Abstract
The effects of stressful life experience on learning are pervasive and vary greatly both within and between individuals. It is therefore unlikely that any one mechanism will underlie these complicated processes. Nonetheless, without identifying the necessary and sufficient circuitry, no complete mechanism or set of mechanisms can be identified. In this review, we provide two anatomical frameworks through which stressful life experience can influence processes related to learning and memory. In the first, stressful experience releases stress hormones, primarily from the adrenals, which directly impact brain areas engaged in learning. In the second, stressful experience indirectly alters the circuits used in learning via intermediary brain regions. Importantly, these intermediary brain regions are not integral to the stress response or learning itself, but rather link the consequences of a stressful experience with circuits used to learn associations. As reviewed, the existing literature provides support for both frameworks, with somewhat more support for the first but sufficient evidence for the latter which involves intermediary structures. Once we determine the circumstances that engage each framework and identify which framework is most predominant, we can begin to focus our efforts on describing the neuronal and hormonal mechanisms that operate within these circuits to influence cognitive processes after stressful life experience.
Indexing terms: Associative learning, stress, amygdala, hippocampus, bed nucleus of the stria terminalis, post-traumatic stress disorder, depression, eyeblink conditioning, sex difference
Introduction
It is not surprising that stressful life events can affect processes of learning and memory. In fact, it is easy to think of many everyday examples wherein a stressful experience alters our ability to acquire or remember new information. In some situations, stress impairs learning. For example, one often forgets the names associated with faces while nervously attending a social event. However, in other situations, stress increases our ability to learn and remember, as may occur when one is asked to recall the details of a car accident or personal trauma. After an extremely stressful event, some people develop enduring psychopathology. The most poignant example is post-traumatic stress disorder (PTSD), a disease marked by ruminations or flashbacks of the trauma which prevent the person from leading a healthy productive life. The neuronal and hormonal markers of stress have been studied for decades and numerous important reviews have been presented. These reviews have focused on the specific biological consequences of stress and how they relate to learning and memory (e.g., Conrad, 2008; Hains and Arnsten, 2008; Howland and Wang, 2008; Joels et al., 2006; Lupien et al., 2005; Lupien and Lepage, 2001; Sandi and Pinelo-Nava, 2007). In this review, we instead focus on the overall anatomical framework through which stressful life experience can modify processes of learning and memory. In doing so, we propose two models. In the first more traditional model, exposure to a stressful event initiates the stress response, which results in the release of stress-related hormones. These hormones, via their receptors, act directly on the circuitry used to form, store, and/or retrieve memories. In the second model, exposure to a stressful event indirectly modulates learning circuitry through intermediary brain regions. These so-called “intermediary structures” are not necessary for initiating the stress response or for learning in and of themselves, but are capable of enhancing or impairing learning by influencing activity in distant brain regions used in the learning process. This review will provide evidence for both models and describe how each may improve our understanding of the mechanisms associated with stress and learning. Lastly, these models may help lay the groundwork for developing more effective treatments for humans suffering from stress-related psychiatric disorders.
Various effects of stress on learning
Before addressing the models, it should be noted that stress does not always have the same effect on learning and memory. For example, sometimes stress enhances learning, while other times stress impairs learning. Properties of the stressor itself, such as intensity, are important, as is its duration (Cordero et al., 1998; Diamond, 2005; Joels, 2006; Sandi and Pinelo-Nava, 2007). In general, longer duration stressors (i.e., chronic) tend to result in memory impairments (Conrad et al., 1996; Joels et al., 2004; Luine et al., 1996; McEwen, 2005). The source of the stressor is also important (Sandi and Pinelo-Nava, 2007). When the act of training is intrinsically stressful, as it is during fear conditioning, the learning process tends to be facilitated by stressful experience. However, when the training is not as stressful or the stressful experience occurs at a time distant from training, the consequences become less predictable (Sandi and Pinelo-Nava, 2007). The stage of the learning process is also important. Stressful experiences tend to enhance processes related to acquisition but often impair those related to recall (Roozendaal, 2002, 2003). Finally, demographic factors, such as sex and age, can alter the way stress modulates learning (Jackson et al., 2006; Lupien et al., 2005; Shors, 2006; Zorawski et al., 2005). Regardless of these many variables, most published studies implicate similar brain regions at the intersection between stress and learning. These regions include but are not limited to the hippocampus, amygdala, and prefrontal cortex. Thus, it would appear that the degree to which stress affects learning and the direction of that effect does not necessarily result from differences in brain circuitries, but rather from differences in physiological and cellular processes within the same or similar circuitries. It is under this premise that we propose the two general anatomical frameworks.
Stress hormones modulate learning and memory
There is a large and overwhelming literature indicating that glucocorticoids (cortisol in humans and corticosterone in rodents) modulate processes related to learning and memory. These stress hormones are released from the adrenal glands following activation of the hypothalamic-pituitary-adrenal (HPA) axis, after which they enter the brain to act on their respective receptors. One clear example of such hormonal modulation is Cushing’s syndrome. People with this disease release excessive amounts of cortisol, and as a consequence, they have difficulty learning and performing during training on various cognitive tasks (Starkman et al., 1992; Starkman et al., 2001). The types of learning that are affected include declarative memory and other tasks such as, visual-spatial tests and trace conditioning (Grillon et al., 2004; Starkman et al., 1992; Starkman et al., 2001). Importantly, the learning deficits expressed by Cushing’s syndrome patients can be reversed when the cortisol concentrations are managed within a normal physiological range (Starkman et al., 2003). Thus, the learning deficits are likely mediated by the presence of excessive amounts of endogenous glucocorticoids. In healthy humans, the release of stress hormones from the adrenals can also influence processes of learning, again typically expressed as deficits in declarative memory. For example, high concentrations of glucocorticoids as well as stressful manipulations elicit poor retrieval of declarative information in healthy participants (Kirschbaum et al., 1996; Kuhlmann et al., 2005; Maheu et al., 2004). In rodents, exposure to either chronic or acute stressors tends to impair the recall of spatial memories (Conrad et al., 1996; Diamond et al., 1999), although there are also a number of reports showing that stress or stress hormones can enhance learning and/or memory. For instance, exposure to either an acute or a chronic stressor enhances an animal’s ability to remember the context associated with a stressful stimulus such as a foot shock, a type of learning referred to as contextual fear conditioning (Conrad et al., 1999; Cordero et al., 2003a; Cordero et al., 2003b; Sandi et al., 2001). Many of these effects are mediated by corticosterone. For example, injecting corticosterone peripherally enhances the acquisition of a classically conditioned eyeblink response, in which an animal learns to associate an auditory stimulus with an aversive stimulation to the eyelid (Beylin and Shors, 2003). If the training conditions themselves are intrinsically stressful learning can be affected, such that animals trained in a cold water maze task learn better than those trained in warmer water (Conboy and Sandi, 2009; Sandi et al., 1997). The enhanced learning and memory after training in colder water is mediated by the presence of glucocorticoids, as is the increase in classical eyeblink conditioning that occurs after exposure to an acute stressful event (Beylin and Shors, 2003; Conboy and Sandi, 2009) (Fig. 1). Thus, glucocorticoids play a central role in regulating learning after stressful life experience.
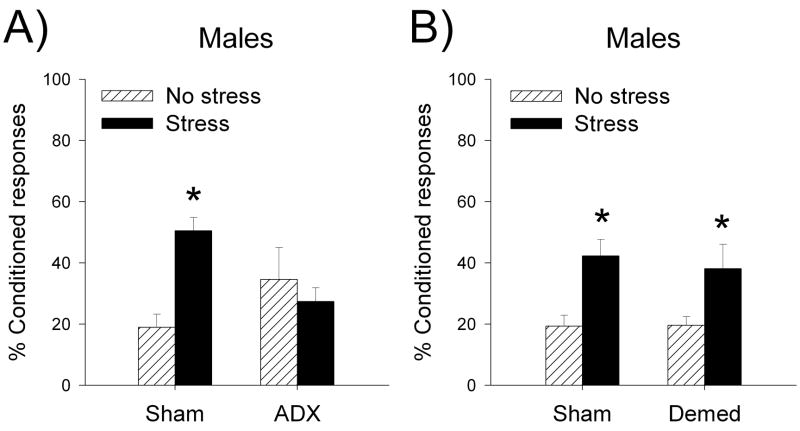
Figure 1. Adrenalectomy, but not demedullation, prevents the stress-induced enhancement of hippocampal dependent trace eyeblink conditioning (Beylin and Shors, 2003).
Stress increased the percentage of CRs emitted in sham operated but not in adrenalectomized (ADX) male rats (A). Note that basal levels of corticosterone were provided in drinking water. Demedullation (Demed), which leaves the adrenal cortex and corticosterone production intact, while removing adrenal catecholamine production, failed to prevent the stress-induced enhancement of trace conditioning, indicating that these effects are specific to corticosterone (B). Data are represented as Mean ± SEM percentage of CRs averaged across 300 training trials. Asterisks indicate a significant difference (p<0.05).
