Abstract
The properties of retrograde amnesia after damage to the hippocampus have been explicated with some success using a rat model of human medial temporal lobe amnesia. We review the results of this experimental work with rats focusing on several areas of consensus in this growing literature. We evaluate the theoretically significant hypothesis that hippocampal retrograde amnesia normally exhibits a temporal gradient, affecting recent, but sparing remote memories. Surprisingly, the evidence does not provide much support for the idea that there is a lengthy process of systems consolidation following a learning episode. Instead, recent and remote memories tend to be equally affected. The extent of damage to the hippocampus is a significant factor in this work since it is likely that spared hippocampal tissue can support at least partial memory retrieval. With extensive hippocampal damage gradients are flat or, in the case of memory tasks with flavour/odour retrieval cues, the retrograde amnesia covers a period of about 1 – 3 days. There is consistent evidence that at the time of learning the hippocampus interferes with or overshadows memory acquisition by other systems. This contributes to the breadth and severity of retrograde amnesia relative to anterograde amnesia in the rat. The fact that multiple, distributed learning episodes can overcome this overshadowing is consistent with a parallel dual-store theory or a Distributed Reinstatement Theory in which each learning episode triggers a short period of memory replay that provides a brief hippocampal-dependent systems consolidation.
Keywords: temporal gradient, anterograde amnesia, memory replay, Distributed Reinstatement Theory, memory overshadowing, remote memory
Retrograde amnesia (RA) is one of the central features of temporal lobe amnesia in humans. Milner and Penfield at the Montreal Neurological Institute were the first to describe RA as a symptom following selective medial temporal lobe resection. A patient (PB) in 1951 and a second (FC) a year later presented with anterograde amnesia (AA) after surgery, in addition, both exhibited partial RA. The pattern of RA they described was severe for recent memories, but it seemed to them that remote memories were relatively spared (Milner, 2005). Moreover, a few years later Scoville and Milner (1957) described similar RA in patient H.M., which is undoubtedly the most well known and studied case of partial RA. This pattern of partial RA involving a disruption of recent memories and sparing of relatively more remote memories is termed a temporal gradient (equivalent disruption of recent and remote memory is term a flat gradient). In addition, it was noted that motor skills were unaffected. In the following years many more amnesics who had sustained bilateral medial temporal lobe damage were described, all of who displayed similar partial amnesia. At that time the affected presurgical interval covered by RA was estimated to be in the range of months to years (Penfield & Milner, 1958; Milner, 1959). This is not the appropriate place to exhaustively review the key issues in human RA, but we note the following three issues that are of direct relevance to us.
First, the measurement of RA in humans has become progressively more refined. One can see a trend for increasing precision in the methods over the past 5 decades. Compare, for example Scoville and Milner (1957) with Squire et al. (1975) and with Gilboa et al. (2006). There is a very wide range of estimates of the length of the pre-injury interval from which memories are lost. We notice a trend for the duration of this RA interval to lengthen in proportion to the increasing rigour in methods to measure it. For example, patient HM's RA interval initially was suggested to affect only the preceding few years. In subsequent studies this interval lengthened to involve two decades (Corkin, 1984) and in the most recent evaluation the investigators conclude that severe RA for autobiographical memories shows no temporal gradient (Steinvorth, et al., 2005). Indeed, our field may not have fully appreciated the fact that: “H.M. was unable to supply an episodic memory of his mother or his father — he could not narrate even one event that occurred at a specific time and place.” (Corkin, 2002).
Second, across studies reporting on RA there is great diversity in which temporal lobe structures are damaged and in the extent of damage to each structure. A number of generalizations have been offered based upon comparing cases. Examples include, the RA interval is longer with more extensive hippocampal damage (Nadel & Moscovitch, 1997) or damage extending beyond the hippocampal system is responsible for loss of more remote memories (Squire & Bayley, 2007). Perhaps there is too much diversity across studies in methods to measure memory and to quantify damage for the relatively small number of patients to convincingly resolve these issues.
Third, memories can be assessed in many ways, using recall vs recognition, with or without time and place specificity, with first-person detailed re-experiencing of events or with gist-like report, with or without emotional/affective valence, and so on. The mnemonic processes supporting performance in these different modes of assessment are likely to be diverse. There is no consensus on the extent to which these diverse processes are affected by temporal lobe RA. For example, several theorists have emphasized a distinction between declarative (explicit) and non declarative (implicit) memory processes and within the declarative category between episodic and semantic memory processes. There are sharp differences among views on whether all types of declarative memory processes have the same dependence upon the hippocampus (Squire, 1992) or if episodic memories have a special relationship (Nadel & Moscovitch, 1997). Generally, it is assumed that performance is disrupted in the same memory tasks in the retrograde direction and in the anterograde direction. That is if a memory task is readily solvable without an intact hippocampus then performance in the same task will be unaffected by hippocampal damage if the supporting memory was acquired prior to hippocampal damage.
Several fundamental aspects of contemporary theories on the organization of long-term memories turn on how these three controversies about the properties of RA are resolved. Consider for a moment the issue of temporal gradients. There is a relatively short period of time after a learning episode when memories are easily disrupted by a wide range of manipulations of activity in networks that are essential in supporting the memory. Manipulations include electroconvulsive shock, protein synthesis blockade, various neuromodulators, transcription inhibitors, etc. The most common view is that there is a cascade of molecular events taking place in essential circuitry that progressively stabilizes the memory trace. This process of cellular consolidation is complete on the time scale of hours. In contrast theorists have explained he lengthy temporal gradient of RA by invoking another memory consolidation process, systems consolidation, that operates on a much longer time-scale of months to decades. The Standard Model of Systems Consolidation (SMSC) holds that declarative memory traces are created at distributed cortical sites. For some period of time after a learning episode, memory retrieval requires reactivation of cortical sites by the hippocampus. Retrieval of memory from learning episodes sufficiently remote in time does not require the hippocampus. The period of support of the memory by hippocampus is necessary for the strengthening of connections among non hippocampal cortical sites to enable them to support hippocampus-independent retrieval. As anomalous results emerged showing highly variable temporal gradient time-scales and some cases of RA with flat gradients Nadel & Moscovitch (1997) proposed a modification of SMSC. On their view, termed Multiple Trace Theory (MTT), RA for the two major subtypes of declarative (explicit) memories, episodic and semantic is different. Semantic memories undergo systems consolidation exactly as described by SMSC. Therefore there should be temporal gradients to RA for semantic memories. Episodic memories always depend upon the hippocampus according to MTT. Without the hippocampus even the most remote episodic memories, if they involve the time and place of the learning episode, cannot be retrieved. RA for episodic memories exhibits flat temporal gradients. MTT has an additional process that explains temporal gradients for episodic memories. Each successive retrieval of an episodic memory lays down an additional memory trace involving the hippocampus. Episodic memories that have been retrieved more often, likely older memories, will have more traces. Thus, if the hippocampus is partially damaged more remote memories will be more likely to have a sufficient spared representation in hippocampus to enable retrieval. So, according to MTT, RA for the category of episodic memory should have a temporal gradient if the hippocampus is partially damaged.
It is not definitively settled if rats have the equivalent of human episodic memory. As such, it would be unwise to attempt to adjudicate among rival theories that contain important postulates linking the episodic/non episodic distinction to systems consolidation. Dealing properly with the issue of the status of episodic memory in rats is beyond the scope of this review. At some point this set of controversies will be resolved. Imagine that episodic and non episodic memory categories cover all of the memories that a rat may have, even if at the moment we cannot diagnose a specific memory as belonging to one category or the other. The resolution will end up being one of three possibilities, rats have only non episodic memories, only episodic memories, or both. In all three cases both MTT and the standard model predict that for every memory task it should be possible to demonstrate a lengthy temporally graded retrograde amnesia. For MTT, with episodic memories, partial HPC damage should produce a temporal gradient; for non episodic memories even complete HPC damage should produce a temporal gradient. It should be seen that even with the uncertainty about the applicability of the episodic category, the effects of partial vs. complete HPC damage on a wide range of memory tasks, with a wide range of learning-damage intervals are not irrelevant.
Regardless of the status of controversies over these issues consideration of the evidence from human RA has led to a rich set of hypotheses that can be tested experimentally in nonhuman animals. In these experiments it is possible to control with greater precision the location and extent of injury, the exact time of the learning episodes, the strength of memory, the type of memory testing, and the nature of intervening experiences between learning and onset of amnesia. The intention of this paper is to review experimental work on RA with rats, the most commonly used nonhuman species for this type of work, involving hippocampal permanent or temporary inactivations. Our attention is focused primarily on those experiments that enable an evaluation of memory in a well-designed retention test rather than over sessions of relearning. We discuss the potential factors that determine if a temporal gradient in hippocampal RA is observed, the nature of RA after complete vs partial hippocampal damage, the task specificity of hippocampal RA, and the effects of the strength and distribution of learning episodes on RA.
Temporal gradients?
The first two papers to evaluate temporal gradient in RA after hippocampal damage in rats provide a useful counter-point, highlighting the principal variants in work in this field. The first, Kim & Fanselow (1992), provided the first experimental demonstration of a temporal gradient. They tested the effects on memory retention of small electrolytic lesions in dorsal hippocampus (dHPC), 1, 7, 14, or 28 days after a single session of fear conditioning involving 15 pairings of context+tone with foot shock. Rats with partial dHPC damage made 1 day after learning showed no evidence of contextual fear. Rats with the same damage made 7 – 28 days after learning showed substantial retention of contextual fear and across those learning-surgery intervals retention performance is very similar. The control group did not show statistically significant changes in retention of contextual fear across the same time intervals. There was no effect of their partial dHPC damage on retention of fear of the tone conditioned stimulus. The authors conclude that the data support the concept of systems consolidation and conclude that the “hippocampus has a temporally restricted role in memory storage that is assumed by other structures over time.” Bolhuis et al. (1994) used the selective neurotoxin, ibotenic acid, to extensively damage dorsal and ventral hippocampus 3 or 98 days after the end of training in the hidden platform version of the Morris water task. In contrast to the control rats' good retention, rats with damage to the hippocampus showed no evidence of place memory on the post-surgical retention test at either training-surgery interval. Bolhuis et al. (1994) concluded that their findings scored against the idea of a temporal gradient in RA after extensive selective hippocampal damage.
The two experiments lead to opposite conclusions and consideration of the differences in their methods points to some of the major factors that have some theoretical importance in this field. The differences include partial vs. extensive damage and navigation vs. non navigational responding. In addition, the hippocampus is necessary for learning to solve the hidden platform version of the Morris water task but not for contextual fear conditioning – the standard version of the water task is hippocampus-dependent in the anterograde direction (Morris et al., 1982; Sutherland et al., 1982), whereas contextual fear conditioning is not (Lehmann, et al., 2009; Maren et al. 1997; Wiltgen et al. 2006).
