Abstract
Imaging studies play an important role in the diagnosis and management of patients with pancreatic adenocarcinoma. Computed tomography (CT) is the most widely available and best validated modality for imaging these patients. Meticulous technique following a well-designed pancreas protocol is essential for maximizing the diagnostic efficacy of CT. After the diagnosis of pancreatic adenocarcinoma is made, the key to management is staging to determine resectability. In practice, staging often entails predicting the presence or absence of vascular invasion by tumor, for which several radiologic grading systems exist. With advances in surgical techniques, the definition of resectability is in evolution, and it is crucial that radiologists have an understanding of the implications of findings that are relevant to the determination of resectability.
Keywords: Pancreatic cancer, CTA, Diagnosis, Staging, Resectability, CT
Pancreatic adenocarcinoma is the second most common gastrointestinal malignancy and the fourth leading cause of cancer deaths in the United States. Imaging studies have an important role in the diagnosis and management of patients with pancreatic adenocarcinoma. Early tumor detection and accurate radiological staging are crucial for identifying patients with potentially resectable disease, and avoiding unnecessary surgery in patients with unresectable disease. Computed tomography (CT) is the imaging modality of choice in this setting because it is widely available and the best validated tool for the diagnosis and staging of pancreatic adenocarcinoma [1]. In this article, we review the technical aspects of a pancreas protocol CT and the findings relevant to diagnosis and staging of pancreatic adenocarcinoma.
The pancreas protocol CT
The goals of a CT protocol for imaging of patients with pancreatic adenocarcinoma are to (1) maximize the attenuation difference between tumor and normal pancreas to increase the conspicuity of the lesion, (2) adequately opacify peripancreatic arteries and veins to facilitate evaluation for vascular involvement by tumor, and (3) optimize enhancement of the liver to aid in detection of hepatic metastases. Although the details of a “pancreas protocol” vary from institution to institution, several basic principles should always be observed.
The pancreas is supplied by splanchnic arteries, whereas the liver is largely supplied by the portal vein. Therefore, peak enhancement of the pancreas occurs after peak enhancement of the aorta (which occurs during the arterial phase), but before peak enhancement of the liver (which occurs during the portal venous phase). Lu et al. described the pancreatic phase as an intermediate between the arterial phase and venous phase, during which there is maximal enhancement of the pancreas, resulting in maximal contrast between tumor and pancreatic parenchyma. At the same time, there is sufficient enhancement of peripancreatic arteries and veins to assess for vascular involvement by tumor [2, 3]. Thus, images obtained in the pancreatic phase are ideal for both detection and local staging of pancreatic adenocarcinoma.
To maximize the attenuation difference between hypovascular metastases and hepatic parenchyma, images are then obtained in the portal venous phase, when there is peak hepatic enhancement [4].
Early studies using multiphase CT for imaging of patients with pancreatic adenocarcinoma often made use of arterial phase images. Recent studies found that images obtained in the arterial phase are unnecessary, as they are inferior to those obtained in the pancreatic phase or the hepatic phase for detection and staging of pancreatic adenocarcinoma [3, 5, 6].
Thus, a pancreas protocol CT should be a dual-phase exam with images obtained during the pancreatic phase and hepatic phase. Noncontrast images that are useful for detecting calcifications and confirming that enhancement is present on postcontrast images may also be obtained.
Zamboni et al. reported that there is no difference in the diagnostic efficacy of different generations of helical CT scanners (i.e., 4-, 8-, 16-, and 64-row scanners) [7].
Using the volumetric data acquired with modern CT scanners, a variety of postprocessing (including multiplanar reformation, maximum intensity projection, minimum intensity projection, volume rendering, and curved planar reformation) are possible, and may be performed at the discretion of the radiologist. Ichikawa et al. reported that coronal and sagittal images improve the performance of CT for evaluating patients with pancreatic cancer, especially when assessing for local extension [5]. The utility of other postprocessing techniques for routine imaging of patients with pancreatic adenocarcinoma has yet to be established.
The pancreas protocol used at our institution is given in Table 1. Patients fast for at least 4 h before the exam and are given 500 mL of water orally as negative contrast agent just before the scan. About 40–45 g of iodine (e.g., 125 cc of a contrast agent containing 350 mg of iodine per mL) is administered intravenously to ensure adequate enhancement. Contrast is power-injected at a rate of 3 cc/s. We prefer this relatively low injection rate because it results in longer imaging windows, thus reducing the possibility of timing errors.
Table 1.
Pancreatic multidetector row computed tomography (MDCT) protocol at UCLA
| MDCT generation | 16 row | 64 row |
|---|---|---|
| Contrast | 125 mL of 350 mg I/mL contrast agent infused at 3 cc/s | |
| Scan timing | ||
| Pancreatic phase (PP) | ||
| Fixed delay | 50 s | 55 s |
| Bolus tracking | 150 HU trigger + 25 s | 150 HU trigger + 30 s |
| Hepatic phase (HP) | ||
| Fixed delay | 70 s | 75 s |
| Bolus tracking | End of PP + 10 s | End of PP + 10 s |
| Scan volume | Diaphragm to iliac crests | Diaphragm to iliac crests |
| Scan direction | Cranial to caudal | Cranial to caudal |
| Collimation (mm) | PP: 0.75, HP: 1.5 | PP: 0.6, HP: 1.5 |
| Table feed/rotation (mm) | PP: 12, HP: 24 | PP: 38, HP: 76 |
| Rotation time (s) | 0.5 | 0.5 |
| KVp | 120 | 120 |
| MAs | 200–250 | 200–250 |
| Image reconstruction | ||
| Routine (thickness/interval) (mm) | PP axial: 2/2 | PP axial: 2/2 |
| PP coronal: 2/1 | PP coronal: 2/1 | |
| PP sagittal: 2/2 | PP sagittal: 2/2 | |
| HP axial: 5/2.5 | HP axial: 5/2.5 | |
| Optional (thickness/interval) (mm) | PP axial: 1/1 | PP axial: 1/1 |
A variety of methods for timing of image acquisition can be used. The simplest, but least reliable, is a fixed delay. With the contrast volume and injection rate described above, we acquire pancreatic phase images 40–45 s after beginning of contrast injection on a 1- or 4-detector scanner and 50–55 s after beginning of contrast injection on a 16- or 64-detector scanner. Note that the faster the scanner, the shorter the acquisition time, and therefore the longer the delay before image acquisition. The use of a fixed delay is not recommended with 16- or 64-detector scanners because the pancreas is imaged over only a few seconds and accurate timing becomes critical.
