Abstract
Purpose
To investigate the relationship between known risk factors for Age-related macular degeneration (AMD) progression and foveolar choroidal circulation in eyes with non-exudative AMD.
Design
A cross-sectional study in non-exudative AMD.
Methods
Laser Doppler Flowmetry (LDF) measurements of relative choroidal blood velocity (ChBVel), volume (ChBVol) and flow (ChBFlow) were obtained in the center of the fovea of 273 study eyes of 204 AMD patients investigated at the Scheie Eye Institute of the University of Pennsylvania Medical School. All study eyes had visual acuity of 20/40 or better, good fixation, no other intraocular pathology and no evidence of choroidal neovascularization. RPE hypertrophy was determined from color fundus photographs by trained masked graders at the Scheie Image Reading Center. Correlation analysis and multivariate linear regression analysis with adjustments for significant covariates were carried out.
Results
A significant inverse correlation was observed between age and ChBFlow (r=− 0.36, p<0.0001), and ChBVol (r=−0.28, p<0.0001), but not for ChBVel. A significant inverse correlation was observed between spherical equivalent and ChBFlow (r=−0.21, p=0.006), and ChBVol (r=−0.14, p=0.04) but not for ChBVel. ChBFlow and ChBVol were significantly lower in patients with a history of hypertension (p≤0.003) and in eyes with RPE hypertrophy (p≤0.04), respectively.
Conclusions
All the above described risk factors for AMD development and progression are associated with decreased choroidal circulatory parameters, suggesting that decreases in choroidal circulatory parameters may be involved in the development of AMD.
Age-related macular degeneration (AMD) is the leading cause of severe visual impairment and blindness among the elderly worldwide and in the developed countries 1–3. In the United States, 1.75 million people are affected by AMD, and its incidence increases with age. By the year 2020, an estimated of 2.95 million people are expected to be affected by AMD 3–5.
AMD is a multifactorial disease that involves a complex interaction of metabolic and functional changes and risk factors that affect the progression of the disease and its response to various treatment modalities 6–7.
The risk factors for AMD can be categorized as demographic, environmental, and genetic 6–7. The strongest risk factor associated with the development and progression of AMD is increasing age; all population based studies confirm that the incidence, prevalence, and progression of AMD increase with advancing age 2–5.
Another established risk factor is cigarette smoking; numerous case-control studies, cohort studies, and cross-sectional studies have found a statistically significant association between smoking and the development and progression of AMD 8.
The association between AMD and Systemic Hypertension (SHTN) has also been investigated. Some studies have showed that SHTN is a strong risk factor for AMD and choroidal neovascularization 9–13, while other studies have failed to demonstrate statistically significant associations 14–15. History of kidney disease 16, hyperopia17, retinal pigment epithelium (RPE) abnormalities, light iris color, and female gender have been shown to be associated with AMD or choroidal neovascularization (CNV) in different studies 10, 18–19.
Previous cross-sectional studies performed in our laboratory have suggested that foveolar choroidal blood flow progressively decreases with increasing severity of AMD 20–21. In addition, a longitudinal study has reported that lower baseline choroidal blood flow parameters are associated with future development of CNV and decrease in vision 22. Furthermore, decreases in choroidal circulatory parameters were detected prior to the development of CNV 22. All these findings have suggested a role for circulatory abnormalities in the development and progression of AMD. In an attempt to further elucidate this role, we have investigated the relationship between the above mentioned risk factors for AMD and foveolar choroidal circulation.
Methods
We included in the study AMD patients examined at Scheie Eye Institute, Department of Ophthalmology, University of Pennsylvania, who were 50 years or older. All subjects were informed about the study and were asked to sign appropriate consent forms prior to the enrollment. All study eyes had visual acuity equal to or better than 20/40, and had no evidence of CNV. A visual acuity of 20/40 or better was required in order to ensure appropriate fixation for optimal foveolar blood flow measurements. All subjects had clear ocular media, pupillary dilatation of 5 mm or more, steady fixation and intraocular pressure (IOP) < 21mmHg in the study eyes. Patients with intraocular pathology or history of intraocular disease other than AMD, as well as patients with Diabetes Mellitus were excluded from the study.
A structured questionnaire was used to obtain information on age, gender, race, history of smoking, medical history, including history of SHTN, cardiovascular disease and kidney disease; ocular history, including eye color, family history of AMD. All subjects had a complete ocular examination, including visual acuity, applanation tonometry, and slit lamp biomicroscopy. After pupillary dilatation with tropicamide 1% (Alcon, Fort Worth, TX) and phenylephrine hydrochloride 10% (Sanofi Winthrop, New York, NY), a fundus examination was performed by an opthalmologist, and stereo fundus photography of the disc and macula were obtained by a trained photographer.
