Abstract
Background
Postprandial symptoms in irritable bowel syndrome (IBS) have been associated with increased bowel contractility.
Aim
To compare ileocolonic and colonic responses to feeding in health and IBS.
Methods
We prospectively analyzed data from separate research trials in122 IBS patients and 41 healthy volunteers. Ileocolonic transit (ICT) was evaluated before [colonic filling (CF)3h] and immediately after (CF4h) a standard lunch at 3h45min, and 2h thereafter. Colonic geometric center (GC) was calculated 2h (GC6h) after lunch ingested at 4h (GC4h) and directly after (GC8h) a standard dinner ingested at 7h45min.
Results
ICT immediately after eating was higher in IBS-D than health (23.1±2.4% vs. 17.5±2.8%, p=0.059). ICT 2h after lunch was similar between groups (p=0.55). There was significant overall group difference in colonic transit 2h post-lunch (p=0.045), particularly in IBS-C (GC6-GC4, Δ 0.29±0.08) vs. health (Δ 0.56±0.12 GC units).
Conclusions
After feeding, ICT is increased in IBS-D, whereas colonic transit is blunted in IBS-C.
Keywords: colonic, gastrocolic reflex, gastroileal reflex, ileocolonic, response to food
INTRODUCTION
Irritable bowel syndrome (IBS) is a common functional disorder in which abdominal pain or discomfort is associated with a change in bowel habits. The prevalence of IBS in the general population is approximately 10% (1). The pathophysiological mechanisms are diverse. By Rome III consensus criteria, patients’ IBS phenotype can be classified according to the predominant bowel pattern: diarrhea predominant (IBS-D), constipation predominant (IBS-C) or mixed IBS (IBS-M).
Symptoms may occur or be exacerbated after a meal in patients with IBS (2–4), including abdominal pain, gas, abdominal distension and urgency (5).
The colonic response to food, sometimes termed the ‘gastrocolic reflex’ (6), results in an integrated increase in colonic tone (7) and phasic pressure activity (8) following meal ingestion. The mechanisms mediating this response involve cholinergic pathways (9,10), as well as mediators such as serotonin (7,11), gastrin (8), prostaglandin E1 (12) and cholecystokinin (8).
The first description of an impaired colonic response to food in patients with IBS is attributed to Connell et al. (13) in the 1960s. Using colonic manometry, they demonstrated that patients with IBS had an exaggerated increase in postprandial colonic activity. In a more recent study, Bouchoucha et al. (14) compared colonic transit of radiopaque markers at rest and after eating a standard test meal; segmental differences in the patterns of colonic filling and emptying were observed in response to eating in healthy volunteers and patients with IBS. The generalizability of the study was limited by the sample size, the propensity for included IBS patients to have delayed colonic transit, and the lack of assessment of ileocolonic transit. Ileocolonic transit is characterized by bolus movements (15,16) that coincide with simultaneous prolonged propagated contractions (17) and may result in abdominal discomfort.
The aim of this study was to quantify ileocolonic and colonic transit in response to meal ingestion in healthy volunteers and different subgroups of IBS patients.
METHODS
Data Source
Data were retrieved from a database of prospectively performed motor, sensory, psychological and autonomic function tests in 122 patients with IBS and 41 healthy volunteers (4). Patients residing within 200 miles of the Mayo Clinic were recruited based on their primary presentation with IBS, identified by the Rome II criteria (18). Volunteers were included as healthy controls if they had less than 4 positive gastrointestinal symptoms out of 19 on the Bowel Disease Questionnaire [BDQ (19)], scored as mild severity at worst or “sometimes” for greatest frequency.
Exclusion criteria included organic disease that might explain the patient’s symptoms and any structural or metabolic disease or condition that affects the gastrointestinal system, including diabetes. In addition, participants were ineligible if they had participated in another clinical trial within the preceding 30 days. The use of any IBS or anti-constipation medication within at least 7 days prior to gastrointestinal transit measurements was not permitted. Participants were allowed to continue low, stable doses of thyroid replacement, low-dose aspirin (81mg/d), SSRIs, estrogen replacement and birth control pills or depot estrogen injections.
All participants had provided written informed consent before participating in the research study. The current analysis was approved by the Mayo Clinic Institutional Review Board. Patients, who had withdrawn authorization to use their records for future research purposes, had their data removed from the analysis.
Scintigraphic Transit Studies
An adaptation of our established scintigraphic method was used to evaluate the ileocolonic and colonic response to food. After an overnight fast, a capsule containing 111In adsorbed on activated charcoal was administered. The methacrylate coating (Eudragit S-100, The Dow Chemical Company) of the capsule dissolves in a pH-sensitive manner upon reaching the alkaline terminal ileum, releasing the radio-isotope into the lumen, thus allowing assessment of colonic transit. After the capsule had emptied from the stomach, documented by its relative position to a radioisotope marker placed on the right anterior iliac crest, participants ingested a 99mTc-labeled breakfast (315kcal; two scrambled eggs, one slice of whole wheat bread and one glass of whole milk). The 99mTc-sulphur colloid in the breakfast was used estimate the colonic filling (CF) at different time points as a surrogate for ileocecal transit.
