Abstract
Autoimmune hepatitis (AIH), primary biliary cirrhosis, and primary sclerosing cholangitis are the three major autoimmune diseases affecting the liver, and of these three, AIH is the most typical autoimmune disease being characterized by a T-cell-rich infiltrate, raised circulating γ-globulins, autoantibodies, HLA associations, and links with other autoimmune diseases. It is the only one, of the three diseases, that responds well to immunosuppressive therapy. AIH is caused by dysregulation of immunoregulatory networks and the consequent emergence of autoreactive T cells that orchestrate a progressive destruction of hepatocytes leading untreated to liver failure. T cells play a major role in the immunopathogenesis, and both CD4+ and CD8+ T cells are involved together with effector responses mediated by NK cells, γδ T cells, and macrophages. A number of triggering factors have been proposed including viruses, xenobiotics, and drugs, but none have been conclusively shown to be involved in pathogenesis.
Keywords: Autoimmune liver disease, Regulatory T cell, Th17, Lymphocytes, Recruitment, Mycophenolate mofetil
Presentation
The clinical presentation of autoimmune hepatitis (AIH) is protean and the clinical course may be characterized by fluctuating periods of increased and decreased activity. As with most autoimmune diseases, AIH is more common in females (70%) and it affects all ages [1] and ethnic groups [2], although with different incidences. The prevalence in developed countries is 1:5,000–1:10,000. It can occur at any age, although type 2 AIH is much more common in childhood. More than 20% of adults with AIH develop disease after the age of 60 years [1, 3]. At one extreme, patients may present with fulminant hepatitis and liver failure [4], whereas other patients, particularly the elderly, may be asymptomatic with an indolent form of the disease. Patients with acute hepatitis have symptoms of jaundice, arthralgia, anorexia, and fatigue that are indistinguishable from those seen with acute viral hepatitis. In such patients, liver biopsy may show features of an acute hepatitis without evidence of significant fibrosis or cirrhosis. In other patients who present with clinical features suggesting acute hepatitis, liver biopsy shows changes of underlying chronic liver disease including bridging fibrosis or cirrhosis [5, 6]. The disease may also have an indolent course, presenting only when the patient develops symptoms of decompensated cirrhosis, with the diagnosis being made for the first time at the end of the disease process. This pattern of presentation is more frequently seen in older patients [1]. Increasingly, patients are being picked up with abnormal liver biochemistry on routine testing for other symptoms or conditions.
AIH is frequently associated with other autoimmune diseases, commonly thyroiditis, ulcerative colitis, type 1 diabetes, rheumatoid arthritis, or celiac disease. Physical examination may be normal or reveal hepatomegaly, splenomegaly, jaundice, and signs of chronic liver disease. AIH may first become evident during pregnancy or in the early postpartum period [7–9]. Furthermore, postpartum exacerbations may occur in patients whose condition improved during pregnancy as a consequence of the natural pregnancy-associated immunosuppressed state [10, 11].
Laboratory abnormalities
AIH is characterized by elevated serum transaminase levels reflecting hepatocyte damage with typically modest elevations in alkaline phosphatase levels. Patients with acute presentation may have jaundice, but otherwise this is a manifestation of severe exacerbations or a feature of end-stage disease. Immunology testing reveals hypergammaglobulinemia and high-titre circulating autoantibodies indicating immune activation. AIH has been subdivided into three main categories according to the autoantibodies detected:
Type 1 AIH, characterized by the presence of anti-smooth muscle antibodies (SMA) and/or anti-nuclear antibodies (ANA). This is the most common form.
Type 2 AIH, characterized by anti-liver kidney microsomal antibodies. This form is rare in adults.
Type 3 AIH, characterized by antibodies to soluble liver or liver-pancreas antigens.
Antibodies against cyclic citrullinated peptides (anti-CCPs) have been validated as specific diagnostic and prognostic markers of rheumatoid arthritis. Anti-CCPs have been found in 9% of patients with AIH [12]. These patients have been reported to have a higher frequency of histological cirrhosis at presentation and to be at greater risk of dying from liver failure [13]. Other autoantibodies that may be found include autoantibodies to asialoglycoprotein receptor and anti-neutrophil cytoplasmic antibodies (ANCA). Approximately 20% of patients will not have any of these autoantibodies, and they often have high immunoglobulin (IgG) and sometimes develop autoantibodies at a later stage.
Histological findings
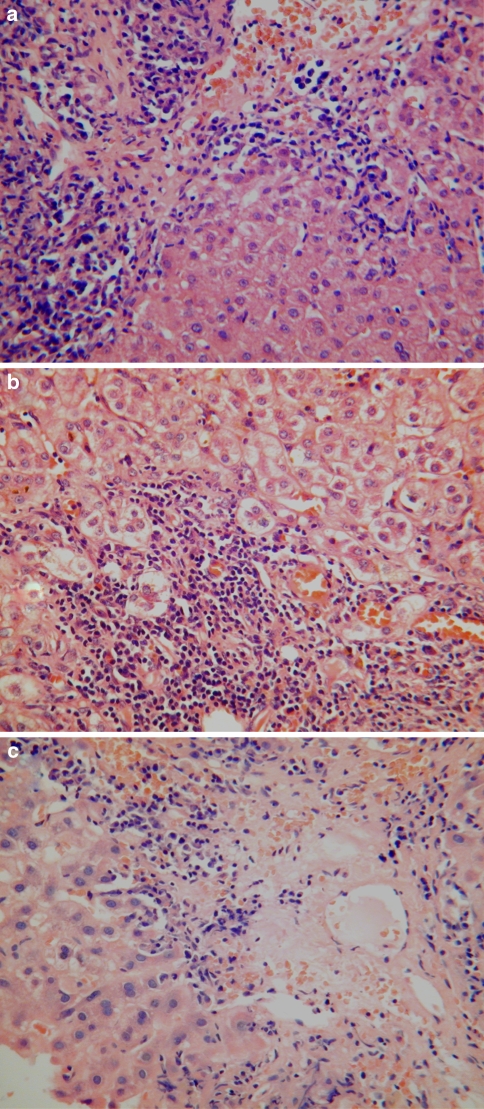
Patients presenting with chronic AIH typically have a plasma cell-rich mononuclear infiltrate mainly involving portal and periportal regions (Fig. 1a). Plasma cells are not invariably present and a paucity of plasma cells does not therefore exclude a diagnosis of AIH. Interface hepatitis is also a key diagnostic feature of AIH and is typically associated with ballooning and rosetting of periportal hepatocytes (Fig. 1b). These changes lead to the development of periportal fibrosis, initially as delicate strands of immature collagen enveloping small clusters of entrapped hepatocytes. Subsequently, there is formation of broader fibrous septa associated with bridging and nodule formation. Progression to cirrhosis occurs in 40–80% of cases. Inflammatory activity often subsides when cirrhosis has developed, making it difficult to distinguish end-stage AIH from other causes of cirrhosis.
Fig. 1.
Histological features of autoimmune hepatitis. a A dense plasma cell-rich portal mononuclear inflammatory infiltrate is associated with moderate interface hepatitis. b Interface hepatitis with ballooning and rosetting of entrapped periportal hepatocytes. c A plasma cell-rich centrilobular mononuclear inflammatory infiltrate is associated with confluent and bridging hepatocyte necrosis
Varying degrees of lobular necroinflammatory activity are also commonly seen in AIH. These range from mild spotty inflammation with acidophil body formation to more severe lesions associated with confluent or bridging necrosis (Fig. 1c). Giant cell transformation of hepatocytes is also quite common—this usually occurs to a minor degree, but in some cases is sufficiently extensive to warrant the term “giant cell hepatitis” [14, 15]. Lobular necroinflammatory changes tend to be more prominent in patients with an acute presentation. In patients presenting with fulminant hepatic failure, there are typically large areas of panacinar necrosis. Many patients with an apparently acute presentation of AIH have histological features of an underlying chronic hepatitis, including evidence of bridging fibrosis or cirrhosis [5, 6].
Liver biopsy plays an important role in establishing a diagnosis of AIH. This is based both on the presence of typical features supporting a diagnosis of AIH and on the absence of atypical features (e.g., biliary abnormalities or steatosis) that might point to an alternative diagnosis. Liver biopsy should be performed to confirm the diagnosis unless there is a good reason or contraindication. Liver biopsy also provides valuable information about disease severity, which has implications for instigating and monitoring responses to immunosuppressive therapy [16]. The pattern and severity of inflammatory activity at the time of presentation are predictive for subsequent progression to fibrosis and cirrhosis [17]. Interface hepatitis generally responds well to immunosuppression, whereas bridging necrosis more frequently progresses to fibrosis or cirrhosis. The presence of cirrhosis at the time of presentation has also been associated with an adverse prognosis [18].
However, such patients frequently have ongoing inflammatory activity and still benefit from immunosuppression. Suppression of inflammatory activity is associated with reduction in fibrosis including some cases in which there appears to be reversal of an established diagnosis of cirrhosis [19, 20]. Conversely, worsening of inflammatory activity during corticosteroid therapy is associated with progression of fibrosis [21].
Diagnosis of AIH
There is no single diagnostic test for AIH. Instead, the diagnosis is based on a combination of clinical, biochemical, immunological, and histological findings, which are summarized in Table 1. In an attempt to develop uniform diagnostic criteria, a working classification for the diagnosis of AIH was devised by the International Autoimmune Hepatitis Working Group, which generated a scoring system based on a combination of clinical, serological, and histological criteria. The original scoring system in 1993 [22] was revised in 1999 [23]. According to the revised system, a definite diagnosis can be made when the aggregate score is more than 15 before treatment and more than 17 after treatment; scores of 10–15 before treatment and 12–17 after treatment imply probable AIH [23]. Points in favor of the diagnosis are female gender, elevated transaminase levels, elevated IgG, non-organ-specific antibodies (ANA or SMA or LKM-1 antibodies), and a family history of autoimmune disease. Supportive features on liver histology include interface hepatitis, lymphoplasmacytic infiltrate, hepatocyte rosetting, and absence of biliary changes, Mallory’s hyaline, or steatosis. Points against the diagnosis are male gender, cholestatic enzymes, recent medications, alcohol consumption of more than 60 g/day, anti-mitochondrial antibodies, or viral hepatitis markers. This scoring system is useful for defining patients in research studies but is not practical for day-to-day clinical use. The system was further revised in 2008 with the view to provide a simple scoring system that could be used in clinical practice. The simplified scoring system incorporates results of autoantibody testing (ANA, SMA, and LKM), IgG levels, histology, and the absence of viral etiologies. [24]. Each of these four features is scored on a scale of 0–2, and a total score of 7 or more is defined as definite AIH [24]. Although it has good sensitivities and specificities for diagnosing AIH, it does not perform well in patients with concomitant fatty liver disease, biliary diseases, or fulminant hepatitis, just the situations where diagnosis can be most challenging [25–27]. The criteria to diagnose AIH in clinical practice are shown in Table 1.
Table 1.
Diagnostic criteria for AIH
| Parameters | Diagnosis of autoimmune hepatitis |
|---|---|
| Clinical | Female gender Associated with other autoimmune diseases |
| Autoantibodies | ANA or SMA >1:40 (type 1) |
| LKM >1:40 (type 2) | |
| SLA+ (type 3) | |
| Immunoglobulin | IgG > upper normal limit |
| Biochemistry | Hepatitic picture (raised AST/ALT levels) |
| Histology | Plasma cell-rich mononuclear infiltrate |
| Interface hepatitis with ballooning and rosetting of periportal hepatocyte; +/− periportal fibrosis | |
| Lobular necroinflammatory activity | |
| No bile duct loss or chronic cholestasis | |
| Radiology and ERCP | Normal |
| Exclusion of other etiology | Exclude viral hepatitis, metabolic, drug, and alcoholic etiology |
| Response to steroid | Good |
Types of AIH
Attempts have been made to divide AIH into subtypes, depending primarily on the pattern of autoantibodies. Although useful for research purposes, these distinctions are not ideal. Some clinical differences are seen between the subtypes, particularly in response to treatment and outcome between type 1 and type 2 AIH, but it must be appreciated that these are not distinct disease entities. Type 1 AIH is most common. It can present at any age, is characterized by ANA, SMA [23], and in some cases perinuclear antineutrophil cytoplasmic antibodies (pANCA) [28, 29]; treatment failure is rare. ANA and SMA titers of more than 1:80 are generally accepted as positive, although positive autoantibodies of uncertain clinical significance are more frequently detected in the elderly [23]. Type 2, which occurs in a younger age group, is associated with positive anti-liver kidney microsomal antibodies and is a more aggressive disease [30, 31]. Treatment failure is more frequent and relapse after drug withdrawal is almost inevitable; most of these patients need lifelong immunosuppressive therapy [1]. The putative type 3 autoimmune hepatitis (AIH type 3) is characterized by autoantibodies against SLA/LP (with or without ANA or SMA) and has been associated with more frequent relapse [32, 33].
Natural history
The seminal trials of immunosuppression in AIH were published more than 25 years ago. They revealed that untreated AIH has a poor prognosis with 5- and 10-year survival rates of 50 and 10%, respectively, whereas treatment with prednisolone is associated with excellent short- and long-term survival [34, 35]. Up to 30% of adult patients have histological features of cirrhosis at diagnosis [18, 36] and progression of fibrosis can occur in patients with inflammatory activity despite steroid therapy [18]. The presence of cirrhosis at baseline significantly increases the risk of subsequent death or liver transplantation [18, 37]. The risk of hepatocellular carcinoma (HCC) in autoimmune liver disease is associated with the development of cirrhosis and is no greater than in other non-viral causes of cirrhosis [38]. The 10-year overall survival of patients with AIH ranges between 80 and 93% [18]. The increasing diagnosis of AIH on routine blood testing, particularly in the elderly, has uncovered a group of patients with asymptomatic AIH who appear to have a very indolent course. These patients are often elderly, and it is not clear what their long-term outcome is or whether they always require immunosuppressive therapy.
