Abstract
Integrins are transmembrane heterodimeric receptors responsible for transducing and modulating signals between the extracellular matrix and cytoskeleton, ultimately influencing cell functions such as adhesion and migration. Integrin α9β1 is classified within a two member sub-family of integrins highlighted in part by its specialized role in cell migration. The importance of this role is demon-strated by its regulation of numerous biological functions including lymphatic valve morphogenesis, lymphangiogenesis, angiogenesis and hematopoietic homeostasis. Compared to other integrins the signaling mechanisms that transduce α9β1-induced cell migration are not well described. We have recently shown that Src tyrosine kinase plays a key proximal role to control α9β1 signaling. Specifically it activates inducible nitric oxide synthase (iNOS) and in turn nitric oxide (NO) production as a means to transduce cell migration. Furthermore, we have also described a role for FAK, Erk and Rac1 in α9β1 signal transduction. Here we provide an over view of known integrin α9β1 signaling pathways and highlight its roles in diverse biological conditions.
Key words: integrin, α9β1, nitric oxide, VEGF, cell migration, cell adhesion
Introduction
Integrins are transmembrane heterodimers that consist of an α and β subunit, and serve most commonly as receptors to extra cellular matrix (ECM) proteins.1 In doing so, they promote numerous cellular functions including their prototypical roles of cell adhesion and migration.2 Since integrins do not possess intrinsic enzymatic activity they transduce these complex functions through intricate, multi-protein and highly regulated intracellular signaling pathways.3,4 A recent review outlined some 156 networks and 690 protein interactions that makeup just the integrin adhesome.5 Cell migration is a clear example of the complex interplay between extracellular matrix, integrin and intracellular proteins; and presumably the “migrasome” network of proteins mediating cell migration is at least as complex as the adhesome if not more so.
ECM proteins are the prototypical integrin ligands, and although many are integrin specific, they can also serve as common binding partners for more than one integrin.1,2 The tri-amino acid sequence of arginine-glycine-aspartic acid (RGD), which serves as a cell attachment site in numerous ECM proteins is a common binding motif for several integrins6 and facilitates this integrin-ligand redundancy. Activation of the integrin and initiation of subsequent signaling cascades can be achieved by both intracellular “ligand” binding such as the actin adaptor protein talin (inside-out activation), and by binding a large array of extracellular ligands (outside-in activation).7 Likewise, the protein-protein signaling networks that transduce these communications are both common and unique to integrins, but in both cases the signaling network defines the nature of their biological effects.2
Integrin α9β1 is a relatively new addition to the β1 integrin subfamily7 and through homologous sequence it forms a unique subfamily with α4β1.9 Compared to other integrins, α9β1 has distinguished its functionality by facilitating accelerated cell migration.10 Despite an extensive description of its ligands7,10–15 the signaling mechanisms that transduce its migratory effect are not well understood. Recently, we have shown that to transduce cell migration α9β1 utilizes a number of common integrin signaling intermediates, in particular Src; but also α9β1-specific intermediates, iNOS and nitric oxide.16 Herein we discuss these findings and highlight the unique nature of α9β1 as demonstrated through the nature of its ligands and diversity of its signal transduction mechanisms.
Integrin α9β1 Utilizes the Common Integrin Signaling Proteins: Src, FAK and Erk
FAK auto-phosphorylation at tyrosine-397 is an early event that prompts association of FAK and Src and in turn initiates a signaling cascade that may result in activation of the small GTPases rac-1 and cdc42.17 We found that specific ligation of integrin α9β1, as with other integrins activates Src, FAK and also promotes their association (Fig. 1A). Similar to its subfamily member α4β1 and in contrast to other β1 integrins, we found that FAK played a redundant role in α9β1-mediated cell migration.16–18 Furthermore, we also found that Src activation was a proximal and dominant signaling protein regulating α9β1 led cell migration.16 Although FAK played a redundant role during cell migration, we postulate that it may serve to amplify migratory signaling; and may play a non-redundant role for non-migratory α9β1 functions such as cell survival or invasion.17
Figure 1.
