Abstract
Migration of cells is one of the most essential prerequisites to form higher organisms and depends on a strongly coordinated sequence of processes. Early migratory events include substrate sensing, adhesion formation, actin bundle assembly and force generation. While substrate sensing was ascribed to filopodia, all other processes were believed to depend mainly on lamellipodia of migrating cells. In this work we show for motile keratinocytes that all processes from substrate sensing to force generation strongly depend on filopodial focal complexes as well as on filopodial actin bundles. In a coordinated step by step process, filopodial focal complexes have to be tightly adhered to the substrate and to filopodial actin bundles to enlarge upon lamellipodial contact forming classical focal adhesions. Lamellipodial actin filaments attached to those focal adhesions originate from filopodia. Upon cell progression, the incorporation of filopodial actin bundles into the lamellipodium goes along with a complete change in actin cross-linker composition from filopodial fascin to lamellipodial α-actinin. α-Actinin in turn is replaced by myosin II and becomes incorporated directly behind the leading edge. Myosin II activity makes this class of actin bundles with their attached FAs the major source of force generation and transmission at the cell front. Furthermore, connection of FAs to force generating actin bundles leads to their stabilization and further enlargement. Consequently, adhesion sites formed independently of filopodia are not connected to detectable actin bundles, transmit weak forces to the substrate and disassemble within a few minutes without having been increased in size.
Key words: filopodia, focal complexes, cell migration, focal adhesion, myosin II, force, actin flow, maturation
Introduction
Migration plays a vital role for most cells and is essential for processes as immune response, wound healing or embryogenesis. In cell migration, a complex mechanism must be strictly regulated. Many processes in substrate sensing, adhesion formation and generation of tracking forces are just some of the early events that need to be interactively combined to an overall working system.1–3 Most essential for migration of mammalian cells is the formation of new adhesion sites. These sites are typically composed of integrins as transmembrane proteins connecting the extracellular matrix with intracellular adhesion molecules. Up to now more than 100 different molecules have been identified in adhesion structures.4 Although their roles are diverse, their main function is the formation of a stable but still dynamic connection between integrins and the actin cytoskeleton enabling the transmission of cytoskeletal forces to the cell environment.
In locomoting cells, new adhesions are formed at the cell front and disassemble at the cell’s rear. This is regulated by cell polarity which guides cell movement. Filopodia and the lamellipodium are the two main structures forming membrane extensions with first contact to new substrate areas in direction of migration. The protrusion of the thin sheet-like lamellipodium is driven by actin polymerization.5,6 Furthermore, for many cell types the lamellipodium is described as the origin of adhesion site formation.7 In addition, for cell types forming filopodia latest research has shown an important role of these finger-like extensions in cell spreading8 and adhesion assembly during migration.9,10 Here, nearly all lamellipodial focal adhesions (FAs) evolve from small focal complexes (FXs) originally assembled within filopodia and just increased in size when overgrown by the lamellipodial leading edge. Filopodia are dynamic structures that enclose a bundle of tightly packed actin fibers arising from the tip complex and cross-linked by fascin.11 In addition to filopodial FX stabilization and maturation during migration, filopodial actin bundles also become incorporated into the lamellipodial actin meshwork where they provide most of all centripetally directed actin filaments within the lamellipodium.12 Even unstable and therefore retracted filopodia have been shown to play an important role in concave lamellipodial actin bundle formation.9
The connection of the cellular cytoskeleton with the extracellular matrix via FAs provides an anchorage for force transmission to pull the cell body forward. During migration cell forces are mainly concentrated at the rearmost sides of the cell directed centripetally. In addition, traction forces could also be identified within the lamellipodium pointing towards the center of the cell.13,14 Most important for traction force generation are molecular motors, i.e., myosin molecules, which are incorporated into actin bundles connected to FAs.15 Myosin containing actin bundles are often denoted as stress fibers although stress fibers in motile and sessile cells are structured very differently.16 Many specific proteins are known to organize the structure of stress fibers in sessile cells as e.g., palladin, myosin and α-actinin displaying a periodic distribution along the filaments.17–20 Upon formation mainly through actin polymerization at adhesion sites,21 these proteins become integrated hierarchically. Here, α-actinin functions as a placeholder for myosin which partially replaces α-actinin slightly after assembly.19
Cell forces additionally seem to play an important role in FA maturation which in turn is essential for regulating cell migration. No matter whether FXs were originally assembled in the lamellipodium or within filopodia, they exhibit a distinct protein composition. Upon maturation this set of proteins changes for lamellipodial FXs7,22,23 whereas filopodial FX protein composition seems to be rather complete and stable during maturation.10 However, independent from their point of origin FXs undergo shape changes from small dot-like structures to large elongated FAs transmitting forces that pull on these structures.24 This suggestion is encouraged by experiments applying external forces on FXs leading to an increase in area.25,26 Additionally, experiments on migrating keratinocytes showed a correlation between the maturation state of FAs and the amplitudes of applied forces.13 Other studies discuss additional mechanisms for assembly and maturation of nascent adhesions. They postulate a key role of actin polymerization at adhesion sites for accumulation of α-actinin and myosin II during FA maturation.27
In this study our goals were to reveal the mechanism and structures underlying the maturation of FAs during migration and to determine the role of cell forces in this process. We could show that stable FAs always originate from filopodial FXs whereas unstable FAs are mostly formed within the lamellipodium. Furthermore, stable FAs are permanently connected to distinct filopodially derived actin bundles. In the filopodium these bundles are cross-linked by fascin which is later replaced by incorporating α-actinin, palladin and myosin II in a coordinated step by step process. Incorporation of myosin II in turn leads to increased force application to the substrate via stable FAs. We present for the first time direct evidences for a force dependent FA maturation as a naturally appearing mechanism during migration. On the basis of these data, we developed an expanded model for FAs maturation depending on filopodially originated actin bundle contraction.
Results
Recent experiments have proven that many lamellipodial focal adhesions (FAs) derive from stably attached filopodial focal complexes (FXs).9,10,28 Unfortunately, structural as well as functional analyses over the whole lifetime of lamellipodial structures of filopodial origin are rare. For this reason we first analyzed stably adhered filopodial FXs in locomoting keratinocytes over time. As already shown before,10 filopodial FX remained in place when overgrown by the lamellipodial leading edge to form classical FAs just by enlargement (Fig. 1A, Suppl. movie 1, white arrows). 65% of all analyzed FAs (120/184) grew from FXs directly behind filopodia. These FAs displayed a constant increase in size and persisted over several minutes. In contrast, 35% of lamellipodial FAs were formed without filopodial influence. These adhesions developed within the lamellipodium but remained small and disassembled already after a few minutes (Fig. 1B, Suppl. movie 1, black arrows).
Figure 1.
Dependence of stable FAs on filopodia. Motile keratinocytes were transfected with GFP-vinculin and analyzed by TIRF combined with differential interference contrast (DIC) microscopy. Stable adhesions showed a clear dependence on the localization of filopodia (A) whereas unstable ones were always formed in a filopodia independent manner (B). Squares in the overview images indicate the zoomed areas in the adjoining time series. White arrows indicate the origin of adhesions. The last images show the time point of adhesion disassembly. Time is given in seconds. Note the short life span of filopodia independent FAs. Scale bars = 10 µm.
