Abstract
α-Synuclein has been linked to the pathogenesis of Parkinson's disease and other synucleinopathies through its propensity to form toxic oligomers. The exact mechanism for oligomeric synuclein-directed cell vulnerability has not been fully elucidated but one hypothesis portends the formation of synuclein-containing pores within cell membranes leading to leak channel-mediated calcium influx and subsequent cell death. Here we demonstrate synuclein-induced formation of SDS-stable oligomers, intracellular synuclein-positive aggregates, alterations in membrane conductance reminiscent of leak channels and subsequent cytotoxicity in a dopaminergic-like cell line. Furthermore we demonstrate that the synuclein-induced membrane conductance changes are blocked by direct extracellular application of an anti-synuclein antibody. The work presented here confirms that synuclein overexpression leads to membrane conductance changes and demonstrates for the first time through antibody blocking studies that synuclein plays a direct role in the formation of leak channels.
Introduction
Parkinson's disease (PD) is the second most common age-related neurodegenerative disease with the classic motoric symptoms of resting tremor, rigidity, akinesia/bradykinesia and postural instability. Although not restricted to the dopaminergic system, all PD cases manifest the invariant loss of substantia nigra pars compacta dopamine neurons (SNpc DAN), dystrophic projections to the striatum and a reduction in the attendant neurotransmitter, dopamine. Furthermore the few remaining SNpc DANs contain large intracytoplasmic proteinaceous inclusions called Lewy bodies, which are replete with the 140-amino acid protein, α-synuclein (Syn) (Spillantini et al., 1997). Syn was etiologically linked to familial PD following the identification of point mutations in and multiplication of the Syn gene (SNCA) in a subset of susceptible individuals (Polymeropoulos et al., 1997; Kruger et al., 1998; Singleton et al., 2003; Zarranz et al., 2004). However the majority of PD cases are sporadic arising from a yet unknown etiology but thought to involve multiple insults many of which converge on Syn. In further support of Syn playing a central role in PD, recent genome-wide association studies (GWAS) demonstrate that variations in SNCA are associated with an increased risk of developing sporadic PD (Satake et al., 2009; Simon-Sanchez et al., 2009).
Syn-induced toxicity has been linked to its proclivity to form oligomeric species, however the exact mechanism(s) through which misfolded Syn mediates dopaminergic neuron death remains unclear (Goldberg & Lansbury, 2000; Volles & Lansbury, 2003). Cell culture and animal models of Syn overexpression link this protein to dysregulation of the proteosome, lysosome, mitochondria as well as augmented proinflammatory responses while Syn-deficient mice point to a normal role for this protein in the regulation of the readily releasable pool of dopamine (Abeliovich et al., 2000; Elkon et al., 2002; Gosavi et al., 2002; Junn & Mouradian, 2002; Meredith et al., 2002; Volles & Lansbury, 2002; Cuervo et al., 2004; Zhang et al., 2005; Martin et al., 2006; Periquet et al., 2007; Cole et al., 2008; Devi et al., 2008; Fasano & Lopiano, 2008; Martinez-Vicente et al., 2008; Parihar et al., 2008; Shavali et al., 2008; Su et al., 2008; Parihar et al., 2009; Su et al., 2009). Genetic data and overexpression models indicate that Syn toxicity is closely associated with the amount of intracellular Syn either via increased expression or decreased degradation of the protein. One leading hypothesis suggests that the ability of Syn to form pentameric pore-like structures in cell membranes results in cell vulnerability and neurodegeneration (Crews et al., 2009). In support of this hypothesis, in vitro evidence from atomic force and electron microscopy studies demonstrates that Syn forms pore-like structures in synthetic membranes (Conway et al., 2000b; Goldberg & Lansbury, 2000; Lashuel et al., 2002; Danzer et al., 2007). Furthermore, computer modeling predicts that Syn forms propagating conformations that dock to the surface of membranes leading to pore-like structures (Tsigelny et al., 2007). The link between Syn membrane localization and permeability resulting in toxicity was first demonstrated in cultured SH-SY5Y cells engineered for Syn overexpression (Furukawa et al., 2006). In addition, cultured HEK293T cells transduced with lentivirus-expressing Syn demonstrated an increase in Zn2+-sensitive whole cell currents further supporting a role for Syn in membrane conductance perturbations (Tsigelny et al., 2007). Here we use a dopaminergic-like cell model with regulated Syn expression and report the formation of oligomeric Syn structures, increased membrane conductance due to leak channel formation and Syn-induced cell death. Importantly we show for the first time that Syn-induced membrane permeability can be blocked by extracellular application of an anti-Syn antibody demonstrating a direct role for Syn in leak channel formation and a potentially novel therapeutic approach to synucleinopathies.
Materials and Methods
Antibodies
The following antibodies were used: A11 (rabbit polyclonal antibody; immunogen: synthetic molecular mimic of soluble oligomers; Invitrogen, catalog no. AHB0052, Camarillo, CA, USA), anti-GAPDH (mouse monoclonal antibody; immunogen: rabbit muscle glyceraldehyde-3-phosphate dehydrogenase; Chemicon/Millipore, catalog no. MAB374, Billerica, MA, USA), anti-α-synuclein for immunoblotting and electrophysiology (Syn-1; mouse monoclonal antibody; immunogen: rat synuclein-1 amino acids 15-123; BD Biosciences, catalog no. 610787, San Jose, CA, USA), anti-α-synuclein antibody for immunocytochemistry (Ab-2; mouse monoclonal antibody; immunogen: recombinant human α-synuclein; NeoMarkers, catalog no. MS-1572-PIABX, Fremont, CA, USA), anti-α-tubulin (rabbit polyclonal antibody; immunogen: synthetic peptide conjugated to KLH derived from residues 1 - 100 of human alpha-tubulin; Abcam, catalog no. ab4074, Cambridge, MA, USA), Alexa Fluor 594 conjugated goat anti-mouse secondary antibody (catalog no. A11005; Invitrogen), horseradish peroxidase conjugated goat anti-rabbit IgG antibody (catalog no. AP132P; Chemicon/Millipore), horseradish peroxidase conjugated goat anti-mouse antibody (catalog no. AP124P; Chemicon/Millipore).
Cell Culture
MN9D cells are derived from mouse embryonic mesencephalon and were a kind gift of Dr. A. Heller (Choi et al., 1991). MN9DwtsynIRESgfp (MN9Dsyn) and MN9DIRESgfp (MN9Dgfp) cells were engineered and cultured as previously described (Luo et al., 2007; Su et al., 2008). MN9Dsyn and MN9Dgfp cells are immortalized dopaminergic-like cell lines that harbor an integrated transgene affording doxycycline [(DOX; 2.0 μg/mL media); Sigma-Aldrich Co., St. Louis, MO, USA] regulated human wildtype α-synuclein (Syn) expression and separately, using an internal ribosome entry site (IRES), green fluorescent protein (GFP) expression (MN9Dsyn). MN9D cells with regulated expression of GFP alone (MN9Dgfp) were used as a control for whole cell patch clamp recordings. The time of induction for each experiment is indicated in the respective figure legends.
MTT (3-[4,5-Dimethylthiazol]-2,5-diphenyltetrazolium) Assay
MN9Dsyn cells (1 × 104 cells/well) were grown in 96-well plates (Nunclon™) in the absence or presence of DOX to induce transgene expression. At various times post-induction cell viability was quantified using an MTT assay as described by the manufacturer (Roche, Indianapolis, IN, USA). Each experiment was performed with an n of 8 and repeated at least three times. Data are expressed as percentage of cell death with respect to control untreated cells (−DOX). Values are the mean ± SEM.