Signaling mechanisms of integrin α9β1. (A) Selective ligation of integrin α9β1 activates proximal tyrosine kinase Src. Src coordinates subsequent signaling pathways; first through activation of FAK, tyrosine phosphorylation of the adaptor protein p130Cas and ultimately co-ordinates activation of small GTPase rac1, which translocates to the cell membrane and causes lamellipodial protrusion; second, Src activates iNOS resulting in increased NO production, activation of cGMP and protein kinase G signaling cascades to enhance cell migration (as reported by Gupta et al.16). (B) Integrin α9β1 can regionally recruit SSAT, an enzyme involved in the catabolism of higher order polyamines spermidine and spermine, which are potent blockers of inward rectifier K+ (Kir) channels. SSAT (along with PAO, polyamine oxidase) facilitates localized enzymatic processing of spermidine and spermine in to the smaller polyamine, putrescine and thus relieving outward efflux of K+ to co-ordinate cell migration (as reported by deHart et al.37).
The molecular details of α9β1-induced Src activation remains to be elucidated, but it appears that unlike β3, its β1 cytoplasmic tail does not associate with Src.19 We postulate that Src may directly interact with the cytoplasmic tail of α9, subsequently recruiting other signaling proteins to form an associated multimeric signaling complex. Normally, Src is maintained in an inactive state by tyrosine phosphorylation (pY527) at its C-terminus, and can be reversibly activated by auto-tyrosine phosphorylation (pY416) in the kinase domain by phosphatases that dephosphorylate pY-527.20 Genetic mutations at the C-terminus that uncouple Src from the PDZ domain of certain membrane proteins have also been shown to result in Src activation.21 Many human cancers especially those at advanced stages express constitutively active Src tyrosine kinase;22 whether or not α9β1, which is expressed in a number of cancer cell lines,9,23–25 is involved in a similar mode of Src activation is also yet to be determined.
It has been clearly shown that extra cellular signal-regulated kinase (Erk) is a central component of integrin signaling and can be activated in a number of ways including through FAK association with adaptor proteins such as Shc and Grb2 and is involved in tumorigenesis and cancer cell proliferation.26,27 In preliminary studies we found that specific ligation of α9β1-induced Erk phosphorylation was Src dependent and contributed to cancer cell migration. However, compared to other α9β1-induced (non-Erk) signaling, Erk-induced cancer cell migration was not robust. This highlights another important aspect of integrin signaling; its cell type variability and that defining integrin-specific signaling within cancer cell types will serve as a means to develop targeted pharmacotherapy. Further studies will also need to define the role of Erk in α9β1 regulation of other tumorogenic processes such as cancer cell proliferation or survival.
Integrin α9β1 Signals in a Unique Fashion through iNOS and NO
In novel findings, we recently showed that integrin α9β1 regulates iNOS activity via Src tyrosine kinase resulting in increased NO production and NO-induced cell migration16 (Fig. 1A). Of note, Erk activation in our in vitro system remains unaffected by inhibition of iNOS activity. Like Src, iNOS is another cytoplasmic protein maintained at low levels in almost all mammalian cells which can be either transcriptionaly upregulated or activated by various stimuli including cytokines and growth factors.16,28–31 Of note, increased iNOS expression also serves as a cancer associated prognostic factor in addition to regulating several other biological processes.28,32 The specialized role of integrin α9β1 in cell migration, its expression in cancer cells and iNOS signaling identifies this integrin as potentially playing a key role in carcinogenesis.
Activation of iNOS in malignant (and non-malignant) cells can promote cell migration via multiple mechanisms.33,34 NO generated from iNOS activation can act as a cofactor to guanylyl cyclase (GC) to promote synthesis of the second messenger cGMP, which regulates cell migration in both a PKG dependent and independent fashion.33,34 Furthermore, NO can regulate both the expression of MMP9 and its activation through cGMP dependent or independent mechanisms.33,34 Relevant to integrin function NO released in to the cellular microenvironment can impact assembly of focal adhesions. NO-induced delay of focal adhesion assembly or their premature destabilization has significant effects on cell migratory responses. Furthermore, interactions between iNOS and rac-GTPase,35 an important mediator of cell migration are now known to affect Rac-GTPase activation or its membrane translocation. We found that α9β1 induces activation of rac1 in cancer cells but there was no significant modulatory effect of iNOS.16 Despite no significant impact on rac1 activation we found a robust interaction between iNOS and rac1 (unpublished findings). Inhibition of iNOS activity however, delayed the spreading of cells on a α9 specific ligand which may be due to a slower accumulation of rac1 at the cell membrane and thus affecting the gener-ation of lamellipodia or actin assembly required for cell spreading. Whether or not iNOS has any role in membrane translocation of activated rac1 is yet to be determined. Regardless of molecular mechanism, it appears that α9β1-induced iNOS activation and NO production may modulate the mechanisms of cancer cell migration and invasion.