Since filopodial FX were attached to filopodial actin bundles we also analyzed attachment to actin bundles of FAs originated from filopodial FX. Immunofluorescent staining against actin and vinculin revealed a structural extension of filopodial actin bundles into the lamellipodium (Fig. 2, white arrows). Furthermore, all FAs originated from filopodial FX remained connected to prominent actin bundles. In contrast, lamellipodial adhesions formed independently from filopodia were not connected to such distinct actin bundles (Suppl. movie 4, red arrowhead).
Figure 2.
Localization of FAs along filopodial originated actin bundles. Keratinocytes were cultured for one day and subsequently fixed and stained for actin (red) and vinculin (green). Red fluorescence was recorded by epi-fluorescence microscopy and green fluorescence in TIRF mode. The overview image is additionally overlaid with a DIC image. White arrows indicate adhesions with direct conjunction to filopodial actin. The box marks the zoomed inset. Note here the connection of FAs with actin bundles originated from filopodia. Scale bar = 10 µm.
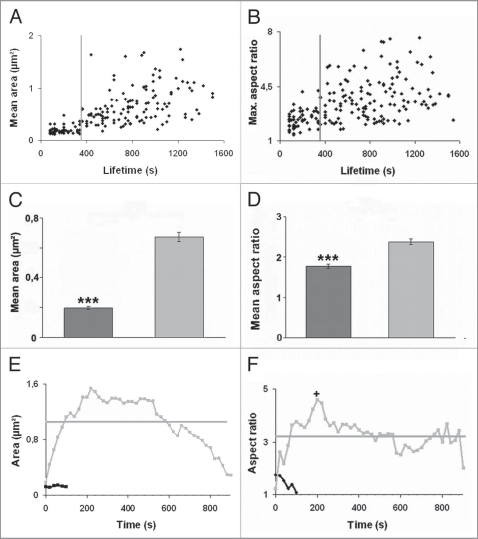
Given data argue for the presence of two different types of lamellipodial adhesion sites. We therefore analyzed all lamellipodial adhesions in more detail by measuring their area and aspect ratio over their whole lifespan. Mean FA areas (Fig. 3A) and maximal FA aspect ratios (Fig. 3B) revealed, when plotted against FA lifetimes, an increasing mean area as well as enhanced aspect ratio the longer FAs persist (Fig. 3A and B). Interestingly, FAs with lifetimes of not more than 6 min were almost constant in mean area as well as aspect ratio. Taking this lifetime as threshold (grey line in Fig. 3A and B) to classify the two groups of FAs as stable and unstable ones, we found that 86% (111/129) of stable focal adhesion were originated from filopodial FXs. In contrast, 84% (46/55) of these lamellipodial FAs with lifetimes below 6 min were formed without filopodial attendance. These results demonstrate the high value of FA lifetime to discriminate between the two groups of lamellipodial FAs. Sorted like this, stable adhesions (n = 129, Table 1) exhibited a mean lifetime of about 13.9 min (s.e. = 0.5 min), a mean area of 0.68 µm2 (s.e. = 0.03 µm2) and a mean aspect ratio of 2.38 (s.e. = 0.07). In contrast, unstable FAs (n = 55) revealed a mean lifetime of about 2.9 minutes (s.e. = 0.2 min), a mean area of 0.20 µm2 (s.e. = 0.01 µm2) and a mean aspect ratio of 1.77 (s.e. = 0.05). All mean values for the two groups of FAs were significantly different (p value < 0.001) (Fig. 3C and D). Exemplarily, areas (Fig. 3E) and aspect ratios (Fig. 3F) of a single stable as well as unstable FA are shown over their whole lifetime corroborating the strong difference in morphological behavior between stable (i.e., mostly originated from filopodial FXs) and unstable (i.e., assembled without filopodial contact) lamellipodial FAs.
Figure 3.
Correlation between focal adhesion growth and stability. Keratinocytes were grown for 24 h and transfected with GFP-vinculin. Afterwards cells were motility stimulated and mean area (A) as well as maximal aspect ratio (B) of each FA (n = 184, out of 4 cells) were analyzed by TIRF microscopy over 30 min and plotted against their lifetimes. Enhanced areas (A) and aspect ratios (B) went along with a long lifetime. Grey lines indicate the threshold for classification into stable and unstable FAs. Mean areas (C) of stable (light gray, n = 124) and unstable (dark gray, n = 55) FAs were compared as well as mean aspect ratios (D) of stable (light gray, n = 129) and unstable (dark gray, n = 55) ones. Asterisks indicate a significant difference between both groups (p value < 0.001). Error bars display the standard error (s.e.). The evolution of areas (E) and aspect ratios (F) of a stable (gray) and an unstable (black) adhesion are given exemplarily for comparison. Mean values for area and aspect ratio (horizontal lines in E and F) and the maximal aspect ratio (+ in F) used for evaluations given in (A-D) are exemplarily indicated for stable FAs.
Table 1.
Analysis of stable and unstable adhesions
| Stable | Unstable | p value | |
| mean lifetime (min) | 13.9 (n = 129) | 2.90 (n = 55) | <0.001 |
| s.e. (min) | 0.5 | 0.20 | |
| mean area (µm2) | 0.68 (n = 124) | 0.20 (n = 55) | <0.001 |
| s.e. (µm2) | 0.03 | 0.01 | |
| mean aspect ratio | 2.38 (n = 129) | 1.77 (n = 55) | <0.001 |
| s.e. | 0.07 | 0.05 |
Migrating keratinocytes were transfected with GFP-vinculin and analyzed via TIRF microscopy. FAs were visualized and automatically tracked over time. A lifetime of 6 min was selected as threshold for discrimination between unstable and stable FAs. Mean lifetime, mean area and mean aspect ratio of unstable (n = 55) and stable (n = 129) FAs were calculated for four independent cells. All data were tested for normal distribution using the Kolmogoroff-Smirnoff test. This test failed for mean area analysis of stable FAs. Therefore, data were tested for outliers who were subsequently erased (5 out of 129). This resulted in a different number of data compared to the other data sets. Removal of these data sets even reduced observed differences between stable and unstable FAs. Differences between stable and unstable FAs were tested for significance using a two-sided t-test. s.e., standard error.
Figure 4.
Protein content of filopodial actin fibers. (A) After transfection with GFP-α-actinin cells were fixed and stained for fascin. White arrows show overlap of fascin and α-actinin. Motility induced keratinocytes were transfected with GFP-α-actinin (B) or GFP-palladin (C) and analyzed in phase contrast (top) and fluorescence (bottom) over time. Palladin and α-actinin are located behind filopodial extensions and showed a clear signal at filopodial shafts (white arrows). White arrowheads indicate incorporation center over time. Time points are given in seconds. Keratinocytes transfected with GFP-α-actinin (D) and with GFP-palladin (E) were fixed and actin was stained. White arrows in (D and E) highlight co-localization of tested proteins with actin. Overlays are additionally superimposed with phase contrast images. Scale bars in (A, D and E) = 10 µm and in (B and C) = 3 µm.