Laser Doppler Flowmetry measurements (Oculix Instrument, Huntingdon Valley, PA) of the choroidal circulation were performed in the center of the foveola to assess relative Choroidal blood velocity, choroidal blood volume and choroidal blood flow. These parameters mainly represent choriocapillaris flow. Details about the method used have been described in previous publications 23–24. Subjects were seated in front of the Laser Doppler Flowmetry camera in a darkened room, and were asked to fixate for three periods of 30 seconds on the aiming laser beam. A 200μm diameter diode laser beam (670μm) with an intensity of 20microWatts at the cornea was delivered through a fundus camera (model TRC; Topcon, Tokyo, Japan). To enable the observation of the position of the laser on the foveola, light with a retinal irradiance of approximately 0.03mW/cm2 and a wavelength of 570nm constantly illuminated the posterior retina (30° in diameter). Choroidal blood velocity and choroidal blood volume were independently obtained with the Laser Doppler Flowmetry instrument. Choroidal blood velocity was proportional to the mean velocity of the red blood cells (RBCs) within the volume sampled by the laser light, and choroidal blood volume was proportional to the number of RBCs in the measured sample. Choroidal blood flow was calculated by the instrument according to the formula: Choroidal blood flow= Constant × Choroidal blood velocity x Choroidal blood volume25. Choroidal blood velocity, choroidal blood volume and choroidal blood flow are all shown in arbitrary units (A.U.)
Immediately after blood flow measurements, while subjects were still seated, brachial artery systolic and diastolic blood pressures were determined by sphygmomanometry (Accutorr 1A; Datascope, Paramus, NJ), using a standardized protocol. Intraocular pressure (IOP) was then measured by Goldmann applanation tonometry. The mean of brachial artery pressure (BPm) was calculated according to the formula: BPm = BPd + 1/3 (BPs − BPd).
Analysis of the blood flow data was performed by a masked observer using software specifically developed for the analysis of Doppler signals from ocular tissues 24. Only parts of the recordings that showed stable circulatory parameters were selected for analysis. An average of approximately 12 seconds of measurements for each study eye was analyzed. We calculated a coefficient of variability (CV) for each study eye derived from three subsequent measurements to assess the reproducibility of the blood flow data. CV was calculated by the formula: CV = (SD/mean) × 100%, where SD represents standard deviation from three replicated measurements. The mean CV was 12.8% (SD=8.4%) for choroidal blood volume, 8.7% (SD=6.8%) for choroidal blood velocity, and 9.9% (SD=6.4%) for choroidal blood flow.
An average of the three measurements for each choroidal circulatory parameter was calculated and was used in data analysis. Correlation analysis and multivariate linear regression analysis with adjustment for significant covariates were carried out. The association between each blood flow parameter and a specific risk factor was first tested separately by a univariate analysis. Risk factors with a p-value < 0.10 from univariate analysis were included in the multivariate regression analysis, which was further simplified by including only the significant risk factors with p<0.05. The association of circulatory parameters with each of studied risk factors was assessed by using the generalized estimating equations (GEE) approach to linear regression to adjust for the correlation between eyes of the same patient 26. Calculations were executed by using PROC GENMOD (SAS Version 9.1, SAS, Cary, NC), and exchangeable working correlation structure was used to describe the correlation in circulatory measurements between paired eyes of each patient 21.
Fundus photographs were graded in a masked fashion at the Scheie Image Reading Center at the University of Pennsylvania by trained graders. Gradings of drusen characteristics and Retinal Pigment Epithelium (RPE) hypertrophic changes in the study eyes were performed according to the Complications of Age-Related Macular Degeneration Prevention Trial (CAPT) protocol 27.
Results
A total of 269 non-exudative AMD eyes from 204 participants were included in this study. Participant’s age ranged from 50 to 87 years (mean=71, SD=8.9 years). One hundred ninety six (96.1%) were Caucasian, 6 were African American, 1 was Asian and 1 was Hispanic. One hundred fourteen (55.9%) Participants were female. Thirteen participants (6.4%) were currently smokers at the time of Laser Doppler Flowmetry measurement. A history of SHTN was present in 91 (44.6%) study participants; 126 (61.8%) participants had a history of cardiac disease, and 18 (8.8%) participants had a history of kidney disease. RPE hypertrophy was observed in 157 (58.4%) study eyes (Table 1).
Table 1.