Overlap syndromes with other autoimmune liver diseases
Overlap syndromes are classified as autoimmune liver diseases that are difficult to classify within a single diagnostic category, such as primary biliary cirrhosis (PBC), primary sclerosing cholangitis (PSC), or AIH, because they have biochemical, immunological, clinical, or histological features suggestive of more than 1 disease process [39–42]. The common forms seen are PBC with features of AIH and biliary features suggestive of PSC in AIH. They usually occur at the same time (true overlap syndromes), but in some cases, patients present with features of one disease and subsequently develop features of the other, sometimes after intervals of several years (sequential overlap syndromes). Sequential syndromes involving PBC and AIH typically present with features of PBC first, whereas in those involving PSC and AIH, features of AIH usually occur first [43].
In the context of PBC, the “overlap syndrome” probably represents one end of a spectrum of immune-mediated injury in which damage to hepatocytes is more prominent and takes the form of interface hepatitis. Even in a biliary disease such as PBC, a degree of parenchymal inflammation is seen in the form of interface or lobular hepatitis. This could reflect antigen-specific T-cell destruction of hepatocytes, but as both the antigen and the cellular target in PBC are so well characterized, this seems unlikely. Hepatic inflammation may therefore be mediated by bystander mechanisms that cause collateral damage to hepatocytes [44, 45]. Collateral hepatocyte damage can result in the release of intracellular antigens and the development of autoantibodies. This categorization is of more than academic importance because it has implications for outcome and treatment. There is now good evidence that patients with PBC associated with florid interface hepatitis respond to immunosuppressive therapy whereas those in whom the biliary features predominate do not and should be treated with ursodeoxycholic acid alone [42, 46, 47]. Why hepatitis should be more steroid sensitive than biliary inflammation is not clear. One possibility is that biliary epithelial cells maintain effector cell survival by providing anti-apoptotic signals through cell-surface integrin ligands such as VCAM-1 and by secreting cytokines (E. Humphreys, D. H. Adams, S. C. Afford, B. Eksteen, unpublished data).
Both AIH and PSC can be associated with inflammatory bowel disease (IBD) [40, 48], and there is evidence that they can occur sequentially in patients who present with typical AIH subsequently developing PSC [43, 49–51]. Evidence from our laboratory suggests that the liver disease is driven by the recruitment of effector lymphocytes that were activated in the gut [52]. When lymphocytes are activated in gut-associated lymphoid tissues, they are not only programmed to respond to antigen but are also imprinted with a homing phenotype that directs their subsequent trafficking back to the gut [53]. After antigen clearance, long-lived memory cells remain that retain gut tropism and thereby provide immune surveillance against the same pathogen entering the gut in the future. The molecular basis of this tissue-specific homing has recently been elucidated and involves interactions with tissue-specific adhesion molecules and chemokines on the endothelium lining the vessels in the target tissue, which are recognized by lymphocytes with appropriate receptors. Endothelium in the gut expresses a unique adhesion molecule called mucosal addressin cell adhesion molecule-1 (MADCAM-1), which is absent from other vascular beds, and a unique chemokine CCL25, which is restricted to the small bowel. Activation of lymphocytes in the gut imprints them with the receptors for these gut-specific molecules: the integrin α4β7 and the chemokine receptor CCR9, respectively. Twenty percent of the T cells infiltrating the liver in AIH/PSC complicating IBD are α4β7+CCR9+ and thus of gut origin, whereas these cells are found at very low frequencies in other liver diseases [54]. Furthermore, these memory/effector T cells secrete IFN-γ, suggesting that they are effector cells capable of promoting liver inflammation. Both MADCAM-1 and CCL25, which are absent from normal liver, are present on hepatic endothelium in liver diseases associated with IBD, providing a mechanism for recruiting these cells. Thus, some mucosal lymphocytes can bind liver endothelium, allowing them to recirculate between the liver and the gut to provide immune surveillance across both sites. If these cells are activated by cross-reactive liver antigens or gut antigens that have entered through the portal circulation, this can lead to their local expansion and the establishment of chronic inflammation in the form of AIH or PSC [44].
The link between AIH and PSC is further supported by the observations that they can occur together, or sequentially, particularly in children. The King’s liver unit reported the presence of histological and/or radiological biliary features compatible with PSC in 50% of children who otherwise fulfilled criteria for AIH [55]. Czaja and Carpenter [56] reported that 20 of 84 adult patients with AIH had biliary changes on liver biopsy in the absence of anti-mitochondrial autoantibodies. In other respects, they behaved like AIH and responded to immunosuppressive therapy [56]. Current evidence suggests that PSC may be preceded in some patients by AIH, particularly in children and adolescents. The pediatric patients usually respond to corticosteroids, unlike patients with classical PSC, although as the biliary features progress, response to immunosuppression may be lost [55]. The reasons for the relative treatment resistance of AIH with biliary features may involve the same mechanisms discussed earlier under PBC. All this evidence suggests that in some patients, particularly those with IBD, autoimmune sclerosing cholangitis and AIH may be part of the same disease process, with AIH progressing to a biliary syndrome resembling PSC. The implications from these findings are that AIH/PSC overlap is more common than previously suspected and biliary features should be sought carefully in patients with AIH both histologically and, if indicated, MR cholangiography. This is particularly true if an elevated alkaline phosphatase level is noted [57]. Addition of ursodeoxycholic acid rather than an increase in immunosuppression may improve biochemistry in such patients. Interestingly, patients with overt AIH who test positive for anti-mitochondrial antibodies (AMAs) at initial presentation and are treated with corticosteroid therapy have shown no clinical or histological evidence of PBC despite the continued detection of AMAs over a long-term follow-up [58].
Genetic susceptibility
AIH is a polygenic disorder and although the heritable component is relatively small, there is good evidence that several genes can affect the risk of developing the disease. The strongest genetic association is with the major histocompatibility complex (MHC). Because T-cell-dependent immune responses are MHC restricted, this implicates T-cell-mediated mechanisms in the etiology of AIH. Molecular mimicry between foreign and self-antigens may explain the loss of self-tolerance and the autoimmunity in anatomically distant organs.
However, such associations are complicated by differences in susceptibility between racial and geographical groups. In whites, AIH susceptibility is associated with HLA-DRB1*0301 and HLA-DRB1*0401 alleles, with other susceptibility alleles sharing a similar motif in DRβ71 and the CTLA4*G allele. HLA DRB1*0301 and DRB1*0401 alleles encode a 6-amino acid sequence at positions 67–72 in the DRβ polypeptide chain of MHC class II. A lysine at position DRβ71 is the key determinant and disease severity is associated with the number of alleles encoding lysine at DRβ71 [59, 60]. Elderly patients have a higher frequency of HLA DRB1*04 than young adults [1]. In children, type 1 AIH is commonly associated with the HLA-DRB1*03 and HLA-DRB1*13 alleles [61]. The presence of DR3 is associated with a higher incidence of treatment failure and a lower probability of going into remission [62].
The peripheral T-cell repertoire is shaped by events in the thymus when autoreactive T cells are deleted. Rare mutations that affect thymic function can lead to AIH, providing further evidence of a T-cell-mediated autoimmune etiology. Medullary thymic epithelial cells present self-antigens on MHC molecules to thymocytes (developing T lymphocytes) and those that respond too strongly to these self-antigens are deleted [62]. The effective presentation of self-antigens by thymic epithelial cells is regulated by the autoimmune regulator gene AIRE, and functional mutations in this gene lead to autoimmune diseases in both mice and humans [63, 64]. AIRE mutations classically result in the autoimmune polyglandular syndrome type 1 (APS type 1), also known as autoimmune polyendocrinopathy, candidosis, and ectodermal dystrophy, and about 20% of these patients have AIH [65]. However, sporadic cases of AIH both in children and in adults are not associated with the common AIRE mutations, although different defects in the AIRE gene may result in phenotypically distinct syndromes of autoimmunity [64–66]. Thymic selection cannot provide complete protection against autoimmunity, particularly in individuals who express HLA haplotypes that make them more likely to recognize self-antigens despite adequate thymic selection; these include HLA-DR3 (A1-B8-DR3) and DR4, which increase susceptibility to type 1 AIH [67] and DR7 associated with type 2 AIH [68], and the development of immune responses against the hepatocyte enzyme, CYP2D6 [69, 70].
Etiology and immunopathogenesis
Molecular mimicry
AIH is characterized by a loss of immune tolerance to antigens on hepatocytes, leading to the destruction of hepatic parenchyma by autoreactive T cells [23, 71]. T cells play a major role in the immunopathogenesis and both CD4+ and CD8+ T cells are involved together with effector responses mediated by NK cells and γδT cells [72]. A number of triggering factors have been proposed including viruses, xenobiotics, and drugs, but none has been shown to be involved in the pathogenesis and it is still a disease of unknown cause and diverse clinical manifestations [73]. Molecular mimicry involving cross-reactivity between epitopes of viruses or xenobiotics and certain liver antigens has been proposed. Mice infected with adenovirus expressing human cytochrome P450 2D6, an autoantigen in type II AIH, developed persistent autoimmune liver disease progressing to fibrosis associated with autoantibodies recognizing P450 2D6. This demonstrates that, at least in mice, viral infection can break tolerance, resulting in autoimmune liver damage [74]. A critical factor in the development of autoimmunity in viral infections may be a permissive proinflammatory environment stimulated by the virus that overwhelms regulatory networks resulting in the generation of self-perpetuating autoimmune reactions [75].
Role of Th17
Th17 T cells, a recently described subset of T-helper cells characterized by the production of IL-17, IL-22, TNF-α, and CCL20, play a crucial role in autoimmunity in both mice and humans [76–78]. They differentiate from naive T cells in the presence of polarizing cytokine such as IL-1β, IL-6, IL-23, and TGF-β, with IL-21 as an autocrine feedback loop [77–79]. These cytokines are secreted by innate immune cells such as dendritic cells [80] and their presence during T-cell activation induces expression of the transcription factors RORγt and RORα that are required to drive the Th17 program [81, 82]. In addition, activation of the aryl hydrocarbon receptor (AHR) is required for Th17 cell development and survival. AHR is a ligand-dependent transcription factor best known for mediating the toxicity of dioxin. Activation of AHR by a high-affinity ligand during Th17 cell development markedly increases the proportion of Th17 T cells and their production of cytokines, suggesting that AHR ligands may be cofactors in the development of autoimmunity. Endogenous ligands for AHR [83, 84] include bilirubin [85], suggesting another mechanism by which liver disease could support Th17 development. Th17 responses have been implicated in several autoimmune models in mice including experimental allergic encephalomyelitis (the murine equivalent of multiple sclerosis) and collagen-induced arthritis (murine model of rheumatoid arthritis). In both diseases, the IL-23 (p-19 subunit) and Th17 pathways have been implicated [86].
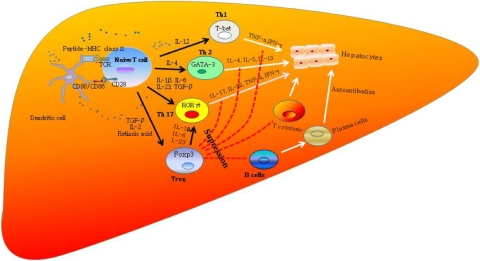
Th17 immunity has also been implicated in human autoimmune diseases including IBD, multiple sclerosis, rheumatoid arthritis, and psoriasis [87–90]. More recently, Th17 cells have been described in human autoimmune liver disease including primary biliary cirrhosis [91], and our own unpublished studies show 5% of the T cell infiltrate in AIH consists of IL-17 secreting CD4 T cells (classical Th17 cells) and CD8 T cells (so-called Tc17 cells) (Y. H. Oo, V. Banz, B. Eksteen, D. H. Adams, unpublished data). The differentiation of different lymphocyte subsets and their role in AIH is described in Fig. 2.
Fig. 2.
