Abstract
Glycated albumin, an early-glycation Amadori-modified protein, stimulates transforming growth factor- β (TGF-β) expression and increases the production of the extracellular matrix proteins in mesangial cells, contributing to the pathogenesis of diabetic nephropathy. Glycated albumin has been shown to increase NADPH oxidase-dependent superoxide formation in mesangial cells. However, the mechanisms are not well understood. Therefore, in the present studies, we determined the mechanisms by which glycated albumin activates NADPH oxidase in primary rat mesangial cells and its contribution to glycated albumin-induced TGF-β expression and extracellular matrix protein production. Our data showed that glyated albumin treatment stimulated NADPH oxidase activity and increased the formation of superoxide formation in rat mesangial cells. Moreover, glycated albumin treatment stimulated the expression and phosphorylation of p47phox, one of the cytosolic regulatory subunits of the NADPH oxidase. However, the levels of other NADPH oxidase subunits including Nox1, Nox 2, Nox4, p22phox, and p67phox were not altered by glycated albumin. Moreover, siRNA-mediated knockdown of p47phox inhibited glycated albumin-induced NADPH oxidase activity and superoxide formation. Glycated albumin-induced TGF-β expression and extracellular matrix production (fibronectin) was also inhibited by p47phox knock down. Taken together, these data suggest that up-regulation of p47phox is involved in glycated albumin mediated activation of NADPH oxidase, leading to glycated albumin-induced expression of TGF-β and extracellular matrix proteins in mesangial cells and contributing to the development of diabetic nephropathy.
Keywords: glycated albumin, NADPH oxidase, p47phox, mesangial cells, TGF-β, extracellular matrix protein
INTRODUCTION
Diabetic nephropathy (DN) is the most common cause of end stage renal failure. About 20–30% of people with type 1 and type 2 diabetes develop DN. DN is characterized by both glomerulosclerosis with thickening of the glomerular basement membrane and mesangial matrix expansion, and tubulointerstitial fibrosis. Mesangial cells produce excessive amount of extracellular matrix proteins, contributing to the glomerulosclerosis.
Diabetes is associated with increased modification of proteins. Typically, glucose reacts non-enzymatically with amino groups of proteins to produce intermediate Amadori products (such as glycated albumin) and finally form a class of irreversibly cross-linked, fluorescent moieties termed advanced glycation end products (AGE) [1]. Glycated albumin is the major form of circulating glycated proteins in vivo and its levels are increased in diabetes [2; 3]. Accumulating evidence suggests that elevated concentrations of glycated albumin or AGE play a role in DN [2; 4; 5], possibly through up-regulation of TGF-β and stimulation of collagen and fibronectin expression in mesangial cells [6; 7; 8; 9].
Studies have shown that glycated albumin or AGE stimulates reactive oxygen species (ROS) generation in mesangial cells [9; 10]. It is known that an important source of ROS generation in kidneys of diabetes is NADPH oxidase [11]. NADPH oxidase is composed of two membrane-associated components, p22phox and gp91phox (Nox-2), and four cytosolic components, p47phox, p67phox, p40phox and rac-1/2 [12]. There are seven homologues of phagocytic Nox-2 proteins that have been detected in nonphagocytic cells (nox1-5; Duox1-2). In the kidney, Nox-1, Nox-2, Nox-4, p22phox, p47phox, and p67phox have been found to be expressed in renal cortex as well as in mesangial cells [13; 14]. Enhanced expression of renal NADPH oxidase including p47phox, p91phox or p22phox has been observed in the kidneys from diabetic animals or patients [11; 15; 16]. Moreover, inhibition of NADPH oxidase by apocynin prevented the development of early DN [13] or AGEs-mediated kidney damage in a type 1 diabetes animal model [17], suggesting that activation of NADPH oxidase contributes to DN. Glycated albumin has been shown to increase NADPH oxidase-dependent superoxide formation in mesangial cells [10]. However, the mechanisms by which glycated albumin-induced activation of NADPH oxidase in mesangial cells are not well understood.
In the present studies, we determined the mechanisms of glycated albumin-mediated activation of NADPH oxidase in primary rat mesangial cells. The results showed that glycated albumin up-regulates p47phox expression/phosphorylation, resulting in increased NADPH oxidase activity and extracellular matrix production in mesangial cells. These studies establish a molecular link between glycated albumin, altered NADPH oxidase activity, and extracellular matrix production in mesangial cells key to diabetic nephropathy through control of p47phox expression.
METHODS AND MATERIALS
Materials
Lucigenin, NADH, NADPH, Tiron, diphenylene iodonium (DPI), and anti-phospho-serine antibody were purchased from Sigma (St Louis, Missouri, USA). Apocynin was purchased from Acros Organics (Morris plains, NJ). MnTBAP was purchased from Calbiochem (La Jolla, CA, USA). Dihydroethidium was purchased from Molecular Probes (Eugene, OR, USA). All drug solutions were prepared fresh before each experiment. Antibodies against Nox 1, Nox 2, p22 phox, p47 phox, Nox 4 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-fibronectin antibody was purchased from Life technologies (Gaithersburg, MD).