Model 1: Stressful experience directly affects learning circuitry via stress hormones
Structures required for learning contain stress hormone receptors
The above examples link stress and stress hormones, particularly glucocorticoids, with altered learning and memory processes. Based on these studies and others, a model has been proposed which assumes that stress affects learning directly by acting on brain regions used for learning and memory itself (Fig. 2a). The learning circuit most often includes the hippocampus because it is necessary for many of the types of learning that are affected by stress, including declarative and spatial memory tasks. Also, for decades it has been well documented that there is a high concentration of corticosterone and density of its receptors within the hippocampus (McEwen et al., 1968; Veldhuis et al., 1982). From these findings, the hippocampus is considered a primary site for regulating learning after stressful experience. However, other regions, such as the amygdala and the prefrontal cortex, have been implicated (Patel et al., 2008; Patel et al., 2000; Sarrieau et al., 1986). Like the hippocampus, these brain regions are necessary for various types of learning that are affected by stress, and they also possess receptors for glucocorticoids and other stress-related compounds such as corticotropin-releasing factor (CRF) and norepinephrine (Arnsten, 1997; Gray and Bingaman, 1996; Roozendaal et al., 2002).
Figure 2. This is a schematic representation of the two models of stress and learning interactions.
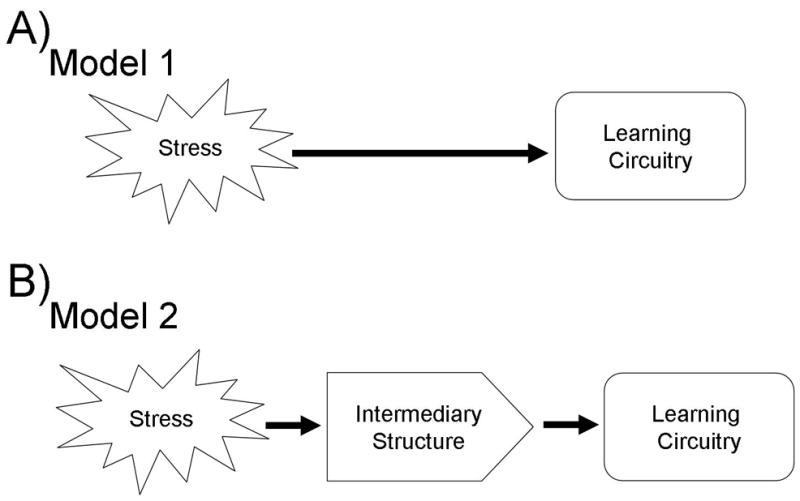
In model 1 stress hormones directly impact learning circuitry (A). In model 2 stress hormones act via intermediary structures to impact learning circuitry (B).
Stressful experience induces physiological, morphological, and cellular changes in learning circuitry
The fact that many stress compounds and their receptors are located in regions involved in learning has led to the general hypothesis that stress hormones and neurotransmitters act directly on learning circuitry to modify processes of learning and memory. The hypothesis is strengthened by overwhelming evidence for physiological, morphological, and cellular changes within those structures as a result of a stressful experience. For example, exposure to an acute stressful experience and peripheral exposure to exogenous glucocorticoids persistently decreases the expression of long-term potentiation (LTP)—a form of synaptic plasticity often promoted as a model of learning in the mammalian brain—in the hippocampus and amygdala (Kavushansky et al., 2006; Shors et al., 1989; Smriga et al., 1996). Furthermore, acute stress increases neuronal excitability in the hippocampus, which is also associated with enhanced learning (Weiss et al., 2005). Stress and glucocorticoid release also alter the production of new neurons in the hippocampus (Gould and Tanapat, 1999; Mirescu and Gould, 2006), and these new cells have been linked to some processes of learning (Leuner et al., 2006; Shors, 2009; Shors et al., 2001b). Additionally, stress can modify the morphology of dendrites in the hippocampus as well as the prefrontal cortex (Radley et al., 2004; Watanabe et al., 1992). In the hippocampus, chronic stress induces dendritic retraction within the CA3 region, an effect associated with deficits in performance on a spatial learning procedure (Lupien et al., 2005; Watanabe et al., 1992; Wright and Conrad, 2005). Additionally, chronic stress induces dendritic retraction and decreases volumetric measurements in the prefrontal cortex (Cerqueira et al., 2007; Radley et al., 2004; Wellman, 2001). These changes are accompanied by deficits in working memory, behavioral flexibility, and attentional set shifting (Cerqueira et al., 2007; Liston et al., 2006). Chronic stress also alters frontostriatal circuits, which then changed decision-making strategies (Dias-Ferreira et al., 2009). At a more reduced level, dendritic spines in these brain regions have been implicated in stress/learning interactions. Acute stressor exposure changes the density of spines on apical dendrites in the CA1 region of the hippocampus in a sex-specific manner (Leuner and Shors, 2004; Shors et al., 2001a; Shors et al., 2004) (Fig. 3). Whereas stress increases the density of dendritic spines in the male hippocampus, it decreases density in the female hippocampus. These changes in spine density are consistent with the effects of stress on learning, as assessed with trace eyeblink conditioning, a task that requires the hippocampus (Leuner and Shors, 2004; Wood and Shors, 1998). Thus, the animals that have more dendritic spines after a stressful experience tend to learn faster, whereas those that have fewer spines tend to be learning impaired. It has been suggested that the presence of dendritic spines provide a biological substrate for rapid encoding of associations after stressful experience and learning in general (Leuner and Shors, 2004).
Figure 3. Opposite effects on stress on hippocampal-dependent trace conditioning and dendritic spines in the hippocampus in males vs. females (Shors et al., 2001a; (Waddell et al., 2008).
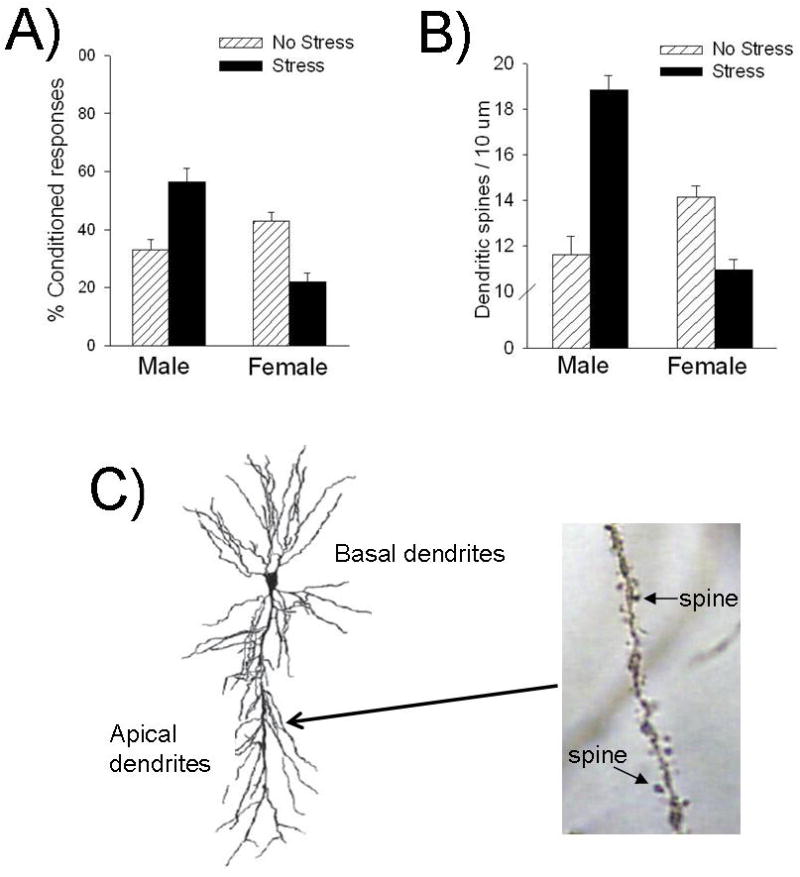
Under unstressed conditions, females emit more CRs than males. However, stressor exposure enhances trace conditioning in males, but impairs it in females (A). Similarly under unstressed conditions, females in proestus have greater spin density than males. Stressful experience increases spine density in males, while it decreases spine density in females (B). The altered spine density following stress is observed on apical dendrites of CA1 pyramidal neurons (C).
In addition to anatomical substrates, molecular responses to stress are likewise prevalent in brain regions that are involved in learning and memory. Stress and stress hormones alter the expression of receptors within these structures, and these alterations in turn influence learning ability. For example, acute stressful experience increases the expression of both AMPA and NMDA receptor subunits in pyramidal neurons of the prefrontal cortex which thereby increases glutamatergic transmission (Yuen et al., 2009). This same stressor facilitates performance on working memory tasks, which depend on the prefrontal cortex (Yuen et al., 2009). The increase in neurotransmission and the increase in working memory are both mediated by activation of glucocorticoid receptors (Yuen et al., 2009). Another cellular mechanism implicated in stress/learning interactions is neural cell adhesion molecule (NCAM). Chronic restraint stress decreases NCAM expression in the hippocampus (Sandi et al., 2001; Venero et al., 2002), and mice without hippocampal NCAM have difficulty learning a spatial orientation task (Bukalo et al., 2004). Thus, a decrease in NCAM in the hippocampus may impair learning directly because the hippocampus is necessary for this type of learning (Bisaz et al., 2009; Sandi, 2004). Similarly, MAPK has been implicated in stress/learning interactions. Revest et al. (2005) reported blocking MAPK activation specifically within the hippocampus prevented the enhancement of contextual fear in response to glucocorticoids (Revest et al., 2005). Thus, it would appear that specific molecular events within the hippocampus itself are necessary to enhance learning in response to glucocorticoids. Overall, these studies (and others not discussed here) support the first model, which proposes that stress hormones act directly on molecular and cellular processes within brain structures that are used for learning itself.