Western Blot Analysis
MN9Dsyn cells (5 × 106 cells/plate) were grown for two days in 10cm plates (Nunclon™, Thermo Scientific, Waltham, MA, USA) in the absence or presence of DOX (Syn induction). Following experimental treatment, cells were washed with PBS and subsequently lysed on ice in modified RIPA buffer (50 mM Tris HCl pH 7.4, 1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl) supplemented with protease inhibitor cocktail (Sigma-Aldrich), PMSF (Sigma-Aldrich) and Halt Phosphatase Inhibitor (Thermo Scientific) using a hand-held homogenizer. Lysates were centrifuged at 13,300 rpm × 15 minutes at 4°C. Supernatants were retained as the soluble lysate while the pellets were washed once with modified RIPA, resuspended in denaturing sample buffer (62.5 mM Tris-HCl pH 6.8, 2% sodium dodecyl sulfate, 10% glycerol, 5% beta-mercaptoethanol and 0.001% bromophenol blue) boiled for 5 minutes and centrifuged as above for 1 minute. The supernatant was retained as SDS-soluble protein. All protein samples were subjected to denaturing SDS-polyacrylamide gradient gel electrophoresis (4-16% PAG gradient) followed by transfer to polyvinylidene difluoride (PVDF) membranes (Perkin Elmer, Waltham, MA, USA) and western blot analysis for α-synuclein (Syn-1; 1:1000). Immunoreactive complexes were visualized using Super Signal West Pico Chemiluminescent Substrate (Thermo Scientific) and exposure to Hyperfilm ECL (Amersham Biosciences, Piscataway, NJ, USA). Membranes were re-probed with a primary antibody against α-tubulin (1:1000) as a loading control (n = 3 for each condition).
Slot Blot Analysis
MN9Dsyn cells (5 × 106 cells/plate) were grown for two days in 10cm plates (Nunclon™) in the absence or presence of DOX to induce SYN expression. Analysis of oligomers was adapted from methods described previously by Shin and colleagues [A11; (Shin et al., 2008)]. Briefly, MN9Dsyn whole cells lysates (0.625 μg; described above in Western Blot Analysis) were loaded onto a nitrocellulose membrane (Whatman, Florham Park, NJ, USA) which was subsequently probed for A11. The same membrane was stripped and re-probed with an anti-α-synuclein antibody (Syn-1) and GAPDH (1:8000) antibody as a loading control (n = 3 for each condition). Quantification of protein levels was performed using the EC3 Imaging System (UVP, LLC., Upland, CA, USA).
Immunocytochemistry
MN9Dsyn cells (4 × 104 cells/well) were grown on polyethyleneimine coated (PEI) glass coverslips (diameter = 12mm, Deckglaser, Germany) in 24-well plates (Nunclon™) in the absence or presence of DOX for 2 days. Syn: Following experimental manipulation cells were gently rinsed with TBS (100 mM Tris-HCl pH 7.5, 0.9% sodium chloride) and fixed in 4% paraformaldehyde/4% sucrose/PBS for 15 minutes at room temperature. Fix was removed and cells rinsed three times with TBS. TBS/4.5% non-fat dry milk (NFDM)/0.1% Triton-X 100 was subsequently used for blocking and permeabilization. The cells were then probed with anti-α-synuclein antibody (NeoMarkers; 1:1000) for 1 hour at room temperature. After washing with TBS/4.5% NFDM/0.05% Triton-X 100 antibody:antigen complexes were visualized following incubation with Alexa Fluor 594 conjugated goat anti-mouse secondary antibody (1:2000). After two washes in TBS/4.5% NFDM/0.05% Triton-X 100, cells were counterstained with DAPI (4′,6′-diamidino-2-phenylindole; Invitrogen; catalog no. D1306; 100nM) and mounted in mowiol (Calbiochem, La Jolla, CA, USA) and imaged. Immunocytochemistry of antibody used to block membrane conductance: MN9Dsyn cells were induced with DOX for 2 days, rinsed with TBS and fixed as described above. Following three washes with TBS, the cells were blocked with TBS/4.5% NFDM and probed with the anti-α-synuclein antibody (Syn-1; 1:1000) for 1 hour in the absence of permeabilization. After washing with TBS/4.5% NFDM/0.05% Triton-X 100 to remove unbound antibody, the cells were incubated with Alexa Fluor 594 conjugated goat anti-mouse secondary antibody (1:2000) for 1 hour at room temperature and imaged. All fluorescent complexes were imaged using a Zeiss Axioskop microscope (Thornwood, NY, USA) with a Photometrics Coolsnap-fx (Roper Scientific, Tucson, AZ, USA) camera and Scanalytic's IPLab software (Fairfax, VA, USA). Confocal microscopy images were captured with a Fluoview FV300 confocal laser-scanning unit on an Olympus IX-70 microscope (Melville, NY, USA). Fluoview software was used for both image acquisition and processing.
Electrophysiological Analysis/Whole Cell Patch Clamp Recordings
MN9Dsyn and MN9Dgfp cells (4 × 104 cells/well) were grown on PEI-coated coverslips in 24-well plates (Nunclon™) in the absence or presence of DOX. Following 2 days of treatment, MN9Dsyn- or MN9Dgfp-containing coverslips (diameter = 12mm, Deckglaser, Germany) were placed on the stage of a Nikon TM2000 inverted microscope. The cells were continuously perfused with an extracellular solution containing 145 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 5 mM Hepes, 5 mM glucose, 0.25 mg/l phenol red, 10 μM D-serine and 23.4 mM sucrose pH 7.4 (all reagents obtained from Sigma-Aldrich). Electrodes were pulled on a vertical pipette puller to a resistance of 4-6 MΩ from borosilicate glass capillaries (Wiretrol II, Drummond, Broomall, PA, USA) and filled with intracellular recording solution containing 145 mM CsCl, 10 mM Hepes, 5 mM MgATP, 0.2mM NaGTP and 10 mM 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) pH 7.2. Whole cell voltage clamp recordings were performed at room temperature using an Axopatch-1D amplifier (Molecular Devices, Downingtown, PA, USA). To measure input resistance, the membrane potential was held at 0 mV and stepped to levels from −45 mV to 0 mV in 5 mV increments and 0 mV to 90 mV in 10 mV increments. While recording, an anti-α-synuclein antibody (250 ng/ml; Syn-1) or horseradish peroxidase conjugated goat anti-rabbit IgG antibody (control; 250 ng/ml) were diluted in extracellular solution to a final concentration of 250 ng/ml and applied locally via a Y-tubing device (Murase et al., 1989). Recording continued following antibody application for 10 minutes. Currents were filtered at 2 kHz with an 8-pole low pass Bessel filter (Frequency Devices, Haverhill, MA), digitized at 5–10 kHz with Digidata 1322 A data acquisition board and pCLAMP9.2 software (both from Molecular Devices). All data were analyzed with Clampfit 9.2 software (Molecular Devices) and are the average of 7-10 cells/treatment condition.
