Abstract
Background
Recurrent gram-negative bloodstream infection (BSI) has not been evaluated in a population-based setting; therefore, we performed a population-based retrospective cohort study to examine the incidence, recurrence, and mortality rates of gram-negative BSI.
Methods
We identified 944 episodes of gram-negative BSI, including 98 recurrent episodes, among Olmsted County, Minnesota, residents from 1/1/1998 to 12/31/2007. Kaplan-Meier method was used to estimate the cumulative incidence rate of recurrence and 28-day all-cause mortality rate of gram-negative BSI. Cox proportional hazard regression was used to determine risk factors for recurrence.
Results
The overall age- and gender-adjusted incidence rate of gram-negative BSI per 100,000 person-years was 84.5 (95% confidence interval [CI]: 79.1–90.0), including 75.7 (95% CI: 70.6–80.8) for first episodes and 8.8 (95% CI: 7.1–10.6) for recurrent episodes. Among 846 patients with first episodes of gram-negative BSI, the cumulative incidence rates of recurrence after 1, 5, and 10 years of the initial episode were 5.6%, 9.2%, and 14.6%, respectively, with death treated as a competing risk. Patients with Klebsiella species were more likely than those with Escherichia coli BSI to develop recurrent gram-negative BSI (hazard ratio: 2.33 [95% CI: 1.34–3.92], p=0.003). The 28-day all-cause mortality rates following the initial and second episodes of gram-negative BSI were 10.0% (95% CI: 8.0–12.0) and 11.3% (95% CI: 4.4–18.2), respectively.
Conclusions
Even though recurrent gram-negative BSI was relatively uncommon in the general population, up to 15% of patients with gram-negative BSI developed a recurrent episode within 10 years of the initial episode.
Keywords: gram-negative, recurrent, bacteremia, epidemiology, mortality, incidence, risk factors, Rochester Epidemiology Project
INTRODUCTION
Recurrence is a known complication of gram-negative bloodstream infection (BSI).1–5 Because previous studies of gram-negative BSI are derived from tertiary care centers, it is difficult to estimate the true rate of recurrence since long-term follow-up after discharge from the hospital is not usually available in such studies. A population-based design provides a unique opportunity to study a well-defined population over a long period of time.6,7 A recent population-based study of the Danish population estimated that 12% of patients with BSI develop recurrence within one year of the initial episode.8 Since gram-negative BSI is microbiologically, clinically and epidemiologically distinct from BSI due to other microorganisms such as gram-positive bacteria, Candida species and polymicrobials, we focused on monomicrobial gram-negative BSI in this study. The aims of this population-based retrospective cohort study were to: (i) determine the incidence and mortality rates of gram-negative BSI; (ii) estimate the cumulative incidence rate of recurrence among patients with first episodes of gram-negative BSI after accounting for the competing risk of death; (iii) examine the microbiology and risk factors of recurrent gram-negative BSI in Olmsted County, Minnesota, over a 10-year period.
MATERIALS AND METHODS
Setting
Olmsted County is located in southeastern Minnesota and has a population of 124,277 according to the 2000 census.9 With the exception of a lower prevalence of persons who inject illegal drugs, a higher prevalence of middle-class persons and a higher proportion of persons employed in the healthcare industry, the population characteristics of Olmsted County residents are similar to those of USA non-Hispanic whites.10, 11 The Rochester Epidemiology Project (REP) is a unique medical records-linkage system that encompasses care delivered to residents of Olmsted County, Minnesota. The microbiology laboratories at Mayo Medical Center and Olmsted Medical Center are the only two laboratories in Olmsted County. These two medical centers are geographically isolated from other urban centers as previously described,6, 10, 12, which increases the likelihood that residents get their healthcare at the local facilities, rather than seeking healthcare at a distant geographic location.
Case ascertainment
All residents of Olmsted County, Minnesota, were eligible for inclusion in the study as we used complete enumeration of Olmsted County population from 1 January 1998 to 31 December 2007. After the institutional review boards of Mayo Medical Center Rochester and Olmsted Medical Center approved the study, we used the microbiology laboratory databases at both institutions to identify all episodes of gram-negative BSI during the study period. Using the REP tools, we identified residents of Olmsted County, Minnesota, for inclusion in the study and excluded those who lived outside Olmsted County at the time of BSI and those with polymicrobial BSI. Among 846 unique Olmsted County residents with first episodes of monomicrobial gram-negative BSI, 80 patients subsequently developed a total of 98 recurrent episodes of gram-negative BSI. The primary investigator (M.N.A.) reviewed the medical records of all patients to confirm the diagnosis, determine patient residency status and obtain clinical features and outcome.