Table 1 presents a list of tasks and independent groups of rats with different extents of damage to the hippocampus in published papers that involve an attempt to experimentally demonstrate a temporal gradient in RA after hippocampal damage in rats. Readers should be heartened by the fact that there are two areas of broad multi-laboratory agreement. First, there is a consensus in studies using memories involving flavour/odours as explicit cues. All results are consistent with a temporal RA gradient, with memory stabilization being complete between 24 – 100 hr. It has been argued that a gradient is obtained because of a disruption of cellular consolidation rather than systems consolidation (Rudy & Sutherland, 2008), but at face value and with further consideration (Tse, et al., 2008), these studies, together with Takehara et al. (2003), are the most compelling cases to date of a temporal gradient in RA in the rat. Second, there is a consensus across laboratories and spatial memory tasks that there is no temporal gradient with spatial navigation memories at least out to 100 days after learning.
TABLE 1.
| Memory task | Damage | RA Duration** (days) | Reference |
|---|---|---|---|
| Context fear | 25% dorsal | 1 (27) | Kim & Fanselow, 1992 |
| Context fear | 25% dorsal | 1 (49) | Anagnostaras et al., 1999 |
| Context fear | 40% dorsal | 28 (71)*** | Maren et al., 1997 |
| Context fear | 40% dorsal | flat (180) | Lehmann et al., 2007 |
| Context fear | 85% d + v | flat (180) | Lehmann et al., 2007 |
| Context fear | 40% dorsal | flat (84) | Sutherland et al., 2008 |
| Context fear | 40% ventral | flat (84) | Sutherland et al., 2008 |
| Context fear | 85% d + v | flat (84) | Sutherland et al., 2008 |
| Tone fear | 25% dorsal | none (28) | Kim & Fanselow, 1992 |
| Tone fear | 25% dorsal | none (50) | Anagnostaras et al., 1999 |
| Tone fear | 40% dorsal | flat (100) | Maren et al., 1997 |
| Tone fear | 40% dorsal | flat (84) | Sutherland et al., 2008 |
| Tone fear | 40% ventral | flat (84) | Sutherland et al., 2008 |
| Tone fear | 85% d + v | flat (84) | Sutherland et al., 2008 |
| Light fear | 85% d + v | flat (42) | Lehmann et al., 2009a |
| Spatial navigation | 80% d + v | flat (98) | Bolhuis et al., 1994 |
| Spatial navigation | 80% d + v | flat (90) | Mumby et al., 1999 |
| Spatial navigation | 75% d + v | flat (105) | Sutherland et al., 2001 |
| Spatial navigation**** | 85% d + v | flat (98) | Clark et al., 2005 |
| Spatial navigation | 95% d + v | flat (42) | Martin et al., 2005 |
| Spatial navigation**** | 40% dorsal | flat (98) | Clark et al., 2005 |
| Spatial navigation | 45% dorsal | flat (42) | Martin et al., 2005 |
| Spatial navigation | 45% ventral | flat (42) | Martin et al., 2005 |
| Object discrimination | 75% d + v | flat (105) | Sutherland et al., 2001 |
| Object discrimination | 80% d + v | none (83) | Mumby et al., 1999 |
| Object discrimination | 75% d + v | none (3) | Lehmann et al., 2007 |
| Object exploration | 75% d + v | flat (35) | Gaskin et al., 2003 |
| Shock-probe | 75% d + v | flat (14) | Lehmann et al., 2006 |
| Picture memory | 75% d + v | flat (90) | Epp et al., 2008 |
| Trace eyeblink | 45% dorsal | 1 (6) | Takehara, et al., 2003 |
| Trace fear | 45% dorsal | 1 (199) | Quinn, et al., 2008a |
| Flavour/odour | 25% d + v | 2 (3) | Winocur, 1990 |
| Flavour/odour | 80% d + v | 2 (3) | Winocur et al., 2001 |
| Flavour/odour | 85% d + v | 1 (9) | Clark et al., 2002 |
| Flavour/odour | 75% d + v | 1 (20) | Ross & Eichenbaum, 2006 |
| Flavour/odour | 90% d + v | 1 (1) | Tse et al., 2007 |
This table summarizes experiments on RA with rats that attempt to evaluate a temporal gradient using the first post-lesion memory retention test, as opposed to post-lesion relearning or retraining.
RA duration is the number of days between learning and surgery at which there is significant RA. In brackets is a measure of uncertainty in the duration of RA indexed by the difference in days between the interval at which there is significant RA and the first remote interval when retention is significantly better. Flat means that RA was equivalent at all learning-surgery intervals with the number in brackets being the longest interval tested. None means no detectable RA with the number in brackets being the longest interval tested.
Even with the most remote memory (100 days) RA was large and statistically significant.
Multiple spatial memory tasks were used in this paper.
Extent of hippocampal damage
Inspection of Table 1 show that the extent of damage to the hippocampus varies widely across studies. This variance is important because a substantial region of spared hippocampus may be able to support memory retrieval. Investigators could erroneously infer that memory retrieval has successfully occurred independently of the hippocampus when in fact it is still hippocampus-dependent. Such functionality of a region of spared hippocampus has been convincingly demonstrated in the case of anterograde acquisition of place memory in the Morris water task. Sparing of as little as 26% of the hippocampus enables place learning to proceed efficiently (Moser, et al., 1995). Inspection of Table 1, as well as our two counter-posed examples, prompts consideration of the hypothesis that partial damage may be associated with a temporal gradient and extensive damage with flat gradients. Care should be taken however in not over-interpreting comparisons across laboratories given the different methodologies used. Better are comparisons between experiments in the same laboratory. Winocur has measured the effects of small dHPC electrolytic lesions and much more extensive, selective neurotoxic lesions on memory for socially transmitted flavour preferences (Winocur, 1990; Winocur et al., 2001). He found very similar RA with temporal gradients showing that memory was stabilized between 48 – 100 hr after learning. This comparison does not support the idea that the extent of damage is important for observing a temporal gradient and further that at least by 100 hr after learning, retrieval of the flavour memory may proceed independently of the hippocampus. More directly, four papers report experiments to explicitly test the effects of partial vs. extensive hippocampal damage on RA gradients (Clark, et al., 2005; Lehmann, et al., 2007 Martin, et al., 2005; Sutherland, et al., 2008). Two of these use spatial navigation memories and two use contextual fear conditioning, but the conclusions are the same. No temporal gradient was observed in any of the experiments with either memory type involving partial or extensive damage. There is some indication that partial damage may be associated with less severe effects on memory, but in all cases remote memories supporting context conditioning or place navigation were lost. Thus, the results of all direct tests and the inference from a cross-experiment test from the same laboratory are in agreement: the extent of damage to the hippocampus is not an important factor in determining whether a temporal gradient is observed.
Another related approach is to examine the relationship between the amount of spared hippocampal tissue and memory retention test performance. We have evaluated this association statistically in three RA experiments, two involving contextual fear conditioning (Lehmann, et al., 2007; Sutherland, et al., 2008) and the other picture discrimination memory (Epp, et al., 2008), using a quantitative assessment of hippocampal volume. In all three cases the association between memory retention and amount of spared hippocampal tissue was statistically significant and suggests that, at least in the case of picture memory and contextual fear memory, spared hippocampal tissue, certainly in the range of 30 – 40%, can support memory retrieval. Many of the experiments summarized in Table 1 have regions of spared hippocampus in this range that could support memory retrieval. Setting aside our own work, experiments reporting temporal gradients of RA for contextual fear conditioning (Anagnostaras, et al., 1999; Kim & Fanselow, 1992; Maren, et al., 1997) spare more than 50% of the hippocampus. Given that spared hippocampal regions can support memory retrieval, if experimenters insist on partial lesion methods, then it is imperative that spared tissue be quantified and that the interaction effect involving amount of spared hippocampal tissue and learning-surgery interval be statistically evaluated as in Sutherland, et al. (2008). If the association between spared tissue and memory performance observed by Lehmann, et al. (2007) with partial lesions is generally true, then differences of as little as 10 –15% in lesion extent could lead to significant memory retention effects between groups of rats tested at different learning-surgery intervals. This may not be a problem if damage exceeds 70%.
If we focus attention on the aforementioned three contextual fear conditioning papers (Anagnostaras, et al., 1999; Kim & Fanselow, 1992; Maren, et al., 1997), the paper by Maren, et al. (1997) is somewhat different from the other two in that the lesion extent is greater and there is a large deficit in retention performance even at the 100 day learning-surgery interval. The hippocampus was damaged 1, 28, or 100 days after learning and as a percentage of control levels of freezing during the first time-bin of retention testing the lesion group are performing at approximately 30, 35, and 50% respectively. Perhaps this degree of retention variability is within the range that could be attributed to differences in the extent of hippocampal damage. Because of the significant and similar RA at all learning-surgery intervals these results are clearly different than Anagnostaras, et al. (1999) and Kim & Fanselow (1992) with smaller lesions who report RA only at a 1 day learning-surgery interval and normal remote memory.
We also take note of the work of Takehara et al. (2003) with rats using trace eyeblink conditioning that is often cited as evidence for a lengthy period of hippocampal dependence of the task memory. First, a very large proportion of the ventral, posterior hippocampus is spared, these are nearly complete dorsal hippocampal lesions. Second, there is no relatively pure post-operative memory retention measure provided. Instead, the data are presented as percentage of trials with properly timed conditioned eyeblinks across whole sessions of retraining, that is with continuing pairings of CS and US. If one inspects the data from the first post-lesion retention (or more accurately retraining) session (Fig. 2, Takehara et al., 2003) there is a clear deficit in conditioned responding in the group with a one-day interval between training and dorsal hippocampal lesion. At one-, two- and four-week intervals performance is better in the first post-lesion session but at a very similar level across those intervals. Thus, the results of this experiment are not compelling of a lengthy RA interval because partial lesions were used, retraining was used during post-lesion testing, and the performance across 1- to 4-wk training-lesion intervals is approximately the same.
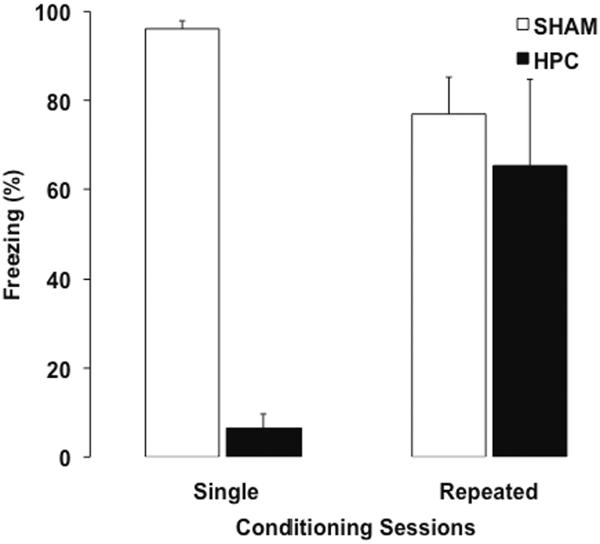
Figure 2.