Statistical Analysis
Each experiment was independently performed at least three times. All data are expressed as mean ± SEM. Statistical analyses for multiple comparisons was performed using a one-way analysis of variance (ANOVA) with Tukey HSD post-hoc test for observations of Syn-induced cell death (Fig. 5). To assess the effects of antibody treatment on cell membrane conductance (i.e. treatment, time, and treatment by time interactions) a two-way repeated-measures ANOVA was employed (Fig. 4). For this analysis the between-subject factors were defined as the presence/absence of DOX (±DOX) and antibody treatment (either the control antibody or the anti-α-synuclein antibody) while the within-subject factor was defined as time (0 minute, 5 minutes and 10 minutes). The sphericity assumption was tested with Mauchly's test and significant effects of antibody treatment at each time point were determined by non-directional Student's t-test with Bonferroni corrections for multiple comparisons. Non-directional Student's t-tests were performed for comparisons involving only two groups (Figs. 1 & 3). All statistical analyses were conducted using SAS9.2 (SAS Statistical Institute, Cary, NC) and SPSS18.0 (SPSS Inc., Chicago, IL). Results were considered statistically significant at P ≤ 0.05.
Figure 5.
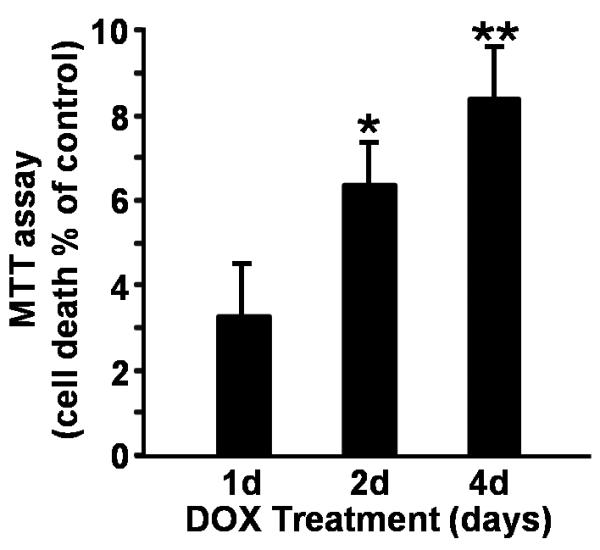
Syn overexpression results in cytotoxicity in a dopaminergic-like cells. MTT assay of MN9Dsyn cells in the presence and absence of DOX treatment overtime. Cell death as a percent of control was calculated as the percentage of mitochondrial activity reduction following Syn overexpression (+DOX; +Syn) compared to the control (−DOX; −Syn) [percent cell death ± SEM; n = 8 wells/treatment]. Syn overexpression for 4 days resulted in ~8% cell death. Each experiment was repeated at least three times. One-way ANOVA and Tukey HSD post-hoc test, */** significant difference as compared with uninduced (−DOX; −Syn) controls following 2 and 4 days of Syn expression respectively; *P = 0.004, **P = 0.0003.
Figure 4.
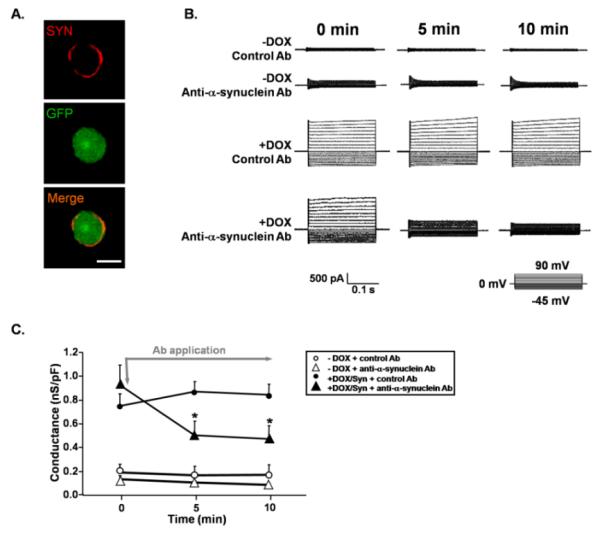
Increased membrane conductance in MN9Dsyn cells is blocked following treatment with an anti-synuclein antibody. (A.) Immunocytochemistry demonstrating that the monoclonal anti-Syn antibody (red) used to block leak currents recognizes Syn on the surface of non-permeabilized MN9Dsyn cells induced to overexpress Syn and GFP (green). Scale bar = 10 μm. (B.) Representative traces showing current changes in MN9Dsyn cells over time in response to local application of either a monoclonal antibody against Syn (250 ng/ml; Anti-α-synuclein Ab) or an antibody that does not recognize Syn (250ng/ml; control Ab; horseradish peroxidase conjugated goat anti-rabbit IgG antibody from Chemicon Inc.; inset: step voltage protocol). 0 min indicates the recording just prior to antibody application while recordings at 5 and 10 minutes (min) occurred during antibody exposure. (C.) Attenuation of Syn-induced conductance changes following local application of an anti-α-synuclein antibody. Values expressed as mean conductance ± SEM (n = 7 - 10 cells/treatment) indicate the slope of each I-V plot. The data shown are normalized to capacitance to eliminate differences due to cell size. The grey arrow indicates the initial application of antibody while the grey line indicates the continuous application of antibody. White circles: −DOX + control antibody; white triangle: −DOX + anti-α-synuclein antibody; black circle: +DOX/Syn + control antibody; black triangle: +DOX/Syn + anti-α-synuclein antibody. The control Ab and the anti-α-synuclein antibody did not affect whole cell membrane conductance in non-induced (−DOX) MN9Dsyn cells. However the anti-α-synuclein antibody significantly decreased membrane conductance of Syn overexpressing MN9Dsyn (+DOX/Syn) over time. Two-way repeated-measures ANOVA and post-hoc test (non-directional Student's t-test with Bonferroni corrections for multiple comparisons), *significant difference of the anti-α-synuclein antibody compared with control antibody at 5 minutes (P = 0.02) and at 10 minutes (P = 0.01).
Figure 1.
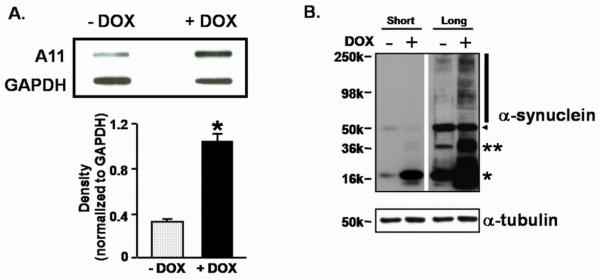
Syn overexpression in a dopaminergic cell line forms oligomers. (A.) Slot blot analysis and quantitative densitometry using the A11 antibody demonstrates an increase of soluble amyloid structures in DOX-induced MN9Dsyn whole cell lysates (+DOX). Blots were reprobed for GAPDH as a loading control. Immunoprotein complexes were quantified by densitometric analysis and values normalized to GAPDH. All data are expressed as mean band density ± SEM from three samples. *non-directional Student's t-test, significant difference as compared with uninduced (−DOX; −Syn) controls, P =0.0007. (B.) Representative western blot analysis of MN9Dsyn lysates. MN9Dsyn cells were grown in the absence (−) and presence (+) of DOX for 2 days (n = 3/treatment). Protein lysates were prepared and subjected to 4-16% SDS-PAGE and immunoblotted for Syn revealing the presence of monomeric (*), dimeric (**) and SDS-resistant high molecular weight Syn oligomers (vertical line). Arrowhead indicates a nonspecific band. Blots were reprobed for α-tubulin as a loading control. “Short” indicates a 3 second exposure time for the film while “Long” represents a 30 second exposure time.
Figure 3.
