Abstract
Single-chain receptors and multi-chain immune recognition receptors (SRs and MIRRs, respectively) represent families of structurally related but functionally different surface receptors expressed on different cells. In contrast to SRs, a distinctive and common structural characteristic of MIRR family members is that the extracellular recognition domains and intracellular signaling domains are located on separate subunits. How extracellular ligand binding triggers MIRRs and initiates intracellular signal transduction processes is not clear. A novel model of immune signaling, the Signaling Chain HOmoOLigomerization (SCHOOL) model, suggests that the homooligomerization of receptor intracellular signaling domains represents a necessary and sufficient condition for receptor triggering. In this review, I demonstrate striking similarities between a consensus model of SR signaling and the SCHOOL model of MIRR signaling and show how these models, together with the lessons learned from viral pathogenesis, provide a molecular basis for novel pharmacological approaches targeting inter- and intrareceptor transmembrane interactions as universal therapeutic targets for a diverse variety of immune and other disorders.
Key words: multichain immune recognition receptor, TCR, single-chain receptor, RTK, transmembrane interactions, immune system, therapeutic targets, receptors, cell signaling, immunotherapy
Single- and Multi-chain Receptors
Cells express at their surface a repertoire of receptors that recognize individual stimuli and transduce this information across the cell membrane, thus activating intracellular signaling pathways. The importance of receptors in health and disease1,2 makes the molecular understanding of transmembrane (TM) signal transduction critical in influencing and controlling this process for therapeutic purposes. Unrelated and functionally diverse receptors can be structurally classified as single- and multi-chain activating receptors. By definition, single-chain receptors (SRs) are receptors with binding and signaling domains located on the same protein chain (Fig. 1A). Examples include receptor tyrosine kinases (RTKs) that are TM glycoproteins consisting of a variable extracellular N-terminal domain, a single membrane spanning domain and a large cytoplasmic portion composed of a juxtamembrane domain, the highly conserved tyrosine kinase domain and a C-terminal regulatory region. In contrast, multi-chain receptors, key among which is the family of multi-chain immune recognition receptors (MIRRs) that are expressed on many different immune cells and mediate antigen recognition,3,4 are characterized by the most intriguing common structural feature: their extracellular recognition (binding) domains and intracellular signaling domains containing immunoreceptor tyrosine-based activation motifs (ITAMs) or the YxxM module are located on separate subunits (Fig. 2A). The association of the subunits in resting cells is mostly driven by the noncovalent TM interactions between recognition and signaling components (Fig. 2A) and plays a key role in receptor assembly and integrity. Typical examples of MIRRs include the T-cell receptor (TCR) complex, the B-cell receptor (BCR) complex, Fc receptors (e.g., FcɛRI, FcαRI, FcγRI and FcγRIII), NK receptors (e.g., NKG2D, CD94/NKG2C, KIR2DS, NKp30, NKp44 and NKp46), immunoglobulin (Ig)-like transcripts and leukocyte Ig-like receptors (ILTs and LIRs, respectively), signal regulatory proteins (SIRPs), dendritic cell immunoactivating receptor (DCAR), myeloid DNAX adapter protein of 12 kD (DAP12)-associating lectin 1 (MDL-1), novel immune-type receptor (NITR), triggering receptors expressed on myeloid cells (TREMs), and the platelet collagen receptor, glycoprotein VI (GPVI).2
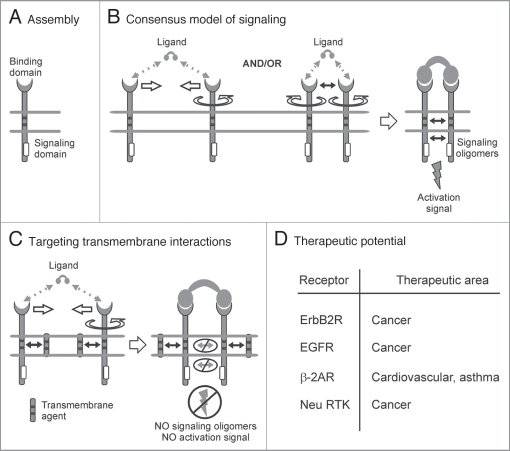
Figure 1.
Single-chain receptors. Single activating receptor assembly and signaling. (A) Single-chain receptor (SR) assembly. Extracellular recognition domain and intracellular signaling domain with a signaling sequence (shown by empty rectangles) are located on the same protein chain. (B) Consensus model of SR signaling. The model proposes that receptor homooligomerization in the cytoplasmic milieu plays a central role in triggering SRs. Ligand-induced SR clustering and reorientation (and/or receptor reorientation in preexisting SR clusters) results in SR oligomerization mediated by transmembrane interactions. In these oligomers, receptors are in sufficient proximity and adopt a correct (permissive) relative orientation and geometry to promote homointeractions between cytoplasmic domains. Formation of competent signaling oligomers results in generation of the activation signal (for receptor tyrosine kinases, this means trans-autophosphorylation of Tyr residues in cytoplasmic signaling sequences) and thus triggers downstream signaling pathways. Protein-protein interactions are shown by solid black arrows. Empty arrows illustrate ligand-induced receptor clustering (oligomerization). Circular arrow indicates ligand-induced receptor reorientation. (C) Interreceptor transmembrane interactions. Within the consensus model of SR signaling, specific blockade of interreceptor transmembrane interactions prevents ligand-induced SR oligomerization. Competent signaling oligomers in cytoplasmic milieu are not formed, thus preventing generation of the activation signal. (D) Therapeutic potential of SR transmembrane inhibitors.