Glycated and nonglycated bovine serum albumin were purchased from Sigma (St Louis, Missouri). Glycated albumin contained 3 mol of fructoselysine per mol albumin. The absence of AGE in the glycated albumin was assessed by measuring of AGE-related fluorescence at excitation maximum of 370 nm and emission maximum of 440 nm as described previously [18]. Endotoxin (LPS) was not detectable in the glycated or nonglycated albumin by the use of Limulus Amebocyte Lysate test kit (Sigma).
Cell Culture
Primary rat mesangial cells (RMCs) were the generous gift from Dr. Anne Woods, University of Alabama at Birmingham as described previously [19]. Cells were cultured in RPMI bovine serum, 5 m 1640 medium supplemented with 20% heat-activated fetal M D-glucose, 2 mM L-glutamine, 1% (v/v) nonessential amino acids, 2 mM sodium pyruvate, 10 μg/ml transferrin, 5 ng/ml sodium selenite, and 0.6 international units/ml insulin. Experiments in this study were performed on cells between the 5th and 10th passages.
Measurement of NAD(P)H-dependent oxidase activity
NADPH oxidase activity in cell homogenates was measured as described previously [20]. Briefly, RMCs were cultured and made quiescent by culturing in serum and insulin-free RPMI 1640 media for 48 h. Cells were then treated with serum-free RPMI 1640 media (with 5 mM glucose) containing control or different concentrations of glycated albumin in the presence or absence of DPI (10 μM) or apocynin (20 μM) for 24 h. After treatment, cells were homogenized and used immediately to measure NADPH dependent superoxide generation. To start the assay, 100 μl of homogenates were added to 900 μl of 50 mM phosphate buffer, pH 7.0, containing 1 mM EGTA, 150 mM sucrose, 5 μM lucigenin, 100 μM NADPH and 100 μM NADH. Photo emission was measured every 15 seconds for 10 minutes in a luminometer (Centro LB 960 microplate reader, Berthold technologies). A buffer blank was subtracted from each reading. The superoxide generation was expressed as relative chemiluminescence (light) units (RLU) × 104/per μg protein.
Dihydroethidium (DHE) staining
RMCs were cultured and treated with control or glycated BSA (200 μg/ml) in the absence or presence of DPI (10 μM) or apocynin (20 μM) for 24 h. This concentration of glycated albumin (200 μg/ml) is similar to that found in clinical specimens (200 to 600 μg/ml) and has been observed to stimulate extracellular matrix production in mesangial cells [7]. After treatment, cells were treated with the superoxide-sensitive fluorescent dye dihydroethidium (DHE) (5 μM in PBS) and were incubated in the dark for 15 minutes at 37°C. Fluorescence was visualized under a fluorescence microscope.
Real-time PCR
After treatments, total RNA from RMCs was extracted. Total RNA (2 μg) was converted to cDNA using MLV reverse transcriptase (Promega). Real-time quantitative RT-PCR analyses were performed using SYBR Green PCR Master Mix kit with a MyiQ real-time PCR thermal cycler (Bio-Rad). Standard curves were generated using 18S RNA primers. The mRNA expression levels of test genes were normalized to 18S levels. Primers were synthesized by Integrated DNA Technologies. The primers for the components of NADPH oxidase including p22phox, Nox1, Nox 4, Nox 2(gp91), p47phox and p67phox were synthesized as described previously [21].
Immunoblotting Analysis
RMCs were treated with control or glycated albumin (200μg/ml) for 24 h. After treatment, conditioned media were collected. Fibronectin levels in the conditioned media were determined by immunoblotting. In addition, cells were harvested and cell homogenates were prepared. Equal amounts of proteins in cell homogenates were subjected to SDS-PAGE and transferred to nitrocellulose membranes to detect p47phox levels with anti-p47phox antibody. The enhanced chemiluminescence detection system (pierce) was used for visualization of immunoreactive bands. β-actin was used as a loading control. Immunoblots were analyzed by scanning densitometry and quantified by Quantity One Gel analysis (Bio-Rad).
For detection of phosphorylation of p47phox, after treatment, cell lysates were immunoprecipitated (IP) using anti-p47phox antibody and immunoblotted with anti-phospho-serine antibody. The protein levels of p47phox in the immunoprecipitates were also determined using anti-p47phox antibody. The phosphorylated p47phox levels were normalized to the total p47phox protein levels.
TGF-β1 measurement
RMCs were treated with control or glycated albumin (200 μg/ml) in the absence or presence of DPI (10 μM), apocynin (20 μM), Tiron (5 mM), or MnTBAP (50 μM) for 24 h. After treatment, conditioned media were collected. Total TGF-β1 levels in the conditioned media were measured by TGF-β1 ELISA kit from R&D system. The mean values of triplicate samples were converted into concentrations of TGF-β (p M) using a standard curve obtained with standard TGF-β1 provided from the kit.