Further Evidence of the Unique and Diverse Nature of α9β1 Signaling Mechanisms
Spermidine, spermine acetyl transferase (SSAT) is an enzyme that acetylates its polyamine substrates spermidine and spermine, ultimately resulting in the generation of the lower order polyamine, putrescine. Spermidine and spermine are potent blockers of outward potassium currents from inward rectifier K+ (Kir) channels.36 Catabolism of spermidine and spermine into putrescine is thus considered to result in rapid efflux of K+ ions, which can modulate cell migration.37 The cytoplasmic tail of α9 interacts with SSAT and facilitates a localized enzymatic process-ing of spermine and spermidine in to putrescine, thereby creating a spatially restricted impairment of K+ ion channel rectification leading to increased K+ ion efflux, and enhanced cell migration37,38 (Fig. 1B). Inwardly rectifying K+ (Kir) channels significantly contribute towards maintaining membrane potential and to cause blood vessel dilation in response to extracellular K+ or shear stress.39 Although the role of α9β1 mediated K+ channel rectification in tumor cells is not yet clear; gene expression profiling has shown ∼3-fold increase in SSAT expression in a late stage (metastatic) colon carcinoma cell line, compared to early stage carcinomas.40 Whether the interaction of α9β1 and SSAT plays a significant role in carcino-genesis remains to be determined.
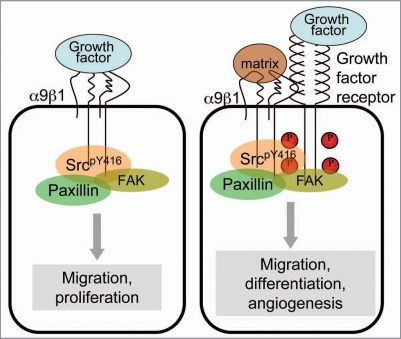
Integrin α9β1 also associates with and modulates signaling from several growth factor receptors.7,10,41 Evidence suggests that integrins and growth factors can synergize signaling for proliferation, survival and migration, since both are able to utilize common signaling mediators such as Src, FAK and MAP kinase.42,43 The molecular basis of functional synergy between integrins and growth factor receptors continues to be defined but appears to fit two separate paradigms (Fig. 2): first, that both integrin and growth factor receptor bind their respective ligands, inducing their clustering within distinct membrane micro domains, and synergistic activation of downstream signaling; second, that ligand binding to integrins alone can trans-activate growth factor receptors to induce downstream signaling.43 Integrin α9β1 plays an essential role in granulopoiesis, wherein it synergizes signaling through the receptor for granulocyte-colony stimulating factor.40 Furthermore, endothelial cell α9β1 appears to play an important role in both lymphangiogenesis and angiogenesis.7,10,44,45 The physiological role of α9β1 was clearly evident in α9-deficient mice, as described by Huang et al.46 α9-deficient mice die 7-10 d post-partum from chylous pleural effusions and demonstrate defective lymphatic valve morphogenesis and function.46,47 In addition, it is now well established that expression of α9β1 is part of the protein signature of lymphatic endothelium.44,48 Similar to its ability to directly bind the lymphangiogenic proteins VEGF-C and D,7 α9β1 also binds VEGF-A and promotes the angiogenic response of VEGF in part through synergy with the VEGF-R2 receptor.10 Through these biological functions, α9β1 positions itself as an important regulatory factor in patho-physiological processes such as wound healing and carcinogenesis.
Figure 2.