The given data strongly indicate that FA stability depends on coupling of actin bundles originated from filopodia. In order to analyze whether these actin bundles changed in protein composition and therefore function depending on their interaction with filopodial FXs and subsequently lamellipodial FAs, we determined protein localization of typical actin cross-linking proteins. Special focus was given to fascin as filopodial actin cross-linker as well as to α-actinin and palladin which are present in lamellipodial actin bundles. For this purpose GFP-α-actinin transfected keratinocytes were fixed and immunofluorescently stained for fascin (Fig. 4A). For every filopodial actin bundle extending into the lamellipodium (basically all, as also given in Fig. 2) we found an amazingly separated localization of both proteins. While fascin was present only in filopodial extensions of the actin bundles, α-actinin could be localized exclusively in the lamellipodial part of the bundle (Fig. 4A). A small overlap region of both proteins at the shafts of filopodia argues for a transition zone where fascin becomes exchanged by α-actinin within the same actin bundle. This transition zone was found at the identical position also by time lapse analyses. Here, GFP-α-actinin as well as GFP-palladin (Fig. 4B and C) revealed a permanent localization of both proteins right behind filopodial extensions (white arrowheads) while they were fully excluded from filopodia (Suppl. movies 2 and 3). To confirm that α-actinin and palladin localization really depended on incorporation into actin bundles, α-actinin (Fig. 4D) and palladin (Fig. 4E) transfected cells were fixed and additionally stained for actin. These experiments prove the co-localization of α-actinin and palladin with actin bundles originated from filopodia (highlighted by white arrowheads).
To directly analyze the chronological order of FA enlargement along actin bundles originated from filopodia in live cell experiments, keratinocytes were double-transfected with GFP-α-actinin and DsRed-vinculin and afterwards motility stimulated (Fig. 5). These time lapse analyses confirm the strong interlink between filopodial originated actin bundles and stable FAs. The data additionally indicate that actin bundles have already exchanged their cross-linking protein fascin against α-actinin when FAs become enlarged and elongated (Fig. 5, white arrows in the overlay, Suppl. movie 4, white arrowheads). Interestingly, FAs along actin fibers of filopodia unable to connect stably to the substrate disassembled instantaneously (Suppl. movie 4, white arrow).
Figure 5.
Formation of stable FAs within filopodial actin. Keratinocytes were grown for 24 h and subsequently double-transfected with GFP-α-actinin and DsRed-vinculin. EGF stimulated cells were analyzed in phase contrast and fluorescence over time. Time series illustrate the assembly of stable FAs along actin bundles originated from filopodia and cross-linked via α-actinin. White arrowheads show co-localization of stable FAs (vinculin) and actin bundles (α-actinin) during elongation. Time points are given in seconds. Scale bar = 5 µm. Slightly affected image quality is caused by the Avalanche photo diode used and a thereby reduced resolution for time lapse analyses.
α-Actinin is known to open actin bundles for myosin II incorporation. At sites of incorporation α-actinin is subsequently replaced by inserted myosin II to form a periodic distribution along stress fibers.19 As shown before within the lamellipodium, actin fibers originating from filopodia contain α-actinin yet lack fascin which is known to bundle actin filaments very tightly29 abolishing incorporation of myosin II. Therefore, α-actinin could function as a placeholder within actin fibers originated from filopodia for further myosin II integration. This was tested by immunofluorescence staining against actin and myosin IIA (Fig. 6A). In the lamellipodium we found a clear signal for myosin II within and directly behind filopodial actin fibers (Fig. 6A, left, white arrows) whereas filopodia totally lack myosin II. Furthermore, the dynamic integration of myosin II was analyzed by live cell imaging on motile keratinocytes simultaneously transfected with YFP-MLC (myosin light chain) and DsRed-α-actinin. These experiments revealed a continuous incorporation of myosin II into α-actinin cross-linked actin bundles originated from filopodia (Fig. 6B, time series). Moreover we observed partial replacement of α-actinin by myosin II visible by intensity reductions of α-actinin upon myosin II incorporation (Fig. 6B, white arrowheads).
Figure 6.
Myosin localization and assembly in filopodial actin. (A) Keratinocytes were fixed and stained for myosin II (green) and actin (red). The overview image is additionally overlaid with a DIC-image. The box indicates the zoomed area shown on the right side. Here, white arrowheads highlight the localization of myosin II in actin bundles emerging from filopodia. (B) Double-transfection with YFP-MLC and DsRed-α-actinin was performed on motile keratinocytes. Cells were analyzed in fluorescence and phase contrast over time. The overview image in phase contrast was recorded slightly after time series acquisition. Myosin could be identified within α-actinin containing actin bundles originated from filopodia. White arrowheads point to regions where α-actinin is partially exchanged against myosin. Time points are given in seconds. Scale bars = 10 µm.
Incorporation of myosin II pointed at an important function of actin bundles originated from filopodia in force formation. This was tested in first place by actin retrograde flow measurements since the contractile activity of myosin II is known to control actin retrograde flow in lamellipodia as well as in filopodial actin bundles.12,30,31 Furthermore, an effective connection of actin filaments to FAs can cause a deceleration of actin flow.32,33 Keratinocytes were transfected with GFP-actin. Defined, small regions of GFP-labeled filopodial actin bundles were bleached to visualize retrograde flow (Fig. 7A and B). Flow rates of filopodial actin fibers with FAs (Fig. 7A) directly behind the filopodium were determined and compared with actin flow rates of filopodia missing FAs (Fig. 7B). FAs behind filopodial fibers were identified via reflection microscopy (Fig. 7A). Only stably adhered filopodia were analyzed. Interestingly, we found a significant difference (p-value < 0.001) between retrograde flow of filopodial actin associated with FAs and filopodial actin without FAs (Fig. 7C). Actin flow associated with FAs exhibited a velocity of 1.11 µm/min (s.e. = 0.07 µm/min, n = 18, out of 9 cells) whereas the velocity of actin flow without FAs averaged 2.52 µm/min (s.e. = 0.18 µm/min, n = 15, out of 6 cells). The significant deceleration of actin flow due to the attendance of FAs therefore clearly confirms an effective connection of stable FAs with filopodial actin fibers.
Figure 7.
Effective connection of stable FAs with filopodial actin fibers. Keratinocytes were grown for 24 h and transfected with GFP-actin. Regions of filopodial actin were bleached to visualize retrograde flow within filopodia with (A) and without (B) FAs conjunction. White boxes in the overview images indicate zoomed areas shown in the time series. Red boxes illustrate bleached regions. Bleaching was performed at time point 10 s. Red lines highlight the progression of bleached regions in the GFP-images. For a better visualization GFP-images were background subtracted in the time series. Time points are given in seconds. Scale bars = 10 µm. (C) Averaged retrograde flow rates for filopodial actin bundles with (light gray, n = 18, out of 9 cells) and without (dark gray, n = 15, out of 6 cells) connection to FAs. Asterisks indicate a significant difference between both mean values (p value < 0.001). Error bars display the standard error (s.e.).