Transfection of cells with siRNA-p47 phox
RMCs were transiently transfected with siRNA targeting to p47 phox (sc-45918 from Santa Cruz) by using the siRNA tranfection reagent (sc-29528). For a negative control, cells were transfected with a control siRNA duplex (sc-37007). After 48 h of transfection, cells were treated with control or glycated BSA (200 μg/ml) for 24 h. After treatment, conditioned media were collected. Fibronectin and total TGF-β levels in the conditioned media were determined by immunoblotting and ELISA, respectively. The cells were harvested and cell homogenates were prepared. The NADPH oxidase activity was measured by lucigenin assay as described above. The efficient knock down of p47phox was determined by immunoblotting.
Statistical Analysis
Data are expressed as the mean ± S.E. Statistical evaluation of the data was performed using ANOVA or student t test as appropriate, considering the p value of <0.05 as significant.
RESULTS
Glycated albumin activated NADPH oxidase and resulted in increased superoxide formation in rat mesangial cells (RMCs)
It has been shown that glycated albumin induced NADPH oxidase-dependent superoxide production in human mesangial cells [10]. However, the mechanisms are not well understood. Therefore, in the following studies, we first confirmed the effect of glycated albumin on NADPH oxidase activation and superoxide production in RMCs and further determined the involved mechanisms.
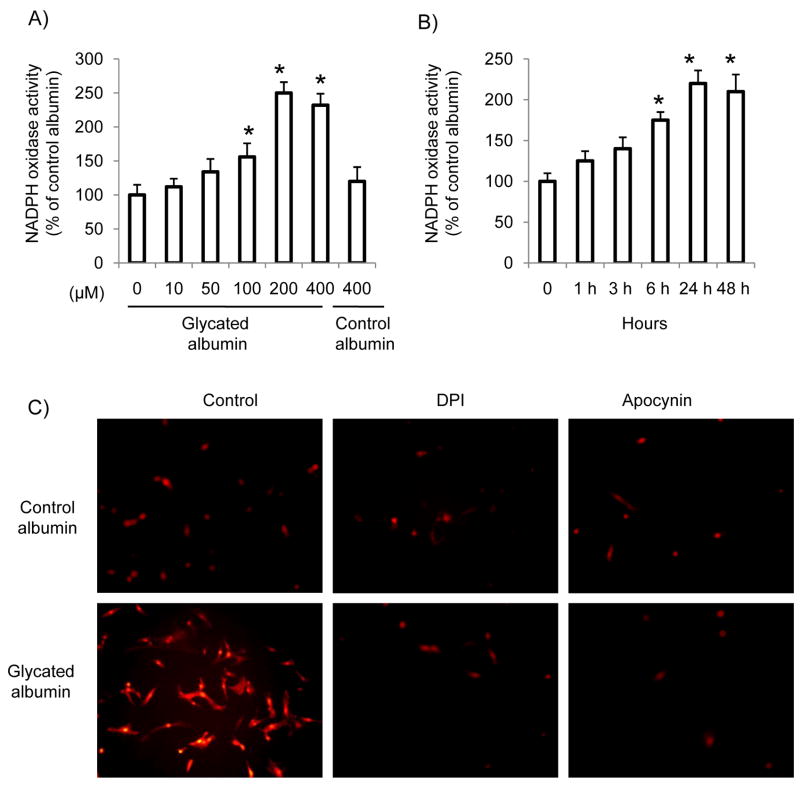
Using lucigenin (5 μM)-enhanced chemiluminescence assay with NADH/NADPH (100 μM) as substrates, we measured NADPH oxidase activity in the homogenates of RMCs after glycated albumin treatment. As shown in Figure 1A and B, glycated albumin increased NADPH oxidase activity in mesangial cells in a dose and time-dependent manner. The maximum effect was achieved after 24 h of glycated albumin treatment at the concentration of 200 μg/ml. Control albumin treatment has no effect on NADPH oxidase activity. Moreover, intracellular superoxide levels were measured using the superoxide-sensitive dye dihydroethidium (DHE) staining and fluorescence microscopy. As shown in Figure 1C, glycated albumin treatment (24 h) increased DHE staining as compared to control albumin, which was inhibited by NADPH oxidase inhibitors, DPI (10 μM) and apocynin (20 μM). The concentration of apocynin used for the current study is far below that needs for its antioxidant effect [22]. Therefore, our data suggest that glycated albumin increases the formation of NADPH oxidase-driven superoxide in mesangial cells.
Figure 1. NADPH oxidase activity and superoxide levels were increased in primary rat mesangial cells (RMCs) after glycated albumin treatment.
(A). RMCs were treated with control albumin or glycated albumin at different concentrations for 24 h. (B) RMCs were treated with glycated albumin or control albumin (200 μg/ml) for different time periods. After treatment, RMCs were harvested and NADPH oxidase activity in cell homogenates was measured as described in Materials and Methods. The experiments were repeated three times. The results shown are means ± SE. *p<0.05 vs. control (0). (C). RMCs were treated with glycated albumin or control albumin (200 μg/ml) in the presence or absence of DPI (10 μM) or apocynin (20 μM) for 24 h. Cells were stained with the superoxide-sensitive dye dihydroethidium (DHE) and observed under a fluorescence microscope. The experiments were repeated three times. The images were acquired with identical acquisition parameters and representative images are shown.