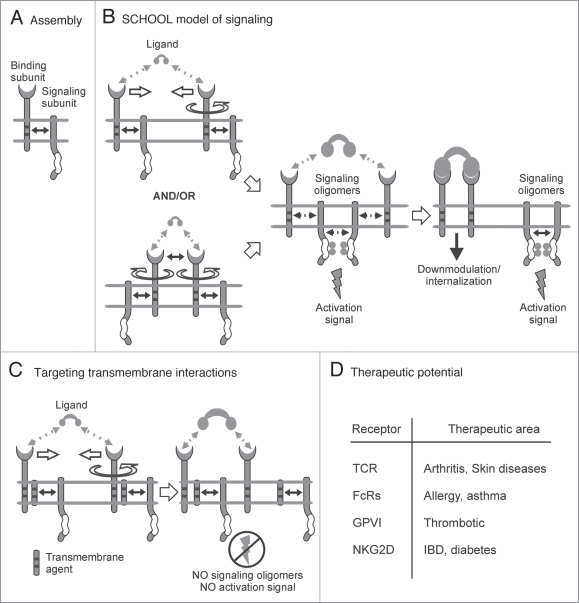
Figure 2.
Multi-chain immune recognition receptors. Multi-chain activating receptor assembly and signaling. (A) Structural and functional organization of multi-chain immune recognition receptors (MIRRs). Immunoreceptor tyrosine-based activation motif (ITAM) is indicated by empty box. Transmembrane interactions between MIRR ligand-binding and signaling components (shown by solid arrow) play a key role in receptor assembly and integrity on resting cells. (B) The signaling chain homooligomerization (SCHOOL) model, proposing that the homooligomerization of signaling subunits plays a central role in triggering MIRR-mediated signal transduction. Small solid black arrows indicate specific intersubunit hetero- and homointeractions between transmembrane and cytoplasmic domains, respectively. Circular arrow indicates ligand-induced receptor reorientation. Empty arrows illustrate ligand-induced receptor clustering (oligomerization). All interchain interactions in a dimeric intermediate are shown by dotted black arrows reflecting their transition state. Curved lines depict disorder of the cytoplasmic domains of MIRR signaling subunits. Phosphate groups are shown as filled gray circles. (C) Molecular mechanisms underlying proposed intervention by transmembrane-targeted agents. Specific blockade of transmembrane interactions between MIRR recognition and signaling subunits results in “pre-dissociation” of the receptor complex, thus preventing formation of signaling oligomers and inhibiting ligand-dependent immune cell activation. In contrast, stimulation of these “pre-dissociated” MIRRs with cross-linking antibodies to signaling subunit(s) should still lead to receptor triggering and cell activation (not shown). (D) Therapeutic potential of MIRR transmembrane inhibitors.
Assuming that the similar architecture of the receptors within the single- and multi-chain receptor families dictates similar mechanisms of receptor triggering, I suggest that transmembrane signal transduction mediated by SRs and MIRRs is based on similar mechanistic principles which in turn provide the similarity of the revealed therapeutic targets. My hypothesis is that signaling chain homooligomerization in cytoplasmic milieu provides the necessary and sufficient event to trigger members of both receptor families. As a consequence of this hypothesis, receptor oligomerization induced or tuned upon ligand binding outside the cell is suggested to be translated across the membrane into protein oligomerization inside the cell, thus providing a general platform for receptor-mediated signaling. Together with the lessons learned from viral pathogenesis, this builds the structural basis for the development of novel pharmacological approaches targeting inter- and intra-receptor transmembrane interactions as universal therapeutic targets for a diverse variety of immune and other disorders. Importantly, this also allows transferring our current and future clinical knowledge, experience and therapeutic strategies between seemingly unrelated diseases.
Transmembrane Signal Transduction: Basic Principles
The molecular mechanisms underlying SR (i.e., receptor tyrosine kinases, RTKs) signaling have been fairly well delineated and suggest that intracellular formation of competent signaling oligomers plays a crucial role in receptor triggering (Fig. 1B). Ligand binding is believed to stimulate SR dimerization and trans-autophosphorylation at defined tyrosine residues through intrinsic kinase activity.5–8 Formation of competent signaling oligomers in cytoplasmic milieu has been shown to be a critical event in Fas receptor signaling.9 Some SRs, such, for example, as members of the tumor necrosis factor (TNF) receptor superfamily10,11 exist as pre-assembled oligomers on the cell surface. In these cases, binding to multivalent ligand results in reorientation of receptors in these oligomers to adopt an interunit geometry permissive for further receptor activation (Fig. 1B).
For multi-chain activating receptors that signal through ITAM/YxxM modules,2,3 the mechanism by which the MIRR transduces ordered information such as antigen recognition from outside the cell via receptor TM and juxtamembrane regions into intracellular biochemical events has been a long-standing open issue until recently, when, in the novel model of immune signaling, the Signaling Chain HOmoOLigomerization (SCHOOL) model, formation of ITAM-containing cytoplasmic signaling oligomers was for the first time suggested to play a critical role in TM signaling mediated by these receptors.12 The model suggests that formation of competent MIRR signaling subunit oligomers mediated by homotypic interactions in cytoplasmic milieu, rather than receptor clusters/oligomers per se, is necessary and sufficient to trigger the receptors and induce transmembrane signal transduction and the downstream signaling sequence (Fig. 2B). Similar to SRs, some MIRRs, such, for example, as TCR and major platelet collagen receptor GPVI can exist as pre-assembled oligomers on the cell surface.13,14 Within the SCHOOL model,4,12,15,16 binding to multivalent ligand results in reorientation of receptors in these oligomers to adopt an interunit geometry permissive for further receptor activation (Fig. 2B), again highlighting the mechanistic generality of receptor-mediated transmembrane signaling.