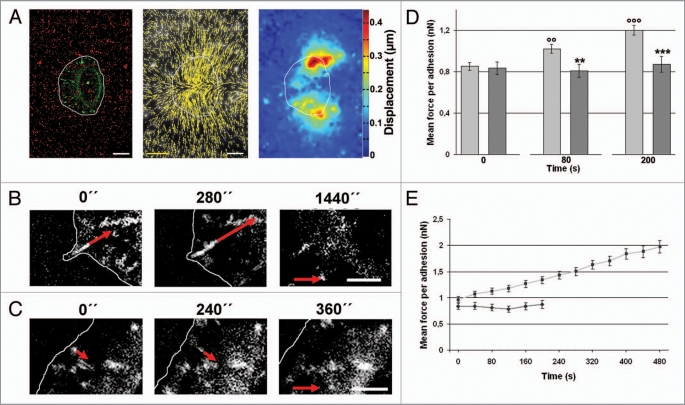
Based on the shown protein composition of actin bundles originated from filopodia and the strong interaction of stable FAs with these bundles we assumed the existence of a FA stabilization process depending on the contractility of attached actin bundles. This hypothesis was tested by performing traction force measurements on GFP-vinculin expressing keratinocytes migrating on micropatterned elastomeric silicone rubber substrates (Fig. 8A). Substrate deformation was visualized by tracking displacements of beads embedded in the very top layer of the substrate (∼0.5 µm thin) over time. Displacement arrows pointed towards the center of the cell and were concentrated at the cells rearmost sides (Fig. 8A). For force calculation, only cells with a constant migration speed and only FAs which newly assembled at the cell front were taken into consideration. This selection avoided misinterpretation due to a general increase in force generation when cells slow down. It additionally allowed the classification of every newly formed FA into the two classes of stable and unstable FAs. Here, the same threshold of 6 min was used to classify those two groups as before. Based on the results given above, stable FAs were assumed to be attached to myosin containing actin bundles originated from filopodia since localization of filopodia on silicone rubber substrates was infeasible. Morphological behaviour of stable as well as unstable FAs in respect to changes in area as well as aspect ratio over time revealed identical results (Fig. 8B and C) as shown on stiff substrates (Fig. 3) indicating the system to be reliable.
Figure 8.
FA stability depends on force transmission. Motile keratinocytes were seeded onto elastic silicone rubber substrates and transfected with GFP-vinculin (A, left). Substrate displacements were analyzed by tracking fluorescent beads (A, left, red dots). Bead displacements are exemplary indicated for the same cell as presented on the left by displacement vectors (A, middle) and as color scale image (A, right). Displacement scale bar (yellow) = 0.5 µm and scale bars (white) = 10 µm. Cell position is indicated by the white line. Force application on every newly formed FA was calculated over time (B and C). FA lifetime was additionally used to classify all FAs as stable (B) or unstable (C, lifetime below 6 min) ones. Red arrows denote measured forces at these adhesions. Last images of the time series show the time point of FA disassembly. Force scale bar (red arrow) = 3 nN. Time points are given in seconds. Scale bars in (B and C) = 5 µm. Cell edges are indicated by white lines. GFP-images were background subtracted. (D) Mean forces per adhesion of stable (light gray) and unstable (dark gray) FAs at different time points. Asterisks indicate significant differences between both values at given time points. Significant increase of force transmission on stable FAs is indicated by circles comparing time points 80 and 200 s with time point 0. (E) Mean force transmission of stable (light gray) and unstable (dark gray) FAs for the first 8 min. Error bars display the standard error (s.e.).
Analyses allowed us to show for the first time a direct correlation between FAs stability and force transmission. Stable FAs experienced an increasing force whereas force transmission for unstable FAs levelled off (Fig. 8B–E). This is shown exemplarily for stable and unstable FAs in a time series starting from FA assembly and leading to the final time point of disassembly (Fig. 8B and C). Statistical analysis of these data substantiated these findings and revealed significant differences of force values between stable and unstable FAs (Fig. 8D and E, Table 2). The significant difference in mean force application (p value < 0.01) became apparent already 80 s after FA assembly. Additionally, mean force values of stable FAs increased constantly with time while force values of unstable FAs remained on a low level (Fig. 8D, Table 2). This increase is demonstrated even more precisely in Figure 8E. Here, the time course of mean force values (n(unstable, t = 0) = 38, n(stable, t = 0) = 124) for stable and unstable FAs out of one measurement on one cell is illustrated. Identical results were achieved in three independent measurements.
Table 2.
Mean force values of stable and unstable FAs
| Stable FAs (nN) | Unstable FAs (nN) | p value | |
| t = 0 s | 0.85 (s.e.: 0.04, n = 116) | 0.84 (s.e.: 0.06, n = 38) | − |
| t = 80 s | 1.02 (s.e.: 0.04, n = 118) | 0.81 (s.e.: 0.06, n = 35) | <0.01 |
| t = 200 s | 1.20 (s.e.: 0.05, n = 116) | 0.87 (s.e.: 0.08, n = 19) | <0.001 |
Traction force measurements were performed on migrating keratinocytes. A threshold of 6 min lifetime was used to differentiate between stable and unstable FAs. Mean forces for stable and unstable FAs were calculated for every time point. t = 0 describes the time point of assembly. All data were tested for normal distribution using the Kolmogoroff-Smirnoff test. This test failed for the data sets obtained for stable FAs. Therefore, outliners were removed (6–8 out of 124). This resulted in a different number of datasets for all three time points. Mean values were barely affected by the removal. The number per data sets of unstable FAs decreased over time due to FA disassembly. Differences between stable and unstable FAs were tested for significance using a two-sided t-test. s.e.: standard error.
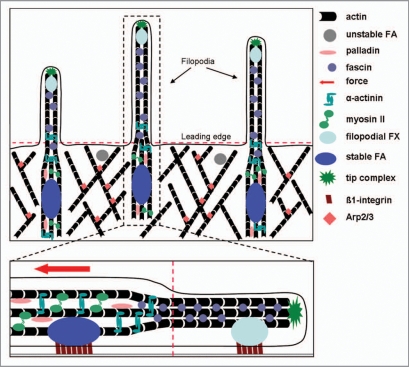
All together, these data clearly show the strong correlation of FA stabilization and force transmission. They additionally prove that, not only stable lamellipodial FAs, but also force generating actin bundles and their connection to the substrate strongly depend on adhesions as well as on cytoskeletal structures originated already during filopodia formation. Maturation of small FXs to stable FAs depends on filopodia and more precisely on the effective connection to force producing actin fibers. This force depended maturation leads to stable FAs and thus to mandatory structures for efficient cell migration (Fig. 9).
Figure 9.
A new model for force induced FA stabilization and maturation. Analysis of stable and unstable FA revealed structural differences. Stable FAs evolve from filopodial FXs with direct connection to parallel actin fibers originated from filopodia. These fibers establish the basis for a force induced maturation of filopodial FXs to stable FAs. Filopodial actin bundles are compacted by fascin, while the same bundles when reached by the lamellipodium (dotted red line) lose fascin and incorporate α-actinin, palladin and myosin II. Force induced stabilization and maturation from FXs to FAs is indicated by the red arrow. Unstable FAs are formed within the dense actin network of the lamellipodium and so miss the connection to the structural machinery provided by filopodia (overview image). This soon leads to disassembly and thus to unstable FAs.