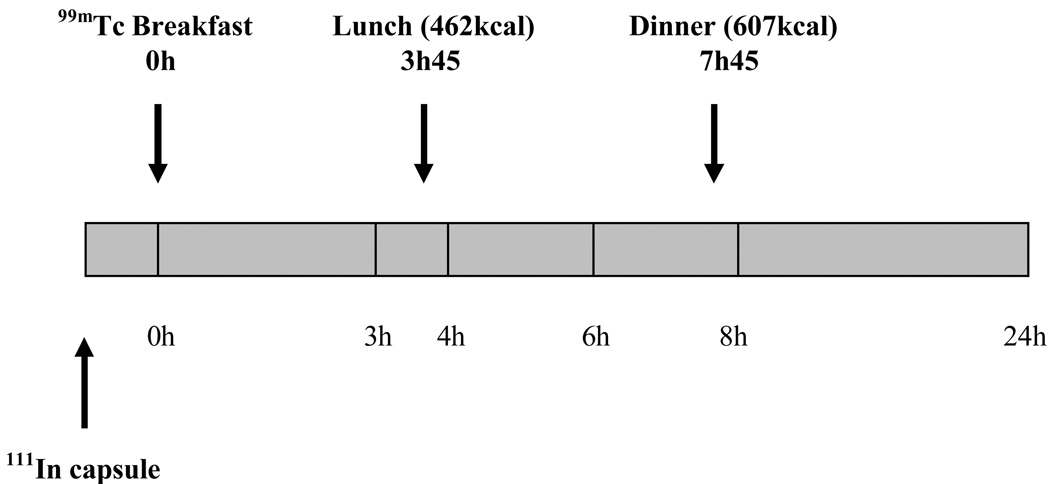
Using a gamma camera, anterior and posterior abdominal scans of 2 minutes duration were acquired immediately following ingestion of the radiolabeled breakfast and at 3, 4, 6 and 8 hours post-breakfast. Additional scans were completed at 24 and 48 hours. An illustration of the experimental design is provided in Figure 1.
Figure 1.
Experimental design.
Three hours and 45 minutes after ingestion of the 99mTc-labeled breakfast, participants were served a standardized lunch of 462 kcal, which comprised one chicken breast, one potato, one pudding cup, three pads of butter and 300ml of water (19% protein, 43% carbohydrates and 38% fat). Transfer of radioisotope from the ileum to the colon following meal ingestion was measured to assess the ileocolonic response to food. To quantify the magnitude of the colonic response to food, a standardized 607kcal dinner was served 7 hours and 45 minutes after the 99mTc-labeled breakfast. This dinner consisted of a roast beef sandwich, milk and a cookie (19% protein, 47% carbohydrates and 34% fat).
Data Analysis
99mTc counts were quantified within a 140 keV (±20%) window and 111In counts within a 247 keV (±20%) window. Count corrections were made for isotope decay, tissue attenuation and downscatter of the 111In in the 99mTc window. A variable region of interest program was used to quantitate counts in each colonic segment.
The ileocolonic response to food, during and following lunch, was quantified by the transfer of 99mTc-labeled chyme from the ileum to the colon and expressed by the difference in colonic filling (CF) at different time points. CF, the proportion of the 99mTc-labeled breakfast that has accumulated in the colon, was measured before the 462kcal chicken lunch (3h), immediately after finishing lunch (4h), and 2 hours thereafter (6h). The differences in CF were used to quantify the ileocolonic transit in response to food: the immediate response was expressed as CF4h − CF3h and the delayed ileocolonic response as CF6h − CF4h.
The colonic response to food was estimated by the progression of 111In-labeled content through the colon, with respect to the different colonic segments: ascending colon (AC), transverse colon (TC), descending colon (DC) and rectosigmoid (RS), numbered as segments 1 to 4, respectively. Segment 5 signifies the expelled stool. Colonic response to food was expressed as the difference in geometric center (GC) before and after the roast beef dinner. The GC is the weighted average of the isotope distribution within the colon and is calculated by the sum of the products of the proportion of 111In counts in each colonic segment and the segment’s weighting factor:
The immediate colonic response to food was expressed as GC8h − GC6h, as the 8h-scan was acquired directly after finishing dinner. We assessed the delayed colonic response to food 2 hours post-meal based on the chicken lunch by comparing GC6h to GC4h.
Statistical Analysis
Kruskal-Wallis tests were used compare IBS-D, IBS-C, IBS-M and healthy controls. Wilcoxon rank sum test was used to compare IBS-D or IBS-C and healthy controls. Results are expressed as mean ± SEM.
RESULTS
Participants
Participants’ demographics are summarized in Table 1. One patient became ill during transit measurements and dropped out. Of the remaining 163 participants, 160 were female and 3 were male. All groups were similar in age, but body mass indices (BMI) were higher in patients with IBS-D and IBS-M. The relationships of meal-related symptoms and participant subgroups are shown in Table 1.
Table 1.
Participants’ Characteristics
| Healthy | IBS-C | IBS-D | IBS-M | |
|---|---|---|---|---|
| Number (males) | 41 (0) | 47 (0) | 45 (3) | 30 (0) |
| Age, years | 34.0 ± 1.6 | 38.4 ± 1.5 | 35.3 ± 1.5 | 38.7 ± 2.3 |
| BMI, kg/m2 | 25.1 ± 0.7 | 25.3 ± 0.5 | 28.8 ± 1.0 | 27.6 ± 0.8 |
| Any meal related symptoms, % | 2 | 67 | 71 | 79 |
BMI, body mass index.
Ileocolonic Response to Food Ingestion
For healthy volunteers, the mean immediate ileocolonic response to food ingested at 3 hours 45 minutes was 17.5 ± 2.8%; the delayed response 2 hours post-meal was 29.3 ± 3.2% (Table 2, Figure 2).
Table 2.