Immunopathogenesis of AIH. A specific autoantigenic peptide is presented to an uncommitted naive T lymphocyte within the HLA class II molecule of dendritic cells/antigen-presenting cell (APC). Naive T cells will differentiate into different T-cell lineages, Th1, Th2, or Th17, depending on cytokine milieu (IL-12 for Th1, IL-4 for Th2, and IL-1β, IL-6, and IL-23, and TGF-β for Th17) and these cells recruit T-cytotoxic (Tc) cells and B cells to initiate a series of immune reactions. Regulatory T cells (Treg) control these effector T cells to prevent excessive damage and they differentiate from naive T cells by TGF-β. Th1 cells secrete TNF-α and IFN-γ, which stimulate Tc lymphocytes. Th2 cells secrete IL-4, IL-5, IL-10, and IL-13 and direct autoantibody production by B lymphocytes. Th17 cells secrete IL17A&F, IL-22, TNF-α, IFN-γ, and CCL20. Regulatory T cells suppress these effector cells to maintain immune homeostasis. If regulatory T cells do not intervene, a variety of effector mechanisms are triggered and hepatocytes destruction/autoimmune hepatitis occur. Th17 cells, which play a major role in murine models of autoimmune disease, may also be involved in autoimmune liver diseases. In some cases, this leads to uncontrolled inflammation and fulminant hepatic failure. Treg could also transform to Th17 cells in inflamed hepatic microenvironment under the influence of inflammatory cytokines
Regulatory T cells
The immune system discriminates between self and non-self, establishing and maintaining unresponsiveness to self (self-tolerance) to suppress harmful immune responses against self-antigens, thereby preventing autoimmunity [92]. Peripheral tolerance is maintained by a subset of CD4 T cells that express high levels of the IL-2 receptor CD25 and low levels of the Il-7 receptor (CD127) known as regulatory T cells (Treg) [93–96]. They are characterized by expression of the transcription factor FoxP3, which is critical for their function [97]. Treg express several costimulatory molecules including cytotoxic T-lymphocyte antigen (CTLA4), an important negative regulator of the immune response [98]. A polymorphism of this gene has been linked to type 1 AIH, suggesting that a defect of Treg might underlie the pathogenesis of AIH [99]. The frequency and function of Treg are reduced in AIH [100–102], suggesting that a defect of Treg could underlie the pathogenesis of AIH. Thus, complex networks of effector and regulatory lymphocytes maintain inflammatory homeostasis in the liver and dysregulation of these networks results in a local environment that favors the development of autoimmunity and hepatitis.
Autoantibodies in AIH pathogenesis
High titers of IgG are characteristic of AIH, which is also associated with the generation of autoantibodies, some of which are implicated directly in disease pathogenesis. Thus, the levels of antibodies against the hepatocyte protein liver-specific protein correlate with disease severity and liver injury. Type 1 AIH is characterized by the presence of anti-SMA, and type 2 AIH is defined by the presence of LKM-1 antibodies that recognize a hepatic cytochrome P450, CYP2D6, expressed on the hepatocyte membrane.
The high levels of IgG seen in AIH may be a consequence of sustained B-cell activation in the liver as elevated intrahepatic levels of CD154, a critical activation signal for B cells, are detected in the liver of patients with AIH [103]. Expanded portal tracts take on a tertiary lymphoid structure as portal-associated lymphoid tissue and such structures include B cells [104]. It is possible that these intrahepatic B cells are responsible for local autoantibody secretion, although it is unknown whether they have any significant role in disease pathogenesis or progression. Several studies implicate B cells in fibrogenesis [105], but it is likely that they mediate this effect either by cytokine secretion or by direct effects on other intrahepatic cells including T cells and stellate cells rather than being mediated by antibody.
Therapy
Standard therapy is based on 2 phases, induction of remission using high doses of corticosteroids and then maintenance of control using low-dose corticosteroids and azathioprine with subsequent reduction of prednisolone to a minimum maintenance dose. AIH usually responds to immunosuppression and was the first chronic liver disease in which a significant improvement in patient survival was noted following drug treatment [73]. Most patients require long-term treatment with corticosteroids and/or azathioprine. The absence of symptoms at presentation may identify some patients who do not require treatment, but therapeutic decisions must be based on disease activity and not symptoms, especially since 26–70% of asymptomatic patients become symptomatic and may progress to cirrhosis [106]. Elderly patients have more advanced disease at presentation, but they respond well to treatment [1].
Inducing and maintaining remission
Corticosteroids are the drug of choice for remission induction, and azathioprine is the drug of choice for maintenance. Bone marrow toxicity must be actively checked after starting azathioprine. It is appropriate in young individuals to start with high doses of prednisolone at 1 mg/kg or 60 mg of total dose, and once the transaminase levels have begun to fall, reduce it by 10-mg steps weekly to 20 mg/day with a slower subsequent reduction to 10 mg/day. Further reduction should be delayed until the liver function tests show normal results. Some people advocate delaying the introduction of azathioprine for 2–3 weeks because (1) it allows the diagnosis to be confirmed by demonstrating steroid responsiveness before adding in a second immunosuppressive agent and (2) liver toxicity due to azathioprine can be confused with nonresponsiveness if azathioprine is started immediately. However, in older patients or those who have comorbidities such as hypertension, osteoporosis, or diabetes in whom prednisolone dosage should be kept to a minimum, it is possible to start with a lower dose of 20–30 mg/day together with azathioprine at 1–2 mg/kg. Maintenance therapy usually involves azathioprine alone or together with low-dose prednisolone. For patients who develop decompensated liver disease despite therapy, orthotopic liver transplantation (OLT) is the treatment of choice. The rare patients who present with fulminant AIH often fail to respond to immunosuppression and require liver transplantation. Although it is worth starting immunosuppressive therapy, it is also important not to delay referral to a transplant center if there is evidence of liver failure as demonstrated by the development of encephalopathy, coagulopathy, or renal failure.
Complications of long-term immunosuppressive drug therapy should be foreseen and treated appropriately, such as blood pressure monitoring, urine examination for glucose, and monitoring of whole blood cell counts in patients on azathioprine to detect bone marrow suppression. All patients on prednisolone at more than 7.5 mg/day should be advised to take calcium and vitamin D supplements or bisphosphonate to prevent osteoporosis.
Remission, relapse and when to stop therapy
Remission is defined as a lack of symptoms with normal serum aminotransferase levels, a normal level of IgG, and inactive liver histology. Remission will be achieved in more than 80% of patients on prednisolone and azathioprine [107]. There is a high risk of relapse following discontinuation of therapy, particularly in type 2 LKM-positive disease in which relapse is almost universal. Most relapses occur within 12 weeks of stopping treatment; however, some may take months or even years to develop and patients must be closely monitored for at least a year after stopping therapy. Steroid withdrawal should not be attempted before 12 months of treatment and maximizing the azathioprine dose to 2 mg/kg per day reduces risk of reactivation. In some series, even patients with histological proven remission have relapse rates of 80% after withdrawal of therapy. Following symptomatic relapse, full-dose treatment needs to be reintroduced and although induction of a second remission is the norm, not all patients can be brought back under control. A common cause of relapse in adolescent patients is a lack of adherence with therapy and evidence of nonadherence or psychosocial problems should always be sought in young patients who unexpectedly relapse.
When to repeat liver biopsy?
Normalization of liver biochemistry during corticosteroid therapy does not necessarily indicate suppression of histological inflammatory activity—confirmation of histological remission (defined by the absence of interface hepatitis) is therefore recommended before immunosuppressive therapy is withdrawn [16, 108–110]. Mild portal inflammatory changes frequently persist and do not appear to be associated with an adverse outcome. However, the presence of portal plasma cells may be associated with an increased risk of relapse after drug withdrawal [37, 109].
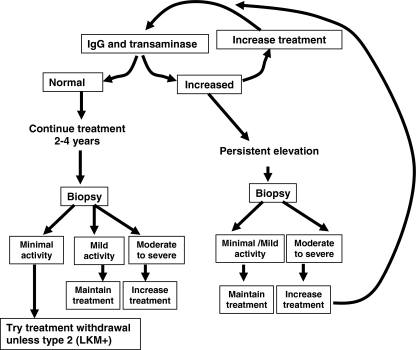
Follow-up biopsies should be considered because there is 5% per year progression to cirrhosis and this is much higher in patients with continuing inflammation. Twenty-five percent of patients with normal transaminase levels have inflammatory lesions on biopsy. Conversely normal IgG and transaminase levels are associated with minimal histological inflammation in 90% of patients. The other scenario in which repeat biopsy is invaluable is confirmation of remission prior to a trial of treatment withdrawal because patients in remission with minimal inflammatory activity on biopsy have a higher chance of successful treatment withdrawal. Figure 3 is an algorithm that outlines the strategy to follow in deciding the level of immunosuppression in AIH.
Fig. 3.
Algorithm for adjusting therapy in patients with autoimmune hepatitis
Treatment failure and alternative therapies for AIH
As mentioned previously, remission is achieved in more than 80% of patients receiving prednisolone and azathioprine [107, 111]. During the first 3–6 months, clinical and biochemical normalization usually occurs, although histological improvement may lag behind. Alternative therapy may be required because of failure to achieve remission or because of adverse effects. However, before deciding that treatment has failed, it is important to review the diagnosis, make sure that immunosuppression is not suboptimal, and, particularly in young people, ensure that they are compliant with therapy. Some patients require high doses of corticosteroids to maintain remission or a second cycle of induction therapy. Intolerance of adverse effects is a common reason for considering alternative therapy. Long-term use of glucocorticoids is complicated by many adverse effects, including diabetes, osteoporosis, and hypertension, and younger patients in particular may find the cosmetic adverse effects unacceptable. A small number of patients cannot tolerate azathioprine because of symptomatic adverse effects, including nausea, rash, abdominal discomfort, or severe and potentially life-threatening complications such as pancreatitis, cholestatic hepatotoxicity, and bone marrow suppression. Routine screening for thiopurine methyltransferase activity before starting azathioprine therapy has not reduced the frequency of adverse effects [112]. Several alternative treatments have been attempted but the quality of evidence is poor, based on a small, noncontrolled series, and there have been few prospective studies [113].
Alternative agents used in the treatment of AIH
Some agents proposed as alternatives to standard treatment are listed in the following:
Budesonide: This is a second-generation glucocorticoid with high (90%) first-pass clearance by the liver. A potential advantage over prednisolone is the theoretical ability to achieve high local tissue levels without systemic adverse effects. Budesonide is effective in both inducing and maintaining remission [114], although there is currently no good evidence that budesonide provides a benefit over prednisolone and care must be taken when using it in patients with cirrhosis in whom shunting may reduce hepatic first pass metabolism [115, 116].
Deflazacort is an oxazoline derivative of prednisolone with both anti-inflammatory and immunosuppressive activities and a lower incidence of corticosteroid complications. Again, there is little evidence for its efficacy in AIH [117].
Cyclosporine [118, 119] and tacrolimus [120] are chemically distinct calcineurin inhibitors (CNIs) that have been successfully used as both induction and rescue therapy in AIH. Adverse effects include neurotoxicity, hypertension, and hyperlipidemia, but the major drawback with both these agents is the high risk of nephrotoxicity. If they are used at the AIH drug level, renal function must be closely monitored and the minimal effective dose should be used. It is probably safest if the use of CNIs is confined to transplant units in which there is extensive experience in their use.
Cyclophosphamide is a cytotoxic agent commonly used to treat vasculitis. Experience in AIH is limited to small uncontrolled series, suggesting that patients may respond well. Remission can be induced with 1–1.5 mg/kg of cyclophosphamide in combination with a tapering dose of corticosteroids, initiated at 1 mg/kg after which patients are maintained with low doses of corticosteroids (2.5–10 g/day), together with 50 mg/day of cyclophosphamide (A. W. Lohse, personal communication) [121]. The ability of this drug to inhibit antibody production is of theoretical benefit but the risk of toxicity means its use should be restricted to units with extensive experience of immunosuppressive therapy.
d-Penicillamine [122, 123] is a modified amino acid with anti-inflammatory activity and metal-chelating properties. Toxicity includes mucocutaneous, gastrointestinal, renal, hematological, pulmonary, and autoimmune complications. There is no notable evidence of efficacy in AIH.
Methotrexate is effective in the normalization of liver enzymes, improved liver histology, and steroid sparing during maintenance of therapy. However, it is teratogenic in women of childbearing age and has been associated with liver fibrosis when used to treat psoriasis [124].
Ursodeoxycholic acid (UDCA) may improve liver biochemistry, but its use does not permit a reduction in the dose of steroids, nor does it affect clinical outcome or histological activity so that it cannot be routinely recommended [125]. There may be a role for UDCA in patients with AIH and biliary features, but this has not been analyzed systematically.
Mycophenolate mofetil (MMF) is an ester prodrug of mycophenolic acid (MPA) and is converted into MPA after oral absorption. MPA is a noncompetitive inhibitor of inosine monophosphate dehydrogenase, which blocks the rate-limiting enzymatic steps in de novo purine nucleotide synthesis, thereby arresting DNA replication in T and B lymphocytes. MMF is well tolerated, with leucopenia and diarrhea as the main adverse effects. It is teratogenic in animals, which must be taken into account when considering it for treatment of women of childbearing age. Studies have shown that MMF is an alternative therapy for those patients who are intolerant of or refractory to azathioprine [126, 127]. A recent study demonstrated that MMF is an effective rescue therapy for children with AIH but not for those with autoimmune sclerosing cholangitis [128]. The experience of MMF in a joint study from Birmingham and Hamburg was disappointing. It was beneficial in only around 50% of patients who were switched from azathioprine because of adverse effects and even less effective for azathioprine nonresponders. We evaluated data of 36 patients with AIH who received MMF as second-line therapy. One patient did not tolerate MMF and had to stop treatment. Median duration of MMF treatment was 16 months (range 1–92 months). Eleven of 36 patients (31%) experienced adverse effects with MMF. Four patients had to stop treatment because of these adverse effects after less than 3 months; their response was considered as treatment failure. Adverse effects included nausea and vomiting (n = 4), abdominal pain (n = 6), and diarrhea (n = 1). Of 36 patients, 14 patients (39%) experienced remission, being defined as aspartate aminotransferase levels of less than twice the upper normal limit after at least 4 months of MMF therapy. Twenty-two patients (61%) did not respond to MMF. The response rate was dependent on the reason for stopping azathioprine. Of 8 patients with prior nonresponse to azathioprine, 6 (75%) did not respond to MMF and only 2 (25%) reached a biochemical remission. Of 28 patients with azathioprine intolerance, only 12 patients (43%) went into remission on MMF. During follow-up, 4 patients underwent liver transplantation. One of these patients responded well to MMF, as defined by normalization of liver biochemistry but despite this still progressed to end-stage cirrhosis, the other 3 failed to respond. Most patients tolerated MMF well [129]. Treatment strategy for patients with AIH is summarized in Table 2.
