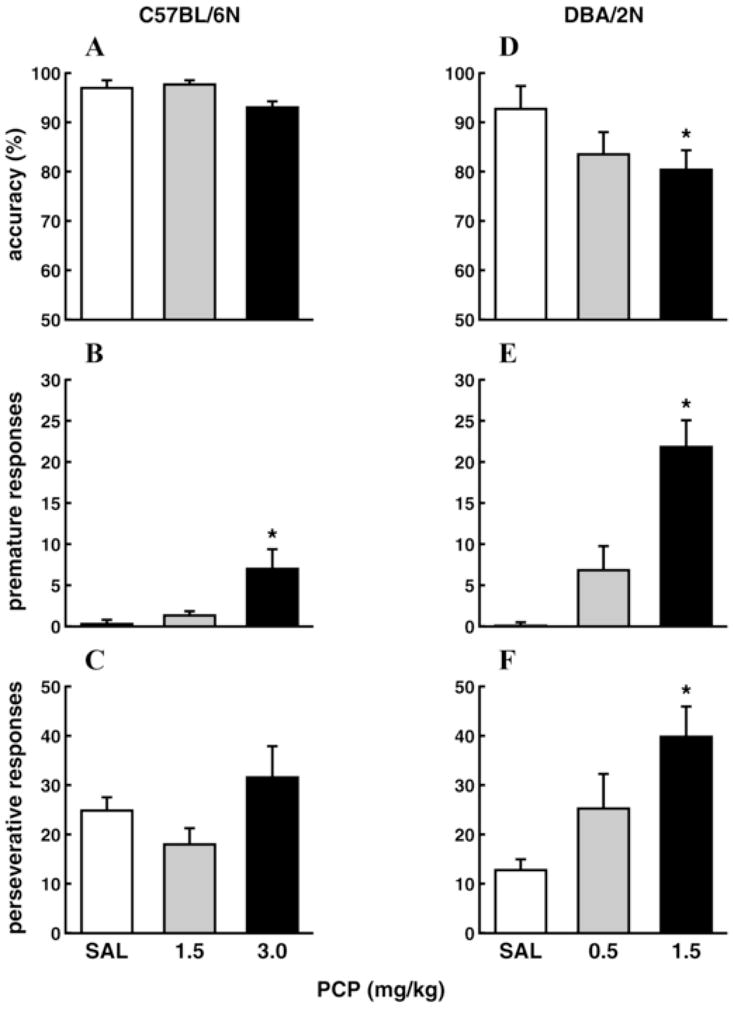
Effects of PCP on 5-CSRTT performance of C57BL/6N and DBA/2N mice. Accuracy (A), premature responses, (B) and perseverative responses (C) of C57BL/6N mice are shown as mean±SEM; (D), (E), (F) same parameters in DBA/2N mice. *P<0.05 vs. saline group. SAL = saline. Adapted from [25], © Springer-Verlag 2005, with kind permission from Springer Science+Business Media.
At doses that decrease accuracy in the 5-CSRTT, PCP and dizocilpine induce hyperlocomotion in rats and mice [84,96,123,175]. These findings raise the question whether 5-CSRTT performance may have simply been disrupted by locomotor hyperactivity rather than a true attentional deficit. However, other manipulations that increase locomotor activity, such as amphetamine, leave accuracy in the 5-CSRTT unaffected [176]. Moreover, in most studies discussed here, latency to reward retrieval was unchanged and latency to correct response actually increased after NMDA antagonist exposure [71,84,96,100,167,168]. These findings suggest that potential increases in locomotor activity were not severe enough to lead to indiscriminate, rapid responding that disrupted performance. Importantly, the deficit in 5-CSRTT accuracy after intracerebral injection of 3-(R)-2-carboxypiperazin-4-propyl-1-phosphonic acid (CPP), a competitive NMDA antagonist, was significantly reduced when the attentional load in the task was lowered by increasing the duration of the visual stimulus [177], suggesting that the observed decrease in accuracy was indeed attributable to impaired attention.
Disruption of attentional performance in the 5-CSRTT induced by NMDA antagonists mirrors the observation of similar disruptions in other attentional tasks after the same manipulation. Decreased attentional performance after acute administration of PCP or dizocilpine was observed in the open-field stimulus object test [76], two-lever and three-lever choice tasks [94,98], lateralized reaction time task [88], lateralized visual signal detection task [60,103,104], and operant ratio discrimination task [64,65]. However, performance in the lateralized visual signal detection task was not altered after a period of withdrawal from repeated dizocilpine administration [120], again suggesting that attentional performance is less affected in the drug-free state after NMDA antagonist treatment than during acute drug exposure. Similar to the 5-CSRTT, single acute or repeated administration of ketamine did not affect attentional performance in the lateralized visual signal detection task up to doses that produced profound nonspecific performance disruptions [99], again pointing toward less reliable effects of ketamine on attentional performance.
Impulsivity: premature responses
Premature responses (sometimes also called “anticipatory responses”; i.e., nosepoke responses performed before presentation of the visual stimulus) are widely accepted as a measure of impulsivity in the 5-CSRTT [152,178–181], and reflect loss of impulse control and disinhibition of inappropriate responding. Some controversy exists regarding whether this type of impulsivity is strictly an aspect of cognition, or should be more properly considered a type of motor impulsivity [180,182]. Schizophrenia patients do exhibit impulsivity characterized by response disinhibition, exemplified by increased errors of commission on Go/No Go tasks or similar tests of response inhibition [16,183–186]. This disinhibition of impulsive responding correlates with impaired overall cognitive performance and other clinical symptoms [186–188], and exhibits similarities to premature 5-CSRTT responding. It should be noted, however, that 5-CSRTT premature responding constitutes failure to inhibit inappropriate responding before the presentation of any stimulus, while errors of commission on Go/No Go tasks or the human CPT reflect failure to inhibit inappropriate responding after the presentation of an irrelevant stimulus when responding should be inhibited. Recent findings suggest that these two types of response inhibition may be differentially mediated [J. W. Young, personal communication].
Most versions of the 5-CSRTT “punish” premature responses with a timeout period, during which the animal is unable to initiate a new trial or otherwise perform the task [152]. Some researchers, however, use a version of the task in which premature responses are merely recorded and have no consequences for the animal [189,190]. This alteration of the task changes the nature of premature responses, because it decreases the incentive for the animal to withhold premature responding. However, a literature overview shows very similar effects of NMDA antagonist administration on both punished or unpunished premature responses. Furthermore, while the original version of the 5-CSRTT requires the animal to initiate each trial through a head entry into the reward magazine [152], some studies employ a version of the task in which new trials start automatically; that is, the ITI begins directly after a correct or incorrect response or at the end of the limited hold period [189,190]. This version of the task does not allow a distinction between premature responses and another 5-CSRTT measure, perseverative responses (discussed below). Instead, any responses that occur during the ITI (which, in this version of the task, encompasses any time period other than stimulus presentation or limited hold) are recorded as “anticipatory responses” or “inappropriate responses.” This lack of distinction is not optimal for the assessment of the effects of NMDA antagonists because evidence suggests that premature and perseverative responses reflect different aspects of response inhibition that have been shown to be dissociable by pharmacological and other manipulations (see below).
Premature responding in the 5-CSRTT is increased by exposure to NMDA antagonists. Single or repeated administration of PCP increased premature responses in the 5-CSRTT in rats (see Figure 2B) [71,96; but see 67] and mice (see Figure 3B, E) [25]. At doses that disrupted accuracy (0.05–0.06 mg/kg), acute administration of dizocilpine also increased premature responses in the 5-CSRTT [83]. Decreases in accuracy and increases in premature responding sometimes co-occur in the 5-CSRTT [179], and it may be argued that the decreases in attention observed after these doses of PCP and dizocilpine were secondary to disruption of task performance by excessive premature responding. However, a number of studies have shown that effects on accuracy and premature responding can be dissociated [25,176,189,190]. Increases in premature responding without concurrent effects on accuracy have been observed after acute administration of a low dose of dizocilpine (0.03 mg/kg) in some studies [84; but see 83,123]. Higher doses induced general, nonspecific behavioral disruption [123,168; but see 66,100]. Increases in premature responses were also observed after acute ketamine administration in CD1, but not C57/BL6, mice [124]. Similar to PCP-induced decreases in accuracy, chronic or acute clozapine, but not acute haloperidol, attenuated the increase in premature responses induced by repeated PCP [71](see Figure 2B) or single acute administrations of dizocilpine [66] in the 5-CSRTT.
Increases in impulsive responding were also observed after single acute or repeated PCP administration, as well as acute dizocilpine administration, during differential reinforcement of low rates (DRL) in rats [75,84,85,109,123,125–131] or mice [81,132,133]. Acute administration of PCP also increased impulsive responding in the lateralized reaction time task in rats [88]. Subchronic administration of PCP, followed by several days of drug washout, delayed extinction of operant responding and increased responding for a conditioned reinforcer in rats [91], and increased impulsive responding in the object retrieval/detour task in monkeys [89,90,92,118], indicating that impulsivity may be more reliably observed in the drug-free state after NMDA antagonist treatment than attentional deficits. Acute ketamine administration did not affect DRL performance in rats [134]. It is possible that impulsivity, like attentional disruption, is less reliably induced by ketamine than by PCP or dizocilpine; however, the paucity of studies investigating ketamine effects on impulsive responding does not permit definite conclusions.
