Abstract
Systemic delivery of multipotent mesenchymal stem cells (MSC) may be of benefit in the treatment of neurological diseases, including multiple sclerosis (MS). Certainly, animal studies have demonstrated functional benefits following MSC transplantation, although the mechanisms by which MSCs migrate to lesions and stimulate repair remain unknown. Chemokines stimulate migration in other settings. In this study, we systematically explore the migratory and proliferative responses of human MSCs (hMSC) to chemokines expressed in MS lesions. We demonstrate that these chemokines trigger hMSC migration. In addition, we show that RANTES and IP-10 promote hMSC proliferation.
Key words: migration, proliferation, multipotent mesenchymal stromal cells, chemokines, demyelination
Introduction
Homing to sites of injury is an important property of any putative reparative cell. Intravenous delivery of bone marrow-derived cells including MSCs1 is of functional benefit in animal models of neurological disease, including demyelination.2–6 The mechanisms by which these cells affect lesion repair are unknown, as are the means by which circulating MSCs enter lesions.
Chemokines are small (8–10 kDa), chemoattractant cytokines. Although their association with inflammation was recognized many years ago, their ability to recruit leucocytes was only more recently appreciated. More recently still, it has been recognized that chemokines also play important roles in cell migration in other contexts, including development, infection, angiogenesis, angiostasis and metastasis.7
Chemokines are known to have a role in recruitment of cells in CNS inflammation, including multiple sclerosis (MS).8–13 Differential expression of a number of chemokines and their receptors has been demonstrated in both acute and chronic MS lesions including monocyte chemotactic protein-1 (MCP-1; CCL2), macrophage inflammatory protein-1α (MIP-1α; CCL3), MIP-1β (CCL4), regulated on activation, normal T cell expressed and secreted (RANTES; CCL5), interferon-inducible protein-10 (IP-10; CXCL10), and stromal cell-derived factor-1 (SDF-1; CXCL12) (reviewed in ref. 14).
We hypothesized that chemokines implicated in the pathogenesis of demyelinating disease have significant effects on the behavior of MSCs. Here we explore the ability of adult hMSCs to migrate and proliferate in vitro in response to various chemokines (MCP-1, RANTES, MIP-1α, MIP-1β, IP-10 and SDF-1).
Results
Human MSCs migrate in response to chemokines expressed in demyelinated lesions.
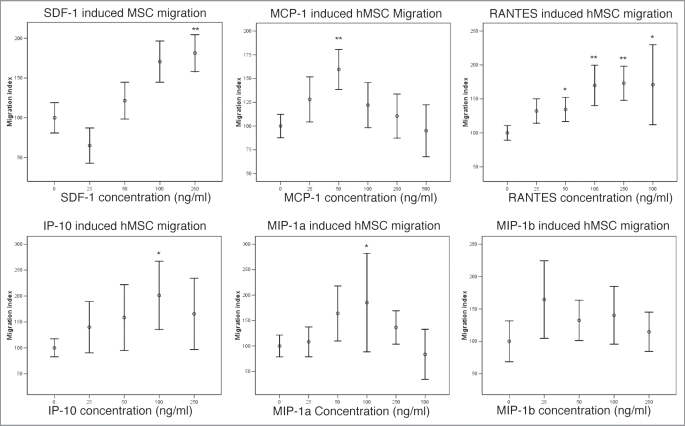
Using the agarose drop migration assay, we assessed the migratory responses of human mesenchymal stem cells in relation to a range of relevant cytokines, quantifying migration as described above. With most chemokines, the results were consistent with a dose-response effect (Fig. 3). MIP-1β showed a positive effect at a concentration of 25 ng/ml but this did not reach statistical significance (p = 0.14).
Figure 3.
Migration of hMSCs in response to chemokines expressed in demyelinated lesions (*p < 0.05 **p < 0.005). SDF-1, MCP-1, RANTES, IP-10 and MIP-1α triggered statistically significant migration of hMSCs. There was a trend towards a positive effect of MIP-1β on migration of hMSCs at 25 ng/ml but this did not reach statistical significance.
Effect of chemokines on hMSC proliferation.