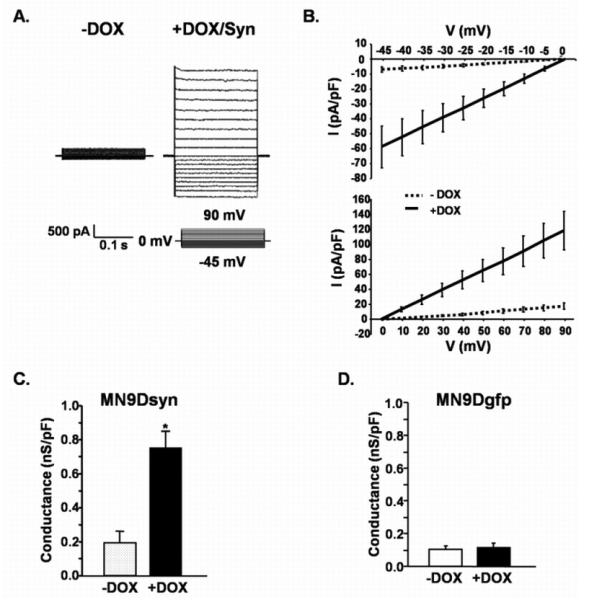
Syn overexpression increases membrane conductance. (A.) Representative traces from DOX-induced (+DOX/Syn) and uninduced (−DOX) MN9Dsyn cells showing currents elicited by stepping membrane voltage from a holding potential of 0 mV to levels between −45 mV and 90 mV (inset: step voltage protocol). (B.) I-V plots demonstrating the current-voltage relationship determined by plotting mean current amplitudes ± SEM (n = 7 – 10 cells/treatment) from Syn expressing (+DOX, solid lines) and control (−DOX, dotted lines) cells at each voltage. (C.) Conductance changes following Syn overexpression. Data were normalized to capacitance to eliminate differences due to cell size. Mean conductance ± SEM (n = 7 - 10 cells/treatment) indicate the slope of each I-V plot. *non-directional Student's t-test, significant increase in membrane conductance in Syn overexpressing MN9Dsyn cells (+DOX) compared with control cells (−DOX; P = 0.004). (D.) GFP overexpression alone (i.e. in the absence of Syn overexpression; MN9Dgfp cells) does not affect membrane conductance. Values expressed as mean conductance ± SEM (n = 6 - 8 cells/treatment) indicate the slope of each I-V plot. No significant change in membrane conductance was observed when GFP-induced (+DOX; +GFP) MN9Dgfp cells were compared with uninduced (−DOX; −GFP) control cells (non-directional Student's t-test, P = 0.68).
Results
α-Synuclein forms oligomers and localizes to the cytoplasmic membrane
Accumulating evidence suggests that Syn conformers corresponding to amyloid oligomers are the most pathogenic species. Therefore we first sought to determine whether Syn overexpression in a dopaminergic-like cell line (MN9Dsyn) engenders the formation of higher molecular weight oligomers and amyloid conformers. We utilized an immortalized dopaminergic cell line that harbors an integrated transgene affording doxycycline (DOX) regulated human wildtype α-synuclein (Syn) expression and separately, using an internal ribosome entry site (IRES), green fluorescent protein (GFP) detection (MN9DwtsynIRESgfp referred to here as MN9Dsyn) (Choi et al., 1991; Strathdee et al., 1999; Su et al., 2008). MN9Dsyn cells were treated for two days with doxcycline (DOX) to induce Syn expression. Using a previously characterized antibody reported to recognize soluble amyloid structures (A11) we observed a 3-fold increase in β-sheet oligomers in cell lysates from Syn overexpressing cells (+DOX) (Shin et al., 2008) as compared with the non-induced control (−DOX) (Fig. 1A; non-directional Student's t-test, P = 0.0007). We then asked whether SDS-resistant oligomeric Syn was present in lysates from induced dopaminergic cells overexpressing Syn (+DOX; Fig. 1B). Following 2 days of DOX induction protein lysates were prepared in modified RIPA buffer and subjected to polyacrylamide gel electrophoresis under denaturing conditions followed by Syn western blot analysis. Monomeric SYN was present following DOX induction (Fig. 1B; *). In addition, dimeric (**) and higher SDS-stable oligomeric Syn conformers (black vertical line) were apparent following a longer film exposure of the Syn western blot (Long, +DOX; Fig. 1B). Furthermore, when analyzed at the cellular level, immunocytochemical studies reveal that Syn and Syn-positive aggregates accumulate at the cell membrane following DOX induction (Fig. 2; white arrows).
Figure 2.
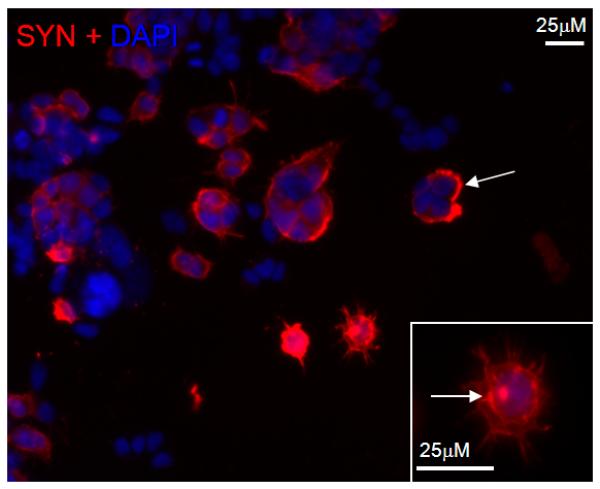
Overexpressed Syn localizes to cell membranes and forms aggregates. MN9Dsyn cells were treated with DOX for two days to induce Syn expression prior to immunocytochemical analysis. Membrane localized and cytosolic Syn as well as inclusion bodies (white arrows) immunostained for Syn (red) are present in the induced MN9Dsyn cells. DAPI (blue) was used to counterstain nuclei. Scale bar = 25 μm. The Syn-specific aggregates are more apparent in the higher magnification inset photomicrograph (white box; arrow).
Membrane conductance is increased in MN9D cells overexpressing α-synuclein
Since we observed oligomeric Syn conformers and membrane localization of this protein, we further undertook an investigation of membrane conductance changes induced by Syn overexpression in our dopaminergic-like cells. First, we performed whole-cell patch-clamp recordings on MN9Dsyn cells in the presence (+DOX) and absence (−DOX) of Syn using an intracellular solution containing cesium chloride and a holding potential of 0 mV to eliminate any contribution from voltage-gated potassium or sodium channels in an attempt to link conductance changes directly to Syn overexpression. An increase in membrane conductance, indicating the presence of leak channels was observed in MN9Dsyn cells overexpressing Syn (Fig. 3A-C). Representative traces demonstrate that changes in conductance are apparent in the full range tested (−45mV to 90mV) and the I/V plots (mean current amplitude ± SEM plotted against voltage) are typical of the presence of leak channels (Fig. 3A & B). Importantly, when conductance changes were normalized to capacitance to adjust for any differences in cell size there was an overall nearly 4-fold increase in membrane conductance in dopaminergic cells that overexpress Syn (Fig. 3C; −DOX compared to +DOX, non-directional Student's t-test, P = 0.004). This effect was Syn specific and not due to general protein overexpression, as MN9Dgfp cells, which were engineered using the same DOX-responsive construct to overexpress GFP only (no Syn), did not display similar conductance changes (MN9Dgfp; Fig. 3D; −DOX compared to +DOX; non-directional Student's t-test, P = 0.68).