Effect of glycated albumin on the expression of NADPH oxidase subunits in RMCs
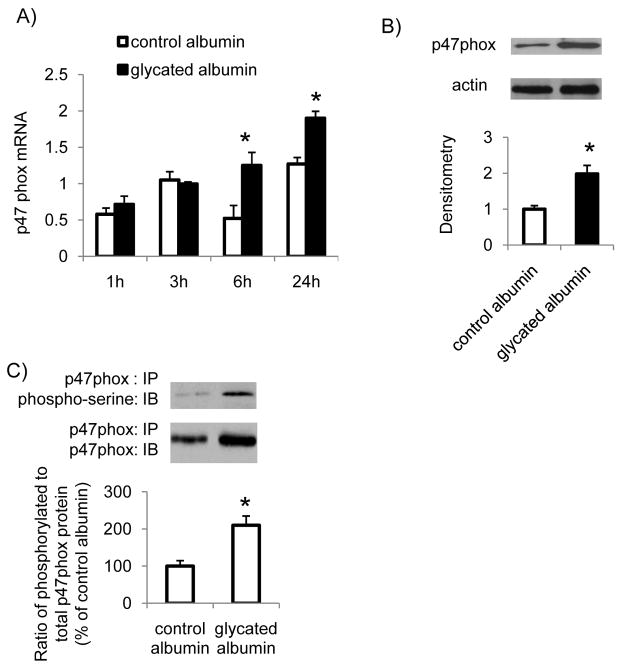
To further determine the mechanisms of glycated albumin mediated activation of NADPH oxidase in RMCs, the effect of glycated albumin on mRNA levels of the components of NADPH oxidase was determined. The results showed that treatment with glycated albumin did not significantly alter the mRNA levels of p22phox, Nox1, Nox2, Nox4, or p67phox in mesangial cells (Supplemental S1). However, p47phox mRNA levels were significantly increased after 6 h and 24 h of glycated albumin treatment (Figure 2A). p47phox protein levels in the cell homogenates were also significantly increased after glycated albumin treatment (Figure 2B). In addition, the ratio of phosphorylated p47phox to total p47phox protein levels was significantly increased in RMCs after glycated albumin treatment (Figure 2C). Together, these studies indicate that glycated albumin up-regulated p47phox expression and stimulated p47phox phosphorylation in mesangial cells, which may contribute to glycated albumin-mediated activation of NADPH oxidase.
Figure 2. Glycated albumin stimulated p47phox expression and phosphorylation in mesangial cells.
RMCs were treated with glycated albumin or control albumin (200 μg/ml) for different time periods. After treatment, cells were harvested. (A). The mRNA levels of p47phox were determined by real-time PCR and normalized to 18S RNA. (B). The protein levels of p47phox after 24 h of glycated albumin treatment were determined by immunoblotting. β-actin was used as an internal control. (C). Cell lysates were immunoprecipitated (IP) using anti-p47phox antibody and immunoblotted (IB) using anti-phospho-serine antibody to detect phosphorylated p47phox at serine residues or using anti-p47phox antibody to determine total p47phox protein levels. The immunoblots were analyzed by densitometry. Results were normalized to total p47phox levels. Results are the mean ± SE (n=3). * P<0.05 v.s. control albumin.
Glycated albumin stimulated TGF-β and extracellular matrix (fibronectin) production in RMCs, which was inhibited by NADPH oxidase inhibitors and superoxide scavengers
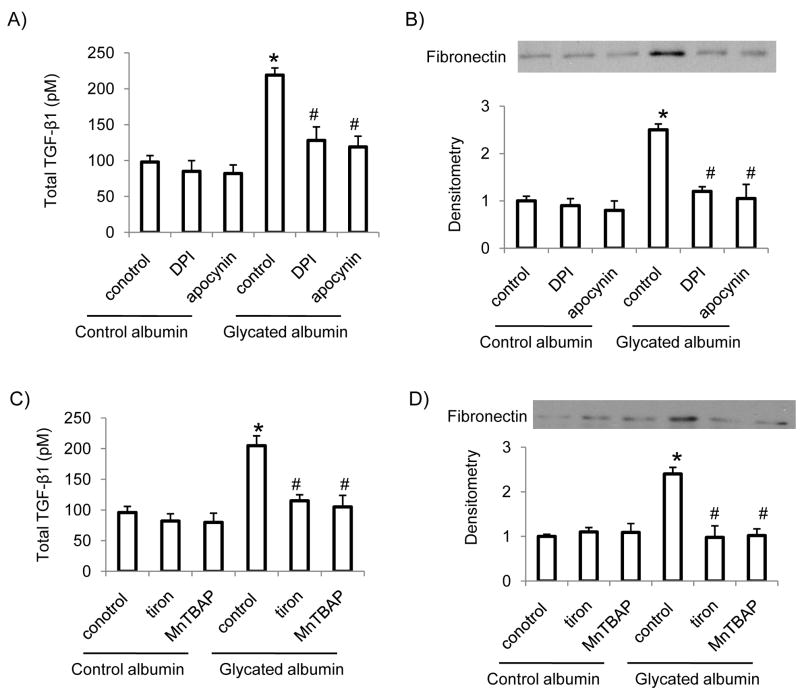
Glycated albumin has been shown to stimulate TGF-β and fibronectin expression in mesangial cells [7]. In the present studies, we determined whether NADPH oxidase induced superoxide formation involves in glycated albumin-induced TGF-β and fibronectin expression. Our results showed that glycated albumin treatment stimulated TGF-β and fibronectin expression in RMCs. Control albumin had no significant effect on TGF-β and fibronectin levels in RMCs. Furthermore, administration of NADPH oxidase inhibitors-DPI and apocynin (Figure 3A and B) or superoxide scavenger-Tiron (5 mM) or SOD mimic-MnTBAP (50 μM) (Figure 3C and D) prevented glycated albumin mediated increases in TGF-β and fibronectin levels in mesangial cells, suggesting that NADPH oxidase-dependent superoxide generation involves in glycated albumin-mediated increases in TGF-β levels and extracellular matrix protein production in mesangial cells.