Association between AMD risk factors and circulatory parameters: Univariate analysis.
| Risk Factors | Number and distribution (%) of study patients or eyes | ChBVol | ChBVel | ChBFlow | |||
|---|---|---|---|---|---|---|---|
| Mean(SE) a | P-value | Mean(SE)a | P-value | Mean(SE)a | P-value | ||
| Age (years) | 0.03 | 0.91 | 0.002 | ||||
| 50–60 | 22 (10.8) | 0.29 (0.02) | 0.39 (0.02) | 9.58 (0.76) | |||
| 61–70 | 63 (30.9) | 0.24 (0.01) | 0.38 (0.01) | 7.65 (0.44) | |||
| 71–80 | 86 (42.2) | 0.21 (0.01) | 0.38 (0.01) | 6.90 (0.28) | |||
| 80 + | 33 (16.2) | 0.21 (0.01) | 0.38 (0.01) | 6.30 (0.38) | |||
| Gender | 0.40 | 0.45 | 0.87 | ||||
| Male | 90 (44.1) | 0.23 (0.01) | 0.38 (0.01) | 7.33 (0.29) | |||
| Female | 114 (55.9) | 0.24 (0.01) | 0.38 (0.01) | 7.40 (0.33) | |||
| Eye color | 0.51 | 0.43 | 0.80 | ||||
| Blue | 65 (31.9) | 0.22 (0.01) | 0.39 (0.01) | 7.01 (0.28) | |||
| Brown | 71 (34.8) | 0.24 (0.01) | 0.37 (0.01) | 7.55 (0.39) | |||
| Hazel | 41 (20.1) | 0.23 (0.02) | 0.38 (0.03) | 7.54 (0.53) | |||
| Green | 20 (9.8) | 0.24 (0.02) | 0.38 (0.01) | 7.30 (0.69) | |||
| Gray/Unknown | 7 (3.4) | 0.24 (0.05) | 0.37 (0.02) | 7.96 (1.88) | |||
| Current smoking | 0.08 | 0.11 | 0.39 | ||||
| No | 191 (93.6) | 0.23 (0.01) | 0.38 (0.01) | 7.32 (0.23) | |||
| Yes | 13 (6.4) | 0.27 (0.02) | 0.36 (0.01) | 7.97 (0.69) | |||
| Systemic Hypertension | 0.009 | 0.48 | 0.005 | ||||
| No | 112 (54.9) | 0.25 (0.01) | 0.38 (0.01) | 7.86 (0.31) | |||
| Yes | 91 (44.6) | 0.21 (0.01) | 0.38 (0.01) | 6.67 (0.27) | |||
| Cardiac disease | 0.80 | 0.55 | 0.44 | ||||
| No | 78 (38.2) | 0.23 (0.01) | 0.38 (0.01) | 7.56 (0.34) | |||
| Yes | 126 (61.8) | 0.23 (0.01) | 0.38 (0.01) | 7.23 (0.28) | |||
| Kidney disease | 0.09 | 0.41 | 0.15 | ||||
| No | 186 (91.2) | 0.24 (0.01) | 0.38 (0.01) | 7.44 (0.23) | |||
| Yes | 18 (8.8) | 0.20 (0.02) | 0.39 (0.01) | 6.58 (0.52) | |||
| Systolic BP (mmHg) | −0.0002 (0.0003) | 0.53 | 0.0005 (0.0002) | 0.047 | 0.003 (0.01) | 0.77 | |
| Diastolic BP (mmHg) | −0.0001 (0.0005) | 0.84 | 0.0014 (0.0004) | 0.001 | 0.02 (0.02) | 0.16 | |
| Mean BP (mmHg) | −0.0002 (0.0004) | 0.65 | 0.0008 (0.0004) | 0.04 | 0.01 (0.01) | 0.31 | |
| RPE Hypertrophy | 0.02 | 0.62 | 0.03 | ||||
| No | 112 (41.6) | 0.25 (0.01) | 0.38 (0.01) | 7.98 (0.39) | |||
| Yes | 157 (58.4) | 0.22 (0.01) | 0.39 (0.01) | 6.91 (0.29) | |||
| Mean (SD) of risk factor | |||||||
| Systolic BP (mmHg) (as continuous) | 136 (21.6) | −0.0002 (0.0003) | 0.53 | 0.0005 (0.0002) | 0.047 | 0.003 (0.01) | 0.77 |
| Diastolic BP (mmHg) (as continuous) | 74 (12.2) | −0.0001 (0.0005) | 0.84 | 0.0014 (0.0004) | 0.001 | 0.02 (0.02) | 0.16 |
| Mean BP (mmHg) (as continous) | 95 (14.0) | −0.0002 (0.0004) | 0.65 | 0.0008 (0.0004) | 0.04 | 0.01 (0.01) | 0.31 |
| Spherical equivalent (D) (as continuous) | 0.51 (2.06) | −0.007 (0.003) | 0.04 | −0.004 (0.003) | 0.16 | −0.31 (0.11) | 0.006 |
ChBVol, ChBVel and ChBFlow are shown in arbitrary units (A.U.).
A statistically significant association was observed between the risk factors; age, SHTN, spherical equivalent or RPE hypertrophy and choroidal blood volume or choroidal blood flow (Table 1, Figures 1 through 6). No significant associations were detected between the above risk factors and choroidal blood velocity (Table 1).
Figure 1.