The detailed blood culture methods used were described elsewhere.13 Briefly, the Mayo Clinic microbiology laboratory used an automated blood culture system (BACTEC 9240, Becton Dickinson Diagnostic Instrument Systems, Sparks, MD) throughout the study period. The Olmsted Medical Center microbiology laboratory used Septi-Chek manual bottles (PML Microbiologics, Wilsonville, OR) from 1998 to 2000 and an automated blood culture system (BACTEC 9050, Becton Dickinson Diagnostic Instrument Systems, Sparks, MD) from 2001 to 2007. Blood cultures were processed using standard microbiology techniques according to the Clinical and Laboratory Standards Institute (CLSI). The microbiology laboratories at the Mayo Medical Center Rochester and Olmsted Medical Center are certified by the College of American Pathologists.
Case definition
Gram-negative BSI was defined as the growth of any aerobic gram-negative bacillus in a blood culture. Monomicrobial gram-negative BSI was defined as the growth of only one species of gram-negative bacillus in a blood culture and polymicrobial BSI was defined as the growth of more than one microorganism in a blood culture, excluding coagulase-negative staphylococci, Corynebacterium spp. and Propionibacterium spp. The growth of one of the latter three microorganisms in a blood culture in addition to gram-negative bacilli was considered skin contamination, rather than polymicrobial BSI. Recurrent gram-negative BSI was defined clinically as the occurrence of a subsequent episode of gram-negative BSI during the study period at least one week after resolution of a previous episode (based on resolution of symptoms and subsequent negative blood cultures). Episodes of recurrent gram-negative BSI were not necessarily caused by the same gram-negative bacillus as the first episode; rather, subsequent growth of any gram-negative bacillus in a blood culture was considered a recurrence regardless of gram-negative bacillus that caused the initial episode. Subsequent growth of the same gram-negative bacillus in a blood culture within one week of the initial episode was not considered a recurrence; rather, it was considered a duplicate of the same initial episode and was not accounted for in the analysis. Subsequent growth of a different gram-negative bacillus in a blood cultures within one week of the initial episode was considered polymicrobial BSI and, therefore, was excluded. Cases were classified according to the site of acquisition into nosocomial, healthcare-associated, and community-acquired as previously defined.14 Briefly, nosocomial BSI was defined as a positive blood culture obtained from patients who had been hospitalized for 48 hours or longer. Healthcare-associated (community-onset) BSI was defined as BSI acquired outside the hospital or within 48 hours of hospital admission in the setting of recent contact with the healthcare system (residents in a nursing home or long-term care facility, those who received intravenous therapy, including antibiotics and chemotherapy, or underwent hemodialysis within 30 days of BSI, and those hospitalized for 2 or more days within 90 days of BSI). Community-acquired BSI was defined as BSI that does not meet the criteria for either one of the above two definitions. The primary source of BSI was defined using the Centers for Disease Control and Prevention criteria.15
Statistical analysis
Incidence rates were estimated separately for first and recurrent episodes of gram-negative BSI that occurred during the study period. The incidence rate, expressed as the number of gram-negative BSI episodes per 100,000 person-years, was calculated assuming that the entire population of Olmsted County was at risk of BSI. The 2000 Olmsted County census figures were used to compute the age- and gender-specific person-years denominator with a projected population growth rate after 2000 of 1.9% per year. Age was categorized into five groups (0–18, 19–39, 40–59, 60–79, and ≥ 80 years). The incidence rates for first and recurrent episodes of gram-negative BSI were directly adjusted to the USA 2000 white population.9 The 95% confidence intervals (CI) for the incidence rate were estimated using a Poisson distribution.
The Kaplan-Meier method was used to estimate the cumulative incidence rate of recurrent gram-negative BSI for up to 10 years following the first episode. Patients were followed from the date of the first episode of gram-negative BSI until the second episode, death, last healthcare encounter or end of study. Patients who were lost to follow-up were censored on the date of their last healthcare encounter. We used two models to estimate the cumulative incidence rate of recurrence. In one model, patients who died during the study period were censored on the date of their death. However, this approach might have overestimated the true incidence rate of recurrence. Therefore, we used an alternative model where death was treated as a competing risk.16
Cox proportional hazard regression was used to evaluate the risk of recurrence in patients with BSI due to a particular gram-negative bacillus compared to others. We only included patients with BSI due to Escherichia coli and Klebsiella spp., the two most common microorganisms causing gram-negative BSI, in this analysis. The number of patients with BSI due to other gram-negative bacilli was relatively small to be analyzed; therefore, they were excluded from this risk factor analysis. To ensure that the results were not affected by confounding, the following variables were also evaluated as potential risk factors for recurrence: age (as continuous variable), gender (male vs. female), site of infection acquisition (community-acquired vs. nosocomial or healthcare-associated), and primary source of BSI (urinary vs. non-urinary).