Ileocolonic and Colonic Response to Food
| Parameter | Healthy | All IBS | IBS-C | IBS-D | IBS-M | |
|---|---|---|---|---|---|---|
| Immediate ileocolonic response |
ΔCF4h−CF3h | 17.5 ± 2.8 | 19.7 ± 1.46 | 19.4 ± 2.4 | 23.1 ± 2.4 | 15.1 ± 2.8 |
| Delayed ileocolonic response | ΔCF6h−CF4h | 29.3 ± 3.2 | 27.34 ± 1.57 | 29.5 ± 2.6 | 24.2 ± 2.3 | 28.4 ± 3.6 |
| Immediate colonic response | ΔGC8h−GC6h | 0.16 ± 0.04 | 0.18 ± 0.02 | 0.18 ± 0.04 | 0.18 ± 0.03 | 0.20 ± 0.06 |
| Delayed colonic response | ΔGC6h-GC4h | 0.56 ± 0.12 | 0.35 ± 0.05 | 0.29 ± 0.08 | 0.50 ± 0.09 | 0.26 ± 0.05 |
CF, colonic filling. GC, geometric center
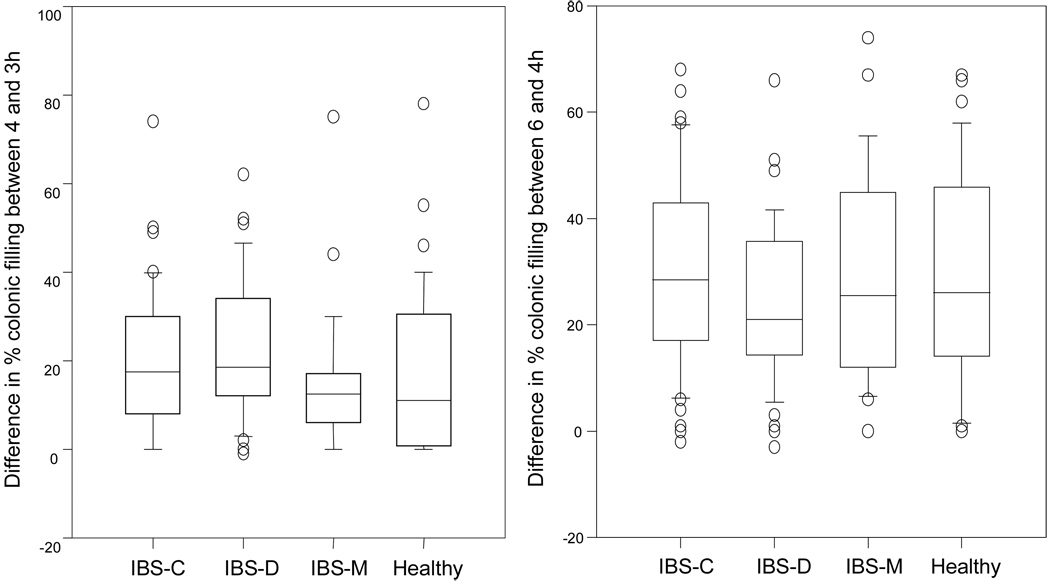
Figure 2.
Box and whisker plots showing the immediate and later ileocolonic response to food as the difference in % of colonic filling between 3 and 4 hours and 4 and 6 hours, respectively. Note the trend towards an increased immediate ileocolonic response in IBS-D compared to healthy volunteers.
There was no statistical difference in the immediate ileocolonic response to food between groups (p=0.075). However, ileocolonic transit immediately following meal ingestion was higher in IBS-D and healthy volunteers (23.1 ± 2.4% vs 17.5 ± 2.8%, p=0.059, Figure 3).
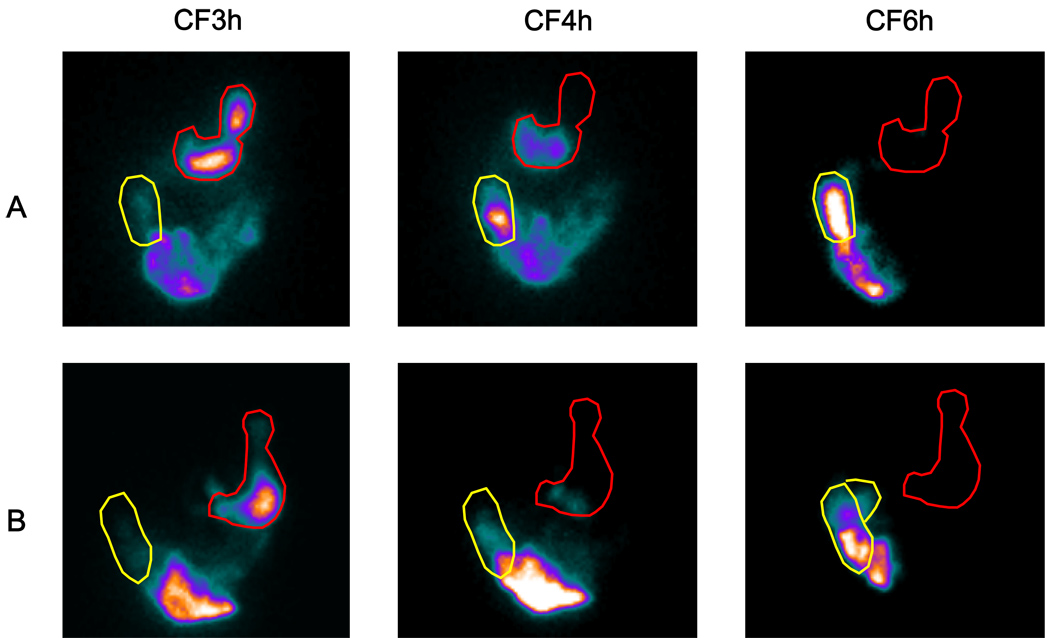
Figure 3.