Scatterplot of Choroidal Blood Flow (ChBFlow) with Age. The linear correlation coefficient between Choroidal blood flow (ChBFlow) and Age is −0.36 (p<0.0001). The linear equation regression between Choroidal blood flow (ChBFlow) and Age is: Choroidal blood flow (ChBFlow) =16.3 − 0.13 × Age. Choroidal blood flow (ChBFlow) is shown in arbitrary units (A.U.), and age is in years.
Figure 6.
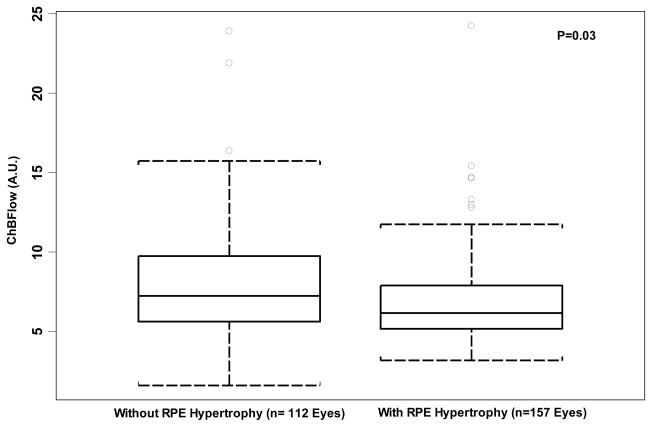
Comparison of Choroidal Blood Volume (ChBVol) between eyes without and with retinal pigment epithelium (RPE) hypertrophy. Boxplot shows a statistically significant difference between the two groups in and choroidal blood flow (ChBFlow) (P=0.03). Each boxplot includes the upper extreme (whisker, excluding outliers indicated as circles outside the box), upper quartile (top portion of box), median (horizontal line in box), lower quartile (bottom portion of box), and lower extreme (whisker).
A significant inverse correlation was observed between age and choroidal blood flow (r=− 0.36, p<0.0001) (Figure 1), and choroidal blood volume(r=−0.28, p<0.0001) (Figure 2). In other words, patients with increasing age tended to have lower choroidal blood flow and ChBVol. These significant associations were still maintained for choroidal blood flow (p=0.004), and for choroidal blood volume (p=0.02) after adjustments for other significant risk factors (Table 2).
Figure 2.
Scatterplot of Choroidal Blood Volumn (ChBVol) with Age. The linear correlation coefficient between Choroidal blood volume (ChBVol) and Age is −0.28 (p<0.0001). The linear regression equation between Choroidal blood volume (ChBVol) and Age is: Choroidal blood volume (ChBVol) =0.44 −0.0029 × Age. Choroidal blood volume (ChBVol) is shown in arbitrary units (A.U.), and age is in years.
Table 2.
Association between AMD risk factors and Choroidal Blood Volume (ChBVol) and Flow (ChBFlow): Multivariate analysis b.
| Risk Factors | ChBVol |
ChBFlow |
||
|---|---|---|---|---|
| Mean (SE)c | P-value | Mean (SE)c | P-value | |
| Age | 0.02 | 0.004 | ||
| 50–60 | 0.29 (0.03) | 9.41 (0.84) | ||
| 61–70 | 0.23 (0.01) | 7.31 (0.35) | ||
| 71–80 | 0.21 (0.01) | 6.71 (0.26) | ||
| 80 + | 0.20 (0.01) | 6.01 (0.32) | ||
| Systemic Hypertension | 0.007 | 0.003 | ||
| No | 0.25 (0.01) | 7.97 (0.32) | ||
| Yes | 0.21 (0.01) | 6.94 (0.31) | ||
| RPE hypertrophy | 0.01 | 0.04 | ||
| No | 0.25 (0.01) | 7.78 (0.32) | ||
| Yes | 0.22 (0.01) | 6.94 (0.31) | ||
| Spherical equivalent (D) (as continuous) | −0.21 | 0.02 | ||
The risk factors in the multivariate model include age, systemic hypertension and RPE hypertrophy for ChBVol, and include age, systemic hypertension, RPE hypertrophy, and spherical equivalent for ChBFlow.
ChBVol and ChBFlow are shown in arbitrary units (A.U.).
A significant inverse correlation was observed between spherical equivalent and choroidal blood flow (r=−0.21, p=0.006) (Figure 3), and choroidal blood volume(r=−0.14, p=0.04) (Figure 4). In other words, patients with more hyperopia tended to have lower choroidal blood flow and choroidal blood volume. These significant associations were still maintained for choroidal blood flow (p=0.02), but not for choroidal blood volume (p=0.28) after adjustments by age, systemic hypertension, and RPE hypertrophy (Table 2).
Figure 3.
