Abstract
Snail has moved into the fast lane of development and cancer biology with the epithelial-mesenchymal transition (EMT) emerging as one of the hottest topics in medical science within the past few years. Snail not only acts primarily as a key inducer of EMT but also plays an important role in cell survival, immune regulation and stem cell biology. This review focuses on the regulation of Snail and discusses the EMT-dependent and -independent functions of Snail in development and disease. Understanding the regulation and functional roles of Snail will shed new light on the mechanism of tumor progression and the development of novel cancer therapies.
Key words: Snail, EMT, stem cell, apoptosis, immunosuppression
The Snail family of zinc-finger transcription factors consist of Snail1 (Snail), Snail2 (Slug) and Snail3 (Smuc), which shares an evolutionary conserved role in mesoderm formation in vertebrates.1 These molecules are composed of a highly conserved carboxy-terminal region containing four to six C2H2-type zinc fingers, which mediate sequence-specific interactions with DNA promoters containing an E-box sequence (CAGGTG). The amino termini of all vertebrate Snail family members contain the evolutionarily conserved SNAG (Snail/Gfi) domain, which is essential for transcriptional repression. Drosophila Snail, however, lacks a SNAG domain; it has a consensus PxDLSx motif and exerts its repressive function through the interaction with the co-repressor CtBP. Thus, vertebrate and Drosophila Snail use either a SNAG domain or a CtBP binding motif to repress gene expression.1 Snail also directly recruits repressor complex to repress gene expression. For example, Peinado et al. demonstrated that Snail interacted with a co-repressor complex SIN3A, and histone deacetylases HDAC1 and HDAC2, to repress E-cadherin expression by modification of local chromatin structure.2 Moreover, other co-repressors, like CtBP,3 can also regulate the activity of Snail proteins. Snail exerts global effects on epithelial cell gene expression profiles, and as a result, it is involved in regulating EMT, cell survival or apoptosis, cell polarity and stem cell-like properties. Importantly, some epithelial cell genes are direct targets of Snail (Table 1).
Table 1.
Known targeted genes of Snail
| EMT-related genes | Cell polarity-related genes | Apoptosis-related genes | Tumor suppressor genes | Others |
| CDH1 | ||||
| CK17/18 | ||||
| SNAIL1 | Crumbs3 | TPS3 | PFKP | RAB25 |
| VDR | Lgl2 | BID | RKIP | SLC27A2 |
| Occludin | dlg3 | DFF40 | CYLD | EGR1 |
| HNF4a | Runx2 | |||
| HNF1b |
Snail is mainly expressed in neoplastic epithelial cells. However, recent studies show that Snail is also expressed in other cell types, such as fibroblasts localized in damaged or carcinomatous tissues, neoplastic mesenchyme cells and macrophages in wounded and inflamed tissues.4–6
Regulation of Snail
Snail is a highly unstable protein and is dually regulated by protein stability and cellular location. Expression of Snail is regulated by an integrated and complex signaling network at the transcriptional and post-transcriptional level; this network includes integrin-linked kinase (ILK), phosphatidylinositol 3-kinase (PI3-K), mitogen-activated protein kinases (MAPKs), glycogen synthase kinase 3-beta (GSK-3β) and NFκB pathways.7 Receptor tyrosine kinase signaling, such as fibroblast growth factor (FGF) or epidermal growth factor (EGF) induces Snail expression by suppressing the activity of GSK-3β. Interestingly, many signaling pathways involved in embryonic development can regulate the expression of Snail. For example, the TGFβ/Smad pathway, which induces EMT in hepatocytes, epithelial and mesothelial cells, transcriptionally induces Snail expression by directly binding to the Snail promoter. In addition, Notch signaling deploys two distinct mechanisms that act in synergy to control the expression of Snail.8 First, Notch directly upregulates Snail expression by recruiting the Notch intracellular domain to the Snail promoter; and second, Notch potentiates hypoxia-inducible factor 1α (HIF1α) recruitment to the lysyl oxidase (LOX) promoter and elevates the hypoxia-induced upregulation of LOX, which stabilizes Snail by protecting it against protein degradation. LOXL2 also attenuates GSK-3β-dependent Snail degra-dation through oxidation of K98 and/or K137 of Snail to induce a conformational change that masks the GSK-3β-dependent regulatory motif.9 Furthermore, Wnt can suppress the activity of GSK-3β, and thus stabilize Snail and β-catenin at the protein level.10,11 Snail expression and protein level can also be regulated by the NFκB pathway via transcriptional and post-translational mechanisms. First, Snail expression is directly activated by the NFκB homologue Dorsal in drosophila.12 NFκB also binds the human Snail promoter between −194 and −78 bp and increases the transcription of Snail.13 In addition, GSK-3β inhibition stimulates the transcription of Snail by activating the NFκB pathway.14 In our recent study, we found that the inflammatory cytokine TNFα is the major signal that induces Snail stabilization.15 TNFα/NFκB-stabilized Snail is mediated by the transcriptional induction of CSN2, which inhibits the phosphorylation and ubiquitylation of Snail by disrupting the binding of Snail with GSK-3β and β-Trcp, and results in the stabilization of Snail in a non-phosphorylated and non-ubiquitylated functional state.
