Abstract
Purpose
Although infection with hepatitis C virus (HCV) affects 32 million individuals from Southeast Asia, little is known about the mode of HCV acquisition and the epidemiology of chronic hepatitis C (CHC) in these individuals. Our goal was to examine risk factors for HCV acquisition, prevalence, and clinical characteristics of HCV genotype 6 compared with genotypes 1 and 2/3 in Southeast Asian (SEA) patients.
Methods
We performed a cross-sectional study of 308 consecutive SEA Americans with CHC evaluated by five gastroenterologists from January 2000 to December 2008 at two community clinics in northern California via medical record review, using a case report form.
Results
A significant proportion of patients (41%) could not recall any specific risk factors for HCV acquisition. The most commonly reported risk factor in patients who reported at least one risk factor was history of surgeries (34%), followed by blood transfusion (25%) and acupuncture (13%). Among patients with core sequence testing for HCV genotype (n = 181), the most common HCV genotypes were genotype 1 (42%) and genotype 6 (41%), followed by genotype 2/3 (17%). There were no major differences in the clinical and virological characteristics between the different genotype groups (1 vs. 2/3 vs. 6).
Conclusion
HCV genotype 6 is as common as genotype 1 in SEAs. Commonly known risk factors for HCV acquisition were not readily identifiable in a large proportion of SEA Americans (41%) and may not be useful in identifying at-risk individuals for HCV screening in this population.
Keywords: HCV, Southeast Asians, Genotype 6, Epidemiology, Risk factors
Introduction
Hepatitis C virus (HCV) is one of the most common bloodborne pathogens globally [1]. In the United States, HCV is the leading cause of end-stage liver disease and the most frequent indication for liver transplantation [2]. HCV is also the primary etiology of hepatocellular carcinoma (HCC) in approximately 30% of patients in the United States [3, 4].
The National Health and Nutrition Examination Survey from 1999 to 2002 estimated that 3.2 million individuals in the United States have chronic hepatitis C (CHC); however, this survey might have underestimated the prevalence of this disease in many recent immigrants including those from Southeast Asia, an area with a high HCV disease prevalence (5.6% in Thailand and 6.1% in Vietnam, according to the World Health Organization) [5, 6]. The HCV disease burden from Southeast Asia (approximately 32 million) is in fact higher than the total HCV burden from Europe, North America, and South America combined (approximately 22 million) [5, 6]. According to Bosch and colleagues [7, 8], 15% of patients with primary liver cancer in Asia were positive for anti-HCV. HCV accounts for as many as 25 and 70% of liver cancer cases in Taiwan and Japan, respectively [9, 10].
It is important to identify affected patients because peginterferon plus ribavirin treatment has been shown to decrease disease progression rate to cirrhosis and possibly the risk of developing HCC [11]. Current guidelines from the Centers for Disease Control and Prevention recommend screening for HCV in only those patients with known HCV risk factors such as injection drug use and blood transfusion before 1992 [12, 13]. However, these guidelines may not be applicable to patients who have immigrated from developing countries, as several studies have speculated that patients from developing countries may be exposed to HCV during routine medical or dental care from exposure to contaminated needles or surgical equipment [14–17]. As a result, these patients may not be offered HCV screening because of the lack of commonly known exposure risks seen in those from Western countries. A small study by Dev and colleagues [17] comparing risk factor histories between white and Southeast Asian (SEA) patients residing in Australia have suggested that many of these patients might have contracted HCV with unsafe therapeutic injections such as immunizations, dental therapy, or surgery whereby the patients were often exposed to reused needles rather than the other commonly identified risk factors.
In addition, SEA patients with CHC may have the lesser known HCV genotype 6. Although there have been a few studies reporting the presence of HCV genotype 6 in patients from Southeast Asia, the sample size in these studies are generally small and thus limiting their conclusions on the prevalence and clinical characteristics of HCV genotype 6 relative to the other commonly known genotypes such as genotypes 1 and 2/3 [16, 18–21].