Scatterplot of Choroidal Blood Flow (ChBFlow) with Spherical Equivalent. The linear correlation coefficient between Choroidal blood flow (ChBFlow) and Spherical Equivalent is −0.21 (p=0.006). The linear regression equation between Choroidal blood flow (ChBFlow) and Spherical Equivalent is: Choroidal blood flow (ChBFlow) =7.44 −0.31 × Spherical Equivalent. Choroidal blood flow (ChBFlow) is shown in arbitrary units (A.U.), and Spherical Equivalent is in Diopters (D).
Figure 4.
Scatterplot of Choroidal Blood Volumn (ChBVol) with Spherical Equivalent. The linear correlation coefficient between Choroidal blood volume (ChBVol) and Spherical Equivalent is −0.14 (p=0.04). The linear regression equation Choroidal blood volume (ChBVol) and Spherical Equivalent is: ChBVol=0.23 −0.0065 × Spherical Equivalent. Choroidal blood volume (ChBVol) is shown in arbitrary units (A.U.), and Spherical Equivalent is in Diopters (D).
Multivariate analysis also confirmed all other statistically significant associations observed in the univariate analysis. Patients with a history of SHTN had choroidal blood volume(Mean ± SE) of 0.21 ± 0.01 A.U. and choroidal blood flow 6.94 ± 0.31 A.U. that were significantly lower than those observed in patients without SHTN (choroidal blood volume 0.25 ± 0.01, P-value = 0.007; and choroidal blood flow 7.97 ± 0.32, P-value = 0.003). Patients with RPE hypertrophy had average choroidal blood volume (0.22 ± 0.01) and choroidal blood flow (6.94 ± 0.31), which were significantly lower than those of eyes without RPE hypertrophy (choroidal blood volume0.25 ± 0.01, P-value = 0.01 and choroidal blood flow 7.78 ± 0.32, P-value = 0.04) respectively (Table 2).
We detected no significant associations between the risk factors: current smoking status, brachial artery systolic and diastolic blood pressures, gender, eye color, cardiac or kidney diseases, and choroidal blood flow parameters (Table 1).
Discussion
AMD is a multifactorial disease that affects primarily the photoreceptors and RPE, Bruch’s membrane, and choriocapillaries. The pathophysiology of AMD is complex and includes genetic factors and other processes such as oxidative stress, inflammation and hypoxia 6, 28.
The results of our current study in AMD patient’s showing decreasing choroidal blood flow and volume, but not velocity, with increasing age are consistent with our previous study in normal subjects 29. The decreases in choroidal blood volume and flow may be caused by age-associated changes in capillary vessel structure and a decline in choriocapillary density and lumen diameter 29–31.
Systemic hypertension (SHTN) has been shown by many studies to be associated with an increased risk for the development of AMD or CNV 9–13, The presence of decreased choroidal blood volume and choroidal blood flow in the patients with history of SHTN in our current study is consistent with our previous findings in a smaller sample size 32, suggesting that an ischemic mechanism may be involved in the etiology of this disease. Possibly, choroidal blood flow decreases could lead to imbalances in choroidal watershed vascular filling zones; the areas most prone to develop ischemia and hypoxia 33. As reported by Ross et al 33 CNV often develops in close proximity to choroidal watershed vascular filling zones, supporting our hypothesis that hypertension may contribute to the progression of AMD and the development of CNV 32, through a decrease in choroidal blood flow.
Consistent with our previous report 32, we found in our current study no significant correlation between choroidal blood flow and actual blood pressure at the time of the flow measurements (Table 1). This could be due to the fact that blood pressure was well controlled in the majority of the study patients and blood pressure in most cases was within the normal range at the time of the blood flow measurements. It is possible that a history of SHTN or the presence of high systemic blood pressure in the past may produce chronic effects on the choroidal vasculature that may have a stronger effect on flow than the actual blood pressure detected at the time of flow measurements 32.
The presence of focal RPE hyperpigmentation in the macula has been associated with a greater risk of AMD and development of CNV 21. RPE serves a variety of metabolic and supportive functions to retinal photoreceptors. The impairment of RPE cell function is probably a crucial initiatory factor that drives slowly to progressive accumulation of lipofuscin formation, to local chronic inflammation and then to drusen formation 28.
Our present results showing that the RPE hyperpigmentation is associated with decreased choroidal blood flow suggests that hypoxia may have a role in the formation of these RPE changes. Hyperpigmentation of the RPE is caused by hypertrophy of the RPE and the formation of clusters of pigmented cells in the subretinal space and the outer nuclear area 35. Possibly, ischemia may modulate RPE proliferation and migration leading to these changes. Because we demonstrate an association and not a causal relationship we cannot conclude with certainty whether reduced flow may cause RPE hyperpigmentation or the reverse.