Snail has to translocate to the nucleus to exert its function, and cytoplasmic Snail has a very short half-life as it is targeted for ubiquitin-mediated proteasome degradation by GSK-3β-induced phosphorylation. The subcellular localization of Snail can be modulated by phosphorylation involving the p21-activated kinase 1 (PAK1).16 Pak1 phosphorylates Snail at S246 and favors the nuclear localization of Snail and, thus enhances its transcription activity. The expression of the zinc transporter LIV1, downstream of signal transducer and activator of transcription 3 (STAT3), controls the nuclear import of Snail in zebrafish embryos.17 Export of Snail is controlled by phosphorylation of a ser-rich sequence adjacent to the nuclear export sequence (NES). GSK-3β phosphorylates the NES of Snail and induces its export to the cytoplasm.18 Exportins, like CRM1, are involved in exporting phosphorylated Snail from the nucleus to the cytoplasm.19
Snail in Development
Snail was first described in Drosophila melanogaster, where it acts as a repressor to inhibit the expression of neurocetodermal genes such as single-minded and shotgun and as such it is essential for the formation of the mesoderm and neural crest.20 As in fly, Snail is a crucial factor in primary mesenchyme cells (PMCs) ingression. Snail inhibits E-cadherin transcription and promotes cadherin endocytosis as well as delamination of PMCs by EMT.21 The Snail gene is critical for gastrulation in normal development of mice.22 Homozygous knockout of Snail in mice is lethal as embryos fail to produce mesoderm, which results in a failure to gastrulate during development. Human Snail has been mapped to chromosome 20q13, an amplicon that is commonly found in patients with breast cancer.
Snail and EMT
EMT is a complex stepwise phenomenon that occurs during embryonic development and tumor progression, and it also plays a crucial role in chronic inflammatory and fibrogenic disease.23 The loss of E-cadherin is the hallmark of EMT. Several transcription factors have been implicated in the transcriptional repression of E-cadherin, including zinc finger proteins of the Snail/Slug family, Twist, ZEB1, SIP1, and the basic helix-loop-helix factor E12/E47. Snail is the first discovered and most important transcriptional repressor of E-cadherin. It functions as a suppressor of the transcription of shotgun (an E-cadherin homologue) to control embryogenesis in Drosophila.1,24 Snail also plays a fundamental role in EMT by suppressing E-cadherin expression in mammalian cells. Overexpression of Snail was recently found in both epithelial and endothelial cells of invasive breast cancer but was undetectable in normal breast.25,26 The expression of Snail in breast carcinomas is associated with metastasis, tumor recurrence and poor prognosis.3 Snail also downregulates the expression of other epithelial molecules, including Claudins, Occludins and Muc1 and induces the expression of genes associated with a mesenchymal and invasive phenotype, such as fibronectin and MMP9.