The Kaplan-Meier method was also used to estimate the 28-day all-cause mortality rates. Patients were followed from the date of gram-negative BSI until death or last healthcare encounter. Patients lost to follow-up were censored on the date of their last healthcare encounter.
JMP (version 8.0, SAS Institute Inc, Cary, NC) was used for statistical analysis. The level of significance for statistical testing was defined as p<0.05 (2-sided).
RESULTS
We identified 846 unique patients with a first episode of monomicrobial gram-negative BSI during the study period. The median age of patients with gram-negative BSI was 68 years (interquartile range [IQR]: 47–81); and 478 (56.5%) were female (Table 1). The median follow-up time for these patients was over 2 years (792 days [IQR: 211–1790]). Nearly one-half (50.5%) of first episodes of gram-negative BSI were community-acquired; 36.2% were healthcare-associated, and 13.4% were nosocomial. The urinary tract was the most common primary source of initial gram-negative BSI (57.2%), followed by the gastrointestinal tract (11.5%), the respiratory tract (7.6%), skin and soft tissue infections (2.5%), central venous catheter-related (1.9%) and other (0.8%). The primary source was not identified for 18.6% of BSI.
Table 1.
Clinical characteristics of patients with first and recurrent episodes of gram-negative bloodstream infection.
| Variable | First episodes n=846 |
Recurrent episodes n=98 |
|---|---|---|
| Age: median (IQR) | 68 (47–81) | 67 (51–80)* |
| Female gender, n (%) | 478 (56.5) | 45 (56)* |
| Site of acquisition, n (%) | ||
| Community-acquired | 427 (50.5) | 43 (44) |
| Healthcare-associated | 306 (36.2) | 51 (52) |
| Nosocomial | 113 (13.4) | 4 (4) |
| Primary source, n (%) | ||
| Urinary tract | 484 (57.2%) | 60 (61) |
| Gastrointestinal tract | 97 (11.5) | 9 (9) |
| Respiratory tract | 64 (7.6) | 2 (2) |
| Skin and soft tissue | 21 (2.5) | 3 (3) |
| Central venous catheter-related | 16 (1.9) | 1 (1) |
| Other | 7 (0.8) | 2 (2) |
| Unknown | 157 (18.6) | 21 (21) |
| Microbiology, n (%) | ||
| Escherichia coli | 457 (54.0) | 51 (52) |
| Klebsiella pneumoniae | 102 (12.1) | 16 (16) |
| Pseudomonas aeruginosa | 55 (6.5) | 7 (7) |
| Proteus mirabilis | 25 (3.0) | 1 (1) |
| Enterobacter cloacae | 24 (2.8) | 4 (4) |
| Klebsiella oxytoca | 18 (2.1) | 6 (6) |
| Other | 165 (19.5) | 13 (13) |
| 28-day mortality, % (95% CI) | 10.0 (8.0–12.0) | 11.3 (4.4–18.2)* |
IQR: interquartile range, CI: confidence intervals.
Results for 80 unique patients with recurrent gram-negative bloodstream infection.
Among this cohort, 80 patients subsequently developed a total of 98 recurrent episodes of gram-negative BSI during the study period (65 patients had one recurrence, 14 patients had 2 recurrences, and one patient had 5 recurrences). The median age of these 80 patients at the time of the first episode of gram-negative BSI was 67 years (IQR: 51–80), and 45 (56%) were female (Table 1). The median time between the first and second episodes of gram-negative BSI was 315 days (IQR: 89–938).
Most recurrent episodes of gram-negative BSI were healthcare-associated (52%); 44% were community-acquired and 4% were nosocomial. The urinary tract was the most common primary source of infection in recurrent episodes of gram-negative BSI (61%), followed by the gastrointestinal tract (9%), skin and soft tissue infections (3%), the respiratory tract (2%), central venous catheter-related (1%), bone and joint infections (1%), and infective endocarditis (1%). The primary source of infection was not identified for the remaining 21% of BSI episodes.
Among the 98 recurrent episodes of gram-negative BSI, E. coli was the most common microorganism accounting for 52% of the episodes, followed by Klebsiella pneumoniae (16%), Pseudomonas aeruginosa (7%), Klebsiella oxytoca (6%), Enterobacter cloacae (4%), Citrobacter freundii (2%), Salmonella spp. (2%). The remaining 10 episodes were due to the following microorganisms: Serratia marcescens, Proteus mirabilis, Stenotrophomonas maltophilia, Haemophilus influenzae, Campylobacter fetus, Cardiobacterium hominis, Moraxella nonliquefaciens, Acinetobacter spp., Chryseobacterium spp., and Methylobacterium spp.