A clear explanation for the relationship between hyperopia and decreased choroidal blood flow and their association with an increased risk of AMD remains elusive. Hyperopic eyes are smaller and have thicker, more rigid and compact sclerae 36. This generalized scleral stiffness may cause an increased choroidal vasculature resistance leading to perfusion abnormalities. This process could adversely affect the transportation of oxygen, nutrients, and metabolic products from the photoreceptors across the RPE, and result in accumulation of metabolic debris and drusen formation and thickening of Bruch’s membrane 17, 30, 37–39.
Interestingly, we did not observe significant correlations between choroidal circulatory parameters and race, eye color or kidney disease. This lack of significant correlation may be due to the smaller number of patients with some of these attributes, a situation that limits our ability to detect associations.
We also did not find a correlation between smoking, cardiac disease or gender and choroidal blood flow. This lack of association could be due to the involvement of other pathogenetic mechanisms that are not related to ischemia or hypoxia. For example, chronic cigarette smoke has been associated with increased oxidative damage and ultrastructural degeneration of the RPE and Bruch membrane, and RPE cell apoptosis 40. In addition, because smoking has an effect on the immune system, it is possible that part of the risk conferred by smoking funnels through inflammatory pathways 6.
Control of modifiable risk factors such as smoking, SHTN, and dietary supplementation with antioxidants and minerals can reduce the risk of developing AMD and CNV 7, 28. Our current results show that several important demographic and environmental risk factors for AMD are associated with decreased choroidal circulatory parameters. This suggests that decreases in choroidal circulatory parameters may be involved in the etiology of AMD or CNV. This kind of information may be useful in both understanding the pathophysiology of AMD and in determining possible targets and strategies for intervention in the prevention and progression of AMD development.
In summary, our current results demonstrate an association between decreased choroidal circulation and a number of risk factor for the development of AMD. All these findings suggest a possible role for choroidal circulatory abnormalities in the development of AMD and CNV. The precise mechanism by which these AMD risk factors and blood flow parameters affect each other remains unclear and needs to be further investigated. Finally, interventions aimed at improving the foveolar choroidal circulation may perhaps have a role in the prevention of AMD development and progression.
Figure 5.
Comparison of Choroidal Blood Volume (ChBVol) between eyes without and with retinal pigment epithelium (RPE) hypertrophy. Boxplot shows a statistically significant difference between the two groups in Choroidal blood volume (ChBVol) (P=0.02). Each boxplot includes the upper extreme (whisker, excluding outliers indicated as circles outside the box), upper quartile (top portion of box), median (horizontal line in box), lower quartile (bottom portion of box), and lower extreme (whisker).
Acknowledgments
A). This project was supported by the National Institutes of Health grant NEI EY12769 and 5 P30 EY 01583, the Vivian Simkins Lasko Research Fund, the Nina C. Mackall Trust, and an unrestricted grant from Research to Prevent Blindness. B). The authors have no financial conflict of interest. C). Involved in conception and design as well as obtaining funding of the study (J.E.G.); conduct of study (J.E.G., W.X., T.I.M., J.C.D., G.S.Y., E.R.M., J.L.D., A.J.B.); collection of data (J.E.G., W.X., T.I.M., J.C.D., J.L.D., A.J.B.); management of data (J.E.G., W.X., G.S.Y., E.R.M.); analysis and interpretation of data (J.E.G., W.X., T.I.M., J.C.D., G.S.Y., E.R.M.); preparation and revision of the manuscript (W.X., J.E.G.); review of the manuscript (J.E.G., T.I.M., J.C.D., G.S.Y., E.R.M., J.L.D., A.J.B.); and final approval of the manuscript (J.E.G., W.X., T.I.M., J.C.D., G.S.Y., E.R.M., J.L.D., A.J.B.). D). The study was approved by the Institutional Review Boards of the University of Pennsylvania in accordance with Health Insurance Portability and Accountability Act regulations and the tenets of the Declaration of Helsinki.
Biographies

Dr. Juan E. Grunwald, MD, Professor of Ophthalmology at the University of Pennsylvania, is the Director of the Vivian Lasko Retinal Vascular Research Laboratory at the Scheie Eye Institute dedicated to research on ocular vascular physiology and pathology. One of his main interests is the elucidation of the role of blood flow abnormalities in the development of eye diseases. Dr. Grunwald is a clinician, researcher and educator who specializes in the treatment of retinal diseases.