Snail in Tumor Recurrence
The propensity of breast cancers to recur following treatment is the most important determinant of clinical outcome. In fact, breast cancer recurrence is typically an incurable disease. For women with breast cancer, disseminated tumor cells are frequently present at the time of diagnosis. Residual tumor cells can be detected in the bone marrow of more than 30% of breast cancer patients lacking any clinical or histopathological signs of metastasis. These residual tumor cells have the ability to survive in a dormant state following treatment. Almost a quarter of breast cancer patients without overt lymph node metastases and that appear cured by surgery, radiation, and adjuvant therapy may have tumor recurrence at local or distant lesions 10 to 20 years later. Using a mammary-specific tetracycline-inducible HER2/neu transgenic mouse model, Moody et al. found that high tumor recurrence correlated with strong upregulation of the transcription factor Snail.27 Primary breast cancer cells transduced with Snail and grafted subcutaneously in mice show rapid tumor recurrence upon inactivation of the HER2/neu pathway, indicating that Snail contributes functionally to tumor recurrence in vivo.27 In agreement with this finding, relapsed tumors are associated with robust Snail expression and loss of E-cadherin in the Wnt1 transgenic mouse model.28 This is further confirmed by clinical data that high expression of Snail is a strong predictor of tumor recurrence and enhances risk stratification and prognostication in superficial bladder tumors and LSCC tumors.29,30
Snail in Immune Regulation
Snail also induces immunosuppression and immunoresistance through immunosuppressive cytokines, regulatory T cells, impaired dendritic cells, and cytotoxic T lymphocyte resistance. Snail+ melanoma cells appear to generate immunosuppressive nTreg-like CD4+CD25− cells rather than to increase CD4+CD25+ nTreg cells, partly by mediating Treg-inducible cytokines, such as TGFβ and TSP1.31 Snail can directly regulate the immune reaction. For example, TGFβ induces the expression of Snail in macrophages, which are recruited to sites of injury and inflammation to mediate the inflammatory response. Therefore, the expression of Snail is a potent marker for migrating macrophages during acute inflammation and early wound healing.6 Oral keratinocytes that express Snail upregulate the production of proinflammatory cytokines and cyclooxygenase 2 (COX2) and acquire an enhanced ability to attract monocytes and to invade a dense interstitial collagen matrix.32 This implies that Snail contributes to malignancy by impeding terminal differentiation at the early stages while increasing invasion and inflammation in later stages.
Snail in Survival
Snail plays an important role in cell cycle and survival. During embryonic development, Snail represses the transcription of Cyclin D2 and increases the expression of p21Cip1/WAF1 to regulate the early to late G1 transition and the G1/S checkpoint.33 Snail also confers resistance to cell death induced by serum depletion or TNFα administration by activating the MAPK and PI3K survival pathways. Conversely, knockdown of Snail expression by an antisense construct increases cell death in colon cancer in a mouse model.34 Moreover, Snail expression enhances resistance to cell death elicited by DNA damage. The expression of Snail family members has been associated with the acquisition of resistance to several types of programmed cell death. For instance, both Snail and Slug protect hematopoietic cells from γ radiation-induced apoptosis.35,36 Snail and Slug promote cell survival after genotoxic stress through direct transcriptional repression of genes involved in programmed cell death, such as BID and caspase-6.37 Snail suppresses the expression of a subset of genes, which are required for p53-mediated apoptosis under stress condition, to promote cell survival.38 In addition, Snail binds to the PTEN promoter to repress its expression and thus results in resistance to gamma radiation-induced apoptosis.39 Expression of Snail also induces resistance to anoikis—the form of apoptosis provoked by a loss of anchorage to appropriate substrates.
Snail and Stem Cells
Physiologically, stem cells are the basis for tissue homeostasis in the adult organism.40 Recently, a number of studies have provided evidence of a role for Snail in the preservation of stem cell function. Through several elegant experiments, Snail was shown to play a fundamental role in controlling bone mass and bone homeostasis by acting as a repressor of both Runx2 and VDR transcription.41 The expression of Snail is tightly regulated in bone development and its activity on osteoblasts regulates the course of bone cell differentiation to ensure normal bone remodeling. In agreement with this, Snail, along with the transcription factors, Asense and Deadpan, control genes involved in neural stem cell self-renewal and multipotency.42 Cumulatively, these studies identify that Snail is an important factor to the preservation of stem cell function.