Discussion
Migration of cells is a complex and highly regulated process essential for almost every cell type during parts of their lifetime. A constantly increasing body of publications has revealed the importance of substrate sensing and adhesion site formation with subsequent force application over these sites as early events in migration of animal cells. Blocking or interfering with any of these processes affects in the mildest case just migration speed but typically inhibits motility or even leads to cell death.34,35
Adhesion molecules as integrins or cadherins are located in filopodia and are the first molecules getting in contact with the environment upon migration. Interestingly, depending on the analyzed cell type filopodial, sensing does not necessarily have to end up in cell matrix adhesions as described, for example, 3T3 fibroblasts during cell spreading.8 Instead, filopodia can also mediate the interaction between macrophages and pathogens36 or between two cells as shown for embryonic epithelial cells37 or the formation of functional neuronal interactions.38 These examples show the overall importance of filopodial sensing. We further believe that, although the molecules involved in mediating the first filopodial contact will be certainly different for the given functions, the underlying mechanism of filopodia dependent force generation identified here and discussed below, describes a more general aspect of filopodia function.
Former results by Schäfer et al.10 argue for the formation of filopodial focal contacts upon filopodia binding to matrix molecules. Although the exact hierarchical recruitment of adhesion proteins still needs to be solved, latest findings now argue for a rather complete set of adhesion proteins already at these very early time points.8,10,39,40 These findings provide interesting additional aspects for adhesion site maturation since for focal adhesions developed from FXs formed in the lamellipodium of filopodia lacking endothelial cells, a clear step by step formation process is described.7,22 Proteins that are completely missing in FXs originated in the lamellipodium as zyxin or tensin are clearly present in filopodial FXs.10 Consistent for both types of FXs is their ability to mature to lamellipodial FAs. As we could show here, for filopodial FXs this transition also depends on their tight connection to actin bundles. However, in contrast to lamellipodial FXs,19 filopodial FXs are already connected to distinct actin bundles upon formation and keep these filopodial actin fibers pickaback when overgrown by the leading edge (shown here). Adhesion sites developed in the lamellipodium could be also identified in this work in migrating keratinocytes but, as for filopodia lacking cell types, these adhesion sites disassembled without any sign of maturation when no contact to actin bundles could be formed.41 The difference in FX association to actin bundles might also be the reason why filopodial FXs already contain FA proteins as zyxin or tensin absent in FXs originated in the lamellipodium.
While maturation of filopodial FXs to classical FAs presumably does not go along with major changes in the protein inventory, different results were found for filopodial actin bundles. These actin bundles typically extend for several µm into the lamellipodium due to filopodial integrity upon incorporation into the lamellipodium during migration. Directly at the base of filopodia, fascin, a filopodial actin cross-linker, becomes efficiently exchanged against α-actinin (Fig. 9). We believe that this incorporation leads to an opening up within the actin bundle as also described for actin bundles originated in the lamellipodium. Additionally, integration of α-actinin into actin bundles, irrespective whether derived from the lamellipodium or from filopodia, is discussed also to be an important prerequisite for FA maturation.27 Subsequently, both types of actin bundles seem to follow the same functional direction by incorporating myosin II which substitutes for α-actinin.19 Present data imply that incorporation of both, α-actinin as well as myosin II, into lamellipodial originated actin bundles occurs only if these bundles are connected to focal adhesions.16 Whether this is also the case for filopodial originated actin bundles remains to be shown in the future. Our data indicate that at least tight adhesion of filopodial tips does not seem to be necessary for the exchange of fascin against α-actinin since growing and even retracting filopodia show a distinct α-actinin signal in the lamellipodial part of their actin bundles. In contrast, myosin II was detected only in a fraction of all filopodia arguing for an additional prerequisite for myosin II incorporation into filopodial originated actin bundles. Nevertheless, although myosin II was not present in all actin bundles originated from filopodia, these bundled filaments were the only ones in direction of migration with incorporated myosin II motor proteins. These observations are consistent with other findings in migrating fibroblasts.30 Here, myosin II was also associated with filopodia at their base which indicated an important role for myosin II in relocating F-actin from the leading edge to the lamella. Several publications argue for a strong interplay between adhesion site maturation and force application to these sites. No matter whether adhesion sites become stressed by internal activation of myosin or by external substrate stretch, FAs enlarge in size allowing them to transmit larger forces.24,42 Furthermore, adaptation to applied forces seems to be a regulatory mechanism especially important for young not fully matured FAs because most present work depends on low serum cell growth conditions to keep FA dynamics high and to detect growth upon force application.25,26 Our results fully confirm the correlation between FA maturation and growth with increasing force application for the first time under unaffected growth conditions (Fig. 9). From our data we cannot fully conclude whether forces applied by stable FAs increase because of FA growth or whether vice versa applied forces are the signal for FA enlargement. However, FA growth upon external force application can be explained only by the second possibility and should be therefore assumed also for our case. Results on the same as well as other cell types have indicated that FA maturation additionally goes along with changes in phospho-tyrosine levels.7,22,23 These changes regulate protein exchange dynamics within the first minutes of FA maturation13 and might therefore also be connected with the force dependent FA maturation analyzed here. Furthermore, former data by Schäfer et al.10 have shown that filopodia elongate repetitively leading to a consecutive alignment of filopodial originated FAs along the myosin II containing actin bundles upon migration. Such a system would be relatively unaffected by the continuous disassembly of filopodial originated actin bundles at their minus-ends in the lamella and the observed disassembly of attached oldest FAs in the same region and would allow a continuous generation of pulling forces in direction of the leading edge. This hypothesis is confirmed by a strongly reduced retrograde flow for filopodia with stable FAs at their base right behind the lamellipodial leading edge. Here, myosin II activity applies a traction force to the substrate while on filopodia without base localized FAs the same myosin II activity is transferred into enhanced filopodial actin retrograde flow.
Additional interesting correlations between filopodia properties and migration speeds of cells or cell fronts were predicted by mathematical modelling.43–45 The authors simulated the effect of diffusion, actin polymerization speeds as well as number of actin filaments within filopodia for stable filopodia formation. From these theoretical considerations they were able to predict several correlations between migratory speeds and filopodia properties. Increasing numbers of filopodia, enhanced filopodial growth rates as well as enhanced overall lengths of filopodia with increasing migration speeds were theoretically predicted and could be confirmed by us via quantifying filopodial properties in our time lapse analyses (data not shown). This agreement between theory and experiment argues that several mechanisms identified here are driven by physical processes. It furthermore indicates that filopodia formation and migration speed are two intrinsically tied processes.
Taking all data together we could show for keratinocytes that not only substrate sensing but also FA formation and maturation as well as lamellipodial actin bundles and force generation in direction of movement strongly depend on filopodia (Fig. 9). All of these early events in migration are heavily interwoven and describe a step by step process.
Material and Methods
Cell culture.