Figure 3. Glycated albumin-induced increases in TGF-β and fibronectin levels were inhibited by NADPH oxidase inhibitors and superoxide scanvengers.
RMCs were treated with glycated albumin or control albumin (200 μg/ml) for 24 h in the presence or absence of NADPH inhibitors-DPI (10 μM) or apocynin (20 μM) (A, B) or superoxide scavenger-Tiron (5 mM) or SOD mimic-MnTBAP (50 μM) (C, D). After treatment, cell conditioned media were collected. Total TGF-β levels and fibronectin levels in the conditioned media were determined by ELISA and immunoblotting, respectively. Results are the mean ± SE (n=4). *p<0.05 vs. control in control albumin group; # p<0.05 vs. control in glycated albumin group.
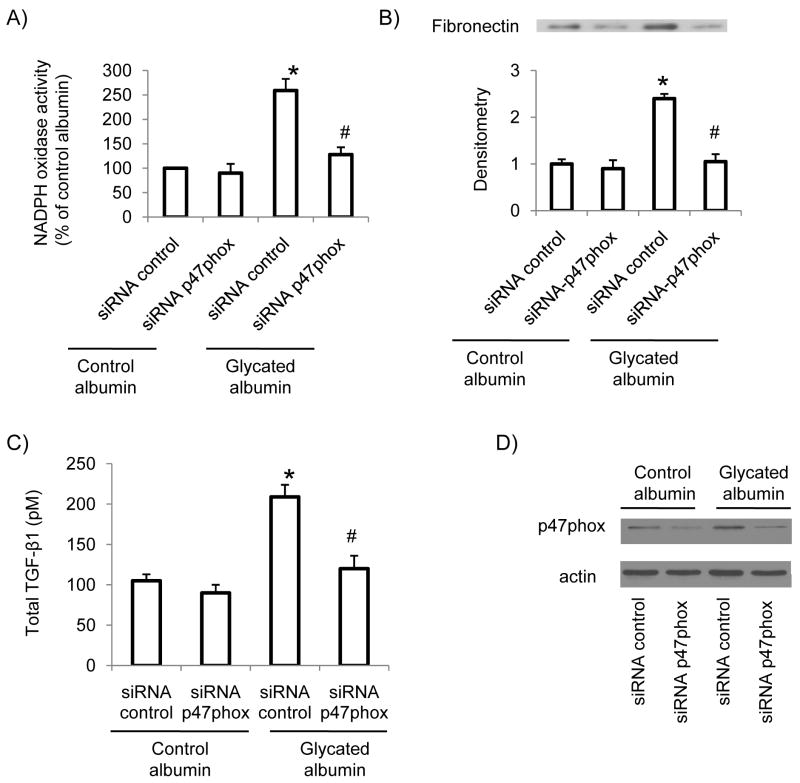
siRNA-mediated p47phox knock down prevented glycated albumin stimulated NADPH oxidase activity and TGF-β and fibronectin levels in RMCs
To further investigate the potential importance of glycated albumin-mediated increase in p47phox levels in superoxide production in RMCs, cells were transiently transfected with siRNA-p47phox to knock down p47phox. The results showed that inhibition of p47 phox abolished glycated albumin mediated increase in superoxide production in mesangial cells (Figure 4A). In addition, glycated albumin-induced increase in TGF-β levels (Figure 4C) and fibronectin expression (Figure 4B) was also inhibited by p47phox knock down. The efficient knock down of p47phox in mesangial cells was confirmed by immunoblotting (Figure 4D). Taken together, these data suggest that up-regulation of p47phox is involved in glycated albumin induced NADPH oxidase activation and increased extracellular matrix production in mesangial cells.
Figure 4. siRNA mediated p47phox knock down inhibited NADPH oxidase activity and TGF-β and fibronectin levels in mesangial cells after glycated albumin treatment.