Increasing evidence suggests that tumors are generated and maintained by a small subset of undifferentiated cells with the ability to self-renew and differentiate into the bulk tumor population.43,44 Such cells are called cancer stem cells (CSCs) and are considered to be critically important for tumor proliferation, invasion and metastasis. Many cancers, including colon, breast, brain, head and neck, pancreatic and hematopoietic malignancies contain caner stem cells. The cancer stem cell concept also proposes the existence of two forms of CSCs in tumor progression, a stationary and an invasive CSC. Stationary CSCs remain embedded in the epithelial tissue and cannot disseminate. In contrast, invasive CSCs are located predominantly at the tumor-host interface. A similar correlation has been observed between EMT induction and the acquisition of certain stem cell-like traits in immortalized nontumorigenic mammary epithelial cells. Furthermore, EMT creates cells that act as progenitors of many different tissues during development. For example, EMT generates mesoderm, which gives rise to a wide range of cell types, including muscle, bone and connective tissues; EMT also creates neural crest which produces glial and neuronal cells, adrenal glandular tissues, pigment-containing cells of the epidermis, and skeletal and connective tissues. These findings indicate that EMT acquires traits reminiscent of those expressed by stem cells and the genetic program of EMT possesses the ability to generate various CSCs in solid tumors. In addition, CD44+/CD24− cells, purified from normal and malignant breast cancer tissue, exhibit features of an EMT, such as reduced expression of E-cadherin, increased expression of fibronectin and vimentin, and robust levels of FOXC2, Twist, Snail and Slug. Weinberg’s group demonstrated recently that EMT conferred stem cell-like properties on tumor cells.45,46 Ectopic expression of Snail greatly increases the tumor cells’ ability to form mammospheres, a property associated with mammary epithelial stem cells, tumor-seeding ability and stem cell surface marker expression. Furthermore, breast tumors resistant to chemo- and endocrine therapies are enriched with cells bearing CSC signatures and EMT markers in post-treatment specimens. In ovarian cancer cells, Snail effectively mediates cell survival and is involved in the acquisition of stem cell-like characteristics.38 Snail and Slug indirectly regulate the activation of a self-renewal stemness program by upregulating NANOG, HDAC1, TCF4, KLF4, HDAC3 and GPC3; by inducing the expression of other “stem cell markers,” including Oct4, Bmi1 and nestin; and by stimulating a 4–5 fold increase in the number of ovarian CSC marker CD44+CD117+ cells.
CSC representation may be a function of cell type of origin, stromal microenvironment, accumulated somatic mutations and stage of malignant progression reached by a tumor. Carcinoma cells at the invasive edges of tumors have been observed to undergo EMT under the influence of microenvironmental signals that they receive from closely apposed stromal tissue. This is reminiscent of embryogenesis, during which microenvironmental signals include TGFβ, Wnt and Notch. Thus, these embryonic development pathways link two important components of tumor progression: the existence of cancer stem cells and the initiation of EMT. These findings inferring an essential role for Snail are in perfect agreement with the properties of CSCs.
Future Prospects
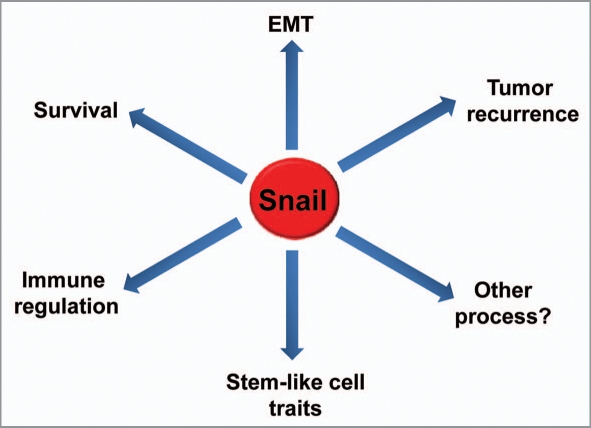
Snail displays a broad spectrum of biological functions (Fig. 1). In addition to the regulation of cell movements and adhesion, cell proliferation and survival, immune suppression and generation of stem cell properties, there are probably several more Snail-regulated processes waiting to be discovered. EMT and MET (mesenchymal-epithelial transition) are dynamic and reversible processes that are influenced by microenvironmental signals and stimuli. EMT provides considerable degree of plasticity and reversibility for cells to differ-entiate or de-differentiate between cancer epithelial cells and mesenchymal cells (or CSCs). One major challenge is how the EMT and MET programs are epigenetically regulated by extrinsic signals. For example, it is known that Snail recruits specific chromatin-modifying complexes to the E-cadherin promoter to silence the expression of E-cadherin during tumor progression and EMT, however, how these factors cooperate and whether these factors also control the expression of other genes during EMT remains to be established. Further investigations will shed new insight on the regulation of Snail in development and disease and will provide us with novel therapeutic approaches for treating metastatic cancer.