Using bolus tracking technique, timing of image acquisition is tailored to the patient’s cardiac output. Attenuation of the aorta at the level of the celiac artery is monitored with continuous low-dose scans. When the attenuation of the aorta reaches a defined threshold (we use 150 HU), image acquisition is triggered after a fixed delay, the length of which depends on scanner speed. On a 64-detector scanner, we use a 30-s delay for pancreatic phase images. Hepatic phase images are obtained 10 s after completion of the acquisition of pancreatic phase images.
In our protocol, unenhanced images are reconstructed at 5 mm thickness in the axial plane, pancreatic phase images are reconstructed at 2 mm thickness in the axial, coronal, and sagittal planes, and portal venous phase images are reconstructed at 5 mm thickness in the axial plane. One mm images are sent to a 3D-capable workstation, where a variety of postprocessing can be performed.
With volumetric acquisition and the capability to perform 3D postprocessing, our pancreas protocol CT is essentially a CT angiogram (CTA). An important difference is that unlike a traditional CTA, our pancreatic CTA images are acquired in the pancreatic phase, which simultaneously captures both the arterial and venous systems. We routinely obtain multiplanar reformations of pancreatic phase images in coronal and sagittal planes, but additional postprocessing is largely reserved for specific problem-solving situations.
Diagnosis of pancreatic adenocarcinoma by CT
CT has a high sensitivity for detecting pancreatic adenocarcinoma, ranging from 89% to 97% [8]. As expected, CT has a higher sensitivity for larger lesions than smaller ones. Legmann et al. reported that CT detected 100% of tumors >15 mm in size, but only 67% of tumors ≤15 mm in size [9]. Similarly, a recent study by Bronstein et al. found that CT detected only 77% of pancreatic tumors ≤2 cm in size [10].
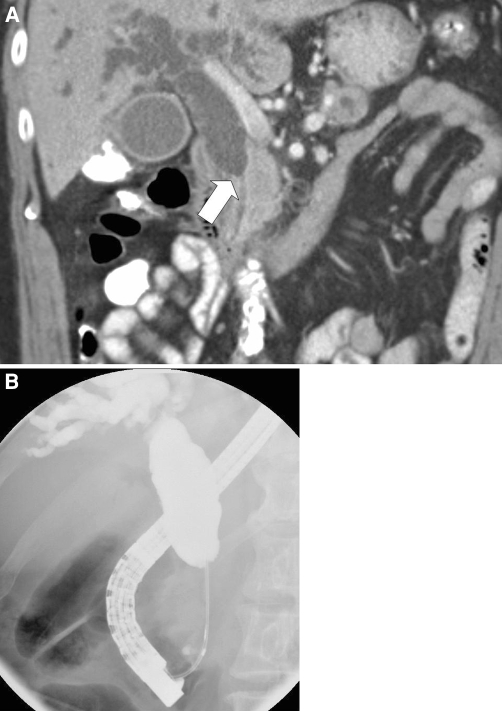
Pancreatic adenocarcinomas typically manifest as ill-defined hypoattenuating masses, but can be isoattenuating to the surrounding parenchyma. Prokesch et al. reported that 11% of pancreatic cancers are isoattenuating on both pancreatic phase and hepatic phase images. In most cases, the presence and location of a pancreatic mass could still be inferred from secondary signs, such as interruption and dilation of the pancreatic duct and/or the common bile duct, mass effect, convex abnormality of the contour of the pancreas, and atrophy of the pancreas [11] (Fig. 1).
Fig. 1.
Sixty-nine-year-old male with an isodense pancreatic head mass. A Coronal CT image demonstrates marked biliary ductal dilation, with abrupt narrowing of the common bile duct in the pancreatic head (arrow), where no mass is identified. B Endoscopic retrograde cholangiopancreaticography demonstrates similar findings. Endoscopic ultrasound performed at the same time revealed a 3 × 2 cm mass in the head of the pancreas that was biopsied and found to be an adenocarcinoma.
Although radiologic signs used to diagnose pancreatic adenocarcinoma are not pathognomonic of the condition, biopsy is usually unnecessary before surgical intervention in patients with potentially resectable disease. If there are confounding factors (such as clinical findings that suggest pancreatitis), biopsy may be performed endoscopically, percutaneously, or surgically. If the patient is not a surgical candidate, confirmation of the diagnosis by biopsy is usually necessary prior to chemotherapy and/or radiation therapy.
Ideally, CT should be performed before biliary stenting, as the stent may cause artifact in the pancreatic head that can mask the lesion, and the trauma of stent insertion often produces inflammatory changes that can be indistinguishable from tumor.
Staging and resectability
Complete surgical resection is the only potentially curative treatment for pancreatic adenocarcinoma, but only 15–20% of patients present with resectable disease. Survival rate after resection is about 12–20% at 5 years, compared to <1% for those with unresectable disease. In many patients, the only obstacle to resection is involvement of the portal vein or superior mesenteric vein, which was once considered an absolute contraindication to pancreaticoduodenectomy. However, venous resection and reconstruction are now commonly performed, with morbidity, mortality, and survival similar to pancreaticoduodenectomy without vascular reconstruction [12].