Intriguingly, in contrast to well-structured cytoplasmic signaling domains of SRs, cytoplasmic domains of MIRR signaling subunits belong to a novel class of intrinsically disordered proteins (IDPs; i.e., proteins that lack a well-defined ordered structure under physiological conditions in vitro). The recently discovered unusual biophysical phenomenon, the ability of these IDPs to homooligomerize,17,18 represents a missing key piece of the MIRR triggering puzzle (Fig. 2B). This raises an interesting question: Why for MIRRs, the receptors with extracellular recognition and intracellular signaling domains located on separate protein chains, nature selected to use an unusual and unique functional link between protein disorder and oligomericity? One can expect that further multidisciplinary studies will clarify this question of great interest and practical utility.
Thus, formation of competent signaling oligomers mediated by homointeractions between well-structured (in SRs) or intrinsically disordered (in MIRRs) cytoplasmic signaling domains is a necessary and sufficient to trigger receptor function. This dictates several important mechanistic principles of receptor-mediated transmembrane signaling:
sufficient interreceptor proximity in receptor dimers/oligomers.
correct (permissive) relative orientation of the receptors in receptor dimers/oligomers.
long enough duration of the receptor-ligand interaction that generally correlates with the strength (affinity/avidity) of the ligand.
sufficient lifetime of an individual receptor in receptor dimers/oligomers.
Interestingly, these general principles are common for SRs and MIRRs, thus linking mechanistically numerous structurally and functionally diverse receptors.
Transmembrane Interactions as Therapeutic Targets
Because of the ubiquitous nature of protein-protein interactions and the knowledge that inappropriate protein-protein binding can lead to disease, the specific and controlled inhibition and/or modulation of these interactions provides a promising novel approach for rational drug design, as revealed by recent progress in the design of inhibitory antibodies, peptides and small molecules.19–21 Suggesting important role of TM interactions that mediate ligand-induced SR dimerization (oligomerization) and homointeractions between cytoplasmic domains that result in formation of competent signaling oligomers (Fig. 1B), the consensus model of SR signaling reveals these interactions as important points for intervention by relevant targeted agents to modulate SR-mediated signaling. In this review, I focus mainly on TM interactions (Fig. 1C) as universal therapeutic targets for SR-related pathologies. Within the model, specific blockade of the ligand binding-induced TM interactions prevents oligomerization of these domains upon ligand stimulation (Fig. 1C). As a result, signaling oligomers are not formed, Tyr residues do not become phosphorylated and the signaling cascade is not initiated (Fig. 1C). Peptides and their derivatives, small-molecule disrupters of protein-protein interactions, site-specific mutations, and other similar agents/modifications can be used to affect these TM interactions.
Similarly, considering MIRR triggering as an outcome of the interplay between three crucially important interactions: (1) antigen/ligand-MIRR extracellular interactions, (2) intrareceptor TM interactions that stabilize and maintain receptor integrity in resting cells and (3) interreceptor cytoplasmic homointeractions that lead to the formation of competent signaling oligomers, the SCHOOL model reveals these specific protein-protein interactions as points of intervention to inhibit and/or modulate MIRR-mediated TM signaling, thus inhibiting and/or modulating the immune response. While antigen/ligand-receptor interactions are a well-known target for drug design and development,22–36 the last two protein-protein interactions that are critically involved in MIRR triggering/signaling, represent promising novel therapeutic targets as revealed by the model.4,12,15,37–39 In this review, I focus mainly on intra-MIRR TM interactions (Fig. 2C) as universal therapeutic targets for MIRR-related pathologies. As suggested by the model (Fig. 2C),4,12,15,37–39 specific blockade or disruption of the TM interactions between MIRR recognition and signaling subunits causes a physical and functional disconnection of the subunits. In this context, the term “physical disconnection” means “pre-dissociation” rather than full dissociation of the subunits because in the absence of stimulus, they can still remain together. Antigen/ligand stimulation of these “pre-dissociated” receptors leads to reorientation and clustering of the recognition but not signaling subunits. As a result, signaling oligomers are not formed, ITAM Tyr residues do not become phosphorylated and the signaling cascade is not initiated (Fig. 2C). These are common targets for all members of the MIRR family, which means that a general pharmaceutical strategy may be used to treat seemingly disparate disorders such, for example, as T cell-mediated skin diseases and platelet disorders.4,37,40,41 As with SRs, peptides and their derivatives, small molecule disruptors of protein-protein interactions, site-specific mutations, and other similar agents/modifications can be used to affect the MIRR TM interactions. Importantly, our current understanding of the MIRR structure and the nature and specificity of TM interactions between receptor recognition and signaling subunits allows us not only to block or disrupt these protein-protein interactions but to also modulate the interactions by sequence-based approach with using corresponding peptides and/or their derivatives. Strengthening/weakening and/or selective disruption of the association between particular recognition and signaling subunits might allow us not to inhibit, but rather to modulate the ligand-induced cell response. In addition, selective “disconnection” of particular signaling subunits from their recognition partner would be invaluable in studies of MIRR-mediated cell activation.
In summary, despite the difference in details of the molecular mechanisms of action, the use of the suggested SR- and MIRR-targeted TM agents represents a general therapeutic strategy for disorders mediated by members of both receptor families: SRs and MIRRs. By revealing specific protein-protein interactions critically involved in receptor-mediated signaling, current mechanistic models of transmembrane signal transduction (Figs. 1B and 2B) not only provide molecular explanations for many biological phenomena and processes and powerful tools for fundamental and applied research but also suggest novel avenues for drug discovery.16,37,40,42,43 Selected receptor-related therapeutic applications are illustrated in Figures 1D and 2D.
