Abstract
Lissencephaly is a devastating neurological disorder caused by to defective neuronal migration. LIS1 (or PAFAH1B1), the gene mutated in lissencephaly patients and its binding protein NDEL1 were found to regulate cytoplasmic dynein function and localization. LIS1 and NDEL1 also play a pivotal role on a microtubule regulation and determination of cell polarity. For example, LIS1 is required for the precise control of mitotic spindle orientation in both neuroepithelial stem cells and radial glial progenitor cells. On the other hand, NDEL1 is essential for mitotic entry as an effector molecule of Aurora-A kinase. In addition, an atypical protein kinase C (aPKC)-Aurora-A-NDEL1 pathway is critical for the regulation of microtubule organization during neurite extension. These findings suggest that physiological functions of LIS1 and NDEL1 in neurons have been ascribed for proteins fundamentally required for cell cycle progression and control. In turn, cell cycle regulators may exert other functions during neurogenesis in a direct or an indirect fashion. Thus far, only a handful of cell cycle regulators have been shown to play physiological cell cycle-independent roles in neurons. Further identification of such proteins and elucidation of their underlying mechanisms of action will likely reveal novel concepts and/or patterns that provide a clear link between their seemingly distinct cell cycle and neuronal functions.
Key words: microtubule, mitotic kinase, neurite, cell polarity, migration
During the development of the mammalian central nervous system, the self-renewal of neural stem cells can occur either by symmetric cell divisions, which generate two daughter cells with the same fate, or by asymmetric cell divisions, which generate one daughter cell that is identical to the mother cell and a second, different non-stem-cell progenitor (reviewed in refs. 1 and 2). Neural non-stem-cell progenitors typically undergo symmetric, differentiating divisions, each of which generates two neurons, which are terminally differentiated, post-mitotic cells. These post-mitotic neural progenitors migrate from their birth place at the ventricular zone to their final destinations in cortical plate (reviewed in ref. 3). Coinciding with the proper positioning of post-mitotic neurons, neurons project neurite and dendrites to targets with the assistant of molecular guidance cues in the local environment. Proper navigations of neurite and dendrite processes ensure synapse formations, which are the basis of brain function. In the series of developmental steps, the determination of neuronal polarity is critically important (reviewed in refs. 4 and 5). A polarity complex of Par3, Par6, and atypical protein kinase C (aPKC) functions in various cell-polarization events including axon formation.6,7 GTPases that regulate actin cytoskeletal dynamics have been implicated in cell polarization. Recent findings provide insights into polarization mechanisms and show intriguing crosstalk between small GTPases and members of polarity complexes in regulating cell polarization (reviewed in ref. 8). Thus, determination of neuronal polarity and regulation of cytoskeletal organization are intimately related.
LIS1 was identified as the first gene mutated in isolated lisssencephaly sequence (ILS), a human neuronal migration defect.9,10 LIS1 and its binding protein, NDEL1 regulate the function and localization of cytoplasmic dynein11,13 as part of an evolutionarily conserved pathway.14,15 Genetic analysis of fungi displaying defective nuclei migration led to the identification of a number of genes and their protein products involved in this process. For example, mutations of nudA (coding for cytoplasmic dynein heavy chain) and genes coding for other subunits of the dynein complex inhibit nuclear migration, including nudC (mammalian NudC, mNudC), nudE (Ndel1 and Nde1) and nudF (Lis1). We recently demonstrate that LIS1 suppresses the motility of cytoplasmic dynein on microtubules in vitro (Suppl. movies 1 and 2), whereas NDEL1 releases the blocking effect of LIS1 on cytoplasmic dynein.16 We demonstrated anterograde co-migration of cytoplasmic dynein and LIS1 (Suppl. movies 3 and 4). When LIS1 function was suppressed by a blocking antibody, anterograde movement of cytoplasmic dynein was severely impaired. Lis1 KO cells exhibited biased distribution around the centrosome and aberrant distribution of organelles. Our favorite model is that LIS1 fixes cytoplasmic dynein on soluble microtubules in an “idling” state, thereby creating a microtubule-LIS1-dynein complex, which could be transported by kinesin to the plus-end of microtubules.