Representative ileocolonic transit scintiscans at 3, 4 and 6 hours in a healthy volunteer (A) and a patient with IBS-D (B). A region of interest is drawn around the ascending colon. The intensity of the image reflects the concentration of counts in each region. Note that there is increased colonic filling in the patient with IBS-D at the 4h scan, reflecting increased ileocolonic transfer in response to the meal.
There were no differences between subgroups in the delayed ileocolonic response, 2 hours post-meal (Table 2).
Colonic Response to Food Ingestion
The mean change in GC after the dinner at 7 hours 45 minutes was 0.16 ± 0.04 GC units for healthy volunteers (Table 2, Figure 4). This immediate postprandial change in GC was not significantly different between IBS patients and healthy volunteers. Two patients, one with IBS-D and one with IBS-M, showed net retrograde movement of colonic content after finishing the meal, as GC8h−GC6h was negative (Figure 4).
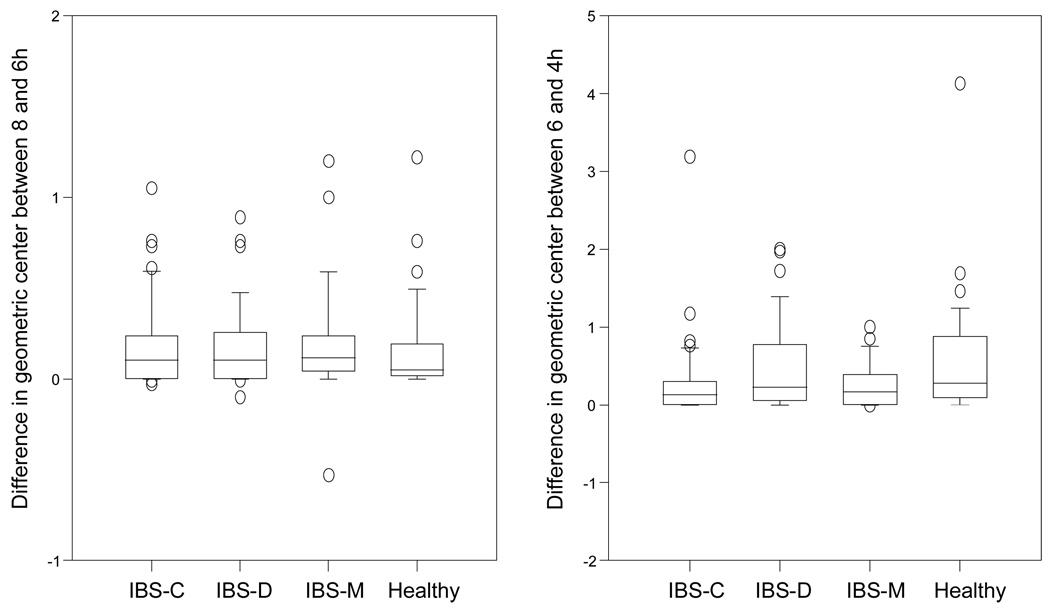
Figure 4.
Box and whisker plots showing the immediate and later colonic response to food as the change in geometric center between 6 and 8 hours and 4 and 6 hours, respectively. Note the impaired colonic response 2 hours post-lunch in IBS-C.
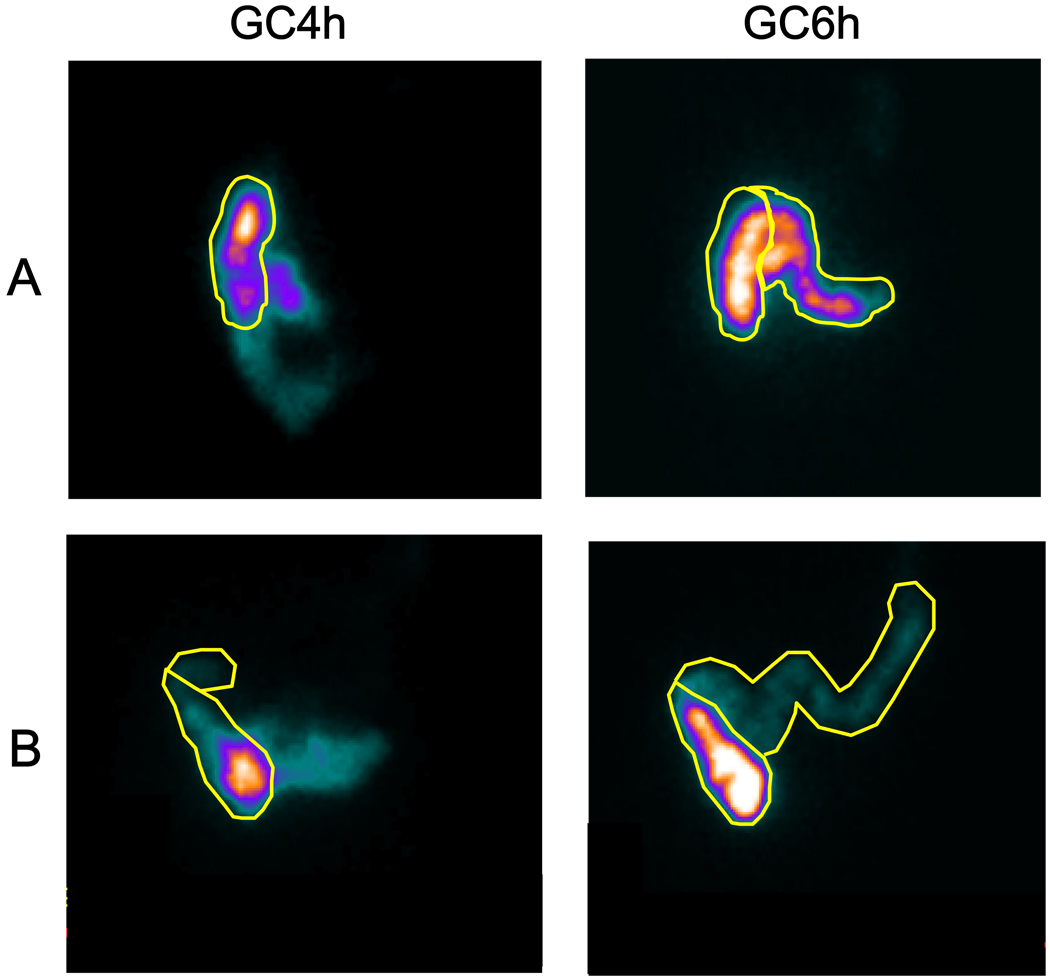
The group difference for the delayed colonic motor response 2 hours post-lunch (GC6h−GC4h) was significant (p=0.045); in particular, it was lower for IBS-C patients compared to healthy volunteers (0.29 ± 0.08 vs 0.56 ± 0.12 GC units, respectively, Figure 5).