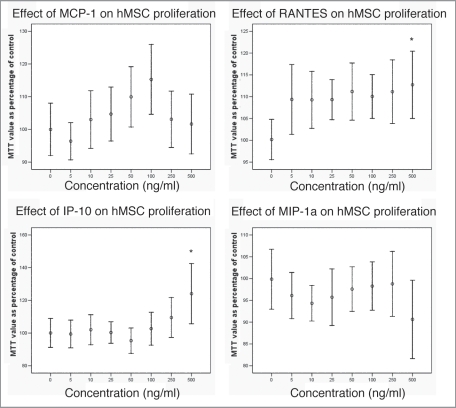
We also assessed the effects of these chemokines on hMSC proliferation. Higher concentrations of RANTES and IP-10 (500 ng/ml) did produce a significant increase in proliferation of hMSCs (Fig. 4). With MCP-1, there was a trend towards a significant effect on proliferation of hMSCs at 50 ng/ml and 100 ng/ml. MIP-1α did not have a statistically significant effect on hMSC proliferation although there was a trend towards a toxic effect at high concentration (500 ng/ml).
Figure 4.
Effect of chemokines on hMSC proliferation (*p < 0.05). A statistically significant effect was seen at high (500 ng/ml) concentrations of RANTES and IP-10.
Discussion
This is the first systematic study, using a dose-response approach, exploring the responses of human MSCs to chemokines known to be expressed in the lesions of multiple sclerosis.
We have demonstrated that hMSCs migrate in response to chemokines expressed in demyelinated lesions including SDF-1, MCP-1, RANTES, MIP-1α and IP-10. In addition, hMSCs proliferate in response to high concentrations of RANTES and IP-10.
These experiments are the first to explore the migration of hMSCs using the agarose drop assay which allows for quantitative analysis of migration and assessment of cell morphology in response to changes in culture medium over 72 h, although direction and velocity of migration are not assessed.21 The results broadly concur with the findings of other investigators who have studied chemokine-induced migration of hMSCs (Table 1).22–31 A notable exception was the failure to demonstrate migration of hMSCs in response to MCP-1 by Ringe et al.26 and Croitoru-Lamoury et al.22 although the latter did demonstrate chemotaxis following pre-treatment with interferon-β. This apparent discrepancy and the reported variations in the concentration of chemokine triggering migration are likely to be explained by the inhomogeneous nature of hMSC cultures, differences in culture conditions (including passage number) and assay method, as well as donor variability and the level of ‘pre-stimulation’ of hMSCs. The finding that, under certain conditions, chemokines exert a proliferative effect on hMSCs is a novel result.
Table 1.
Summary of published results regarding migration of human MSCs in response to chemokines
| Reference | Assay method | SDF-1 | MCP-1 | MIP-1α | RANTES | IP-10 |
| Wang, et al. Hematology 2002; 7:113–7. | Boyden, 5 h | Increased to max tested 300 ng/ml | Increased to max tested 40 ng/ml | |||
| Wynn, et al. Blood 2004; 104:2643–5. | Transwell | Max 30 ng/ml | ||||
| Sordi, et al. Blood 2005; 106:419–27. | Boyden, overnight | Increasing concentrations to max 1,000 ng/ml | Max 300 ng/ml | |||
| Honczarenko, et al. Stem Cells 2006; 24:1030–41. | Chemotaxis chamber, 45 min | 1 µg/ml; ‘bell-shaped’ dose response curves | ||||
| Son, et al. Stem Cells 2006; 24:1254–64. | Tranmatrigel, 24 h | 100 ng/ml | ||||
| Dwyer, et al. Clin Can Res 2007; 13:5020–7. | Transwell 18 h | Increased between 150–600 pg/ml | ||||
| Ringe, et al. J Cell Biochem 2007; 101:135–46. | ChemoTx, 20 h | 250–1,000 nM, min 100 nM | No migration 1–1,000 nM | |||
| Schmal, et al. Cytotherapy 2007; 9:69–79. | Minichambers, 1.5 h | Max 1–10 ng/ml | ||||
| Ponte, et al. Stem cells 2007; 25:1737–45. | Transwell, overnight | 150 ng/ml | 100 ng/ml | 100 ng/ml | 150 ng/ml | |
| Croitoru-Lamoury, et al. J Interf Cyto Res 2007; 27:53–64. | Transwell, 48 h | 50 & 500 ng/ml | 1,000 ng/ml only with IFNb | 1,000 ng/ml |
The possibility that circulating MSCs, exposed to chemokines, migrate into lesions, may have implications for spontaneous repair processes in MS. MSCs in experimental models have been shown to influence neural stem cell differentiation,32–34 remyelination,2,5,6 axon loss35,36 and, particularly, immune activity.5,37
Our findings may also have implications for the development of new therapeutic interventions designed to mobilize endogenous cells to enhance repair. Potentially, small molecules may be designed to mobilize endogenous cell populations—analogous to the current clinical use of granulocyte-colony stimulating factor (G-CSF) to mobilize CD34-positive cells during the work-up for peripheral stem cell collection. Clinical trials employing chemokine receptor antagonists in MS are already in progress14 although the scientific rationale for these trials has been anti-inflammatory rather than mobilisation of specific populations of bone marrow cells. The relatively low concentration of MSCs in normal marrow may mean that cytokine-mobilisation would be insufficient for therapeutic purposes; nonetheless there are significant potential advantages compared with the exacting requirements of ex vivo proliferation.