Lis1 is also essential for the precise control of mitotic spindle orientation in both neuroepithelial stem cells and radial glial progenitor cells.17 Controlled gene deletion of Lis1 in vivo in neuroepithelial stem cells, where cleavage is uniformly vertical and symmetrical, provokes rapid apoptosis of those cells, while radial glial progenitors are less affected. We believe the role of LIS1 in promoting the anterograde transport of cytoplasmic dynein on kinesin as part of a microtubule-LIS1-dynein complex, as described in the previous paragraph, is responsible for controlling spindle orientation, since when LIS1 is reduced, cortical dynein fixed on the surface of the cell is also reduced. Impaired cortical microtubule capture via loss of cortical dynein causes astral and cortical microtubules to be greatly reduced in Lis1-deficient cells.17 Thus, Lis1 is intimiately involved in the determination of cell polarity as an effector molecule, which regulates dynein localization and/or function as well as microtubule organization.
Interestingly, more than half of LIS1 protein is degraded at the cell cortex after transport to the plus-end of MTs via calpain-dependent proteolysis. We recently demonstrated that inhibition or knockdown of calpain protects LIS1 from proteolysis resulting in the augmentation of LIS1 levels in Lis1+/− mouse embryonic fibroblast (MEF) cells, which leads to rescue of the aberrant distribution of cytoplasmic dynein and intracellular components including mitochondria and β-COP positive vesicles.18 We also showed that presence of calpain inhibitors improves neuronal migration of Lis1+/− cerebellar granular neurons.18 This study demonstrates that stabilization of proteins in disorders caused by haploinsufficiency is a potential therapeutic strategy and provides a proof-of-principle for this notion.
NDEL1, a binding partner of LIS1 is also essential for the regulation of cytoplasmic dynein and microtubule organization.12,13 In particular, NDEL1 is phosphorylated by cyclin dependent kinase1 (CDK1) in mitotic cells, or CDK5 in post-mitotic neurons, and this phosphorylation is essential for proper targeting of NDEL1 binding proteins to the centrosome.19 NDEL1 is also a substrate of the mitotic kinase Aurora-A, by which NDEL1 connects Aurora-A to other target molecules for the regulation of microtubule organization.20 Interestingly, NDEL1 is differentially phosphorylated by Aurora-A and CDK1. It is possible that distinct pools of NDEL1 may be targeted by each kinase, or conversely the affects of each kinase may counteract each other within the same pool of NDEL1.
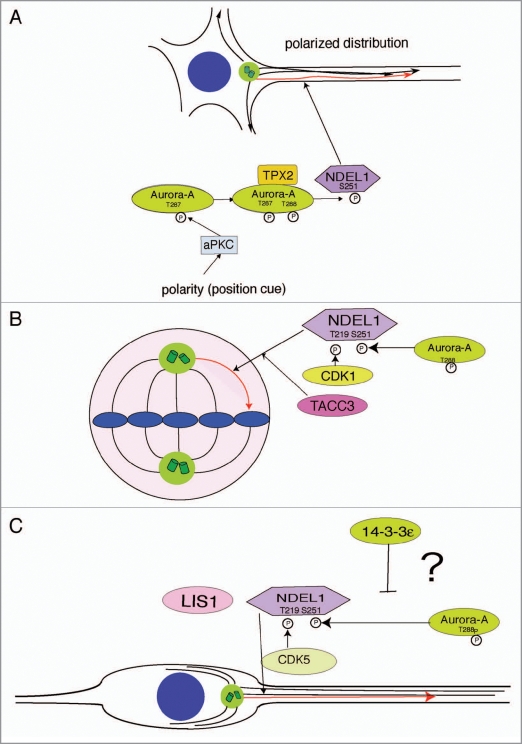
Aurora-A is a one of representative mitotic kinase, whose homologues have been reported in various organisms including yeast, nematodes, fruit flies and vertebrates (reviewed in ref. 21). The three human homologues of Aurora kinases (A, B and C) are essential for proper execution of various mitotic events and are important for maintaining genomic integrity. Aurora-A is mainly localized at spindle poles and the mitotic spindle during mitosis, where it regulates the functions of centrosomes, spindles and kinetochores required for proper mitotic progression. In particular, Aurora-A plays a pivotal role on microtubule reorganization during remodeling from interphase microtubules to mitotic microtubules, i.e., the mitotic spindle. We recently reported molecular and cell biological data that support a unique role of aPKC-Aurora-A-NDEL1 pathway on microtubule dynamics at the neurite hillock during neurite extension.22 PKCζ phosphorylates Aurora-A at T287 and activates it, which augments interaction with TPX2 and facilitates activation of Aurora-A at the neurite hillock, followed by S251 phosphorylation of NDEL1 and recruitment. Inhibition of PKCζ/λ, depletion of Aurora-A and disruption of Ndel1 severely affected neurite extension and microtubule dynamics, suggesting that the aPKC-Aurora-A-NDEL1 pathway is an important regulatory system of microtubule oranization within neurite processes (Fig. 1A).
Figure 1.