Cognitive flexibility: perseverative responses
Perseverative responses, defined as continued nosepokes after a correct response has been performed, are considered an indicator of compulsivity. Cognitive inflexibility, i.e. the inability to alter behavior in reaction to changing situational demands, is hypothesized to contribute to compulsivity [191]. Because perseverative responses constitute persistence in an initially rewarded behavior (nosepoke) despite the fact that it is no longer rewarded, they are often considered a measure of cognitive inflexibility [192]. Cognitive inflexibility is a characteristic deficit in schizophrenia [193–199; but see 200].
The interpretation of perseverative responses as an indicator of cognitive inflexibility assumes that perseverative nosepokes occur in the same aperture as the initial correct responses, thus constituting repeated maladaptive enactment of a previously rewarded action. Unfortunately, 5-CSRTT studies do not typically record the actual aperture in which perseverative responses occur. However, informal observations in the authors’ laboratory suggest that, indeed, perseverative responses are usually made in the same aperture as the original correct response. Different versions of the 5-CSRTT either record perseverative responses without consequences for the animal or punish them with a timeout. The latter option means that only one perseverative response can be recorded per trial, even if the animal performs a large number of repetitive nosepokes, because the initial perseverative response triggers the timeout period and any remaining nosepokes are recorded as timeout responses. This may lead to underestimation of perseveration.
Acute administration of PCP or dizocilpine increased 5-CSRTT perseverative responses in rats [83,84,96,123]. Acute administration of PCP also increased perseverative responses in DBA, but not C57/BL6, mice (see Figure 3C, F) [25], while acute ketamine increased perseverative responses in C57/BL6, but not CD1, mice [124]. Increases in perseverative responding sometimes coincided with decreases in 5-CSRTT accuracy [25,83,96,123], but were also observed at doses that did not affect accuracy, omissions, or response latencies [84,96,124]. Importantly, although NMDA antagonists often increased both premature and perseverative responding [83,84,96,123], effects on the two types of disinhibited responding could be dissociated. For example, while acute PCP did not affect perseverative responses in C57/BL6 mice in a study by Greco and coworkers, the same doses increased premature responses (see Figure 3C, F) [25], suggesting that different mechanisms of inhibitory response control may be implicated. Similarly, although acute administration of ketamine increased perseverative responses in C57/BL6 mice, premature responses remain unaffected in these mice; conversely, acute ketamine increased premature responses, but not perseverative responses, in CD1 mice [124].
In other tasks assessing compulsivity and cognitive flexibility, NMDA antagonists also induced performance disruption indicative of increased perseveration. Such deficits were observed in the reversal learning task in rats after single or repeated administration of PCP [61,69,77,87], or during the drug-free state following subchronic PCP [68,70,72,86,91,108] or ketamine [140] administration. However, it should be noted that one study using acute dizocilpine found impairments in reversal learning only at doses that also disrupted simple odor discrimination [82]. The extradimensional shift phase of the set shifting task was likewise disrupted during the drug-free state after PCP administered subchronically [62,63,105,107,110,115], chronically [116], once 24 hours before testing [79], or postnatally [114,115], though not after withdrawal from subchronic ketamine treatment [140]. Similar deficits were seen in the set shifting task in rats given an acute administration of dizocilpine [111] and mice given repeated PCP [95]. Perseverative errors were also observed in the repeated acquisition task after acute administration of PCP or dizocilpine to rats [74,113] or monkeys [73,80], and in the object retrieval/detour task after a period of withdrawal from subchronic PCP administered to monkeys [89,90,92].
The persistent increase in perseverative errors in a variety of tasks in the drug-free state after NMDA antagonist treatment in both rats and monkeys [62,63,68,70,72,79,86,89–92,105,107,108,110,115,116,140] suggests that this administration regimen reliably induces some types of cognitive inflexibility, and may be expected to increase perseverative responding in the 5-CSRTT also. However, one study found no impairment of reversal learning after a period of withdrawal from subchronic PCP exposure in mice [119], possibly indicating that this species is less sensitive to this manipulation. A similar PCP regimen also produced no disruption in the fixed/variable goal location task in rats [78]; this task may be less suited to detect cognitive deficits induced by subchronic NMDA antagonist treatment. Increases in perseverative errors in both the 5-CSRTT [124] and other tasks [140] after ketamine treatment suggest that cognitive flexibility may be more susceptible to disruption with ketamine than attention.
Cognitive flexibility/stimulus control: timeout responses
Timeout responses (i.e., nosepokes made during the timeout interval) are not as well studied as other 5-CSRTT parameters. Unfortunately, most studies do not even report this measure. Timeout responses constitute continued responding past the point when responding is no longer rewarded, and thus may represent an additional measure of compulsivity related to cognitive inflexibility. Indeed, as outlined above, when perseverative responses are punished by a timeout period, the majority of perseverative responses may be recorded as timeout responses. This may explain why, in studies performed in our laboratory, repeated PCP administration did not increase perseverative responding but significantly increased timeout responding [26,71,121,122]. In addition, the measure of timeout responses may record disorganized responses during the timeout that are not tied to stimulus presentation. Such responses may reflect a loss of stimulus control over responding. Timeout responses could therefore constitute a measure of stimulus control impairment, especially in studies that do not punish perseverative responding (thus reducing the influence of perseveration on the number of timeout responses recorded). To better assess this possibility, 5-CSRTT response patterns could be analyzed to determine whether timeout responses in a given experiment occur in close temporal and/or spatial proximity to the stimulus, or are largely unrelated to stimulus presentation.
Like perseverative responses, timeout responses appear to reflect a different aspect of response disinhibition than premature responses. Chronic clozapine treatment, at doses that successfully attenuated increases in premature responding induced by repeated PCP administration, did not affect the PCP-induced increases in timeout responding [71]. Conversely, chronic treatment with a metabotropic glutamate receptor antagonist attenuated increases in timeout responding induced by repeated PCP, but did not affect increases in premature responding [26] (see Supplement 1).
Speed of processing: response latencies
The average latency of the animal to make a correct response in the 5-CSRTT represents a measure of its processing speed in the task, as long as motor slowing or lack of motivation is ruled out. Increases in correct response latency in the absence of changes in another latency measure, the average latency to retrieve the reward, suggest that the animal’s locomotor function and motivation for the reward are unaffected; such a pattern of effects is therefore likely to reflect a true slowing of processing speed. The average latency to make an incorrect response is rarely reported, because it is generally affected similarly to the latency to a correct response [26,71,121,122], despite exhibiting somewhat less reliable drug effects [176].
Increases in correct response latency in the 5-CSRTT, without alteration of reward latency, were observed after single or repeated PCP administration [67,71,96]. In contrast, one study found decreases in latency to a correct response after an acute low dizocilpine dose (0.03 mg/kg) [83] with no effect on other 5-CSRTT measures, suggesting that the decrease in correct response latency was not simply due to a gross increase in locomotor activity. These results may therefore indicate that the effects of NMDA antagonists on processing speed are dose-dependent, with low doses subtly enhancing processing speed. Indeed, increases in the latency to a correct response in the 5-CSRTT were found after administration of an acute high dose of dizocilpine (0.25 mg/kg), as well as in the drug-free state after subchronic dizocilpine administration [66]. However, during withdrawal from subchronic dizocilpine treatment, reward latencies were also significantly increased; therefore, nonspecific locomotor impairment cannot be ruled out in this case.
Single acute or repeated PCP administrations also increased response latencies in a number of other cognitive tasks, including the three-lever choice task [94] and the reversal learning task [77]. Acute administration of ketamine tended to increase response latency in a simple reaction time task in monkeys [112]. Increases in response latency were also found after acute dizocilpine administration in the lateralized visual signal detection task [103,104].
Conclusion
In summary, NMDA antagonist administration induces a number of disruptions of performance in the 5-CSRTT with relevance to cognitive dysfunction in schizophrenia, including impaired attention, increased impulsivity, cognitive inflexibility, and slowed processing speed. These deficits are partially responsive to treatments that show some effectiveness in ameliorating cognitive schizophrenia symptoms, suggesting that NMDA antagonist-induced disruption of 5-CSRTT performance has predictive validity as an animal model of cognitive disruptions in schizophrenia.
Investigation of neurochemical changes after NMDA antagonist administration, and examination of the effects of various neurotransmitter manipulations on NMDA antagonist-induced disruption of 5-CSRTT performance, have implicated a number of brain areas and neurotransmitter systems in schizophrenia-like cognitive deficits as assessed by the 5-CSRTT. These findings are discussed in the Supplement 1, and summarized in Tables S2 and S3.
Disruption of 5-CSRTT performance via NMDA antagonist administration therefore presents a promising model of cognitive dysfunction that may be used to: (i) explore the role of different brain circuits and neurotransmitter systems in the cognitive symptoms of schizophrenia, (ii) ensure benign cognitive profiles of novel antipsychotic medications, and (iii) investigate the effectiveness of proposed procognitive treatments for schizophrenia patients.