Mean (+ SEM) percent time freezing during a retention test by Sham and HPC rats that received either a single or repeated contextual fear conditioning sessions before surgery. Complete HPC damage after a single (massed) contextual fear conditioning session that involved 12 foot shocks given within 17 min caused a significant freezing impairment (p < .05), suggesting that the damage caused severe retrograde amnesia and that the memory is dependent on the HPC. Oppositely, rats that received complete HPC damage after repeated conditioning sessions distributed across 6 days, which also in total involved 12 shocks and 17 minutes within the conditioning context, did not freeze significantly less than their respective control group (p > .05). Moreover, the HPC rats from the repeated condition showed significantly more freezing than the HPC rats from the single condition (p < .05). Hence, repeated conditioning sessions prevented the retrograde amnesic effects of complete HPC damage, implying that non-HPC systems were able to establish an HPC-independent memory and that repeated learning sessions mitigated the HPC overshadowing. The findings are adapted from and are fully described in Lehmann et al. (2009).
The MTT makes an explicit prediction that complete damage to the hippocampus should lead to flat gradients, whereas partial damage should lead to temporal gradients in RA for episodic and semantic memories. The observations that extensive hippocampal damage causes flat gradients lends partial support to the MTT over the SMSC. However, all of the experimental tests on the effect of partial damage fail to support the MTT.
Temporary Inactivation
An alternative tool used to investigate hippocampal function is a temporary lesion. Temporary inactivation involves “turning off” neural transmission in discrete regions of circuitry for a prescribed period of time from as little as 15 minutes to as long as 2 weeks (Lomber, 1999; Riedel et al., 1999). While the tissue is inactivated, basic cellular mechanisms continue to function maintaining the health of the network so that when the network “turns on”, then normal function may resume (Riedel et al., 1999). Though maintenance processes persist during inactivation, the overall ability of the network to encode or retrieve stored information is lost or significantly diminished. Temporary inactivations of discrete neural networks provide powerful advantages that are not possible with permanent lesion techniques, such as: (a) avoiding recovery of function, (b) flexibility in research design, and (c) control over temporal parameters (Lomber, 1999). With these techniques, the role of the hippocampus during memory acquisition, consolidation, and retrieval can be systematically investigated within specific learning and memory tasks. It is important in considering the diverse results with temporary inactivation that in most cases the extent of hippocampal inactivation has not been established.
The use of temporary inactivations to investigate hippocampal function has a long history stretching back to pioneering work by Avis & Carlton (1968) and Hughes (1969). In these studies, temporary hippocampal spreading depression, induced by intra-hippocampal infusions of potassium chloride, was used to determine hippocampal involvement in various components of a conditioned emotional response task Inactivating the hippocampus 24 hours following training disrupted performance during a retention test 4 days later. Hughes (1969) tested this finding further by inactivating the hippocampus at multiple time points following learning (i.e., 1, 3, 7, and 21 days) and found similar results--- hippocampal inactivation at recent or remote time points produced RA.
Since this time, a range of inactivation techniques have been used to investigate the role of the hippocampus in memory, including infusions of local anaesthetics (e.g., tetrodotoxin and lidocaine) (Sacchetti, Lorenzini, Baldi, Tassoni, & Bucherelli, 1999; Atkins, Mashhoon, & Kantak, 2008), the GABAa agonist muscimol (Holt & Maren, 1999; Matus-Amat, Higgins, Barrientos, & Rudy, 2004), and AMPA/kainate receptor antagonists (Riedel, et al., 1999; Micheau, Riedel, Roloff, Inglis, & Morris, 2004). In general studies using temporary inactivations of the hippocampus to investigate retrograde effects show comparable results to those found with permanent lesions. RA induced by hippocampal inactivation during memory retention has been reported in the Morris water task (Broadbent, Squire, & Clark, 2009; Teixeira, Pomedli, Maei, Kee, & Frankland, 2006; Micheau et al., 2004; Riedel et al., 1999), a working memory variant of the Morris water task (Bohbot, Otáhal, Liu, Nadel, & Bures, 1996), radial arm maze (Maviel, Durkin, Menzaghi, & Bontempi, 2004), conditioned emotional response (Hughes, 1969; Avis & Carlton, 1968), auditory fear conditioning (Hobin, Ji, & Maren, 2006; Corcoran, Desmond, Frey, & Maren, 2005; Corcoran & Maren, 2001; Maren & Holt, 2000; Holt & Maren, 1999), and the Fanselow immediate shock variant of contextual fear conditioning (Biedenkapp, & Rudy, 2009; Matus-Amat et al., 2004). It is important to note that most of the work done with temporary inactivation of the hippocampus has targeted, with a single bilateral infusion site, the dorsal portion of the hippocampus. The exceptions to this trend have focused on a single bilateral infusion site in the ventral hippocampus (Hobin et al., 2006), ventral subiculum (Biedenkapp & Rudy, 2009), and posterior hippocampus (Hughes, 1969; Avis & Carlton, 1968).
Although comparable RA effects are found when using permanent lesions and temporary inactivation techniques, there are a number of findings that could be interpreted as inconsistent. Most recently, Resstel et al. (2008) found that inactivation of the dorsal hippocampus (using cobalt chloride) during testing for contextual fear memory did not cause a memory impairment. Similarly, Holt & Maren (1999) did not find an effect of dorsal hippocampal inactivation (using muscimol) during retention testing in the standard context fear paradigm. These results are not surprising considering that permanent lesions of the dorsal hippocampus need to be very extensive in order to impair expression of fear memory in a similar task. With a similarly short learning-lesion interval Lehmann, et al., 2007 found only a very small effect of bilateral dHPC damage. Apparent contradictions arise in the results of lesion methods (permanent vs. temporary) prompts the question of whether comparable amounts of the hippocampal network are affected. It is quite possible that the inactivation parameters used in the contradictory studies did not inactivate enough of the hippocampal network to produce RA.
Given the advantages of temporary inactivation of the hippocampus, it is interesting that its application has been quite limited, not widely applied to diverse memory tasks at multiple time points after a learning episode. This limit could result from inadequate characterization of the temporal, spatial, and physiological effects of inactivations (i.e., duration of inactivation, extent and spread of network inactivation, and possible long-term physiological modifications due to inactivation). It will be necessary to elucidate optimal parameters of inactivating the hippocampus, possibly through electrophysiological recording and/or imaging of immediate early gene activity (Kubik, Miyashita, & Guzowski, 2008). There is currently good agreement in the results of RA experiments using permanent or temporary inactivation, but the range of parameters for comparison is too small to be definitive.
Type of memory task
The hippocampus obviously does not contribute equally to all memory tasks. This has been overwhelmingly supported by experiments assessing the effect of extensive hippocampal damage in the anterograde direction (O'Keefe & Nadel, 1978; Squire, 1992). For example, tasks that require remembering spatial/contextual information tend to be disrupted but those requiring simple discrimination between discrete cues or discrete stimulus-response learning tend to be unaffected (O'Keefe & Nadel, 1978). It would be very surprising if the nature of the memory task was not also important in RA, or in determining whether a temporal RA gradient is observed. In the realm of place navigation tasks in rats all experiments find flat RA gradients. At face value this scores against the generality of the systems consolidation process. However, it has been argued that navigation tasks do not offer a fair test of memory retention because in order to express a potentially intact place memory the rat must navigate using a process that requires up-dating of current position through a long trajectory (Knowlton & Fanselow, 1998). This hypothetical up-dating process may normally depend upon an intact hippocampal acquisition mechanism. Thus, it is possible that the rat has an intact remote place memory, but because the hippocampal lesion disrupts the up-dating process, it cannot be demonstrated through accurate open-field navigation. If this were true then none of the place navigation experiments would count against the SMSC. Only one experiment was explicitly designed to test this hypothesis. Clark, et al. (2007) modified the Morris water task by placing 4 identical visible beacons over the centre of each quadrant – one quadrant held the hidden platform. This clever modification would provide a navigational prop obviating the need for continuous up-dating during a trajectory to a remembered location in the pool. According to the hypothesis rats now should be able to express an intact, systems-consolidated, remote spatial memory by simply swimming to the correct beacon. Instead the beacons conferred no benefit during memory retention testing. The hypothesis is disconfirmed. The outcome of Clark, et al.'s (2007) experiment supports the idea that the RA gradient for spatial information really is flat. In addition, the authors make the important observation that during the retention test the rats did not use the beacons at all. For many this is surprising because learning to swim to visible beacons after hippocampal damage is unaffected. Clearly the RA after hippocampal damage is not limited to place memory and in the case of visible beacons (and contextual fear conditioning) can involve task performances that are unaffected in the anterograde direction. Sutherland, et al. (2001) have also reported a flat RA gradient, from 1 day to 15 wk, for visible platform discrimination memory in the Morris water task. To further reinforce the notion that continuous up-dating is not the reason that place navigation memory has a flat RA gradient, we note that there is growing list of memory tasks that exhibit flat RA gradients after extensive hippocampus damage. The list includes not only contextual fear conditioning (Lehmann, et al., 2007; Sutherland, et al., 2008) and visible beacon/landmark memory (Clark, et al., 2007; Sutherland, et al., 2001), but also fear conditioning to a discrete tone cue (Sutherland, et al., 2008; Maren, et al., 1997), picture discrimination memory (Epp, et al., 2006), shock-probe conditioning (Lehmann, et al., 2006a), and fear-potentiated startle memory (Lehmann, et al., 2006b). If this list continues to grow it should certainly compel a reexamination of the general applicability of the systems consolidation concept. At the present time, through examining the outcome of RA experiments with extensive hippocampal damage since the time of our two counterpoints, it is clear that the preponderance of the more recent results closely resemble those of Bolhuis, et al. (1994) showing flat gradients – no sparing of remote memories. None show a lengthy systems consolidation interval. The two notable task exceptions to flat gradients are social-transmission of flavour preferences (Winocur, 1990; Winocur et al., 2001; Clark et al., 2002; Ross and Eichenbaum, 2006) and flavour map memory (Tse et al., 2007). Both show very short intervals after learning (3 – 72 hr) when either small or large hippocampal lesions can unequivocally disrupt memory retention. Parenthetically, Clark et al. (2002) have an ambiguous result at 10 days learning-lesion interval. They show that the HPC lesion group is not different from chance at this interval, but unfortunately they are not significantly worse than the control group. It is intriguing that both have flavour/odour cues triggering memory retrieval. We note that Kim and Fanselow (1991) and Anagnostaras, et al. (1999) provided a potent odour cue in their context fear task by placing 5% ammonium hydroxide into the conditioning chambers. Thus, the evidence available from the current diverse memory tasks, especially those with extensive hippocampal damage which provide the most rigorous examination of the systems consolidation hypothesis, prompts the following generalizations: that memories that can be retrieved with an odour/flavour cue may have a short interval of vulnerability to hippocampal damage and that there is no evidence for a lengthy hippocampus dependent systems consolidation process. The hippocampus typically continues to make a necessary contribution to memory retrieval even for remote memories.