Table 2.
Treatment strategy for AIH
| Standard therapy | Alternative therapy (relapse or failure of standard therapy or intolerant of steroid or azathioprine) |
| Induction | Induction |
| Prednisolone 1 mg/kg per day weekly reduction (by 10-mg steps) to 20 mg/day over 6 weeks and then add azathioprine when AST level decreases to 2–3 times the normal range | Calcineurin inhibitors (cyclosporine/tacrolimus) |
| Cyclophosphamide | |
| Budesonide | |
| or | |
| Prednisolone 20 mg/day and azathioprine 1 mg/kg per day | |
| Maintenance of remission | Maintenance of remission |
| Aim: normal transaminases, normal IgG | |
| Increase azathioprine to 1.5 mg/kg per day | Cyclosporine or tacrolimus |
| Mycophenolate mofetil | |
| Steroid reduction below 10 mg/day once biochemical remission is achieved | Cyclophosphamide |
| Methotrexate |
Biological agents in AIH
In theory, immunomodulating biologic agents should be effective in AIH. Anti-B cell therapy with the anti-CD20 antibody, rituximab, has been tried in other autoimmune diseases and is theoretically attractive because of the ability to inhibit autoantibody production. It has been used in patients with AIH and other autoimmune complications, including ITP and hemolytic anemia, and individual case reports are promising [105, 130]. The most experience in hepatology has been to treat mixed cryoglobulinemia in the context of HCV infection [131, 132] and mixed cryoglobulinemia in patients with renal transplant [133, 134]. It probably works by inducing immune reconstitution rather than a simple effect on autoantibody production. Although largely well tolerated and safe, there are potential long-term adverse effects related to dysregulated immune reconstitution and opportunistic infections, and if given inadvertently to patients with hepatitis B, it can lead to reactivation of infection [135, 136]. Our own limited experience in 3 cases is that it is safe and effective in patients who are resistant to conventional immunosuppressive therapy, but a controlled clinical trial is required to evaluate these promising anecdotal reports.
There is increasing clinical experience with anticytokine therapies, particularly anti-TNFα therapy in rheumatoid arthritis and IBD. T-cell-mediated mechanisms induce liver injury in AIH and injection of concanavalin A (Con A) into mice causes a T-cell-mediated hepatitis driven by TNF-α. All this evidence suggests that anti-TNF-α therapy might be effective in AIH [137]. However, recent experience cautions against this because infliximab therapy has been associated with the induction of severe de novo AIH in some patients treated for other diseases [138–141]. The explanation for these observations is unclear but might be related to the ability of TNF-α to promote liver regeneration, although the fact that some patients respond to etanercept suggest a direct toxicity related to infliximab [142–144].
Biologic agents aimed at other cytokines have been developed and are being assessed in human disease, usually rheumatoid arthritis. These include anti-IL-1β and IL-6 [145–151]. Two humanized monoclonal antibodies targeting IL-17A showed positive results in patients with rheumatoid arthritis [152, 153]. All these biologic agents are at various stages of clinical evaluation and may have a role to play in AIH.
FTY720 (Fingolimod), a new class of immunomodulator, inhibits lymphocyte egress from secondary lymphoid tissues and thymus by agonistic activity at sphingosine 1-phosphate receptors [154–161]. In vivo studies suggest that both FTY720 and another more recently developed S1P agonist, KRP203, promote lymphocyte sequestration in secondary lymph nodes and significantly reduce the number of CD4 T cells infiltrating the liver in the Con A hepatitis model [162]. These agents may be of potential interest in treating AIH in the future, especially if they are proven safe and effective in current clinical trials in transplantation.
Because a defect in regulatory T-cell function appears to contribute to the breakdown in self-tolerance, in AIH manipulation of regulatory T cells might be another therapeutic option [163]. The ability to generate antigen-specific Tregs in type 2 AIH suggests that this may be a good clinical model for developing Treg therapy. However, such therapy is expensive and there is a risk that adoptively transferred Tregs may either cease to function or even differentiate into IL-17 secreting cells in the chronically inflamed environment so that such studies must be developed with caution [164–166] (Fig. 2).
AIH in special circumstances
AIH and pregnancy
Rarely, AIH may present during pregnancy or more commonly after delivery. AIH affects young women, and it is common to be faced with a patient with established AIH who either wants to become pregnant or has already done so. Most patients with AIH can expect a good outcome, whereas higher rates of maternal and fetal problems are reported particularly in patients with type 2 or 3 AIH [9]. Unexplained adverse pregnancy outcomes were associated with antibodies to SLA/LP and Ro/SSA in the affected mothers [9]. In addition, the disease may flare after delivery, as the pregnancy-associated immunosuppressive state is lost [167, 168]. However, there is no reason to counsel against pregnancy, provided the disease is well controlled at the time of conception. Patients with cirrhosis need careful monitoring to make sure that complications of portal hypertension do not develop during late pregnancy [10]. It used to be claimed that fertility was greatly reduced in women with cirrhosis, but this is not the case, at least for compensated cirrhosis, and patients should be advised about contraception. The immunosuppression of pregnancy may promote remission of autoimmune diseases; however, this does not mean that immunosuppressive therapy can be omitted and particular care is required postdelivery when the effect is rapidly lost. Both azathioprine and corticosteroids are relatively safe, although MMF is teratogenic in animals and wherever possible should be avoided [130, 169]. Patients on MMF who plan to get pregnant should be switched to another immunosuppressant or be covered by higher dose of steroids during pregnancy [10].
AIH in the elderly
Of adult patients with AIH, more than 20% develop the disease after the age of 60 years [1, 3] and some studies have shown a greater degree of hepatic fibrosis and cirrhosis at presentation in elderly patients [2, 3, 170]. Elderly patients treated with steroids have similar outcomes compared with AIH patients younger than 60 years [170] and they have a higher frequency of HLA DRB1*04 than young adults [1]. Increasingly, elderly patients in particular are being diagnosed by routine screening tests. Many of these patients are asymptomatic with minimal biochemical liver abnormalities, and it is unclear what the prognosis is for such patients and whether it is affected by immunosuppressive therapy.
AIH and liver transplantation
Liver transplantation is the only life-saving option in approximately 10% of AIH patients who reach end-stage cirrhosis despite therapy. Liver transplantation may also be indicated in cases of severe acute AIH presenting as fulminant hepatic failure [171, 172]. The survival rate among patients and grafts 5 years after liver transplantation is approximately 80–90% [171, 173]. The overall success rate of liver transplantation is high, but a proportion of patients, up to 25% in some series [173–177], are at risk of developing recurrent disease despite maintenance immunosuppression to prevent graft rejection. Recurrent AIH is characterized by the persistence of autoantibodies, elevated IgG levels, and typical features on liver biopsy [173, 178]. The diagnostic utility of autoantibody testing in the liver allograft is uncertain. Autoantibodies have been found in some patients transplanted for conditions other than AIH (discussed further later), whereas others have histological features compatible with AIH in the absence of demonstrable autoantibodies [179]. In some cases, histological features suggestive of recurrent AIH can precede recurrence of clinical and biochemical symptoms by several years [176].
It is interesting that an autoimmune disease can recur in the context of allogeneic HLA after transplantation. This has led to the concept that this is in fact a form of graft rejection rather than autoimmunity. However, autoantigens can be presented by allogeneic HLA providing a mechanism for true autoimmune disease to recur. The long-term use of low-dose steroids posttransplant has been claimed to reduce the incidence of recurrent disease [180].
De novo AIH
The term “de novo AIH” has been used to describe new type of graft dysfunction affecting patients transplanted for diseases other than AIH, who subsequently develop classical biochemical, serological, and histological features of AIH [180–185]. A higher prevalence has been reported in children (5–10%) than in adults (1–2%), possibly reflecting immunosuppressive drugs interfering with normal T-cell maturation. Most patients respond to increased immunosuppression, although rare cases have progressed to cirrhosis and graft failure.
Several studies have identified areas of overlap between de novo AIH and late cellular rejection, and it has been postulated that an immune response directed against graft antigens rather than self-antigens might be a form of alloimmune injury rather than true autoimmune disease. In support of this suggestion, autoantibodies have been described transiently in association with episodes of acute and chronic rejection [187–190], previous episodes of acute rejection have been reported as having predictive value for the subsequent development of de novo AIH [183, 184, 186] and de novo AIH may arise in the setting of suboptimal immunosuppression [191] or as a consequence of non-specific stimulation of the immune system by interferon therapy for HCV infection [192–194]. Perhaps, the most convincing evidence for an alloimmune response is the observation that the development of de novo AIH was closely associated with antibodies to glutathione-S-transferase T1 (GSTT1) developing in a GSST1-negative recipient of a GSTT1-positive graft [195–198]. As the GSST1 enzyme is expressed by hepatocytes in the liver allograft, this could represent a form of alloimmune injury (i.e., rejection) directed against hepatocytes. However, the association between de novo AIH and a donor/recipient GSST1 mismatch has not been confirmed in other studies [199, 200]. Autoantibodies are frequently present in children without biochemical signs of graft dysfunction [187, 190, 201], and liver biopsy is required to establish an accurate diagnosis.
Ten percent to 50% of late posttransplant biopsies have unexplained predominantly portal-based inflammatory changes that have been referred to as “idiopathic” chronic hepatitis [202] or “interface hepatitis” [203]. A higher prevalence has been documented in children, many of whom also have autoantibodies but lack the biochemical changes required to make a diagnosis of de novo AIH [204]. Recognition of this entity is important, as it may lead to bridging fibrosis or cirrhosis in 50–70% of children 10 years posttransplant [203, 204]. Autoantibodies have also been identified in adults with “idiopathic” chronic hepatitis, which has been associated with the development of progressive fibrosis [205]. Given the areas of overlap alluded to above, it seems likely that de novo AIH and “idiopathic” chronic hepatitis” are part of a spectrum of alloimmune damage in the liver allograft (late rejection with “hepatitic features”) [205, 206]. In support of this suggestion, a number of studies have suggested that late acute rejection may have features that more closely resemble chronic hepatitis than early acute rejection [200].
Conclusions
AIH occurs as a consequence of a breakdown in self-tolerance. Naturally occurring CD4+CD25highCD127lowFoxP3+ regulatory T cells (Treg) actively engage in the maintenance of immune homeostasis, and defects in both the number and function of Treg are associated with AIH. Recent evidence suggests that Th17 immune response also plays a major role in autoimmune diseases including AIH. It is now firmly established that regulatory T cells constitute an indispensable component of the immune homeostasis and further elucidation of the molecular and cellular bases of Treg function or development may provide ways to control autoimmune diseases including AIH. Diagnosis of AIH is made by a combination of clinical, biochemical, serological, and histological criteria and exclusion of other etiology. Most patients with AIH are well controlled with corticosteroids and azathioprine. However, some patients require alternative therapy because of drug intolerance, development of significant adverse effects, or failure to control disease activity. Elderly patients have an indolent but often aggressive disease that responds well to corticosteroid therapy. Adverse outcomes in pregnancy are associated with antibodies to soluble liver antigen/liver pancreas and Ro/SSA. Liver transplantation is the treatment of choice for AIH patients who had end-stage decompensated liver diseases or HCC. Recurrence of AIH is common in the graft after liver transplantation, and these patients normally require low-dose steroids.