Supplementary Material
Acknowledgments
The authors wish to thank Dr. Jared Young for insightful comments and input on this review; Drs. Barbara Greco, Roberto Invernizzi, and Mirjana Carli for permission to use Figure 3; Mr. Mike Arends for editorial assistance; and Ms. Janet Hightower for assistance with figure preparation. NA was supported by Tobacco-Related Disease Research Program (TRDRP) Individual Pre-doctoral Fellowship 15DT-0048 from the State of California, and AM was supported by National Institute of Mental Health grant 2R01MH062527.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Elvevåg B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol. 2000;14:1–2. [PubMed] [Google Scholar]
- 2.McGurk SR, Meltzer HY. The role of cognition in vocational functioning in schizophrenia. Schizophr Res. 2000;45:175–184. doi: 10.1016/s0920-9964(99)00198-x. [DOI] [PubMed] [Google Scholar]
- 3.Sharma T, Antonova L. Cognitive function in schizophrenia: deficits, functional consequences, and future treatment. Psychiatr Clin North Am. 2003;26:25–40. doi: 10.1016/s0193-953x(02)00084-9. [DOI] [PubMed] [Google Scholar]
- 4.Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res. 2004;72:41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Bilder RM, Lieberman JA, Kim Y, Alvir JM, Reiter G. Methylphenidate and neuroleptic effects on oral word production in schizophrenia. Neuropsychiatry Neuropsychol Behav Neurol. 1992;5:262–271. [Google Scholar]
- 6.Gallhofer B, Bauer U, Lis S, Krieger S, Gruppe H. Cognitive dysfunction in schizophrenia: comparison of treatment with atypical antipsychotic agents and conventional neuroleptic drugs. Eur Neuropsychopharmacol. 1996;6:S13–S20. doi: 10.1016/0924-977x(96)00010-7. [DOI] [PubMed] [Google Scholar]
- 7.Mortimer AM. Cognitive function in schizophrenia: do neuroleptics make a difference? Pharmacol Biochem Behav. 1997;56:789–795. doi: 10.1016/s0091-3057(96)00425-x. [DOI] [PubMed] [Google Scholar]
- 8.Meltzer HY, McGurk SR. The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull. 1999;25:233–255. doi: 10.1093/oxfordjournals.schbul.a033376. [DOI] [PubMed] [Google Scholar]
- 9.Bilder RM, Goldman RS, Volavka J, Czobor P, Hoptman M, Sheitman B, et al. Neurocognitive effects of clozapine, olanzapine, risperidone, and haloperidol in patients with chronic schizophrenia or schizoaffective disorder. Am J Psychiatry. 2002;159:1018–1028. doi: 10.1176/appi.ajp.159.6.1018. [DOI] [PubMed] [Google Scholar]
- 10.Bender S, Dittmann-Balcar A, Schall U, Wolstein J, Klimke A, Riedel M, et al. Influence of atypical neuroleptics on executive functioning in patients with schizophrenia: a randomized, double-blind comparison of olanzapine vs. clozapine. Int J Neuropsychopharmacol. 2005;9:135–145. doi: 10.1017/S1461145705005924. [DOI] [PubMed] [Google Scholar]
- 11.Keefe RSE, Bilder RM, Davis SM, Harvey PD, Palmer BW, Gold JM, et al. Neurocognitive Effects of Antipsychotic Medications in Patients With Chronic Schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64:633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- 12.Keefe RS, Seidman LJ, Christensen BK, Hamer RM, Sharma T, Sitskoorn MM, et al. Comparative effect of atypical and conventional antipsychotic drugs on neurocognition in first-episode psychosis: a randomized, double-blind trial of olanzapine versus low doses of haloperidol. Am J Psychiatry. 2004;161:985–995. doi: 10.1176/appi.ajp.161.6.985. [DOI] [PubMed] [Google Scholar]
- 13.Young JW, Powell SB, Risbrough VB, Marston HM, Geyer MA. Using the MATRICS to guide development of a preclinical cognitive test battery for research in schizophrenia. Pharmacol Ther. 2009;122:150–202. doi: 10.1016/j.pharmthera.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Laurent A, Saoud M, Bougerol T, d’Amato T, Anchisi AM, Biloa-Tang M, et al. Attentional deficits in patients with schizophrenia and in their non-psychotic first-degree relatives. Psychiatry Res. 1999;89:147–159. doi: 10.1016/s0165-1781(99)00109-2. [DOI] [PubMed] [Google Scholar]
- 16.Wykes T, Reeder C, Corner J. The prevalence and stability of an executive processing deficit, response inhibition, in people with chronic schizophrenia. Schizophr Res. 2000;46:241–253. doi: 10.1016/s0920-9964(99)00233-9. [DOI] [PubMed] [Google Scholar]
- 17.Morice R. Cognitive inflexibility and pre-frontal dysfunction in schizophrenia and mania. Br J Psychiatry. 1990;157:50–54. doi: 10.1192/bjp.157.1.50. [DOI] [PubMed] [Google Scholar]
- 18.Nelson HE, Pantelis C, Carruthers K, Speller J, Baxendale S, Barnes TRE. Cognitive functioning and symptomatology in chronic schizophrenia. Psychol Med. 1990;20:357–365. doi: 10.1017/s0033291700017670. [DOI] [PubMed] [Google Scholar]
- 19.Sharma T, Mockler D. The cognitive efficacy of atypical antipsychotics in schizophrenia. J Clin Psychopharmacol. 1998;18:12S–19S. doi: 10.1097/00004714-199804001-00004. [DOI] [PubMed] [Google Scholar]
- 20.Green MF, Marshall BD, Jr, Wirshing WC, Ames D, Marder SR, McGurk S, et al. Does risperidone improve verbal working memory in treatment-resistant schizophrenia? Am J Psychiatry. 1997;154:799–804. doi: 10.1176/ajp.154.6.799. [DOI] [PubMed] [Google Scholar]
- 21.Stip E, Lussier I. The effect of risperidone on cognition in patients with schizophrenia. Can J Psychiatry. 1996;41:S35–S40. doi: 10.1177/070674379604100802. [DOI] [PubMed] [Google Scholar]
- 22.Stip E, Lussier I, Lalonde P, Luyet A, Fabian J. Atypical neuroleptics and selective attention. Encephale. 1999;25:260–264. [PubMed] [Google Scholar]
- 23.Weiser M, Shneider-Beeri M, Nakash N, Brill N, Bawnik O, Reiss S, et al. Improvement in cognition associated with novel antipsychotic drugs: a direct drug effect or reduction of EPS? Schizophr Res. 2000;46:81–89. doi: 10.1016/s0920-9964(00)00025-6. [DOI] [PubMed] [Google Scholar]
- 24.Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- 25.Greco B, Invernizzi RW, Carli M. Phencyclidine-induced impairment in attention and response control depends on the background genotype of mice: reversal by the mGLU2/3 receptor agonist LY379268. Psychopharmacology (Berl) 2005;179:68–76. doi: 10.1007/s00213-004-2127-9. [DOI] [PubMed] [Google Scholar]
- 26.Amitai N, Kuczenski R, Markou A. Role of glutamate and metabotropic glutamate receptors in schizophrenia-like cognitive deficits in rats. Neuropharmacology. 2008;55:585. [Google Scholar]
- 27.Ossowska K, Pietraszek M, Wardas J, Nowak G, Zajaczkowski W, Wolfarth S, et al. The role of glutamate receptors in antipsychotic drug action. Amino Acids. 2000;19:87–94. doi: 10.1007/s007260070037. [DOI] [PubMed] [Google Scholar]
- 28.Galici R, Echemendia NG, Rodriguez AL, Conn PJ. A selective allosteric potentiator of metabotropic glutamate (mGlu) 2 receptors has effects similar to an orthosteric mGlu2/3 receptor agonist in mouse models predictive of antipsychotic activity. J Pharmacol Exp Ther. 2005;315:1181–1187. doi: 10.1124/jpet.105.091074. [DOI] [PubMed] [Google Scholar]
- 29.Imre G, Salomons A, Jongsma M, Fokkema DS, Den Boer JA, Ter Horst GJ. Effects of the mGluR2/3 agonist LY379268 on ketamine-evoked behaviours and neurochemical changes in the dentate gyrus of the rat. Pharmacol Biochem Behav. 2006;84:392–399. doi: 10.1016/j.pbb.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 30.Henry SA, Lehmann-Masten V, Gasparini F, Geyer MA, Markou A. The mGluR5 antagonist MPEP, but not the mGluR2/3 agonist LY314582, augments PCP effects on prepulse inhibition and locomotor activity. Neuropharmacology. 2002;43:1199–1209. doi: 10.1016/s0028-3908(02)00332-5. [DOI] [PubMed] [Google Scholar]
- 31.Schreiber R, Lowe D, Voerste A, De Vry J. LY354740 affects startle responding but not sensorimotor gating or discriminative effects of phencyclidine. Eur J Pharmacol. 2000;388:R3–R4. doi: 10.1016/s0014-2999(99)00844-4. [DOI] [PubMed] [Google Scholar]
- 32.Krystal JH, Abi-Saab W, Perry E, D’Souza DC, Liu N, Gueorguieva R, et al. Preliminary evidence of attenuation of the disruptive effects of the NMDA glutamate receptor antagonist, ketamine, on working memory by pretreatment with the group II metabotropic glutamate receptor agonist, LY354740, in healthy human subjects. Psychopharmacology (Berl) 2005;179:303–309. doi: 10.1007/s00213-004-1982-8. [DOI] [PubMed] [Google Scholar]
- 33.Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry. 2001;159:1367–1377. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- 34.Konradi C, Heckers S. Molecular aspects of glutamate dysregulation: implications for schizophrenia and its treatment. Pharmacol Ther. 2003;97:153–179. doi: 10.1016/s0163-7258(02)00328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duncan GE, Sheitman BB, Lieberman JA. An integrated view of pathophysiological models of schizophrenia. Brain Res Brain Res Rev. 1999;29:250–256. doi: 10.1016/s0165-0173(99)00002-8. [DOI] [PubMed] [Google Scholar]