The overall age- and gender-adjusted incidence rate of gram-negative BSI was 84.5 (95% CI: 79.1–90.0) per 100,000 person-years, including 75.7 (95% CI: 70.6–80.8) per 100,000 person-years for first episodes and 8.8 (95% CI: 7.1–10.6) per 100,000 person-years for recurrent episodes. The age-adjusted incidence rates of all, first, and recurrent episodes of gram-negative BSI per 100,000 person-years were 83.4 (95% CI: 76.3–90.6), 74.7 (95% CI: 68.0–81.5), and 8.7 (95% CI: 6.4–11.0), respectively, in females; and 89.3 (95% CI: 80.3–98.2), 80.0 (95% CI: 71.6–88.5), and 9.2 (95% CI: 6.4–12.1), respectively, in males. The incidence rates of all, first, and recurrent episodes of gram-negative BSI increased with age in both females (Figure 1A) and males (Figure 1B). There was no apparent change in the age- and gender-adjusted incidence rate of gram-negative BSI throughout the 10-year study period (Figure 2).
Figure 1.
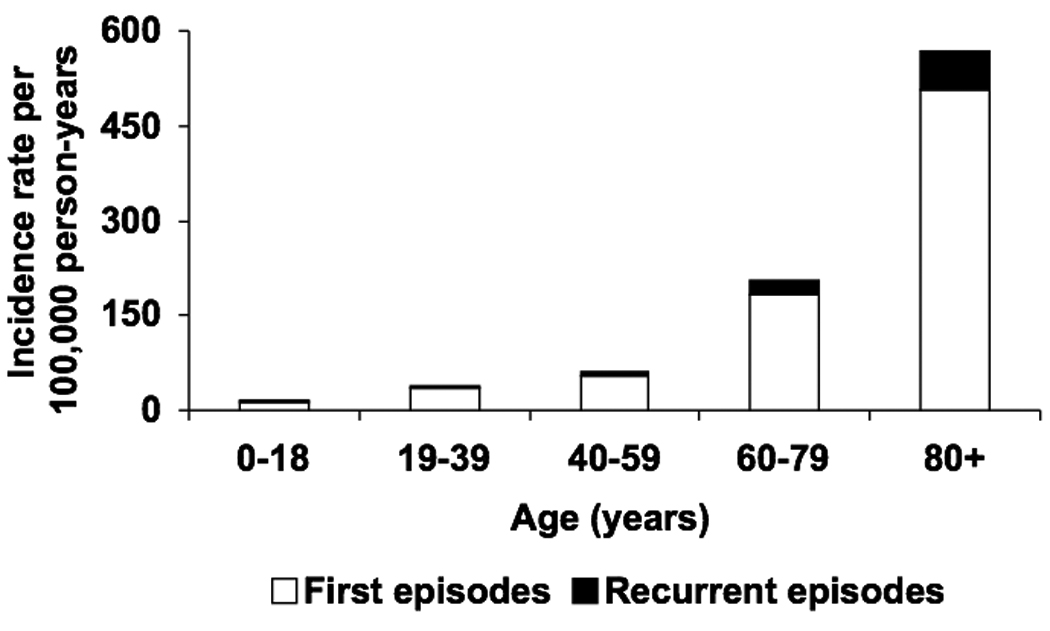
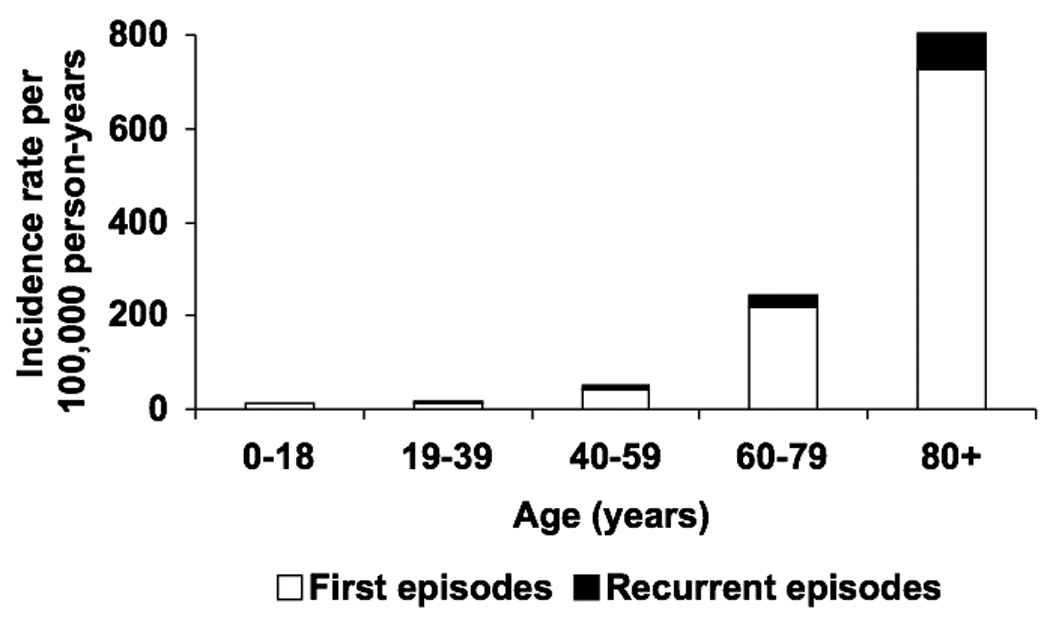
Incidence rate of first and recurrent episodes of gram-negative bloodstream infection by age in females (A) and males (B), 1998–2007.