Materials and Methods
Recombinant human chemokines were obtained from Peprotech; SDF-1 (300-28A), MCP-1 (300-04), MIP-1α (300-08), MIP-1β (300-09), RANTES (300-06) and IP-10 (300-12).
Marrow collection.
Adult human marrow was obtained from the discarded femoral head of patients undergoing total hip replacement at the Avon Orthopaedic Centre (AOC), Southmead Hospital, Bristol with the approval of the local ethics committee and following formal patient consent.
Isolation and proliferation of hMSCs.
The marrow was broken up with a scalpel and washed in Hanks medium (Sigma H9269) until only white spicules of bone remained. The cell suspension was layered onto an equal volume of Lymphoprep (Axis-Shield PoC AS) and spun (1,620 g) for 30 min. A red cell lysis step was performed (10 min incubation at 4°C with 0.15 M ammonium chloride, 0.01 M potassium bicarbonate and 0.15 mM EDTA in ddH2O) and the mononuclear cells were re-suspended in Dulbecco’s modified eagle’s medium (DMEM, D5523, Sigma) following a further wash with Hanks. Cell number and viability were assessed using trypan blue exclusion (Sigma, T8154). Initially, cells were plated at a density of 4 × 105/cm2 in standard hMSC medium [DMEM supplemented with 10% foetal bovine serum (FBS, StemCell Technologies Inc., 06471)] in vented tissue culture flasks (Falcon). Cultures were incubated in a humidified, 5% CO2 atmosphere at 37°C and the medium exchanged every 5–7 d. Upon reaching a minimum of 80% confluence, adherent cells were passaged with trypsin-EDTA (Cambrex, BE17-161E) and re-plated in T75 flasks with fresh medium at 0.5 × 106 cells per flask. MSCs were routinely differentiated into adipogenic, chondrogenic and osteogenic cell types according to previously published methods.15–18 The immunophenotype as determined by FACS was consistent with the defining criteria for MSCs.1
Agarose drop migration assay.
The chosen method of assessing hMSC migration in vitro was the agarose drop method, modified from Frost et al.19 Human MSCs between second and fifth passage were trypsinised and resuspended in 2% FCS/DMEM at approximately 1 × 106 cells/40 µl. The concentration of FCS was a balance; higher concentrations induced significant migration independent of the chemokine added but some FCS was required to maintain cell viability. The cells were mixed with 20 µl 1% low melting point agarose (Sigma A-9045) in PBS which was pre-warmed to 95°C then cooled to 37°C. The cell/agarose mixture (2 µl/well) was aliquoted quickly onto the center of pre-cooled wells coated with poly-L-lysine. The mixture was then allowed to set at 4°C for 10 min. Subsequently, 750 µl of medium appropriate to the experiment was added to the wells around the cell suspension/agarose droplet. In some experiments, 20 µg/ml aphidicolin (Sigma A0781) was added to the flood to inhibit cellular proliferation. Following 72 h incubation at 37°C, migration between the edge of the agarose drop and the penumbra of outwardly migrating cells was measured at 0, 90, 180 and 270° using a phase contrast microscope and calibrated graticule (Fig. 1). To correct for systematic differences between trials, migration distances were converted to a migration index: distance migrated in test wells divided by the mean distance migrated in the control wells. Results are presented as a percent of control. Each experiment was repeated at least in triplicate.