- 36.Breier A, Malhotra AK, Pinals DA, Weisenfeld NI, Pickar D. Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. Am J Psychiatry. 1997;154:805–811. doi: 10.1176/ajp.154.6.805. [DOI] [PubMed] [Google Scholar]
- 37.Krystal JH, D’Souza DC, Mathalon D, Perry E, Belger A, Hoffman R. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl) 2003;169:215–233. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- 38.Snyder SH. Phencyclidine. Nature. 1980;285:355–356. doi: 10.1038/285355a0. [DOI] [PubMed] [Google Scholar]
- 39.Cho HS, D’Souza DC, Gueorguieva R, Perry EB, Madonick S, Karper LP, et al. Absence of behavioral sensitization in healthy human subjects following repeated exposure to ketamine. Psychopharmacology (Berl) 2005;179:136–143. doi: 10.1007/s00213-004-2066-5. [DOI] [PubMed] [Google Scholar]
- 40.Pradhan SN. Phencyclidine (PCP): some human studies. Neurosci Biobehav Rev. 1984;8:493–501. doi: 10.1016/0149-7634(84)90006-x. [DOI] [PubMed] [Google Scholar]
- 41.Luby ED, Cohen BD, Rosenbaum G, Gottlieb JS, Kelley R. Study of a new schizophrenomimetic drug: sernyl. AMA Arch Neurol Psychiatry. 1959;81:363–369. doi: 10.1001/archneurpsyc.1959.02340150095011. [DOI] [PubMed] [Google Scholar]
- 42.Bakker CB, Amini FB. Observations on the psychotomimetic effects of Sernyl. Compr Psychiatry. 1961;2:269–280. doi: 10.1016/s0010-440x(61)80033-3. [DOI] [PubMed] [Google Scholar]
- 43.Allen RM, Young SJ. Phencyclidine-induced psychosis. Am J Psychiatry. 1978;135:1081–1084. doi: 10.1176/ajp.135.9.1081. [DOI] [PubMed] [Google Scholar]
- 44.Castellani S, Giannini AJ, Boeringa JA, Adams PM. Phencyclidine intoxication: assessment of possible antidotes. J Toxicol Clin Toxicol. 1982;19:313–319. doi: 10.3109/15563658209025737. [DOI] [PubMed] [Google Scholar]
- 45.Javitt DC. Negative schizophrenic symptomatology and the PCP (phencyclidine) model of schizophrenia. Hillside J Clin Psychiatry. 1987;9:12–35. [PubMed] [Google Scholar]
- 46.Steinpreis RE. The behavioral and neurochemical effects of phenyclidine in humans and animals: some implications for modeling psychosis. Behav Brain Res. 1996;74:45–55. doi: 10.1016/0166-4328(95)00162-x. [DOI] [PubMed] [Google Scholar]
- 47.Itil T, Keskiner A, Kiremitci N, Holden JM. Effect of phencyclidine in chronic schizophrenics. Can Psychiatr Assoc J. 1967;12:209–12. doi: 10.1177/070674376701200217. [DOI] [PubMed] [Google Scholar]
- 48.Lahti AC, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13:9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- 49.Malhotra AK, Adler CM, Kennison SD, Elman I, Pickar D, Breier A. Clozapine blunts N-methyl-D-aspartate antagonist-induced psychosis: a study with ketamine. Biol Psychiatry. 1997;42:664–668. doi: 10.1016/s0006-3223(96)00546-x. [DOI] [PubMed] [Google Scholar]
- 50.Malhotra AK, Pinals DA, Adler CM, Elman I, Clifton A, Pickar D, et al. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology. 1997;17:141–150. doi: 10.1016/S0893-133X(97)00036-5. [DOI] [PubMed] [Google Scholar]
- 51.Newcomer JW, Farber NB, Jevtovic-Todorovic V, Selke G, Melson AK, Hershey T, et al. Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology. 1999;20:106–118. doi: 10.1016/S0893-133X(98)00067-0. [DOI] [PubMed] [Google Scholar]
- 52.Rosenbaum G, Cohen BD, Luby ED, Gottlieb JS, Yelen D. Comparison of sernyl with other drugs: simulation of schizophrenic performance with sernyl, LSD-25, and amobarbital (amytal) sodium: I. Attention, motor function, and proprioception. Arch Gen Psychiatry. 1959;1:651–656. doi: 10.1001/archpsyc.1959.03590060113013. [DOI] [PubMed] [Google Scholar]
- 53.Meltzer HY, Holzman PS, Hassan SZ, Guschwan A. Effects of phencyclidine and stress on plasma creatine phosphokinase (CPK) and aldolase activities in man. Psychopharmacologia. 1972;26:44–53. doi: 10.1007/BF00421917. [DOI] [PubMed] [Google Scholar]
- 54.Meltzer HY, Fessler RG, Simonovic M, Sturgeon D. PCP: neurochemistry, treatment, and more. Am J Psychiatry. 1979;136:235–237. doi: 10.1176/ajp.136.2.235. [DOI] [PubMed] [Google Scholar]
- 55.Pearlson GD. Psychiatric and medical syndromes associated with phencyclidine (PCP) abuse. Johns Hopkins Med J. 1981;148:25–33. [PubMed] [Google Scholar]
- 56.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 57.Malhotra AK, Pinals DA, Weingartner H, Sirocco K, Missar CD, Pickar D, et al. NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology. 1996;14:301–307. doi: 10.1016/0893-133X(95)00137-3. [DOI] [PubMed] [Google Scholar]
- 58.Krystal JH, Bennett A, Abi-Saab D, Belger A, Karper LP, D’Souza DC, et al. Dissociation of ketamine effects on rule acquisition and rule implementation: possible relevance to NMDA receptor contributions to executive cognitive functions. Biol Psychiatry. 2000;47:137–143. doi: 10.1016/s0006-3223(99)00097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guillermain Y, Micallef J, Possamaï C, Blin O, Hasbroucq T. N-methyl-D-aspartate receptors and information processing: human choice reaction time under a subanesthetic dose of ketamine. Neurosci Lett. 2001;303:29–32. doi: 10.1016/s0304-3940(01)01695-0. [DOI] [PubMed] [Google Scholar]
- 60.Rezvani AH, Kholdebarin E, Cauley MC, Dawson E, Levin ED. Attenuation of pharmacologically-induced attentional impairment by methylphenidate in rats. Pharmacol Biochem Behav. 2009;92:141–146. doi: 10.1016/j.pbb.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 61.Idris NF, Neill JC, Large CH. Comparison of the efficacy of two anticonvulsants, phenytoin and valproate to improve PCP and d-amphetamine induced deficits in a reversal learning task in the rat. Front Behav Neurosci. 2009;3:8. doi: 10.3389/neuro.08.008.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodefer JS, Nguyen TN, Karlsson JJ, Arnt J. Reversal of subchronic PCP-induced deficits in attentional set shifting in rats by sertindole and a 5-HT6 receptor antagonist: comparison among antipsychotics. Neuropsychopharmacology. 2008;33:2657–2666. doi: 10.1038/sj.npp.1301654. [DOI] [PubMed] [Google Scholar]
- 63.Egerton A, Reid L, McGregor S, Cochran SM, Morris BJ, Pratt JA. Subchronic and chronic PCP treatment produces temporally distinct deficits in attentional set shifting and prepulse inhibition in rats. Psychopharmacology (Berl) 2008;198:37–49. doi: 10.1007/s00213-008-1071-5. [DOI] [PubMed] [Google Scholar]
- 64.Willmore CB, Bespalov AY, Beardsley PM. Competitive and noncompetitive NMDA antagonist effects in rats trained to discriminate lever-press counts. Pharmacol Biochem Behav. 2001;69:493–502. doi: 10.1016/s0091-3057(01)00568-8. [DOI] [PubMed] [Google Scholar]
- 65.Willmore CB, Krall DM, Spears FM, Makriyannis A, Elmer GI. Cognitive effects of psychotomimetic drugs in rats discriminating number cues. Psychopharmacology (Berl) 2009;206:653–664. doi: 10.1007/s00213-008-1339-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paine TA, Carlezon WA., Jr Effects of antipsychotic drugs on MK-801-induced attentional and motivational deficits in rats. Neuropharmacology. 2009;56:788–797. doi: 10.1016/j.neuropharm.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Auclair AL, Besnard J, Newman-Tancredi A, Depoortère R. The five choice serial reaction time task: comparison between Sprague-Dawley and Long-Evans rats on acquisition of task, and sensitivity to phencyclidine. Pharmacol Biochem Behav. 2009;92:363–369. doi: 10.1016/j.pbb.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 68.Abdul-Monim Z, Neill JC, Reynolds GP. Sub-chronic psychotomimetic phencyclidine induces deficits in reversal learning and alterations in parvalbumin-immunoreactive expression in the rat. J Psychopharmacol. 2007;21:198–205. doi: 10.1177/0269881107067097. [DOI] [PubMed] [Google Scholar]
- 69.Abdul-Monim Z, Reynolds GP, Neill JC. The atypical antipsychotic ziprasidone, but not haloperidol, improves phencyclidine-induced cognitive deficits in a reversal learning task in the rat. J Psychopharmacol. 2003;17:57–65. doi: 10.1177/0269881103017001700. [DOI] [PubMed] [Google Scholar]
- 70.Abdul-Monim Z, Reynolds GP, Neill JC. The effect of atypical and classical antipsychotics on sub-chronic PCP-induced cognitive deficits in a reversal-learning paradigm. Behav Brain Res. 2006;169:263–273. doi: 10.1016/j.bbr.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 71.Amitai N, Semenova S, Markou A. Cognitive-disruptive effects of the psychotomimetic phencyclidine and attenuation by atypical antipsychotic medications in rats. Psychopharmacology (Berl) 2007;193:521–537. doi: 10.1007/s00213-007-0808-x. [DOI] [PubMed] [Google Scholar]