Reflecting this development, the American Joint Commission on Cancer (AJCC) TNM staging system for pancreatic adenocarcinoma was revised in the 6th edition of the AJCC Cancer Staging Manual (Table 2). Isolated venous involvement is now considered T3 disease (locally invasive, but potentially resectable), whereas tumor involvement of the celiac artery or superior mesenteric artery remains T4 disease (locally advanced and unresectable) [13, 14]. Arterial resection and reconstruction is technically difficult, and complete tumor resection is rarely possible due to tumor infiltration along the nerves of the celiac plexus.
Table 2.
The 6th edition of the American Joint Commission on cancer staging system for pancreatic adenocarcinoma [14]
| Definition | Comments | |
|---|---|---|
| Primary tumor (T) | ||
| T0 | No evidence of primary | |
| Tis | In situ | |
| T1 | Limited to pancreas, ≤2 cm | |
| T2 | Limited to pancreas, >2 cm | |
| T3 | Extends beyond the pancreas, but without involvement of the celiac artery or superior mesenteric artery | Emphasis is on factors that determine resectability. In the 6th edition, only extrapancreatic extension to the celiac artery or superior mesenteric artery is T4. In the 5th edition, extrapancreatic extension to the stomach, spleen, colon, portal vein, and superior mesenteric vein was also considered T4. |
| T4 | Involves the celiac artery or superior mesenteric artery | |
| Regional lymph nodes (N) | ||
| N0 | No nodal metastases | In the 6th edition, no distinction is made between the number of nodes involved. In the 5th edition, N1 was subdivided into N1a (involvement of a single node) and N1b (involvement of more than one node) |
| N1 | Regional nodal metastases | |
| Distant metastases (M) | ||
| M0 | No distant metastases | |
| M1 | Distant metastases | |
| Stage groupings | ||
| Stage 0 | Tis N0 M0 | |
| Stage I | IA: T1 N0 M0 | Potentially resectable. |
| IB: T2 N0 M0 | ||
| Stage II | IIA: T3 N0 M0 | Potentially resectable. |
| IIB: T1–3 N1 M0 | ||
| Stage III | T4 N0-1 M0 | Locally advanced and unresectable. |
| Stage IV | T1–4 N0-1 M1 | Metastatic and unresectable. |
Current criteria for resectability include absence of distant metastases, lack of evidence of tumor involvement of major arteries, and (if there is venous invasion) a suitable segment of portal vein (above) and superior mesenteric vein (below) the site of venous involvement to allow for venous reconstruction [15]. Precisely what is considered resectable venous involvement depends on the preferences of the treating surgeon, but ideally the resected segment of vein should be downstream (i.e., closer to the liver) from the entry of the jejunal veins, and the involved segment of portal or superior mesenteric vein should be <2 cm in length [12].
Because there are no universally accepted criteria for determining resectability, the terms “resectable” and “unresectable” should be used only when there is a clear understanding between the radiologist and the referring physician. In radiologic reports, findings relevant to the staging of pancreatic adenocarcinoma are often described, without specifically stating whether or not a patient has resectable disease.
TNM staging: primary tumor (T)
Although patients with T1 disease have a better prognosis than patients with T2 disease [16], differentiation between the two groups has little impact on management since both have potentially resectable disease. On the other hand, distinguishing patients with T3 disease from those with T1 or T2 disease and distinguishing patients with T4 disease from those with T3 disease are of paramount importance. Patients with T3 disease may or may not have resectable disease depending on the skills and preferences of the treating surgeon, while patients with T4 disease have unresectable disease. Thus, the key to T staging of pancreatic adenocarcinoma is identification of vascular invasion by tumor.
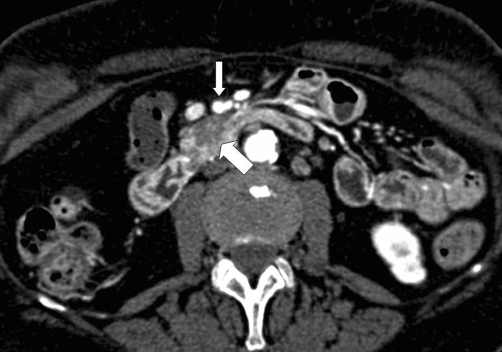
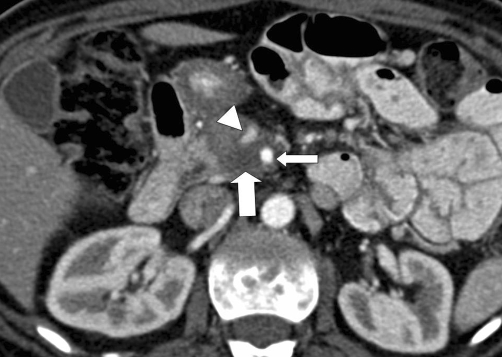
The most commonly used system for predicting vascular invasion by pancreatic adenocarcinoma is a five-grade scale proposed by Lu et al. based on the degree of contact between tumor and a vessel (Table 3). Tumor contiguity with >50% of the perimeter of a vessel was found to be the optimal threshold for predicting vascular invasion, with a sensitivity of 84% and specificity of 98% [17] (Figs. 2, 3).
Table 3.