Normal human epidermal keratinocytes (NHEK) from neonatal foreskin were purchased from Lonza (Verviers, Belgium) and sub-cultured in complete keratinocytes growth medium (KGM, Lonza) at 37°C and 5% CO2. Cells were used for experiments from passage one to four. For sub-culturing or subsequent experiments cells were harvested using 0.025% Trypsin and 0.01% EDTA in Hank’s buffered salt solution (HBSS, Lonza). 20,000–30,000 keratinocytes were seeded into self made glass bottom Petri dishes (glass thickness: 170 µm) for further analysis or on elastic silicone rubber substrates for force measurements. Glass bottom Petri dishes were coated with 2.5 µg/cm2 and elastomeric substrates with 5 µg/cm2 human plasma fibronectin (BD, Bioscience, Palo Alto, CA, USA) in medium for 45 min at 37°C. To induce cell motility keratinocytes were stimulated with 50 nM human epidermal growth factor (EGF, Sigma) at least 1 h before experimental start.
Cloning of cDNA-constructs, plasmids and transfection.
GFP-vinculin was kindly provided by Benjamin Geiger (Weizmann Institute of Science, Rehovot, Israel), GFP-α-actinin by Carol Otey (Department of Cell and Molecular Physiology, Los Angeles, CA, USA) and YFP-MLC by Pekka Lappalainen (Institute of Biotechnology, Helsinki, Finland). GFP-actin was purchased as plasmid pEGFP-actin from BD-Bioscience. GFP-VASP was a gift from Jürgen Wehland (University Braunschweig, Germany). Open reading frame of palladin cDNA (clone AF205078) was incorporated into pEGFP-C1 (Clontech, Saint-Germain-en-Laye, France) resulting in pEGFP-palladin. Open reading frames of vinculin cDNA (clone J04126) was cloned into DsRed-monomer-C1 (Clontech) resulting in DsRed-vinculin. DsRed-α-actinin was created by incorporating open reading frame of α-actinin (clone M95178) into DsRed-monomer-N1 (Clontech).
Transient transfections of keratinocytes were performed using Transit Keratinocyte reagent (Mirus, Madison, WI, USA) according to the manufacturer’s instructions.
Immunofluorescence staining.
Keratinocytes were fixed using 3.7% formaldehyde in cytoskeleton buffer (CB: 150 mM NaCl, 5 mM MgCl2, 5 mM EGTA, 5 mM glucose, 10 mM MES, pH 6.1) for 20 min at 37°C and 5% CO2. In case of fascin staining, fixation was performed using a 1:1 mixture of methanol and acetone at minus 20°C for 5 min. Afterwards, cells were washed three times with 30 mM glycin in CB, permeabilized with 0.2% Triton in CB for 10 min, rinsed in CB and subsequently treated with blocking solution (5% skim milk powder in CB) for 60 min at RT. Fixed cells were treated with primary antibodies (see below) diluted 1:200 in blocking solution for 45 min at 37°C. After wash-ing three times with blocking solution and 0.2% Tween20, cells were incubated with appropriate secondary antibodies diluted 1:200 in blocking solution. For actin staining Alexa Fluor546 Phalloidin (Molecular Probes, Eugene, OR, USA) was used in a 1:200 dilution for 45 min at 37°C. Finally, samples were washed three times with blocking solution and 0.2% Tween20 and rinsed with water. Used primary antibodies were against vinculin (clone hVIN-1, Sigma), fascin (clone 55K2, Chemicon, Temecula, CA, USA) and myosin IIa (M8064, Sigma). Cy2 goat anti mouse, Cy3 goat anti mouse and Cy2 goat anti rabbit (Dianova, Hamburg, Germany) were used as secondary anti-bodies, respectively.
Microscopy.
For adhesion shape and stability analysis, GFP-transfected cells were observed with a Total Internal Reflection Fluorescence (TIRF) microscope (Observer.Z1, Carl Zeiss, MicroImaging, Germany). Time lapse images in Differential Interference Contrast (DIC) and TIRF mode were acquired using a 100x/1.46 DIC α-plan apochromat oil objective (Carl Zeiss) and a CCD camera (AxioCam MRm, Carl Zeiss) under live cell conditions. TIRF was excited with the 488 nm line of an argon-ion-laser. Immunofluorescence images presented in Figures 3 and 6 were additionally recorded in epi-fluorescence using the 43HE Cy3 filter (Zeiss). All other immunofluorescence as well as time lapse images except sequences for force measurements were acquired with a confocal microscope (LSM710, Zeiss) using the 63x/1.4 Ph3 plan apochromat oil objective (Zeiss). All time lapse and actin flow analyses were performed at 37°C and 5% CO2. The 488 nm argon ion laser line was used for imaging GFP, phase contrast and reflection with appropriate filter settings. Excitation of DsRed, Cy3 and Alexa Fluor546 was performed using the 543 nm HeNe laser. Emission was recorded with a long pass filter. For bleaching filopodial actin the 488 nm argon ion laser line of the LSM710 was operated at 100% with ten iterations (40 µsec per pixel). Regions of interest to be bleached were selected using the LSM 710 software tool V.5.0 SP1.1 (Carl Zeiss, Jena). The avalanche photodiode system of the LSM710 was used for imaging Figures 4A and C, 5 and 6B (only the green channel). For cell force measurements, GFP-transfected cells were analyzed with a Cell Observer system (Carl Zeiss) equipped with a CCD camera (AxioCam MRm, Carl Zeiss) and a 40x/1.3 Ph3 plan neofluar oil objective (Carl Zeiss). Live cell imaging was performed by phase contrast simultaneously with fluorescence microscopy using filter sets appropriate for GFP and DsRed visualization.
Actin flow measurements.
GFP-actin of transfected migrating keratinocytes was recorded simultaneously with phase contrast and reflection in time lapse movies over a period of 46–240 s. Bleached stripes were used to reveal actin flow in filopodial actin fibers (see microscopy). Width of bleach stripes was always in the range of 1–2 µm. Reflection images were used to determine FAs within filopodial actin. Actin flow speeds were measured by manual tracking the edges of bleached stripes with the point tool of the ImageJ software (Wayne Rasband, U.S. National Institute of Health). For better visualization, image background was calculated by a mean filter (filter box size: 30 pixel (0.0081 µm2/pixel) and subtracted from original images using ImageJ. Only stable filopodial actin bundles with long extensions into the lamellipodium were analyzed.
Adhesion shape analysis.
TIRF time lapse images of GFP-vinculin transfected keratinocytes were recorded over a period of 30 min. Sequences of four cells were analyzed with a routine for automated FA tracking implemented in Matlab (The Mathworks, version 7.6, Natick, MA, USA) which is briefly described in the following: To correct for photobleaching, the intensity distribution of every frame was normalized between 0 and 1 and cytoplasmic background was reduced by highpass filtering as described by Zamir et al.46 (Filter box size: 2 µm). FA regions were detected by an intensity threshold Imin=0.1. To track individual FAs over time, temporally and spatially overlapping regions were assigned to the same patch by the connected component labeling method “union-find” from Sedgewick.49 All patches larger than a minimum area Amin=0.1 µm2 were assigned to FAs, all remaining patches were refused. Miscalculated FAs were manually deleted. Mean area of each FA over time was measured and plotted against lifetime. Additionally, maximal aspect ratios of every FA were plotted against their corresponding lifetime. FAs which disassembled within 6 min were defined as unstable. All remaining FAs were classified as stable. Mean lifetime, mean area and mean aspect ratio were calculated for stable and for unstable FAs and compared using a two-sided t-test.