- 72.Auclair AL, Newman-Tancredi A, Depoortère R. Comparative analysis of typical, atypical, and novel antipsychotics with preferential D2/D3 and 5-HT1A affinity in rodent models of cognitive flexibility and sensory gating: (II) the reversal learning task and PPI of the startle reflex. Int J Neuropsychopharmacol. 2006;9:S145–S146. [Google Scholar]
- 73.Buffalo E, Gilliam MP, Allen RM, Paule MG. Acute behavioral effects of MK-801 in rhesus monkeys: assessment using an operant test battery. Pharmacol Biochem Behav. 1994;48:935–940. doi: 10.1016/0091-3057(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 74.Cohn J, Ziriax JM, Cox C, Cory-Slechta DA. Comparison of error patterns produced by scopolamine and MK-801 on repeated acquisition and transition baselines. Psychopharmacology (Berl) 1992;107:243–254. doi: 10.1007/BF02245144. [DOI] [PubMed] [Google Scholar]
- 75.Compton AD, Slemmer JE, Drew MR, Hyman JM, Golden KM, Balster RL, et al. Combinations of clozapine and phencyclidine: effects on drug discrimination and behavioral inhibition in rats. Neuropharmacology. 2001;40:289–297. doi: 10.1016/s0028-3908(00)00126-x. [DOI] [PubMed] [Google Scholar]
- 76.Dai H, Carey RJ. The NMDA antagonist MK-801 can impair attention to exteroceptive stimuli. Behav Brain Res. 1994;62:149–156. doi: 10.1016/0166-4328(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 77.Depoortère R, Auclair AL, Bardin L, Bruins Slot L, Kleven MS, Colpaert F, et al. F15063, a compound with D2/D3 antagonist, 5-HT1A agonist and D4 partial agonist properties: III. Activity in models of cognition and negative symptoms. Br J Pharmacol. 2007;151:266–277. doi: 10.1038/sj.bjp.0707160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Deschênes A, Goulet S, Doré FY. Rule shift under long-term PCP challenge in rats. Behav Brain Res. 2006;167:134–140. doi: 10.1016/j.bbr.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 79.Egerton A, Reid L, McKerchar CE, Morris BJ, Pratt JA. Impairment in perceptual attentional set-shifting following PCP administration: a rodent model of set-shifting deficits in schizophrenia. Psychopharmacology (Berl) 2005;179:77–84. doi: 10.1007/s00213-004-2109-y. [DOI] [PubMed] [Google Scholar]
- 80.Frederick DL, Gilliam MP, Allen RR, Paule MG. Acute behavioral effects of phencyclidine on rhesus monkey performance in an operant test battery. Pharmacol Biochem Behav. 1995;52:789–797. doi: 10.1016/0091-3057(95)00182-v. [DOI] [PubMed] [Google Scholar]
- 81.Freeman AS, Martin BR, Balster RL. Relationship between the development of behavioral tolerance and the biodisposition of phencyclidine in mice. Pharmacol Biochem Behav. 1984;20:373–377. doi: 10.1016/0091-3057(84)90273-9. [DOI] [PubMed] [Google Scholar]
- 82.Galizio M, Miller L, Ferguson A, McKinney P, Pitts RC. Olfactory repeated discrimination reversal in rats: effects of chlordiazepoxide, dizocilpine, and morphine. Behav Neurosci. 2006;120:1175–1179. doi: 10.1037/0735-7044.120.5.1175. [DOI] [PubMed] [Google Scholar]
- 83.Grottick AJ, Higgins GA. Effect of subtype selective nicotinic compounds on attention as assessed by the five-choice serial reaction time task. Behav Brain Res. 2000;117:197–208. doi: 10.1016/s0166-4328(00)00305-3. [DOI] [PubMed] [Google Scholar]
- 84.Higgins GA, Enderlin M, Haman M, Fletcher PJ. The 5-HT2A receptor antagonist M100,907 attenuates motor and “impulsive-type” behaviours produced by NMDA receptor antagonism. Psychopharmacology (Berl) 2003;170:309–319. doi: 10.1007/s00213-003-1549-0. [DOI] [PubMed] [Google Scholar]
- 85.Hudzik TJ, Slifer BL. Interaction of sigma and PCP-like drugs on operant behaviors in the rat. Psychopharmacology (Berl) 1992;108:115–122. doi: 10.1007/BF02245295. [DOI] [PubMed] [Google Scholar]
- 86.Idris NF, Large CH, Reynolds GP, Neill JC. Lamotrigine and clozapine reverse an established cognitive deficit induced by sub-chronic PCP (phencyclidine) in the rat. pA2 Online: E-journal of the British Pharmacology Society. 2003;1:056. http://www.pa2online.org/
- 87.Idris NF, Repeto P, Neill JC, Large CH. Investigation of the effects of lamotrigine and clozapine in improving reversal-learning impairments induced by acute phencyclidine and D-amphetamine in the rat. Psychopharmacology (Berl) 2005;179:336–348. doi: 10.1007/s00213-004-2058-5. [DOI] [PubMed] [Google Scholar]
- 88.Jentsch JD, Anzivino LA. A low dose of the alpha2 agonist clonidine ameliorates the visual attention and spatial working memory deficits produced by phencyclidine administration to rats. Psychopharmacology (Berl) 2004;175:76–83. doi: 10.1007/s00213-004-1772-3. [DOI] [PubMed] [Google Scholar]
- 89.Jentsch JD, Redmond DE, Jr, Elsworth JD, Taylor JR, Youngren KD, Roth RH. Enduring cognitive deficits and cortical dopamine dysfunction in monkeys after long-term administration of phencyclidine. Science. 1997;277:953–955. doi: 10.1126/science.277.5328.953. [DOI] [PubMed] [Google Scholar]
- 90.Jentsch JD, Roth RH, Taylor JR. Object retrieval/detour deficits in monkeys produced by prior subchronic phencyclidine administration: evidence for cognitive impulsivity. Biol Psychiatry. 2000;48:415–424. doi: 10.1016/s0006-3223(00)00926-4. [DOI] [PubMed] [Google Scholar]
- 91.Jentsch JD, Taylor JR. Impaired inhibition of conditioned responses produced by subchronic administration of phencyclidine to rats. Neuropsychopharmacology. 2001;24:66–74. doi: 10.1016/S0893-133X(00)00174-3. [DOI] [PubMed] [Google Scholar]
- 92.Jentsch JD, Taylor JR, Redmond DE, Jr, Elsworth JD, Youngren KD, Roth RH. Dopamine D4 receptor antagonist reversal of subchronic phencyclidine-induced object retrieval/detour deficits in monkeys. Psychopharmacology (Berl) 1999;142:78–84. doi: 10.1007/s002130050865. [DOI] [PubMed] [Google Scholar]
- 93.Jentsch JD, Tran A, Le D, Youngren KD, Roth RH. Subchronic phencyclidine adminstration reduces mesoprefrontal dopamine utilization and inpairs cortical-dependent cognition in the rat. Neuropsychopharmacology. 1997;17:92–99. doi: 10.1016/S0893-133X(97)00034-1. [DOI] [PubMed] [Google Scholar]
- 94.Jin J, Yamamoto T, Watanabe S. The involvement of receptors in the choice reaction performance deficits induced by phencyclidine. Eur J Pharmacol. 1997;319:147–152. doi: 10.1016/s0014-2999(96)00858-8. [DOI] [PubMed] [Google Scholar]
- 95.Laurent V, Podhorna J. Subchronic phencyclidine treatment impairs performance of C57BL/6 mice in the attentional set-shifting task. Behav Pharmacol. 2004;15:141–148. doi: 10.1097/00008877-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 96.Le Pen G, Grottick AJ, Higgins GA, Moreau J. Phencyclidine exacerbates attentional deficits in a neurodevelopmental rat model of schizophrenia. Neuropsychopharmacology. 2003;28:1799–1809. doi: 10.1038/sj.npp.1300208. [DOI] [PubMed] [Google Scholar]
- 97.McClure GYH, McMillan DE. Effects of drugs on response duration differentiation: VI. Differential effects under differential reinforcement of low rates of responding schedules. J Pharmacol Exp Ther. 1997;281:1368–1380. [PubMed] [Google Scholar]
- 98.Mishima K, Fujii M, Aoo N, Yoshikawa T, Fukue Y, Honda Y, et al. The pharmacological characterization of attentional processes using a two-lever choice reaction time task in rats. Biol Pharm Bull. 2002;25:1570–1576. doi: 10.1248/bpb.25.1570. [DOI] [PubMed] [Google Scholar]
- 99.Nelson CL, Burk JA, Bruno JP, Sarter M. Effects of acute and repeated systemic administration of ketamine on prefrontal acetylcholine release and sustained attention performance in rats. Psychopharmacology (Berl) 2002;161:168–179. doi: 10.1007/s00213-002-1004-7. [DOI] [PubMed] [Google Scholar]
- 100.Paine TA, Tomasiewicz HC, Zhang K, Carlezon WA., Jr Sensitivity of the five-choice serial reaction time task to the effects of various psychotropic drugs in Sprague-Dawley rats. Biol Psychiatry. 2007;62:687–693. doi: 10.1016/j.biopsych.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 101.Podhorna J, Didriksen M. Performance of male C57BL/6J mice and Wistar rats in the water maze following various schedules of phencyclidine treatment. Behav Pharmacol. 2005;16:25–34. doi: 10.1097/00008877-200502000-00003. [DOI] [PubMed] [Google Scholar]
- 102.Presburger G, Robinson JK. Spatial signal detection in rats is differentially disrupted by -9-tetrahydrocannabinol, scopolamine, and MK-801. Behav Brain Res. 1999;99:27–34. doi: 10.1016/s0166-4328(98)00065-5. [DOI] [PubMed] [Google Scholar]