RMCs were transiently transfected with siRNA-p47phox or control siRNA for 48 h. Then cells were treated with glycated albumin or control albumin for 24 h. The NADPH oxidase activity in cell homogenates was measured by lucigenin assay (A). Fibronectin protein levels in cell lysates were determined by immunoblotting (B). Total TGF-β levels were determined by ELISA (C). The efficient knockdown of p47phox was confirmed by immunoblotting (D). Results are the mean ± SE (n=4). * P<0.05 vs. siRNA control in control albumin group; # p<0.05 vs. siRNA control in glycated albumin group
DISCUSSION
Our studies showed that glycated albumin stimulated p47phox expression and phosphorylation, leading to activation of NADPH oxidase and increased formation of superoxide in rat mesangial cells. Moreover, siRNA-mediated knockdown of p47phox prevented glycated albumin induced superoxide production and extracellular matrix production in mesangial cells. Together, our studies provide first evidence that glycated albumin stimulates TGF-β and resultant extracellular matrix production in rat mesangial cells through p47phox-dependent NADPH oxidase activation and superoxide formation.
Increased modification of proteins has been observed under diabetic conditions. Typically, glucose reacts non-enzymatically with amino groups of proteins to produce intermediate Amadori products (such as glycated albumin) and finally form a class of irreversibly cross-linked, fluorescent moieties termed advanced glycation end products [1]. Accumulating evidence suggests that elevated concentrations of glycated albumin play a role in the development of diabetic nephropathy [23; 24; 25; 26]. One mechanism is through glycated albumin-mediated generation of reactive oxygen species (ROS) in mesangial cells [10]. It is known that NADPH oxidase is one of the major sources of ROS generation in diabetic kidneys [11]. Glycated albumin has been shown to induce NADPH oxidase-dependent superoxide production in human mesangial cells [10]. Consistently, our studies demonstrated that glycated albumin stimulated NADPH oxidase activity and increased the formation of superoxide in rat mesangial cells. Moreover, we found that the mRNA levels of NADPH oxidase subunits including Nox 1, Nox 2, Nox 4, p22 phox or p67phox were not significantly altered by glycated albumin treatment. However, p47phox mRNA levels as well as protein levels in the cell homogenates were significantly increased by glycated albumin. In addition, glycated albumin also stimulated phosphorylation of p47phox in mesangial cells. Together, these studies suggest that increased p47 phox expression and phosphorylation may be a mechanism involving in glycated albumin-mediated activation of NADPH oxidase in mesangial cells. It appears that the effect of glycated albumin on p47phox expression is cell type specific, since no changes in p47phox levels were revealed in glycated albumin treated cardiomyocytes [27]. In addition, glycated albumin has been shown to stimulate PKC activity in mesangial cells [6; 10]. Increased PKC activity may up-regulate p47phox expression and contributes to glycated albumin-induced NADPH oxidase activity. At this time, the relevant receptor and down-stream signaling pathways that mediate the glycated albumin response in mesangial cells is not clear and needs to be further investigated.
Our studies showed that glycated albumin treatment stimulated TGF-β levels and increased the expression of extracellular matrix protein (fibronectin) in mesangial cells, which is consistent with the studies from Cohen et al [23; 24; 25; 26]. Furthermore, we demonstrated that p47phox-depdendent NADPH oxidase activity mediates glycated albumin-induced stimulation of TGF-β and resultant extracellular matrix protein production in mesangial cells. It is known that glycated albumin activated NF-κB in a variety of cell types [28; 29; 30; 31; 32; 33]. NF-κB is an important redox-sensitive transcription factor and has been implicated in the development of renal diseases [34]. Therefore, it is possible that glycated albumin-mediated increase in NADPH-oxidase dependent superoxide formation activates NF-κB, resulting in increased expression of TGF-β in mesangial cells. Further studies need to be performed to investigate the above possibility.
In summary, our data indicated that glycated albumin up-regulated p47phox expression/phosphorylation and resulted in activation of NADPH oxidase in rat mesangial cells. In addition, NADPH oxidase-driven superoxide formation mediates glycated albumin-induced increase in TGF-β levels and extracellular matrix production, contributing to the development of diabetic nephropathy.
Supplementary Material
RMCs were treated with glycated albumin or control albumin (200 μg/ml) for different time periods. After treatment, cells were harvested. The mRNA levels of p22phox, NOX1, NOX4, NOX2 and p67phox were determined by real-time PCR and normalized to 18s RNA. Results are the mean ± SE (n=3).
Acknowledgments
This work was supported by an award P20RR021954 from the NCRR and grants from AHA (to S. W) and JDRF (to S. W).