Changes in membrane conductance are directly linked to the presence of α-synuclein
To explore whether the increase in membrane conductance was directly related to membrane-associated Syn, while recording, we locally applied a monoclonal antibody specific for Syn and continued to monitor conductance changes. First we established that the anti-α-synuclein monoclonal antibody was capable of recognizing membrane localized Syn. MN9Dsyn cells were induced with DOX and 2 days later prepared for immunocytochemistry in the absence of detergents to limit membrane permeabilization. SYN:antibody complexes (red) were visualized “rimming” the induced cell while GFP which is co-expressed with Syn is localized within the cell (Fig. 4A; red vs. green). Next the change in whole cell membrane conductance was monitored before and during local antibody administration via a Y-tubing device. Here we show that extracellular application of the anti-α-synuclein antibody significantly decreased membrane conductance changes of Syn-overexpressing cells over time [Fig. 4B & C; black triangles; two-way repeated-measures ANOVA; F2,30 = 9.4, P = 0.001; Mauchly's test of sphericity was not significant in the repeated-measures ANOVA models (P > 0.05).] with the most significant effect occurring at later time points (Fig. C; non-directional Student's t-test with Bonferroni corrections; significant difference of the anti-α-synuclein antibody compared with control antibody at 5 minutes P = 0.02 and at 10 minutes P= 0.01), whereas a control antibody (horseradish peroxidase-conjugated goat anti-rabbit IgG antibody) that does not recognize Syn did not rescue the Syn-induced increase in membrane permeability (Fig. 4B & C; black circles). Neither the control antibody nor the anti-α-synuclein antibody affected membrane conductivity in non-induced MN9Dsyn cells (Fig. 4B & C; open circles and triangles respectively). We have shown previously that Syn localizes to the membrane as well as the cytosol (Fig. 2). Importantly, in the absence of detergents, we did not observe any intracellular Syn staining (Fig. 4A) indicating that fixation during the immunocytochemical analysis did not sufficiently alter membrane permeability to allow detectable levels of antibody to enter the cells. The observed effect of the anti-α-synuclein antibody on membrane conductance was therefore likely a consequence of interaction with Syn on the outer surface of the cell membrane. To our knowledge this is the first direct demonstration of Syn-induced leak channel production which can be blocked by a Syn-specific antibody.
α-Synuclein overexpression causes cell death in a dopamingeric neuronal cell line
To investigate whether Syn overexpression leads to dopaminergic cell death in addition to oligomer formation and membrane conductance changes, MN9Dsyn cells were exposed to DOX for 1, 2 and 4 days and the reduction of 3-[4,5-Dimethylthiazol]-2,5-diphenyltetrazolium (MTT) was quantified. Here we demonstrate a modest but significant time-dependent increase in MN9Dsyn cell death following Syn induction [Fig. 5; mean percent cell death ± SEM from eight samples per group as compared to non-induced control (−DOX); ~3% after 1 day; ~6% following 2 days of Syn induction (one-way ANOVA and Tukey HSD post-hoc test, P = 0.004); ~8% following 4 days of Syn induction, (one-way ANOVA and Tukey HSD post-hoc test, P = 0.0003)].
Discussion
Syn is a natively unfolded protein which can readily form protofibrils/oligomers, fibrils and large aggregates following overexpression, exposure to changes in pH, oxidative stress, or by interaction with dopamine (Conway et al., 2001; Uversky et al., 2001; Shtilerman et al., 2002; Ischiropoulos, 2003; Maguire-Zeiss et al., 2006). Interestingly, in vitro data demonstrates that dopamine inhibits the conversion of Syn to the fibrillar form leading to an increase in the purported toxic/protofibillar conformation, which has been proposed to contribute to the selective vulnerability of SNpc DANs in PD (Conway et al., 2001). Emerging evidence supports a role for oligomeric Syn in PD substantia nigra pars compacta dopamine neuron (SNpc DAN) vulnerability. For example, point mutations in Syn, which are linked to autosomal dominant forms of familial PD, accelerate the formation of oligomers (Conway et al., 1998; 2000a; Volles & Lansbury, 2002). Several mechanisms have been proposed for oligomeric Syn-induced DAN toxicity including increased oxidative stress, changes in protein degradation and altered membrane permeability (Betarbet et al., 2000; Sharon et al., 2001; Volles et al., 2001; Gosavi et al., 2002; Lee et al., 2002; Hashimoto et al., 2003; Cuervo et al., 2004; Rochet et al., 2004; Fornai et al., 2005; Furukawa et al., 2006; Martin et al., 2006; Bellucci et al., 2008; Borland et al., 2008). One current hypothesis suggests that the formation of Syn oligomer-containing ring-like structures cause leakage in cell membranes resulting in a disruption of cellular ionic balance and ultimately cell death. Both wild-type and mutant Syn have been shown to form ring-like pores in synthetic vesicles and artificial lipid bilayer membranes (Goldberg & Lansbury, 2000; Volles & Lansbury, 2002; Danzer et al., 2007; Tsigelny et al., 2008). The experiments presented in this paper, however, are focused on the effect of intracellular Syn expression, subsequent transport to the cell membrane and attendant membrane permeability alterations in a model more akin to dopaminergic neurons, MN9D cells.
MN9D cells were derived from mouse embryonic ventral mesencephalic neurons and express tyrosine hydroxylase and dopamine transporter which are key features of SNpc DANs (Choi et al., 1991; Choi et al., 1992; Rick et al., 2006). Previously, we engineered the regulated gene expression of Syn and/or GFP in MN9D cells by placing SCNA under the direction of a tetracycline/doxcycline responsive autoregulated bi-directional promoter and GFP downstream of an internal ribosome entry site (Strathdee et al., 1999; Luo et al., 2007; Su et al., 2008). The co-expression of GFP allows for easy determination of Syn-expressing cells during whole cell patch clamp recordings. In addition, this dopaminergic milieu is critical since DANs are particularly vulnerable in PD and Syn conformation is affected by the microenvironment, including the presence of dopamine. Therefore, although MN9Dsyn cells do not completely recapitulate SNpc DAN they do allow us to examine the effects of Syn overexpression in a simplified dopaminergic environment.
We first determined in our model the presence of oligomeric Syn, Syn-immunopositive aggregates and membrane localized Syn following overexpression. We hypothesized that pores formed by Syn would act as non-selective leak channels and contribute to Syn-induced toxicity. Pores or leak channels allow ions to flow across the cytoplasmic membrane freely and result in an increase in membrane conductance. Whole cell patch clamp recordings of Syn overexpressing MN9D cells revealed increased membrane conductance that was not attributable to either voltage-gated potassium or sodium channels as they were inactivated by the presence of cesium chloride and a membrane holding potential at 0 mV. Using cultured HEK293T cells transduced with lentivirus-expressing wild-type Syn, Tsigelny et al. demonstrated an increase in Zn2+-sensitive whole cell currents (Tsigelny et al., 2007) while Furukawa et al. showed increased plasma membrane permeability and cell death in cultured cells overexpressing only mutant forms of Syn (Furukawa et al., 2006). Our data extend these previous findings by demonstrating Syn-induced cell death in concert with a voltage-independent increase in membrane conductance, reminiscent of leak channels. The lack of concordance between the Furukawa study and our findings, which comport with Tsigelny et al., could derive from multiple factors, such as the differences in the inherent properties of each cell line (i.e. plasma membrane properties and intracellular microenvironment) and/or variations in Syn protein expression levels. Significantly, we also demonstrate for the first time that the intracellular production of Syn leads concurrently to increased oligomeric Syn and membrane conductance changes that are blocked by local extracellular application of an anti-Syn antibody (Syn-1). Since this antibody detected only membrane-localized Syn (i.e. no detectable intracellular Syn) when tested under detergent-free conditions, it is likely that our antibody treatment dampened Syn-induced membrane conductance changes by interacting with Syn on the outer surface of MN9Dsyn cells. However, we do not know which amino acids are exposed and available for antibody interaction in our model. In vitro experiments demonstrate that depending on the curvature of the lipid membranes Syn may adopt a broken α-helix or even an extended α-helix conformation demonstrating the flexibility of the interactions between Syn and lipid membranes (Davidson et al., 1998; Chandra et al., 2003; Chandra et al., 2004; Bortolus et al., 2008; Georgieva et al., 2008; Jao et al., 2008). Little is known about the structure of Syn in situ and most efforts use anti-Syn antibodies that recognize linear epitopes in conjunction with purified protein, fixed cells or tissue. Previous mapping studies identified amino acid residues 91-99 as the Syn epitopes recognized by Syn-1 [the same antibody used in these studies; (Perrin et al., 2003; Li et al., 2005; Liu et al., 2005)]. Even though amino acids 1-99 are considered the lipid binding portion of Syn, it is possible that when Syn forms propagating multimers in the plasma membrane, structural residues become available for the Syn-1 antibody to bind (Tsigelny et al., 2007; Perlmutter et al., 2009). Importantly, the epitope mapping studies for this antibody used Syn deletions examined under denaturing conditions while little is known about the ability of this antibody to recognize native (nondenatured) Syn as would be present in our electrophysiological studies. In fact, the conformation of membrane or pore-like Syn in vivo is yet unknown.