- 103.Rezvani AH, Kholdebarin E, Dawson E, Levin ED. Nicotine and clozapine effects on attentional performance impaired by the NMDA antagonist dizocilpine in female rats. Int J Neuropsychopharmacol. 2008;11:63–70. doi: 10.1017/S1461145706007528. [DOI] [PubMed] [Google Scholar]
- 104.Rezvani AH, Levin ED. Nicotinic–glutamatergic interactions and attentional performance on an operant visual signal detection task in female rats. Eur J Pharmacol. 2003;465:83–90. doi: 10.1016/s0014-2999(03)01439-0. [DOI] [PubMed] [Google Scholar]
- 105.Rodefer JS, Murphy ER, Baxter MG. PDE10A inhibition reverses subchronic PCP-induced decifits in attentional set-shifting in rats. Eur J Neurosci. 2005;21:1070–1076. doi: 10.1111/j.1460-9568.2005.03937.x. [DOI] [PubMed] [Google Scholar]
- 106.Thompson DM, Winsauer PJ, Mastropaolo J. Effects of phencyclidine, ketamine and MDMA on complex operant behavior in monkeys. Pharmacol Biochem Behav. 1986;26:401–405. doi: 10.1016/0091-3057(87)90136-5. [DOI] [PubMed] [Google Scholar]
- 107.McLean SL, Beck JP, Woolley ML, Neill JC. A preliminary investigation into the effects of antipsychotics on sub-chronic phencyclidine-induced deficits in attentional set-shifting in female rats. Behav Brain Res. 2008;189:152–158. doi: 10.1016/j.bbr.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 108.McLean SL, Idris NF, Woolley ML, Neill JC. D1-like receptor activation improves PCP-induced cognitive deficits in animal models: implications for mechanisms of improved cognitive function in schizophrenia. Eur Neuropsychopharmacol. 2009;19:440–450. doi: 10.1016/j.euroneuro.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 109.Ardayfio PA, Benvenga MJ, Chaney SF, Love PL, Catlow J, Swanson SP, et al. The 5-hydroxytryptamine2A receptor antagonist R-(+)- -(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl-4-piperidinemethanol (M100907) attenuates impulsivity after both drug-induced disruption (dizocilpine) and enhancement (antidepressant drugs) of differential-reinforcement-of-low-rate 72-s behavior in the rat. J Pharmacol Exp Ther. 2008;327:891–897. doi: 10.1124/jpet.108.143370. [DOI] [PubMed] [Google Scholar]
- 110.Goetghebeur P, Dias R. Comparison of haloperidol, risperidone, sertindole, and modafinil to reverse an attentional set-shifting impairment following subchronic PCP administration in the rat: a back translational study. Psychopharmacology (Berl) 2009;202:287–293. doi: 10.1007/s00213-008-1132-9. [DOI] [PubMed] [Google Scholar]
- 111.Darrah JM, Stefani MR, Moghaddam B. Interaction of N-methyl-D-aspartate and group 5 metabotropic glutamate receptors on behavioral flexibility using a novel operant set-shift paradigm. Behav Pharmacol. 2008;19:225–234. doi: 10.1097/FBP.0b013e3282feb0ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Taffe MA, Davis SA, Gutierrez T, Gold LH. Ketamine impairs multiple cognitive domains in rhesus monkeys. Drug Alcohol Depend. 2002;68:175–187. doi: 10.1016/s0376-8716(02)00194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shannon HE, Love PL. Within-session repeated acquisition behavior in rats as a potential model of executive function. Eur J Pharmacol. 2004;498:125–134. doi: 10.1016/j.ejphar.2004.04.054. [DOI] [PubMed] [Google Scholar]
- 114.Broberg BV, Dias R, Glenthøj BY, Olsen CK. Evaluation of a neurodevelopmental model of schizophrenia: early postnatal PCP treatment in attentional set-shifting. Behav Brain Res. 2008;190:160–163. doi: 10.1016/j.bbr.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 115.Broberg BV, Glenthøj BY, Dias R, Larsen DB, Olsen CK. Reversal of cognitive deficits by an ampakine (CX516) and sertindole in two animal models of schizophrenia-sub-chronic and early postnatal PCP treatment in attentional set-shifting. Psychopharmacology (Berl) 2009 doi: 10.1007/s00213-009-1540-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 116.Pedersen CS, Goetghebeur P, Dias R. Chronic infusion of PCP via osmotic mini-pumps: a new rodent model of cognitive deficit in schizophrenia characterized by impaired attentional set-shifting (ID/ED) performance. J Neurosci Methods. 2009;185:66–69. doi: 10.1016/j.jneumeth.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 117.Didriksen M, Skarsfeldt T, Arnt J. Reversal of PCP-induced learning and memory deficits in the Morris’ water maze by sertindole and other antipsychotics. Psychopharmacology (Berl) 2007;193:225–233. doi: 10.1007/s00213-007-0774-3. [DOI] [PubMed] [Google Scholar]
- 118.Jentsch JD, Taylor JR, Elsworth JD, Redmond DE, Jr, Roth RH. Altered frontal cortical dopaminergic transmission in monkeys after subchronic phencyclidine exposure: involvement in frontostriatal cognitive deficits. Neuroscience. 1999;90:823–832. doi: 10.1016/s0306-4522(98)00481-3. [DOI] [PubMed] [Google Scholar]
- 119.Brigman JL, Ihne J, Saksida LM, Bussey TJ, Holmes A. Effects of subchronic phencyclidine (PCP) treatment on social behaviors, and operant discrimination and reversal learning in C57BL/6J mice. Front Behav Neurosci. 2009;3:2. doi: 10.3389/neuro.08.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rezvani AH, Tizabi Y, Getachew B, Hauser SR, Caldwell DP, Hunter C, et al. Chronic nicotine and dizocilpine effects on nicotinic and NMDA glutamatergic receptor regulation: interactions with clozapine actions and attentional performance in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1030–1040. doi: 10.1016/j.pnpbp.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 121.Amitai N, Markou A. Chronic nicotine improves cognitive performance in a test of attention but does not attenuate cognitive disruption induced by repeated phencyclidine administration. Psychopharmacology (Berl) 2009;202:275–286. doi: 10.1007/s00213-008-1246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Amitai N, Markou A. Increased impulsivity and disrupted attention induced by repeated phencyclidine are not attenuated by chronic quetiapine treatment. Pharmacol Biochem Behav. 2009;93:248–257. doi: 10.1016/j.pbb.2008.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Higgins GA, Ballard TM, Huwyler J, Kemp JA, Gill R. Evaluation of the NR2B-selective NMDA receptor antagonist Ro63–1908 on rodent behaviour: evidence for an involvement of NR2B NMDA receptors in response inhibition. Neuropharmacology. 2003;44:324–341. doi: 10.1016/s0028-3908(02)00402-1. [DOI] [PubMed] [Google Scholar]
- 124.Oliver YP, Ripley TL, Stephens DN. Ethanol effects on impulsivity in two mouse strains: similarities to diazepam and ketamine. Psychopharmacology (Berl) 2009;204:679–692. doi: 10.1007/s00213-009-1500-0. [DOI] [PubMed] [Google Scholar]
- 125.Poling A, Cleary J, Jackson K, Wallace S. d-amphetamine and phencyclidine alone and in combination: effects on fixed-ratio and interresponse-time-greater-than-t responding of rats. Pharmacol Biochem Behav. 1981;15:357–361. doi: 10.1016/0091-3057(81)90262-8. [DOI] [PubMed] [Google Scholar]
- 126.Sanger DJ, Jackson A. Effects of phencyclidine and other N-methyI-D-aspartate antagonists on the schedule-controlled behavior of rats. J Pharmacol Exp Ther. 1989;248:1215–1221. [PubMed] [Google Scholar]
- 127.Sanger DJ. NMDA antagonists disrupt timing behaviour in rats. Behav Pharmacol. 1992;3:593–600. [PubMed] [Google Scholar]
- 128.Wiley JL, Willmore CB. Effects of nitric oxide synthase inhibitors on timing and short-term memory in rats. Behav Pharmacol. 2000;11:421–429. doi: 10.1097/00008877-200008000-00008. [DOI] [PubMed] [Google Scholar]
- 129.Welzl H, Berz S, Battig K. The effects of the noncompetitive NMDA receptor antagonist MK 801 on DRL performance in rats. Psychobiology. 1991;19:211–216. [Google Scholar]
- 130.Stephens DN, Cole BJ. AMPA antagonists differ from NMDA antagonists in their effects on operant DRL and delayed matching to position tasks. Psychopharmacology (Berl) 1996;126:249–259. doi: 10.1007/BF02246455. [DOI] [PubMed] [Google Scholar]
- 131.Tonkiss J, Almeida SS, Galler JR. Prenatally malnourished female but not male rats show increased sensitivity to MK-801 in a differential reinforcement of low rates task. Behav Pharmacol. 1998;9:49–60. [PubMed] [Google Scholar]
- 132.Balster RL, Baird JB. Effects of phencyclidine, d-amphetamine and pentobarbital on spaced responding in mice. Pharmacol Biochem Behav. 1979;11:617–623. doi: 10.1016/0091-3057(79)90252-1. [DOI] [PubMed] [Google Scholar]
- 133.Horwood JM, Ripley TL, Stephens DN. Evidence for disrupted NMDA receptor function in tissue plasminogen activator knockout mice. Behav Brain Res. 2004;150:127–138. doi: 10.1016/S0166-4328(03)00248-1. [DOI] [PubMed] [Google Scholar]