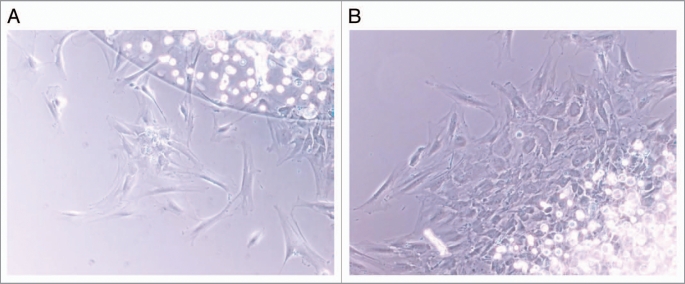
Figure 1.
Human MSCs migrating out of an agarose droplet under control conditions (A; 0 ng/ml MCP-1) and following stimulation of migration (B; 100 ng/ml MCP-1). Under conditions of low levels of migration, individual cells or small clusters could be seen (A) but with higher levels of migration, sheets of migrating MSCs were observed (B).
Cellular proliferation assay.
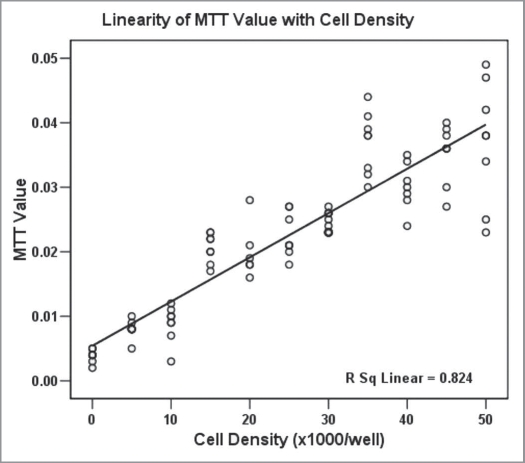
Cellular proliferation was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay (M5655, Sigma, UK).20 Human MSCs between second and fifth passage were plated in 50 µl 2% FCS in DMEM into 96 well plates (Nunc or Falcon) at a cell density of 1,000 cells per well. The total volume in the wells was made up to 100 µl by the addition of × 2 concentration of chemokine in 2% FCS in DMEM. At 72 h, 20 µl of 5 mg/ml MTT in PBS was added and incubated at 37°C for 30 min. Subsequently, the medium was carefully aspirated and 50 µl isopropanol added to each well. A Multiskan Ascent spectrophotometer was used to measure the specific absorbance at 540 nm. Each experiment was repeated at least in duplicate. Results are presented as the MTT value as a percentage of control. The linear relationship between cell density and MTT signal was established in preliminary experiments where the MTT signal was recorded from wells plated at a defined cell density [Fig. 2, Pearson correlation 0.907 (significant at the 0.01 level)].
Figure 2.
MTT signal correlated with cell density [Pearson correlation 0.907 (significant at the 0.01 level)].
Statistical analysis.
Microsoft Excel was used to process data. For comparison between test conditions and controls, a one-way ANOVA was performed, followed by Dunnett’s post-hoc test (SPSS version 12). Error bars show 95% confidence intervals.