Grading system proposed by Lu et al. [17] for predicting vascular invasion by tumor based on the degree of tumor contiguity with a vessel
| Category | Description | Comment |
|---|---|---|
| Grade 0 | No contiguity of tumor with a vessel | Vascular invasion in 0% of cases |
| Grade 1 | Tumor contiguous with <25% of the circumference of a vessel | Vascular invasion in 0% of cases |
| Grade 2 | Tumor contiguous with 25–50% of the circumference of a vessel | Vascular invasion in 57% of cases |
| Grade 3 | Tumor contiguous with 50–75% of the circumference of a vessel | Vascular invasion in 88% of cases |
| Grade 4 | Tumor contiguous with >75% of the circumference of a vessel or any vessel constriction | Vascular invasion in all cases |
Fig. 2.
Eighty-two-year-old female with resectable pancreatic adenocarcinoma. Axial CT image demonstrates a hypovascular mass in the head of the pancreas (large arrow). The tumor abuts the posterior aspect of the superior mesentery artery (small arrow), but the area of contact encompasses only 25% of the circumference of the vessel and there is no deformity of the vessel. The patient underwent successful pancreaticoduodenectomy and was well at the time of this article was written, 11 months after surgery.
Fig. 3.
Fifty-four-year-old female with locally advanced pancreatic adenocarcinoma. Axial CT image demonstrates a hypovascular mass in the head of the pancreas (large arrow) that encircles both the superior mesenteric vein (arrowhead) and superior mesenteric artery (small arrow). There is deformity of the superior mesenteric vein, which is an indication of vascular invasion, regardless of the degree of contact between the vessel and tumor.
An important, but often overlooked, component of the grading scale proposed by Lu et al. is vessel deformity, which is considered a sign of vascular invasion regardless of the degree of contact between the vessel and tumor. Illustrating the importance of this, Hough et al. described the teardrop superior mesenteric vein sign as a specific sign of unresectable pancreatic adenocarcinoma. The teardrop sign is present when the normal round shape of the vessel is changed to a teardrop shape on axial images [18]. Because venous resection was not an option at the authors’ institution at the time of the study, the teardrop sign should be considered a sign of superior mesenteric venous invasion by tumor rather than unresectability.
Modifications to the grading system proposed by Lu et al. have been proposed to increase sensitivity for detecting venous invasion and specificity for detecting arterial invasion [19–21] (Table 4). Higher sensitivity for predicting venous invasion is desirable because patients with undiagnosed venous invasion may be deemed to have “unresectable” disease at surgery if resection is attempted at an institution where venous reconstruction is not performed. On the other hand, higher specificity for predicting arterial invasion reduces the risk of overstaging patients with T3 disease and denying them the chance for surgical resection. Additional studies are needed to confirm the validity of these recommendations.
Table 4.
Sensitivity and specificity for predicting vascular invasion by tumor using 50% tumor contiguity with the circumference of a vessel as threshold
| Authors | Sensitivity (%) | Specificity (%) | Comment/Proposed modification(s) |
|---|---|---|---|
| Lu et al. [17] | 84 | 98 | In this study, vessel constriction was considered a sign of vascular invasion, regardless of the degree of contact between vessel and tumor. Tumor contiguity with 50% of the circumference of a vessel was used as a criterion only in cases without vessel constriction. |
| O’Malley et al. [19] | 46 | 99 | In this study, the grading system proposed by Lu et al. was modified to exclude vessel constriction as a sign of vascular invasion. This may account for the lower sensitivity reported. Raising the threshold to 75% decreased sensitivity to 38%, but increased specificity to 100%. |
| Nakayama et al. [20]—veins only | 71 | 86 | The criteria used by Lu et al. were found to be optimal. |
| Nakayama et al. [20]—arteries only | 78 | 79 | Raising the threshold to 75% decreased sensitivity to 61%, but increased specificity to 92%. |
| Li et al. [21]—veins only | 49 | 100 | Including venous obliteration, venous stenosis, or teardrop SMV as signs of venous invasion increased sensitivity to 92% while specificity remained 100%. Note that these criteria are identical to those proposed by Lu et al. |
| Li et al. [21]—arteries only | 97 | 91 | Using a combination of tumor involvement of >50% of the circumference of an artery and irregularity of the arterial wall or stenosis of the artery reduced sensitivity to 79%, but increased specificity to 99%. |
Other systems for predicting vascular invasion by pancreatic adenocarcinoma have been proposed. Although not widely used, radiologists should be familiar with them, as they are occasionally encountered in the literature (Tables 5, 6). Loyer et al. found that a tumor that abuts a vessel usually does not invade it if the points of contact form a convexity against the vessel (i.e., the mass displaces or compresses, but does not wrap around, the vessel). On the other hand, if the points of contact form a concavity against the vessel (i.e., the mass wraps around the vessel), vascular invasion is often present [22]. Raptopoulos et al. found that a vessel that is encased and narrowed by tumor is likely to be invaded by it [23]. Encasement was originally defined by the authors as tumor extending around least two-thirds of the perimeter of a vessel, but in a recent article published by the same group, encasement was defined as extension of tumor around at least 50% of the perimeter of a vessel [24].
Table 5.
Grading system proposed by Loyer et al. [22] for predicting vascular invasion by tumor
| Category | Description | Comment |
|---|---|---|
| Type A | Fat plane separates the tumor and/or the normal pancreatic parenchyma from adjacent vessels | Overall resection rate: 100%. Resection rate without venous resection: 95%. Conclusion: “Lesions with type A and B appearances are likely to be resectable lesions” |
| Type B | Normal parenchyma separates the hypodense tumor from adjacent vessels | Overall resection rate: 100%. Resection rate without venous resection: 95%. Conclusion: “Lesions with type A and B appearances are likely to be resectable lesions” |
| Type C | Hypodense tumor is inseparable from adjacent vessels, and the points of contact form a convexity against the vessels | Overall resection rate: 89%. Resection rate without venous resection: 55%. Conclusion: “Lesions of type C vascular involvement should be operated on with an intention to resect the tumor, but the tumor may or may not adhere to the wall of the vessels” |
| Type D | Hypodense tumor is inseparable from adjacent vessels, and the points of contact form a concavity against the vessels or partially encircle the vessels | Overall resection rate: 47%. Resection rate without venous resection: 7%. Conclusion: “Lesions of type D vascular involvement would require pancreatic resection with a plan to perform venous resection and venous graft or patch or would be unresectable for surgeons who do not have that appearance” |
| Type E | Hypodense tumor encircles adjacent vessels, and no fat plane is identified between the tumor and the vessels | Overall resection rate: 0%. Resection rate without venous resection: 0%. Conclusion: “Lesions of the type E and F vascular involvement are not likely to be resectable” |
| Type F | Tumor occludes the vessels | Overall resection rate: 0%. Resection rate without venous resection: 0%. Conclusion: “Lesions of the type E and F vascular involvement are not likely to be resectable” |
Table 6.