Figure 1.
Snail displays a broad spectrum of biological functions in the epithelial-mesenchymal transition (EMT), cell survival, immune regulation, tumor recurrence and stem cell biology.
Acknowledgements
We thank all the investigators that contribute to the Snail field and we apologize to those whose work is important but that we are unable to cite here. Our study is supported by grants from NIH (RO1CA125454), the Susan G. Komen Foundation (KG081310) and the Mary Kay Ash Foundation (to B.P. Z.).
Abbreviations
- EMT
epithelial-mesenchymal transition
- CtBP
corepressor C-terminal binding protein
- CSN2
COP9 signalosome 2
- ILK
integrin-linked kinase
- PI3-K
phosphatidylinositol 3-kinase
- MAPKs
mitogen-activated protein kinases
- GSK-3β
glycogen synthase kinase 3-beta
- FGF
fibroblast growth factor
- EGF
epidermal growth factor
- HIF1α
hypoxia-inducible factor 1α
- LOX
lysyl oxidase
- PAK1
p21-activated kinase 1
- STAT3
signal transducer and activator of transcription 3
- NES
nuclear exports sequence
- CSCs
cancer stem cells
- HDAC
histone deacetylases
- TCF4
transcription factor 4
- KLF4
krüppel-like factors 4
- GPC3
glypican-3
- CDH1
E-cadherin
- DFF
DNA fragmentation factor
- EGR1
early growth response 1
- PFKP
phosphofructokinase, platelet type
- PMCs
primary mesenchyme cells
- SCL27A2
solute carrier family 27, member2
- CYLD
cylindromatosis
- RKIP
Raf kinase inhibitor protein
- VDR
vitamin D receptor
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/10943
References
- 1.Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–166. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 2.Peinado H, Ballestar E, Esteller M, Cano A. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol. 2004;24:306–319. doi: 10.1128/MCB.24.1.306-319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 4.Rowe RG, Li XY, Hu Y, Saunders TL, Virtanen I, Garcia de Herreros A, et al. Mesenchymal cells reactivate Snail1 expression to drive three-dimensional invasion programs. J Cell Biol. 2009;184:399–408. doi: 10.1083/jcb.200810113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franci C, Takkunen M, Dave N, Alameda F, Gomez S, Rodriguez R, et al. Expression of Snail protein in tumor-stroma interface. Oncogene. 2006;25:5134–5144. doi: 10.1038/sj.onc.1209519. [DOI] [PubMed] [Google Scholar]
- 6.Hotz B, Visekruna A, Buhr HJ, Hotz HG. Beyond epithelial to mesenchymal transition: A novel role for the transcription factor snail in inflammation and wound healing. J Gastrointest Surg. 2010;14:388–397. doi: 10.1007/s11605-009-1068-3. [DOI] [PubMed] [Google Scholar]
- 7.de Craene B, van Roy F, Berx G. Unraveling signalling cascades for the Snail family of transcription factors. Cell Signal. 2005;17:535–547. doi: 10.1016/j.cellsig.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci USA. 2008;105:6392–6397. doi: 10.1073/pnas.0802047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peinado H, Del Carmen Iglesias-de la Cruz M, Olmeda D, Csiszar K, Fong KS, Vega S, et al. A molecular role for lysyl oxidase-like 2 enzyme in snail regulation and tumor progression. EMBO J. 2005;24:3446–3458. doi: 10.1038/sj.emboj.7600781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yook JI, Li XY, Ota I, Fearon ER, Weiss SJ. Wnt-dependent regulation of the E-cadherin repressor snail. J Biol Chem. 2005;280:11740–11748. doi: 10.1074/jbc.M413878200. [DOI] [PubMed] [Google Scholar]
- 11.Yook JI, Li XY, Ota I, Hu C, Kim HS, Kim NH, et al. A Wnt-Axin2-GSK3beta cascade regulates Snail1 activity in breast cancer cells. Nat Cell Biol. 2006;8:1398–1406. doi: 10.1038/ncb1508. [DOI] [PubMed] [Google Scholar]
- 12.Ip YT, Park RE, Kosman D, Yazdanbakhsh K, Levine M. Dorsal-twist interactions establish snail expression in the presumptive mesoderm of the Drosophila embryo. Genes Dev. 1992;6:1518–1530. doi: 10.1101/gad.6.8.1518. [DOI] [PubMed] [Google Scholar]