We hypothesized that a significant proportion of SEA patients infected with HCV do not have any identifiable or commonly known exposure risks. Our goal was to describe the epidemiology of CHC including risk factors for HCV acquisition, distribution of HCV genotypes, and clinical characteristics of SEA patients with CHC such as those with genotype 6 in a large consecutive sample of SEA patients with CHC.
Materials and methods
We conducted a cross-sectional study of all patients with CHC identified via ICD-9 electronic query and seen by five gastroenterologists at two community-based clinics in northern California between January 2000 and December 2008. All patients had positive anti-HCV (Roche Amplicor HCV test, version 2.0; Roche Molecular Diagnostics Systems, Branchburg, NY) and positive HCV ribonucleic acid (RNA) polymerase chain reaction (Roche Monitor HCV test; Roche Molecular Diagnostics Systems). All clinical records were reviewed using a patient case report form. A total of 424 patients with CHC were identified. We excluded patients with coinfection with hepatitis B virus (n = 9), patients who were not SEAs (n = 78), or patients who did not have HCV genotype testing (n = 29). A total of 308 patients were included in the study analysis.
HCV genotype testing was performed by core sequencing technique from 1999 to 2003 (HCV Genotype Test, Quest Diagnostics, San Juan Capistrano, CA) and the line probe assay INNO-LiPA (version 1.0 from August 2003 to November 2006 and version 2.0 from November 2006 to 2008; Innogenetics, Ghent, Belgium). As previous studies have observed that HCV genotype 6 subtypes (previously known as genotypes 7, 8, and 9) can be incorrectly classified as genotype 1 or 1b because of a similar nucleotide homology in the 5′-untranslated region of the HCV genome [18, 22, 23], our primary HCV genotype analysis included only those 181 patients whose HCV genotype was determined by core sequencing. Patient baseline characteristics were analyzed according to HCV genotypes: 1, 2/3, and 6. Patients whose HCV genotype testing was performed with the line probe assay INNO-LiPA (n = 122) were analyzed in a secondary analysis comparing HCV genotype distribution among patients who had HCV genotype testing by core sequencing versus patients who had HCV genotype testing by INNO-LiPA assay.
Cirrhosis was defined by the presence of portal hypertension or clinical hepatic decompensation (thrombocytopenia, splenomegaly, ascites, hepatic encephalopathy, varices) or by the presence of stage 4 fibrosis on liver histology.
Questions regarding potential HCV exposure history were asked by treating physicians who were prompted by an electronic medical record template containing a detailed list of risk factors (Table 1). Patients without a record of their exposure history were not included in our risk factor identification analysis. Data concerning exposure to risk factors was collected to study the frequency of commonly known risk factor exposure in the United States in patients with known CHC and not the identification of risk factors that are associated with or the cause of CHC, as this has already been described in a study by Alter [24].
Table 1.
Risk factor questionnaire embedded in electronic medical record template for new patients with chronic hepatitis C
| Embedded risk factor questionnaire |
| History of injection drug use |
| Blood or blood product transfusion |
| Tattoos |
| Body piercing |
| Acupuncture |
| Prior surgeries |
| Exposure to contaminated needles |
| Sexual contact with a person who had hepatitis |
Statistical analysis
Comparison of categorical variables across the different genotype groups was analyzed using chi-square (χ2) tests. Analysis of variance was applied to normally distributed continuous variables, whereas nonparametric statistics was applied to other variables. Differences with a two-tailed P < 0.05 were considered statistically significant. All statistical analysis was performed using Stata version 10.0 (Stata Corporation, College Station, TX). The study was approved by the institutional review board at Stanford University.