- 134.Cheng R, MacDonald CJ, Meck WH. Differential effects of cocaine and ketamine on time estimation: implications for neurobiological models of interval timing. Pharmacol Biochem Behav. 2006;85:114–122. doi: 10.1016/j.pbb.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 135.Adams B, Moghaddam B. Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J Neurosci. 1998;18:5545–5554. doi: 10.1523/JNEUROSCI.18-14-05545.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Adams BW, Moghaddam B. Effect of clozapine, haloperidol, or M100907 on phencyclidine-activated glutamate efflux in the prefrontal cortex. Biol Psychiatry. 2001;50:750–757. doi: 10.1016/s0006-3223(01)01195-7. [DOI] [PubMed] [Google Scholar]
- 137.Stefani MR, Groth K, Moghaddam B. Glutamate receptors in the rat medial prefrontal cortex regulate set-shifting ability. Behav Neurosci. 2003;117:728–737. doi: 10.1037/0735-7044.117.4.728. [DOI] [PubMed] [Google Scholar]
- 138.Melnick SM, Rodriguez JS, Bernardi RE, Ettenberg A. A simple procedure for assessing ataxia in rats: effects of phencyclidine. Pharmacol Biochem Behav. 2002;72:125–130. doi: 10.1016/s0091-3057(01)00727-4. [DOI] [PubMed] [Google Scholar]
- 139.Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- 140.Floresco SB, Zhang Y, Enomoto T. Neural circuits subserving behavioral flexibility and their relevance to schizophrenia. Behav Brain Res. 2009;204:396–409. doi: 10.1016/j.bbr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 141.Curran HV, Morgan CJ. Cognitive, dissociative and psychotogenic effects of ketamine in recreational users on the night of drug use and 3 days later. Addiction. 2000;95:575–590. doi: 10.1046/j.1360-0443.2000.9545759.x. [DOI] [PubMed] [Google Scholar]
- 142.Morgan CJ, Riccelli M, Maitland CH, Curran HV. Long-term effects of ketamine: evidence for a persisting impairment of source memory in recreational users. Drug Alcohol Depend. 2004;75:301–308. doi: 10.1016/j.drugalcdep.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 143.Morgan CJ, Muetzelfeldt L, Curran HV. Consequences of chronic ketamine self-administration upon neurocognitive function and psychological wellbeing: a 1-year longitudinal study. Addiction. 2009 doi: 10.1111/j.1360-0443.2009.02761.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 144.Morgan CJ, Muetzelfeldt L, Curran HV. Ketamine use, cognition and psychological wellbeing: a comparison of frequent, infrequent and ex-users with polydrug and non-using controls. Addiction. 2009;104:77–87. doi: 10.1111/j.1360-0443.2008.02394.x. [DOI] [PubMed] [Google Scholar]
- 145.Cosgrove J, Newell TG. Recovery of neuropsychological functions during reduction in use of phencyclidine. J Clin Psychol. 1991;47:159–169. doi: 10.1002/1097-4679(199101)47:1<159::aid-jclp2270470125>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 146.Uhlhaas PJ, Millard I, Muetzelfeldt L, Curran HV, Morgan CJ. Perceptual organization in ketamine users: preliminary evidence of deficits on night of drug use but not 3 days later. J Psychopharmacol. 2007;21:347–352. doi: 10.1177/0269881107077739. [DOI] [PubMed] [Google Scholar]
- 147.Morgan CJ, Huddy V, Lipton M, Curran HV, Joyce EM. Is persistent ketamine use a valid model of the cognitive and oculomotor deficits in schizophrenia? Biol Psychiatry. 2009;65:1099–1102. doi: 10.1016/j.biopsych.2008.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Adler CM, Goldberg TE, Malhotra AK, Pickar D, Breier A. Effects of ketamine on thought disorder, working memory, and semantic memory in healthy volunteers. Biol Psychiatry. 1998;43:811–816. doi: 10.1016/s0006-3223(97)00556-8. [DOI] [PubMed] [Google Scholar]
- 149.Oranje B, van Berckel BNM, Kemner C, van Ree JM, Kahn RS, Verbaten PD. The effects of a sub-anaesthetic dose of ketamine on human selective attention. Neuropsychopharmacology. 2000;22:293–302. doi: 10.1016/S0893-133X(99)00118-9. [DOI] [PubMed] [Google Scholar]
- 150.Morgan CJ, Mofeez A, Brandner B, Bromley L, Curran HV. Ketamine impairs response inhibition and is positively reinforcing in healthy volunteers: a dose-response study. Psychopharmacology (Berl) 2004;172:298–308. doi: 10.1007/s00213-003-1656-y. [DOI] [PubMed] [Google Scholar]
- 151.Morgan CJ, Mofeez A, Brandner B, Bromley L, Curran HV. Acute effects of ketamine on memory systems and psychotic symptoms in healthy volunteers. Neuropsychopharmacology. 2004;29:208–218. doi: 10.1038/sj.npp.1300342. [DOI] [PubMed] [Google Scholar]
- 152.Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- 153.Carli M, Robbins TW, Evenden JL, Everitt BJ. Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats: implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res. 1983;9:361–380. doi: 10.1016/0166-4328(83)90138-9. [DOI] [PubMed] [Google Scholar]
- 154.Leonard JA. Medical Research Council, Applied Psychology Unit Reports. Cambridge, UK: 1959. Five-choice serial reaction apparatus. Vol. Report 326. [Google Scholar]
- 155.Rosvold HE, Mirsky AF, Saranson I, Bransome EB, Beck LH. A continuous performance test of brain damage. J Consult Psychol. 1956;20:343–350. doi: 10.1037/h0043220. [DOI] [PubMed] [Google Scholar]
- 156.Humby T, Laird FM, Davies W, Wilkinson LS. Visuospatial attentional functioning in mice: interactions between cholinergic manipulations and genotype. Eur J Neurosci. 1999;11:2813–2823. doi: 10.1046/j.1460-9568.1999.00701.x. [DOI] [PubMed] [Google Scholar]
- 157.Young JW, Finlayson K, Spratt C, Marston HM, Crawford N, Kelly JS, et al. Nicotine improves sustained attention in mice: evidence for involvement of the alpha7 nicotinic acetylcholine receptor. Neuropsychopharmacology. 2004;29:891–900. doi: 10.1038/sj.npp.1300393. [DOI] [PubMed] [Google Scholar]
- 158.Young JW, Light GA, Marston HM, Sharp R, Geyer MA. The 5-choice continuous performance test: evidence for a translational test of vigilance for mice. PLoS One. 2009;4:e4227. doi: 10.1371/journal.pone.0004227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kurtz MM, Ragland JD, Bilker W, Gur RC, Gur RE. Comparison of the continuous performance test with and without working memory demands in healthy controls and patients with schizophrenia. Schizophr Res. 2001;48:307–316. doi: 10.1016/s0920-9964(00)00060-8. [DOI] [PubMed] [Google Scholar]
- 160.Orzack MH, Kornetsky C. Attention dysfunction in chronic schizophrenia. Arch Gen Psychiatry. 1966;14:323–326. doi: 10.1001/archpsyc.1966.01730090099015. [DOI] [PubMed] [Google Scholar]
- 161.Earle-Boyer EA, Serper MR, Davidson M, Harvey PD. Continuous performance tests in schizophrenic patients: stimulus and medication effects on performance. Psychiatry Res. 1991;37:47–56. doi: 10.1016/0165-1781(91)90105-x. [DOI] [PubMed] [Google Scholar]
- 162.Finkelstein JR, Cannon TD, Gur RE, Gur RC, Moberg P. Attentional dysfunctions in neuroleptic-naive and neuroleptic-withdrawn schizophrenic patients and their siblings. J Abnorm Psychol. 1997;106:203–212. doi: 10.1037//0021-843x.106.2.203. [DOI] [PubMed] [Google Scholar]
- 163.Elvevåg B, Weinberger DR, Suter JC, Goldberg TE. Continuous performance test and schizophrenia: a test of stimulus-response compatibility, working memory, response readiness, or none of the above? Am J Psychiatry. 2000;157:772–780. doi: 10.1176/appi.ajp.157.5.772. [DOI] [PubMed] [Google Scholar]
- 164.Spratt C, Marston HM, Sharkey J, Kelly JS. Comparison of rat and mouse attentional performance in the 5-choice serial reaction task. BNA Abstracts. 2000;17:49.03. [Google Scholar]
- 165.Semenova S, Markou A. The effects of the mGluR5 antagonist MPEP and the mGluR2/3 antagonist LY341495 on rats’ performance in the 5-choice serial reaction time task. Neuropharmacology. 2007;52:863–872. doi: 10.1016/j.neuropharm.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Carli M, Samanin R. Serotonin2 receptor agonists and serotonergic anorectic drugs affect rats’ performance differently in a five-choice serial reaction time task. Psychopharmacology (Berl) 1992;106:228–234. doi: 10.1007/BF02801977. [DOI] [PubMed] [Google Scholar]
- 167.Terry AV, Risbrough VB, Buccafusco JJ, Menzaghi F. Effects of (±)-4-{[2-(1-methyl-2-pyrrolidinyl)ethyl]thio}phenol hydrochloride (SIB-1553A), a selective ligand for nicotinic acetylcholine receptors, in tests of visual attention and distractibility in rats and monkeys. J Pharmacol Exp Ther. 2002;301:284–292. doi: 10.1124/jpet.301.1.284. [DOI] [PubMed] [Google Scholar]