Integrin crosstalk with growth factors and their cognate receptors. Left: shows direct ligation of integrin α9β1 by growth factors such as VEGF-A, C, D and NGF triggering the necessary signaling pathways to transduce cell attachment and migration (reported by Vlahakis et al.7,10 and Staniszewska et al.15). Right: shows an alternative mechanism, whereby growth factor stimulation (with or without) integrin ligation can promote co-association of growth factor receptor with integrins, where they synergize their respective effects. Specifically integrin α9β1 associates with VEGF-R2 or GCSF-R following co-stimulation of the integrin and the growth factor receptors with VEGF-A or GCSF, respectively to co-ordinate signaling for cell migration, proliferation, angiogenesis and granulopoiesis (as reported by Vlahakis et al.10 and Chen et al.41).
Summary
As described here integrin α9β1 has been implicated in diverse biological functions including angiogenesis, lymphangiogenesis, lymphatic valve morphogenesis, granulopoiesis and tumorigenesis. Like other integrins α9β1 ligation can activate signaling through Src and FAK mediated tyrosine phosphorylation of multiple proteins including p130Cas16 and paxillin.49 Redundancy of FAK and paxillin16,49 and a less robust migratory stimulus through Erk MAP kinase demonstrates the complexity of α9β1’s signaling network involving several parallel pathways. Recent reports showing activation of outward potassium ion channels via localized modulation of polyamines37 and nitric oxide16 production through modulating iNOS activity are two specific signaling pathways assigned to α9β1 thus far. The potential role of these α9β1 specific signaling pathways and their significance in such processes as carcinogenesis or wound healing are unclear at this point.
In human cancers, integrins have been shown to contribute to tumor growth and metastasis, and thus have been identified as legitimate therapeutic targets with anti-integrin αv targeted therapies already evaluated.50,51 It is worth noting that due to the redundancy in expression and function of integrins, inhibiting a single integrin may not result in a clinically significant effect. Therefore, strategies to target multiple integrins and/or growth factor receptors will be more useful. Since integrin α9β1 not only orchestrates biologically significant signaling in isolation, but also associates with growth factor receptors to modulate their function, it represents a novel potential pharmacotherapeutic target.
Acknowledgements
This work was supported by the National Institutes of Health grants 1K08 HL076455-01A2 and Mayo Foundation to N.E.V. Authors are thankful to Saji Oommen for helpful suggestions.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/10900
References
- 1.Hynes RO. Integrins: bidirectional allosteric signaling machines. Cell. 2002;110:16–18. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 2.Stupack DG, Cheresh DA. Get a ligand, get a life: integrins, signaling and cell survival. J Cell Sci. 2002;115:3729–3738. doi: 10.1242/jcs.00071. [DOI] [PubMed] [Google Scholar]
- 3.Gahmberg CG, Fagerholm SC, Nurmi SM, Chavakis T, Marchesan S, Grönholm M. Regulation of integrin activity and signaling. Biochimica et Biophysica Acta. 2009;1790:431–444. doi: 10.1016/j.bbagen.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harburger DS, Calderwood DA. Integrin signalling at a glance. J Cell Sci. 2009;122:159–163. doi: 10.1242/jcs.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9:858–867. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 7.Vlahakis NE, Young BA, Atakilit A, Sheppard D. The lymphangiogenic vascular endothelial growth factors VEGF-C and -D are ligands for the integrin alpha9beta1. J Biol Chem. 2005;280:4544–4552. doi: 10.1074/jbc.M412816200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmer EL, Ruegg C, Ferrando R, Pytela R, Sheppard D. Sequence and tissue distribution of the integrin alpha9 subunit, a novel partner of beta1 that is widely distributed in epithelia and muscle. J Cell Biol. 1993;123:1289–297. doi: 10.1083/jcb.123.5.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hibi K, Yamakawa K, Ueda R, Horio Y, Murata Y, Tamari M, et al. Aberrant upregulation of a novel integrin alpha subunit gene at 3p21.3 in small cell lung cancer. Oncogene. 1994;9:611–619. [PubMed] [Google Scholar]