Figure 5.
Representative colonic transit scintiscans at 4 and 6 hours in a healthy volunteer (A) and a patient with IBS-C (B). Regions of interest are drawn around the ascending and transverse colon. The intensity of the image reflects the concentration of counts in each region. Note that there is less movement of radio-isotope in the patient with IBS-C compared to the healthy volunteer, reflecting a blunted delayed colonic response to food to the chicken lunch.
DISCUSSION
In this study, we assessed the ileocolonic and colonic transit responses to food ingestion in patients with IBS and healthy volunteers by measuring the progression of chyme through the ileocolonic regions following meal ingestions that occurred 4 and 8 hours after the intake of a radiolabeled meal and a capsule that delivered activated charcoal particles to the ileocolonic junction. We demonstrated increased ileocolonic transit in IBS-D and decreased colonic transit in IBS-C in response to meal ingestion.
Ileocolonic Transit
Transfer of chyme from the terminal ileum to the colon occurs in bolus movements (15) that coincide with prolonged propagating ileal contractions (17,20). After meal ingestion, the number of boluses increases (21) with concurrent filling of the colon (15), suggesting a ‘gastroileal’ reflex with propulsion of content (21). This may be a neural reflex or it may result from augmented ileal flow as the residue of the first meal reaches the terminal ileum approximately 4 hours after ingestion and its movement into the colon coincides with the second meal ingestion (22,23); alternatively, specific nutrients may activate ileocolonic transit.
Neural reflexes may contribute to the ileocolonic transit. The cholinergic mechanisms involved in increased ileocecal transit have been investigated in a canine model of the ileocolonic region in vivo, with evidence for involvement of muscarinic M1 and M3 mechanisms (26,27). If symptoms are aggravated postprandially in IBS, these data suggest that further studies of selective antimuscarinic agents in patients with IBS are indicated.
Disturbances of ileocolonic transfer have been proposed as a pathophysiological mechanism for symptoms in IBS patients (24,25). We observed a numerical difference in ileocolonic transit during meal ingestion in patients with IBS-D. Since there was no difference in small bowel transit between IBS subgroups [data published in detail in (4)], it seems unlikely that the numerical difference in ileocolonic transit observed in the current study results from a difference in arrival of residue from the breakfast meal.
The arrival of products of digestion of fat or complex carbohydrate [such as short chain fatty acids (SCFA)] in the ileum may trigger ileal motility (15,28) and ileocolonic transfers; the presence of fat concentrations associated with moderate steatorrhea can overwhelm this capacitance and result in diarrhea (29,30). This activation of ileal motility by SCFA is inhibited by the nonselective opiate blocker, naloxone, and calcium channel blocker. Our studies are limited by the relatively low fat content of the diet. However, Steed et al. (31) showed that, in normal subjects, equicaloric high and low fat meals did not induce different transit profiles. Further evaluation of higher concentrations of fat or SCFAs delivered to the ileocolonic region in IBS patients would be of significant interest.
Colonic Transit and Motility and Food Ingestion
We were not able to demonstrate a difference in the immediate colonic response following meal ingestion between IBS patients and healthy volunteers. Bazzocchi et al. (32) had reported absence of an immediate colonic response following meal ingestion in patients with chronic functional diarrhea, in contrast to healthy volunteers. However, there are several differences in these studies. First, our scintigraphic measurement evaluates the unprepared colon, whereas Bazzocchi et al. performed colonic cleansing, which may have affected the residual content and its propulsion. Second, we used a 462 kcal lunch and a 607 kcal dinner (each consisting of approximately 20% protein, 45% carbohydrates and 35% fat), whereas the other study used a high-fat, 1000 kcal meal. The caloric content and meal composition may be important determinants of the magnitude of colonic response to food (8,33–36). Nevertheless, the meal calorie content in our studies was sufficient to stimulate colonic motility, since colonic motor responses to food have been documented with a calorie load comparable to ours (37) or lower [200 kcal (33)].
Electromyographic and manometric observations have demonstrated an increase in colonic tone and spike activity in IBS patients after eating (9,14,38–40). Studies of propulsion or colonic transit of radiopaque markers after meals (14) in healthy controls showed emptying of the cecum and ascending colon, and filling of the rectosigmoid. In IBS patients (14), eating resulted in emptying of the cecum-ascending colon, the left transverse colon and the splenic flexure, without filling of the distal colon. Di Stefano et al. showed a normal tonic response to 200 kcal in health and to 400 kcal in IBS-C; in contrast, patients with IBS-D required 1000 kcal to induce the normal increase in rectosigmoid tone (33). The reduced colonic response to food reported by Bouchoucha et al. may reflect colonic function in patients with IBS-C (14). The blunted colonic response to food in IBS-C patients 2 hours postprandially in our study is consistent with the overall results of Bouchoucha et al. and with other reports of impaired colonic response to food in constipated patients (41–45).