Contributor Information
Ye H. Oo, Email: y.h.oo@bham.ac.uk
David H. Adams, Phone: +44-121-4158702, FAX: +44-121-4158701, Email: d.h.adams@bham.ac.uk
References
- 1.Czaja AJ, Carpenter HA. Distinctive clinical phenotype and treatment outcome of type 1 autoimmune hepatitis in the elderly. Hepatology. 2006;43(3):532–538. doi: 10.1002/hep.21074. [DOI] [PubMed] [Google Scholar]
- 2.Miyake Y, Iwasaki Y, Sakaguchi K, Shiratori Y. Clinical features of Japanese male patients with type 1 autoimmune hepatitis. Aliment Pharmacol Ther. 2006;24(3):519–523. doi: 10.1111/j.1365-2036.2006.03013.x. [DOI] [PubMed] [Google Scholar]
- 3.Al Chalabi T, Boccato S, Portmann BC, Mcfarlane IG, Heneghan MA. Autoimmune hepatitis (AIH) in the elderly: a systematic retrospective analysis of a large group of consecutive patients with definite AIH followed at a tertiary referral centre. J Hepatol. 2006;45(4):575–583. doi: 10.1016/j.jhep.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Miyake Y, Iwasaki Y, Terada R, Onishi T, Okamoto R, Sakai N, et al. Clinical characteristics of fulminant-type autoimmune hepatitis: an analysis of eleven cases. Aliment Pharmacol Ther. 2006;23(9):1347–1353. doi: 10.1111/j.1365-2036.2006.02894.x. [DOI] [PubMed] [Google Scholar]
- 5.Nikias GA, Batts KP, Czaja AJ. The nature and prognostic implications of autoimmune hepatitis with an acute presentation. J Hepatol. 1994;21(5):866–871. doi: 10.1016/s0168-8278(94)80251-3. [DOI] [PubMed] [Google Scholar]
- 6.Burgart LJ, Batts KP, Ludwig J, Nikias GA, Czaja AJ. Recent onset autoimmune hepatitis. Biopsy findings and clinical correlations. Am J Surg Pathol. 1995;19(6):699–708. doi: 10.1097/00000478-199506000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Terrabuio DR, Abrantes-Lemos CP, Carrilho FJ, Cancado EL. Follow-up of pregnant women with autoimmune hepatitis: the disease behavior along with maternal and fetal outcomes. J Clin Gastroenterol. 2009;43(4):350–356. doi: 10.1097/MCG.0b013e318176b8c5. [DOI] [PubMed] [Google Scholar]
- 8.Werner M, Bjornsson E, Prytz H, Lindgren S, Almer S, Broome U, et al. Autoimmune hepatitis among fertile women: strategies during pregnancy and breastfeeding? Scand J Gastroenterol. 2007;42(8):986–991. doi: 10.1080/00365520601155266. [DOI] [PubMed] [Google Scholar]
- 9.Schramm C, Herkel J, Beuers U, Kanzler S, Galle PR, Lohse AW. Pregnancy in autoimmune hepatitis: outcome and risk factors. Am J Gastroenterol. 2006;101(3):556–560. doi: 10.1111/j.1572-0241.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 10.Heneghan MA, Norris SM, O’Grady JG, Harrison PM, Mcfarlane IG. Management and outcome of pregnancy in autoimmune hepatitis. Gut. 2001;48(1):97–102. doi: 10.1136/gut.48.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5(3):266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 12.Fusconi M, Vannini A, Dall’Aglio AC, Pappas G, Cassani F, Ballardini G, et al. Anti-cyclic citrullinated peptide antibodies in type 1 autoimmune hepatitis. Aliment Pharmacol Ther. 2005;22(10):951–955. doi: 10.1111/j.1365-2036.2005.02686.x. [DOI] [PubMed] [Google Scholar]
- 13.Montano-Loza A, Czaja AJ, Carpenter HA, Piette A, Murphy D, Shums Z, et al. Frequency and significance of antibodies to cyclic citrullinated peptide in type 1 autoimmune hepatitis. Autoimmunity. 2006;39(4):341–348. doi: 10.1080/08916930600783348. [DOI] [PubMed] [Google Scholar]
- 14.Ben-Ari Z, Broida E, Monselise Y, Kazatsker A, Baruch J, Pappo O, et al. Syncytial giant-cell hepatitis due to autoimmune hepatitis type II (LKM1+) presenting as subfulminant hepatitis. Am J Gastroenterol. 2000;95(3):799–801. doi: 10.1111/j.1572-0241.2000.01863.x. [DOI] [PubMed] [Google Scholar]
- 15.Devaney K, Goodman ZD, Ishak KG. Postinfantile giant-cell transformation in hepatitis. Hepatology. 1992;16(2):327–333. doi: 10.1002/hep.1840160208. [DOI] [PubMed] [Google Scholar]
- 16.Carpenter HA, Czaja AJ. The role of histologic evaluation in the diagnosis and management of autoimmune hepatitis and its variants. Clin Liver Dis. 2002;6(3):685–705. doi: 10.1016/s1089-3261(02)00022-3. [DOI] [PubMed] [Google Scholar]
- 17.Burt AD, Portmann BC, Ferrell LD, MacSween R. Autoimmune hepatitis. In Pathology of the Liver. Edinburgh: Churchill Livingstone; 2007. 493–517
- 18.Feld JJ. Autoimmune hepatitis: effect of symptoms and cirrhosis on natural history and outcome. Hepatology. 2005;42(1):53–62. doi: 10.1002/hep.20732. [DOI] [PubMed] [Google Scholar]
- 19.Cotler SJ, Jakate S, Jensen DM. Resolution of cirrhosis in autoimmune hepatitis with corticosteroid therapy. J Clin Gastroenterol. 2001;32(5):428–430. doi: 10.1097/00004836-200105000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Czaja AJ, Carpenter HA. Decreased fibrosis during corticosteroid therapy of autoimmune hepatitis. J Hepatol. 2004;40(4):646–652. doi: 10.1016/j.jhep.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Czaja AJ, Carpenter HA. Progressive fibrosis during corticosteroid therapy of autoimmune hepatitis. Hepatology. 2004;39(6):1631–1638. doi: 10.1002/hep.20235. [DOI] [PubMed] [Google Scholar]
- 22.Johnson PJ, Mcfarlane IG. Meeting report: International Autoimmune Hepatitis Group. Hepatology. 1993;18(4):998–1005. doi: 10.1002/hep.1840180435. [DOI] [PubMed] [Google Scholar]
- 23.Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31(5):929–938. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 24.Hennes EM, Zeniya M, Czaja AJ, Pares A, Dalekos GN, Krawitt EL, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48(1):169–176. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]
- 25.Czaja AJ. Performance parameters of the diagnostic scoring systems for autoimmune hepatitis. Hepatology. 2008;48(5):1540–1548. doi: 10.1002/hep.22513. [DOI] [PubMed] [Google Scholar]
- 26.Yeoman AD, Westbrook RH, Al-Chalabi T, Carey I, Heaton ND, Portmann BC, et al. Diagnostic value and utility of the simplified International Autoimmune Hepatitis Group (IAIHG) criteria in acute and chronic liver disease. Hepatology. 2009;50(2):538–545. doi: 10.1002/hep.23042. [DOI] [PubMed] [Google Scholar]
- 27.Wiegard C, Schramm C, Lohse AW. Scoring systems for the diagnosis of autoimmune hepatitis: past, present, and future. Semin Liver Dis. 2009;29(3):254–261. doi: 10.1055/s-0029-1233532. [DOI] [PubMed] [Google Scholar]
- 28.Roozendaal C, Jong MA, Berg AP, Wijk RT, Limburg PC, Kallenberg CG. Clinical significance of anti-neutrophil cytoplasmic antibodies (ANCA) in autoimmune liver diseases. J Hepatol. 2000;32(5):734–741. doi: 10.1016/s0168-8278(00)80241-x. [DOI] [PubMed] [Google Scholar]
- 29.Roozendaal C, Kallenberg CG. Anti-neutrophil cytoplasm autoantibodies (ANCA) in autoimmune liver diseases. Hepatogastroenterology. 1999;46(30):3034–3040. [PubMed] [Google Scholar]
- 30.Duchini A, McHutchison JG, Pockros PJ. LKM-positive autoimmune hepatitis in the western United States: a case series. Am J Gastroenterol. 2000;95(11):3238–3241. doi: 10.1111/j.1572-0241.2000.03207.x. [DOI] [PubMed] [Google Scholar]
- 31.Bridoux-Henno L, Maggiore G, Johanet C, Fabre M, Vajro P, Dommergues JP, et al. Features and outcome of autoimmune hepatitis type 2 presenting with isolated positivity for anti-liver cytosol antibody. Clin Gastroenterol Hepatol. 2004;2(9):825–830. doi: 10.1016/s1542-3565(04)00354-4. [DOI] [PubMed] [Google Scholar]
- 32.Wies I, Brunner S, Henninger J, Herkel J, Kanzler S, Meyer zum Buschenfelde KH, et al. Identification of target antigen for SLA/LP autoantibodies in autoimmune hepatitis. Lancet. 2000;355(9214):1510–1515. doi: 10.1016/s0140-6736(00)02166-8. [DOI] [PubMed] [Google Scholar]
- 33.Kanzler S, Weidemann C, Gerken G, Lohr HF, Galle PR, Meyer zum Buschenfelde KH, et al. Clinical significance of autoantibodies to soluble liver antigen in autoimmune hepatitis. J Hepatol. 1999;31(4):635–640. doi: 10.1016/s0168-8278(99)80342-0. [DOI] [PubMed] [Google Scholar]
- 34.Kirk AP, Jain S, Pocock S, Thomas HC, Sherlock S. Late results of the Royal Free Hospital prospective controlled trial of prednisolone therapy in hepatitis B surface antigen negative chronic active hepatitis. Gut. 1980;21(1):78–83. doi: 10.1136/gut.21.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murray-Lyon IM, Stern RB, Williams R. Controlled trial of prednisone and azathioprine in active chronic hepatitis. Lancet. 1973;1(7806):735–737. doi: 10.1016/s0140-6736(73)92125-9. [DOI] [PubMed] [Google Scholar]
- 36.Kogan J, Safadi R, Ashur Y, Shouval D, Ilan Y. Prognosis of symptomatic versus asymptomatic autoimmune hepatitis: a study of 68 patients. J Clin Gastroenterol. 2002;35(1):75–81. doi: 10.1097/00004836-200207000-00016. [DOI] [PubMed] [Google Scholar]
- 37.Verma S, Gunuwan B, Mendler M, Govindrajan S, Redeker A. Factors predicting relapse and poor outcome in type I autoimmune hepatitis: role of cirrhosis development, patterns of transaminases during remission and plasma cell activity in the liver biopsy. Am J Gastroenterol. 2004;99(8):1510–1516. doi: 10.1111/j.1572-0241.2004.30457.x. [DOI] [PubMed] [Google Scholar]
- 38.Yeoman AD, Al-Chalabi T, Karani JB, Quaglia A, Devlin J, Mieli-Vergani G, et al. Evaluation of risk factors in the development of hepatocellular carcinoma in autoimmune hepatitis: implications for follow-up and screening. Hepatology. 2008;48(3):863–870. doi: 10.1002/hep.22432. [DOI] [PubMed] [Google Scholar]
- 39.Lohse AW, zum Buschenfelde KH, Franz B, Kanzler S, Gerken G, Dienes HP. Characterization of the overlap syndrome of primary biliary cirrhosis (PBC) and autoimmune hepatitis: evidence for it being a hepatitic form of PBC in genetically susceptible individuals. Hepatology. 1999;29(4):1078–1084. doi: 10.1002/hep.510290409. [DOI] [PubMed] [Google Scholar]
- 40.Kaya M, Angulo P, Lindor KD. Overlap of autoimmune hepatitis and primary sclerosing cholangitis: an evaluation of a modified scoring system. J Hepatol. 2000;33(4):537–542. doi: 10.1034/j.1600-0641.2000.033004537.x. [DOI] [PubMed] [Google Scholar]
- 41.Talwalkar JA, Keach JC, Angulo P, Lindor KD. Overlap of autoimmune hepatitis and primary biliary cirrhosis: an evaluation of a modified scoring system. Am J Gastroenterol. 2002;97(5):1191–1197. doi: 10.1111/j.1572-0241.2002.05703.x. [DOI] [PubMed] [Google Scholar]
- 42.Beuers U, Rust C. Overlap syndromes. Semin Liver Dis. 2005;25(3):311–320. doi: 10.1055/s-2005-916322. [DOI] [PubMed] [Google Scholar]
- 43.Abdo AA, Bain VG, Kichian K, Lee SS. Evolution of autoimmune hepatitis to primary sclerosing cholangitis: a sequential syndrome. Hepatology. 2002;36(6):1393–1399. doi: 10.1053/jhep.2002.37200. [DOI] [PubMed] [Google Scholar]
- 44.Adams DH, Eksteen B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nat Rev Immunol. 2006;6(3):244–251. doi: 10.1038/nri1784. [DOI] [PubMed] [Google Scholar]
- 45.Bertolino P, Klimpel G, Lemon SM. Hepatic inflammation and immunity: a summary of a conference on the function of the immune system within the liver. Hepatology. 2000;31(6):1374–1378. doi: 10.1053/jhep.2000.8376. [DOI] [PubMed] [Google Scholar]
- 46.Chazouilleres O, Wendum D, Serfaty L, Rosmorduc O, Poupon R. Long term outcome and response to therapy of primary biliary cirrhosis-autoimmune hepatitis overlap syndrome. J Hepatol. 2006;44(2):400–406. doi: 10.1016/j.jhep.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 47.Poupon R, Chazouilleres O, Corpechot C, Chretien Y. Development of autoimmune hepatitis in patients with typical primary biliary cirrhosis. Hepatology. 2006;44(1):85–90. doi: 10.1002/hep.21229. [DOI] [PubMed] [Google Scholar]
- 48.Saich R, Chapman R. Primary sclerosing cholangitis, autoimmune hepatitis and overlap syndromes in inflammatory bowel disease. World J Gastroenterol. 2008;14(3):331–337. doi: 10.3748/wjg.14.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perdigoto R, Carpenter HA, Czaja AJ. Frequency and significance of chronic ulcerative colitis in severe corticosteroid-treated autoimmune hepatitis. J Hepatol. 1992;14(2–3):325–331. doi: 10.1016/0168-8278(92)90178-r. [DOI] [PubMed] [Google Scholar]