Grading system proposed by Raptopoulos et al. [23] for predicting vascular invasion by tumor
| Category | Description | Comment |
|---|---|---|
| Grade 0 | Normal, with a fat plane or normal pancreas between the tumor and the vessel | 100% resectable |
| Grade 1 | Loss of fat plane between the tumor and the vessel, with or without smooth displacement of the vessel | 100% resectable |
| Grade 2 | Flattening or slight irregularity of one side of the vessel | 92% resectable |
| Grade 3 | Encased vessel with tumor extending around at least two sides (two-thirds of the perimeter), altering its contour and producing concentric or eccentric narrowing of the lumena | The recommended threshold for predicting vascular invasion. In this study, resection was performed in 1 of 10 patients with grade 3 findings, but tumor along perivascular neural bundles was present at resection margins. |
| Grade 4 | Occluded vessel | No attempted surgery |
aIn a recent article by the same group, encasement was defined as tumor extending around 50% of the perimeter of a vessel [24]
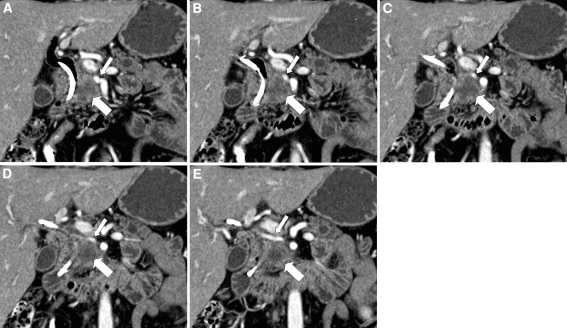
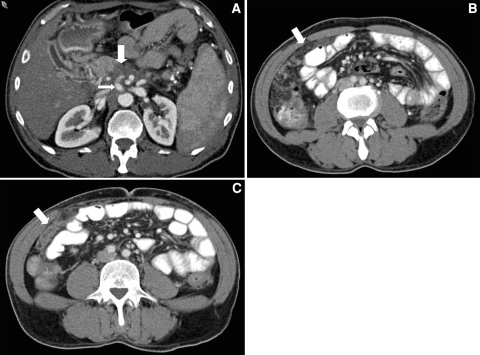
It is important to note any variant vascular anatomy that may be present. Anatomic variants may render resectable a tumor that would otherwise be unresectable (e.g., a celiac artery that does not give rise to the common hepatic artery may be sacrificed) or render unresectable a tumor that would otherwise be unresectable (e.g., isolated involvement of a replaced right hepatic artery by tumor) (Fig. 4).
Fig. 4.
Forty-three-year-old male with locally advanced pancreatic adenocarcinoma. (A–E) Sequential coronal CT images demonstrate a replaced right hepatic artery (small arrow) arising from the superior mesenteric artery. The aberrant vessel is mildly deformed by a hypovascular mass in the pancreatic head (large arrow), suggesting tumor invasion of the vessel. This was not prospectively identified, and the patient was found at surgery to have unresectable disease due to invasion of a replaced right hepatic artery.
TNM staging: regional lymph nodes (N)
The accuracy of CT for predicting nodal metastases is low. Lymph nodes that are normal in size may harbor micrometastases, whereas enlarged lymph nodes are often reactive. Roche et al. reported that CT diagnosed regional nodal metastases in patients with pancreatic adenocarcinoma with a sensitivity of 14% and a specificity of 85% if a short-axis diameter of 10 mm is used as the sole criterion for tumor involvement. Using a short-axis diameter of 5 mm as threshold increased sensitivity to 71%, but reduced specificity to 64%. Morphologic features (rounded nodes, clustered nodes, nodes with no fatty hilum) were not found to be helpful. The authors concluded that in a patient with pancreatic adenocarcinoma, the finding of enlarged peripancreatic lymph nodes on CT should not preclude attempted resection [25].
In practice, peripancreatic lymph nodes are resected en bloc with the primary tumor, and preoperative detection of peritumoral lymphadenopathy is not essential for assessing the resectability of pancreatic adenocarcinomas. Illustrating this, DeWitt et al. reported that 72% of resectable tumors had regional nodal involvement, compared to only 50% of unresectable pancreatic tumors [26].
TNM staging: distant metastases (M)
Because distant metastases (M1 disease) contraindicates tumor resection, their detection is an important part of preoperative imaging. In the abdomen, the liver and peritoneal surfaces are common sites for distant metastases.
Hepatic metastases from pancreatic adenocarcinoma appear as solid hypovascular masses with imaging features similar to the primary tumor (Fig. 5). When a lesion is large, the diagnosis of metastatic disease to the liver is usually straightforward. If imaging findings are equivocal, a large lesion can be biopsied percutaneously.
Fig. 5.