- 13.Barbera MJ, Puig I, Dominguez D, Julien-Grille S, Guaita-Esteruelas S, Peiro S, et al. Regulation of Snail transcription during epithelial to mesenchymal transition of tumor cells. Oncogene. 2004;23:7345–7354. doi: 10.1038/sj.onc.1207990. [DOI] [PubMed] [Google Scholar]
- 14.Bachelder RE, Yoon SO, Franci C, de Herreros AG, Mercurio AM. Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: implications for the epithelial-mesenchymal transition. J Cell Biol. 2005;168:29–33. doi: 10.1083/jcb.200409067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM, Zhou BP. Stabilization of snail by NFkappaB is required for inflammation-induced cell migration and invasion. Cancer Cell. 2009;15:416–428. doi: 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Z, Rayala S, Nguyen D, Vadlamudi RK, Chen S, Kumar R. Pak1 phosphorylation of snail, a master regulator of epithelial-to-mesenchyme transition, modulates snail’s subcellular localization and functions. Cancer Res. 2005;65:3179–3184. doi: 10.1158/0008-5472.CAN-04-3480. [DOI] [PubMed] [Google Scholar]
- 17.Yamashita S, Miyagi C, Fukada T, Kagara N, Che YS, Hirano T. Zinc transporter LIVI controls epithelial-mesenchymal transition in zebrafish gastrula organizer. Nature. 2004;429:298–302. doi: 10.1038/nature02545. [DOI] [PubMed] [Google Scholar]
- 18.Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, et al. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 19.Dominguez D, Montserrat-Sentis B, Virgos-Soler A, Guaita S, Grueso J, Porta M, et al. Phosphorylation regulates the subcellular location and activity of the snail transcriptional repressor. Mol Cell Biol. 2003;23:5078–5089. doi: 10.1128/MCB.23.14.5078-5089.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alberga A, Boulay JL, Kempe E, Dennefeld C, Haenlin M. The snail gene required for mesoderm formation in Drosophila is expressed dynamically in derivatives of all three germ layers. Development. 1991;111:983–992. doi: 10.1242/dev.111.4.983. [DOI] [PubMed] [Google Scholar]
- 21.Wu SY, McClay DR. The Snail repressor is required for PMC ingression in the sea urchin embryo. Development. 2007;134:1061–1070. doi: 10.1242/dev.02805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carver EA, Jiang R, Lan Y, Oram KF, Gridley T. The mouse snail gene encodes a key regulator of the epithelial-mesenchymal transition. Mol Cell Biol. 2001;21:8184–8188. doi: 10.1128/MCB.21.23.8184-8188.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 24.Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–3161. doi: 10.1242/dev.01907. [DOI] [PubMed] [Google Scholar]
- 25.Martin TA, Goyal A, Watkins G, Jiang WG. Expression of the transcription factors snail, slug and twist and their clinical significance in human breast cancer. Ann Surg Oncol. 2005;12:488–496. doi: 10.1245/ASO.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 26.Parker BS, Argani P, Cook BP, Liangfeng H, Chartrand SD, Zhang M, et al. Alterations in vascular gene expression in invasive breast carcinoma. Cancer Res. 2004;64:7857–7866. doi: 10.1158/0008-5472.CAN-04-1976. [DOI] [PubMed] [Google Scholar]
- 27.Moody SE, Perez D, Pan TC, Sarkisian CJ, Portocarrero CP, Sterner CJ, et al. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005;8:197–209. doi: 10.1016/j.ccr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Debies MT, Gestl SA, Mathers JL, Mikse OR, Leonard TL, Moody SE, et al. Tumor escape in a Wnt1-dependent mouse breast cancer model is enabled by p19Arf/p53 pathway lesions but not p16 Ink4a loss. J Clin Invest. 2008;118:51–63. doi: 10.1172/JCI33320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruyere F, Namdarian B, Corcoran NM, Pedersen J, Ockrim J, Voelzke BB, et al. Snail expression is an independent predictor of tumor recurrence in superficial bladder cancers. Urol Oncol. 2009 doi: 10.1016/j.urolonc.2008.11.005. In press. [DOI] [PubMed] [Google Scholar]