- 50.McNair AN, Moloney M, Portmann BC, Williams R, Mcfarlane IG. Autoimmune hepatitis overlapping with primary sclerosing cholangitis in five cases. Am J Gastroenterol. 1998;93(5):777–784. doi: 10.1111/j.1572-0241.1998.224_a.x. [DOI] [PubMed] [Google Scholar]
- 51.Buuren HR, Hoogstraten HJE, Terkivatan T, Schalm SW, Vleggaar FP. High prevalence of autoimmune hepatitis among patients with primary sclerosing cholangitis. J Hepatol. 2000;33(4):543–548. doi: 10.1034/j.1600-0641.2000.033004543.x. [DOI] [PubMed] [Google Scholar]
- 52.Grant AJ, Lalor PF, Salmi M, Jalkanen S, Adams DH. Homing of mucosal lymphocytes to the liver in the pathogenesis of hepatic complications of inflammatory bowel disease. Lancet. 2002;359(9301):150–157. doi: 10.1016/S0140-6736(02)07374-9. [DOI] [PubMed] [Google Scholar]
- 53.Agace WW. T-cell recruitment to the intestinal mucosa. Trends Immunol. 2008;29(11):514–522. doi: 10.1016/j.it.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 54.Eksteen B, Grant AJ, Miles A, Curbishley SM, Lalor PF, Hubscher SG, et al. Hepatic endothelial CCL25 mediates the recruitment of CCR9+ gut-homing lymphocytes to the liver in primary sclerosing cholangitis. J Exp Med. 2004;200(11):1511–1517. doi: 10.1084/jem.20041035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gregorio GV, Portmann B, Karani J, Harrison P, Donaldson PT, Vergani D, et al. Autoimmune hepatitis/sclerosing cholangitis overlap syndrome in childhood: a 16-year prospective study. Hepatology. 2001;33(3):544–553. doi: 10.1053/jhep.2001.22131. [DOI] [PubMed] [Google Scholar]
- 56.Czaja AJ, Carpenter HA. Autoimmune hepatitis with incidental histologic features of bile duct injury. Hepatology. 2001;34(4 Pt 1):659–665. doi: 10.1053/jhep.2001.27562. [DOI] [PubMed] [Google Scholar]
- 57.Abdalian R, Dhar P, Jhaveri K, Haider M, Guindi M, Heathcote EJ. Prevalence of sclerosing cholangitis in adults with autoimmune hepatitis: evaluating the role of routine magnetic resonance imaging. Hepatology. 2008;47(3):949–957. doi: 10.1002/hep.22073. [DOI] [PubMed] [Google Scholar]
- 58.O’Brien C, Joshi S, Feld JJ, Guindi M, Dienes HP, Heathcote EJ. Long-term follow-up of antimitochondrial antibody-positive autoimmune hepatitis. Hepatology. 2008;48(2):550–556. doi: 10.1002/hep.22380. [DOI] [PubMed] [Google Scholar]
- 59.Donaldson PT, Czaja AJ. Genetic effects on susceptibility, clinical expression, and treatment outcome of type 1 autoimmune hepatitis. Clin Liver Dis. 2002;6(3):707–725. doi: 10.1016/s1089-3261(02)00023-5. [DOI] [PubMed] [Google Scholar]
- 60.Czaja AJ. Genetic factors affecting the occurrence, clinical phenotype, and outcome of autoimmune hepatitis. Clin Gastroenterol Hepatol. 2008;6(4):379–388. doi: 10.1016/j.cgh.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 61.Pando M, Larriba J, Fernandez GC, Fainboim H, Ciocca M, Ramonet M, et al. Pediatric and adult forms of type I autoimmune hepatitis in Argentina: evidence for differential genetic predisposition. Hepatology. 1999;30(6):1374–1380. doi: 10.1002/hep.510300611. [DOI] [PubMed] [Google Scholar]
- 62.Anderson G, Jenkinson WE, Jones T, Parnell SM, Kinsella FA, White AJ, et al. Establishment and functioning of intrathymic microenvironments. Immunol Rev. 2006;209:10–27. doi: 10.1111/j.0105-2896.2006.00347.x. [DOI] [PubMed] [Google Scholar]
- 63.Su MA, Anderson MS. Aire: an update. Curr Opin Immunol. 2004;16(6):746–752. doi: 10.1016/j.coi.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 64.Su MA, Giang K, Zumer K, Jiang H, Oven I, Rinn JL, et al. Mechanisms of an autoimmunity syndrome in mice caused by a dominant mutation in Aire. J Clin Invest. 2008;118(5):1712–1726. doi: 10.1172/JCI34523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Djilali-Saiah I, Renous R, Caillat-Zucman S, Debray D, Alvarez F. Linkage disequilibrium between HLA class II region and autoimmune hepatitis in pediatric patients. J Hepatol. 2004;40(6):904–909. doi: 10.1016/j.jhep.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 66.Vogel A, Liermann H, Harms A, Strassburg CP, Manns MP, Obermayer-Straub P. Autoimmune regulator AIRE: evidence for genetic differences between autoimmune hepatitis and hepatitis as part of the autoimmune polyglandular syndrome type 1. Hepatology. 2001;33(5):1047–1052. doi: 10.1053/jhep.2001.24031. [DOI] [PubMed] [Google Scholar]
- 67.Czaja AJ, Santrach PJ, Breanndan MS. Shared genetic risk factors in autoimmune liver disease. Dig Dis Sci. 2001;46(1):140–147. doi: 10.1023/a:1005670111068. [DOI] [PubMed] [Google Scholar]
- 68.Mieli-Vergani G, Vergani D. Immunological liver diseases in children. Semin Liver Dis. 1998;18(3):271–279. doi: 10.1055/s-2007-1007163. [DOI] [PubMed] [Google Scholar]
- 69.Lohse AW, Lohr H, Bilo K, Zumbuschenfelde KHM. Recognition and regulation of LKM-specific T-cell clones in LKM-positive autoimmune hepatitis. Hepatology. 1994;20:A144. [Google Scholar]
- 70.Donaldson PT. Genetics in autoimmune hepatitis. Semin Liver Dis. 2002;22(4):353–364. doi: 10.1055/s-2002-35705. [DOI] [PubMed] [Google Scholar]
- 71.Vergani D, Mieli-Vergani G. Autoimmune hepatitis. Autoimmun Rev. 2003;2(5):241–247. doi: 10.1016/s1568-9972(03)00017-x. [DOI] [PubMed] [Google Scholar]
- 72.Strassburg CP, Obermayer-Straub P, Manns MP. Autoimmunity in liver diseases. Clin Rev Allergy Immunol. 2000;18(2):127–139. doi: 10.1385/CRIAI:18:2:127. [DOI] [PubMed] [Google Scholar]
- 73.Krawitt EL. Autoimmune hepatitis. N Engl J Med. 2006;354(1):54–66. doi: 10.1056/NEJMra050408. [DOI] [PubMed] [Google Scholar]
- 74.Holdener M, Hintermann E, Bayer M, Rhode A, Rodrigo E, Hintereder G, et al. Breaking tolerance to the natural human liver autoantigen cytochrome P450 2D6 by virus infection. J Exp Med. 2008;205(6):1409–1422. doi: 10.1084/jem.20071859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Horwitz MS, Sarvetnick N. Viruses, host responses, and autoimmunity. Immunol Rev. 1999;169:241–253. doi: 10.1111/j.1600-065X.1999.tb01319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Costa-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8(6):639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 77.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 78.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8(9):950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 79.Costa-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8(9):942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 80.Gerosa F, Baldani-Guerra B, Lyakh LA, Batoni G, Esin S, Winkler-Pickett RT, et al. Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. J Exp Med. 2008;205(6):1447–1461. doi: 10.1084/jem.20071450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 82.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28(1):29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci USA. 2008;105(28):9721–9726. doi: 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Veldhoen M, Hirota K, Christensen J, O’Garra A, Stockinger B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J Exp Med. 2009;206(1):43–49. doi: 10.1084/jem.20081438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol. 2008;21(1):102–116. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201(2):233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314(5804):1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103(9):1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8(5):500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 90.Pene J, Chevalier S, Preisser L, Venereau E, Guilleux MH, Ghannam S, et al. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J Immunol. 2008;180(11):7423–7430. doi: 10.4049/jimmunol.180.11.7423. [DOI] [PubMed] [Google Scholar]
- 91.Harada K, Shimoda S, Sato Y, Isse K, Ikeda H, Nakanuma Y. Periductal interleukin-17 production in association with biliary innate immunity contributes to the pathogenesis of cholangiopathy in primary biliary cirrhosis. Clin Exp Immunol. 2009;157(2):261–270. doi: 10.1111/j.1365-2249.2009.03947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101(5):455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 93.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 94.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6(4):345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 95.Ruprecht CR, Gattorno M, Ferlito F, Gregorio A, Martini A, Lanzavecchia A, et al. Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J Exp Med. 2005;201(11):1793–1803. doi: 10.1084/jem.20050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203(7):1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 98.Boulougouris G, McLeod JD, Patel YI, Ellwood CN, Walker LS, Sansom DM. Positive and negative regulation of human T cell activation mediated by the CTLA-4/CD28 ligand CD80. J Immunol. 1998;161(8):3919–3924. [PubMed] [Google Scholar]
- 99.Agarwal K, Czaja AJ, Jones DE, Donaldson PT. Cytotoxic T lymphocyte antigen-4 (CTLA-4) gene polymorphisms and susceptibility to type 1 autoimmune hepatitis. Hepatology. 2000;31(1):49–53. doi: 10.1002/hep.510310110. [DOI] [PubMed] [Google Scholar]
- 100.Longhi MS, Hussain MJ, Bogdanos DP, Quaglia A, Mieli-Vergani G, Ma Y, et al. Cytochrome P450IID6-specific CD8 T cell immune responses mirror disease activity in autoimmune hepatitis type 2. Hepatology. 2007;46(2):472–484. doi: 10.1002/hep.21658. [DOI] [PubMed] [Google Scholar]
- 101.Longhi MS, Meda F, Wang P, Samyn M, Mieli-Vergani G, Vergani D, et al. Expansion and de novo generation of potentially therapeutic regulatory T cells in patients with autoimmune hepatitis. Hepatology. 2008;47(2):581–591. doi: 10.1002/hep.22071. [DOI] [PubMed] [Google Scholar]
- 102.Longhi MS, Hussain MJ, Mitry RR, Arora SK, Mieli-Vergani G, Vergani D, et al. Functional study of CD4+CD25+ regulatory T cells in health and autoimmune hepatitis. J Immunol. 2006;176(7):4484–4491. doi: 10.4049/jimmunol.176.7.4484. [DOI] [PubMed] [Google Scholar]
- 103.Mayo MJ, Mosby JM, Jeyarajah R, Combes B, Khilnani S, Al-halimi M, et al. The relationship between hepatic immunoglobulin production and CD154 expression in chronic liver diseases. Liver Int. 2006;26(2):187–196. doi: 10.1111/j.1478-3231.2005.01211.x. [DOI] [PubMed] [Google Scholar]
- 104.Grant AJ. Hepatic expression of secondary lymphoid chemokine (CCL21) promotes the development of portal-associated lymphoid tissue in chronic inflammatory liver disease. Am J Pathol. 2002;160(4):1445–1455. doi: 10.1016/S0002-9440(10)62570-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moritoki Y, Lian ZX, Ohsugi Y, Ueno Y, Gershwin ME. B cells and autoimmune liver diseases. Autoimmun Rev. 2006;5(7):449–457. doi: 10.1016/j.autrev.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 106.Czaja AJ, Carpenter HA, Santrach PJ, Moore SB. Autoimmune cholangitis within the spectrum of autoimmune liver disease. Hepatology. 2000;31(6):1231–1238. doi: 10.1053/jhep.2000.7878. [DOI] [PubMed] [Google Scholar]
- 107.Sanchez-Urdazpal L, Czaja AJ, van Hoek B, Krom RA, Wiesner RH. Prognostic features and role of liver transplantation in severe corticosteroid-treated autoimmune chronic active hepatitis. Hepatology 1992;15(2):215–221 [DOI] [PubMed]
- 108.Montano-Loza AJ, Carpenter HA, Czaja AJ. Consequences of treatment withdrawal in type 1 autoimmune hepatitis. Liver Int. 2007;27(4):507–515. doi: 10.1111/j.1478-3231.2007.01444.x. [DOI] [PubMed] [Google Scholar]
- 109.Czaja AJ, Carpenter HA. Histological features associated with relapse after corticosteroid withdrawal in type 1 autoimmune hepatitis. Liver Int. 2003;23(2):116–123. doi: 10.1034/j.1600-0676.2003.00810.x. [DOI] [PubMed] [Google Scholar]