There is a final point of consensus in the work on task specificity of RA, a point that was not predicted by either of the aforementioned theoretical contenders. Some early papers noted that certain discrimination memory tasks were disrupted by hippocampal damage in the retrograde direction and are unaffected in the anterograde direction (Sara, 1981; Ross, et al., 1984). These were largely ignored. We now have numerous examples of the same phenomenon, memory tasks exhibiting severe RA without AA. These include contextual fear conditioning (Maren, et al., 1997), learned fear of a discrete tone cue (Sutherland, et al., 2008), visible platform discrimination (Sutherland, et al., 2001), visible beacon memory (Clark, et al., 2007), picture discrimination memory (Driscoll, et al., 2006; Epp, et al., 2008), object recognition (Gaskin et al., 2003), discrimination memory (Broadbent, Clark, & Squire, 2007), and fear-potentiated startle memory (Lehmann, et al., 2006b). Clearly, there are non hippocampal networks that are sufficient to enable storage and retrieval of the memories supporting performance in these tasks. Why then, if the hippocampus is intact at the time of learning, do these networks not establish an independently retrievable memory to support performance? Maren, et al. (1997) suggest that a competitive process exists at the time of learning between the hippocampal-based memory network and these other systems and normally the hippocampal network wins. This process of inhibition, interference, or competition involving the hippocampus dominating other networks has not received much experimental or theoretical attention. The dominated networks almost certainly involve amygdala circuitry (in the case of fear conditioning) and the cortico-striatal system (in the case of cue-discrimination memories). In many ways this interference or inhibition resembles the process of overshadowing in learning when one very salient cue acquires nearly all of the available associative strength despite the presence of other equally predictive, but very low salience, cues.
Memory Systems and Competition – Hippocampal Overshadowing
As previously mentioned the contributions of the hippocampus to long-term memory are not all-encompassing. In humans, it generally seems that explicit or declarative memories, which include information about personal events, are dependent on the hippocampus. In contrast, implicit or procedural memories, which include habits and skills, are independent of the hippocampus. This dissociation was initially described by Scoville and Milner (1957) when discussing the amnesia of patient H.M and has been reported in many other amnesics with hippocampal damage since then (Rempel-Clower, et al., 1996; Squire & Knowlton, 1995). A fictional example of this dissociation would be that the hippocampus is required to report on details about time, place, etc, concerning the first time we were able to ride a bicycle without training wheels or similar details about the last time we went for a bike ride – both being distinct personal events. If we were asked to demonstrate how to ride a bicycle, however, the hippocampus would not be required. Hence, responding to the different ways of probing memory about bicycle riding depends on different subsets of distributed brain regions, sometimes necessarily involving the hippocampus. Dissociations of this type have led theorists to posit that there are multiple memory systems in which different brain networks support different types of memory. Each system would be responsible for encoding, storing, and retrieving information involving qualitatively different representations, suited to the types of actions they support (Milner, 1959; Nadel & Moscovitch, 1997; Sherry & Schacter, 1987; Squire & Zola, 1996).
In line with this approach, several memory systems have also been demonstrated in rats (see O'Keefe & Nadel, 1978; White & McDonald, 2002). For instance, in a clever study, McDonald and White (McDonald & White, 1993) dissociated three memory systems: the hippocampus, the amygdala, and the caudate system. Specifically, they tested rats in a radial arm maze according to different test versions that each tap into different types of memory or associations among stimuli. The first was the win-shift task in which the animal need not return into an arm of the maze in which it previously retrieved food. The second task was win-stay, which required the animal to remember and return to specific arms of the maze that predicted food location. And the third task, conditioned cue preference, required the animal to approach a cue (light) in the maze that marked food location. Damage to the hippocampus impaired performance on the win-shift task, but not the win-stay or conditioned cue preference. Damage to the amygdala impaired performance on the conditioned cue preference test, but not the other two tests. Finally, damage to the caudate impaired performance on the win-stay task, but not the conditioned cue preference or win-shift tasks. Based on this triple dissociation they concluded that the mammalian brain included at least three independent memory systems, one supporting spatial map memory, a second supporting cue-outcome memory, and a third supporting cue-response memory.
It is unclear as to how many memory systems exist and describing each system that has been proposed is beyond the scope of this review. Nevertheless, it is important to acknowledge that not all memory systems require the hippocampus. For the remainder of this section we will consider two categorizations of memory systems: 1) a hippocampal memory system, and 2) non-hippocampal memory systems.
Compelling studies and several theories suggest that the hippocampal memory system interacts in significant ways with non- hippocampal systems (Frank, et al., 2003; McClelland, et al., 1995; Sherry & Schacter, 1987; White & McDonald, 2002). During some learning episodes, the hippocampal and non-hippocampal memory systems seem to function in parallel (McDonald & White, 1994; Sherry & Schacter, 1987), each system simultaneously creating its own record from the experience. We do not mean to imply that the memories formed in different systems are the “same memories”, instead they almost certainly represent different aspects of the same learning episode. In other situations, the different memory systems seem to engage in an antagonistic interaction or compete in the acquisition and retention of information (Frank, et al., 2003; McDonald & White, 1995). The existence of competition is strongly supported by the fact that damage to the hippocampus causes retrograde, but not anterograde, amnesia in a wide range of tasks. For instance, rats with extensive hippocampal damage made after learning, but not before, are impaired in object recognition (Broadbent, Clark, & Squire, 2007; Gaskin, Tremblay, & Mumby, 2003), shock-probe conditioning (Lehmann, Carfagnini, Yamin, & Mumby, 2005; Lehmann, Lecluse, Houle, & Mumby, 2006), contextual fear conditioning (Maren, et al., 1997; Wiltgen, et al., 2006), visual discrimination (Driscoll, et al., 2005; Sutherland, et al., 2001), fear-potentiated startle (Sparks, et al., 2005), pattern discrimination (roadbent, Clark, & Squire, 2007), and home base memory (Lehmann, et al., 2007; unpublished data). That these types of memories can be readily acquired and retained in absence of the hippocampus implies that non-hippocampal systems now have a record that is independently retrievable of a memory that is normally hippocampus-dependent. It is important to acknowledge that we are not implying that the memories created when the hippocampus is intact represent the same information as when the hippocampus is absent. The anterograde vs. retrograde dissociation is explained if the hippocampus, when present, interferes with the non-hippocampal systems establishing a strong enough representation to support successful retrieval during a retention test. This interfering effect could be instantiated in a number of ways: there could be a direct inhibition of other systems by the output from the hippocampus, there could be a competitive interaction at the time of action or response selection, there could be a competition for associative strength mediated by a third structure, and so. A view that we consider below is that the hippocampus wins a competition during a learning episode thereby preventing other systems from acquiring an independent memory. If the hippocampus is absent, then other systems are released from this interference and are able to acquire independent memories. One way that this interference from the hippocampus on the other memory systems has been characterized is as overshadowing (Driscoll, et al., 2005; Fanselow & Poulos, 2004; Lehmann, et al., 2006; Maren, et al., 1997; Sutherland, et al., 2006).
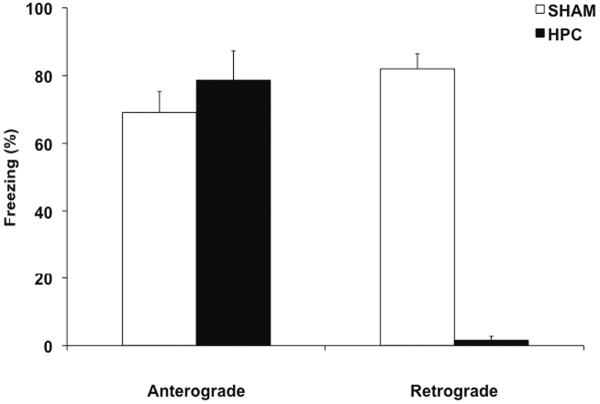
Several research groups have demonstrated the dissociable effects of hippocampal damage on anterograde and retrograde memory in contextual fear conditioning (Lehmann, et al., 2009; Maren, et al., 1997; Wiltgen, et al., 2006). Perhaps it is becoming a classic example of the hippocampus overshadowing non-hippocampal memory systems. Contextual fear conditioning involves rats learning and remembering that a configuration of static cues or context (e.g., a small chamber in a specific environment) is associated with an aversive event (e.g., foot shock). When tested for retention, rats are returned to the context in which they received the aversive event and display several species-specific defensive responses, including freezing which is defined as complete immobility except for breathing. The amount of freezing expressed during the retention test can be quantified and used as an index of memory. Rats that receive complete damage to the hippocampus days or months after contextual fear conditioning show very little freezing during the retention test compared to the control rats (Lehmann, et al., 2007; Sutherland, et al., 2008), suggesting that memory for contextual fear conditioning is dependent on the hippocampus. In contrast, rats that received complete damage to the hippocampus before conditioning show as much freezing as control rats (Wiltgen, et al., 2006), suggesting that contextual fear conditioning can be acquired and retained in absence of the hippocampus. This absence of AA for contextual fear conditioning in rats with extensive hippocampal damage suggests that this memory is now necessarily dependent on non- hippocampal systems. Figure 1 illustrates the dissociable effects of complete hippocampal damage made before and after learning in contextual fear conditioning in our laboratory. It is important to note, that the learning parameters (i.e., time in the conditioning chamber and the number of shocks) were identical in both instances. Hence, the absence of AA in the group that received hippocampal damage before conditioning is not due to a greater learning experience. Thus, normally the memory that is formed from the learning episode necessarily depends on the hippocampus, as supported by the RA findings, but a different memory from the same learning episode can readily be acquired and retained by non-hippocampal systems, but only when the hippocampus is absent. The latter implies that an intact hippocampus normally competes and overshadows non-hippocampal memory systems during contextual fear conditioning.
Figure 1.
Evidence from our laboratory that complete HPC damage in rats has dissociable effects on anterograde and retrograde memory in contextual fear conditioning. Complete damage made to the HPC before a single contextual fear conditioning session (Anterograde) did not cause anterograde amnesia, suggesting that, in absence of the HPC, non-HPC memory systems can readily acquire and support the memory for successful performance on the retention test. However, complete HPC damage induced 1–3 days after a single contextual fear conditioning session (Retrograde) almost abolished freezing during the retention test, suggesting profound retrograde amnesia and that contextual fear conditioning is normally dependent on the HPC. That contextual fear conditioning is normally dependent on the HPC, but can be supported by non-HPC memory systems in absence of the HPC implies that in the intact brain the HPC and non-HPC systems interact and that the HPC overshadows or prevents the non-HPC systems from acquiring an independent memory.
Hippocampal overshadowing is analogous to the concept of overshadowing presented in the Pavlovian conditioning literature and initially described by Pavlov (Pavlov & Anrep, 1927). If a compound of two neutral stimuli is paired with an unconditioned stimulus, typically the most salient of the two neutral stimuli will gain more associative strength and elicit a stronger conditioned response than the less salient stimulus. Yet, if the neutral stimuli are paired independently with the unconditioned stimulus, then both acquire similar associative strength and elicit similar conditioned responses. Hence, as a compound, the dominant stimulus overshadows the weaker stimulus. Similarly, we suggest that an analogous process occurs between memory systems. When two or more memory systems are active during an event, then a competition occurs between them and the dominating system will acquire the memory and overshadow the weaker system pertaining to that event. Thus, for the types of memories we have been studying, the hippocampus is the dominant system and overshadows the non-hippocampal systems. But, if the hippocampus is extensively damaged, the other systems are freed from this competition and readily acquire independently retrievable memories. An alternative view is that this interfering effect could involve the hippocampal output being a significant modulator of the representations in neocortical perceptual association areas at the time of the learning, such that at the time of memory retrieval, in the absence of the hippocampus, the original representation of cues is not reinstated, and thus, the target memory is not contacted. There are few data to constrain the possible processes that mediate this overshadowing. One experiment by Biedenkapp & Rudy (2009) studied overshadowing in the contextual fear conditioning task. They showed that infusion of a D1 dopamine agonist into the amygdala, a treatment that is thought to enhance associative plasticity in the amygdala, was effective in overcoming hippocampal overshadowing of the other system. At face value this outcome favours the idea that overshadowing is based upon a competitive process. Overshadowing could also be accomplished by multiple processes.