Dr. Wei Xu, MD, received her medical degree from Hunan Medical College and completed her medical residency at St. Luke Hospital in Shanghai, China. Dr. Xu has devoted her efforts to a career in biomedical research, both basic and clinical science. She has been working at the University of Pennsylvania School of Medicine for 6 years, and has been the first author for publications in national scientific journals. Her current interest is in clinical research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Congdon N, O’Colmain B, Klaver CC, et al. Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 2.Augood CA, Vingerling JR, de Jong PT, et al. Prevalence of age-related maculopathy in older Europeans: the European Eye Study (EUREYE) Arch Ophthalmol. 2006;124:529–35. doi: 10.1001/archopht.124.4.529. [DOI] [PubMed] [Google Scholar]
- 3.Friedman DS, O’Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell P, Wang JJ, Foran S, Smith W. Five-year incidence of age-related maculopathy lesions: the Blue Mountains Eye Study. Ophthalmology. 2002;109:1092–7. doi: 10.1016/s0161-6420(02)01055-2. [DOI] [PubMed] [Google Scholar]
- 5.Mukesh BN, Dimitrov PN, Leikin S, et al. Five-year incidence of age-related maculopathy: the Visual Impairment Project. Ophthalmology. 2004;111:1176–82. doi: 10.1016/j.ophtha.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 6.Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Prog Retin Eye Res. 2009 Jan;28(1):1–18. doi: 10.1016/j.preteyeres.2008.10.001. Epub 2008 Nov 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman HR, Chan CC, Ferris FL, 3rd, Chew EY. Age-related macular degeneration. Lancet. 2008 Nov;22(372):9652, 1835–45. doi: 10.1016/S0140-6736(08)61759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein R. Overview of progress in the epidemiology of age-related macular degeneration. Ophthalmic Epidemiol. 2007;14:184–187. doi: 10.1080/09286580701344381. [DOI] [PubMed] [Google Scholar]
- 9.Hyman L, Schachat AP, He Q, et al. Hypertension, cardiovascular disease, and age-related macular degeneration. Arch Ophthalmol. 2000;118:351–358. doi: 10.1001/archopht.118.3.351. [DOI] [PubMed] [Google Scholar]
- 10.Age-Related Eye Disease Study Group. Risk factors associated with age-related macular degeneration: a case- control study in the age related eye disease study. Age-Related Eye Disease Study Report Number 3. Ophthalmology. 2000;107:2224–2232. doi: 10.1016/s0161-6420(00)00409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein R, Klein BE, Tomany SC, Cruickshanks KJ. The association of cardiovascular disease with the long-term incidence of age-related maculopathy. Ophthalmology. 2003;110:1273–80. doi: 10.1016/S0161-6420(03)00599-2. [DOI] [PubMed] [Google Scholar]
- 12.Hogg RE, Woodside JV, Gilchrist SE, et al. Cardiovascular disease and hypertension are strong risk factors for choroidal neovascularization. Ophthalmology. 2008 Jun;115(6):1046–1052.e2. doi: 10.1016/j.ophtha.2007.07.031. Epub 2007 Oct 22. [DOI] [PubMed] [Google Scholar]
- 13.Tomany SC, Wang JJ, van Leeuwen R, et al. Risk factors for incidentage-related macular degeneration. Pooled findings from 3 continents. Ophthalmology. 2004;111:1280–87. doi: 10.1016/j.ophtha.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Delcourt C, Michel F, Colvez A, et al. Associations of cardiovascular disease and its risk factors with age-related macular degeneration: the POLA study. Ophthalmic Epidemiol. 2001;8:237–49. doi: 10.1076/opep.8.4.237.1613. [DOI] [PubMed] [Google Scholar]
- 15.Chaine G, Hullo A, Sahel J, et al. Case-control study of the risk factors for age related macular degeneration. Br J Ophthalmol. 1998;82:996–1002. doi: 10.1136/bjo.82.9.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nitsch D, Evans J, Roderick PJ, Smeeth L, Fletcher AE. Associations between chronic kidney disease and age-related macular degeneration. Ophthalmic Epidemiol. 2009 May–Jun;16(3):181–6. doi: 10.1080/09286580902863064. [DOI] [PubMed] [Google Scholar]
- 17.Ikram MK, van Leeuwen R, Vingerling JR, et al. Relationship between refraction and prevalent as well as Incident age-related maculopathy: the Rotterdam Study. Invest Ophthalmol Vis Sci. 2003;44:3778–3782. doi: 10.1167/iovs.03-0120. [DOI] [PubMed] [Google Scholar]
- 18.Au Eong KG, Haller JA. Risk factors for age-related macular degeneration and choroidal neovascularization. In: Lim JI, editor. Age-Related Macular Degeneration. New York, NY: Marcel Dekker Inc; 2002. pp. 389–395. [Google Scholar]