Applications in Biology and Medicine:Single-Chain Receptors
Studies with the epidermal growth factor (EGF) and ErbB2 receptors have shown that synthetic peptides encompassing the TM domains of these receptors inhibit the autophosphorylation and signaling pathway of their cognate receptor.44,45 These peptides are thought to block/disrupt specific TM interactions, thereby inhibiting receptor dimerization and activation.44,45
Using differential epitope tagging, it has been demonstrated that β2-adrenergic receptors form homodimers and that TM domain VI of the receptor may represent part of an interface for receptor dimerization.46 As shown, a peptide derived from this domain inhibits both dimerization and β-adrenergic agonist-promoted stimulation of adenylyl cyclase activity.46 In contrast, a peptide based on the sequence of transmembrane domain 6 of the D1 dopamine receptor (D1DR) has been found to specifically inhibit D1DR binding and function without affecting receptor oligomerization.47 One possible explanation for this finding is that in addition to ligand-stimulated dimerization of receptors, the correct (permissive) relative orientation in the receptor dimers formed can also play an important role in D1DR signaling. The importance of the relative orientation has been shown for other SRs such as, for example, EGF receptors,48 Epo receptor,49–52 toll-like receptors (TLRs)53 and the integral membrane receptor LuxPQ.54 Thus, the presence of the TM peptide bound to the D1DR TM domain is likely to prevent ligand-induced formation of receptor dimers with correct intermolecular orientation, thus preventing generation of the activation signal.
Another example of SR-targeted TM inhibitory peptides, the short peptide sequences corresponding to the Neu RTK TM domain, have been also reported to independently fold in membranes, interact with the full length receptor and inhibit transformation of cells in vitro and in vivo.55
Thus, the sequence-based blockade of the interreceptor TM protein interactions as applied to SR signaling provides evidence for the importance and clinical significance of the SR-related TM-targeted strategy.
Applications in Biology and Medicine: Multi-chain Activating Receptors
The vast majority of multi-chain activating receptors are immune receptors that recognize foreign antigens and initiate a variety of biological responses.2 For this reason, the most commonly used name for this family is multi-chain immune recognition receptors (MIRRs), the term that was first introduced in 1992 by Keegan and Paul.3
T-cell antigen receptor.
TCR provides an intriguing ability of T cells to discern and differentially respond to major histocompatibility complex (MHC)-bound peptides that can differ by only a single amino acid. Structurally, TCR is a member of the MIRR family with the α and β antigen-binding subunits that are bound by electrostatic transmembrane interactions with three signaling homo- and heterodimers: ζζ, CD3ɛδ and CD3ɛγ (Fig. 3), thus maintaining the integrity of TCR in resting T cells.56,57 Within the SCHOOL model of TCR-mediated TM signal transduction (Fig. 3), distinct TCR signaling is achieved through ζ and CD3 signaling oligomers (Fig. 3),12,15,16 and intrareceptor transmembrane interactions represent not only promising therapeutic targets but also an important point of viral attack (Fig. 4).4,16,37,40,58
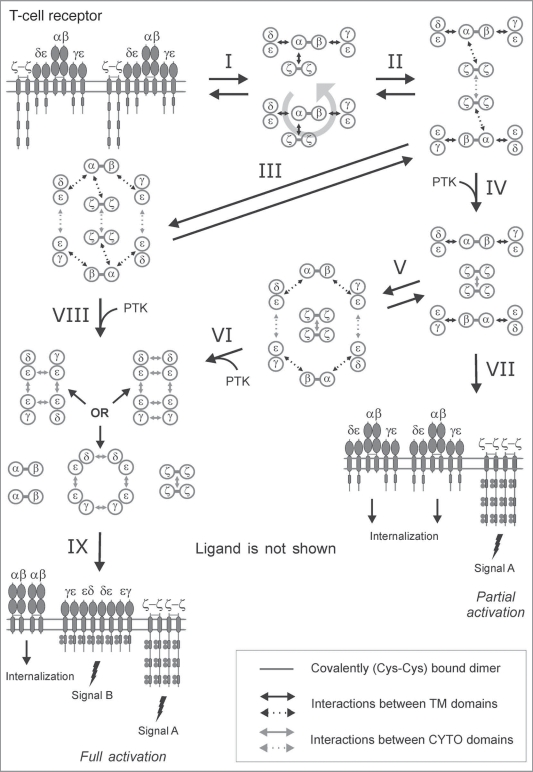
Figure 3.
SCHOOL model of T-cell receptor signaling. A schematic representation of the SCHOOL-based molecular mechanisms of T-cell receptor (TCR) signaling. Immunoreceptor tyrosine-based activation motifs (ITAMs) are shown as gray rectangles. TCR-CD3-ζ components are represented as whole polypeptides and as a simplified axial view. All interchain interactions in intermediate complexes are shown by dotted arrows reflecting their transition state. Circular arrow indicates ligand-induced receptor reorientation. Interaction with multivalent ligand (not shown) clusters the receptors and pushes them to reorientate (I) and bring signaling subunits into a correct relative orientation and in sufficient proximity in the formed receptor oligomer (for illustrative purposes, receptor dimer is shown), thus starting the trans-homointeractions between ζ molecules (II). Then, two alternative pathways can take a place depending on the nature of activating stimuli. First is going through a stage IV resulting in formation of ζ2 dimer (dimer of dimers) and phosphorylation of the ζ ITAM tyrosines, thus triggering downstream signaling events. Then, the signaling ζ oligomers formed subsequently dissociate from the TCR-CD3 complex, resulting in internalization of the remaining engaged TCR-CD3 complexes (VII). This pathway leads to partial (or incomplete) T cell activation. Alternatively, the intermediate complex formed at the stage II can undergo further rearrangements, starting trans-homointeractions between CD3 proteins (III) and resulting in formation of an oligomeric intermediate. Again, the stages I, II and III can be reversible or irreversible depending on interreceptor proximity and relative orientation of the receptors in TCR dimers/oligomers as well as on time duration of the TCR-ligand contact and lifetime of the receptor in TCR dimers/oligomers that generally correlate with the nature of the stimulus and its specificity and affinity/avidity. Next, in the signaling oligomers formed (III), the ITAM tyrosines undergo phosphorylation by PTKs that leads to generation of the activation signal, dissociation of signaling oligomers and internalization of the remaining engaged TCRαβ chains (VIII, XI). This pathway provides at least two different activation signals from the ζ and CD3 signaling oligomers (signals A and B), respectively, and results in full T-cell activation. The distinct signaling through ζ and CD3 oligomers (or through various combinations of signaling chains in CD3 oligomeric structures) might be also responsible for distinct functions such as T-cell proliferation, effector functions, T-cell survival, pathogen clearance, TCR anergy, etc. In addition, the signaling oligomers formed can sequentially interact with the signaling subunits of nonengaged TCRs resulting in formation of higher-order signaling oligomers, thus amplifying and propagating the activation signal (not shown). Also, this leads to the release and subsequent internalization of the remaining nonengaged TCR complexes and/or TCRαβ chains (not shown). Abbreviations: PTK, protein tyrosine kinase. Phosphate groups are shown as filled gray circles.