Results
Demographic and HCV acquisition risk factors
Almost all SEA patients in this study were foreign-born (n = 307/308). The majority of our patients (98%) were Vietnamese or Vietnamese Chinese, whereas a small minority (2%) was from Cambodia. The majority of our patients were also male (65%). Table 2 describes the demographic characteristics for the entire sample of 308 patients, whereas Table 3 describes the demographic characteristics for the subgroup of 181 patients who underwent HCV genotype testing with the core sequencing assay, the preferred method for accurate identification of patients with genotype 6 and its subtypes. There were no significant differences among the different genotype groups with regard to age, history of smoking or alcohol use, and family history of CHC, chronic hepatitis B, HCC, or liver-related death. There was a trend for higher proportion of males in the genotype 1 group than in genotype 2/3 and genotype 6 (78 vs. 57 and 68%, P = 0.08, respectively).
Table 2.
Demographic characteristics and family history of Southeast Asians with chronic hepatitis C
| All patients (N = 308) | |
|---|---|
| Mean age ± SD (years) (n = 308) | 50 ± 10 |
| Male (n = 308) | 201 (65%) |
| History of smoking (n = 228) | 81 (36%) |
| History of significant alcohol use (n = 231) | 81 (35%) |
| Family history of chronic hepatitis B (n = 227) | 6 (3%) |
| Family history of chronic hepatitis C (n = 227) | 4 (2%) |
| Family history of hepatocellular carcinoma (n = 228) | 16 (7%) |
| Family history of liver-related death (n = 228) | 6 (3%) |
| Family history of any liver disease (n = 228) | 8 (4%) |
Table 3.
Demographic characteristics and family history of Southeast Asians with chronic hepatitis C by hepatitis C virus genotypes
| Patients with HCV genotypes by core sequencing (N = 181) | ||||
|---|---|---|---|---|
| Genotype 1 (N = 77) | Genotype 2/3 (N = 30) | Genotype 6 (N = 74) | Pa | |
| Mean age ± SD (years) (n = 308, 77/30/74) | 49 ± 8.9 | 50 ± 10 | 51 ± 11 | 0.46 |
| Male (n = 308, 77/30/74) | 60 (78%) | 17 (57%) | 50 (68%) | 0.08 |
| History of smoking (n = 228, 40/23/52) | 13 (33%) | 8 (35%) | 18 (35%) | 0.97 |
| History of significant alcohol use (n = 231, 43/22/52) | 9 (21%) | 5 (23%) | 13 (25%) | 0.90 |
| Family history of chronic hepatitis B (n = 227, 44/23/49) | 0 (0%) | 0 (0%) | 1 (2%) | 0.50 |
| Family history of chronic hepatitis C (n = 227, 44/23/49) | 1 (2%) | 2 (9%) | 5 (10%) | 0.30 |
| Family history of hepatocellular carcinoma (n = 228, 44/23/49) | 1 (2%) | 0 (0%) | 0 (0%) | 0.44 |
| Family history of liver-related death (n = 228, 44/23/49) | 0 (0%) | 0 (0%) | 1 (2%) | 0.50 |
| Family history of any liver disease (n = 228, 44/23/49) | 2 (5%) | 1 (4%) | 1 (2%) | 0.78 |
aP value comparing the different genotype groups
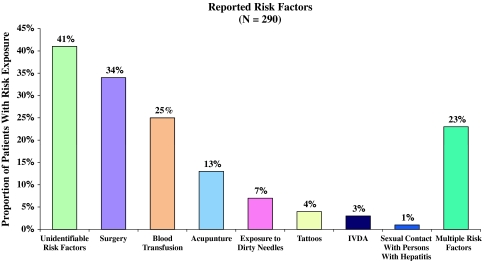
A total of 290 patients (94%) had a risk factor interview. Of these, 119 (41%) were unable to recall any exposure risks (Fig. 1). For patients who were able to recall an exposure risk (n = 171, 59%), the most commonly reported risk factor in this group was a history of surgery (34%), followed by a history of blood transfusion (25%), acupuncture (13%), exposure to contaminated needles (7%), tattoos (4%), intravenous drug abuse (IVDA) (3%), and a history of sexual contacts with individuals with hepatitis (1%). Approximately one-fifth of the patients reported more than one risk factor (n = 67, 23%). The mean age and gender of patients with identifiable risk factors were similar to those without (49 ± 10 vs. 50 ± 9 and 66 vs. 63% male, respectively).