- 30.Peinado H, Moreno-Bueno G, Hardisson D, Perez-Gomez E, Santos V, Mendiola M, et al. Lysyl oxidase-like 2 as a new poor prognosis marker of squamous cell carcinomas. Cancer Res. 2008;68:4541–4550. doi: 10.1158/0008-5472.CAN-07-6345. [DOI] [PubMed] [Google Scholar]
- 31.Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell. 2009;15:195–206. doi: 10.1016/j.ccr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 32.Lyons JG, Patel V, Roue NC, Fok SY, Soon LL, Halliday GM, et al. Snail upregulates proinflammatory mediators and inhibits differentiation in oral keratinocytes. Cancer Res. 2008;68:4525–4530. doi: 10.1158/1078-0432.CCR-07-6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vega S, Morales AV, Ocana OH, Valdes F, Fabregat I, Nieto MA. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 2004;18:1131–1143. doi: 10.1101/gad.294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roy HK, Iversen P, Hart J, Liu Y, Koetsier JL, Kim Y, et al. Downregulation of SNAIL suppresses MIN mouse tumorigenesis: modulation of apoptosis, proliferation and fractal dimension. Mol Cancer Ther. 2004;3:1159–1165. [PubMed] [Google Scholar]
- 35.Perez-Mancera PA, Perez-Caro M, Gonzalez-Herrero I, Flores T, Orfao A, de Herreros AG, et al. Cancer development induced by graded expression of Snail in mice. Hum Mol Genet. 2005;14:3449–3461. doi: 10.1093/hmg/ddi373. [DOI] [PubMed] [Google Scholar]
- 36.Inoue A, Seidel MG, Wu W, Kamizono S, Ferrando AA, Bronson RT, et al. Slug, a highly conserved zinc finger transcriptional repressor, protects hematopoietic progenitor cells from radiation-induced apoptosis in vivo. Cancer Cell. 2002;2:279–288. doi: 10.1016/s1535-6108(02)00155-1. [DOI] [PubMed] [Google Scholar]
- 37.Kajita M, McClinic KN, Wade PA. Aberrant expression of the transcription factors snail and slug alters the response to genotoxic stress. Mol Cell Biol. 2004;24:7559–7566. doi: 10.1128/MCB.24.17.7559-7566.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurrey NK, Jalgaonkar SP, Joglekar AV, Ghanate AD, Chaskar PD, Doiphode RY, et al. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells. 2009;27:2059–2068. doi: 10.1002/stem.154. [DOI] [PubMed] [Google Scholar]
- 39.Escriva M, Peiro S, Herranz N, Villagrasa P, Dave N, Montserrat-Sentis B, et al. Repression of PTEN phosphatase by Snail1 transcriptional factor during gamma radiation-induced apoptosis. Mol Cell Biol. 2008;28:1528–1540. doi: 10.1128/MCB.02061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 41.de Frutos CA, Dacquin R, Vega S, Jurdic P, Machuca-Gayet I, Nieto MA. Snail1 controls bone mass by regulating Runx2 and VDR expression during osteoblast differentiation. EMBO J. 2009;28:686–696. doi: 10.1038/emboj.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Southall TD, Brand AH. Neural stem cell transcriptional networks highlight genes essential for nervous system development. EMBO J. 2009;28:3799–3807. doi: 10.1038/emboj.2009.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 44.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, et al. Cancer stem cells-perspectives on current status and future directions: AACR workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 45.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mani SA, Yang J, Brooks M, Schwaninger G, Zhou A, Miura N, et al. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc Natl Acad Sci USA. 2007;104:10069–10074. doi: 10.1073/pnas.0703900104. [DOI] [PMC free article] [PubMed] [Google Scholar]