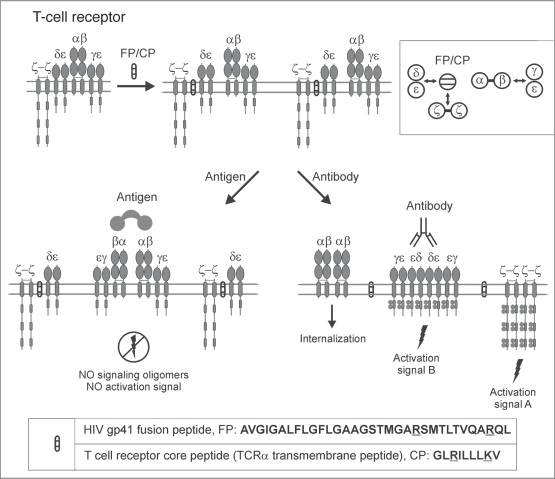
Figure 4.
SCHOOL model of T-cell receptor signaling in the presence of transmembrane peptides. A schematic representation of the SCHOOL-based mechanisms of action of T-cell receptor transmembrane inhibitors such as the T-cell receptor core peptide (CP) and HIV-1 gp41 fusion peptide (FP). Considering the close similarity in patterns of inhibition of T-cell activation and immunosuppressive activity observed for CP and FP, the SCHOOL model reasonably suggests a similar molecular mechanism of action for both peptides. Within the model, these peptides compete with the TCRα chain for binding to the CD3δɛ and ζ signaling subunits, thus disrupting the transmembrane (TM) interactions between these subunits and resulting in disconnection and predissociation of the relevant signaling subunits from the remaining receptor complex (also shown in the inset as a simplified axial view). This prevents formation of signaling oligomers upon multivalent antigen stimulation, thus inhibiting antigen-mediated T-cell activation. In contrast, stimulation of these “predissociated” MIRRs with cross-linking antibodies to signaling subunit(s) should still lead to receptor triggering and cell activation. The model predicts that the same mechanisms of inhibitory action can be applied to TCR TM peptides corresponding to the TM regions of not only the TCRαβ recognition subunits but the corresponding CD3ɛ, CD3δ, CD3γ and ζ signaling subunits as well.
Transmembrane peptides capable of inhibiting TCR-mediated cell activation, including the TCR core peptide (CP), a synthetic peptide corresponding to the sequence of the TCRα TM domain and known to interact with the TM domains of CD3δɛ and ζ,56,57 were first reported in 1997.59 Interestingly, T-cell activation via anti-CD3 antibodies is not affected by this peptide. As shown, TCR CP might be a proper treatment for human T-cell-mediated dermatoses substituting for corticosteroids60 and a novel potential therapy for rheumatoid arthritis and other T-cell-mediated disorders.59,61,62 However, despite extensive studies,61,62 the mode of action of this clinically relevant peptide has not been elucidated until 2004 when the SCHOOL model of TCR signaling (Fig. 3) was first introduced.12
Briefly, as suggested by the SCHOOL model (Fig. 4),4,12,15,37,38 TCR CP competes with the TCRα chain for binding to CD3δɛ and ζ hetero- and homodimers, respectively, thus resulting in disconnection/pre-dissociation of the signaling subunits from the remaining receptor complex (Fig. 4). This leads to inhibition of antigen- but not antibody-mediated TCR triggering and cell activation (Fig. 4). Interestingly, the proposed mechanism is the only mechanism consistent with all experimental and clinical data reported up to date for TCR and other MIRR TM peptides and their lipid and/or sugar conjugates.39,60,62–72
The SCHOOL model predicted that the same mechanisms of inhibitory action can be applied to MIRR TM peptides corresponding to the TM regions of not only the MIRR recognition subunits but to the corresponding signaling subunits as well.12,15 This was recently confirmed experimentally by showing that the synthetic peptides corresponding not only to the TM sequence of the antigen recognition TCRα subunit (i.e., TCR CP) but also to the sequences of the TM regions of the signaling CD3 (δ, ɛ or γ) and ζ subunits are able to inhibit the immune response in vivo (CD3 TM peptides) and NK-cell cytolytic activity in vivo (ζ TM peptide).62,73
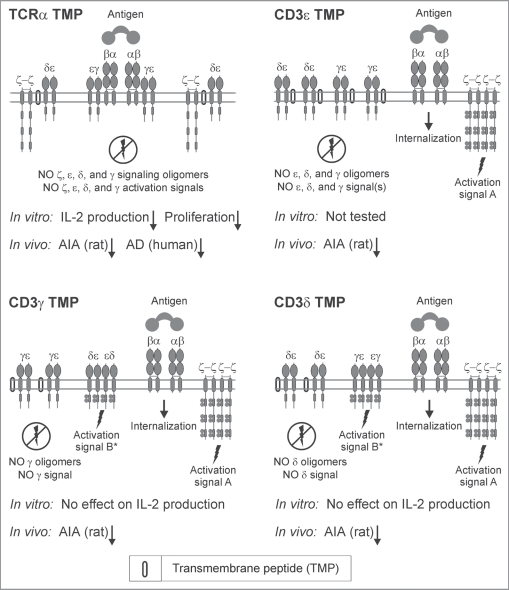
Interestingly, the model suggests a molecular explanation for the apparent discrepancy in CD3 TM peptide activity between in vitro and in vivo T-cell inhibition (Fig. 5).62 It has been shown that the CD3δ and CD3γ TM peptides do not impact T cell function in vitro (the CD3ɛ TM peptide has not been used in the reported in vitro experiments because of solubility issues) but that all three CD3 TM peptides decrease signs of inflammation in the adjuvant-induced arthritis rat model in vivo and inhibit an immune response.62 Within the SCHOOL model, the CD3δ and CD3γ TM peptides disconnect the corresponding signaling subunits (CD3δ and CD3γ, respectively) from the remaining receptor complex (Fig. 5). Thus, these subunits do not participate in further signaling processes upon antigen stimulation. On the other hand, the previously reported in vitro activation studies with T cells lacking CD3γ and/or CD3δ cytoplasmic domains indicate that antigen-stimulated induction of cytokine secretion and T-cell proliferation are intact,74 thus explaining the absence of inhibitory effect of the CD3δ and CD3γ TM peptides in the in vitro activation assays used.62 However, in vivo deficiency either of CD3δ or CD3γ results in severe immunodeficiency disorders.75 This could explain the inhibitory effect observed in the in vivo studies for all three CD3δ, γ and ɛ TM peptides (Fig. 5).62 Thus, these experimental data confirm that our ability to selectively “disconnect” specific signaling subunits using the MIRR TM peptides in line with the SCHOOL model can provide a powerful tool to study MIRR functions and immune cell signaling as well as to rationally design novel inhibitors and/or modulators of the immune response.4,12,15,16,37,40