Not all types of memories affected by hippocampal damage are subject to overshadowing. Damage to the hippocampus can cause profound AA in certain tasks, suggesting that non-hippocampal systems fail to acquire and support these memories. For instance, the hidden platform version of the Morris Water Task (Martin, de Hoz, & Morris, 2005; Morris, Garrud, Rawlins, & O'Keefe, 1982; Sutherland, et al., 1982), paired-associate learning (Tse, et al., 2007), socially transmitted food preference (Clark, Broadbent, Zola, & Squire, 2002; Winocur, 1990; Winocur, McDonald, & Moscovitch, 2001), certain versions of transverse patterning discrimination (Driscoll, et al., 2005), and object-context pairing (Mumby, Gaskin, Glenn, Schramek, & Lehmann, 2002) are all impaired with hippocampal damage induced before learning. An important subset of memories that is dependent on hippocampus and cannot independently be acquired by non-hippocampal systems involves configural/conjunctive associations (Driscoll, et al., 2005; Sutherland & Rudy, 1989). Therefore, it seems that the acquisition and retention of a subset of configural/conjunctive associations may not involve a competition amongst memory systems or, at very least, hippocampal overshadowing.
When considering hippocampal overshadowing, the issue is not whether the non-hippocampal system, in absence of the hippocampus, acquires and retains the same information as the hippocampus normally does. Most likely, a rat with hippocampal damage does not retrieve the same information about an event as a rat that has an intact hippocampus. The memories leading to the successful performance on a memory task are obviously supported by different neural systems in an intact rat and an hippocampal -damaged rat and it is very likely that the memory in each rat is also qualitatively different. Therefore, we are not suggesting that there is a “back-up system” in case of injury to the hippocampus. The key feature in the overshadowing model is that the hippocampal system and non-hippocampal systems can both independently acquire and retain qualitatively different representations to support successful performance on a memory task. However, for many types of memory tasks, when both systems are fully functional, then a competition occurs and the hippocampus dominates in acquiring the supporting memory for task performance, which in turn makes the memory hippocampal -dependent. For example, in contextual fear conditioning it is believed that the RA observed following hippocampal damage is the result of a configural/conjunctive representation of the context being lost (Maren, et al., 1997; Rudy, Barrientos, & O'Reilly, 2002; Rudy & O'Reilly, 1999). It is also argued that the absence of AA in contextual fear conditioning in hippocampal-damaged rats is not due to the ability to establish a configural/conjunctive representation of the context in non-hippocampal systems. Rather the anterograde memory for contextual fear conditioning would be supported by elemental associations – associations between discrete elements of the context (e.g., the walls, floor, and odour of the chamber) being paired with the aversive experience (Maren, et al., 1997; Wiltgen, et al., 2006). Why can discrete elements of the context be readily associated with the aversive event in absence of the hippocampus, but not when it is present? Again, the simplest explanation is that non-hippocampal systems are released from the hippocampal overshadowing (Driscoll, et al., 2005), otherwise, the non-hippocampal systems, despite the presence of the hippocampus, would establish an elemental association that would support performance on the retention test and no RA would be observed following hippocampal damage. Therefore, the hippocampal overshadowing phenomenon implies that the hippocampal system prevents non-hippocampal systems from fully establishing the type of memory that they are normally involved in and not preventing a copy of an hippocampal system memory.
Overcoming Hippocampal Overshadowing – Learning Parameters
The hippocampal overshadowing phenomenon raises a pivotal hypothesis: Despite an intact hippocampus and the occurring overshadowing, can the non-hippocampal systems be driven to establish a strong enough representation to support successful retention? In other words, can we manipulate the learning parameters to establish a strong memory in both the hippocampal system and non-hippocampal systems that could each independently support retention? If yes, then damaging the entire hippocampus should no longer cause RA because memory could then be supported by the non-hippocampal systems. A positive outcome would point to another explanation, other than systems consolidation, for why some remote memories are unaffected by hippocampal RA.
We have started to answer the above question by examining the effects of repeated learning episodes. Following the premise that distributed learning establishes “stronger” memories than massed learning (Fanselow, DeCola, & Young, 1993; Fanselow & Tighe, 1988), we hypothesized that establishing a memory through distributed learning sessions might incrementally establish an independently retrievable memory in the non-hippocampal networks. Consequently, a memory normally dependent on the HPC would now survive complete hippocampal damage.
In support of this hypothesis, we recently demonstrated that contextual fear conditioning distributed across 11 sessions, rather than a single learning session, creates a context fear memory that is immune to RA after very extensive hippocampal damage (Lehmann, et al., 2009b). The most important comparison involved rats that received all of their context-foot shock pairings in a single episode vs those that received the same number of pairings distribute across 6 days. Rats that received very extensive hippocampal damage one week after a single 17 min contextual fear conditioning session during which they received 12 foot shocks, showed dramatically less context fear on the retention test than control rats, consistent with complete RA. In contrast, rats that received very extensive hippocampal damage after 11 conditioning sessions distributed across 6 days, which matched the number of foot shocks and context exposure time as the single episode procedure, showed the same level of context fear as control rats. The latter findings are illustrated in Figure 2. Importantly, the interval between initial learning and surgery was identical in both conditions controlling for an hypothetical, time-based systems consolidation process. Moreover, the absence of a context fear deficit in the hippocampal-damaged rats experiencing distributed learning episodes was not due to a generalized fear response. The hippocampal-damaged rats did not show fear when tested in a novel context. Thus, repeated contextual fear conditioning sessions enabled non-hippocampal systems to acquire and retain a discrete contextual fear conditioning memory, despite the hippocampus being present during learning. The repeated learning sessions mitigated the hippocampal overshadowing typically observed in contextual fear conditioning.
This result is significant because it is the first demonstration that a recent contextual fear memory can survive very extensive post-training hippocampal damage. Indeed, there is a consensus across laboratories that damage to the hippocampus causes RA for recent, single episode of contextual fear conditioning (Anagnostaras, et al., 1999; Kim & Fanselow, 1992; Lehmann, et al., 2007; Maren, et al., 1997; Sutherland, et al., 2008). There are diverse results on whether there is a temporal gradient for contextual fear conditioning, with some findings that more remote memory is spared (Anagnostaras, et al., 1999; Kim & Fanselow, 1992; Maren, et al., 1997). Nevertheless, it is uniformly agreed that a recent memory for a single contextual fear-conditioning episode is dependent on the hippocampus. In contrast, distributing the contextual fear conditioning across 6 days established a recent hippocampus-independent memory. The memory created by distributed learning is assuredly as recent as in the single learning episode memory because the interval between initial learning and the induction of hippocampal damage in both conditions was matched; yet only the single session memory did not survive hippocampal damage. There are no other reports of intact retrograde memory for contextual fear conditioning following damage induced at an learning-surgery interval that is as short.
We have obtained a similar pattern of findings when manipulating the number learning sessions in object recognition (Lehmann, Sparks, Spanswick, & Sutherland, 2008). Object recognition in rats can be assessed using the novel object preference test, which relies on the rat's natural propensity to investigate novelty (Ennaceur & Delacour, 1988). Briefly, rats are given a certain number of sample/learning sessions during which they are free to investigate a pair of identical objects that is fixed to the floor of an open field. Rats are then given, at a later time, a retention test in the same open field with one of the two sample objects having been changed to an object they have never encountered before (i.e., novel object). Typically, rats will spend a greater proportion of their object investigation time with the novel object, suggesting that they remember the sample object. Others (Gaskin, Tremblay, & Mumby, 2003) have found that hippocampal damage causes RA for object recognition when rats are given up to five sample/learning sessions and we have observed the same result (Lehmann, et al., 2008). Moreover, memory for object recognition does not seem to undergo time-based systems consolidation because hippocampal damage has caused RA for this memory even when the damage is caused up to 5 weeks after learning (Gaskin, et al., 2003; Lehmann, et al., 2008). Therefore, object recognition memory may normally dependent on the hippocampus for at least a period of five weeks.
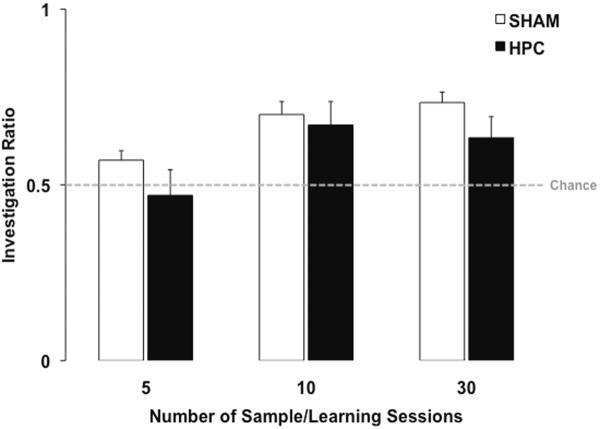
Object recognition memory, however, can be established in non- hippocampal systems in absence of the HPC (Ainge, et al., 2006; Gaskin, et al., 2003; Lehmann, et al., 2008; Mumby, Gaskin, Glenn, Schramek, & Lehmann, 2002; Mumby, Tremblay, Lecluse, & Lehmann, 2005). The dissociation between the effects of hippocampal damage on anterograde and retrograde memory, as previously described, suggests that the hippocampus overshadows non- hippocampal systems for object recognition. Again, we examined whether increasing the number of learning sessions can overcome the overshadowing, establish an independent memory in non-hippocampal systems, and prevent the RA resulting from hippocampal damage. Our preliminary evidence indicates that increasing the number of sample sessions from 5 to 10 is sufficient to make recent object recognition memory hippocampus-independent (Lehmann, et al., 2008). As shown in Figure 3, rats that received complete hippocampal damage 3–5 days after 5 sample/learning sessions did not show a preference for the novel object during the retention test, whereas their control group did show a preference for the novel object. Hence, the hippocampal damage caused RA in the 5-sample session condition as found in previous studies (Broadbent, et al., 2008; Gaskin, et al., 2003; Lehmann, et al., 2008). However, if rats received very extensive hippocampal damage 3–5 days after having received 10 or 30 sample sessions, then they showed a reliable preference for the novel object on the retention test. This finding is consistent with the idea that after 10 sample sessions the memory had become established in non-hippocampal systems. Thus, in object recognition, as in contextual fear conditioning, the hippocampal-overshadowing can be overcome by an increase in the number of learning sessions.
Figure 3.