Footnotes
DISCLOSURES
No conflicts of interest are declared by authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lapolla A, Fedele D, Reitano R, Arico NC, Seraglia R, Traldi P, Marotta E, Tonani R. Enzymatic digestion and mass spectrometry in the study of advanced glycation end products/peptides. J Am Soc Mass Spectrom. 2004;15:496–509. doi: 10.1016/j.jasms.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 2.Cohen MP, Ziyadeh FN. Role of Amadori-modified nonenzymatically glycated serum proteins in the pathogenesis of diabetic nephropathy. J Am Soc Nephrol. 1996;7:183–90. doi: 10.1681/ASN.V72183. [DOI] [PubMed] [Google Scholar]
- 3.Guthrow CE, Morris MA, Day JF, Thorpe SR, Baynes JW. Enhanced nonenzymatic glucosylation of human serum albumin in diabetes mellitus. Proc Natl Acad Sci U S A. 1979;76:4258–61. doi: 10.1073/pnas.76.9.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukami K, Yamagishi S, Ueda S, Okuda S. Role of AGEs in diabetic nephropathy. Curr Pharm Des. 2008;14:946–52. doi: 10.2174/138161208784139710. [DOI] [PubMed] [Google Scholar]
- 5.Tanji N, Markowitz GS, Fu C, Kislinger T, Taguchi A, Pischetsrieder M, Stern D, Schmidt AM, D’Agati VD. Expression of advanced glycation end products and their cellular receptor RAGE in diabetic nephropathy and nondiabetic renal disease. J Am Soc Nephrol. 2000;11:1656–66. doi: 10.1681/ASN.V1191656. [DOI] [PubMed] [Google Scholar]
- 6.Cohen MP, Ziyadeh FN, Lautenslager GT, Cohen JA, Shearman CW. Glycated albumin stimulation of PKC-beta activity is linked to increased collagen IV in mesangial cells. Am J Physiol. 1999;276:F684–90. doi: 10.1152/ajprenal.1999.276.5.F684. [DOI] [PubMed] [Google Scholar]
- 7.Ziyadeh FN, Han DC, Cohen JA, Guo J, Cohen MP. Glycated albumin stimulates fibronectin gene expression in glomerular mesangial cells: involvement of the transforming growth factor-beta system. Kidney Int. 1998;53:631–8. doi: 10.1046/j.1523-1755.1998.00815.x. [DOI] [PubMed] [Google Scholar]
- 8.Fukami K, Ueda S, Yamagishi S, Kato S, Inagaki Y, Takeuchi M, Motomiya Y, Bucala R, Iida S, Tamaki K, Imaizumi T, Cooper ME, Okuda S. AGEs activate mesangial TGF-beta-Smad signaling via an angiotensin II type I receptor interaction. Kidney Int. 2004;66:2137–47. doi: 10.1111/j.1523-1755.2004.66004.x. [DOI] [PubMed] [Google Scholar]
- 9.Lal MA, Brismar H, Eklof AC, Aperia A. Role of oxidative stress in advanced glycation end product-induced mesangial cell activation. Kidney Int. 2002;61:2006–14. doi: 10.1046/j.1523-1755.2002.00367.x. [DOI] [PubMed] [Google Scholar]
- 10.Yoo CW, Song CY, Kim BC, Hong HK, Lee HS. Glycated albumin induces superoxide generation in mesangial cells. Cell Physiol Biochem. 2004;14:361–8. doi: 10.1159/000080346. [DOI] [PubMed] [Google Scholar]
- 11.Li JM, Shah AM. ROS generation by nonphagocytic NADPH oxidase: potential relevance in diabetic nephropathy. J Am Soc Nephrol. 2003;14:S221–6. doi: 10.1097/01.asn.0000077406.67663.e7. [DOI] [PubMed] [Google Scholar]
- 12.Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–76. [PubMed] [Google Scholar]
- 13.Gill PS, Wilcox CS. NADPH oxidases in the kidney. Antioxid Redox Signal. 2006;8:1597–607. doi: 10.1089/ars.2006.8.1597. [DOI] [PubMed] [Google Scholar]
- 14.Miyata K, Rahman M, Shokoji T, Nagai Y, Zhang GX, Sun GP, Kimura S, Yukimura T, Kiyomoto H, Kohno M, Abe Y, Nishiyama A. Aldosterone stimulates reactive oxygen species production through activation of NADPH oxidase in rat mesangial cells. J Am Soc Nephrol. 2005;16:2906–12. doi: 10.1681/ASN.2005040390. [DOI] [PubMed] [Google Scholar]
- 15.Onozato ML, Tojo A, Goto A, Fujita T, Wilcox CS. Oxidative stress and nitric oxide synthase in rat diabetic nephropathy: effects of ACEI and ARB. Kidney Int. 2002;61:186–94. doi: 10.1046/j.1523-1755.2002.00123.x. [DOI] [PubMed] [Google Scholar]
- 16.Matsunaga-Irie S, Maruyama T, Yamamoto Y, Motohashi Y, Hirose H, Shimada A, Murata M, Saruta T. Relation between development of nephropathy and the p22phox C242T and receptor for advanced glycation end product G1704T gene polymorphisms in type 2 diabetic patients. Diabetes Care. 2004;27:303–7. doi: 10.2337/diacare.27.2.303. [DOI] [PubMed] [Google Scholar]
- 17.Thallas-Bonke V, Thorpe SR, Coughlan MT, Fukami K, Yap FY, Sourris KC, Penfold SA, Bach LA, Cooper ME, Forbes JM. Inhibition of NADPH oxidase prevents advanced glycation end product-mediated damage in diabetic nephropathy through a protein kinase C-alpha-dependent pathway. Diabetes. 2008;57:460–9. doi: 10.2337/db07-1119. [DOI] [PubMed] [Google Scholar]