Evaluation of evidence for direct effect of stress hormones on learning circuitry
Although there is much support for this model, there are some exceptions. For one, it is well established that stressful experience impairs the induction of LTP in the hippocampus (Shors et al., 1989; Smriga et al., 1996). Because LTP is often considered a physiological model (if not a mechanism) for learning, it would seem reasonable that stress would impair learning that depends on the hippocampus. But it does not always do so. In fact, stress tends to enhance trace eyeblink conditioning, which depends on the hippocampus for learning (Shors and Matzel, 1996; Shors et al., 1992). As another example, stressful experience reduces the number of new cells that are produced in the hippocampus but again tends to enhance rather than impair learning which is associated with those new cells (Leuner et al., 2004; Shors et al., 2007). Minimally, these two examples rule out any overarching rule indicating that the modulation of learning is necessarily mediated by an observed change in response to stress at the neurophysiological or cellular level. That said, we have so far discussed several lines of evidence which suggest that stress does modulate learning via the direct actions of stress hormones on learning circuitry. First, structures involved in learning contain stress hormones receptors, which make direct effects of hormones possible. Second, exposure to a stressful event induces physiological, morphological, and cellular changes within regions critical for learning, and these changes often mirror the effects on learning (e.g., decreases in dendritic spines are associated with impaired learning and vice versa).
Although compelling, these lines of evidence do not directly test the model that stress hormones act within specific brain regions to alter mnemonic processes. Establishing a direct and causal connection is difficult and in some cases impossible because of technical limitations. There are not many, if any, methods to transiently and selectively prevent morphological changes that occur as a result of stress. Brain lesions or inactivation techniques are also ineffective if the brain region is necessary for learning. However, other techniques do exist and are being used. With remarkable success, drugs that mimic or block stress hormones are being injected directly into brain regions that are used during learning tasks that are intrinsically stressful, like fear conditioning and passive avoidance (e.g., Donley et al., 2005; Ferry and McGaugh, 1999, 2000; Ji et al., 2003; Liang et al., 1986; McGaugh, 2004; Mueller et al., 2008; Rodrigues et al., 2009; Yang et al., 2006). Other studies have used local drug infusions to examine how stress or stress hormones modulate hippocampal-dependent learning. Roozendaal et al. (2003) found that infusing a glucocorticoid agonist into the hippocampus impaired the recall of spatial memory. As noted, Revest et al. (2005) reported that a decrease in MAPK activation in the hippocampus prevented an increase in contextual fear in response to glucocorticoids (Revest et al., 2005). These studies suggest that stress hormones directly modify activity within the hippocampus to influence learning. However, not all of the aforementioned effects have been examined with local drug infusions, so it remains to be determined whether these same principles hold in all instances.
Model 2: Stress hormones affect learning circuitry via intermediary structures
In the second proposed model, we suggest that stressful experience affects learning indirectly though intermediary structures (Fig. 2b). These intermediary structures are not required for stress responses or learning itself, but instead link the consequences of a stressful experience with a specific learning circuitry. Recent work from our laboratory has identified the amygdala, hippocampus, and bed nucleus of the stria terminalis (BNST) as critical components within the circuit. In the standard version of these experiments, adult rats are exposed to an acute stressor of restraint and periodic low-intensity tailshocks or swim stress for 30 minutes (Servatius and Shors, 1994; Shors, 2001; Shors et al., 1992). Twenty-four hours later, rats are trained for the first time on a classical eyeblink conditioning task in which a white noise conditioned stimulus (CS) precedes and predicts a periorbital eyelid stimulation, the unconditioned stimulus (US). After many training trials, the rats learn to emit an eyeblink response in anticipation of the US, a response referred to as the conditioned response (CR). The number of CRs emitted over trials is an indirect measure of an animal’s ability to associate the CS with the US and correctly time the CR within milliseconds of the US. Using this procedure, the acute stressful event reliably alters learning and does so very differently in male vs. female rats. In all male species tested (rats, mice, and humans), acute stressor exposure enhances subsequent eyeblink conditioning (Duncko et al., 2007; Shors et al., 1992; Weiss et al., 2005), whereas the same stressful event reduces and in most instances, prevents learning in females (Wood et al., 2001; Wood and Shors, 1998). This phenomenon has been reviewed elsewhere (Shors, 2004, 2006) and will be discussed here only as it relates to brain circuitry.
The learning circuit used to associate the CS with the US in eyeblink conditioning is well characterized and includes the cerebellum (Christian and Thompson, 2003; Mauk and Thompson, 1987; Thompson, 2005). Specifically, mossy fibers from the pontine nucleus and climbing fibers from the inferior olive carry information about the CS and US respectively to the interpositus nucleus of the cerebellum, wherein the plasticity occurs. From there, efferents from the interpositus project to motor areas necessary for generating the CR. During a slightly different version of the task, a trace interval (or temporal gap) is placed between the CS and the US. When trained under these conditions, animals without a hippocampus cannot learn (Bangasser et al., 2006; Beylin et al., 2001; Solomon et al., 1986). Moreover, neuronal activity in the hippocampus predicts the acquisition of the learned response during trace conditioning (McEchron and Disterhoft, 1999). Thus, the cerebellum and its brainstem connections are necessary to learn both the delay and trace conditioned response, whereas the hippocampus is not necessary to learn the delay response.
The hippocampus as an intermediary structure
Because the circuitry is known, eyeblink conditioning can be used to identify intermediary structures that potentially link stress with learning. Recently, we demonstrated that the hippocampus is used in this way to connect a stressful event with learning. We were able to test this hypothesis because the hippocampus is not necessary for the simple delay version of the task, though performance of this task is still modulated by stress. Males and females were given complete hippocampal lesions and then tested for the effects of stress on learning (Bangasser and Shors, 2007) (Fig. 4). These lesions prevented both the enhanced conditioning after stress in males, as well as the deficit in females after stress. Importantly, neither learning itself nor the corticosterone response to stress was disrupted by the lesion procedure (Bangasser and Shors, 2007). Thus, the role of the hippocampus in learning and HPA activation was dissociated from its role in the modulation of learning by stress. To our knowledge, this was the first lesion study to demonstrate that the hippocampus can serve as an intermediary structure that links stressful experience with learning circuitry.
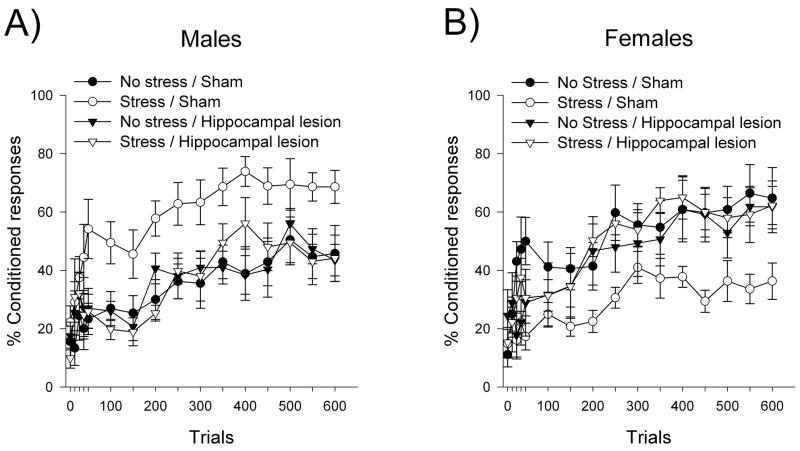
Figure 4. The hippocampus is required for stress to modulate learning, even when learning itself is independent of the hippocampus (Bangasser and Shors, 2007).
Hippocampal lesions prevented the stress-induced enhancement of delay eyeblink conditioning in male rats (A) and the stress-induced impairment of conditioning in female rats (B). Data are represented as Mean ± SEM percentage of CRs over 600 training trials (150 trials per day).
The amygdala as an intermediary structure
Studies suggest that the basolateral nucleus of the amygdala (BLA) is also an intermediary brain structure between stress and learning. Rodriguez Manzanares et al. (2005) found that blocking BLA activity during stress prevented the subsequent enhancement of contextual fear conditioning (Rodriguez Manzanares et al., 2005). Similarly, Waddell et al. (2008) found that neuronal activity within the BLA during the stressor was necessary in order for male rats to express the enhanced learning after stress, as well as for females to express a deficit in performance (Fig. 5). Excitatory neuronal activity within the BLA was temporarily prevented with a GABA agonist during the stressor, and animals were trained the next day. Because training occurred in the absence of the compound, the treatment could not have altered the learning process itself. It also does not disrupt the release of corticosterone during the stressor (Kim et al., 2005). These results support the idea that the BLA serves as an intermediary structure linking the consequences of stressful experience with the anatomical circuitry used to acquire new information.
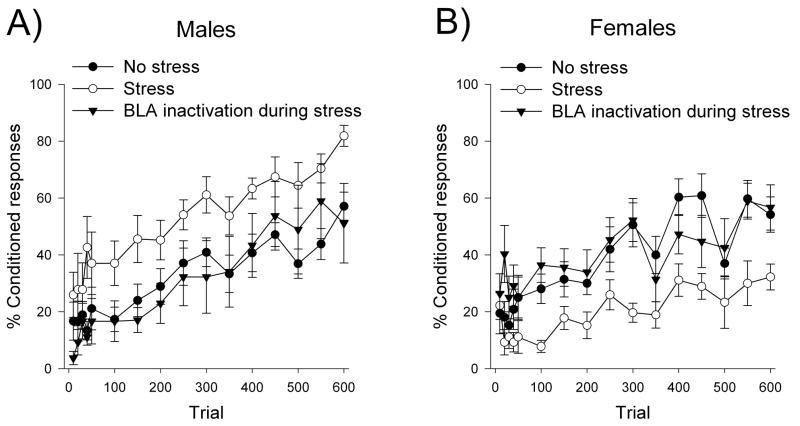
Figure 5. Activation of the amygdala during stressor exposure is required for stress to modulate learning (Waddell et al., 2008).