Models of microtubule remodeling. (A) Neurite extension: an unknown upstream cue polarity activates aPKC followed by T287 phosphorylation of Aurora-A. T287 phosphorylation of Aurora-A facilitates binding of the Aurora-A activator, TPX2 resulting in activation of Aurora-A at the neurite hillock, which leads to phosphorylation of NDEL1, one of effector molecules of Aurora-A. Finally, phosphorylation of NDEL1 triggers remodeling microtubules during neurite extension. (B) Spindle formation: NDEL1 is differentially phosphorylated at T219 and Ser251 by CDK1 and Aurora-A, respectively at the beginning of mitotic entry. NDEL1 is required for centrosome targeting of TACC3 through the interaction with TACC3. (C) Neuronal migration: during neuronal migration, NDEL1 may be differentially phosphorylated at T219 and Ser251 by CDK5 and Aurora-A, respectively. 14-3-3ɛ might negatively regulate Aurora-A kinase.
Our preliminary data suggest that Aurora-A is also activated by neurons during migration, and may further link signaling components already implicated in neuronal migration. Mice deficient in Ywhae that encondes 14-3-3ɛ have defects in brain development and neuronal migration, similar to defects observed in mice heterozygous with respect to Lis1.23 Mice heterozygous with respect to both genes have more severe migration defects than single heterozygotes. Heterozygous deletions of 17p13.3 in human result in the human neuronal migration disorders isolated lissencephaly sequence (ILS) and the more severe Miller-Dieker syndrome (MDS). Mice carrying double heterozygous mutations of Ywhae and Lis1 are therefore thought to be a mouse model of MDS. Intriguingly, 14-3-3ɛ binds to NDEL1 after phosphorylation by CDK1/CDK5, protecting phospho-NDEL1 from phosphatase attack.
14-3-3 proteins mediate multiple cellular events, including scaffolding of signaling molecules, regulation of enzyme catalysis, and subcellular targeting. In the C. elegans, 14-3-3 homolog, Par5 is required for correct anterior-posterior zygote polarization.24 In addition, phosphorylation-dependent interactions between 14-3-3ɛ, and the tight junction-associated protein Par3 had been reported.25 Intriguingly, 14-3-3ɛ is a centrosomal protein,26 suggesting that 14-3-3ɛ, Aurora-A and NDEL1 might create a complex at the centrosome, which may then be involved in the determination of polarity and neuronal migration. These findings might be the result of the known role of Aurora-A as a regulator of microtubule network. Microtubules are emanated from MTOC, and are extended into the chromosome, nucleus or the cell periphery (Fig. 1). These microtubule flows associated with the dynamic remodeling will provide enough force to maintain a neurite process, a spindle body or a leading process.
Post-mitotic neurons, however, lose their mitotic competence permanently. Intuitively, once a neural progenitor differentiates into a neuron, the post-mitotic neurons have severed all ties with the cell cycle, in which the expression of cell cycle proteins are assumed to be not expressed. Emerging evidence reveals that this holds true for a handful of core cell cycle regulators, which facilitate the differentiation and maturation of neurons, suggesting that “core“ cell cycle regulators serve diverse postmitotic functions that span various developmental stages of a neuron, including neuronal migration, axonal elongation, axon pruning, dendrite morphogenesis and synaptic maturation and plasticity (reviewed in ref. 27). Among the essential kinases that function in mitosis are Aurora kinases, evolutionarily conserved serine-threonine kinases that maintain genomic stability and are required for mitotic progression. Although they share conserved regions, each member (Aurora A, B and C) contributes distinctly to cell cycle progression. Aurora-A is essential for mitotic entry, centrosome maturation during late G2 and prophase, centrosome separation during bipolar spindle assembly and mitotic spindle organization (reviewed in refs. 21 and 28). During mitotic progression, Aurora-A loss of function prevents centrosomal separation prior to mitotic spindle formation and results in monopolar spindles.29 We reported an essential role of Aurora-A during neurite extension. Wirtz-Peitz et al. reported that Aurora-A phosphorylates Par-6.30 This phosphorylation cascade triggered by the activation of Aurora-A is responsible for the asymmetric localization of Numb in mitosis, which provides further evidence for crosstalk of PAR proteins and Aurora-A.30 Apart from neurons, the interactions between the prometastatic scaffolding protein HEF1/Cas-L/NEDD9 and the oncogenic Aurora-A kinase at the basal body of cilia had been reported.31 This pathway is both necessary and sufficient for ciliary resorption and constitutes an unexpected non-mitotic activity of Aurora-A in vertebrates.