Figure 5.
SCHOOL mechanisms of selective modulation of T-cell receptor signaling by different transmembrane peptides. A schematic representation of the SCHOOL-based mechanisms of action of different T-cell receptor transmembrane inhibitors. Within the SCHOOL model, upon antigen stimulation of T cells, T-cell receptor α-chain (TCRα) transmembrane peptide (TMP) prevents formation of all signaling oligomers, including ζ, CD3ɛ, CD3δ and CD3γ. This inhibits T-cell activation in both in vitro and in vivo. In contrast, other TMPs prevent formation of signaling oligomers (and therefore signaling) of selected signaling subunits. This inhibits T-cell activation in vivo whereas inhibition in vitro depends on the evaluation method used. For example, antigen-stimulated induction of cytokine secretion and T-cell proliferation in T cells lacking CD3γ and/or CD3δ cytoplasmic domains,74 thus explaining the absence of inhibitory effect of the CD3δ and CD3γ TM peptides in the in vitro IL-2 production and T-cell proliferation assays used as markers of T-cell activation.62 Abbreviations: AD, atopic dermatitis; AIA, adjuvant-induced arthritis; IL-2, interleukin 2.
Glycoprotein VI receptor.
Activation of circulating platelets by exposed vessel wall collagen is a primary step in the pathogenesis of thrombotic diseases such as heart attack and stroke. However, despite intensive research efforts in antithrombotic drug discovery and development, uncontrolled hemorrhage still remains the most common side effect associated with antithrombotic drugs that are currently in use. The selective inhibition of GPVI, the central platelet collagen receptor on platelets, and/or its signaling may inhibit thrombosis without affecting hemostatic plug formation, thus representing a magic bullet for platelet-mediated diseases.76 However, the mechanism of GPVI signaling has remained unknown till recently,39,41 hindering the further development of this promising antithrombotic strategy.
The GPVI-FcRγ receptor complex is formed by the association of recognition GPVI subunit with signal-transducing FcRγ subunit that contains the ITAM sequence in its intrinsically disordered cytoplasmic domain. Thus, structurally, GPVI belongs to the MIRR family and the SCHOOL model could be and was applied to explain the mechanisms of GPVI-mediated signaling.16,37,40 This resulted in the development of novel mechanistic concept of platelet inhibition and the invention of new platelet inhibitors.39–41
NKG2D receptor.
Inflammatory bowel diseases (IBDs) affect millions of people worldwide with 2.8 million currently diagnosed with ulcerative colitis (UC) or Crohn’s disease (CD) in the United States alone. There is still a great need for additional targets and agents for effectively reducing IBDs, despite advances in immune disorder research.77
In 2007, a unique subset of CD4+ T cells expressing the activating NKG2D receptor has been identified in both the mucosa and peripheral blood of IBD patients, thus suggesting an important role of this receptor in the pathogenesis of IBDs.78 Later, in two independent studies,79,80 regulation of the NKG2D signaling pathway has been proven to represent a new therapeutic target for IBDs and be of key importance in successful treatment of UC and CD. However, further development of this promising strategy has been hindered by the lack of knowledge about the mechanism of NKG2D signaling.
As a member of the MIRR family, the NKG2D activating receptor consists of recognition NKG2D subunit non-covalently associated in transmembrane milieu with signal-transducing DAP10 subunit that contains the YxxM signaling sequence in its cytoplasmic domain predicted to be intrinsically disordered. This suggests that the NKG2D receptor complex signals through the SCHOOL model-based mechanisms and the NKG2D-DAP10 TM interactions represent a promising point of intervention that can be targeted by relevant agents.4,16,37,40
TREM-1 receptor.