Figure 2.
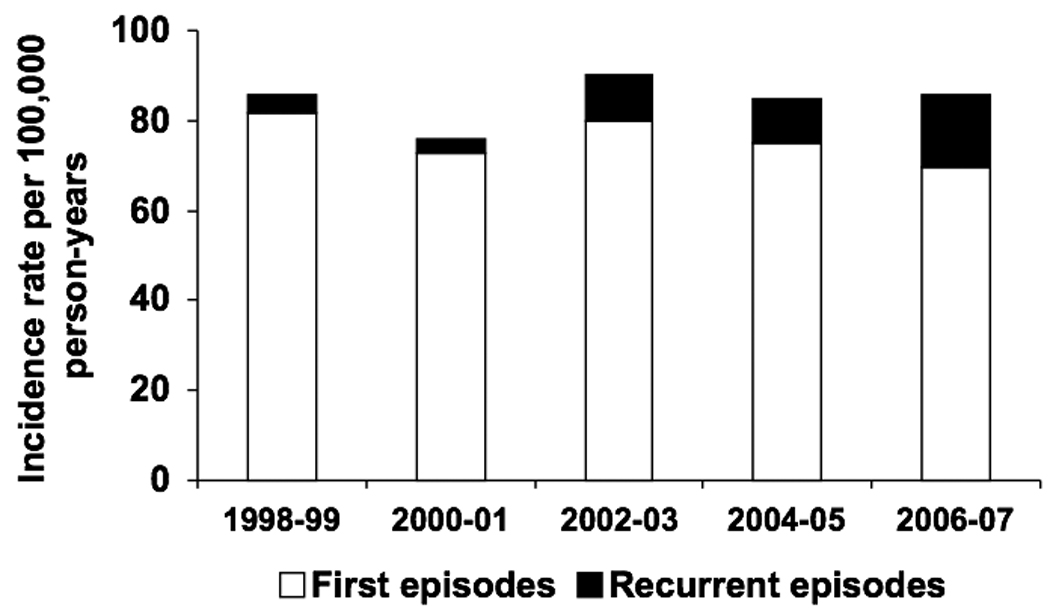
Age- and gender-adjusted incidence rate of gram-negative bloodstream infection by calendar year.
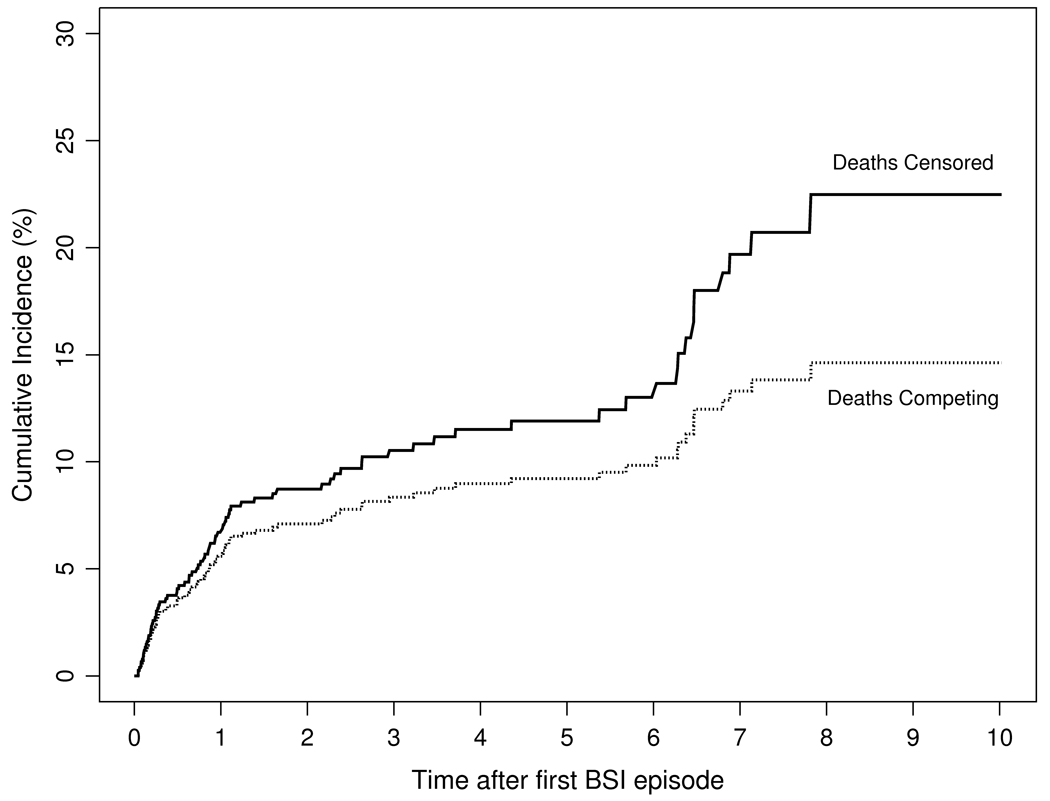
Among 846 patients with first episodes of gram-negative BSI, we estimated the cumulative incidence rate of recurrence after 1, 5, and 10 years of the initial episode to be 5.6%, 9.2%, and 14.6%, respectively, with death treated as a competing risk (Figure 3).