Our data suggest that when Syn interacts with the cell membrane it assumes a topology in which a portion is exposed to the extracellular environment, perhaps through the formation of a pore, and is available for antibody binding. Most importantly this Syn:antibody interaction is sufficient to attenuate membrane conductance changes. Taken together the data presented here support the hypothesis that Syn overexpression results in increased oligomer production and the formation of membrane bound Syn-containing pores which may contribute to cell vulnerability. However, our data cannot exclusively attribute the observed changes to Syn oligomers. In order to establish a direct link between Syn oligomers and changes in membrane conductance conformer-specific antibodies need to be developed which can differentially identify structural forms of Syn in vivo.
In PD the SNpc DANs are particularly vulnerable and invariably die resulting in the classic clinical motoric features. It is interesting to speculate that Syn protofibrils, which are stabilized by the interaction with dopamine quinone, make DANs more susceptible to neurodegeneration. For instance, stable Syn protofibrils could result in an augmentation of pore-like structures in cell membranes resulting in membrane leakage and altered ionic balance engendering oxidative stress and finally cell death. In addition, Syn has been shown to interact with mitochondrial membranes and it is probable that Syn-induced leak channels in this organelle would lead to mitochondrial dysfunction (Nakamura et al., 2008; Parihar et al., 2008; Shavali et al., 2008). Our studies indicate that increased leak channel conductance occurs prior to substantial cell death suggesting that pore formation may be a fundamental event in dopamine cell vulnerability.
Future experiments will be aimed at evaluating membrane conductance changes in primary dopaminergic neurons and nigrostriatal slice cultures that overexpress Syn. These models will be utilized to extend the leak channel blocking effect of anti-Syn antibody therapies. Experimental hurdles currently prevent the identification of leak channels in PD subjects, however, the reduction of leak channel conductance in our cell culture model following extracellular application of an anti-Syn antibody suggests that pore blockers may represent a novel class of therapies for PD as well as other synucleinopathies including multiple system atrophy, dementia with Lewy bodies and pure autonomic failure.
Acknowledgements
We would like to thank Dr. Seung Lim for help with microscopy and Dr. Zhanyan Fu for help with electrophysiology. This work was supported by NIEHS (R01ES014470; KMZ).
Abbreviations
- DAN
dopamine neuron
- DOX
doxycycline
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- GFP
green fluorescent protein
- GWAS
genome-wide association studies
- IRES
internal ribosome entry site
- MTT
3-[4,5-Dimethylthiazol]-2,5-diphenyltetrazolium
- PD
Parkinson's disease
- SNpc
substantia nigra pars compacta
- SYN
human wildtype α-synuclein
References
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- Bellucci A, Collo G, Sarnico I, Battistin L, Missale C, Spano P. Alpha-synuclein aggregation and cell death triggered by energy deprivation and dopamine overload are counteracted by D2/D3 receptor activation. J Neurochem. 2008;106:560–577. doi: 10.1111/j.1471-4159.2008.05406.x. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Borland MK, Trimmer PA, Rubinstein JD, Keeney PM, Mohanakumar K, Liu L, Bennett JP., Jr. Chronic, low-dose rotenone reproduces Lewy neurites found in early stages of Parkinson's disease, reduces mitochondrial movement and slowly kills differentiated SH-SY5Y neural cells. Mol Neurodegener. 2008;3:21. doi: 10.1186/1750-1326-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolus M, Tombolato F, Tessari I, Bisaglia M, Mammi S, Bubacco L, Ferrarini A, Maniero AL. Broken helix in vesicle and micelle-bound alpha-synuclein: insights from site-directed spin labeling-EPR experiments and MD simulations. J Am Chem Soc. 2008;130:6690–6691. doi: 10.1021/ja8010429. [DOI] [PubMed] [Google Scholar]
- Chandra S, Chen X, Rizo J, Jahn R, Sudhof TC. A broken alpha -helix in folded alpha -Synuclein. J Biol Chem. 2003;278:15313–15318. doi: 10.1074/jbc.M213128200. [DOI] [PubMed] [Google Scholar]
- Chandra S, Fornai F, Kwon HB, Yazdani U, Atasoy D, Liu X, Hammer RE, Battaglia G, German DC, Castillo PE, Sudhof TC. Double-knockout mice for alpha- and beta-synucleins: effect on synaptic functions. Proc Natl Acad Sci U S A. 2004;101:14966–14971. doi: 10.1073/pnas.0406283101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HK, Won L, Roback JD, Wainer BH, Heller A. Specific modulation of dopamine expression in neuronal hybrid cells by primary cells from different brain regions. Proc Natl Acad Sci U S A. 1992;89:8943–8947. doi: 10.1073/pnas.89.19.8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HK, Won LA, Kontur PJ, Hammond DN, Fox AP, Wainer BH, Hoffmann PC, Heller A. Immortalization of embryonic mesencephalic dopaminergic neurons by somatic cell fusion. Brain Res. 1991;552:67–76. doi: 10.1016/0006-8993(91)90661-e. [DOI] [PubMed] [Google Scholar]
- Cole NB, Dieuliis D, Leo P, Mitchell DC, Nussbaum RL. Mitochondrial translocation of alpha-synuclein is promoted by intracellular acidification. Exp Cell Res. 2008;314:2076–2089. doi: 10.1016/j.yexcr.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4:1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- Conway KA, Harper JD, Lansbury PT., Jr. Fibrils formed in vitro from alpha-synuclein and two mutant forms linked to Parkinson's disease are typical amyloid. Biochemistry. 2000a;39:2552–2563. doi: 10.1021/bi991447r. [DOI] [PubMed] [Google Scholar]
- Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PT., Jr. Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson's disease: implications for pathogenesis and therapy. Proc Natl Acad Sci U S A. 2000b;97:571–576. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KA, Rochet JC, Bieganski RM, Lansbury PT., Jr Kinetic Stabilization of the alpha -Synuclein Protofibril by a Dopamine- alpha -Synuclein Adduct. Science. 2001;294:1346–1349. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- Crews L, Tsigelny I, Hashimoto M, Masliah E. Role of synucleins in Alzheimer's disease. Neurotox Res. 2009;16:306–317. doi: 10.1007/s12640-009-9073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- Danzer KM, Haasen D, Karow AR, Moussaud S, Habeck M, Giese A, Kretzschmar H, Hengerer B, Kostka M. Different species of alpha-synuclein oligomers induce calcium influx and seeding. J Neurosci. 2007;27:9220–9232. doi: 10.1523/JNEUROSCI.2617-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem. 2008;283:9089–9100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkon H, Don J, Melamed E, Ziv I, Shirvan A, Offen D. Mutant and wild-type alpha-synuclein interact with mitochondrial cytochrome C oxidase. J Mol Neurosci. 2002;18:229–238. doi: 10.1385/JMN:18:3:229. [DOI] [PubMed] [Google Scholar]