Triggering receptor expressed on myeloid cells 1 (TREM-1) is inducible on monocyte/macrophages and neutrophils and accelerates tissue destruction by propagating inflammatory responses in disease related to bacterial infections.81–83 Recently, blockade of TREM-1 has been suggested as a new approach to rheumatic diseases that is safer than the presently available immunosuppressive treatments.83
Structurally, TREM-1 belongs to the MIRR family and signals through the non-covalently bound ITAM-containing adaptor molecule, DAP12. Thus, as predicted by the SCHOOL model of MIRR signaling,4,40 the intrareceptor TM interactions between TREM-1 and DAP-12 subunits represent a promising novel therapeutic target.
What Viral Pathogenesis Teaches Us
To successfully infect, replicate and persist in the host, viruses have evolved numerous strategies to take control of multiple cellular processes including those that target TM signal transduction mediated by immune receptors. Example is the inhibition of T-cell activation that has been reported for the fusion peptide (FP) found in the N terminus of the HIV envelope glycoprotein 41 (gp41).84,85 These data are the first to demonstrate that FP not only functions to fuse the virion with the host cell membrane86,87 but also has immunomodulatory activity. This peptide has been shown to inhibit antigen-specific T cell proliferation and proinflammatory cytokine secretion in vitro.84 This effect is specific: T-cell activation via PMA/ionomycin or mitogenic antibodies to CD3 is not affected by FP. As with TCR-CP, FP shows immunosuppressive activity, inhibiting the activation of arthritogenic T cells in the autoimmune disease model of adjuvant arthritis and reducing the disease-associated IFNγ response.84 Similarly to TCR CP,60,69,88,89 HIV gp41 FP has been suggested for the treatment of T-cell-mediated pathologies.84 However, the mode of action of this peptide has remained unexplained until 2006 when the SCHOOL model was first applied to this area.4
Within the SCHOOL model, HIV gp41 FP prevents formation of signaling oligomers and thus inhibits antigen-dependent T-cell activation (Fig. 4), acting similarly in this respect to TCR CP. However, stimulation with anti-CD3 antibodies of these “pre-dissociated” TCRs still results in receptor triggering and cell activation (Fig. 4).4 More recent studies85 have confirmed the predicted mechanism of action of the HIV FP.
Charge distribution patterns for fusion protein regions are surprisingly conserved in many unrelated viruses and show similarities to those for TCR CP and HIV FP (Fig. 6). Thus, it is highly probable that these proteins would also target the TCR TM interactions using the SCHOOL mechanism. Exploratory sequence investigation of FPs from SARS-CoV, Lassa virus (LASV), lymphocytic choriomeningitis virus (LCMV), Mopeia virus (MOPV) and Tacaribe virus (TACV) reveals a close similarity in the positioning of the electropositive residues (Fig. 6). Intriguingly, analysis of other unrelated viruses has yielded similar correlations in primary structure and function. Earlier studies have reported an inhibitory effect on lymphocyte proliferation by CKS-17 peptide, a synthetic 17-mer peptide with sequence corresponding to a highly conserved region of TM proteins of human and animal retroviruses including the TM protein gp21 of human T lymphotropic virus type 1 (HTLV-1).90–92 Interestingly, peptides corresponding to regions of HIV TM protein gp41 homologous to the highly conserved and immunosuppressive sequence contained within the TM proteins p15E and gp21 of animal and human retroviruses, respectively, have been also reported to inhibit lymphoproliferation.91,92 Recently, filoviral 17-mer peptides corresponding to a 17 amino acid domain in filoviral glycoproteins that resembles an immunosuppressive motif in retroviral envelope proteins have been demonstrated to inhibit TCR-mediated cell activation.93 In all peptides, a striking similarity is observed in the charge distribution patterns with the positioning of the essential positively charged residues almost identical to that for the HIV gp41 FP (Fig. 6), suggesting again a similarity in their mode of action. This clearly demonstrates that different viruses have adopted similar mechanisms of specifically targeting TCR, disrupting receptor architecture and suppressing the immune system.
Figure 6.
Similarities in the charge distribution patterns of different immunomodulatory viral sequences. Charge distribution patterns of different immunomodulatory viral sequences. Primary sequence analysis of proven and predicted immunomodulatory sequences of viral fusion protein regions and other domains shows a similarity in charge distribution pattern with two essential positively charged residues spaced apart by 4 (class I) or 8 (class III) amino acids or with three essential positively charged residues spaced apart by 3 amino acids (class II), suggesting a similarity of the SCHOOL-based mechanisms used by diverse viruses in their pathogenesis to modulate the host immune response. Abbreviations: TCR, T-cell receptor; CP, core peptide; HIV, human immunodeficiency virus; gp, glycoprotein; FP, fusion peptide/protein; TMD, transmembrane domain; CKS-17, a synthetic retroviral envelope heptadecapeptide; Fr-MLV, Friend murine leukemia virus; gp, glycoprotein; HHV-6 U24, human herpesvirus 6 U24 protein; HTLV-1, human T lymphotropic virus type 1; HVA, herpesvirus ateles; HVS, herpesvirus saimiri; ITAM, immunoreceptor tyrosine-based activation motif; LASV, Lassa virus; LCMV, lymphocytic choriomeningitis virus; MARV, Marburg virus; MOPV, Mopeia virus; SARS-CoV, severe acute respiratory syndrome coronavirus; SEBOV, Sudan Ebola virus; TACV, Tacaribe virus; Tip, tyrosine kinase interacting protein; Tio, two-in-one protein; TMD, transmembrane domain; ZEBOV, Zaire Ebola virus.