- 10.Vlahakis NE, Young BA, Atakilit A, Hawkridge AE, Issaka RB, Boudreau N, et al. Integrin α9β1 directly binds to Vascular Endothelial Growth Factor (VEGF)-A and contributes to VEGF-A-induced angiogenesis. J Biol Chem. 2007;282:15187–5196. doi: 10.1074/jbc.M609323200. [DOI] [PubMed] [Google Scholar]
- 11.Yokosaki Y, Palmer EL, Prieto AL, Crossin KL, Bourdon MA, Pytela R, et al. The integrin α9β1 mediates cell attachment to a non-RGD site in third fibronectin type III repeat of Tenascin. J Biol Chem. 1996;269:26691–26696. [PubMed] [Google Scholar]
- 12.Yokosaki Y, Matsuura N, Higashiyama S, Murakami I, Obara M, Yamakido M, et al. Identification of the ligand binding site for the integrin alpha9beta1 in the third fibronectin type III repeat of tenescin-C. J Biol Chem. 1998;273:11423–11428. doi: 10.1074/jbc.273.19.11423. [DOI] [PubMed] [Google Scholar]
- 13.Yokasaki Y, Sheppard D. Mapping of the cryptic integrin-binding site in osteopontin suggests a new mechanism by which thrombin can regulate inflammation and tissue repair. Trends in Cardiovascular Med. 2000;10:155–159. doi: 10.1016/s1050-1738(00)00055-4. [DOI] [PubMed] [Google Scholar]
- 14.Shinde AV, Bystroff C, Wang C, Vogelezang MG, Vincent PA, Hynes RO, et al. Identification of the peptide sequences within the EIIIA (EDA) segment of fibronectin that mediate integrin α9β1-dependent cellular activities. J Biol Chem. 2008;283:2858–2870. doi: 10.1074/jbc.M708306200. [DOI] [PubMed] [Google Scholar]
- 15.Staniszewska I, Sariyer IK, Lecht S, Brown MC, Walsh EM, Tuszynski GP, et al. Integrin α9β1 is a receptor for nerve growth factor and other neurotrophins. J Cell Sci. 2008;121:504–513. doi: 10.1242/jcs.000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta SK, Vlahakis NE. Integrin α9β1 mediates enhanced cell migration through nitric oxide synthase activity regulated by Src tyrosine kinase. J Cell Sci. 2009;122:2043–2054. doi: 10.1242/jcs.041632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Hsia DA, Lim S-T, Bernard-Trifilo JA, Mitra SK, Tanaka S, Hertog Jd, et al. Integrin α4β1 promotes focal adhesion kinase-independent cell motility via α4 cytoplasmic domain-specific activation of c-Src. Mol Cell Biol. 2005;25:9700–9712. doi: 10.1128/MCB.25.21.9700-9712.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arias-Salgado EG, Lizano S, Sarkar S, Brugge JS, Ginsberg MH, Shattil SJ. Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc Natl Acad Sci USA. 2003;100:13298–13302. doi: 10.1073/pnas.2336149100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacAuley A, Cooper JA. Structural differences between repressed and derepressed forms of p60c-src. Mol Cell Biol. 1989;9:2648–2656. doi: 10.1128/mcb.9.6.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baumgartner M, Radziwill G, Lorger M, Weiss A, Moelling K. c-Src-Mediated epithelial cell migration and invasion regulated by PDZ binding site. Mol Cell Biol. 2008;28:642–655. doi: 10.1128/MCB.01024-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giacone G, Zucali PA. Src as a potential thera-peutic target in non-small cell lung cancer. Ann Oncol. 2008;19:1219–1223. doi: 10.1093/annonc/mdn048. [DOI] [PubMed] [Google Scholar]