- Fasano M, Lopiano L. Alpha-synuclein and Parkinson's disease: a proteomic view. Expert Rev Proteomics. 2008;5:239–248. doi: 10.1586/14789450.5.2.239. [DOI] [PubMed] [Google Scholar]
- Fornai F, Lenzi P, Ferrucci M, Lazzeri G, di Poggio AB, Natale G, Busceti CL, Biagioni F, Giusiani M, Ruggieri S, Paparelli A. Occurrence of neuronal inclusions combined with increased nigral expression of alpha-synuclein within dopaminergic neurons following treatment with amphetamine derivatives in mice. Brain Res Bull. 2005;65:405–413. doi: 10.1016/j.brainresbull.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Matsuzaki-Kobayashi M, Hasegawa T, Kikuchi A, Sugeno N, Itoyama Y, Wang Y, Yao PJ, Bushlin I, Takeda A. Plasma membrane ion permeability induced by mutant alpha-synuclein contributes to the degeneration of neural cells. J Neurochem. 2006;97:1071–1077. doi: 10.1111/j.1471-4159.2006.03803.x. [DOI] [PubMed] [Google Scholar]
- Georgieva ER, Ramlall TF, Borbat PP, Freed JH, Eliezer D. Membrane-bound alpha-synuclein forms an extended helix: long-distance pulsed ESR measurements using vesicles, bicelles, and rodlike micelles. J Am Chem Soc. 2008;130:12856–12857. doi: 10.1021/ja804517m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MS, Lansbury PT., Jr. Is there a cause-and-effect relationship between alpha-synuclein fibrillization and Parkinson's disease? Nat Cell Biol. 2000;2:E115–119. doi: 10.1038/35017124. [DOI] [PubMed] [Google Scholar]
- Gosavi N, Lee HJ, Lee JS, Patel S, Lee SJ. Golgi fragmentation occurs in the cells with prefibrillar alpha-synuclein aggregates and precedes the formation of fibrillar inclusion. J Biol Chem. 2002;277:48984–48992. doi: 10.1074/jbc.M208194200. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Rockenstein E, Crews L, Masliah E. Role of protein aggregation in mitochondrial dysfunction and neurodegeneration in Alzheimer's and Parkinson's diseases. Neuromolecular Med. 2003;4:21–36. doi: 10.1385/NMM:4:1-2:21. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H. Oxidative modifications of alpha-synuclein. Ann N Y Acad Sci. 2003;991:93–100. doi: 10.1111/j.1749-6632.2003.tb07466.x. [DOI] [PubMed] [Google Scholar]
- Jao CC, Hegde BG, Chen J, Haworth IS, Langen R. Structure of membrane-bound alpha-synuclein from site-directed spin labeling and computational refinement. Proc Natl Acad Sci U S A. 2008;105:19666–19671. doi: 10.1073/pnas.0807826105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junn E, Mouradian MM. Human alpha-synuclein over-expression increases intracellular reactive oxygen species levels and susceptibility to dopamine. Neurosci Lett. 2002;320:146–150. doi: 10.1016/s0304-3940(02)00016-2. [DOI] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Lashuel HA, Petre BM, Wall J, Simon M, Nowak RJ, Walz T, Lansbury PT., Jr. Alpha-synuclein, especially the Parkinson's disease-associated mutants, forms pore-like annular and tubular protofibrils. J Mol Biol. 2002;322:1089–1102. doi: 10.1016/s0022-2836(02)00735-0. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Shin SY, Choi C, Lee YH, Lee SJ. Formation and removal of alpha-synuclein aggregates in cells exposed to mitochondrial inhibitors. J Biol Chem. 2002;277:5411–5417. doi: 10.1074/jbc.M105326200. [DOI] [PubMed] [Google Scholar]
- Li W, West N, Colla E, Pletnikova O, Troncoso JC, Marsh L, Dawson TM, Jakala P, Hartmann T, Price DL, Lee MK. Aggregation promoting C-terminal truncation of alpha-synuclein is a normal cellular process and is enhanced by the familial Parkinson's disease-linked mutations. Proc Natl Acad Sci U S A. 2005;102:2162–2167. doi: 10.1073/pnas.0406976102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CW, Giasson BI, Lewis KA, Lee VM, Demartino GN, Thomas PJ. A precipitating role for truncated alpha-synuclein and the proteasome in alpha-synuclein aggregation: implications for pathogenesis of Parkinson disease. J Biol Chem. 2005;280:22670–22678. doi: 10.1074/jbc.M501508200. [DOI] [PubMed] [Google Scholar]
- Luo Y, Henricksen LA, Giuliano RE, Prifti L, Callahan LM, Federoff HJ. VIP is a transcriptional target of Nurr1 in dopaminergic cells. Exp Neurol. 2007;203:221–232. doi: 10.1016/j.expneurol.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Maguire-Zeiss KA, Wang CI, Yehling E, Sullivan MA, Short DW, Su X, Gouzer G, Henricksen LA, Wuertzer CA, Federoff HJ. Identification of human alpha-synuclein specific single chain antibodies. Biochem Biophys Res Commun. 2006;349:1198–1205. doi: 10.1016/j.bbrc.2006.08.127. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Pan Y, Price AC, Sterling W, Copeland NG, Jenkins NA, Price DL, Lee MK. Parkinson's disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J Neurosci. 2006;26:41–50. doi: 10.1523/JNEUROSCI.4308-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Vicente M, Talloczy Z, Kaushik S, Massey AC, Mazzulli J, Mosharov EV, Hodara R, Fredenburg R, Wu DC, Follenzi A, Dauer W, Przedborski S, Ischiropoulos H, Lansbury PT, Sulzer D, Cuervo AM. Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J Clin Invest. 2008;118:777–788. doi: 10.1172/JCI32806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Totterdell S, Petroske E, Santa Cruz K, Callison RC, Jr., Lau YS. Lysosomal malfunction accompanies alpha-synuclein aggregation in a progressive mouse model of Parkinson's disease. Brain Res. 2002;956:156–165. doi: 10.1016/s0006-8993(02)03514-x. [DOI] [PubMed] [Google Scholar]