Temporary inactivation of the amygdala during stressor exposure prevented enhanced conditioning in males (A) and impaired conditioning in female rats (B). Data are represented as Mean ± SEM percentage of CRs over 600 training trials (150 trials per day).
The bed nucleus of the stria terminalis as an intermediary structure
The bed nucleus of the stria terminalis (BNST) has long been associated with stress and anxiety, and more recently with processes of learning and memory (Casada and Dafny, 1991; Davis and Shi, 1999; Davis et al., 1997). Because it is the major output structure from the amygdala, it was predicted that the BNST might also be an intermediary structure to link stressful experience with learning (Krettek and Price, 1978; Weller and Smith, 1982). Indeed, permanent lesions of the BNST prevent the enhanced trace eyeblink conditioning that occurs after exposure to a stressful experience in male rats (Bangasser et al., 2005). However, the BNST is only necessary during specific time periods. Using a temporary inactivation technique, we found that inactivation of the BNST during the stressful event did not prevent the enhancement of conditioning. Only inactivation during training was effective (Bangasser et al., 2005). Importantly, BNST inactivation did not disrupt the HPA stress response or conditioning itself. Thus, these data indicate that the BNST acts as an intermediary structure that mediates the lasting effects of stress on conditioning. Interestingly enough, the BNST is not critical under all conditions – or at least not in all animals. Females, which express a profound learning deficit after stress, were unaffected by the BNST inactivation procedure (Bangasser and Shors, 2008) (Fig. 6a). Thus, unlike in males, the BNST does not mediate the effect of stress on conditioning in females.
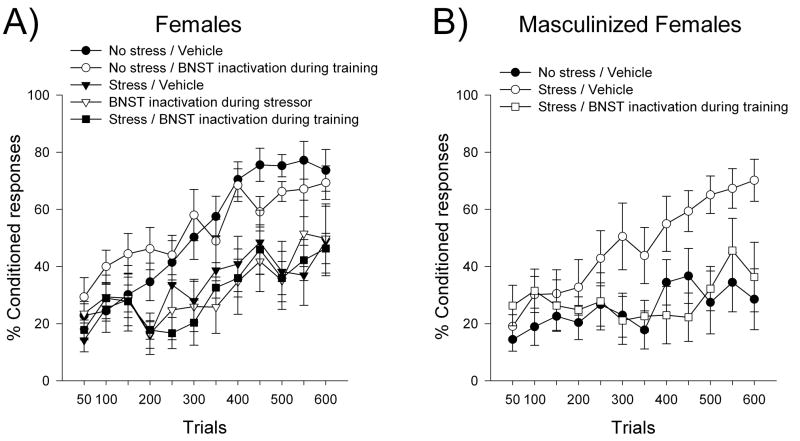
Figure 6. The BNST is required for stress effects on learning in masculinized, but not in cycling (i.e., normal) female rats (Bangasser et al., 2005).
In cycling females, BNST inactivation at any timepoint failed to prevent impaired conditioning (A). Just like in males, masculinized females require their BNST during training for enhanced conditioning after stress (B). Data are represented as Mean ± SEM percentage of CRs over 600 training trials (150 trials per day).
To our knowledge, a specific stress/learning circuit in one sex and not the other is unprecedented. However, given the sexually dimorphic nature of the structure, one might have predicted this outcome. The BNST is masculinized by a perinatal surge in testosterone, which increases its volume and changes its neurochemical profile (del Abril et al., 1987; Han and De Vries, 2003). This same perinatal surge organizes the stress effect on classical eyeblink conditioning (Shors and Miesegaes, 2002). Thus, females that are exposed to testosterone at birth behave like males as adults, i.e. they learn better after the stressor. Moreover, these same females now require activity within the BNST to express the enhancement in learning (Bangasser and Shors, 2008) (Fig. 6b). These results indicate that a masculinized BNST, rather than a feminized one, is required for stress to enhance acquisition of this simple associative response. Notably, a loss of BNST activity did not cause masculinized females or males to respond with a learning deficit, as observed in cycling females. In other words, inactivation of the BNST did not feminize the male response. Note that we did not provide estrogen to masculinized females or males, but the data nonetheless suggest that brain regions other than the BNST are being engaged by females to impair learning after a stressful event. Overall, these studies demonstrate that males and females use different brain regions to modulate learning after a stressful event. Moreover, they underscore the importance of considering sex when identifying the critical brain circuitry used for a given behavioral response.
Intermediary structures at the intersection between stress and learning
In this model system, we have determined that the hippocampus, amygdala, and BNST are necessary to modulate learning after stress. How they interact with one another to do so remains unknown. Each of these brain regions projects to the cerebellar eyeblink conditioning circuit via monosynaptic or polysynaptic connections (Fig. 7). The hippocampus sends afferents to the subiculum, which then projects via the retrosplenial cortex to the pontine nuclei that are critical for CS processing (Berger et al., 1986). The BLA projects via the central nucleus of the amygdala (CeA) to both the lateral tegmental field of the brainstem and the pontine nuclei, parts of the US and CS pathways, respectively (Krettek and Price, 1978; Steinmetz et al., 1987; Tracy et al., 1998; Whalen and Kapp, 1991). The BNST can affect the cerebellar eyeblink circuitry via direct afferents to the pontine nuclei (Holstege et al., 1985). Thus, the three brain regions that we know to be involved at some level in the stress/learning interaction (the amygdala, hippocampus and BNST) interact with the eyeblink circuitry. They could do so independently of one another, but it seems more likely that they form an elaborate network that is used to more generally monitor stressful experiences, remember that experience and relate it to future learning opportunities. One network might begin in the hippocampus, which projects to the BLA via the entorhinal cortex and the subiculum (Aggleton et al., 1987; Canteras and Swanson, 1992). The hippocampus and BLA project to the BNST via the fimbria/fornix and CeA, respectively. In this case, the BNST would serve as a final output to eyeblink conditioning circuitry, at least in males (Cullinan et al., 1993; Krettek and Price, 1978; Weller and Smith, 1982). Alternatively, the network may involve the retrosplenial cortex, since both the hippocampus and BNST send afferents to the retrosplenial cortex, which in turn projects to the pontine nuclei. In this scenario, the retrosplenial cortex would be an intermediary structure much like the BNST (Berger et al., 1986; Swanson and Cowan, 1977). Regardless of the specifics, it appears that an extended circuit of intermediary brain regions, in addition to those used to elicit the stress response and orchestrate learning, are being used to modify learning after stressful experience, at least in some cases.
Figure 7. This figure illustrates how the hippocampus, amygdala, and BNST could affect the circuitry necessary for classical eyeblink conditioning.
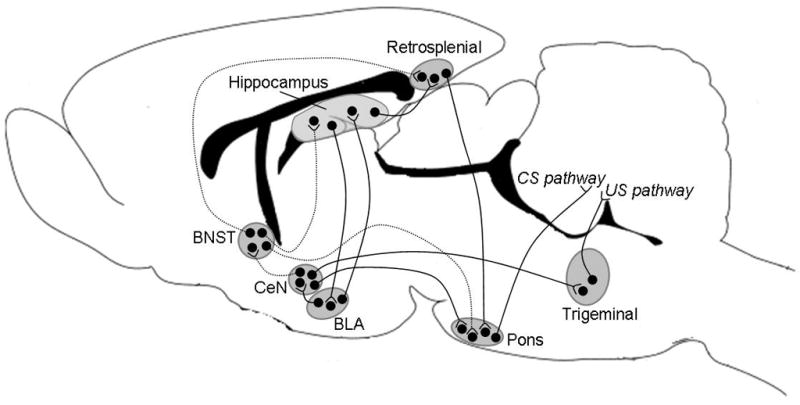
Solid lines represent possible connections in males and females, dashed lines represent possible connections in males only.