- 23.Vantygham SA, Allan AL, Postenka CO, Al-Katib W, Keeney M, Tuck AB, et al. A new model for lymphatic metastasis: development of a variant of the MDA-MB-468 human breast cancer cell line that aggressively metastasizes to lymph nodes. Clin Exp Metastasis. 2005;22:351–361. doi: 10.1007/s10585-005-0745-1. [DOI] [PubMed] [Google Scholar]
- 24.Timoshenko AV, Rastogi S, Lala PK. Migration promoting role of VEGF-C and VEGF-C binding receptors in human breast cancer cells. Br J Cancer. 2007;97:1090–1098. doi: 10.1038/sj.bjc.6603993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown MC, Staniszewska I, Lazarovici P, Tuszynski GP, Del Valle L, Marcinkiewicz C. Regulatory effect of nerve growth factor in α9β1 integrin-dependent progression of glioblastoma. Neuro-Oncol. 2008;10:968–980. doi: 10.1215/15228517-2008-047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wary KK, Mariotti A, Zurzolo C, Giancotti FG. A requirement for caveolin-1 and associated Fyn in integrin signaling and anchorage-dependent cell growth. Cell. 1998;94:625–634. doi: 10.1016/s0092-8674(00)81604-9. [DOI] [PubMed] [Google Scholar]
- 27.Vomastek T, Iwanicki M, Schaeffer H-J, Tarcsafalvi, Parson JT, Weber MJ. RACK1 targets the extracellular signal-regulated kinase/mitogen-activated protein kinase pathway to link integrin engagement with focal adhesion disassembly and cell motility. Mol Cell Biol. 2007;27:8296–8305. doi: 10.1128/MCB.00598-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korhonen R, Lahti A, Kankaaranta H, Moilanen E. Nitric oxide production and signaling in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:471–479. doi: 10.2174/1568010054526359. [DOI] [PubMed] [Google Scholar]
- 29.Kone BC, Kuncewicz T, Zhang W, Yu ZY. Protein interactions with nitric oxide synthases: controlling the right time, the right place, and the right amount of nitric oxide. Am J Physiol Renal Physiol. 2003;285:178–190. doi: 10.1152/ajprenal.00048.2003. [DOI] [PubMed] [Google Scholar]
- 30.Jenei V, Deevi RK, Adams CA, Axellson L, Hirst DG, Andersson T, et al. Nitric oxide produced in response to engagement of β2 integrins on human neutrophils activates the monomeric GTPase Rap1 and rap2 and promotes adhesion. J Biol Chem. 2006;281:35008–35020. doi: 10.1074/jbc.M601335200. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Brovkovych V, Brovkovych S, Tan F, Lee B-S, Sharma T, et al. Dynamic receptor-dependent activation of inducuble nitric oxide synthase by Erk-mediated phosphorylation of Ser745. J Biol Chem. 2007;282:32453–32461. doi: 10.1074/jbc.M706242200. [DOI] [PubMed] [Google Scholar]
- 32.Yang C, Wang Y, Zhao J, Zhang Y-L. Clinical significance of the expression of inducible nitric oxide synthase in non-small cell lung cancer. Chinese J Cancer Res. 2007;19:136–140. [Google Scholar]
- 33.Jadeski LC, Chakraborty C, Lala PK. Nitric oxide-mediated promotion of mammary tumour cell migration requires sequential activation of nitric oxide synthase and mitogen-activated protein kinase. Int J Cancer. 2003;106:496–504. doi: 10.1002/ijc.11268. [DOI] [PubMed] [Google Scholar]
- 34.Ridnour LA, Windhausen AN, Isenberg JS, Yeung N, Thomas DD, Vitek MP, et al. Nitric oxide regulates matrix metalloproteinase-9 activity by guanylyl-cyclase-dependent and independent pathways. Proc Natl Acad Sci USA. 2007;104:16898–16903. doi: 10.1073/pnas.0702761104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuncewicz T, Balakrishnan P, Snuggs MB, Kone BC. Specific association of nitric oxide synthase-2 with Rac isoforms in activated murine macrophages. Am J Physiol Renal Physiol. 2001;281:326–336. doi: 10.1152/ajprenal.2001.281.2.F326. [DOI] [PubMed] [Google Scholar]
- 36.Lopatin AN, Makhina EN, Nichols CG. Potassium channel by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 1994;372:366–369. doi: 10.1038/372366a0. [DOI] [PubMed] [Google Scholar]