Colonic Response and Symptoms after Feeding
An abnormal colonic response to food may trigger postprandial symptoms in patients with IBS, though there is little objective evidence to date. Simren et al. documented the majority of patients with IBS consider their symptoms to be related to meals particularly foods rich in carbohydrates and fat; they did not measure colonic transit in the same study (46). We did not gather symptom data during the transit measurements (8); however, all participants filled out bowel disease questionnaires prior to the transit studies, including assessment of postprandial symptoms such as abdominal pain occurring immediately (0–30 minutes) or 30–120 minutes after a meal and aggravation of symptoms after oral intake. These data, previously published in detail elsewhere (4), show that 95% of our healthy volunteers experience no postprandial symptoms, whereas approximately 70% of IBS patients report postprandial symptoms (Table 1). There was also a higher percentage of IBS-D patients who reported abdominal pain immediately following a meal. These data are consistent with the hypothesis that increased ileocolonic transfer in IBS-D might be associated with postprandial symptoms. However, this has to be tested prospectively with measurement of motor responses and symptoms simultaneously. The lack of any difference in the immediate postprandial colonic response to food ingested at 4 and 8 hours suggests that postprandial urgency may reflect distal colonic stimulation by feeding. This was not detectable in our study since the isotope was located in the proximal colon at 4 to 8 hours.
Strengths and Limitations
One of the strengths of this study is the large number of participants included for assessment of ileocolonic and colonic transit in response to eating in the different subgroups of IBS patients. If confirmed in prospective studies of transit and simultaneous symptom measurement, this may lead to better designed treatments that inhibit postprandial responses such as 5-HT3 antagonists (7,47) and selective muscarinic antagonists (26,27).
As the study was conducted retrospectively, there are some weaknesses: meals of a different caloric content were served at ~4 and ~8 hours after the radiolabeled breakfast meal, and colonic response to food was assessed in response to both meals. The delayed colonic response to feeding was assessed in relation to the smaller lunchtime meal, as no scans were available at the 10 hour time point (2 hours after the larger meal).
CONCLUSION
In summary, this study demonstrates that scintigraphy can assess ileocolonic motor responses to food ingestion and suggests impaired colonic response to food in patients with IBS-C and numerically increased ileocolonic response (p=0.059) in patients with IBS-D. Future studies should explore the relationship of food ingestion to the pathophysiology of IBS and the relationship to symptoms measured simultaneously. These noninvasive studies may provide evidence for the colonic response to food as a potential target for pharmacological treatment to normalize the responses and relieve patients’ symptoms.
Footnotes
Disclosure: Dr. Camilleri’s work in IBS is supported in part by RO1 grant DK-54681 from the National Institutes of Health.
Contributor Information
Annemie Deiteren, Clinical Enteric Neuroscience Translational and Epidemiological Research (CENTER), Mayo Clinic, Rochester, Minnesota.
Michael Camilleri, Clinical Enteric Neuroscience Translational and Epidemiological Research (CENTER), Mayo Clinic, Rochester, Minnesota
Duane Burton, Clinical Enteric Neuroscience Translational and Epidemiological Research (CENTER), Mayo Clinic, Rochester, Minnesota.
Sanna McKinzie, Clinical Enteric Neuroscience Translational and Epidemiological Research (CENTER), Mayo Clinic, Rochester, Minnesota
Archana Rao, Clinical Enteric Neuroscience Translational and Epidemiological Research (CENTER), Mayo Clinic, Rochester, Minnesota
Alan R. Zinsmeister, Division of Biomedical Statistics and Informatics, Department of Health Sciences Research, College of Medicine, Mayo Clinic, Rochester, Minnesota.
REFERENCES
- 1.Saito YA, Schoenfeld P, Locke GR., III The epidemiology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol. 2002;97:1910–1915. doi: 10.1111/j.1572-0241.2002.05913.x. [DOI] [PubMed] [Google Scholar]
- 2.Patients' description of diarrhea, constipation and symptom variation during a prospective 6-week study. Ragnarsson G, Bodemar G. Pain is temporally related to eating but not to defaecation in the irritable bowel syndrome (IBS) Eur J Gastroenterol Hepatol. 1998;10:415–421. doi: 10.1097/00042737-199805000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Lea R, Whorwell PJ. The role of food intolerance in irritable bowel syndrome. Gastroenterol Clin North Am. 2005;34:247–255. doi: 10.1016/j.gtc.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Camilleri M, McKinzie S, Busciglio I, Low PA, Sweetser S, Burton D, Baxter K, Ryks M, Zinsmeister AR. Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008;6:772–781. doi: 10.1016/j.cgh.2008.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simrén M, Månsson A, Langkilde A, Svedlund J, Abrahamsson H, Bengtsson U, et al. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion. 2001;63:108–115. doi: 10.1159/000051878. [DOI] [PubMed] [Google Scholar]