Seventy-year-old male with metastatic pancreatic adenocarcinoma. A Axial CT image demonstrates a hypovascular mass in the pancreatic head (large arrow) and an enlarged peripancreatic lymph node (small arrow). B Axial CT image demonstrates multiple hypovascular lesions in the liver (small arrows), with imaging features similar to the primary tumor in the pancreas.
A commonly encountered problem when staging cancer patients with CT is the presence of small (≤10 mm) hypodense lesions that are often described by radiologists as “too small to characterize” or “indeterminate.” Because of partial volume averaging and/or pseudo-enhancement, the attenuation of these lesions cannot be accurately measured [27]. Although usually benign, Schwartz et al. found that such lesions represent metastases in 11.6% of patients with cancer [28]. Follow-up imaging of these lesions is an option, but there are no established guidelines defining the frequency and duration of follow-up imaging. Furthermore, delaying surgery in patients who are otherwise surgical candidates is impractical. Percutaneous biopsy may yield a definitive diagnosis, but is often technically difficult. In practice, resection is often attempted despite the presence of these lesions. Unexpected hepatic metastases are responsible for 40–60% of aborted resections. Small indeterminate lesions account for many of these cases, but some hepatic metastases are not apparent on CT even in retrospect [8, 29, 30].
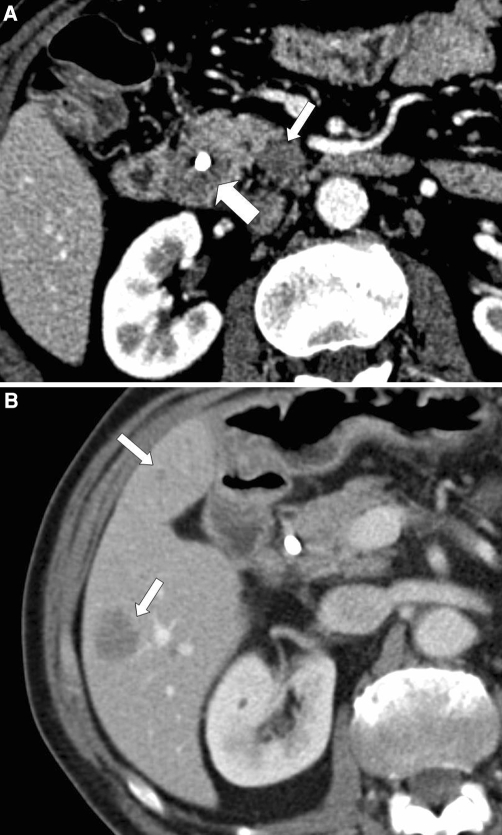
Peritoneal carcinomatosis is common in patients with advanced pancreatic adenocarcinoma. CT findings of peritoneal carcinomatosis include ascites, peritoneal thickening and contrast enhancement, nodular bowel wall thickening representing serosal implants, and soft tissue infiltration of the omentum [31] (Fig. 6). Peritoneal carcinomatosis is easily diagnosed by CT when advanced, but the sensitivity of imaging studies for small peritoneal implants is limited. Diehl et al. reported that CT identified only 80% of patients found to have peritoneal metastases at surgery [25].
Fig. 6.
Fifty-three-year-old male with unresectable pancreatic adenocarcinoma. A Axial CT image demonstrates a hypovascular mass in the pancreatic body (large arrow) that encases the celiac artery (small arrow). B Axial CT image at the time of diagnosis demonstrates ill-defined soft tissue within the greater omentum (arrow), which was interpreted as either representing peritoneal carcinomatosis or edema. C Axial CT image obtained 3 months later demonstrates nodular soft tissue within the greater omentum (arrow) that is unequivocal for peritoneal carcinomatosis.
Performance of CT for staging pancreatic adenocarcinoma
Because of the dismal prognosis of patients who do not undergo complete tumor resection, the criteria used to predict vascular invasion by tumor are biased toward high specificity, at the cost of sensitivity, to avoid denying surgery to patients with potentially resectable disease. Furthermore, the sensitivity of CT for small hepatic and peritoneal metastases is limited. It is, therefore, not surprising that although the positive predictive value of CT for predicting unresectability is high (89–100%), its positive predictive value for predicting resectability is much lower (45–79%) [24]. When interpreting these statistics, it should be remembered that the definition of resectability is evolving, and what was once considered unresectable disease may now be resectable.
Restaging of pancreatic adenocarcinoma after neoadjuvant chemoradiation therapy
Neoadjuvant, or preoperative, chemoradiation therapy is used to downstage patients with locally advanced disease or to improve survival in patients who present with resectable tumors. Restaging after neoadjuvant therapy is an important part of the management of these patients, but local inflammatory changes induced by neoadjuvant therapy may confound assessment for vascular involvement by tumor.
White et al. reported that among patients who received preoperative therapy, 22% with locally advanced disease diagnosed by CT were found to be resectable at surgery. Some of them had positive surgical margins, but at a rate no higher than patients deemed to have potentially resectable disease by CT. The authors recommended that only venous occlusion be considered unequivocal evidence of locally advanced disease, and all other findings that may represent vascular invasion should be confirmed by endoscopic ultrasound and fine needle aspiration [32]. Tamm et al. reported that of four patients with questionable vascular involvement by tumor after neoadjuvant therapy, only one was found to be unresectable at the time of surgery [33].
In a more recent study, Kim et al. reported the positive predictive value of CT for resectability among patients who received neoadjuvant therapy to be 91%. The authors concluded that neoadjuvant therapy does not significantly affect the determination of tumor resectability by CT. A major drawback of the study is that all except one of the patients studied had resectable disease. CT failed to predict the unresectability of the only patient who was unsuccessfully resected (resulting in a specificity of 0% for resectability), and the only patient diagnosed with locally advanced disease by CT was successfully resected (resulting in a negative predictive value for resectability of 0%) [34].