Mean (+ SEM) novel object investigation ratio during an object recognition retention test by Sham and HPC rats that received 5, 10, or 30 sample/learning sessions before surgery. Complete HPC damage induced 3–5 days after 5 sample sessions, lasting 5 minutes each and distributed across 5 consecutive days, caused retrograde amnesia. Indeed the Sham rats showed a significant preference (above chance; p < .05) for the novel object during the 2-minute retention test, but not the HPC rats (p > .05). In contrast, HPC-damaged rats that received 10 sample sessions, each lasting 5 minutes and distributed over 2 weeks, did not show a retrograde impairment as they investigated the novel object significantly more than the sample object (p < .05). A similar preference for the novel object (p < .05) was observed when the HPC rats received 30 sample sessions, distributed across 6 weeks, prior to surgery. Combined, these findings suggest that object recognition memory may be dependent on the HPC, but that additional learning non-HPC systems may also support the memory.
Further support for our hypothesis that initial learning parameters can decrease a memory's vulnerability to hippocampal damage comes from a study by Winocur et al. (2005). In that study, despite receiving hippocampal damage as adults, rats that were reared in a complex environment appeared to remember the spatial organization of the environment. In contrast, rats that were allowed to learn the configuration of the environment, but were not reared in it, exhibited significant RA following the hippocampal damage. One must then conclude that living in the environment allowed non-hippocampal systems to establish an independent memory to support successful performance on their retention test. Moreover, rearing in the environment effectively acts like repeated, distributed learning episodes. Unfortunately, the latter cannot be strongly concluded because the authors did not control for a hypothetical time-based systems consolidation, as they did not match the interval between initial learning and HPC ablation between the reared and non-reared rats in their study. Nevertheless, our position predicts that time would not be a determining factor.
When human amnesic patterns caused by hippocampal damage are discussed the characteristics of initial learning are typically not considered because the retrospective examination of a patient's memory is undermined by an inability to determine the properties of the initial learning episodes (Andersen, 2007, p. 566), how many learning episodes involved the information, and when each of these occurred. These considerations raise the possibility that some remote memories in patients displaying temporally graded RA were less vulnerable to hippocampal damage because of repeated learning episodes or some other property of initial learning that favoured acquisition and retention in non-hippocampal systems. Considering remote memories, it is fair to speculate that they are more likely to be reiterated or re-experienced many times and distributed across episodes with the passage of time. By reiteration, we mean re-experiencing the associated elements of the original learning episode, explicit re-exposure to very similar information, not persistent long-term off-line memory replay. Each of these reiterations may have promoted strengthening in non- hippocampal systems similar to our current findings in contextual fear conditioning and object recognition experiments examining effects of learning parameters. Thus the spared remote memories after HPC damage in patients might be due to more remote memories becoming established in non- hippocampal systems because similar information was reiterated many times in distributed learning episodes. These memories would consequently be less likely to be lost following hippocampal damage because they would be sufficiently established outside the hippocampus. Most importantly, the underlying mechanism for memories becoming independent of the hippocampus could be a change in the strength of the representation in non-hippocampal systems over learning or reiteration, rather than a sustained period of hippocampal dependent systems consolidation process extending long after the learning episode.
Some support for reiteration or additional experience promoting hippocampus-independence of remote memories can be found in a patient with hippocampal damage (Maguire, Nannery, & Spiers, 2006). Patient TT was a taxi driver in London for approximately 40 years before sustaining bilateral hippocampal damage. He learned a layout of about 25,000 streets during his training to become a taxi driver and subsequently used some routes much more frequently than others. Interestingly, the routes that TT used most frequently were remembered after the hippocampus was damaged, whereas he displayed robust RA for the routes he used less frequently. These findings suggest that TT's RA for London routes was dependent on how often he experienced the routes. Knowledge about all routes was approximately 40 years old which certainly qualifies all of them as remote memories, yet TT showed greater amnesia compared to control subjects for the routes less frequently traveled. Therefore, repetition and not time-based consolidation may be the determining factor for memories becoming hippocampus-independent in this patient, congruent with our view that learning parameters, especially distributed reiterations, are critical in making memories become independent of the hippocampus. As a caution here we note that it is possible that frequency of route use might be confounded with recency and with street size.
Combined our present contextual fear conditioning and object recognition findings demonstrate that a memory can be formed that is hippocampus independent through repeated, distributed learning episodes, despite the fact that with one or a few learning episodes a hippocampus dependent memory is necessary (although these two memories likely do not represent identical information from the learning episode). Moreover, this change in dependency can occur rapidly, in just a few days. This is a sharp contrast to the prevailing view that memories become independent of the hippocampus through a lengthy systems consolidation process (Moscovitch, Nadel, Winocur, Gilboa, & Rosenbaum, 2006; Squire, 1992; Squire, Stark, & Clark, 2004). As previously described, it is generally believed that over time, with a lengthy period of off-line hippocampal dependent reinstatement of the memory representation, the essential support for memories is “switched” from dependence on the hippocampus to neocortical networks (Anagnostaras, Gale, & Fanselow, 2001; Frankland & Bontempi, 2005; McClelland, McNaughton, & O'Reilly, 1995; Meeter & Murre, 2004; Squire & Alvarez, 1995; Squire, et al., 2004; Wiltgen, Brown, Talton, & Silva, 2004). We suggest that for many memories each learning episode incrementally establishes a stronger representation in non-hippocampal systems. With a sufficient number of distributed learning episodes, memories can survive complete hippocampal damage because they would be dependent on other systems. In other words, the hippocampus is often in competition with non- hippocampal systems in establishing and supporting a memory. The hippocampus typically dominates and the memory becomes thus hippocampus-dependent. With additional distributed learning trials, more and more of a representation pertaining to the episodes is captured by the non-hippocampal systems. And, at a certain point, the memory would surpass a threshold to be supported independently of the hippocampus. The evidence from our contextual fear conditioning and object recognition experiments, which tap into different types of memories, favour this view and demonstrate that hippocampal independence can be achieved with distributed learning episodes and that demonstrates that a lengthy, time-based systems consolidation is not necessary for making memories become hippocampus-independent.
Conclusions
We have reviewed some of the basic characteristics of the results of a large number of experiments on RA after hippocampal damage in rats. The experimental analysis of RA using these approaches benefits from being able to precisely control when a learning episode takes place, the number of iterations of the same information, the time between learning and hippocampal damage, and the extent and location of the damage. The following generalizations are emerging: 1. If hippocampal damage is very extensive, RA exhibits a flat gradient for at least months, for even the most remote memories supporting most tasks; 2. Tasks that involve odour/flavour retrieval cues exhibit a temporal gradient of RA lasting 24 – 100 hr; 3. Partial hippocampal damage tends to be associated with milder RA, but in direct experimental tests of this factor, sparing of remote memories (temporal gradients) are not more likely to be observed than if damage is very extensive; 4. Spared hippocampal tissue with partial damage contributes to memory retrieval; 5. Where direct comparisons can be made, temporary inactivations tend to produce the same results as permanent lesions; 6. There is a large set of memory tasks that are impaired by RA that are unaffected in anterograde direction by extensive hippocampal damage, consistent with a hippocampal process that interferes with or overshadows independent processing by other systems; 7. Some variables in initial learning, such as distributed learning episodes, can create a memory that survives hippocampal RA.
Recent work on RA after hippocampal damage in rats questions the generality of a lengthy process of hippocampal-dependent systems consolidation. There are no examples of memory tasks with rats having extensive hippocampal damage exhibiting a lengthy interval covered by RA with spared remote memories. We offer an alternative to protracted systems consolidation to explain instances of spared remote memories in hippocampal RA, involving initial learning parameters, such as distributed learning, that favour acquisition by non hippocampal networks.
The fact that there is only very weak evidence (one study with rats) for a long-term (weeks to months time-scale) systems consolidation process, and no experimental evidence from very extensive hippocampal damage, does harm to the idea that creation of a permanent non hippocampal memory depends upon a lengthy period of off-line (post-learning episode) hippocampus-based reinstatement of memories in a distributed cortico-hippocampal network (McClelland, et al., 1995; Squire, 1992). We suggest that the following two possibilities are consistent with the preponderance of the experimental data. The first is a simple dual-store model. Different memory systems acquire information independently, based upon learning rate parameters set by their intrinsic network properties and by modulatory interactions from other systems. McClelland et al. (1995) explicitly rejected this sort of model in favour of a long-term systems consolidation theory, in large part based upon data of the kind reported by Kim & Fanselow (1992). If that type of temporal gradient is not generally observed then the dual-store model may merit reconsideration. In the data we review here the dual-store model runs afoul of the experiments on RA with flavours/odours. A simple interpretation of those results is that there is a short-term systems consolidation process that depends on a short period (on the time-scale of hours) of hippocampal-dependent reiteration of representations of the information from the learning episode. We note that the evidence for hippocampal-cortical reiteration or replay outside of the context of original learning is now extensive. A full review of these data is clearly outside the scope of this paper. From the perspective of our considerations here however it is important to consider that not only is there solid evidence from multiple laboratories confirming patterned replay or reiteration of stored memories in hippocampus and neocortex using ensemble unit recording and immediate early gene activity, but this replay lasts on the time-scale of hours (not weeks to months) (Euston, et al., 2007; Karlson & Frank, 2009; Kudrimoti, et al.,1999; Pavlides & Winson, 1989; Ribeiro, et al., 2004; Wilson & McNaughton, 1994). There is some evidence, albeit very limited, in the data from Kudrimoti, et al. (1999) for replay as long as 24 hr after a learning episode, and there is a controversial report suggesting that replay can occur as long as 48 hr after (Ribeiro, et al., 2004; but see Tatsuno, et al., 2006). These considerations, together with our observations that distributed repetition of a learning episode, involving context fear conditioning or object exploration, uniquely creates a hippocampus-independent memory, prompt consideration of a novel account. The second account, which we term the Distributed Reinstatement Theory, holds that a learning episode rapidly creates a stored memory representation that is quite strong in hippocampus and very weak in non hippocampal networks, bouts of hippocampus-dependent replay incrementally strengthen the non hippocampal memory representation, but the bouts of replay become very infrequent on the time-scale of hours. For nearly all memories this would be insufficient to establish a long-term memory that is independent of the hippocampus. Thus, without additional learning episodes most memories continue in the long-term to depend on the hippocampus for recall. If learning episodes are repeated in a distributed fashion, after each episode there is a series of bouts of off-line replay. Thus, each reiteration of the learning episode would incrementally enhance the non hippocampal representation of the relevant information, potentially through two processes, a small amount of direct associative strength acquired independently by the non hippocampal network, and through a series of replays for some hours after each episode. Memories that differ in complexity or overlapping content with other events likely differ in the number of series of bouts of short-lasting off-line replay needed before they have a chance to be represented strongly in non hippocampal networks. On this view flavour/odour memories must be special in that they may need only a single series of replay events triggered by one learning episode for substantial systems consolidation to occur. Contextual or spatial memories may be at the opposite end of this dimension.