Figure 3.
Cumulative incidence rate of recurrent gram-negative bloodstream infection by time following the first episode (in years) with follow-up censored at death or with death treated as a competing risk.
Patients with Klebsiella spp. BSI were more likely to develop recurrences than were those with E. coli BSI (hazard ratio: 2.33 [95% CI: 1.34–3.92], p=0.003). There was no significant difference in the risk of recurrence across age, gender, site of infection acquisition, or primary source of BSI (Table 2).
Table 3.
Cox proportional hazard regression model results for risk factors of recurrence in patients with gram-negative bloodstream infection.*
| Variable | HR (95% CI) | P-value |
|---|---|---|
| Age (per 10 years) | 1.05 (0.94–1.19) | 0.38 |
| Gender: male vs. female | 1.19 (0.70–1.97) | 0.51 |
| Site of infection acquisition: | ||
| CA vs. HCA or nosocomial | 0.74 (0.45–1.24) | 0.25 |
| Primary source of infection: | 0.76 (0.44–1.39) | 0.37 |
| Urinary vs. non-urinary | ||
| Causative pathogen: | ||
| Klebsiella spp. vs. E. coli | 2.33 (1.34–3.92) | 0.003 |
HR: hazard ratio, CI: confidence interval, CA: community-acquired, HCA: healthcare-associated.
Only patients with E. coli and Klebsiella spp. were included in this analysis.
Only 5 patients (0.6%) were lost to follow up within 28-days of the first episode of gram-negative BSI and no patients were lost to follow up within 28-days of the second episode. The 28-day all-cause mortality rates following the first and second episodes of gram-negative BSI were 10.0% (95% CI: 8.0–12.0) and 11.3% (95% CI: 4.4–18.2), respectively.
DISCUSSION
To our knowledge, this is the first population-based study to examine the incidence rate, microbiology, risk factors and outcome of recurrent gram-negative BSI in North America. On the basis of an age- and gender-adjusted incidence rate of 8.8/100,000 person-years, we conclude that recurrent gram-negative BSI is a relatively uncommon condition at the population level. Nevertheless, among patients with first episode of gram-negative BSI, the cumulative incidence rate of recurrence was approximately 6%, 9%, and 15% after 1, 5, and 10 years of the initial episode, respectively. The 28-day all-cause mortality rate associated with the second episode of gram-negative BSI was comparable to that associated with the first episode (11% vs. 10%).
The reported age- and gender-adjusted incidence rate of gram-negative BSI of 84.5/100,000 person-years from 1998 to 2007 in this study was comparable to a previous estimate from a cross-sectional study of the same population from 2003 to 2005.13 The observed increase in the incidence rate of gram-negative BSI with age was consistent with the results of previous studies of BSI due to individual gram-negative bacilli in the same population.6, 7, 17
The recurrence rate of 10% over 37 months observed by Mylotte and McDermott from a single tertiary care center serving, predominantly, a population of older men was similar to what we observed in our study over the same duration with follow-up censored at death. Nevertheless, it might have been an overestimate when compared with the results of our alternative model, which treated death as a competing event (Figure 3). In addition, our follow-up period of 10 years was longer than that in Mylotte's and McDermott's study and the population-based design of our study allowed us to examine a population that was better defined and more uniform than the population in their tertiary-care center.1 The patients with recurrent gram-negative BSI studied by Wendt et al. had a median age of 39 years,4 which is much lower than the median age of 67 years reported in both our study and a recent population-based study from Denmark.8 We think that the substantial differences in age between population-based studies and those performed at tertiary care centers likely result from referral bias, which affects the patient populations at the latter institutions.18
The urinary tract was the most common primary source of infection in recurrent episodes of gram-negative BSI. This is consistent with the results of previous studies,1,8 but contradictory to those of another that demonstrated that one-half of recurrent episodes of gram-negative BSI were central venous catheter-related.4 Only 1% of episodes of recurrent gram-negative BSI in our study were central venous catheter-related. Patients referred to tertiary care centers might have been more likely to have complex medical conditions requiring placement of central venous catheters than were patients in our population-based study who were treated in their local hospitals, which might have accounted, in part, for the difference in the primary source of infection between the two studies.