Fig. 1.
Exposure risks for infection with hepatitis C virus
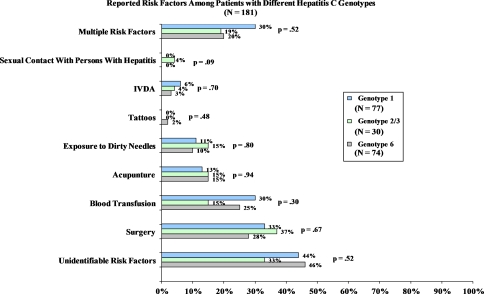
In our subgroup of patients who underwent HCV genotype testing with the core sequencing assay (Fig. 2), we did not observe any statistically significant differences in the distribution of risk factor exposure among the three genotype groups.
Fig. 2.
Exposure risks for infection with hepatitis C virus in Southeast Asians by core sequencing assay
HCV genotype distribution
Results by core sequencing test
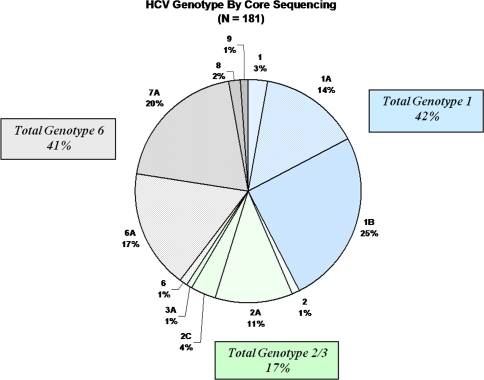
The majority of patients either had genotype 1, 1a, or 1b (n = 77, 42%) or genotype 6 or one of its subtypes (previously known as genotypes 7–9) (n = 73, 41%) (Fig. 3). HCV genotypes 2, 2a, 2c, and 3 were present in only a minority of patients (n = 30, 17%).
Fig. 3.
Hepatitis C genotypes in Southeast Asians by core sequencing assay
Results by INNO-LiPA
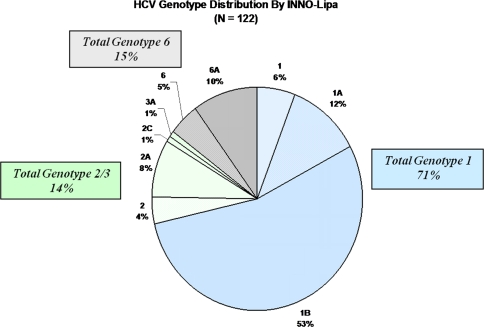
Unlike results seen by core sequencing test, the vast majority of patients tested by INNO-LiPA assay had genotype 1, 1a, or 1b (n = 87, 71%) (Fig. 4). There were 17 patients (14%) with HCV genotype 2, 2a, 2c, or 3a and only 18 patients (15%) with HCV genotype 6.
Fig. 4.
Hepatitis C genotypes in Southeast Asians by INNO-LiPA assay
The distribution of HCV genotypes by core sequencing was significantly different from results by the INNO-LiPA assay (P < 0.0001).
Other clinical/virological characteristics by HCV genotypes
Table 4 describes the clinical and virological characteristics for the entire sample of 308 patients, whereas Table 5 describes the subgroup of 181 patients who underwent HCV genotype testing with the core sequencing assay. For our entire sample, only 36 patients (12%) had cirrhosis and 6 patients (2%) had a diagnosis of HCC at their initial evaluation. Patients who had HCV genotype 1 had a higher prevalence of cirrhosis than patients with HCV genotypes 2/3 and 6; however, this finding was not statistically significant (P = 0.30).
Table 4.