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ainge JA, Heron-Maxwell C, Theofilas P, Wright P, de Hoz L, Wood ER. The role of the hippocampus in object recognition in rats: examination of the influence of task parameters and lesion size. Behavioural Brain Research. 2006;167(1):183–195. doi: 10.1016/j.bbr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11(1):8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Maren S, Fanselow MS. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subjects examination. Journal of Neuroscience. 1999;19(3):1106–1114. doi: 10.1523/JNEUROSCI.19-03-01106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P. The hippocampus book. Oxford University Press; Oxford; New York: 2007. [Google Scholar]
- Atkins A, Mashhoon Y, Kantak K. Hippocampal regulation of contextual cue-induced reinstatement of cocaine-seeking behavior. Pharmacology, Biochemistry and Behavior. 2008;90:481–491. doi: 10.1016/j.pbb.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avis HH, Carlton PL. Amnesia produced by hippocampal spreading depression. Science. 1968;161(3836):73–75. doi: 10.1126/science.161.3836.73. [DOI] [PubMed] [Google Scholar]
- Biedenkapp JC, Rudy JW. Hippocampal and extrahippocampal systems compete for control of contextual fear: Role of ventral subiculum and amygdala. Learning & Memory. 2009;16:38–45. doi: 10.1101/lm.1099109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohbot V, Otáhal P, Liu Z, Nadel L, Bures J. Electroconvulsive shock and lidocaine reveal rapid consolidation of spatial working memory in the water maze. Proceedings of the National Academy of Science USA. 1996;93:4016–4019. doi: 10.1073/pnas.93.9.4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhuis JJ, Stewart CA, Forrest EM. Retrograde amnesia and memory reactivation in rats with ibotenate lesions to the hippocampus or subiculum. Quarterly Journal of Experimental Psychology. 1994;B 47:129–150. [PubMed] [Google Scholar]
- Broadbent NJ, Gaskin S, Squire LR, Clark RE. Recognition memory after hippocampal damage in rats: Anterograde and temporally graded retrograde memory impairment. Society for Neuroscience Abstracts. 2008 791.12. [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Reversible hippocampal lesions disrupt water maze performance during both recent and remote memory tests. Learning & Memory. 2006;13:187–191. doi: 10.1101/lm.134706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Rats depend on habit memory for discrimination learning and retention. Learning & Memory. 2007;14:145–151. doi: 10.1101/lm.455607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Broadbent NJ, Squire LR. Hippocampus and remote spatial memory in rats. Hippocampus. 2005a;15:260–272. doi: 10.1002/hipo.20056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Broadbent NJ, Squire LR. Impaired remote spatial memory after hippocampal lesions despite extensive training beginning early in life. Hippocampus. 2005b;15:340–346. doi: 10.1002/hipo.20076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Broadbent NJ, Squire LR. The hippocampus and spatial memory: Findings with a novel modification of the watermaze. Journal of Neuroscience. 2007;27:6647–6654. doi: 10.1523/JNEUROSCI.0913-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Broadbent NJ, Zola SM, Squire LR. Anterograde amnesia and temporally graded retrograde amnesia for a nonspatial memory task after lesions of hippocampus and subiculum. Journal of Neuroscience. 2002;22(11):4663–4669. doi: 10.1523/JNEUROSCI.22-11-04663.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Maren S. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. Journal of Neuroscience. 2001;21(5):1720–1726. doi: 10.1523/JNEUROSCI.21-05-01720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. Journal of Neuroscience. 2005;25(39):8978–8987. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkin S. What's new with the amnesic patient HM? Nature Reviews Neuroscience. 2002;3:153–60. doi: 10.1038/nrn726. [DOI] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE, Nader K. Cellular and systems reconsolidation in the hippocampus. Neuron. 2002;36(3):527–538. doi: 10.1016/s0896-6273(02)01001-2. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Howard SR, Prusky GT, Rudy JW, Sutherland RJ. Seahorse wins all races: Hippocampus participates in both linear and non-linear visual discrimination learning. Behavioural Brain Research. 2005;164(1):29–35. doi: 10.1016/j.bbr.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behavioural Brain Research. 1988;31(1):47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Epp J, Keith JR, Spanswick SC, Stone JC, Prusky GT, Sutherland RJ. Retrograde amnesia for visual memories after hippocampal damage in rats. Learning & Memory. 15:214–221. doi: 10.1101/lm.788008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euston DR, Tatsuno M, McNaughton BL. Fast-forward playback of recent memory sequences in prefrontal cortex during sleep. Science. 2007;318:1147–1150. doi: 10.1126/science.1148979. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annual Review of Psychology. 2004;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Tighe TJ. Contextual conditioning with massed versus distributed unconditional stimuli in the absence of explicit conditional stimuli. Journal of Experimental Psycholology: Animal Behavior Processes. 1988;14(2):187–199. [PubMed] [Google Scholar]
- Fanselow MS, DeCola JP, Young SL. Mechanisms responsible for reduced contextual conditioning with massed unsignaled unconditional stimuli. Journal of Experimental Psycholology: Animal Behavior Processes. 1993;19(2):121–137. [PubMed] [Google Scholar]
- Frank MJ, Rudy JW, O'Reilly RC. Transitivity, flexibility, conjunctive representations, and the hippocampus. II. A computational analysis. Hippocampus. 2003;13(3):341–354. doi: 10.1002/hipo.10084. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nature Reviews Neuroscience. 2005;6(2):119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Cestari V, Filipkowski RK, McDonald RJ, Silva AJ. The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. Behavioral Neuroscience. 1998;112(4):863–874. doi: 10.1037//0735-7044.112.4.863. [DOI] [PubMed] [Google Scholar]
- Gaskin S, Tremblay A, Mumby DG. Retrograde and anterograde object recognition in rats with hippocampal lesions. Hippocampus. 2003;13(8):962–969. doi: 10.1002/hipo.10154. [DOI] [PubMed] [Google Scholar]
- Gaskin S, Tremblay A, Mumby DG. Retrograde and anterograde object recognition in rats with hippocampal lesions. Hippocampus. 2003;13(8):962–969. doi: 10.1002/hipo.10154. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Winocur G, Rosenbaum RS, Poreh A, Gao F, Black SE, Westmacott R, Moscovitch M. Hippocampal contributions to recollection in retrograde and anterograde amnesia. Hippocampus. 2006;16:966–980. doi: 10.1002/hipo.20226. [DOI] [PubMed] [Google Scholar]
- Hobin JA, Ji J, Maren S. Ventral hippocampal muscimol disrupts context-specific fear memory retrieval after extinction in rats. Hippocampus. 2006;16:174–182. doi: 10.1002/hipo.20144. [DOI] [PubMed] [Google Scholar]
- Holt W, Maren S. Muscimol inactivation of the dorsal hippocampus impairs contextual retrieval of fear memory. Journal of Neuroscience. 1999;19(20):9054–9062. doi: 10.1523/JNEUROSCI.19-20-09054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RA. Retrograde amnesia in rats produced by hippocampal injections of potassium chloride: gradient of effect and recovery. Journal of Comparative and Physiological Psychology. 1969;68(4):637–644. doi: 10.1037/h0027648. [DOI] [PubMed] [Google Scholar]
- Mattias P, Karlsson MP, Loren M, Frank LM. Awake replay of remote experiences in the hippocampus. Nature Neuroscience. 2009;12(7):913–920. doi: 10.1038/nn.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256(5057):675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Fanselow MS. The hippocampus, consolidation and online memory. Current Opinion in Neurobiology. 1998;8:293–296. doi: 10.1016/s0959-4388(98)80154-2. [DOI] [PubMed] [Google Scholar]
- Kubik S, Miyashita T, Guzowski JF. Hippocampal inactivation produces hypoactivity in a cortical circuit: a marked decrease in experience-dependent Arc gene expression in rat retrosplenial cortex. Society for Neuroscience Abstracts. 2008 389.4. [Google Scholar]
- Kudrimoti HS, Barnes CA, McNaughton BL. Reactivation of hippocampal cell assemblies: effects of behavioral state, experience and EEG dynamics. Journal of Neuroscience. 1994;19:4090–4101. doi: 10.1523/JNEUROSCI.19-10-04090.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann H, Carfagnini A, Yamin S, Mumby DG. Context-dependent effects of hippocampal damage on memory in the shock-probe test. Hippocampus. 2005;15(1):18–25. doi: 10.1002/hipo.20024. [DOI] [PubMed] [Google Scholar]
- Lehmann H, Clark BJ, Whishaw IQ. Similar development of cued and learned home bases in control and hippocampal-damaged rats in an open field exploratory task. Hippocampus. 2007;17(5):370–380. doi: 10.1002/hipo.20274. [DOI] [PubMed] [Google Scholar]
- Lehmann H, Lacanilao S, Sutherland RJ. Complete or partial hippocampal damage produces equivalent retrograde amnesia for remote contextual fear memories. European Journal of Neuroscience. 2007;25(5):1278–1286. doi: 10.1111/j.1460-9568.2007.05374.x. [DOI] [PubMed] [Google Scholar]
- Lehmann H, Lecluse V, Houle A, Mumby DG. Retrograde amnesia following hippocampal lesions in the shock-probe conditioning test. Hippocampus. 2006;16(4):379–387. doi: 10.1002/hipo.20159. [DOI] [PubMed] [Google Scholar]
- Lehmann H, Sparks FT, O'Brien J, Robert J, McDonald RJ, Sutherland RJ. Complete, but not dorsal or ventral, hippocampal damage produces retrograde amnesia for fear-potentiated startle in rats. Neuroscience. 2009a doi: 10.1016/j.neuroscience.2010.03.005. submitted. [DOI] [PubMed] [Google Scholar]
- Lehmann H, Sparks FT, Spanswick SC, Sutherland RJ. Learning parameters mitigate retrograde amneisa after hippocampal damage. Society for Neuroscience Abstracts. 2008 295.9. [Google Scholar]
- Lehmann H, Sparks FT, Spanswick SC, Hadikin C, McDonald RJ, Sutherland RJ. Making context memories independent of the hippocampus. Learning & Memory. 2009;16:417–420. doi: 10.1101/lm.1385409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomber S. The advantages and limitations of permanent or reversible deactivation techniques in the assessment of neural function. Journal of Neuroscience Methods. 1999;86:109–117. doi: 10.1016/s0165-0270(98)00160-5. [DOI] [PubMed] [Google Scholar]
- Lomber S, Payne B, Horel J. The cryoloop: an adaptable reversible cooling deactivation method for behavioral or electrophysiological assessment of neural function. Journal of Neuroscience Methods. 1999;86(2):179–194. doi: 10.1016/s0165-0270(98)00165-4. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Nannery R, Spiers HJ. Navigation around London by a taxi driver with bilateral hippocampal lesions. Brain. 2006;129(11):2894–2907. doi: 10.1093/brain/awl286. [DOI] [PubMed] [Google Scholar]