- 168.Shoaib M, Bizarro L. Deficits in a sustained attention task following nicotine withdrawal in rats. Psychopharmacology (Berl) 2005;178:211–222. doi: 10.1007/s00213-004-2004-6. [DOI] [PubMed] [Google Scholar]
- 169.Parsons CG, Panchenko VA, Pinchenko VO, Tsyndrenko AY, Krishtal OA. Comparative patch-clamp studies with freshly dissociated rat hippocampal and striatal neurons on the NMDA receptor antagonistic effects of amantadine and memantine. Eur J Neurosci. 1996;8:446–454. doi: 10.1111/j.1460-9568.1996.tb01228.x. [DOI] [PubMed] [Google Scholar]
- 170.Geyer MA, Markou A. Animal models of psychiatric disorders. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. Raven Press; New York: 1995. pp. 787–798. [Google Scholar]
- 171.Buchanan RW, Holstein C, Breier A. The comparative efficacy and long-term effect of clozapine treatment on neuropsychological test performance. Biol Psychiatry. 1994;36:717–725. doi: 10.1016/0006-3223(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 172.Potkin SG, Fleming K, Jin Y, Gulasekaram B. Clozapine enhances neurocognition and clinical symptomatology more than standard neuroleptics. J Clin Psychopharmacol. 2001;21:479–483. doi: 10.1097/00004714-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 173.Harvey PD, Rabinowitz J, Eerdekens M, Davidson M. Treatment of cognitive impairment in early psychosis: a comparison of risperidone and haloperidol in a large long-term trial. Am J Psychiatry. 2005;162:1888–1895. doi: 10.1176/appi.ajp.162.10.1888. [DOI] [PubMed] [Google Scholar]
- 174.Woodward ND, Purdon SE, Meltzer HY, Zald DH. A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophrenia. Int J Neuropsychopharmacol. 2005;8:457–472. doi: 10.1017/S146114570500516X. [DOI] [PubMed] [Google Scholar]
- 175.Gleason SD, Shannon HE. Blockade of phencyclidine-induced hyperlocomotion by olanzapine, clozapine and serotonin receptor subtype selective antagonists in mice. Psychopharmacology (Berl) 1997;129:79–84. doi: 10.1007/s002130050165. [DOI] [PubMed] [Google Scholar]
- 176.Cole BJ, Robbins TW. Amphetamine impairs the discriminative performance of rats with dorsal noradrenergic bundle lesions on a 5-choice serial reaction time task: new evidence for central dopaminergic-noradrenergic interactions. Psychopharmacology (Berl) 1987;91:458–466. doi: 10.1007/BF00216011. [DOI] [PubMed] [Google Scholar]
- 177.Mirjana C, Baviera M, Invernizzi RW, Balducci C. The serotonin 5-HT2A receptors antagonist M100907 prevents impairment in attentional performance by NMDA receptor blockade in the rat prefrontal cortex. Neuropsychopharmacology. 2004;29:1637–1647. doi: 10.1038/sj.npp.1300479. [DOI] [PubMed] [Google Scholar]
- 178.Puumala T, Sirviö J. Changes in activities of dopamine and serotonin systems in the frontal cortex underlie poor choice accuracy and impulsivity of rats in an attention task. Neuroscience. 1998;83:489–499. doi: 10.1016/s0306-4522(97)00392-8. [DOI] [PubMed] [Google Scholar]
- 179.Puumala T, Ruotsalainen S, Jäkälä P, Koivisto E, Riekkinen P, Jr, Sirviö J. Behavioral and pharmacological studies on the validation of a new animal model for attention deficit hyperactivity disorder. Neurobiol Learn Mem. 1996;66:198–211. doi: 10.1006/nlme.1996.0060. [DOI] [PubMed] [Google Scholar]
- 180.Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- 181.Talpos JC, Wilkinson LS, Robbins TW. A comparison of multiple 5-HT receptors in two tasks measuring impulsivity. J Psychopharmacol. 2006;20:47–58. doi: 10.1177/0269881105056639. [DOI] [PubMed] [Google Scholar]
- 182.Brunner D, Hen R. Insights into the neurobiology of impulsive behavior from serotonin receptor knockout mice. Ann N Y Acad Sci. 1997;836:81–105. doi: 10.1111/j.1749-6632.1997.tb52356.x. [DOI] [PubMed] [Google Scholar]
- 183.Kiehl KA, Smith AM, Hare RD, Liddle PF. An event-related potential investigation of response inhibition in schizophrenia and psychopathy. Biol Psychiatry. 2000;48:210–221. doi: 10.1016/s0006-3223(00)00834-9. [DOI] [PubMed] [Google Scholar]
- 184.Weisbrod M, Kiefer M, Marzinzik F, Spitzer M. Executive control is disturbed in schizophrenia: evidence from event-related potentials in a Go/NoGo task. Biol Psychiatry. 2000;47:51–60. doi: 10.1016/s0006-3223(99)00218-8. [DOI] [PubMed] [Google Scholar]
- 185.Badcock JC, Michie PT, Johnson L, Combrinck J. Acts of control in schizophrenia: dissociating the components of inhibition. Psychol Med. 2002;32:287–297. doi: 10.1017/s0033291701005128. [DOI] [PubMed] [Google Scholar]
- 186.Chan RC, Chen EY, Cheung EF, Chen RY, Cheung HK. The components of executive functioning in a cohort of patients with chronic schizophrenia: a multiple single-case study design. Schizophr Res. 2006;81:173–189. doi: 10.1016/j.schres.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 187.Chan RCK, Chen EY, Chen RY, Poon SP, Cheung HK. Executive disorders in patients with schizophrenia and its correlates with neurological signs. Schizophr Res. 2002;53:137. [Google Scholar]
- 188.Chan RC, Chen EY, Cheung EF, Chen RY, Cheung HK. Executive dysfunctions in schizophrenia: relationships to clinical manifestation. Eur Arch Psychiatry Clin Neurosci. 2004;254:256–262. doi: 10.1007/s00406-004-0492-3. [DOI] [PubMed] [Google Scholar]
- 189.Hahn B, Shoaib M, Stolerman IP. Nicotine-induced enhancement of attention in the five-choice serial reaction time task: the influence of task demands. Psychopharmacology (Berl) 2002;162:129–137. doi: 10.1007/s00213-002-1005-6. [DOI] [PubMed] [Google Scholar]
- 190.Mirza NR, Stolerman IP. Nicotine enhances sustained attention in the rat under specific task condition. Psychopharmacology (Berl) 1998;138:266–274. doi: 10.1007/s002130050671. [DOI] [PubMed] [Google Scholar]
- 191.Chamberlain SR, Fineberg NA, Blackwell AD, Robbins TW, Sahakian BJ. Motor inhibition and cognitive flexibility in obsessive-compulsive disorder and trichotillomania. Am J Psychiatry. 2006;163:1282–1284. doi: 10.1176/ajp.2006.163.7.1282. [DOI] [PubMed] [Google Scholar]
- 192.Dalley JW, Theobald DE, Pereira EA, Li PM, Robbins TW. Specific abnormalities in serotonin release in the prefrontal cortex of isolation-reared rats measured during behavioural performance of a task assessing visuospatial attention and impulsivity. Psychopharmacology (Berl) 2002;164:329–340. doi: 10.1007/s00213-002-1215-y. [DOI] [PubMed] [Google Scholar]
- 193.Fey ET. The performance of young schizophrenics and young normals on the Wisconsin Card Sorting Test. J Consult Psychol. 1951;15:311–319. doi: 10.1037/h0061659. [DOI] [PubMed] [Google Scholar]
- 194.Goldberg TE, Weinberger DR, Berman KF, Pliskin NH, Podd MH. Further evidence for dementia of the prefrontal type in schizophrenia? A controlled study of teaching the Wisconsin Card Sorting Test. Arch Gen Psychiatry. 1987;44:1008–1014. doi: 10.1001/archpsyc.1987.01800230088014. [DOI] [PubMed] [Google Scholar]
- 195.Braff DL, Heaton R, Kuck J, Cullum M, Moranville J, Grant I, et al. The generalized pattern of neuropsychological deficits in outpatients with chronic schizophrenia with heterogeneous Wisconsin Card Sorting Test results. Arch Gen Psychiatry. 1991;48:891–898. doi: 10.1001/archpsyc.1991.01810340023003. [DOI] [PubMed] [Google Scholar]
- 196.Sullivan EV, Mathalon DH, Zipursky RB, Kersteen-Tucker Z, Knight RT, Pfefferbaum A. Factors of the Wisconsin Card Sorting Test as measures of frontal-lobe function in schizophrenia and in chronic alcoholism. Psychiatry Res. 1993;46:175–199. doi: 10.1016/0165-1781(93)90019-d. [DOI] [PubMed] [Google Scholar]
- 197.Murray GK, Cheng F, Clark L, Barnett JH, Blackwell AD, Fletcher PC, et al. Reinforcement and reversal learning in first-episode psychosis. Schizophr Bull. 2008;34:848–855. doi: 10.1093/schbul/sbn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Leeson VC, Robbins TW, Matheson E, Hutton SB, Ron MA, Barnes TR, et al. Discrimination learning, reversal, and set-shifting in first-episode schizophrenia: stability over six years and specific associations with medication type and disorganization syndrome. Biol Psychiatry. 2009;66:586–593. doi: 10.1016/j.biopsych.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Leeson VC, Robbins TW, Franklin C, Harrison M, Harrison I, Ron MA, et al. Dissociation of long-term verbal memory and fronto-executive impairment in first-episode psychosis. Psychol Med. 2009;39:1799–1808. doi: 10.1017/S0033291709005935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hutton SB, Puri BK, Duncan LJ, Robbins TW, Barnes TR, Joyce EM. Executive function in first-episode schizophrenia. Psychol Med. 1998;28:463–473. doi: 10.1017/s0033291797006041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