Preparation of elastomeric silicone rubber substrates.
For force measurements keratinocytes were seeded on elastic silicone rubber substrates (Sylgard 184, Dow Corning GmbH, Wiesbaden, Germany). Preparation and characterization were performed as described before.47,48 Briefly, a 55:1 (w/w) mixture of base and cross-linker was prepared. Red fluorescent beads (0.2 µm diameter, Crimson-FluoSpheres, Invitrogen) were mixed into one part of the mixture and applied on a micro-structured, silanized silicon dioxide surface (2 µm dots arranged in a square lattice of a 3.5 µm lattice constant). Silanization was performed with trichloro(1H,1H,2H,2H-perfluorooctyl)silane (Sigma). The layer thickness was reduced to less than 0.5 µm by wiping the surface with a lint-free tissue. Remaining uncross-linked elastomer without fluorescent beads was deposited on top and the thickness of the substrates was reduced to a defined value (80–100 µm) using glass slices as spacers and a cover slide on top. Cross-linking of silicone rubber was performed at 60°C over night. Elastomeric substrates were glued to the bottom of 3.5 cm Petri dishes to cover predrilled 1.5 cm holes. After curing, substrates exhibited a Poisson’s ratio of 0.5 and a Young’s modulus of 8 and 11 kPa depending on the batch of Sylgard used.
Traction measurements and force evaluation.
Keratinocytes were grown on beads microstructured silicone rubber substrates and subsequently transfected with GFP-vinculin or GFP-VASP. Migration was induced as described above. Time lapse movies of migrating keratinocytes were recorded simultaneously for GFP-marked FAs, red fluorescent beads and cell’s position in phase contrast over time periods of 25–52 min and analyzed using Matlab as software (The Mathworks).47,48 To achieve a reference image without cell induced substrate deformation, cells were removed by trypsination and subsequent treatment with 20 mM NH4OH and 0.1 M NaCl for at least 1 h. Traction force measurements and point force evaluation were performed as described in Merkel et al.48 In brief, bead positions in the first image of the merged image sequence were localized by cross correlation with an arbitrarily chosen bead as template. Bead displacements along the whole sequence were determined by cross-correlation with the images of each bead in the first image as template. Lateral drift was calculated from the average shift of beads in areas far from the cell where no substrate deformations occurred. For evaluation of forces applied at every FA, FAs were tracked manually over time using the GFP-channel. Following parameter values were used for force evaluations: Poisson’s ratio: 0.5, masking radius around each FA: 1 µm, Young’s modulus: 8 kPa or 11 kPa, depending on the batch of Sylgard184 used.
Cell 1 was analyzed over a time period of 25 min and 12 unstable and 44 stable FAs could be identified. Analysis of cell 2 over 52 min revealed 7 unstable and 73 stable FAs. Cell 3 was imaged for 42 min, 38 unstable and 124 stable FAs were observed.
Statistical analysis.
All data used for detailed statistical analysis (Figs. 3C and D, and 8D, Tables 1 and 2) were tested for normal distribution using the Kolmogoroff-Smirnoff test. If this test failed, data were tested for outliers which were subsequently erased. This resulted in a slightly varying number of incorporated data sets. To assess significance of differences a two-sided t-test was performed.
Acknowledgements
We thank I. Lauter for helpful discussions and critical reading of the manuscript. We thank Nils Hersch for helpful work in cell culture and microscopy. We are grateful to Benjamin Geiger for the GFP-vinculin construct, Carol Otey for the GFP-α-actinin, Pekka Lappalainen for the YFP-MLC and Jürgen Wehland for the GFP-VASP construct.
Abbreviations
- MLC
myosin light chain
- FA
focal adhesion
- FX
focal complex
- DIC
differential interference contrast
- TIRF
total internal reflection fluorescence
- s.e.
standard error
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/10745
Supplementary Material
References
- 1.Geiger B, Bershadsky A. Assembly and mechanosensory function of focal contacts. Curr Opin Cell Biol. 2001;13:1–3. doi: 10.1016/s0955-0674(00)00255-6. [DOI] [PubMed] [Google Scholar]
- 2.Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 3.Gupton SL, Waterman-Storer CM. Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell. 2006;125:1361–1374. doi: 10.1016/j.cell.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 4.Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9:858–867. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 6.Ponti A, Machacek M, Gupton SL, Waterman-Storer CM, Danuser G. Two distinct actin networks drive the protrusion of migrating cells. Science. 2004;305:1782–1786. doi: 10.1126/science.1100533. [DOI] [PubMed] [Google Scholar]
- 7.Zaidel-Bar R, Ballestrem C, Kam Z, Geiger B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J Cell Sci. 2003;116:4605–4613. doi: 10.1242/jcs.00792. [DOI] [PubMed] [Google Scholar]
- 8.Partridge MA, Marcantonio EE. Initiation of attachment and generation of mature focal adhesions by integrin-containing filopodia in cell spreading. Mol Biol Cell. 2006;17:4237–4248. doi: 10.1091/mbc.E06-06-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nemethova M, Auinger S, Small JV. Building the actin cytoskeleton: filopodia contribute to the construction of contractile bundles in the lamella. J Cell Biol. 2008;180:1233–1244. doi: 10.1083/jcb.200709134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schäfer C, Borm B, Born S, Möhl C, Eibl EM, Hoffmann B. One step ahead: role of filopodia in adhesion formation during cell migration of keratinocytes. Exp Cell Res. 2009;315:1212–1224. doi: 10.1016/j.yexcr.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Vignjevic D, Kojima S, Aratyn Y, Danciu O, Svitkina T, Borisy GG. Role of fascin in filopodial protrusion. J Cell Biol. 2006;174:863–875. doi: 10.1083/jcb.200603013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medeiros NA, Burnette DT, Forscher P. Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat Cell Biol. 2006;8:215–226. doi: 10.1038/ncb1367. [DOI] [PubMed] [Google Scholar]
- 13.Möhl C, Kirchgessner N, Schäfer C, Küpper K, Born S, Diez G, et al. Becoming stable and strong: the interplay between vinculin exchange dynamics and adhesion strength during adhesion site maturation. Cell Motil Cytoskeleton. 2009;66:350–364. doi: 10.1002/cm.20375. [DOI] [PubMed] [Google Scholar]
- 14.Oliver T, Dembo M, Jacobson K. Separation of propulsive and adhesive traction stresses in locomoting keratocytes. J Cell Biol. 1999;145:589–604. doi: 10.1083/jcb.145.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- 16.Deguchi S, Sato M. Biomechanical properties of actin stress fibers of non-motile cells. Biorheology. 2009;46:93–105. doi: 10.3233/BIR-2009-0528. [DOI] [PubMed] [Google Scholar]