- 110.Czaja AJ, Carpenter HA. Autoimmune hepatitis. In: Portmann BC, Ferrell LD, editors. MacSween’s Pathology of the Liver. Edinburgh: Churchill Livingstone; 2007. pp. 493–517. [Google Scholar]
- 111.Czaja AJ, Davis GL, Ludwig J, Baggenstoss AH, Taswell HF. Autoimmune features as determinants of prognosis in steroid-treated chronic active hepatitis of uncertain etiology. Gastroenterology. 1983;85(3):713–717. [PubMed] [Google Scholar]
- 112.Czaja AJ, Carpenter HA. Thiopurine methyltransferase deficiency and azathioprine intolerance in autoimmune hepatitis. Dig Dis Sci. 2006;51(5):968–975. doi: 10.1007/s10620-006-9336-5. [DOI] [PubMed] [Google Scholar]
- 113.Strassburg CP, Manns MP. Treatment of autoimmune hepatitis. Semin Liver Dis. 2009;29(3):273–285. doi: 10.1055/s-0029-1233534. [DOI] [PubMed] [Google Scholar]
- 114.Wiegand J, Schuler A, Kanzler S, Lohse A, Beuers U, Kreisel W, et al. Budesonide in previously untreated autoimmune hepatitis. Liver Int. 2005;25(5):927–934. doi: 10.1111/j.1478-3231.2005.01122.x. [DOI] [PubMed] [Google Scholar]
- 115.Danielsson A, Prytz H. Oral budesonide for treatment of autoimmune chronic active hepatitis. Aliment Pharmacol Ther. 1994;8(6):585–590. doi: 10.1111/j.1365-2036.1994.tb00334.x. [DOI] [PubMed] [Google Scholar]
- 116.Czaja AJ, Lindor KD. Failure of budesonide in a pilot study of treatment-dependent autoimmune hepatitis. Gastroenterology. 2000;119(5):1312–1316. doi: 10.1053/gast.2000.0010000001. [DOI] [PubMed] [Google Scholar]
- 117.Rebollo BJ, Cifuentes MC, Pinar MA, Caunedo AA, Salas HE, Jimenez-Saenz M, et al. Deflazacort for long-term maintenance of remission in type I autoimmune hepatitis. Rev Esp Enferm Dig. 1999;91(9):630–638. [PubMed] [Google Scholar]
- 118.Sherman KE, Narkewicz M, Pinto PC. Cyclosporine in the management of corticosteroid-resistant type I autoimmune chronic active hepatitis. J Hepatol. 1994;21(6):1040–1047. doi: 10.1016/s0168-8278(05)80615-4. [DOI] [PubMed] [Google Scholar]
- 119.Hyams JS, Ballow M, Leichtner AM. Cyclosporine treatment of autoimmune chronic active hepatitis. Gastroenterology. 1987;93(4):890–893. doi: 10.1016/0016-5085(87)90454-9. [DOI] [PubMed] [Google Scholar]
- 120.Thiel DH, Wright H, Carroll P, Abu-Elmagd K, Rodriguez-Rilo H, McMichael J, et al. Tacrolimus: a potential new treatment for autoimmune chronic active hepatitis: results of an open-label preliminary trial. Am J Gastroenterol. 1995;90(5):771–776. [PMC free article] [PubMed] [Google Scholar]
- 121.Kanzler S, Gerken G, Dienes HP, Meyer zum Buschenfelde KH, Lohse AW. Cyclophosphamide as alternative immunosuppressive therapy for autoimmune hepatitis—report of three cases. Z Gastroenterol. 1997;35(7):571–578. [PubMed] [Google Scholar]
- 122.Stern RB, Wilkinson SP, Howorth PJ, Williams R. Controlled trial of synthetic d-penicillamine and prednisone in maintenance therapy for active chronic hepatitis. Gut. 1977;18(1):19–22. doi: 10.1136/gut.18.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stern RB, Wilkinson SP, Williams R. Proceedings: controlled trial of d-penicillamine therapy in chronic active hepatitis. Gut. 1976;17(5):390. [PubMed] [Google Scholar]
- 124.Burak KW, Urbanski SJ, Swain MG. Successful treatment of refractory type 1 autoimmune hepatitis with methotrexate. J Hepatol. 1998;29(6):990–993. doi: 10.1016/s0168-8278(98)80128-1. [DOI] [PubMed] [Google Scholar]
- 125.Czaja AJ, Carpenter HA, Lindor KD. Ursodeoxycholic acid as adjunctive therapy for problematic type 1 autoimmune hepatitis: a randomized placebo-controlled treatment trial. Hepatology. 1999;30(6):1381–1386. doi: 10.1002/hep.510300603. [DOI] [PubMed] [Google Scholar]
- 126.Devlin SM, Swain MG, Urbanski SJ, Burak KW. Mycophenolate mofetil for the treatment of autoimmune hepatitis in patients refractory to standard therapy. Can J Gastroenterol. 2004;18(5):321–326. doi: 10.1155/2004/504591. [DOI] [PubMed] [Google Scholar]
- 127.Richardson PD, James PD, Ryder SD. Mycophenolate mofetil for maintenance of remission in autoimmune hepatitis in patients resistant to or intolerant of azathioprine. J Hepatol. 2000;33(3):371–375. doi: 10.1016/s0168-8278(00)80271-8. [DOI] [PubMed] [Google Scholar]
- 128.Aw MM, Dhawan A, Samyn M, Bargiota A, Mieli-Vergani G. Mycophenolate mofetil as rescue treatment for autoimmune liver disease in children: a 5-year follow-up. J Hepatol. 2009;51(1):156–160. doi: 10.1016/j.jhep.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 129.Hennes EM, Oo YH, Schramm C, Denzer U, Buggisch P, Wiegard C, et al. Mycophenolate mofetil as second line therapy in autoimmune hepatitis? Am J Gastroenterol. 2008;103(12):3063–3070. doi: 10.1111/j.1572-0241.2008.02180.x. [DOI] [PubMed] [Google Scholar]
- 130.Santos ES, Arosemena LR, Raez LE, O’Brien C, Regev A. Successful treatment of autoimmune hepatitis and idiopathic thrombocytopenic purpura with the monoclonal antibody, rituximab: case report and review of literature. Liver Int. 2006;26(5):625–629. doi: 10.1111/j.1478-3231.2006.01262.x. [DOI] [PubMed] [Google Scholar]
- 131.Zaja F, De VS, Mazzaro C, Sacco S, Damiani D, De MG, et al. Efficacy and safety of rituximab in type II mixed cryoglobulinemia. Blood. 2003;101(10):3827–3834. doi: 10.1182/blood-2002-09-2856. [DOI] [PubMed] [Google Scholar]
- 132.Saadoun D, Delluc A, Piette JC, Cacoub P. Treatment of hepatitis C-associated mixed cryoglobulinemia vasculitis. Curr Opin Rheumatol. 2008;20(1):23–28. doi: 10.1097/BOR.0b013e3282f1330c. [DOI] [PubMed] [Google Scholar]
- 133.Basse G, Ribes D, Kamar N, Mehrenberger M, Esposito L, Guitard J, et al. Rituximab therapy for de novo mixed cryoglobulinemia in renal transplant patients. Transplantation. 2005;80(11):1560–1564. doi: 10.1097/01.tp.0000183749.79424.b4. [DOI] [PubMed] [Google Scholar]
- 134.Basse G, Ribes D, Kamar N, Mehrenberger M, Sallusto F, Esposito L, et al. Rituximab therapy for mixed cryoglobulinemia in seven renal transplant patients. Transplant Proc. 2006;38(7):2308–2310. doi: 10.1016/j.transproceed.2006.06.131. [DOI] [PubMed] [Google Scholar]
- 135.Hamaki T, Kami M, Kusumi E, Ueyama J, Miyakoshi S, Morinaga S, et al. Prophylaxis of hepatitis B reactivation using lamivudine in a patient receiving rituximab. Am J Hematol. 2001;68(4):292–294. doi: 10.1002/ajh.10043. [DOI] [PubMed] [Google Scholar]
- 136.Westhoff TH, Jochimsen F, Schmittel A, Stoffler-Meilicke M, Schafer JH, Zidek W, et al. Fatal hepatitis B virus reactivation by an escape mutant following rituximab therapy. Blood. 2003;102(5):1930. doi: 10.1182/blood-2003-05-1403. [DOI] [PubMed] [Google Scholar]
- 137.Weiler-Normann C, Wiegard C, Schramm C, Lohse AW. A case of difficult-to-treat autoimmune hepatitis successfully managed by TNF-alpha blockade. Am J Gastroenterol. 2009;104(11):2877–2878. doi: 10.1038/ajg.2009.433. [DOI] [PubMed] [Google Scholar]
- 138.Germano V, Picchianti DA, Baccano G, Natale E, Onetti MA, Priori R, et al. Autoimmune hepatitis associated with infliximab in a patient with psoriatic arthritis. Ann Rheum Dis. 2005;64(10):1519–1520. doi: 10.1136/ard.2004.032821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tobon GJ, Canas C, Jaller JJ, Restrepo JC, Anaya JM. Serious liver disease induced by infliximab. Clin Rheumatol. 2007;26(4):578–581. doi: 10.1007/s10067-005-0169-y. [DOI] [PubMed] [Google Scholar]
- 140.Ozorio G, McGarity B, Bak H, Jordan AS, Lau H, Marshall C. Autoimmune hepatitis following infliximab therapy for ankylosing spondylitis. Med J Aust. 2007;187(9):524–526. doi: 10.5694/j.1326-5377.2007.tb01396.x. [DOI] [PubMed] [Google Scholar]
- 141.Fairhurst DA, Sheehan-Dare R. Autoimmune hepatitis associated with infliximab in a patient with palmoplantar pustular psoriasis. Clin Exp Dermatol. 2009;34(3):421–422. doi: 10.1111/j.1365-2230.2008.03088.x. [DOI] [PubMed] [Google Scholar]
- 142.Khanna M, Shirodkar MA, Gottlieb AB. Etanercept therapy in patients with autoimmunity and hepatitis C. J Dermatol Treat. 2003;14(4):229–232. doi: 10.1080/09546630310020470. [DOI] [PubMed] [Google Scholar]
- 143.Becker H, Willeke P, Domschke W, Gaubitz M. Etanercept tolerance in a patient with previous infliximab-induced hepatitis. Clin Rheumatol. 2008;27(12):1597–1598. doi: 10.1007/s10067-008-1000-3. [DOI] [PubMed] [Google Scholar]
- 144.Goldenberg G, Jorizzo JL. Use of etanercept in treatment of pyoderma gangrenosum in a patient with autoimmune hepatitis. J Dermatol Treat. 2005;16(5–6):347–349. doi: 10.1080/09546630500424722. [DOI] [PubMed] [Google Scholar]
- 145.Morgan AW, Hale G, Rebello PR, Richards SJ, Gooi HC, Waldmann H, et al. A pilot study of combination anti-cytokine and anti-lymphocyte biological therapy in rheumatoid arthritis. QJM. 2008;101(4):299–306. doi: 10.1093/qjmed/hcn006. [DOI] [PubMed] [Google Scholar]
- 146.Nixon R, Bansback N, Brennan A. The efficacy of inhibiting tumour necrosis factor alpha and interleukin 1 in patients with rheumatoid arthritis: a meta-analysis and adjusted indirect comparisons. Rheumatology (Oxford) 2007;46(7):1140–1147. doi: 10.1093/rheumatology/kem072. [DOI] [PubMed] [Google Scholar]
- 147.Nishimoto N, Kishimoto T, Yoshizaki K. Anti-interleukin 6 receptor antibody treatment in rheumatic disease. Ann Rheum Dis. 2000;59(Suppl 1):i21–i27. doi: 10.1136/ard.59.suppl_1.i21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Drewe E, Powell RJ. Clinically useful monoclonal antibodies in treatment. J Clin Pathol. 2002;55(2):81–85. doi: 10.1136/jcp.55.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Park JY, Pillinger MH. Interleukin-6 in the pathogenesis of rheumatoid arthritis. Bull NYU Hosp Jt Dis. 2007;65(Suppl 1):S4–S10. [PubMed] [Google Scholar]
- 150.Guignard S, Dien G, Dougados M. Severe systemic inflammatory response syndrome in a patient with adult onset Still’s disease treated with the anti-IL1 drug anakinra: a case report. Clin Exp Rheumatol. 2007;25(5):758–759. [PubMed] [Google Scholar]
- 151.Berg WB. Arguments for interleukin 1 as a target in chronic arthritis. Ann Rheum Dis. 2000;59(Suppl 1):i81–i84. doi: 10.1136/ard.59.suppl_1.i81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Genovese M, Van Den Bosch F, Roberson S, Bojin S, Biagini I, Ryan P, et al. LY2439821, a humanized anti-IL-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis. Arthritis Rheum (Epub 2010 Jan 21) [DOI] [PubMed]
- 153.Durez P, et al. AIN457, an anti-IL-17 antibody, shows good safety and induces clinical responses in patients with active rheumatoid arthritis (RA) despite methotrexate therapy in a randomized, double-blind proof-of-concept trial. Ann Rheum Dis 2010;68(Suppl 3) (abstract LB.002)
- 154.Cinamon G, Matloubian M, Lesneski MJ, Xu Y, Low C, Lu T, et al. Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nat Immunol. 2004;5(7):713–720. doi: 10.1038/ni1083. [DOI] [PubMed] [Google Scholar]
- 155.Kahan BD. FTY720: from bench to bedside. Transplant Proc. 2004;36(2 Suppl):531S–543S. doi: 10.1016/j.transproceed.2004.01.092. [DOI] [PubMed] [Google Scholar]
- 156.Rosen H, Sanna G, Alfonso C. Egress: a receptor-regulated step in lymphocyte trafficking. Immunol Rev. 2003;195:160–177. doi: 10.1034/j.1600-065x.2003.00068.x. [DOI] [PubMed] [Google Scholar]
- 157.Cyster JG. Lymphoid organ development and cell migration. Immunol Rev. 2003;195:5–14. doi: 10.1034/j.1600-065x.2003.00075.x. [DOI] [PubMed] [Google Scholar]
- 158.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008;8(10):753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Massberg S, Andrian UH. Fingolimod and sphingosine-1-phosphate—modifiers of lymphocyte migration. N Engl J Med. 2006;355(11):1088–1091. doi: 10.1056/NEJMp068159. [DOI] [PubMed] [Google Scholar]
- 160.Bohler T, Budde K, Neumayer HH, Waiser J. Novel mediators of FTY720 in human lymphocytes. Transplantation. 2005;79(4):492–495. doi: 10.1097/01.tp.0000151773.43266.17. [DOI] [PubMed] [Google Scholar]