- Maren S, Holt W. The hippocampus and contextual memory retrieval in Pavlovian conditioning. Behavioural Brain Research. 2000;110:97–108. doi: 10.1016/s0166-4328(99)00188-6. [DOI] [PubMed] [Google Scholar]
- Maren S, Holt W. Hippocampus and pavlovian fear conditioning in rats: Muscimol infusions into the ventral, but not dorsal, hippocampus impair the acquisition of conditional freezing to an auditory conditional stimulus. Behavioral Neuroscience. 2004;118(1):97–110. doi: 10.1037/0735-7044.118.1.97. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behavioural Brain Research. 1997;88(2):261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behavioural Brain Research. 1997;88(2):261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Martin SJ, de Hoz L, Morris RG. Retrograde amnesia: neither partial nor complete hippocampal lesions in rats result in preferential sparing of remote spatial memory, even after reminding. Neuropsychologia. 2005;43(4):609–624. doi: 10.1016/j.neuropsychologia.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins E, Barrientos R, Rudy J. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. Journal of Neuroscience. 2004;24(10):2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maviel T, Durkin TP, Menzaghi F, Bontempi B. Sites of neocortical reorganization critical for remote spatial memory. Science. 2004;305:96–99. doi: 10.1126/science.1098180. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychological Review. 1995;102(3):419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychological Review. 1995;102(3):419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. A triple dissociation of memory systems: hippocampus, amygdala, and dorsal striatum. Behavioral Neuroscience. 1993;107(1):3–22. doi: 10.1037//0735-7044.107.1.3. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. Parallel information processing in the water maze: evidence for independent memory systems involving dorsal striatum and hippocampus. Behavioral & Neural Biology. 1994;61(3):260–270. doi: 10.1016/s0163-1047(05)80009-3. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. Information acquired by the hippocampus interferes with acquisition of the amygdala-based conditioned-cue preference in the rat. Hippocampus. 1995;5(3):189–197. doi: 10.1002/hipo.450050305. [DOI] [PubMed] [Google Scholar]
- Meeter M, Murre JM. Consolidation of long-term memory: evidence and alternatives. Psychological Bulletin. 2004;130(6):843–857. doi: 10.1037/0033-2909.130.6.843. [DOI] [PubMed] [Google Scholar]
- Micheau J, Riedel G, Roloff E.v.L., Inglis J, Morris RGM. Reversible hippocampal inactivation partially dissociates how and where to search in the water maze. Behavioral Neuroscience. 2004;118(5):1022–1032. doi: 10.1037/0735-7044.118.5.1022. [DOI] [PubMed] [Google Scholar]
- Milner B. The memory defect in bilateral hippocampal lesions. Psychiatric Research Reports, American Psychiatric Association. 1959;(No 11):43–58. [PubMed] [Google Scholar]
- Milner B. The medial temporal-lobe amnesic syndrome. Psychiatric Clinics of North America. 2005;28:599–611. doi: 10.1016/j.psc.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Moser M-B, Moser EI, Forrest E, Andersen P, Morris RGM. Spatial learning with a minislab in the dorsal hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:9697–9701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Nadel L, Winocur G, Gilboa A, Rosenbaum RS. The cognitive neuroscience of remote episodic, semantic and spatial memory. Current Opinion in Neurobiology. 2006;16(2):179–190. doi: 10.1016/j.conb.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learning & Memory. 2002;9(2):49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learning & Memory. 2002;9(2):49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG, Tremblay A, Lecluse V, Lehmann H. Hippocampal damage and anterograde object-recognition in rats after long retention intervals. Hippocampus. 2005;15(8):1050–1056. doi: 10.1002/hipo.20122. [DOI] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Current Opinion in Neurobiology. 1997;7(2):217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- O'Keefe JM, Nadel L. The hippocampus as a cognitive map. Oxford University Press; Oxford: 1978. [Google Scholar]
- Pavlides C, Winson J. Influences of hippocampal place cell firing in the awake state on the activity of these cells during subsequent sleep episodes. Journal of Neuroscience. 1989;9:2907–2918. doi: 10.1523/JNEUROSCI.09-08-02907.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP, Anrep GV. Conditioned reflexes : an investigation of the physiological activity of the cerebral cortex. Oxford University Press; London: 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W, Milner B. Memory deficits induced by bilateral lesions in the hippocampal zone. Archives of Neurology and Psychiatry. 1958;79:475–97. doi: 10.1001/archneurpsyc.1958.02340050003001. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Ma QD, Tinsley MR, Koch C, Fanselow MS. Inverse temporal contributions of the dorsal hippocampus and medial prefrontal cortex to the expression of long-term fear memories. Learning & Memory. 2008a;15:368–372. doi: 10.1101/lm.813608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JJ, Wied HM, Ma QD, Tinsley MR, Fanselow MS. Dorsal hippocampus involvement in delay fear conditioning depends upon the strength of the tone-footshock association. Hippocampus. 2008b;18(7):640–654. doi: 10.1002/hipo.20424. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL, Zola SM, Squire LR, Amaral DG. Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. Journal of Neuroscience. 1996;16(16):5233–5255. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resstel LB, Joca SR, Corrêa FM, Guimarães FS. Effects of reversible inactivation of the dorsal hippocampus on the behavioral and cardiovascular responses to an aversive conditioned context. Behavioral Pharmacology. 2008;19(2):137–144. doi: 10.1097/FBP.0b013e3282f62c9e. [DOI] [PubMed] [Google Scholar]
- Riedel G, Micheau J, Lam AGM, Roloff E.n.L., Martin SJ, Bridge H, de Hoz L, Poeschel B, McCulloch J, Morris RGM. Reversible neural inactivation reveals hippocampal participation in several memory processes. Nature. 1999;2(10):898–905. doi: 10.1038/13202. [DOI] [PubMed] [Google Scholar]
- Rudy JW, O'Reilly RC. Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behavioral Neuroscience. 1999;113(5):867–880. doi: 10.1037//0735-7044.113.5.867. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Sutherland RJ. The hippocampal formation is necessary for rats to learn and remember configural discriminations. Behavioural Brain Research. 1989;34(1–2):97–109. doi: 10.1016/s0166-4328(89)80093-2. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Barrientos RM, O'Reilly RC. Hippocampal formation supports conditioning to memory of a context. Behavioral Neuroscience. 2002;116(4):530–538. doi: 10.1037//0735-7044.116.4.530. [DOI] [PubMed] [Google Scholar]
- Sacchetti B, Lorenzini C, Baldi E, Tassoni G, Bucherelli C. Auditory thalamus, dorsal hippocampus, basolateral amygdala, and perirhinal cortex role in the consolidation of conditioned freezing to context and to acoustic conditioned stimulus in the rat. Journal of Neuroscience. 1999;19(21):9570–9578. doi: 10.1523/JNEUROSCI.19-21-09570.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neurochemistry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry DF, Schacter DL. The evolution of multiple memory systems. Psychological Review. 1987;94(4):439–454. [Google Scholar]
- Sparks FT, O'Brien J, Lehmann H, Sutherland RJ. Hippocampal damage produces retrograde amnesia for fear potentiated startle. Society for Neuroscience Abstracts. 2005;31 doi: 10.1016/j.neuroscience.2010.03.005. 649.12. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Squire LR, Alvarez P. Retrograde amnesia and memory consolidation: a neurobiological perspective. Current Opinion in Neurobiology. 1995;5(2):169–177. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Squire LR, Bayley PJ. The neuroscience of remote memory. Current Opinion in Neurobiology. 2007;17:185–96. doi: 10.1016/j.conb.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Slater PC, Chace PM. Retrograde Amnesia: Temporal Gradient in Very Long Term Memory following Electroconvulsive Therapy. Science. 1975;187(4171):77–79. doi: 10.1126/science.1109228. [DOI] [PubMed] [Google Scholar]
- Squire LR, Knowlton BJ. Learning about categories in the absence of memory. Proceedings of the National Academy of Science USA. 1995;92(26):12470–12474. doi: 10.1073/pnas.92.26.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Zola SM. Memory, memory impairment, and the medial temporal lobe. Cold Spring Harbor Symposium on Quantitative Biology. 1996;61:185–195. [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annual Review of Neuroscience. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Steinvorth S, Levine B, Corkin S. Medial temporal lobe structures are needed to re-experience remote autobiographical memories: evidence from H.M. and W.R. Neuropsychologia. 2005;43:479–496. doi: 10.1016/j.neuropsychologia.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Kolb B, Whishaw IQ. Spatial mapping: definitive disruption by hippocampal or medial frontal cortical damage in the rat. Neuroscience Letters. 1982;31:271–276. doi: 10.1016/0304-3940(82)90032-5. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Lehmann H, Spanswick SC, Sparks FT, Melvin NR. Growth points in research on memory and hippocampus. Canadian Journal of Experimenta Psychology. 2006;60(2):166–174. doi: 10.1037/cjep20060016. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, O'Brien J, Lehmann H. Absence of systems consolidation of fear memories after dorsal, ventral, or complete hippocampal damage. Hippocampus. 2008;16:417–420. doi: 10.1002/hipo.20431. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, O'Brien J, Lehmann H. Absence of systems consolidation of fear memories after dorsal, ventral, or complete hippocampal damage. Hippocampus. 2008;18:710–718. doi: 10.1002/hipo.20431. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Weisend MP, Mumby D, Astur RS, Hanlon FM, Koerner A, et al. Retrograde amnesia after hippocampal damage: recent vs. remote memories in two tasks. Hippocampus. 2001;11(1):27–42. doi: 10.1002/1098-1063(2001)11:1<27::AID-HIPO1017>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Takehara K, Shigenori Kawahara S, Kirino Y. Time-dependent reorganization of the brain components underlying memory retention in trace eyeblink conditioning. Journal of Neuroscience. 2003;23(30):9897–9905. doi: 10.1523/JNEUROSCI.23-30-09897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuno M, Lipa P, McNaughton BL. Methodological considerations on the use of template matching to study long-lasting memory trace replay. Journal of Neuroscience. 2006;26(42):10727–10742. doi: 10.1523/JNEUROSCI.3317-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, et al. Schemas and memory consolidation. Science. 2007;316(5821):76–82. doi: 10.1126/science.1135935. [DOI] [PubMed] [Google Scholar]
- Tse D, Langston RF, Bethus I, et al. Does assimilation into schemas involve systems or cellular consolidation? It's not just time. Learning & Memory. 2008;89(4):361–365. doi: 10.1016/j.nlm.2007.09.007. [DOI] [PubMed] [Google Scholar]
- White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiology of Learning & Memory. 2002;77(2):125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Brown RA, Talton LE, Silva AJ. New circuits for old memories: the role of the neocortex in consolidation. Neuron. 2004;44(1):101–108. doi: 10.1016/j.neuron.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, Fanselow MS. Context fear learning in the absence of the hippocampus. Journal of Neuroscience. 2006;26(20):5484–5491. doi: 10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G. Anterograde and retrograde amnesia in rats with dorsal hippocampal or dorsomedial thalamic lesions. Behavioural Brain Research. 1990;38(2):145–154. doi: 10.1016/0166-4328(90)90012-4. [DOI] [PubMed] [Google Scholar]
- Winocur G, McDonald RM, Moscovitch M. Anterograde and retrograde amnesia in rats with large hippocampal lesions. Hippocampus. 2001;11(1):18–26. doi: 10.1002/1098-1063(2001)11:1<18::AID-HIPO1016>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Winocur G, Moscovitch M, Fogel S, Rosenbaum RS, Sekeres M. Preserved spatial memory after hippocampal lesions: effects of extensive experience in a complex environment. Nature Neuroscience. 2005;8(3):273–275. doi: 10.1038/nn1401. [DOI] [PubMed] [Google Scholar]