- 6.Duthie HL. Colonic response to eating. Gastroenterology. 1978;75:527–528. [PubMed] [Google Scholar]
- 7.von der Ohe MR, Hanson RB, Camilleri M. Serotonergic mediation of postprandial colonic tonic and phasic responses in humans. Gut. 1994;35:536–541. doi: 10.1136/gut.35.4.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snape WJ, Jr, Matarazzo SA, Cohen S. Effect of eating and gastrointestinal hormones on human colonic myoelectrical and motor activity. Gastroenterology. 1978;75:373–378. [PubMed] [Google Scholar]
- 9.Sullivan M, Cohen S, Snape WJ. Colonic myoelectrical activity in irritable-bowel syndrome. Effect of eating and anticholinergics. N Engl J Med. 1978;298:878–883. doi: 10.1056/NEJM197804202981604. [DOI] [PubMed] [Google Scholar]
- 10.Bassotti G, Imbimbo BP, Gaburri M, Daniotti S, Morelli A. Transverse and sigmoid colon motility in healthy humans: effects of eating and of cimetropium bromide. Digestion. 1987;37:59–64. doi: 10.1159/000199488. [DOI] [PubMed] [Google Scholar]
- 11.Bjornsson ES, Chey WD, Ladabaum U, Woods ML, Hooper FG, Owyang C, et al. Differential 5-HT3 mediation of human gastrocolonic response and colonic peristaltic reflex. Am J Physiol. 1998;275:G498–G505. doi: 10.1152/ajpgi.1998.275.3.G498. [DOI] [PubMed] [Google Scholar]
- 12.Delvaux M, Fioramonti J, Bueno L, Frexinos J. Alterations of colonic motility after oral administration of prostaglandin E1 analogue misoprostol in man. J Gastrointest Motil. 1992;4:33–38. [Google Scholar]
- 13.Connell AM, Jones FA, Rowlands EN. Motility of the pelvic colon. IV. Abdominal pain associated with colonic hypermotility after meals. Gut. 1965;6:105–112. doi: 10.1136/gut.6.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouchoucha M, Odinot J, Devroede G, Landi B, Cugnenc P, Barbier J. Simple clinical assessment of colonic response to food. Int J Colorectal Dis. 1998;13:217–222. doi: 10.1007/s003840050164. [DOI] [PubMed] [Google Scholar]
- 15.Spiller RC, Brown ML, Phillips SF. Emptying of the terminal ileum in intact humans. Influence of meal residue and ileal motility. Gastroenterology. 1987;92:724–729. doi: 10.1016/0016-5085(87)90024-2. [DOI] [PubMed] [Google Scholar]
- 16.Greydanus MP, Camilleri M, Colemont LJ, Phillips SF, Brown ML, Thomforde GM. Ileocolonic transfer of solid chyme in small intestinal neuropathies and myopathies. Gastroenterology. 1990;99:158–164. doi: 10.1016/0016-5085(90)91243-y. [DOI] [PubMed] [Google Scholar]
- 17.Quigley EM, Phillips SF, Dent J, Taylor BM. Myoelectric activity and intraluminal pressure of the canine ileocolonic sphincter. Gastroenterology. 1983;85:1054–1062. [PubMed] [Google Scholar]
- 18.Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Muller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45 Suppl 2:II43–II47. doi: 10.1136/gut.45.2008.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talley NJ, Phillips SF, Wiltgen CM, Zinsmeister AR, Melton LJ., III Assessment of functional gastrointestinal disease: the bowel disease questionnaire. Mayo Clin Proc. 1990;65:1456–1479. doi: 10.1016/s0025-6196(12)62169-7. [DOI] [PubMed] [Google Scholar]
- 20.Kruis W, Azpiroz F, Phillips SF. Contractile patterns and transit of fluid in canine terminal ileum. Am J Physiol. 1985;249:G264–G270. doi: 10.1152/ajpgi.1985.249.2.G264. [DOI] [PubMed] [Google Scholar]
- 21.Camilleri M, Colemont L, Phillips S, Brown M, Thomforde G, Chapman N, Zinsmeister AR. Human gastric emptying and colonic filling of solids characterized by a new method. Am J Physiol. 1989;257:G284–G290. doi: 10.1152/ajpgi.1989.257.2.G284. [DOI] [PubMed] [Google Scholar]
- 22.Hebden JM, Blackshaw PE, Perkins AC, D'Amato M, Spiller RC. Small bowel transit of a bran meal residue in humans: sieving of solids from liquids and response to feeding. Gut. 1998;42:685–689. doi: 10.1136/gut.42.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spiller RC, Brown ML, Phillips SF, Azpiroz F. Scintigraphic measurements of canine ileocolonic transit. Direct and indirect effects of eating. Gastroenterology. 1986;91:1213–1220. doi: 10.1016/s0016-5085(86)80019-1. [DOI] [PubMed] [Google Scholar]
- 24.Phillips SF, Camilleri M. The ileocecal area and the irritable bowel syndrome. Gastroenterol Clin North Am. 1991;20:297–311. [PubMed] [Google Scholar]
- 25.Hutchinson R, Notghi A, Smith NB, Harding LK, Kumar D. Scintigraphic measurement of ileocaecal transit in irritable bowel syndrome and chronic idiopathic constipation. Gut. 1995;36:585–589. doi: 10.1136/gut.36.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leelakusolvong S, Bharucha AE, Sarr MG, Hammond PI, Brimijoin S, Phillips SF. Effect of extrinsic denervation on muscarinic neurotransmission in the canine ileocolonic region. Neurogastroenterol Motil. 2003;15:173–186. doi: 10.1046/j.1365-2982.2003.00399.x. [DOI] [PubMed] [Google Scholar]