Baseline patient clinical and virological characteristics for all patients
| All patients (N = 308) | |
|---|---|
| White blood cells (K/μL) (n = 246) | 5.9 (3.1–11.2) |
| Platelet count (K/μL) (n = 245) | 198 (56–461) |
| Alanine aminotransferase (U/L) (n = 251) | 78 (13–597) |
| Total bilirubin (mg/dL) (n = 251) | 0.8 (0.3–4.1) |
| Albumin (g/dL) (n = 253) | 4.3 (0.4–5.7) |
| HCV RNA (IU/mL) (n = 250) | 1.5 × 106 (2.7 × 103 to 2.7 × 107) |
| Hepatocellular carcinoma (n = 308) | 6 (2%) |
| Cirrhosis (n = 308) | 36 (12%) |
Table 5.
Baseline patient clinical and virological characteristics for those with HCV genotypes tested by core sequencing of hepatitis C virus genotypes
| Patients with HCV genotype by core sequencing (N = 181) | ||||
|---|---|---|---|---|
| Genotype 1 (N = 77) | Genotype 2/3 (N = 30) | Genotype 6 (N = 74) | Pa | |
| White blood cells (K/μL) (n = 246, 56/22/48) | 6.3 (3.1–9.8) | 5.7 (3.6–9.2) | 5.8 (3.2–11.2) | 0.83 |
| Platelet count (K/μL) (n = 245, 55/22/48) | 191 (56–334) | 195 (115–461) | 202 (90–359) | 0.36 |
| Alanine aminotransferase (U/L) (n = 251, 56/25/49) | 102 (34–350) | 75 (17–340) | 90 (28–249) | 0.45 |
| Total bilirubin (mg/dL) (n = 251, 55/24/51) | 0.8 (0.3–2.5) | 0.8 (0.3–3.9) | 0.8 (0.3–2) | 0.81 |
| Albumin (g/dL) (n = 253, 56/24/51) | 4.4 (3.4–5.2) | 4.3 (0.8–5) | 4.3 (1.7–5.2) | 0.36 |
| HCV RNA (IU/mL) (n = 250, 54/21/55) | 1.4 × 106 (2.2 × 104 to 1.1 × 107) | 2.8 × 105 (2.7 × 103 to 6.2 × 106) | 1.1 × 106 (1.2 × 104 to 1.5 × 106) | 0.16 |
| Hepatocellular carcinoma (n = 308, 77/30/74) | 2 (3%) | 0 (0%) | 1 (1%) | 0.63 |
| Cirrhosis (n = 308, 77/30/74) | 10 (13%) | 1 (3%) | 6 (8%) | 0.30 |
Results are expressed in median (range) or proportion (%)
aP value comparing the different genotype groups
No significant differences were observed among the three different HCV genotype groups with regard to alanine aminotransferase (ALT), total bilirubin, albumin, white blood cell count, platelets, and liver histology (Table 5). Patients with HCV genotypes 1 and 6 appeared to have higher baseline median HCV RNA levels than patients with genotype 2/3, although this difference did not reach statistical significance (1.4 × 106 vs. 1.1 × 106 vs. 2.8 × 105, P = 0.16, respectively).
Discussion
Compared with results from studies of Western patients, the epidemiology of CHC appears to be different in our SEA cohort: HCV genotype 6 is as common as genotype 1 (approximately 40% each), with HCV genotype 2/3 making up only a small minority (<20%), and many patients (41%) were unable to recall a risk factor for HCV acquisition. In addition, among those who can recall a risk factor, the majority had history of surgeries and/or blood transfusion and only few (<5%) with a history of IVDA. Patients with HCV genotype 6 appear to present with similar clinical characteristics as those with genotype 1 or 2/3.