- 19.Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study Severity Scale for Age-Related Macular Degeneration AREDS Report No. 17. Arch Ophthalmol. 2005 November;123(11):1484–1498. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grunwald JE, Hariprasad SM, DuPont J, et al. Foveolar choroidal blood flow in age–related macular degeneration. Invest Ophthalmol Vis Sci. 1998;39(2):385–390. [PubMed] [Google Scholar]
- 21.Grunwald JE, Metelitsina TI, Dupont JC, Ying GS, Maguire MG. Reduced Foveolar Choroidal Blood Flow in Eyes with Increasing AMD Severity. Invest Ophthalmol Vis Sci. 2005;46:1033–1038. doi: 10.1167/iovs.04-1050. [DOI] [PubMed] [Google Scholar]
- 22.Metelitsina TI, Grunwald JE, DuPont JC, Ying GS, Brucker AJ, Dunaief JL. Foveolar choroidal circulation and choroidal neovascularization in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008 Jan;49(1):358–63. doi: 10.1167/iovs.07-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riva CE, Cranstoun SD, Grunwald JE, Petrig BL. Choroidal blood flow in the foveal region of the human ocular fundus. Invest Ophthalmol Vis Sci. 1994;35:4273–4281. [PubMed] [Google Scholar]
- 24.Riva CE, Harino S, Petrig BL, Shonat RD. Laser-Doppler flowmetry in the optic nerve. Exp Eye Res. 1992;55:499–506. doi: 10.1016/0014-4835(92)90123-a. [DOI] [PubMed] [Google Scholar]
- 25.Riva CE. Basic principles of laser Doppler flowmetry and application to the ocular circulation. Int Ophthalmol. 2001;23:183–189. doi: 10.1023/a:1014433913240. [DOI] [PubMed] [Google Scholar]
- 26.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 27.The CAPT Research Group. Risk Factors for Choroidal Neovascularization and Geographic Atrophy in the Complications of Age-Related Macular Degeneration Prevention Trial. Ophthalmology. 2008 Sep;115(9) doi: 10.1016/j.ophtha.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Nowak JZ. Age-related macular degeneration (AMD): pathogenesis and therapy. Pharmacol Rep. 2006 May–Jun;58(3):353–63. [PubMed] [Google Scholar]
- 29.Grunwald JE, Hariprasad SM, DuPont J. Effect of aging on foveolar choroidal circulation. Arch Ophthalmol. 1998;116:150–154. doi: 10.1001/archopht.116.2.150. [DOI] [PubMed] [Google Scholar]
- 30.Ramrattan RS, van der Schaft TL, Mooy CM, de Bruijn WC, Mulder PG, de Jong PT. Morphometric analysis of Bruch’s membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci. 1994;35(6):2857–2864. [PubMed] [Google Scholar]
- 31.Ehrlich R, Kheradiya NS, Winston DM, Moore DB, Wirostko B, Harris A. Age-related ocular vascular changes. Graefes Arch Clin Exp Ophthalmol. 2009 May;247(5):583–91. doi: 10.1007/s00417-008-1018-x. Epub 2008 Dec 16. [DOI] [PubMed] [Google Scholar]
- 32.Metelitsina TI, Grunwald JE, DuPont JC, Ying GS. Effect of systemic hypertension on foveolar choroidal blood flow in age related macular degeneration. Br J Ophthalmol. 2006 Mar;90(3):342–6. doi: 10.1136/bjo.2005.082974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ross RD, Barofsky JM, Cohen G, et al. Presumed macular choroidal watershed vascular filling, choroidal neovascularization, and systemic vascular disease in patients with age-related macular degeneration. Am J Ophthalmol. 1998;125:71–80. doi: 10.1016/s0002-9394(99)80237-2. [DOI] [PubMed] [Google Scholar]
- 34.Bressler NM, Silva J, Bressler SB, Fine SL, Green WR. Clinicopathologic correlation of drusen and retinal pigment epithelial abnormalities in age-related macular degeneration. Retina. 1994;14:130–142. [PubMed] [Google Scholar]
- 35.Boker T, Fang T, Steinmetz R. Refractive error and choroidal perfusion characteristics in patients with choroidal neovascularization and age-related macular degeneration. Ger J Ophthalmol. 1993;2:10–13. [PubMed] [Google Scholar]
- 36.Friedman E, Krupsky S, Lane A, et al. Ocular blood flow velocity in age-related macular degeneration. Ophthalmology. 1995;102:640–6. doi: 10.1016/s0161-6420(95)30974-8. [DOI] [PubMed] [Google Scholar]
- 37.Friedman E. A hemodynamic model of the pathogenesis of agerelated macular degeneration. Am J Ophthalmol. 1997;124:677–682. doi: 10.1016/s0002-9394(14)70906-7. [DOI] [PubMed] [Google Scholar]
- 38.Kiel JW, Reiner AJ. Morphometric analysis of the choroid, Bruch’s membrane, and retinal pigment epithelium in eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 1997;38:1290–1292. [PubMed] [Google Scholar]
- 39.Fujihara M, Nagai N, Sussan TE, Biswal S, Handa JT. Chronic cigarette smoke causes oxidative damage and apoptosis to retinal pigmented epithelial cells in mice. PLoS ONE. 2008 Sep 1;3(9):e3119. doi: 10.1371/journal.pone.0003119. [DOI] [PMC free article] [PubMed] [Google Scholar]