Patients with BSI due to Klebsiella spp. were at least twice more likely than those with E. coli BSI to develop recurrent episodes of gram-negative BSI. Although 52% of recurrent episodes of gram-negative BSI were healthcare-associated, compared to only 36% of initial episodes, we did not find as association between recurrence and the site of acquisition or primary source of infection in our study. This is contrary to the observations of Jensen et al. study that identified nosocomial and healthcare-associated acquisition as well as gastrointestinal tract primary source of infection as independent risk factors for recurrence of BSI.8 There are several differences between the two studies that might account for the difference in the results. First, the inclusion of BSI due to microorganisms other than gram-negative bacilli in the latter study, such as gram-positive bacteria, Candida spp. and polymicrobilas, that have different clinical features than gram-negative BSI likely explains the difference in the results between the two studies. Second, Jensen et al. study included only recurrences within one year of the initial episode of BSI, whereas ours included all recurrences throughout the 10-year study period. Since the median time between the first and second episodes of BSI in our study was 315 days, nearly one-half of recurrent episodes of BSI occurred after one year of the initial episode. It is possible that the risk factors are different between early and late recurrences. Finally, since the number of patients with recurrent BSI in Jensen et al. study was larger than that in ours, it remains possible that our model for risk factors of recurrence was underpowered.
The 28-day all-cause mortality rate of recurrent gram-negative BSI of 11% was lower than the 17% mortality rate reported from a single center between 1984 and 1987.1 Similarly, the 28-day all-cause mortality rate of 10% following the first episode of gram-negative BSI is much lower than the 24–33% mortality rates reported from previous population-based and hospital-based investigations performed in the 1990s.19, 20 Nonetheless, the mortality rate in our study is comparable to that reported in a more recent investigation from 2001 to 2006.21
The primary strengths of this study were the population-based design, which decreased the possibility that referral bias affected the results, and the prolonged follow-up facilitated by the REP. Accounting for the competing risk of death when estimating the recurrence rates was another unique advantage of our work.
Our study has limitations. First, we did not perform pulse-field gel electrophoresis to differentiate relapses from re-infections;4, 5 rather, we used a clinical definition for recurrence. Second, the population of Olmsted County consists mainly of middle class whites; therefore, our study results may be generalized only to communities with similar population characteristics. Finally, our data were derived from one geographic area. The results of studies from multiple geographic locations might provide a more generalizable view.
In summary, this is the first population-based study that defined the incidence rate, microbiology, risk factors and outcome of recurrent gram-negative BSI in North America. Although recurrent gram-negative BSI was relatively uncommon in the general population, 6%, 9% and 15% of patients with gram-negative BSI had second episodes 1, 5 and 10 years after the initial episode, respectively. Patients with Klebsiella spp. BSI had a higher risk of recurrent gram-negative BSI than did those with E. coli BSI. Nearly one-half of recurrent episodes of gram-negative BSI were healthcare-associated. E. coli was the most common microorganism and the urinary tract was the most common primary source of recurrent gram-negative BSI. The mortality rate of gram-negative BSI was lower than that previously reported over the past decade.
Acknowledgments
The authors thank Emily Vetter and Mary Ann Butler for providing us with vital data from the microbiology laboratory databases at the Mayo Clinic, Rochester and Olmsted Medical Center.
The authors thank Susan Schrage, Susan Stotz, R.N., and all the staff at the Rochester Epidemiology Project for their administrative help and support.
Funding. The study received funding from the Small Grants Program and the Baddour Family Fund at the Mayo Clinic, Rochester, MN. The funding source had no role in study design.
This work was made possible by research grant R01-AR30582 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (National Institutes of Health, U.S. Public Health Service).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential conflicts of interest. MNA, JEE, and LMB: No conflict.