Although HCV genotype 6 has been reported in patients from Asia, its prevalence and clinical characteristics have not been well described in a large patient sample by using an accurate genotyping method such as core sequencing test [18–21]. Our study included more than 300 SEA patients with CHC and a total of 181 patients with HCV genotype testing by core sequencing. The prevalence of HCV genotype 6 in our patients with core sequencing HCV genotype testing was 41% and similar to that of HCV genotype 1 (42%). In contrast, in our subset of 122 patients who had HCV genotype testing done with the INNO-LiPA assay, the prevalence of HCV genotype 6 was much lower (15%) and the total prevalence of HCV genotype 1 was 71%, consistent with previous studies that reported mistyping of HCV genotype 6 as genotype 1 by the INNO-LiPA assay [18, 22, 23]. Our study, however, was not intended to directly address the discordance rate of HCV genotyping between the core sequencing and the INNO-LiPA test, as this has been previously described [18], but rather to describe the prevalence of the different genotypes in SEA patients with CHC by using the gold standard method, which is the core sequencing test. Accurate HCV genotyping is important, as HCV genotypes have been known to be among the most important predictors for treatment outcomes to standard peginterferon and ribavirin therapy, at least for patients with genotypes 1 and 2/3 [25–28]. More limited data also suggest that patients with lesser known HCV genotypes 4, 5, and 6 may exhibit different treatment outcomes from those with genotype 1 and 2/3 [21]. With regard to baseline demographic, clinical, and virological characteristics, patients with HCV genotype 6 in our study did not seem to exhibit major differences from those with genotype 1 or 2/3.
In addition to the high prevalence of HCV genotype 6, our patient cohort had a lower than expected rate of identifiable risk factors for HCV acquisition. Many of the patients who could not recall their exposure risk when asked by their physicians might have been exposed to HCV via very routine/casual medical and dental practices that they did not relate to, then, as potential exposure risks for viral hepatitis. In addition, patients’ ability to recall exposure risk that did not lead to any immediate sequelae many years or decades later is usually poor. Nevertheless, the majority of Western patients can still recall their exposure risk because they were generally more eventful such as blood transfusion or IVDA, whereas recalling casual exposures such as routine dental cleaning or injections from childhood vaccinations are likely to be much more difficult.
Indeed, a study of SEA patients residing in Australia observed that many of them might have mistakenly believed that therapeutic injections and routine medical practices administered by registered practitioners in their countries are safe and possibly lead many patients to underreport their risk factors [17]. Another retrospective study that included 103 SEA Americans also reported suspected unsafe therapeutic injections to be a major cause of HCV transmission in their patients [16].
Limited studies from Vietnam have identified geographically dependent risk factors that are also not commonly seen in Western countries: ventoused scarifications (also known as cupping, the practice of administering glass cups onto soft skin as part of a therapeutic procedure involving bloodletting) [29] and acupuncture were associated with the transmission of HCV in Ho Chi Minh City in Southern Vietnam, whereas a history of hospitalization and tattoos were identified as risk factors for HCV acquisition in two randomly selected rural districts in Northern Vietnam [15, 30].
In a subanalysis of history of exposure to risk factors in patients who underwent genotype testing by the core sequencing assay, we did not observe any statistically significant differences in the frequency of risk factor exposure among the different genotype groups.
Risk factor data in our study were not obtained with prospective research questionnaire, which might have been more effective in soliciting patients’ recall of potential distant exposure risk than care providers at routine clinical visits; however, as discussed earlier, patients’ ability to recall distant exposure during very casual and routine medical and dental encounters might be quite limited regardless and these results would be more representative of what may be seen in a real-life setting. Our study is not population based, but our patients were recruited from community clinics as opposed to tertiary care or university centers and their results may still be more applicable to the general patient population with CHC (only 12% of our patients had cirrhosis and 2% HCC).
In summary, exposure risk for HCV acquisition was unidentifiable in routine clinical visits in almost half of the SEAs with CHC in our study sample, despite use of preset risk factor questionnaire on electronic medical record template. Many of these patients (41%) also had the lesser known HCV genotype 6 by HCV genotype core sequencing testing. Further studies are needed to examine treatment outcomes in patients with HCV genotype 6 and to prospectively survey risk factors for HCV acquisition to explore other potential routes of viral transmission in this population. Commonly known risk factors for HCV acquisition may not be as useful in identifying at-risk Asian patients for appropriate HCV screening.