- 18.Nevado J, Peiro C, Vallejo S, El-Assar M, Lafuente N, Matesanz N, Azcutia V, Cercas E, Sanchez-Ferrer CF, Rodriguez-Manas L. Amadori adducts activate nuclear factor-kappaB-related proinflammatory genes in cultured human peritoneal mesothelial cells. Br J Pharmacol. 2005;146:268–79. doi: 10.1038/sj.bjp.0706309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang S, Shiva S, Poczatek MH, Darley-Usmar V, Murphy-Ullrich JE. Nitric oxide and cGMP-dependent protein kinase regulation of glucose-mediated thrombospondin 1-dependent transforming growth factor-beta activation in mesangial cells. J Biol Chem. 2002;277:9880–8. doi: 10.1074/jbc.M108360200. [DOI] [PubMed] [Google Scholar]
- 20.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–8. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 21.Chabrashvili T, Kitiyakara C, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Effects of ANG II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase, and SOD expression. Am J Physiol Regul Integr Comp Physiol. 2003;285:R117–24. doi: 10.1152/ajpregu.00476.2002. [DOI] [PubMed] [Google Scholar]
- 22.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–7. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 23.Chen S, Cohen MP, Ziyadeh FN. Amadori-glycated albumin in diabetic nephropathy: pathophysiologic connections. Kidney Int Suppl. 2000;77:S40–4. doi: 10.1046/j.1523-1755.2000.07707.x. [DOI] [PubMed] [Google Scholar]
- 24.Cohen MP, Hud E, Wu VY. Amelioration of diabetic nephropathy by treatment with monoclonal antibodies against glycated albumin. Kidney Int. 1994;45:1673–9. doi: 10.1038/ki.1994.219. [DOI] [PubMed] [Google Scholar]
- 25.Cohen MP, Hud E, Wu VY, Ziyadeh FN. Albumin modified by Amadori glucose adducts activates mesangial cell type IV collagen gene transcription. Mol Cell Biochem. 1995;151:61–7. doi: 10.1007/BF01076897. [DOI] [PubMed] [Google Scholar]
- 26.Cohen MP, Lautenslager GT, Hud E, Shea E, Wang A, Chen S, Shearman CW. Inhibiting albumin glycation attenuates dysregulation of VEGFR-1 and collagen IV subchain production and the development of renal insufficiency. Am J Physiol Renal Physiol. 2007;292:F789–95. doi: 10.1152/ajprenal.00201.2006. [DOI] [PubMed] [Google Scholar]
- 27.Zhang M, Kho AL, Anilkumar N, Chibber R, Pagano PJ, Shah AM, Cave AC. Glycated proteins stimulate reactive oxygen species production in cardiac myocytes: involvement of Nox2 (gp91phox)-containing NADPH oxidase. Circulation. 2006;113:1235–43. doi: 10.1161/CIRCULATIONAHA.105.581397. [DOI] [PubMed] [Google Scholar]
- 28.Hattori Y, Banba N, Gross SS, Kasai K. Glycated serum albumin-induced nitric oxide production in vascular smooth muscle cells by nuclear factor kappaB-dependent transcriptional activation of inducible nitric oxide synthase. Biochem Biophys Res Commun. 1999;259:128–32. doi: 10.1006/bbrc.1999.0736. [DOI] [PubMed] [Google Scholar]
- 29.Salazar R, Brandt R, Krantz S. Binding of Amadori glucose-modified albumin by the monocytic cell line MonoMac 6 activates protein kinase C epsilon protein tyrosine kinases and the transcription factors AP-1 and NF-kappaB. Glycoconj J. 2001;18:769–77. doi: 10.1023/a:1021151417556. [DOI] [PubMed] [Google Scholar]
- 30.De Oliveira C, Colette C, Monnier L, Descomps B, Pares-Herbute N. Insulin alters nuclear factor-lambdaB and peroxisome proliferator-activated receptor-gamma protein expression induced by glycated bovine serum albumin in vascular smooth-muscle cells. J Lab Clin Med. 2005;145:144–50. doi: 10.1016/j.lab.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Higai K, Shimamura A, Matsumoto K. Amadori-modified glycated albumin predominantly induces E-selectin expression on human umbilical vein endothelial cells through NADPH oxidase activation. Clin Chim Acta. 2006;367:137–43. doi: 10.1016/j.cca.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Brandt R, Krantz S. Glycated albumin (Amadori product) induces activation of MAP kinases in monocyte-like MonoMac 6 cells. Biochim Biophys Acta. 2006;1760:1749–53. doi: 10.1016/j.bbagen.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Cohen MP, Shea E, Chen S, Shearman CW. Glycated albumin increases oxidative stress, activates NF-kappa B and extracellular signal-regulated kinase (ERK), and stimulates ERK-dependent transforming growth factor-beta 1 production in macrophage RAW cells. J Lab Clin Med. 2003;141:242–9. doi: 10.1067/mlc.2003.27. [DOI] [PubMed] [Google Scholar]
- 34.Guijarro C, Egido J. Transcription factor-kappa B (NF-kappa B) and renal disease. Kidney Int. 2001;59:415–24. doi: 10.1046/j.1523-1755.2001.059002415.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RMCs were treated with glycated albumin or control albumin (200 μg/ml) for different time periods. After treatment, cells were harvested. The mRNA levels of p22phox, NOX1, NOX4, NOX2 and p67phox were determined by real-time PCR and normalized to 18s RNA. Results are the mean ± SE (n=3).