Acknowledgements
C.M.R. was supported by The Ipsen Trust and The Patrick Berthoud Charitable Foundation. The Burden Chair of Clinical Neurosciences (N.J.S.) is supported by The Burden Trust. We thank Dr. Christopher Halfpenny for his advice in setting up the agarose drop assay and are grateful to the patients and staff of the Avon Orthopaedic Centre for donation and collection of marrow.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/11404
References
- 1.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 2.Akiyama Y, Radtke C, Honmou O, Kocsis JD. Remyelination of the spinal cord following intravenous delivery of bone marrow cells. Glia. 2002;39:229–236. doi: 10.1002/glia.10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J, Li Y, Wang L, Zhang Z, Lu D, Mei Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 4.Lee ST, Chu K, Park JE, Lee K, Kang L, Kim SU, et al. Intravenous administration of human neural stem cells induces functional recovery in Huntington’s disease rat model. Neurosci Res. 2005;52:243–249. doi: 10.1016/j.neures.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Li Y, Chen J, Cui Y, Lu M, Elias SB, et al. Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp Neurol. 2005;195:16–26. doi: 10.1016/j.expneurol.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 8.Balashov KE, Rottman JB, Weiner HL, Hancock WW. CCR5(+) and CXCR3(+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc Natl Acad Sci USA. 1999;96:6873–6878. doi: 10.1073/pnas.96.12.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang D, Han Y, Rani MR, Glabinski A, Trebst C, Sørensen T, et al. Chemokines and chemokine receptors in inflammation of the nervous system: manifold roles and exquisite regulation. Immunol Rev. 2000;177:52–67. doi: 10.1034/j.1600-065x.2000.17709.x. [DOI] [PubMed] [Google Scholar]
- 10.Merrill JE, Benveniste EN. Cytokines in inflammatory brain lesions: helpful and harmful. Trends Neurosci. 1996;19:331–338. doi: 10.1016/0166-2236(96)10047-3. [DOI] [PubMed] [Google Scholar]
- 11.Ransohoff RM. Mechanisms of inflammation in MS tissue: adhesion molecules and chemokines. J Neuroimmunol. 1999;98:57–68. doi: 10.1016/s0165-5728(99)00082-x. [DOI] [PubMed] [Google Scholar]
- 12.Simpson JE, Newcombe J, Cuzner ML, Woodroofe MN. Expression of monocyte chemoattractant protein-1 and other beta-chemokines by resident glia and inflammatory cells in multiple sclerosis lesions. J Neuroimmunol. 1998;84:238–249. doi: 10.1016/s0165-5728(97)00208-7. [DOI] [PubMed] [Google Scholar]
- 13.Babcock AA, Kuziel WA, Rivest S, Owens T. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J Neurosci. 2003;23:7922–7930. doi: 10.1523/JNEUROSCI.23-21-07922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szczucinski A, Losy J. Chemokines and chemokine receptors in multiple sclerosis. Potential targets for new therapies. Acta Neurol Scand. 2007;115:137–146. doi: 10.1111/j.1600-0404.2006.00749.x. [DOI] [PubMed] [Google Scholar]
- 15.Wexler SA, Donaldson C, Denning-Kendall P, Rice C, Bradley B, Hows JM. Adult bone marrow is a rich source of human mesenchymal ‘stem’ cells but umbilical cord and mobilized adult blood are not. Br J Haematol. 2003;121:368–374. doi: 10.1046/j.1365-2141.2003.04284.x. [DOI] [PubMed] [Google Scholar]
- 16.Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312. [PubMed] [Google Scholar]
- 17.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 18.Yoo JU, Barthel TS, Nishimura K, Solchaga L, Caplan AI, Goldberg VM, et al. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am. 1998;80:1745–1757. doi: 10.2106/00004623-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Frost EE, Milner R, Ffrench-Constant C. Migration assays for oligodendrocyte precursor cells. Methods Mol Biol. 2000;139:265–278. doi: 10.1385/1-59259-063-2:265. [DOI] [PubMed] [Google Scholar]
- 20.Loveland BE, Johns TG, Mackay IR, Vaillant F, Wang ZX, Hertzog PJ. Validation of the MTT dye assay for enumeration of cells in proliferative and antiproliferative assays. Biochem Int. 1992;27:501–510. [PubMed] [Google Scholar]