- Murase K, Ryu PD, Randic M. Excitatory and inhibitory amino acids and peptide-induced responses in acutely isolated rat spinal dorsal horn neurons. Neurosci Lett. 1989;103:56–63. doi: 10.1016/0304-3940(89)90485-0. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Nemani VM, Wallender EK, Kaehlcke K, Ott M, Edwards RH. Optical reporters for the conformation of alpha-synuclein reveal a specific interaction with mitochondria. J Neurosci. 2008;28:12305–12317. doi: 10.1523/JNEUROSCI.3088-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar MS, Parihar A, Fujita M, Hashimoto M, Ghafourifar P. Mitochondrial association of alpha-synuclein causes oxidative stress. Cell Mol Life Sci. 2008;65:1272–1284. doi: 10.1007/s00018-008-7589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar MS, Parihar A, Fujita M, Hashimoto M, Ghafourifar P. Alpha-synuclein overexpression and aggregation exacerbates impairment of mitochondrial functions by augmenting oxidative stress in human neuroblastoma cells. Int J Biochem Cell Biol. 2009;41:2015–2024. doi: 10.1016/j.biocel.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Periquet M, Fulga T, Myllykangas L, Schlossmacher MG, Feany MB. Aggregated alpha-synuclein mediates dopaminergic neurotoxicity in vivo. J Neurosci. 2007;27:3338–3346. doi: 10.1523/JNEUROSCI.0285-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter JD, Braun AR, Sachs JN. Curvature dynamics of alpha-synuclein familial Parkinson disease mutants: molecular simulations of the micelle- and bilayer-bound forms. J Biol Chem. 2009;284:7177–7189. doi: 10.1074/jbc.M808895200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin RJ, Payton JE, Barnett DH, Wraight CL, Woods WS, Ye L, George JM. Epitope mapping and specificity of the anti-alpha-synuclein monoclonal antibody Syn-1 in mouse brain and cultured cell lines. Neurosci Lett. 2003;349:133–135. doi: 10.1016/s0304-3940(03)00781-x. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Rick CE, Ebert A, Virag T, Bohn MC, Surmeier DJ. Differentiated dopaminergic MN9D cells only partially recapitulate the electrophysiological properties of midbrain dopaminergic neurons. Dev Neurosci. 2006;28:528–537. doi: 10.1159/000095115. [DOI] [PubMed] [Google Scholar]
- Rochet JC, Outeiro TF, Conway KA, Ding TT, Volles MJ, Lashuel HA, Bieganski RM, Lindquist SL, Lansbury PT. Interactions Among alpha-Synuclein, Dopamine, and Biomembranes: Some Clues for Understanding Neurodegeneration in Parkinson's Disease. J Mol Neurosci. 2004;23:23–34. doi: 10.1385/jmn:23:1-2:023. [DOI] [PubMed] [Google Scholar]
- Satake W, Nakabayashi Y, Mizuta I, Hirota Y, Ito C, Kubo M, Kawaguchi T, Tsunoda T, Watanabe M, Takeda A, Tomiyama H, Nakashima K, Hasegawa K, Obata F, Yoshikawa T, Kawakami H, Sakoda S, Yamamoto M, Hattori N, Murata M, Nakamura Y, Toda T. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson's disease. Nat Genet. 2009;41:1303–1307. doi: 10.1038/ng.485. [DOI] [PubMed] [Google Scholar]
- Sharon R, Goldberg MS, Bar-Josef I, Betensky RA, Shen J, Selkoe DJ. alpha-Synuclein occurs in lipid-rich high molecular weight complexes, binds fatty acids, and shows homology to the fatty acid-binding proteins. Proc Natl Acad Sci U S A. 2001;98:9110–9115. doi: 10.1073/pnas.171300598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavali S, Brown-Borg HM, Ebadi M, Porter J. Mitochondrial localization of alpha-synuclein protein in alpha-synuclein overexpressing cells. Neurosci Lett. 2008;439:125–128. doi: 10.1016/j.neulet.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin TM, Isas JM, Hsieh CL, Kayed R, Glabe CG, Langen R, Chen J. Formation of soluble amyloid oligomers and amyloid fibrils by the multifunctional protein vitronectin. Mol Neurodegener. 2008;3:16. doi: 10.1186/1750-1326-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtilerman MD, Ding TT, Lansbury PT., Jr. Molecular crowding accelerates fibrillization of alpha-synuclein: could an increase in the cytoplasmic protein concentration induce Parkinson's disease? Biochemistry. 2002;41:3855–3860. doi: 10.1021/bi0120906. [DOI] [PubMed] [Google Scholar]
- Simon-Sanchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, Paisan-Ruiz C, Lichtner P, Scholz SW, Hernandez DG, Kruger R, Federoff M, Klein C, Goate A, Perlmutter J, Bonin M, Nalls MA, Illig T, Gieger C, Houlden H, Steffens M, Okun MS, Racette BA, Cookson MR, Foote KD, Fernandez HH, Traynor BJ, Schreiber S, Arepalli S, Zonozi R, Gwinn K, van der Brug M, Lopez G, Chanock SJ, Schatzkin A, Park Y, Hollenbeck A, Gao J, Huang X, Wood NW, Lorenz D, Deuschl G, Chen H, Riess O, Hardy JA, Singleton AB, Gasser T. Genome-wide association study reveals genetic risk underlying Parkinson's disease. Nat Genet. 2009;41:1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Strathdee CA, McLeod MR, Hall JR. Efficient control of tetracycline-responsive gene expression from an autoregulated bi-directional expression vector. Gene. 1999;229:21–29. doi: 10.1016/s0378-1119(99)00045-1. [DOI] [PubMed] [Google Scholar]
- Su X, Federoff HJ, Maguire-Zeiss KA. Mutant alpha-Synuclein Overexpression Mediates Early Proinflammatory Activity. Neurotox Res. 2009;16:238–254. doi: 10.1007/s12640-009-9053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Maguire-Zeiss KA, Giuliano R, Prifti L, Venkatesh K, Federoff HJ. Synuclein activates microglia in a model of Parkinson's disease. Neurobiol Aging. 2008;29:1690–1701. doi: 10.1016/j.neurobiolaging.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsigelny IF, Bar-On P, Sharikov Y, Crews L, Hashimoto M, Miller MA, Keller SH, Platoshyn O, Yuan JX, Masliah E. Dynamics of alpha-synuclein aggregation and inhibition of pore-like oligomer development by beta-synuclein. Febs J. 2007;274:1862–1877. doi: 10.1111/j.1742-4658.2007.05733.x. [DOI] [PubMed] [Google Scholar]
- Tsigelny IF, Crews L, Desplats P, Shaked GM, Sharikov Y, Mizuno H, Spencer B, Rockenstein E, Trejo M, Platoshyn O, Yuan JX, Masliah E. Mechanisms of hybrid oligomer formation in the pathogenesis of combined Alzheimer's and Parkinson's diseases. PLoS One. 2008;3:e3135. doi: 10.1371/journal.pone.0003135. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Uversky VN, Li J, Fink AL. Evidence for a partially folded intermediate in alpha-synuclein fibril formation. J Biol Chem. 2001;276:10737–10744. doi: 10.1074/jbc.M010907200. [DOI] [PubMed] [Google Scholar]
- Volles MJ, Lansbury PT., Jr. Vesicle permeabilization by protofibrillar alpha-synuclein is sensitive to Parkinson's disease-linked mutations and occurs by a pore-like mechanism. Biochemistry. 2002;41:4595–4602. doi: 10.1021/bi0121353. [DOI] [PubMed] [Google Scholar]
- Volles MJ, Lansbury PT., Jr. Zeroing in on the pathogenic form of alpha-synuclein and its mechanism of neurotoxicity in Parkinson's disease. Biochemistry. 2003;42:7871–7878. doi: 10.1021/bi030086j. [DOI] [PubMed] [Google Scholar]
- Volles MJ, Lee SJ, Rochet JC, Shtilerman MD, Ding TT, Kessler JC, Lansbury PT., Jr. Vesicle permeabilization by protofibrillar alpha-synuclein: implications for the pathogenesis and treatment of Parkinson's disease. Biochemistry. 2001;40:7812–7819. doi: 10.1021/bi0102398. [DOI] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhou Y, Hong JS, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson's disease. Faseb J. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]