MNA has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Mylotte JM, McDermott C. Recurrent gram-negative bacteremia. Am J Med. 1988;85:159–163. doi: 10.1016/s0002-9343(88)80335-8. [DOI] [PubMed] [Google Scholar]
- 2.Maslow JN, Mulligan ME, Arbeit RD. Recurrent Escherichia coli bacteremia. J Clin Microbiol. 1994;32:710–714. doi: 10.1128/jcm.32.3.710-714.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capdevila JA, Almirante B, Pahissa A, Planes AM, Ribera E, Martinez-Vazquez JM. Incidence and risk factors of recurrent episodes of bacteremia in adults. Arch Intern Med. 1994;154:411–415. [PubMed] [Google Scholar]
- 4.Wendt C, Messer SA, Hollis RJ, Pfaller MA, Wenzel RP, Herwaldt LA. Recurrent gram-negative bacteremia: incidence and clinical patterns. Clin Infect Dis. 1999;28:611–617. doi: 10.1086/515152. [DOI] [PubMed] [Google Scholar]
- 5.Wendt C, Messer SA, Hollis RJ, Pfaller MA, Wenzel RP, Herwaldt LA. Molecular epidemiology of gram-negative bacteremia. Clin Infect Dis. 1999;28:605–610. doi: 10.1086/515151. [DOI] [PubMed] [Google Scholar]
- 6.Al-Hasan MN, Wilson JW, Lahr BD, Eckel-Passow JE, Baddour LM. Incidence of Pseudomonas aeruginosa bacteremia: a population-based study. Am J Med. 2008;121:702–708. doi: 10.1016/j.amjmed.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Hasan MN, Lahr BD, Eckel-Passow JE, Baddour LM. Epidemiology and outcome of Klebsiella species bloodstream infection: a population-based study. Mayo Clin Proc. 2010;85(2):139–144. doi: 10.4065/mcp.2009.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen US, Knudsen JD, Ostergaard C, Gradel KO, Frimodt-Moller N, Schonheyder HC. Recurrent bacteraemia: A 10-year regional population-based study of clinical and microbiological risk factors. J Infect. 2010;60(3):191–199. doi: 10.1016/j.jinf.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 9. [Accessed December 22, 2007];US Census Bureau. Olmsted County QuickFacts. http://quickfacts.census.gov/qfd/states/27/27109.html.
- 10.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 11.Steckelberg JM, Melton LJ, 3rd, Ilstrup DM, Rouse MS, Wilson WR. Influence of referral bias on the apparent clinical spectrum of infective endocarditis. Am J Med. 1990;88:582–588. doi: 10.1016/0002-9343(90)90521-e. [DOI] [PubMed] [Google Scholar]
- 12.Tleyjeh IM, Steckelberg JM, Murad HS, et al. Temporal trends in infective endocarditis: a population-based study in Olmsted County, Minnesota. JAMA. 2005;293:3022–3028. doi: 10.1001/jama.293.24.3022. [DOI] [PubMed] [Google Scholar]
- 13.Uslan DZ, Crane SJ, Steckelberg JM, et al. Age- and sex-associated trends in bloodstream infection: a population-based study in Olmsted County, Minnesota. Arch Intern Med. 2007;167:834–839. doi: 10.1001/archinte.167.8.834. [DOI] [PubMed] [Google Scholar]
- 14.Friedman ND, Kaye KS, Stout JE, et al. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791–797. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 15.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 16.Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 17.Al-Hasan MN, Lahr BD, Eckel-Passow JE, Baddour LM. Seasonal variation in Escherichia coli bloodstream infection: a population-based study. Clin Microbiol Infect. 2009;15:947–950. doi: 10.1111/j.1469-0691.2009.02877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bower JH, Maraganore DM, McDonnell SK, Rocca WA. Incidence and distribution of parkinsonism in Olmsted County, Minnesota, 1976–1990. Neurology. 1999;52:1214–1220. doi: 10.1212/wnl.52.6.1214. [DOI] [PubMed] [Google Scholar]
- 19.Pedersen G, Schonheyder HC, Sorensen HT. Antibiotic therapy and outcome of monomicrobial gram-negative bacteraemia: a 3-year population-based study. Scand J Infect Dis. 1997;29:601–606. doi: 10.3109/00365549709035903. [DOI] [PubMed] [Google Scholar]
- 20.Kang CI, Kim SH, Park WB, et al. Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob Agents Chemother. 2005;49:760–766. doi: 10.1128/AAC.49.2.760-766.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Hasan MN, Wilson JW, Lahr BD, et al. Beta-lactam and fluoroquinolone combination antibiotic therapy for bacteremia caused by gram-negative bacilli. Antimicrob Agents Chemother. 2009;53:1386–1394. doi: 10.1128/AAC.01231-08. [DOI] [PMC free article] [PubMed] [Google Scholar]