Potential mechanisms within circuits
How these intermediary structures are influenced by stress to affect learning in efferent structures is not known, but several possibilities have been proposed. For example, stress hormones and neurotransmitters released from peripheral targets can alter neurophysiological responses within the BLA and the hippocampus (Buffalari and Grace, 2007; Karst et al., 2002; Kavushansky and Richter-Levin, 2006; Shors et al., 1989; Smriga et al., 1996). These same substances also have relatively dramatic effects of stress on dendritic morphology in these brain regions (Magarinos and McEwen, 1995; Mitra and Sapolsky, 2008). The BNST has a relatively high concentration of receptors for corticosterone, norepinephrine, and corticotropin-releasing factor, activation of which can in turn modify gene expression and cellular responses within the structure (Davis and Shi, 1999; Davis et al., 1997; Egli et al., 2005; Shepard et al., 2006). Thus, these cellular alternations within intermediary structures may connect stress hormones and transmitters to learning circuitry. Alternatively, the process may be more psychological. By this, we mean that the intermediary structures may process information about the stressful event which is then relayed to efferent brain regions. When an animal is experiencing a stressor, it simultaneously records many aspects of the experience, such as contextual information about where and when the stressful event occurred. If the contextual cues that were associated with the stressful event are presented to the animal during training, they can react more intensely to the training experience because the cues serve as reminders of the stressful event. This reminder may actually cause a stress response in the animal during learning. Accordingly, it is not the stress response that occurred days earlier that alters learning but rather the stressful state triggered by the memory of the stressful context. There is some evidence to support this hypothesis. Typically in our procedure, the stressor and the training occur in different contexts (as different as is possible) and the effects of stress on eyeblink conditioning only persist when training begins two days after the stressful event has ceased. However, if the animals are trained in the same context as the stressor, the effects persist for a longer time period (Shors and Servatius, 1997; Wood et al., 2001). Interestingly, the hippocampus and amygdala are known to be critical for encoding the contextual cues associated with emotional events (Anagnostaras et al., 2001; Davis, 1994; Fanselow and Kim, 1994; Phillips and LeDoux, 1992). Moreover, neuronal activity within the amygdala, which decreases during the stressor, is also reduced when the animal is re-exposed to the context in which the stressful event occurred (Shors, 1999). Antagonizing NMDA receptors in the BLA during the stressor prevents enhanced learning and the decrease in neuronal activity within the BLA, indicating that neuronal plasticity is required (Shors and Mathew, 1998). Thus, the amygdala (and potentially the hippocampus) may be critical for encoding contextual information about a stressful event which is later used to modify future learning processes, even when the amygdala is not necessary for learning the specific task. More generally, these results suggest that intermediary structures serve not only as relay stations but rather, as sites for learning about the stressful experience itself.
Implications for the treatment of stress-related mental illness
As noted, PTSD is induced by stressful experience and is expressed as a cognitive disorder. Other stress-related illnesses such as depression and generalized anxiety disorder are also characterized by cognitive disturbance (Austin et al., 2001; Bemelmans et al., 1996). If we could identify the brain circuits used at the intersection between stress and learning, we might be able to better understand how stressful life events impact cognitive function in humans. More critically, we might be able to design interventions that target specific brain structures or even circuits in humans with stress-related illness or, ideally, prevent the illness before it is established. In preclinical animal models, local administration of the β-adrenergic blocker, propranolol, into the amygdala attenuates the enhancing effect of norepinephrine on memory consolidation (Liang et al., 1986; Miranda et al., 2003; Salinas et al., 1997). Similarly, blocking glucocorticoid receptors in the amygdala after reactivation of a fearful memory prevents the consolidation of a recalled memory (Tronel and Alberini, 2007). Others have begun to use similar approaches in humans. For example, Pitman and colleagues (2002) treated people with propranolol with the hope of blocking the consolidation of a traumatic memory. The treatment begins within hours of the trauma and then the drug is continuously administered for days afterward (Pitman et al., 2002). Amazingly, they found that even months later, these humans emitted a blunted autonomic response to images related to the trauma, i.e. contextual cues (Pitman et al., 2002). It would appear that the stress hormones released during the trauma could not access their receptors afterward, and as a consequence, the memory for the trauma was not as intensely consolidated, lessening its impact later in life.
Others have begun to intervene with stress hormones themselves. As noted, stress hormones tend to impair retrieval of fear memories (Roozendaal, 2002, 2003). De Quervain and colleagues administered patients with PTSD and phobias with systemic low doses of cortisol (Aerni et al., 2004; de Quervain and Margraf, 2008; Soravia et al., 2006). They found that treated patients had less salient memories of the event and were less likely to experience cognitive disruption (Aerni et al., 2004; de Quervain and Margraf, 2008; Soravia et al., 2006). With imaging, these researchers observed that the drug had its most notable effects in medial temporal lobe structures, including the hippocampus (de Quervain et al., 2003; Oei et al., 2007; Roozendaal et al., 2003). Although suggestive, it is not possible to verify that the drug is working via activity in this specific brain region. In the meantime, pharmaceutical companies are aggressively pursuing ways to target compounds into discrete brain regions. If successful, it might be useful to target the intermediary structures because, at least in principle, this would lessen the interaction between stress and learning but leave the stress response and learning itself intact.
Finally, we consider a third option for treating stress-related cognitive disruption. Rather than manipulating hormones or their receptors, another approach has been to target the mnemonic processes that occur during cognitive training and/or therapy. For example, there is renewed interest in D-cycloserine, a partial agonist to the NMDA receptor. In general, this drug is used as a cognitive enhancer in normal healthy animals, but it is also being used to facilitate extinction learning during exposure therapy for phobias and other anxiety-related disorders (Hofmann et al., 2006; Ressler et al., 2004; Wilhelm et al., 2008). Recently, we found that the drug not only enhances learning in general but reverses the negative effect of stress on learning. As discussed, stress profoundly disrupts classical eyeblink conditioning in females. However, if they are trained in the presence of D-cycloserine, they learn very well, comparable to female rats that were not stressed (Waddell et al., 2009). Since the drug was given long after the stressful event occurred, it is not just preventing the deficit in learning but reversing it. However, the drug was given systemically, and thus, we do not know where in the brain it is acting to reverse the effects of stress on learning. It may act on the learning circuit directly (i.e. the hippocampus and cerebellum in this case), or it may intervene via intermediary structures (e.g., amygdala or BNST) to modify processes of learning. If the latter case is supported, then intermediary structures may be targeted with pharmaceutical compounds during cognitive behavioral therapy.
Conclusion
This review has examined the anatomical frameworks by which stress modulates learning by delineating two models. In the first model, stressful experience directly impacts the circuits used for learning. In the second model, stressful experience alters processes of learning via activity within intermediary structures, which are neither necessary for learning nor for the stress response. As discussed, the first model has a great deal of empirical support, but the second model is not far behind. If we can determine when each framework is engaged and which one predominates, we can begin to consider how mechanisms operate within these circuits to modify learning after stressful experience. It is certainly possible that neither model is entirely correct and that some combination of circuits and interactions between those circuits act together to modify learning after stressful life events. However, by delineating, defending, and potentially rejecting one or the other of these two models, we may come to a greater understanding about how brain regions interact with one another to influence future learning after stress.
Acknowledgments
This work was supported by to NIH (NIMH 59970) and NSF (IOB-0444364) and ARRA (431552) to TJS, and NIH( NIMH 084423) to DAB. Special thanks to Lisa Maeng for helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aerni A, Traber R, Hock C, Roozendaal B, Schelling G, Papassotiropoulos A, Nitsch RM, Schnyder U, de Quervain DJ. Low-dose cortisol for symptoms of posttraumatic stress disorder. Am J Psychiatry. 2004;161:1488–1490. doi: 10.1176/appi.ajp.161.8.1488. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Friedman DP, Mishkin M. A comparison between the connections of the amygdala and hippocampus with the basal forebrain in the macaque. Experimental Brain Research. 1987;67(3):556–68. doi: 10.1007/BF00247288. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances.[see comment]. [Review] [81 refs] Hippocampus. 2001;11(1):8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine regulation of the prefrontal cortex. J Psychopharmacol. 1997;11:151–162. doi: 10.1177/026988119701100208. [DOI] [PubMed] [Google Scholar]
- Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression: Possible implications for functional neuropathology. [References] British Journal of Psychiatry. 2001;178:206. doi: 10.1192/bjp.178.3.200. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Santollo J, Shors TJ. The bed nucleus of the stria terminalis is critically involved in enhancing associative learning after stressful experience. Behavioral Neuroscience. 2005;119(6):1459–66. doi: 10.1037/0735-7044.119.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Shors TJ. The hippocampus is necessary for enhancements and impairments of learning following stress. Nature Neuroscience. 2007;10:1401–1403. doi: 10.1038/nn1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Shors TJ. The bed nucleus of the stria terminalis modulates learning after stress in masculinized but not cycling females. J Neurosci. 2008;28:6383–6387. doi: 10.1523/JNEUROSCI.0831-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Waxler DE, Santollo J, Shors TJ. Trace conditioning and the hippocampus: the importance of contiguity. Journal of Neuroscience. 2006;26(34):8702–6. doi: 10.1523/JNEUROSCI.1742-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemelmans KJ, Goekoop JG, van Kempen GMJ. Recall performance in acutely depressed patients and plasma cortisol. Biological Psychiatry. 1996;39:752. doi: 10.1016/0006-3223(95)00097-6. [DOI] [PubMed] [Google Scholar]
- Berger TW, Weikart CL, Bassett JL, Orr WB. Lesions of the retrosplenial cortex produce deficits in reversal learning of the rabbit nictitating membrane response: implications for potential interactions between hippocampal and cerebellar brain systems. Behavioral Neuroscience. 1986;100(6):802–9. doi: 10.1037//0735-7044.100.6.802. [DOI] [PubMed] [Google Scholar]
- Beylin AV, Gandhi CC, Wood GE, Talk AC, Matzel LD, Shors TJ. The role of the hippocampus in trace conditioning: temporal discontinuity or task difficulty? Neurobiology of Learning & Memory. 2001;76(3):447–61. doi: 10.1006/nlme.2001.4039. [DOI] [PubMed] [Google Scholar]
- Beylin AV, Shors TJ. Glucocorticoids are necessary for enhancing the acquisition of associative memories after acute stressful experience. Hormones & Behavior. 2003;43(1):124–31. doi: 10.1016/s0018-506x(02)00025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaz R, Conboy L, Sandi C. Learning under stress: a role for the neural cell adhesion molecule NCAM. Neurobiol Learn Mem. 2009;91:333–342. doi: 10.1016/j.nlm.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Buffalari DM, Grace AA. Noradrenergic modulation of basolateral amygdala neuronal activity: opposing influences of alpha-2 and beta receptor activation. J Neurosci. 2007;27:12358–12366. doi: 10.1523/JNEUROSCI.2007-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukalo O, Fentrop N, Lee AY, Salmen B, Law JW, Wotjak CT, Schweizer M, Dityatev A, Schachner M. Conditional ablation of the neural cell adhesion molecule reduces precision of spatial learning, long-term potentiation, and depression in the CA1 subfield of mouse hippocampus. J Neurosci. 2004;24:1565–1577. doi: 10.1523/JNEUROSCI.3298-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras NS, Swanson LW. Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: a PHAL anterograde tract-tracing study in the rat. Journal of Comparative Neurology. 1992;324(2):180–94. doi: 10.1002/cne.903240204. [DOI] [PubMed] [Google Scholar]
- Casada JH, Dafny N. Restraint and stimulation of bed nucleus of the stria terminalis produce similar stress-like behaviors. Brain Research Bulletin. 1991;27(2):207–12. doi: 10.1016/0361-9230(91)90069-v. [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Mailliet F, Almeida OF, Jay TM, Sousa N. The prefrontal cortex as a key target of the maladaptive response to stress. J Neurosci. 2007;27:2781–2787. doi: 10.1523/JNEUROSCI.4372-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. [Review] [289 refs] Learning & Memory. 2003 Dec;10(6):427–55. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Conboy L, Sandi C. Stress at Learning Facilitates Memory Formation by Regulating AMPA Receptor Trafficking Through a Glucocorticoid Action. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD. Chronic stress-induced hippocampal vulnerability: the glucocorticoid vulnerability hypothesis. Rev Neurosci. 2008;19:395–411. doi: 10.1515/revneuro.2008.19.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behavioral Neuroscience. 1996;110(6):1321–34. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, Magarinos AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Cordero MI, Kruyt ND, Sandi C. Modulation of contextual fear conditioning by chronic stress in rats is related to individual differences in behavioral reactivity to novelty. Brain Res. 2003a;970:242–245. doi: 10.1016/s0006-8993(03)02352-7. [DOI] [PubMed] [Google Scholar]
- Cordero MI, Merino JJ, Sandi C. Correlational relationship between shock intensity and corticosterone secretion on the establishment and subsequent expression of contextual fear conditioning. Behav Neurosci. 1998;112:885–891. doi: 10.1037//0735-7044.112.4.885. [DOI] [PubMed] [Google Scholar]
- Cordero MI, Venero C, Kruyt ND, Sandi C. Prior exposure to a single stress session facilitates subsequent contextual fear conditioning in rats. Evidence for a role of corticosterone. Horm Behav. 2003b;44:338–345. doi: 10.1016/s0018-506x(03)00160-0. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Watson SJ. Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. Journal of Comparative Neurology. 1993;332(1):1–20. doi: 10.1002/cne.903320102. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in emotional learning. [Review] [205 refs] International Review of Neurobiology. 1994;36:225–66. doi: 10.1016/s0074-7742(08)60305-0. [DOI] [PubMed] [Google Scholar]
- Davis M, Shi C. The extended amygdala: are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety?. [Review] [40 refs] Annals of the New York Academy of Sciences. 1999;877:281–91. doi: 10.1111/j.1749-6632.1999.tb09273.x. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Amygdala and bed nucleus of the stria terminalis: differential roles in fear and anxiety measured with the acoustic startle reflex. [Review] [75 refs] Philosophical Transactions of the Royal Society of London - Series B: Biological Sciences. 1997;352(1362):1675–87. doi: 10.1098/rstb.1997.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quervain DJ, Henke K, Aerni A, Treyer V, McGaugh JL, Berthold T, Nitsch RM, Buck A, Roozendaal B, Hock C. Glucocorticoid-induced impairment of declarative memory retrieval is associated with reduced blood flow in the medial temporal lobe. Eur J Neurosci. 2003;17:1296–1302. doi: 10.1046/j.1460-9568.2003.02542.x. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Margraf J. Glucocorticoids for the treatment of post-traumatic stress disorder and phobias: a novel therapeutic approach. Eur J Pharmacol. 2008;583:365–371. doi: 10.1016/j.ejphar.2007.11.068. [DOI] [PubMed] [Google Scholar]
- del Abril A, Segovia S, Guillamon A. The bed nucleus of the stria terminalis in the rat: regional sex differences controlled by gonadal steroids early after birth. Brain Research. 1987;429(2):295–300. doi: 10.1016/0165-3806(87)90110-6. [DOI] [PubMed] [Google Scholar]
- Diamond DM. Cognitive, endocrine and mechanistic perspectives on nonlinear relationships between arousal and brain function. Nonlinearity Biol Toxicol Med. 2005;3:1–7. doi: 10.2201/nonlin.003.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond DM, Park CR, Heman KL, Rose GM. Exposing rats to a predator impairs spatial working memory in the radial arm water maze. Hippocampus. 1999;9(5):542–52. doi: 10.1002/(SICI)1098-1063(1999)9:5<542::AID-HIPO8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, Costa RM, Sousa N. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325:621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- Donley MP, Schulkin J, Rosen JB. Glucocorticoid receptor antagonism in the basolateral amygdala and ventral hippocampus interferes with long-term memory of contextual fear. Behav Brain Res. 2005;164:197–205. doi: 10.1016/j.bbr.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Duncko R, Cornwell B, Cui L, Merikangas KR, Grillon C. Acute exposure to stress improves performance in trace eyeblink conditioning and spatial learning tasks in healthy men. Learn Mem. 2007;14:329–335. doi: 10.1101/lm.483807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli RE, Kash TL, Choo K, Savchenko V, Matthews RT, Blakely RD, Winder DG. Norepinephrine modulates glutamatergic transmission in the bed nucleus of the stria terminalis. Neuropsychopharmacology. 2005;30:657–668. doi: 10.1038/sj.npp.1300639. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Kim JJ. Acquisition of contextual Pavlovian fear conditioning is blocked by application of an NMDA receptor antagonist D,L-2-amino-5-phosphonovaleric acid to the basolateral amygdala. Behavioral Neuroscience. 1994;108(1):210–2. doi: 10.1037//0735-7044.108.1.210. [DOI] [PubMed] [Google Scholar]
- Ferry B, McGaugh JL. Clenbuterol administration into the basolateral amygdala post-training enhances retention in an inhibitory avoidance task. Neurobiology of Learning & Memory. 1999;72(1):8–12. doi: 10.1006/nlme.1998.3904. [DOI] [PubMed] [Google Scholar]
- Ferry B, McGaugh JL. Role of amygdala norepinephrine in mediating stress hormone regulation of memory storage. [Review] [131 refs] Acta Pharmacologica Sinica. 2000;21(6):481–93. [PubMed] [Google Scholar]
- Gould E, Tanapat P. Stress and hippocampal neurogenesis. [Review] [55 refs] Biological Psychiatry. 1999;46(11):1472–9. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- Gray TS, Bingaman EW. The amygdala: corticotropin-releasing factor, steroids, and stress. Crit Rev Neurobiol. 1996;10:155–168. doi: 10.1615/critrevneurobiol.v10.i2.10. [DOI] [PubMed] [Google Scholar]
- Grillon C, Smith K, Haynos A, Nieman LK. Deficits in hippocampus-mediated Pavlovian conditioning in endogenous hypercortisolism. Biol Psychiatry. 2004;56:837–843. doi: 10.1016/j.biopsych.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Hains AB, Arnsten AF. Molecular mechanisms of stress-induced prefrontal cortical impairment: implications for mental illness. Learn Mem. 2008;15:551–564. doi: 10.1101/lm.921708. [DOI] [PubMed] [Google Scholar]