Aurora-A kinase, Plk1 or CDK1 has been recognized as a mitotic kinase, which regulates mitotic entry. Cells in which these genes are mutated display defective mitotic entry. Individual proteins however, have multiple functions within specific cellular context. For example, Aurora-A may participate remodeling microtubule in mitotic spindle formation and in remodeling of microtubule organization during neurite extension or neuronal migration. Apart from existing concept, elucidation of multiple functions of cell cycle regulators will provide us with a better understanding of the extent to which they exert physiological cell cycle-independent neuronal functions.
Acknowledgements
We thank Takako Takitho and Yurie Hata for support of manuscript writing. This work was supported by Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan from the Ministry of Education, Science, Sports and Culture of Japan to Shinji Hirotsune. This work was also supported by The Mother and Child Health Foundation, The Naito Foundation, Japan Brain Foundation and The Uehara Memorial Foundation to Shinji Hirotsune, and NIH grants NS41030 and HD47380 to Anthony Wynshaw-Boris.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/10715
Supplementary Material
References
- 1.Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- 2.Siller KH, Doe CQ. Spindle orientation during asymmetric cell division. Nat Cell Biol. 2009;11:365–374. doi: 10.1038/ncb0409-365. [DOI] [PubMed] [Google Scholar]
- 3.Gupta A, Tsai LH, Wynshaw-Boris A. Life is a journey: a genetic look at neocortical development. Nat Rev Genet. 2002;3:342–355. doi: 10.1038/nrg799. [DOI] [PubMed] [Google Scholar]
- 4.Arimura N, Kaibuchi K. Key regulators in neuronal polarity. Neuron. 2005;48:881–884. doi: 10.1016/j.neuron.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Arimura N, Kaibuchi K. Neuronal polarity: from extracellular signals to intracellular mechanisms. Nat Rev Neurosci. 2007;8:194–205. doi: 10.1038/nrn2056. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura T, Kato K, Yamaguchi T, Fukata Y, Ohno S, Kaibuchi K. Role of the PAR-3-KIF3 complex in the establishment of neuronal polarity. Nat Cell Biol. 2004;6:328–334. doi: 10.1038/ncb1118. [DOI] [PubMed] [Google Scholar]
- 7.Shi SH, Jan LY, Jan YN. Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell. 2003;112:63–75. doi: 10.1016/s0092-8674(02)01249-7. [DOI] [PubMed] [Google Scholar]
- 8.Iden S, Collard JG. Crosstalk between small GTPases and polarity proteins in cell polarization. Nat Rev Mol Cell Biol. 2008;9:846–859. doi: 10.1038/nrm2521. [DOI] [PubMed] [Google Scholar]
- 9.Dobyns WB. The neurogenetics of lissencephaly. Neurol Clin. 1989;7:89–105. [PubMed] [Google Scholar]
- 10.Reiner O, Carrozzo R, Shen Y, Wehnert M, Faustinella F, Dobyns WB, et al. Isolation of a Miller-Dieker lissencephaly gene containing G protein beta-subunit-like repeats. Nature. 1993;364:717–721. doi: 10.1038/364717a0. [DOI] [PubMed] [Google Scholar]
- 11.Vallee RB, Tsai JW. The cellular roles of the lissencephaly gene LIS1, and what they tell us about brain development. Genes Dev. 2006;20:1384–1393. doi: 10.1101/gad.1417206. [DOI] [PubMed] [Google Scholar]
- 12.Niethammer M, Smith DS, Ayala R, Peng J, Ko J, Lee MS, et al. NUDEL is a novel Cdk5 substrate that associates with LIS1 and cytoplasmic dynein. Neuron. 2000;28:697–711. doi: 10.1016/s0896-6273(00)00147-1. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki S, Shionoya A, Ishida M, Gambello MJ, Yingling J, Wynshaw-Boris A, et al. A LIS1/NUDEL/cytoplasmic dynein heavy chain complex in the developing and adult nervous system. Neuron. 2000;28:681–696. doi: 10.1016/s0896-6273(00)00146-x. [DOI] [PubMed] [Google Scholar]
- 14.Morris NR, Efimov VP, Xiang X. Nuclear migration, nucleokinesis and lissencephaly. Trends Cell Biol. 1998;8:467–470. doi: 10.1016/s0962-8924(98)01389-0. [DOI] [PubMed] [Google Scholar]
- 15.Morris SM, Albrecht U, Reiner O, Eichele G, Yu-Lee LY. The lissencephaly gene product Lis1, a protein involved in neuronal migration, interacts with a nuclear movement protein, NudC. Curr Biol. 1998;8:603–606. doi: 10.1016/s0960-9822(98)70232-5. [DOI] [PubMed] [Google Scholar]