Conflict of interest statement
None.
Abbreviations
- ALT
Alanine aminotransferase
- CHC
Chronic hepatitis C
- HCC
Hepatocellular carcinoma
- HCV
Hepatitis C virus
- IVDA
Intravenous drug abuse
- RNA
Ribonucleic acid
- SEA
Southeast Asian
References
- 1.Hepatitis C—global prevalence (update). Wkly Epidemiol Rec 1999;74(49):425–427 [PubMed]
- 2.Mallette C, Flynn MA, Promrat K. Outcome of screening for hepatitis C virus infection based on risk factors. Am J Gastroenterol. 2008;103(1):131–137. doi: 10.1111/j.1572-0241.2007.01522.x. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology. 2002;36(5 Suppl 1):S74–S83. doi: 10.1002/hep.1840360710. [DOI] [PubMed] [Google Scholar]
- 4.Hassan MM, Frome A, Patt YZ, El-Serag HB. Rising prevalence of hepatitis C virus infection among patients recently diagnosed with hepatocellular carcinoma in the United States. J Clin Gastroenterol. 2002;35(3):266–269. doi: 10.1097/00004836-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144(10):705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen MH, Keeffe EB. Chronic hepatitis C: genotypes 4 to 9. Clin Liver Dis. 2005;9(3):411–426. doi: 10.1016/j.cld.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Bosch FX, Ribes J, Borras J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999;19(3):271–285. doi: 10.1055/s-2007-1007117. [DOI] [PubMed] [Google Scholar]
- 8.Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127(5 Suppl 1):S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Chen DS. Hepatocellular carcinoma in Taiwan. Hepatol Res. 2007;37(Suppl 2):S101–S105. doi: 10.1111/j.1872-034X.2007.00170.x. [DOI] [PubMed] [Google Scholar]
- 10.Umemura T, Ichijo T, Yoshizawa K, Tanaka E, Kiyosawa K. Epidemiology of hepatocellular carcinoma in Japan. J Gastroenterol. 2009;44(Suppl 19):102–107. doi: 10.1007/s00535-008-2251-0. [DOI] [PubMed] [Google Scholar]
- 11.Veldt BJ, Heathcote EJ, Wedemeyer H, Reichen J, Hofmann WP, Zeuzem S, et al. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med. 2007;147(10):677–684. doi: 10.7326/0003-4819-147-10-200711200-00003. [DOI] [PubMed] [Google Scholar]
- 12.Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Recomm Rep 1998;47(RR-19):1–39 [PubMed]
- 13.Alter MJ, Seeff LB, Bacon BR, Thomas DL, Rigsby MO, Di Bisceglie AM. Testing for hepatitis C virus infection should be routine for persons at increased risk for infection. Ann Intern Med. 2004;141(9):715–717. doi: 10.7326/0003-4819-141-9-200411020-00013. [DOI] [PubMed] [Google Scholar]
- 14.Li CP, Hwang SJ, Lu CL, Chan CY, Wu JC, Lee FY, et al. Risk factor analysis of patients with chronic hepatitis C in Taiwan. Zhonghua Yi Xue Za Zhi (Taipei) 1996;58(4):275–280. [PubMed] [Google Scholar]
- 15.Ngo Y, Maugat S, Duong QT, Nguyen TN, Astagneau P. Risk of hepatitis C related to traditional medicine: a case control study in Ho Chi Minh City, Vietnam. Rev Epidemiol Sante Publ. 2007;55(2):107–112. doi: 10.1016/j.respe.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Cheng JT, Hsien C, Sun HE, Tong MJ. The emerging importance of chronic hepatitis C infection in Asian Americans. Am J Gastroenterol. 2006;101(12):2737–2743. doi: 10.1111/j.1572-0241.2006.00831.x. [DOI] [PubMed] [Google Scholar]