- Han TM, De Vries GJ. Organizational effects of testosterone, estradiol, and dihydrotestosterone on vasopressin mRNA expression in the bed nucleus of the stria terminalis. Journal of Neurobiology. 2003;54(3):502–10. doi: 10.1002/neu.10157. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Meuret AE, Smits JA, Simon NM, Pollack MH, Eisenmenger K, Shiekh M, Otto MW. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch Gen Psychiatry. 2006;63:298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- Holstege G, Meiners L, Tan K. Projections of the bed nucleus of the stria terminalis to the mesencephalon, pons, and medulla oblongata in the cat. Experimental Brain Research. 1985;58(2):379–91. doi: 10.1007/BF00235319. [DOI] [PubMed] [Google Scholar]
- Howland JG, Wang YT. Synaptic plasticity in learning and memory: stress effects in the hippocampus. Prog Brain Res. 2008;169:145–158. doi: 10.1016/S0079-6123(07)00008-8. [DOI] [PubMed] [Google Scholar]
- Jackson ED, Payne JD, Nadel L, Jacobs WJ. Stress differentially modulates fear conditioning in healthy men and women. Biological Psychiatry. 2006;59(6):516–22. doi: 10.1016/j.biopsych.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Ji JZ, Wang XM, Li BM. Deficit in long-term contextual fear memory induced by blockade of beta-adrenoceptors in hippocampal CA1 region. Eur J Neurosci. 2003;17:1947–1952. doi: 10.1046/j.1460-9568.2003.02620.x. [DOI] [PubMed] [Google Scholar]
- Joels M. Corticosteroid effects in the brain: U-shape it. Trends Pharmacol Sci. 2006;27:244–250. doi: 10.1016/j.tips.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Joels M, Karst H, Alfarez D, Heine VM, Qin Y, van Riel E, Verkuyl M, Lucassen PJ, Krugers HJ. Effects of chronic stress on structure and cell function in rat hippocampus and hypothalamus. Stress. 2004;7:221–231. doi: 10.1080/10253890500070005. [DOI] [PubMed] [Google Scholar]
- Joels M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: how does it work? Trends Cogn Sci. 2006;10:152–158. doi: 10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Karst H, Nair S, Velzing E, Rumpff-van Essen L, Slagter E, Shinnick-Gallagher P, Joels M. Glucocorticoids alter calcium conductances and calcium channel subunit expression in basolateral amygdala neurons. European Journal of Neuroscience. 2002;16(6):1083–9. doi: 10.1046/j.1460-9568.2002.02172.x. [DOI] [PubMed] [Google Scholar]
- Kavushansky A, Richter-Levin G. Effects of stress and corticosterone on activity and plasticity in the amygdala. J Neurosci Res. 2006;84:1580–1587. doi: 10.1002/jnr.21058. [DOI] [PubMed] [Google Scholar]
- Kavushansky A, Vouimba RM, Cohen H, Richter-Levin G. Activity and plasticity in the CA1, the dentate gyrus, and the amygdala following controllable vs. uncontrollable water stress. Hippocampus. 2006;16(1):35–42. doi: 10.1002/hipo.20130. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Koo JW, Lee HJ, Han JS. Amygdalar inactivation blocks stress-induced impairments in hippocampal long-term potentiation and spatial memory. Journal of Neuroscience. 2005;25(6):1532–9. doi: 10.1523/JNEUROSCI.4623-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Wolf OT, May M, Wippich W. Stress- and treatment-induced elevations of cortisol levels associated with impaired declarative memory in healthy adults. Life Sciences. 1996;58:1483. doi: 10.1016/0024-3205(96)00118-x. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. Journal of Comparative Neurology. 1978;178(2):225–54. doi: 10.1002/cne.901780204. [DOI] [PubMed] [Google Scholar]
- Kuhlmann S, Piel M, Wolf OT. Impaired Memory Retrieval after Psychosocial Stress in Healthy Young Men. [References] Journal of Neuroscience. 2005;25:2982. doi: 10.1523/JNEUROSCI.5139-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning?. [Review] [96 refs] Hippocampus. 2006;16(3):216–24. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Shors TJ. Males and females respond differently to controllability and antidepressant treatment. Biological Psychiatry. 2004;56(12):964–70. doi: 10.1016/j.biopsych.2004.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Shors TJ. New spines, new memories. [Review] [66 refs] Molecular Neurobiology. 2004;29(2):117–30. doi: 10.1385/MN:29:2:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KC, Juler RG, McGaugh JL. Modulating effects of posttraining epinephrine on memory: involvement of the amygdala noradrenergic system. Brain Research. 1986;368(1):125–33. doi: 10.1016/0006-8993(86)91049-8. [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. Journal of Neuroscience. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, Martinez C, Villegas M, Magarinos AM, McEwen BS. Restraint stress reversibly enhances spatial memory performance. Physiology & Behavior. 1996;59(1):27–32. doi: 10.1016/0031-9384(95)02016-0. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Fiocco A, Wan N, Maheu F, Lord C, Schramek T, Tu MT. Stress hormones and human memory function across the lifespan. [Review] [108 refs] Psychoneuroendocrinology. 2005;30(3):225–42. doi: 10.1016/j.psyneuen.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Lepage M. Stress, memory, and the hippocampus: can’t live with it, can’t live without it. [Review] [224 refs] Behavioural Brain Research. 2001;127(1–2):137–58. doi: 10.1016/s0166-4328(01)00361-8. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Maheu FS, Joober R, Beaulieu S, Lupien SJ. Differential effects of adrenergic and corticosteroid hormonal systems on human short- and long-term declarative memory for emotionally arousing material. Behavioral Neuroscience. 2004;118(2):420–8. doi: 10.1037/0735-7044.118.2.420. [DOI] [PubMed] [Google Scholar]
- Mauk MD, Thompson RF. Retention of classically conditioned eyelid responses following acute decerebration. Brain Research. 1987;403(1):89–95. doi: 10.1016/0006-8993(87)90126-0. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Disterhoft JF. Hippocampal encoding of non-spatial trace conditioning. Hippocampus. 1999;9:385–396. doi: 10.1002/(SICI)1098-1063(1999)9:4<385::AID-HIPO5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005;54:20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Weiss JM, Schwartz LS. Selective retention of corticosterone by limbic structures in rat brain. Nature. 1968;220:911–912. doi: 10.1038/220911a0. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. [Review] [240 refs] Annual Review of Neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Miranda MI, Lalumiere RT, Buen TV, Bermudez-Rattoni F, McGaugh JL. Blockade of noradrenergic receptors in the basolateral amygdala impairs taste memory. European Journal of Neuroscience. 2003;18(9):2605–10. doi: 10.1046/j.1460-9568.2003.03008.x. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Gould E. Stress and adult neurogenesis. [Review] [78 refs] Hippocampus. 2006;16(3):233–8. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- Mitra R, Sapolsky RM. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc Natl Acad Sci U S A. 2008;105:5573–5578. doi: 10.1073/pnas.0705615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D, Porter JT, Quirk GJ. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J Neurosci. 2008;28:369–375. doi: 10.1523/JNEUROSCI.3248-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oei NY, Elzinga BM, Wolf OT, de Ruiter MB, Damoiseaux JS, Kuijer JP, Veltman DJ, Scheltens P, Rombouts SA. Glucocorticoids Decrease Hippocampal and Prefrontal Activation during Declarative Memory Retrieval in Young Men. Brain Imaging Behav. 2007;1:31–41. doi: 10.1007/s11682-007-9003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel PD, Katz M, Karssen AM, Lyons DM. Stress-induced changes in corticosteroid receptor expression in primate hippocampus and prefrontal cortex. Psychoneuroendocrinology. 2008;33:360–367. doi: 10.1016/j.psyneuen.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel PD, Lopez JF, Lyons DM, Burke S, Wallace M, Schatzberg AF. Glucocorticoid and mineralocorticoid receptor mRNA expression in squirrel monkey brain. J Psychiatr Res. 2000;34:383–392. doi: 10.1016/s0022-3956(00)00035-2. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience. 1992;106(2):274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Sanders KM, Zusman RM, Healy AR, Cheema F, Lasko NB, Cahill L, Orr SP. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry. 2002;51:189–192. doi: 10.1016/s0006-3223(01)01279-3. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Revest JM, Di Blasi F, Kitchener P, Rouge-Pont F, Desmedt A, Turiault M, Tronche F, Piazza PV. The MAPK pathway and Egr-1 mediate stress-related behavioral effects of glucocorticoids. Nat Neurosci. 2005;8:664–672. doi: 10.1038/nn1441. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- Rodriguez Manzanares PA, Isoardi NA, Carrer HF, Molina VA. Previous stress facilitates fear memory, attenuates GABAergic inhibition, and increases synaptic plasticity in the rat basolateral amygdala. Journal of Neuroscience. 2005;25(38):8725–34. doi: 10.1523/JNEUROSCI.2260-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B. Stress and memory: opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol Learn Mem. 2002;78:578–595. doi: 10.1006/nlme.2002.4080. [DOI] [PubMed] [Google Scholar]
- Roozendaal B. Systems mediating acute glucocorticoid effects on memory consolidation and retrieval. [Review] [120 refs] Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2003;27(8):1213–23. doi: 10.1016/j.pnpbp.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Brunson KL, Holloway BL, McGaugh JL, Baram TZ. Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(21):13908–13. doi: 10.1073/pnas.212504599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Griffith QK, Buranday J, de Quervain DJ, McGaugh JL. The hippocampus mediates glucocorticoid-induced impairment of spatial memory retrieval: dependence on the basolateral amygdala. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(3):1328–33. doi: 10.1073/pnas.0337480100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas JA, Introini-Collison IB, Dalmaz C, McGaugh JL. Posttraining intraamygdala infusions of oxotremorine and propranolol modulate storage of memory for reductions in reward magnitude. Neurobiology of Learning & Memory. 1997;68(1):51–9. doi: 10.1006/nlme.1997.3776. [DOI] [PubMed] [Google Scholar]
- Sandi C. Stress, cognitive impairment and cell adhesion molecules. Nat Rev Neurosci. 2004;5:917–930. doi: 10.1038/nrn1555. [DOI] [PubMed] [Google Scholar]