- 37.deHart GW, Jin T, McCloskey DE, Pegg AE, Sheppard D. The α9β1 integrin enhances cell migration by polyamine-mediated modulation of an inward-rectifier potassium channel. Proc Natl Acad Sci USA. 2008;105:7188–7193. doi: 10.1073/pnas.0708044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen C, Young BA, Coleman CS, Pegg AE, Sheppard D. Spermidine/spermine N1-acetyltransferase specifically binds to the integrin α9 subunit cytoplasmic domain and enhances cell migration. J Cell Biol. 2004;167:161–170. doi: 10.1083/jcb.200312166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tennant BP, Cui Y, Tinker A, Clapp LH. Functional expression of inward rectifier potassium channels in cultured human pulmonary smooth muscle cells: evidence for a major role of Kir2.4 subunits. J Membrane Biol. 2007;213:19–29. doi: 10.1007/s00232-006-0037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Futschik M, Jeff A, Pattison A, Kasabov N, Sullivan M, Merrie A, et al. Gene expression profiling of metastatic and non-metastatic colorectal cancer cell lines. Genome Letters. 2002;1:26–34. [Google Scholar]
- 41.Chen C, Huang X, Atakilit A, Zhu Q-S, Corey SJ, Sheppard D. The Integrin alpha9beta1 contributes to granulopoiesis by enhancing granulocyte colony-stimulating factor receptor signaling. Immunity. 2006;25:895–906. doi: 10.1016/j.immuni.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 42.Wang SE, Xiang B, Zent R, Quaranta V, Pozzi A, Arteaga CL. Transforming growth factor β induces clustering of HER2 and integrins by activating Src-focal adhesion kinase and receptor association to the cytoskeleton. Cancer Res. 2009;69:475–482. doi: 10.1158/0008-5472.CAN-08-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamada KM, Even-Ram S. Integrin regulation of growth factor receptors. Nat Cell Biol. 2002;4:75–76. doi: 10.1038/ncb0402-e75. [DOI] [PubMed] [Google Scholar]
- 44.Garmy-Susini B, Varner JA. Roles of integrins in tumor angiogenesis and lymphangiogenesis. Lymphatic Res Biol. 2008;6:155–163. doi: 10.1089/lrb.2008.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Staniszewska I, Zaveri S, Del Valle L, Oliva I, Rothman VL, Croul SE, et al. Interaction of α9β1 integrin with throbospondin-1 promotes angiogenesis. Circ Res. 2007;100:1308–1316. doi: 10.1161/01.RES.0000266662.98355.66. [DOI] [PubMed] [Google Scholar]
- 46.Huang XZ, Wu JF, Ferrando R, Lee JH, Wang YL, Farese RV Jr, et al. Fatal bilateral chylothorax in mice lacking the integrin α9β1. Mol Cell Biol. 2000;20:5208–5215. doi: 10.1128/mcb.20.14.5208-5215.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bazigou E, Xie S, Chen C, Weston A, Miura N, Sorokin L, et al. Integrin α9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev Cell. 2009;17:175–186. doi: 10.1016/j.devcel.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koichi M, Tetsuro W, Akira S, Yasuhiro Y, Natsuko I, Shinji M, et al. Prox1 induces lymphatic endothelial differentiation via integrin alpha9 and other signaling cascades. Mol Biol cell. 2007;18:1421–1429. doi: 10.1091/mbc.E06-09-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young BA, Taooka Y, Liu S, Askins KJ, Yokosaki Y, Thomas SM, et al. The cytoplasmic domain of the integrin α9 subunit requires the adaptor protein to inhibit cell spreading but promotes cell migration in a paxillin independent manner. Mol Biol Cell. 2001;12:3214–3225. doi: 10.1091/mbc.12.10.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cai W, Chen X. Anti-angiogenic cancer therapy based on the integrin alphavbeta3 antagonism. Anticancer Agents Med Chem. 2006;6:407–428. doi: 10.2174/187152006778226530. [DOI] [PubMed] [Google Scholar]
- 51.Rust WL, Carper SW, Plopper GE. The promise of integrins as effective targets for anticancer agents. J Biomed Biotech. 2002;2:124–130. doi: 10.1155/S1110724302204015. [DOI] [PMC free article] [PubMed] [Google Scholar]