It is likely that modification of the current criteria for predicting unresectability of pancreatic adenocarcinoma will be needed for the evaluation of patients who received neoadjuvant therapy. For example, Kim et al. did not consider the presence of a perivascular halo (defined as a thin, low-attenuation lesion surrounding a vessel) to be a sign of vascular invasion. The authors speculated that this may be one of the reasons for the high positive predictive value for resectability achieved in the study [34]. Further research on this topic is needed.
Conclusion
Diagnostic imaging plays a vital role in the management of patients with pancreatic adenocarcinoma, and CT is currently the modality of choice. To maximize the diagnostic efficacy of CT, meticulous technique following a well-designed pancreas protocol is essential. Once the diagnosis of pancreatic adenocarcinoma is made, the key to management is determining resectability. Despite advances in nonoperative management of pancreatic adenocarcinoma, complete surgical resection remains the only potentially curative therapy. With development of new surgical techniques, the definition of resectability is in evolution. Patients with tumors that were once considered to be unresectable may now be surgical candidates at some centers. It is, therefore, important that radiologists describe in detail the findings that are relevant for staging of pancreatic adenocarcinoma and have a clear understanding of the implications of these findings.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Bipat SB, Phoa SSKS, van Delden OM, et al. Ultrasonography, computed tomography and magnetic resonance imaging for diagnosis and determining resectability of pancreatic adenocarcinoma. J Comput Assist Tomogr. 2005;29:438–445. doi: 10.1097/01.rct.0000164513.23407.b3. [DOI] [PubMed] [Google Scholar]
- 2.Lu DSK, Vedantham S, Krasny RM, et al. Two-phase helical CT for pancreatic tumors: pancreatic versus hepatic phase enhancement of tumor, pancreas, and vascular structures. Radiology. 1996;199:697–701. doi: 10.1148/radiology.199.3.8637990. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher JG, Wiersema MJ, Farrell MA, et al. Pancreatic malignancy: value of arterial, pancreatic, and hepatic phase imaging with multi-detector row CT. Radiology. 2003;229:81–90. doi: 10.1148/radiol.2291020582. [DOI] [PubMed] [Google Scholar]
- 4.Francis IR, Cohan RH, McNulty NJ. Multidetector CT of the liver and hepatic neoplasms: effect of multiphasic imaging on tumor conspicuity and vascular enhancement. AJR Am J Roentgenol. 2003;180:1217–1224. doi: 10.2214/ajr.180.5.1801217. [DOI] [PubMed] [Google Scholar]
- 5.Ichikawa T, Erturk SM, Sou H, et al. MDCT of pancreatic adenocarcinoma: optimal imaging phases and multiplanar reformatted imaging. AJR Am J Roentgenol. 2006;187:1513–1520. doi: 10.2214/AJR.05.1031. [DOI] [PubMed] [Google Scholar]
- 6.McNulty NJ, Francis IR, Platt JR, et al. Multi-detector row helical CT of the pancreas: effect of contrast-enhanced multiphasic imaging on enhancement of the pancreas, peripancreatic vasculature, and pancreatic adenocarcinoma. Radiology. 2001;220:97–102. doi: 10.1148/radiology.220.1.r01jl1897. [DOI] [PubMed] [Google Scholar]
- 7.Zamboni GA, Kruskal JB, Vollmer CM, et al. Pancreatic adenocarcinoma: value of multidetector CT angiography in preoperative evaluation. Radiology. 2007;245(3):770–778. doi: 10.1148/radiol.2453061795. [DOI] [PubMed] [Google Scholar]
- 8.Valls C, Andia E, Sanchez A, et al. Dual-phase helical CT of pancreatic adenocarcinoma: assessment of resectability before surgery. AJR Am J Roentgenol. 2002;178:821–826. doi: 10.2214/ajr.178.4.1780821. [DOI] [PubMed] [Google Scholar]
- 9.Legmann P, Vignaux O, Dousset B, et al. Pancreatic tumors: comparison of dual-phase helical CT and endoscopic sonography. AJR Am J Roentgenol. 1998;170:1315–1322. doi: 10.2214/ajr.170.5.9574609. [DOI] [PubMed] [Google Scholar]
- 10.Bronstein YL, Loyer EM, Kaur H, et al. Detection of small pancreatic tumors with multiphasic helical CT. AJR Am J Roentgenol. 2004;182:619–623. doi: 10.2214/ajr.182.3.1820619. [DOI] [PubMed] [Google Scholar]
- 11.Prokesch RW, Chow LC, Beaulieu CF, et al. Isoattenuating pancreatic adenocarcinoma at multi-detector row CT: secondary signs. Radiology. 2002;224:764–768. doi: 10.1148/radiol.2243011284. [DOI] [PubMed] [Google Scholar]
- 12.Lall CG, Howard TJ, Skandarajah A, et al. New concepts in staging and treatment of locally advanced pancreatic head cancer. AJR Am J Roentgenol. 2007;189:1044–1050. doi: 10.2214/AJR.07.2131. [DOI] [PubMed] [Google Scholar]
- 13.Bilimoria KY, Bentrem DJ, Ko CY, et al. Validation of the 6th edition AJCC Pancreatic Cancer Staging System. Cancer. 2007;110:738–744. doi: 10.1002/cncr.22852. [DOI] [PubMed] [Google Scholar]
- 14.Katz MHG, Hwang R, Fleming JB. Tumor-node-metastasis staging of pancreatic adenocarcinoma. Cancer J Clin. 2008;58:111–125. doi: 10.3322/CA.2007.0012. [DOI] [PubMed] [Google Scholar]