- 17.Edlund M, Lotano MA, Otey CA. Dynamics of alpha-actinin in focal adhesions and stress fibers visualized with alpha-actinin-green fluorescent protein. Cell Motil Cytoskeleton. 2001;48:190–200. doi: 10.1002/1097-0169(200103)48:3<190::AID-CM1008>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 18.Goicoechea SM, Arneman D, Otey CA. The role of palladin in actin organization and cell motility. Eur J Cell Biol. 2008;87:517–525. doi: 10.1016/j.ejcb.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hotulainen P, Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J Cell Biol. 2006;173:383–394. doi: 10.1083/jcb.200511093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parast MM, Otey CA. Characterization of palladin, a novel protein localized to stress fibers and cell adhesions. J Cell Biol. 2000;150:643–656. doi: 10.1083/jcb.150.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Endlich N, Otey CA, Kriz W, Endlich K. Movement of stress fibers away from focal adhesions identifies focal adhesions as sites of stress fiber assembly in stationary cells. Cell Motil Cytoskeleton. 2007;64:966–976. doi: 10.1002/cm.20237. [DOI] [PubMed] [Google Scholar]
- 22.Zaidel-Bar R, Cohen M, Addadi L, Geiger B. Hierarchical assembly of cell-matrix adhesion complexes. Biochem Soc Trans. 2004;32:416–420. doi: 10.1042/BST0320416. [DOI] [PubMed] [Google Scholar]
- 23.Zaidel-Bar R, Milo R, Kam Z, Geiger B. A paxillin tyrosine phosphorylation switch regulates the assembly and form of cell-matrix adhesions. J Cell Sci. 2007;120:137–148. doi: 10.1242/jcs.03314. [DOI] [PubMed] [Google Scholar]
- 24.Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3:466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 25.Galbraith CG, Yamada KM, Sheetz MP. The relationship between force and focal complex development. J Cell Biol. 2002;159:695–705. doi: 10.1083/jcb.200204153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, et al. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol. 2001;153:1175–1186. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi CK, Vicente-Manzanares M, Zareno J, Whitmore LA, Mogilner A, Horwitz AR. Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat Cell Biol. 2008;10:1039–1050. doi: 10.1038/ncb1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steketee MB, Tosney KW. Three functionally distinct adhesions in filopodia: shaft adhesions control lamellar extension. J Neurosci. 2002;22:8071–8083. doi: 10.1523/JNEUROSCI.22-18-08071.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kureishy N, Sapountzi V, Prag S, Anilkumar N, Adams JC. Fascins, and their roles in cell structure and function. Bioessays. 2002;24:350–361. doi: 10.1002/bies.10070. [DOI] [PubMed] [Google Scholar]
- 30.Anderson TW, Vaughan AN, Cramer LP. Retrograde flow and myosin II activity within the leading cell edge deliver F-actin to the lamella to seed the formation of graded polarity actomyosin II filament bundles in migrating fibroblasts. Mol Biol Cell. 2008;19:5006–5018. doi: 10.1091/mbc.E08-01-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le CC, Carlier MF. Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol Rev. 2008;88:489–513. doi: 10.1152/physrev.00021.2007. [DOI] [PubMed] [Google Scholar]
- 32.Gardel ML, Sabass B, Ji L, Danuser G, Schwarz US, Waterman CM. Traction stress in focal adhesions correlates biphasically with actin retrograde flow speed. J Cell Biol. 2008;183:999–1005. doi: 10.1083/jcb.200810060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu K, Ji L, Applegate KT, Danuser G, Waterman-Storer CM. Differential transmission of actin motion within focal adhesions. Science. 2007;315:111–115. doi: 10.1126/science.1135085. [DOI] [PubMed] [Google Scholar]
- 34.Fazal F, Gu L, Ihnatovych I, Han Y, Hu W, Antic N, et al. Inhibiting myosin light chain kinase induces apoptosis in vitro and in vivo. Mol Cell Biol. 2005;25:6259–6266. doi: 10.1128/MCB.25.14.6259-6266.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salo S, Boutaud A, Hansen AJ, He L, Sun Y, Morales S, et al. Antibodies blocking adhesion and matrix binding domains of laminin-332 inhibit tumor growth and metastasis in vivo. Int J Cancer. 2009;125:1814–1825. doi: 10.1002/ijc.24532. [DOI] [PubMed] [Google Scholar]
- 36.Niedergang F, Chavrier P. Signaling and membrane dynamics during phagocytosis: many roads lead to the phagos(R)ome. Curr Opin Cell Biol. 2004;16:422–428. doi: 10.1016/j.ceb.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 37.Millard TH, Martin P. Dynamic analysis of filopodial interactions during the zippering phase of Drosophila dorsal closure. Development. 2008;135:621–626. doi: 10.1242/dev.014001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sekino Y, Kojima N, Shirao T. Role of actin cytoskeleton in dendritic spine morphogenesis. Neurochem Int. 2007;51:92–104. doi: 10.1016/j.neuint.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 39.Laukaitis CM, Webb DJ, Donais K, Horwitz AF. Differential dynamics of alpha5 integrin, paxillin and alpha-actinin during formation and disassembly of adhesions in migrating cells. J Cell Biol. 2001;153:1427–1440. doi: 10.1083/jcb.153.7.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, et al. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003;302:103–106. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- 41.Zamir E, Geiger B. Molecular complexity and dynamics of cell-matrix adhesions. J Cell Sci. 2001;114:3583–3590. doi: 10.1242/jcs.114.20.3583. [DOI] [PubMed] [Google Scholar]
- 42.Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc Natl Acad Sci USA. 2003;100:1484–1489. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atilgan E, Wirtz D, Sun SX. Mechanics and dynamics of actin-driven thin membrane protrusions. Biophys J. 2006;90:65–76. doi: 10.1529/biophysj.105.071480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mogilner A, Edelstein-Keshet L. Regulation of actin dynamics in rapidly moving cells: a quantitative analysis. Biophys J. 2002;83:1237–1258. doi: 10.1016/S0006-3495(02)73897-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mogilner A, Rubinstein B. The physics of filopodial protrusion. Biophys J. 2005;89:782–795. doi: 10.1529/biophysj.104.056515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zamir E, Katz BZ, Aota S, Yamada KM, Geiger B, Kam Z. Molecular diversity of cell-matrix adhesions. J Cell Sci. 1999;112:1655–1669. doi: 10.1242/jcs.112.11.1655. [DOI] [PubMed] [Google Scholar]
- 47.Cesa CM, Kirchgessner N, Mayer D, Schwarz US, Hoffmann B, Merkel R. Micropatterned silicone elastomer substrates for high resolution analysis of cellular force patterns. Rev Sci Instrum. 2007;78:034301. doi: 10.1063/1.2712870. [DOI] [PubMed] [Google Scholar]
- 48.Merkel R, Kirchgessner N, Cesa CM, Hoffmann B. Cell force microscopy on elastic layers of finite thickness. Biophys J. 2007;93:3314–3323. doi: 10.1529/biophysj.107.111328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sedgewick R. Connectivity-Union-Find Algorithms. In: Harrison MA, editor. Algorithms in C.1. New York: Addison-Wesley publishing company; 1990. pp. 441–449. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.