- 27.Bharucha AE, Seide B, Guan Z, Andrews CN, Zinsmeister AR. Effect of tolterodine on gastrointestinal transit and bowel habits in healthy subjects. Neurogastroenterol Motil. 2008;20:643–648. doi: 10.1111/j.1365-2982.2008.01089.x. [DOI] [PubMed] [Google Scholar]
- 28.Kamath PS, Phillips SF. Initiation of motility in canine ileum by short chain fatty acids and inhibition by pharmacological agents. Gut. 1988;29:941–948. doi: 10.1136/gut.29.7.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spiller RC, Brown ML, Phillips SF. Decreased fluid tolerance, accelerated transit, and abnormal motility of the human colon induced by oleic acid. Gastroenterology. 1986;91:100–107. doi: 10.1016/0016-5085(86)90445-2. [DOI] [PubMed] [Google Scholar]
- 30.Kamath PS, Phillips SF, O'Connor MK, Brown ML, Zinsmeister AR. Colonic capacitance and transit in man: modulation by luminal contents and drugs. Gut. 1990;31:443–449. doi: 10.1136/gut.31.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steed KP, Bohemen EK, Lamont GM, Evans DF, Wilson CG, Spiller RC. Proximal colonic response and gastrointestinal transit after high and low fat meals. Dig Dis Sci. 1993;38:1793–1800. doi: 10.1007/BF01296101. [DOI] [PubMed] [Google Scholar]
- 32.Bazzocchi G, Ellis J, Villanueva-Meyer J, Reddy S, Mena I, Snape WJ. Effect of eating on colonic motility and transit in patients with functional diarrhea. Simultaneous scintigraphic and manometric evaluations. Gastroenterology. 1991;101:1298–1306. doi: 10.1016/0016-5085(91)90080-5. [DOI] [PubMed] [Google Scholar]
- 33.Di Stefano M, Miceli E, Missanelli A, Mazzocchi S, Corazza GR. Meal induced rectosigmoid tone modification: a low caloric meal accurately separates functional and organic gastrointestinal disease patients. Gut. 2006;55:1409–1414. doi: 10.1136/gut.2005.076323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao SS, Kavelock R, Beaty J, Ackerson K, Stumbo P. Effects of fat and carbohydrate meals on colonic motor response. Gut. 2000;46:205–211. doi: 10.1136/gut.46.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright SH, Snape WJ, Jr, Battle W, Cohen S, London RL. Effect of dietary components on gastrocolonic response. Am J Physiol. 1980;238:G228–G232. doi: 10.1152/ajpgi.1980.238.3.G228. [DOI] [PubMed] [Google Scholar]
- 36.Steed K, Bohemen E, Lamont G, Evans D, Wilson C, Spiller R. Proximal colonic response and gastrointestinal transit after high and low fat meals. Dig Dis Sci. 1993;38:1793–1800. doi: 10.1007/BF01296101. [DOI] [PubMed] [Google Scholar]
- 37.Kerlin P, Zinsmeister A, Phillips S. Motor responses to food of the ileum, proximal colon, and distal colon of healthy humans. Gastroenterology. 1983;84:762–770. [PubMed] [Google Scholar]
- 38.Bouchoucha M, Faye A, Devroede G, Arsac M. Effects of oral pinaverium bromide on colonic response to food in irritable bowel syndrome patients. Biomed Pharmacother. 2000;54:381–387. doi: 10.1016/S0753-3322(01)80005-6. [DOI] [PubMed] [Google Scholar]
- 39.Rogers J, Henry MM, Misiewicz JJ. Increased segmental activity and intraluminal pressures in the sigmoid colon of patients with the irritable bowel syndrome. Gut. 1989;30:634–641. doi: 10.1136/gut.30.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Narducci F, Bassotti G, Granata MT, Pelli MA, Gaburri M, Palumbo R, et al. Colonic motility and gastric emptying in patients with irritable bowel syndrome. Effect of pretreatment with octylonium bromide. Dig Dis Sci. 1986;31:241–246. doi: 10.1007/BF01318114. [DOI] [PubMed] [Google Scholar]
- 41.Bouchoucha M, Devroede G, Faye A, Le Toumelin P, Arhan P, Arsac M. Colonic response to food in constipation. Int J Colorectal Dis. 2006;21:826–833. doi: 10.1007/s00384-005-0787-5. [DOI] [PubMed] [Google Scholar]
- 42.Bazzocchi G, Ellis J, Villanueva-Meyer J, Jing J, Reddy S, Mena I, et al. Postprandial colonic transit and motor activity in chronic constipation. Gastroenterology. 1990;98:686–693. doi: 10.1016/0016-5085(90)90289-d. [DOI] [PubMed] [Google Scholar]
- 43.Bjornsson ES, Chey WD, Hooper F, Woods ML, Owyang C, Hasler WL. Impaired gastrocolonic response and peristaltic reflex in slow-transit constipation: role of 5-HT(3) pathways. Am J Physiol. 2002;283:G400–G407. doi: 10.1152/ajpgi.00082.2001. [DOI] [PubMed] [Google Scholar]
- 44.O'Brien MD, Camilleri M, von der Ohe MR, Phillips SF, Pemberton JH, Prather CM, Wiste JA, Hanson RB. Motility and tone of the left colon in constipation: a role in clinical practice? Am J Gastroenterol. 1996;91:2532–2538. [PubMed] [Google Scholar]
- 45.Grotz RL, Pemberton JH, Levin KE, Bell AM, Hanson RB. Rectal wall contractility in healthy subjects and in patients with chronic severe constipation. Ann Surg. 1993;218:761–768. doi: 10.1097/00000658-199312000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simrén M, Månsson A, Langkilde AM, Svedlund J, Abrahamsson H, Bengtsson U, Björnsson ES. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion. 2001;63:108–115. doi: 10.1159/000051878. [DOI] [PubMed] [Google Scholar]
- 47.von der Ohe MR, Camilleri M, Kvols LK. A 5HT3 antagonist corrects the postprandial colonic hypertonic response in carcinoid diarrhea. Gastroenterology. 1994;106:1184–1189. doi: 10.1016/0016-5085(94)90008-6. [DOI] [PubMed] [Google Scholar]