- 15.Tseng JF, Tamm EP, Lee JE, et al. Venous resection in pancreatic cancer surgery. Best Pract Res Clin Gastroenterol. 2006;20(2):349–364. doi: 10.1016/j.bpg.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner MJ (eds) (2007) SEER survival monograph: cancer survival among adults: U.S. SEER Program, 1988–2001, patient and tumor characteristics. National Cancer Institute, SEER Program, NIH Pub. No. 07-6215, Bethesda, MD
- 17.Lu DSK, Reber HA, Krasny RM, et al. Local staging of pancreatic cancer: criteria for unresectability of major vessels as revealed by pancreatic-phase, thin-section helical CT. AJR Am J Roentgenol. 1997;168:1439–1443. doi: 10.2214/ajr.168.6.9168704. [DOI] [PubMed] [Google Scholar]
- 18.Hough TJ, Raptopoulos V, Siewert B, et al. Teardrop superior mesenteric vein: CT sign for unresectable carcinoma of the pancreas. AJR Am J Roentgenol. 1999;173:1509–1512. doi: 10.2214/ajr.173.6.10584793. [DOI] [PubMed] [Google Scholar]
- 19.O’Malley ME, Boland GWL, Wood BJ, et al. Adenocarcinoma of the head of the pancreas: determination of surgical unresectability with thin-section pancreatic-phase helical CT. AJR Am J Roentgenol. 1999;173:1513–1518. doi: 10.2214/ajr.173.6.10584794. [DOI] [PubMed] [Google Scholar]
- 20.Nakayama Y, Yamashita Y, Kadota M, et al. Vascular encasement by pancreatic cancer: correlation of CT findings with surgical and pathologic results. J Comput Assist Tomogr. 2001;25(3):337–342. doi: 10.1097/00004728-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Zeng MS, Zhou KR, et al. Pancreatic adenocarcinoma—the different CT criteria for peripancreatic major arterial and venous invasion. J Comput Assist Tomogr. 2005;29:170–175. doi: 10.1097/01.rct.0000155060.73107.83. [DOI] [PubMed] [Google Scholar]
- 22.Loyer EM, David CL, Dubrow RA, et al. Vascular involvement in pancreatic adenocarcinoma: reassessment by thin-section CT. Abdom Imaging. 1996;21:202–206. doi: 10.1007/s002619900046. [DOI] [PubMed] [Google Scholar]
- 23.Raptopoulos V, Steer ML, Sheiman RG, et al. The use of helical CT and CT angiography to predict vascular involvement from pancreatic cancer: correlation with findings at surgery. AJR Am J Roentgenol. 1997;168:971–977. doi: 10.2214/ajr.168.4.9124153. [DOI] [PubMed] [Google Scholar]
- 24.Brennan DDD, Zamboni GA, Raptopoulos VD, et al. Comprehensive preoperative assessment of pancreatic adenocarcinoma with 64-section volumetric CT. Radiographics. 2007;27:1653–1666. doi: 10.1148/rg.276075034. [DOI] [PubMed] [Google Scholar]
- 25.Roche CJ, Hughes ML, Garvey CJ, et al. CT and pathologic assessment of prospective nodal staging in patients with ductal adenocarcinoma of the head of the pancreas. AJR Am J Roentgenol. 2003;180:475–480. doi: 10.2214/ajr.180.2.1800475. [DOI] [PubMed] [Google Scholar]
- 26.DeWitt J, Devereaux B, Chriswell M, et al. Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann Intern Med. 2004;141:753–763. doi: 10.7326/0003-4819-141-10-200411160-00006. [DOI] [PubMed] [Google Scholar]
- 27.Maki DD, Birnbaum BA, Chakraborty DP, et al. Renal cyst pseudoenhancement: beam-hardening effects on CT numbers. Radiology. 1999;213:468–472. doi: 10.1148/radiology.213.2.r99nv33468. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz LH, Gandras EJ, Colangelo SM, et al. Prevalence and importance of small hepatic lesions found at CT in patients with cancer. Radiology. 1999;210:71–74. doi: 10.1148/radiology.210.1.r99ja0371. [DOI] [PubMed] [Google Scholar]
- 29.Diehl SJ, Lehmann KJ, Sadick M, et al. Pancreatic cancer: value of dual-phase helical CT in assessing resectability. Radiology. 1998;206:373–378. doi: 10.1148/radiology.206.2.9457188. [DOI] [PubMed] [Google Scholar]
- 30.Bluemke DA, Cameron JL, Hruban RH, et al. Potentially resectable pancreatic adenocarcinoma: spiral CT assessment with surgical and pathologic correlation. Radiology. 1998;197:381–385. doi: 10.1148/radiology.197.2.7480681. [DOI] [PubMed] [Google Scholar]
- 31.Walkey MM, Friedman AC, Sohotra P, et al. CT manifestations of peritoneal carcinomatosis. AJR Am J Roentgenol. 1988;150:1035–1041. doi: 10.2214/ajr.150.5.1035. [DOI] [PubMed] [Google Scholar]
- 32.White RR, Paulson EK, Freed KS. Staging of pancreatic cancer before and after neoadjuvant chemoradiation. J Gastrointest Surg. 2001;5:626–633. doi: 10.1016/S1091-255X(01)80105-0. [DOI] [PubMed] [Google Scholar]
- 33.Tamm EP, Loyer EM, Faria S. Staging of pancreatic cancer with multidetector CT in the setting of preoperative chemoradiation therapy. Abdom Imaging. 2006;31:566–574. doi: 10.1007/s00261-005-0194-y. [DOI] [PubMed] [Google Scholar]
- 34.Kim YE, Park MS, Hong HS. Effects of neoadjuvant combined chemotherapy and radiation therapy on the CT evaluation of resectability and staging in patients with pancreatic cancer. Radiology. 2009;250(3):758–765. doi: 10.1148/radiol.2502080501. [DOI] [PubMed] [Google Scholar]