- 16.Yamada M, Toba S, Yoshida Y, Haratani K, Mori D, Yano Y, et al. LIS1 and NDEL1 coordinate the plus-end-directed transport of cytoplasmic dynein. EMBO J. 2008;27:2471–2483. doi: 10.1038/emboj.2008.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yingling J, Youn YH, Darling D, Toyo-Oka K, Pramparo T, Hirotsune S, et al. Neuroepithelial stem cell proliferation requires LIS1 for precise spindle orientation and symmetric division. Cell. 2008;132:474–486. doi: 10.1016/j.cell.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamada M, Yoshida Y, Mori D, Takitoh T, Kengaku M, Umeshima H, et al. Inhibition of calpain increases LIS1 expression and partially rescues in vivo phenotypes in a mouse model of lissencephaly. Nat Med. 2009;15:1202–1207. doi: 10.1038/nm.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toyo-Oka K, Sasaki S, Yano Y, Mori D, Kobayashi T, Toyoshima YY, et al. Recruitment of katanin p60 by phosphorylated NDEL1, an LIS1 interacting protein, is essential for mitotic cell division and neuronal migration. Hum Mol Genet. 2005;14:3113–3128. doi: 10.1093/hmg/ddi339. [DOI] [PubMed] [Google Scholar]
- 20.Mori D, Yano Y, Toyo-oka K, Yoshida N, Yamada M, Muramatsu M, et al. NDEL1 phosphorylation by Aurora-A kinase is essential for centrosomal maturation, separation and TACC3 recruitment. Mol Cell Biol. 2007;27:352–367. doi: 10.1128/MCB.00878-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marumoto T, Zhang D, Saya H. Aurora-A—a guardian of poles. Nat Rev Cancer. 2005;5:42–50. doi: 10.1038/nrc1526. [DOI] [PubMed] [Google Scholar]
- 22.Mori D, Yamada M, Mimori-Kiyosue Y, Shirai Y, Suzuki A, Ohno S, et al. An essential role of the aPKC-Aurora A-NDEL1 pathway in neurite elongation by modulation of microtubule dynamics. Nat Cell Biol. 2009;11:1057–1068. doi: 10.1038/ncb1919. [DOI] [PubMed] [Google Scholar]
- 23.Toyo-oka K, Shionoya A, Gambello MJ, Cardoso C, Leventer R, Ward HL, et al. 14-3-3epsilon is important for neuronal migration by binding to NUDEL: a molecular explanation for Miller-Dieker syndrome. Nat Genet. 2003;34:274–285. doi: 10.1038/ng1169. [DOI] [PubMed] [Google Scholar]
- 24.Morton DG, Shakes DC, Nugent S, Dichoso D, Wang W, Golden A, et al. The Caenorhabditis elegans par-5 gene encodes a 14-3-3 protein required for cellular asymmetry in the early embryo. Dev Biol. 2002;241:47–58. doi: 10.1006/dbio.2001.0489. [DOI] [PubMed] [Google Scholar]
- 25.Hurd TW, Fan S, Liu CJ, Kweon HK, Hakansson K, Margolis B. Phosphorylation-dependent binding of 14-3-3 to the polarity protein Par3 regulates cell polarity in mammalian epithelia. Curr Biol. 2003;13:2082–2090. doi: 10.1016/j.cub.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 26.Andersen JS, Wilkinson CJ, Mayor T, Mortensen P, Nigg EA, Mann M. Proteomic characterization of the human centrosome by protein correlation profiling. Nature. 2003;426:570–574. doi: 10.1038/nature02166. [DOI] [PubMed] [Google Scholar]
- 27.Frank CL, Tsai LH. Alternative functions of core cell cycle regulators in neuronal migration, neuronal maturation and synaptic plasticity. Neuron. 2009;62:312–326. doi: 10.1016/j.neuron.2009.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giet R, Petretti C, Prigent C. Aurora kinases, aneuploidy and cancer, a coincidence or a real link? Trends Cell Biol. 2005;15:241–250. doi: 10.1016/j.tcb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Glover DM, Leibowitz MH, McLean DA, Parry H. Mutations in aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell. 1995;81:95–105. doi: 10.1016/0092-8674(95)90374-7. [DOI] [PubMed] [Google Scholar]
- 30.Wirtz-Peitz F, Nishimura T, Knoblich JA. Linking cell cycle to asymmetric division: Aurora-A phosphorylates the Par complex to regulate Numb localization. Cell. 2008;135:161–173. doi: 10.1016/j.cell.2008.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–1363. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.