- 161.Ledgerwood LG, Lal G, Zhang N, Garin A, Esses SJ, Ginhoux F, et al. The sphingosine 1-phosphate receptor 1 causes tissue retention by inhibiting the entry of peripheral tissue T lymphocytes into afferent lymphatics. Nat Immunol. 2008;9(1):42–53. doi: 10.1038/ni1534. [DOI] [PubMed] [Google Scholar]
- 162.Kaneko T, Murakami T, Kawana H, Takahashi M, Yasue T, Kobayashi E. Sphingosine-1-phosphate receptor agonists suppress concanavalin A-induced hepatic injury in mice. Biochem Biophys Res Commun. 2006;345(1):85–92. doi: 10.1016/j.bbrc.2006.04.067. [DOI] [PubMed] [Google Scholar]
- 163.Andersen KG, Butcher T, Betz AG. Specific immunosuppression with inducible Foxp3-transduced polyclonal T cells. PLoS Biol. 2008;6(11):e276. doi: 10.1371/journal.pbio.0060276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Deknuydt F, Bioley G, Valmori D, Ayyoub M. IL-1beta and IL-2 convert human Treg into T(H)17 cells. Clin Immunol. 2009;131(2):298–307. doi: 10.1016/j.clim.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 165.Voo KS, Wang YH, Santori FR, Boggiano C, Wang YH, Arima K, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci USA. 2009;106(12):4793–4798. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Koenen HJ, Smeets RL, Vink PM, van Rijseen E, Boots AM, Joosten I. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood 2008;112(6):2340–2352 [DOI] [PubMed]
- 167.Trowsdale J, Betz AG. Mother’s little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol. 2006;7(3):241–246. doi: 10.1038/ni1317. [DOI] [PubMed] [Google Scholar]
- 168.Aluvihare VR, Kallikourdis M, Betz AG. Tolerance, suppression and the fetal allograft. J Mol Med. 2005;83(2):88–96. doi: 10.1007/s00109-004-0608-2. [DOI] [PubMed] [Google Scholar]
- 169.Oo YH, Neuberger J. Use of mycophenolate in the treatment of autoimmune hepatitis. Liver Int. 2005;25(4):687–691. doi: 10.1111/j.1478-3231.2005.01123.x. [DOI] [PubMed] [Google Scholar]
- 170.Miyake T, Miyaoka H, Abe M, Furukawa S, Shigematsu S, Furukawa E, et al. Clinical characteristics of autoimmune hepatitis in older aged patients. Hepatol Res. 2006;36(2):139–142. doi: 10.1016/j.hepres.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 171.Tripathi D, Neuberger J. Autoimmune hepatitis and liver transplantation: indications, results, and management of recurrent disease. Semin Liver Dis. 2009;29(3):286–296. doi: 10.1055/s-0029-1233531. [DOI] [PubMed] [Google Scholar]
- 172.Czaja AJ. Special clinical challenges in autoimmune hepatitis: the elderly, males, pregnancy, mild disease, fulminant onset, and nonwhite patients. Semin Liver Dis. 2009;29(3):315–330. doi: 10.1055/s-0029-1233530. [DOI] [PubMed] [Google Scholar]
- 173.Schreuder TC, Hubscher SG, Neuberger J. Autoimmune liver diseases and recurrence after orthotopic liver transplantation: what have we learned so far? Transpl Int. 2009;22(2):144–152. doi: 10.1111/j.1432-2277.2008.00729.x. [DOI] [PubMed] [Google Scholar]
- 174.Vogel A, Heinrich E, Bahr MJ, Rifai K, Flemming P, Melter M, et al. Long-term outcome of liver transplantation for autoimmune hepatitis. Clin Transplant. 2004;18(1):62–69. doi: 10.1111/j.1399-0012.2004.00117.x. [DOI] [PubMed] [Google Scholar]
- 175.Duclos-Vallee JC, Sebagh M. Recurrence of autoimmune disease, primary sclerosing cholangitis, primary biliary cirrhosis, and autoimmune hepatitis after liver transplantation. Liver Transpl. 2009;15(Suppl 2):S25–S34. doi: 10.1002/lt.21916. [DOI] [PubMed] [Google Scholar]
- 176.Duclos-Vallee JC, Sebagh M, Rifai K, Johanet C, Ballot E, Guettier C, et al. A 10 year follow up study of patients transplanted for autoimmune hepatitis: histological recurrence precedes clinical and biochemical recurrence. Gut. 2003;52(6):893–897. doi: 10.1136/gut.52.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gautam M, Cheruvattath R, Balan V. Recurrence of autoimmune liver disease after liver transplantation: a systematic review. Liver Transpl. 2006;12(12):1813–1824. doi: 10.1002/lt.20910. [DOI] [PubMed] [Google Scholar]
- 178.Hubscher SG. Recurrent autoimmune hepatitis after liver transplantation: diagnostic criteria, risk factors, and outcome. Liver Transpl. 2001;7(4):285–291. doi: 10.1053/jlts.2001.23085. [DOI] [PubMed] [Google Scholar]
- 179.Gonzalez-Koch A, Czaja AJ, Carpenter HA, Roberts SK, Charlton MR, Porayko MK, et al. Recurrent autoimmune hepatitis after orthotopic liver transplantation. Liver Transpl. 2001;7(4):302–310. doi: 10.1053/jlts.2001.21449. [DOI] [PubMed] [Google Scholar]
- 180.Salcedo M, Vaquero J, Banares R, Rodriguez-Mahou M, Alvarez E, Vicario JL, et al. Response to steroids in de novo autoimmune hepatitis after liver transplantation. Hepatology. 2002;35(2):349–356. doi: 10.1053/jhep.2002.31167. [DOI] [PubMed] [Google Scholar]
- 181.Kerkar N, Hadzic N, Davies ET, Portmann B, Donaldson PT, Rela M, et al. De-novo autoimmune hepatitis after liver transplantation. Lancet. 1998;351(9100):409–413. doi: 10.1016/S0140-6736(97)06478-7. [DOI] [PubMed] [Google Scholar]
- 182.Heneghan MA, Portmann BC, Norris SM, Williams R, Muiesan P, Rela M, et al. Graft dysfunction mimicking autoimmune hepatitis following liver transplantation in adults. Hepatology. 2001;34(3):464–470. doi: 10.1053/jhep.2001.26756. [DOI] [PubMed] [Google Scholar]
- 183.D’Antiga L, Dhawan A, Portmann B, Francavilla R, Rela M, Heaton N, et al. Late cellular rejection in paediatric liver transplantation: aetiology and outcome. Transplantation. 2002;73(1):80–84. doi: 10.1097/00007890-200201150-00015. [DOI] [PubMed] [Google Scholar]
- 184.Miyagawa-Hayashino A, Haga H, Egawa H, Hayashino Y, Sakurai T, Minamiguchi S, et al. Outcome and risk factors of de novo autoimmune hepatitis in living-donor liver transplantation. Transplantation. 2004;78(1):128–135. doi: 10.1097/01.tp.0000132328.33460.43. [DOI] [PubMed] [Google Scholar]
- 185.Mieli-Vergani G, Vergani D. De novo autoimmune hepatitis after liver transplantation. J Hepatol. 2004;40(1):3–7. doi: 10.1016/j.jhep.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 186.Venick RS, McDiarmid SV, Farmer DG, Gornbein J, Martin MG, Vargas JH, et al. Rejection and steroid dependence: unique risk factors in the development of pediatric posttransplant de novo autoimmune hepatitis. Am J Transplant. 2007;7(4):955–963. doi: 10.1111/j.1600-6143.2006.01717.x. [DOI] [PubMed] [Google Scholar]
- 187.Avitzur Y, Ngan BY, Lao M, Fecteau A, Ng VL. Prospective evaluation of the prevalence and clinical significance of positive autoantibodies after pediatric liver transplantation. J Pediatr Gastroenterol Nutr. 2007;45(2):222–227. doi: 10.1097/MPG.0b013e31805ce219. [DOI] [PubMed] [Google Scholar]
- 188.Duclos-Vallee JC, Johanet C, Bach JF, Yamamoto AM. Autoantibodies associated with acute rejection after liver transplantation for type-2 autoimmune hepatitis. J Hepatol. 2000;33(1):163–166. doi: 10.1016/s0168-8278(00)80175-0. [DOI] [PubMed] [Google Scholar]
- 189.Lohse AW, Obermayer-Straub P, Gerken G, Brunner S, Altes U, Dienes HP, et al. Development of cytochrome P450 2D6-specific LKM-autoantibodies following liver transplantation for Wilson’s disease—possible association with a steroid-resistant transplant rejection episode. J Hepatol. 1999;31(1):149–155. doi: 10.1016/s0168-8278(99)80175-5. [DOI] [PubMed] [Google Scholar]
- 190.Riva S, Sonzogni A, Bravi M, Bertani A, Alessio MG, Candusso M, et al. Late graft dysfunction and autoantibodies after liver transplantation in children: preliminary results of an Italian experience. Liver Transpl. 2006;12(4):573–577. doi: 10.1002/lt.20673. [DOI] [PubMed] [Google Scholar]
- 191.Fiel MI, Agarwal K, Stanca C, Elhajj N, Kontorinis N, Thung SN, et al. Posttransplant plasma cell hepatitis (de novo autoimmune hepatitis) is a variant of rejection and may lead to a negative outcome in patients with hepatitis C virus. Liver Transpl. 2008;14(6):861–871. doi: 10.1002/lt.21447. [DOI] [PubMed] [Google Scholar]
- 192.Cholongitas E, Samonakis D, Patch D, Senzolo M, Burroughs AK, Quaglia A, et al. Induction of autoimmune hepatitis by pegylated interferon in a liver transplant patient with recurrent hepatitis C virus. Transplantation. 2006;81(3):488–490. doi: 10.1097/01.tp.0000196716.07188.c4. [DOI] [PubMed] [Google Scholar]
- 193.Kontorinis N, Agarwal K, Elhajj N, Fiel MI, Schiano TD. Pegylated interferon-induced immune-mediated hepatitis post-liver transplantation. Liver Transpl. 2006;12(5):827–830. doi: 10.1002/lt.20706. [DOI] [PubMed] [Google Scholar]
- 194.Berardi S, Lodato F, Gramenzi A, D’Errico A, Lenzi M, Bontadini A, et al. High incidence of allograft dysfunction in liver transplanted patients treated with pegylated-interferon alpha-2b and ribavirin for hepatitis C recurrence: possible de novo autoimmune hepatitis? Gut. 2007;56(2):237–242. doi: 10.1136/gut.2006.092064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Aguilera I, Sousa JM, Gavilan F, Bernardos A, Wichmann I, Nunez-Roldan A. Glutathione S-transferase T1 mismatch constitutes a risk factor for de novo immune hepatitis after liver transplantation. Liver Transpl. 2004;10(9):1166–1172. doi: 10.1002/lt.20209. [DOI] [PubMed] [Google Scholar]
- 196.Aguilera I, Sousa JM, Gavilan F, Bernardos A, Wichmann I, Nunez-Roldan A. Glutathione S-transferase T1 genetic mismatch is a risk factor for de novo immune hepatitis in liver transplantation. Transplant Proc. 2005;37(9):3968–3969. doi: 10.1016/j.transproceed.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 197.Rodriguez-Mahou M, Salcedo M, Fernandez-Cruz E, Tiscar JL, Banares R, Clemente G, et al. Antibodies against glutathione S-transferase T1 (GSTT1) in patients with GSTT1 null genotype as prognostic marker: long-term follow-up after liver transplantation. Transplantation. 2007;83(8):1126–1129. doi: 10.1097/01.tp.0000259963.47350.da. [DOI] [PubMed] [Google Scholar]
- 198.Salcedo M, Rodriguez-Mahou M, Rodriguez-Sainz C, Rincon D, Alvarez E, Vicario JL, et al. Risk factors for developing de novo autoimmune hepatitis associated with anti-glutathione-S-transferase T1 antibodies after liver transplantation. Liver Transpl. 2009;15(5):530–539. doi: 10.1002/lt.21721. [DOI] [PubMed] [Google Scholar]
- 199.Yoshizawa K, Shirakawa H, Ichijo T, Umemura T, Tanaka E, Kiyosawa K, et al. De novo autoimmune hepatitis following living-donor liver transplantation for primary biliary cirrhosis. Clin Transplant. 2008;22(3):385–390. doi: 10.1111/j.1399-0012.2007.00787.x. [DOI] [PubMed] [Google Scholar]
- 200.Grammatikopoulos. AASLD; 2010 (abstract 706)
- 201.Richter A, Grabhorn E, Helmke K, Manns MP, Ganschow R, Burdelski M. Clinical relevance of autoantibodies after pediatric liver transplantation. Clin Transplant. 2007;21(3):427–432. doi: 10.1111/j.1399-0012.2007.00667.x. [DOI] [PubMed] [Google Scholar]
- 202.Shaikh OS, Demetris AJ. Idiopathic posttransplantation hepatitis? Liver Transpl. 2007;13(7):943–946. doi: 10.1002/lt.21202. [DOI] [PubMed] [Google Scholar]
- 203.Herzog D, Soglio DB, Fournet JC, Martin S, Marleau D, Alvarez F. Interface hepatitis is associated with a high incidence of late graft fibrosis in a group of tightly monitored pediatric orthotopic liver transplantation patients. Liver Transpl. 2008;14(7):946–955. doi: 10.1002/lt.21444. [DOI] [PubMed] [Google Scholar]
- 204.Evans HM. Progressive histological damage in liver allografts following pediatric liver transplantation. Hepatology. 2006;43(5):1109–1117. doi: 10.1002/hep.21152. [DOI] [PubMed] [Google Scholar]
- 205.Syn WK. Natural history of unexplained chronic hepatitis after liver transplantation. Liver Transpl. 2007;13(7):984–989. doi: 10.1002/lt.21108. [DOI] [PubMed] [Google Scholar]
- 206.Hubscher S. What does the long-term liver allograft look like for the pediatric recipient? Liver Transpl. 2009;15(Suppl 2):S19–S24. doi: 10.1002/lt.21902. [DOI] [PubMed] [Google Scholar]