- Sandi C, Loscertales M, Guaza C. Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. Eur J Neurosci. 1997;9:637–642. doi: 10.1111/j.1460-9568.1997.tb01412.x. [DOI] [PubMed] [Google Scholar]
- Sandi C, Merino JJ, Cordero MI, Touyarot K, Venero C. Effects of chronic stress on contextual fear conditioning and the hippocampal expression of the neural cell adhesion molecule, its polysialylation, and L1. Neuroscience. 2001;102:329–339. doi: 10.1016/s0306-4522(00)00484-x. [DOI] [PubMed] [Google Scholar]
- Sandi C, Pinelo-Nava MT. Stress and memory: behavioral effects and neurobiological mechanisms. Neural Plast. 2007;2007:78970. doi: 10.1155/2007/78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrieau A, Dussaillant M, Agid F, Philibert D, Agid Y, Rostene W. Autoradiographic localization of glucocorticosteroid and progesterone binding sites in the human post-mortem brain. J Steroid Biochem. 1986;25:717–721. doi: 10.1016/0022-4731(86)90300-6. [DOI] [PubMed] [Google Scholar]
- Servatius RJ, Shors TJ. Exposure to inescapable stress persistently facilitates associative and nonassociative learning in rats. Behavioral Neuroscience. 1994;108(6):1101–6. doi: 10.1037//0735-7044.108.6.1101. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Schulkin J, Myers DA. Chronically elevated corticosterone in the amygdala increases corticotropin releasing factor mRNA in the dorsolateral bed nucleus of stria terminalis following duress. Behavioural brain research. 2006;174:193–196. doi: 10.1016/j.bbr.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Acute stress and re-exposure to the stressful context suppress spontaneous unit activity in the basolateral amygdala via NMDA receptor activation. Neuroreport. 1999;10:2811–2815. doi: 10.1097/00001756-199909090-00021. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Acute stress rapidly and persistently enhances memory formation in the male rat. Neurobiology of Learning & Memory. 2001;75(1):10–29. doi: 10.1006/nlme.1999.3956. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Learning during stressful times. [Review] [64 refs] Learning & Memory. 2004 Apr;11(2):137–44. doi: 10.1101/lm.66604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ. Stressful experience and learning across the lifespan. [Review] [108 refs] Annual Review of Psychology. 2006;57:55–85. doi: 10.1146/annurev.psych.57.102904.190205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ. Saving new brain cells. Sci Am. 2009;300:46, 52–54. doi: 10.1038/scientificamerican0309-46. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. Journal of Neuroscience. 2001a;21(16):6292–7. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Falduto J, Leuner B. The opposite effects of stress on dendritic spines in male vs. female rats are NMDA receptor-dependent. European Journal of Neuroscience. 2004:145–150. doi: 10.1046/j.1460-9568.2003.03065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Mathew J, Sisti HM, Edgecomb C, Beckoff S, Dalla C. Neurogenesis and helplessness are mediated by controllability in males but not in females. Biol Psychiatry. 2007;62:487–495. doi: 10.1016/j.biopsych.2006.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Mathew PR. NMDA receptor antagonism in the lateral/basolateral but not central nucleus of the amygdala prevents the induction of facilitated learning in response to stress. Learning & Memory. 1998 Aug;5(3):220–30. [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Matzel LD. Long-term potentiation: what’s learning got to do with it?. [Review] [575 refs] Behavioral & Brain Sciences. 1996 doi: 10.1017/s0140525x97001593. discussion–55. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G. Testosterone in utero and at birth dictates how stressful experience will affect learning in adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(21):13955–60. doi: 10.1073/pnas.202199999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories.[see comment][erratum appears in Nature 2001 Dec 20–27;414(6866):938] Nature. 2001b;410(6826):372–6. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Seib TB, Levine S, Thompson RF. Inescapable versus escapable shock modulates long-term potentiation in the rat hippocampus. Science. 1989;244(4901):224–6. doi: 10.1126/science.2704997. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Servatius RJ. The contribution of stressor intensity, duration, and context to the stress-induced facilitation of associative learning. Neurobiology of Learning & Memory. 1997;68(1):92–6. doi: 10.1006/nlme.1997.3763. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Weiss C, Thompson RF. Stress-induced facilitation of classical conditioning. Science. 1992;257(5069):537–9. doi: 10.1126/science.1636089. [DOI] [PubMed] [Google Scholar]
- Smriga M, Saito H, Nishiyama N. Hippocampal long- and short-term potentiation is modulated by adrenalectomy and corticosterone. Neuroendocrinology. 1996;64(1):35–41. doi: 10.1159/000127095. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ. Hippocampus and trace conditioning of the rabbit’s classically conditioned nictitating membrane response. Behavioral Neuroscience. 1986;100(5):729–44. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- Soravia LM, Heinrichs M, Aerni A, Maroni C, Schelling G, Ehlert U, Roozendaal B, de Quervain DJ. Glucocorticoids reduce phobic fear in humans. Proc Natl Acad Sci U S A. 2006;103:5585–5590. doi: 10.1073/pnas.0509184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkman MN, Gebarski SS, Berent S, Schteingart DE. Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing’s syndrome. Biological Psychiatry. 1992;32(9):756–65. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Giordani B, Berent S, Schork A, Schteingart DE. Elevated cortisol levels in Cushing’s disease are associated with cognitive decrements. [References] Psychosomatic Medicine. 2001;63:993. doi: 10.1097/00006842-200111000-00018. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Giordani B, Gebarski SS, Schteingart DE. Improvement in learning associated with increase in hippocampal formation volume. [References] Biological Psychiatry. 2003;53:238. doi: 10.1016/s0006-3223(02)01750-x. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Logan CG, Rosen DJ, Thompson JK, Lavond DG, Thompson RF. Initial localization of the acoustic conditioned stimulus projection system to the cerebellum essential for classical eyelid conditioning. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(10):3531–5. doi: 10.1073/pnas.84.10.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol. 1977;172:49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- Thompson RF. In search of memory traces. [Review] [105 refs] Annual Review of Psychology. 2005;56:1–23. doi: 10.1146/annurev.psych.56.091103.070239. [DOI] [PubMed] [Google Scholar]
- Tracy JA, Thompson JK, Krupa DJ, Thompson RF. Evidence of plasticity in the pontocerebellar conditioned stimulus pathway during classical conditioning of the eyeblink response in the rabbit. Behavioral Neuroscience. 1998;112(2):267–85. doi: 10.1037//0735-7044.112.2.267. [DOI] [PubMed] [Google Scholar]
- Tronel S, Alberini CM. Persistent disruption of a traumatic memory by postretrieval inactivation of glucocorticoid receptors in the amygdala. Biol Psychiatry. 2007;62:33–39. doi: 10.1016/j.biopsych.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis HD, Van Koppen C, Van Ittersum M, De Kloet ER. Specificity of the adrenal steroid receptor system in rat hippocampus. Endocrinology. 1982;110:2044–2051. doi: 10.1210/endo-110-6-2044. [DOI] [PubMed] [Google Scholar]
- Venero C, Tilling T, Hermans–Borgmeyer I, Schmidt R, Schachner M, Sandi C. Chronic stress induces opposite changes in the mRNA expression of the cell adhesion molecules NCAM and L1. Neuroscience. 2002;115:1211–1219. doi: 10.1016/s0306-4522(02)00543-2. [DOI] [PubMed] [Google Scholar]
- Waddell J, Bangasser DA, Shors TJ. The basolateral nucleus of the amygdala is necessary to induce the opposing effects of stressful experience on learning in males and females. 2008 doi: 10.1523/JNEUROSCI.1129-08.2008. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Research. 1992;588(2):341–5. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Weiss C, Sametsky E, Sasse A, Spiess J, Disterhoft JF. Acute stress facilitates trace eyeblink conditioning in C57BL/6 male mice and increases the excitability of their CA1 pyramidal neurons. Learning & Memory. 2005 Apr;12(2):138–43. doi: 10.1101/lm.89005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller KL, Smith DA. Afferent connections to the bed nucleus of the stria terminalis. Brain Research. 1982;232(2):255–70. doi: 10.1016/0006-8993(82)90272-4. [DOI] [PubMed] [Google Scholar]
- Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Kapp BS. Contributions of the amygdaloid central nucleus to the modulation of the nictitating membrane reflex in the rabbit. Behavioral Neuroscience. 1991;105(1):141–53. doi: 10.1037//0735-7044.105.1.141. [DOI] [PubMed] [Google Scholar]
- Wilhelm S, Buhlmann U, Tolin DF, Meunier SA, Pearlson GD, Reese HE, Cannistraro P, Jenike MA, Rauch SL. Augmentation of behavior therapy with D-cycloserine for obsessive-compulsive disorder. Am J Psychiatry. 2008;165:335–341. doi: 10.1176/appi.ajp.2007.07050776. quiz 409. [DOI] [PubMed] [Google Scholar]
- Wood GE, Beylin AV, Shors TJ. The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females. Behavioral Neuroscience. 2001;115(1):175–87. doi: 10.1037/0735-7044.115.1.175. [DOI] [PubMed] [Google Scholar]
- Wood GE, Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(7):4066–71. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RL, Conrad CD. Chronic stress leaves novelty-seeking behavior intact while impairing spatial recognition memory in the Y-maze. Stress. 2005;8:151–154. doi: 10.1080/10253890500156663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YL, Chao PK, Lu KT. Systemic and intra-amygdala administration of glucocorticoid agonist and antagonist modulate extinction of conditioned fear. Neuropsychopharmacology. 2006;31:912–924. doi: 10.1038/sj.npp.1300899. [DOI] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci U S A. 2009;106:14075–14079. doi: 10.1073/pnas.0906791106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorawski M, Cook CA, Kuhn CM, LaBar KS. Sex, stress, and fear: individual differences in conditioned learning. Cogn Affect Behav Neurosci. 2005;5:191–201. doi: 10.3758/cabn.5.2.191. [DOI] [PubMed] [Google Scholar]