- 17.Dev A, Sundararajan V, Sievert W. Ethnic and cultural determinants influence risk assessment for hepatitis C acquisition. J Gastroenterol Hepatol. 2004;19(7):792–798. doi: 10.1111/j.1440-1746.2004.03381.x. [DOI] [PubMed] [Google Scholar]
- 18.Dev AT, McCaw R, Sundararajan V, Bowden S, Sievert W. Southeast Asian patients with chronic hepatitis C: the impact of novel genotypes and race on treatment outcome. Hepatology. 2002;36(5):1259–1265. doi: 10.1053/jhep.2002.36781. [DOI] [PubMed] [Google Scholar]
- 19.Fung J, Lai CL, Hung I, Young J, Cheng C, Wong D, et al. Chronic hepatitis C virus genotype 6 infection: response to pegylated interferon and ribavirin. J Infect Dis. 2008;198(6):808–812. doi: 10.1086/591252. [DOI] [PubMed] [Google Scholar]
- 20.Hui CK, Yuen MF, Sablon E, Chan AO, Wong BC, Lai CL. Interferon and ribavirin therapy for chronic hepatitis C virus genotype 6: a comparison with genotype 1. J Infect Dis. 2003;187(7):1071–1074. doi: 10.1086/368217. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen MH, Trinh HN, Garcia R, Nguyen G, Lam KD, Keeffe EB. Higher rate of sustained virologic response in chronic hepatitis C genotype 6 treated with 48 weeks versus 24 weeks of peginterferon plus ribavirin. Am J Gastroenterol. 2008;103(5):1131–1135. doi: 10.1111/j.1572-0241.2008.01793.x. [DOI] [PubMed] [Google Scholar]
- 22.Bukh J, Purcell RH, Miller RH. Sequence analysis of the core gene of 14 hepatitis C virus genotypes. Proc Natl Acad Sci USA. 1994;91(17):8239–8243. doi: 10.1073/pnas.91.17.8239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stuyver L, Wyseur A, Arnhem W, Hernandez F, Maertens G. Second-generation line probe assay for hepatitis C virus genotyping. J Clin Microbiol. 1996;34(9):2259–2266. doi: 10.1128/jcm.34.9.2259-2266.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alter MJ. Prevention of spread of hepatitis C. Hepatology. 2002;36(5 Suppl 1):S93–S98. doi: 10.1002/hep.1840360712. [DOI] [PubMed] [Google Scholar]
- 25.Davis GL, Lau JY. Factors predictive of a beneficial response to therapy of hepatitis C. Hepatology. 1997;26(3 Suppl 1):122S–1227S. doi: 10.1002/hep.510260721. [DOI] [PubMed] [Google Scholar]
- 26.Martinot-Peignoux M, Marcellin P, Pouteau M, Castelnau C, Boyer N, Poliquin M, et al. Pretreatment serum hepatitis C virus RNA levels and hepatitis C virus genotype are the main and independent prognostic factors of sustained response to interferon alfa therapy in chronic hepatitis C. Hepatology. 1995;22(4 Pt 1):1050–1056. doi: 10.1002/hep.1840220406. [DOI] [PubMed] [Google Scholar]
- 27.McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339(21):1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 28.Poynard T, Marcellin P, Lee SS, Niederau C, Minuk GS, Ideo G, et al. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT) Lancet. 1998;352(9138):1426–1432. doi: 10.1016/S0140-6736(98)07124-4. [DOI] [PubMed] [Google Scholar]
- 29.Dunglison R. Medical Lexicon: a Dictionary of Medical Science. 15th ed. Philadelphia: Blanchard and Lea; 1857. 992
- 30.Nguyen V, McLaws M, Dore G. Prevalence and risk factors for hepatitis C infection in rural north Vietnam. Hepatol Int 2007;1(3):387–393 [DOI] [PMC free article] [PubMed]