- 21.Frost EE, Zhou Z, Krasnesky K, Armstrong RC. Initiation of oligodendrocyte progenitor cell migration by a PDGF-A activated extracellular regulated kinase (ERK) signaling pathway. Neurochem Res. 2009;34:169–81. doi: 10.1007/s11064-008-9748-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Croitoru-Lamoury J, Lamoury FM, Zaunders JJ, Veas LA, Brew BJ. Human mesenchymal stem cells constitutively express chemokines and chemokine receptors that can be upregulated by cytokines, IFNbeta and Copaxone. J Interferon Cytokine Res. 2007;27:53–64. doi: 10.1089/jir.2006.0037. [DOI] [PubMed] [Google Scholar]
- 23.Dwyer RM, Potter-Beirne SM, Harrington KA, Lowery AJ, Hennessy E, Murphy JM, et al. Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin Cancer Res. 2007;13:5020–5027. doi: 10.1158/1078-0432.CCR-07-0731. [DOI] [PubMed] [Google Scholar]
- 24.Honczarenko M, Le Y, Swierkowski M, Ghiran I, Glodek AM, Silberstein LE. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24:1030–1041. doi: 10.1634/stemcells.2005-0319. [DOI] [PubMed] [Google Scholar]
- 25.Ponte AL, Marais E, Gallay N, Langonné A, Delorme B, Hérault O, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 26.Ringe J, Strassburg S, Neumann K, Endres M, Notter M, Burmester GR, et al. Towards in situ tissue repair: human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. J Cell Biochem. 2007;101:135–146. doi: 10.1002/jcb.21172. [DOI] [PubMed] [Google Scholar]
- 27.Schmal H, Niemeyer P, Roesslein M, Hartl D, Loop T, Südkamp NP, et al. Comparison of cellular functionality of human mesenchymal stromal cells and PBMC. Cytotherapy. 2007;9:69–79. doi: 10.1080/14653240601011557. [DOI] [PubMed] [Google Scholar]
- 28.Son BR, Marquez-Curtis LA, Kucia M, Wysoczynski M, Turner AR, Ratajczak J, et al. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24:1254–1264. doi: 10.1634/stemcells.2005-0271. [DOI] [PubMed] [Google Scholar]
- 29.Sordi V, Malosio ML, Marchesi F, Mercalli A, Melzi R, Giordano T, et al. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 2005;106:419–427. doi: 10.1182/blood-2004-09-3507. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Li Y, Chen X, Chen J, Gautam SC, Xu Y, et al. MCP-1, MIP-1, IL-8 and ischemic cerebral tissue enhance human bone marrow stromal cell migration in interface culture. Hematology. 2002;7:113–117. doi: 10.1080/10245330290028588. [DOI] [PubMed] [Google Scholar]
- 31.Wynn RF, Hart CA, Corradi-Perini C, O’Neill L, Evans CA, Wraith JE, et al. A small proportion of mesenchymal stem cells strongly express functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643–2645. doi: 10.1182/blood-2004-02-0526. [DOI] [PubMed] [Google Scholar]
- 32.Croft AP, Przyborski SA. Mesenchymal stem cells expressing neural antigens instruct a neurogenic cell fate on neural stem cells. Exp Neurol. 2008;216:329–341. doi: 10.1016/j.expneurol.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Rivera FJ, Couillard-Despres S, Pedre X, Ploetz S, Caioni M, Lois C, et al. Mesenchymal stem cells instruct oligodendrogenic fate decision on adult neural stem cells. Stem Cells. 2006;24:2209–2219. doi: 10.1634/stemcells.2005-0614. [DOI] [PubMed] [Google Scholar]
- 34.Bai L, Caplan A, Lennon D, Miller RH. Human Mesenchymal Stem Cells Signals Regulate Neural Stem Cell Fate. Neurochem Res. 2006;32:353–362. doi: 10.1007/s11064-006-9212-x. [DOI] [PubMed] [Google Scholar]
- 35.Kassis I, Grigoriadis N, Gowda-Kurkalli B, Mizrachi-Kol R, Ben-Hur T, Slavin S, et al. Neuroprotection and immunomodulation with mesenchymal stem cells in chronic experimental autoimmune encephalomyelitis. Arch Neurol. 2008;65:753–761. doi: 10.1001/archneur.65.6.753. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Li Y, Lu M, Cui Y, Chen J, Noffsinger L, et al. Bone marrow stromal cells reduce axonal loss in experimental autoimmune encephalomyelitis mice. J Neurosci Res. 2006;84:587–595. doi: 10.1002/jnr.20962. [DOI] [PubMed] [Google Scholar]
- 37.Bai L, Lennon DP, Eaton V, Maier K, Caplan AI, Miller SD, et al. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia. 2009;57:1192–203. doi: 10.1002/glia.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]