The generality of the SCHOOL model suggests that TM interactions of other MIRRs can also represent a point of viral attack. As reported,94 the recognition of the human CMV tegument protein pp65 by NKp30, the natural killer (NK) cell activating receptor, does not lead to NK-cell activation but instead results in a general inhibition mediated by the dissociation of the NKp30-ζ complex and loss in the ability of cells to kill virus-infected cells. Within the context of the SCHOOL model, pp65 may target the TM interactions between NKp30 and ζ, leading to functional disconnection of ζ in a manner similar to the action of TCR CP and HIV FP (Fig. 4).
TM interactions can be targeted not only from outside but also from inside the cell. Recently, it has been shown that the HHV-6 U24 protein downregulates TCR surface expression and that U24-expressing T cells are resistant to activation by antigen-presenting cells.95 TCR downregulation activity has been also reported for the highly conserved membrane-proximal sequence of the tyrosine kinase-interacting protein (Tip) of HVS.96,97 Notably, primary sequences of HHV-6 U24,28–60 and HIV FP exhibit a similar pattern with two Arg residues spaced apart by eight amino acids (Fig. 6). The positioning of the essential electropositive residues is remarkably conserved in HVS Tip213–228, the relevant domain of the two-in-one (Tio) protein of herpesvirus ateles (HVA), and HTLV-1 gp21 (Fig. 6). Thus, the SCHOOL mechanisms similar to those applied for TCR CP and HIV gp41 FP (Fig. 4) can be used by HHV-6 and other viruses in their arsenal of immune evasion tactics. Importantly, as predicted, the viral agents prevent only antigen- but not antibody-specific T cell activation (Fig. 4). Indeed, anti-CD3 antibodies activate HHV-6-infected T cells, resulting to great increase of viral replication.98,99 Interestingly, increase of viral replication induced by clinically relevant antibody(OKT3)-mediated activation of HIV-infected T cells is currently used for purging of the latent HIV-1 reservoirs in vivo,100 thus suggesting a potential generality of the SCHOOL mechanism-based antiviral approaches.
There are several important lessons that we can learn from the molecular mechanisms of viral pathogenesis:
using modern methodologies,101–108 it is possible to design and produce TM agents that are able to modulate the immune response as specific and effective as viruses do;
as predicted, TCR CP and many different immunomodulatory viral sequences affect similar TCR TM interactions, suggesting that general principles of designing TM peptides might be readily used at this stage;104,105
antibodies to MIRR signaling subunits can be used to modulate the affected immune cell response during viral infection;
considering our selective ability to functionally disconnect any particular TCR signaling subunits,16,37,40,62 we can use the relevant peptides as a powerful tool to dissect fine mechanisms of viral pathogenesis; and, finally,
two unrelated viruses, HIV and human CMV, use a similar mode of action to modulate the host immune response mediated by two functionally different MIRRs-TCR and NKp30, thus suggesting that similar general mechanisms can be or are used by other viral and possibly non-viral pathogens.
In conclusion, rather than targeting virus-specific proteins or processes, it would be advantageous to transfer therapeutic strategies that target redundant processes found among a number of viruses. In addition, as demonstrated by the similar function of natural HIV FP and synthetically derived clinically relevant TCR CP, viral immune evasion strategies can be transferred to therapeutic strategies that require similar functionalities. Viruses represent years of evolution and the efficiency and optimization that come along with it. Therefore, viral functions should not only be studied as foreign processes but as efficient strategies that we can use in our own attempts at immune evasion or immunomodulation.
Conclusions
Considering growing interest in targeting cell surface receptor signaling as a potential treatment strategy for different diseases, the development of novel pharmacological approaches critically depends on our improved understanding of the molecular mechanisms underlying receptor triggering and subsequent transmembrane signal transduction. Novel general platform for receptor-mediated signaling suggests that receptor oligomerization induced or tuned upon ligand binding outside the cell is translated across the membrane into protein oligomerization inside the cell. Within this platform, homooligomerization of receptor intracellular signaling domains is considered as a necessary and sufficient condition for receptor triggering. This reveals inter- and intrareceptor transmembrane interactions as universal therapeutic targets for a diverse variety of seemingly unrelated immune (and not only immune) disorders, and, together with the lessons learned from viral pathogenesis, opens new horizons in the development of novel therapeutic strategies targeting receptor-mediated transmembrane signal transduction.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/10746
References
- 1.Rudd CE. Disabled receptor signaling and new primary immunodeficiency disorders. N Engl J Med. 2006;354:1874–1877. doi: 10.1056/NEJMp068062. [DOI] [PubMed] [Google Scholar]
- 2.Sigalov AB, editor. Multichain Immune Recognition Receptor Signaling: From Spatiotemporal Organization to Human Disease. New York: Springer-Verlag; 2008. [PubMed] [Google Scholar]
- 3.Keegan AD, Paul WE. Multichain immune recognition receptors: similarities in structure and signaling pathways. Immunol Today. 1992;13:63–68. doi: 10.1016/0167-5699(92)90136-U. [DOI] [PubMed] [Google Scholar]
- 4.Sigalov AB. Immune cell signaling: a novel mechanistic model reveals new therapeutic targets. Trends Pharmacol Sci. 2006;27:518–524. doi: 10.1016/j.tips.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Heldin CH. Dimerization of cell surface receptors in signal transduction. Cell. 1995;80:213–223. doi: 10.1016/0092-8674(95)90404-2. [DOI] [PubMed] [Google Scholar]
- 6.Hubbard SR. Structural analysis of receptor tyrosine kinases. Prog Biophys Mol Biol. 1999;71:343–358. doi: 10.1016/s0079-6107(98)00047-9. [DOI] [PubMed] [Google Scholar]
- 7.Weiss A, Schlessinger J. Switching signals on or off by receptor dimerization. Cell. 1998;94:277–280. doi: 10.1016/s0092-8674(00)81469-5. [DOI] [PubMed] [Google Scholar]
- 8.Schlessinger J. Signal transduction. Autoinhibition control. Science. 2003;300:750–752. doi: 10.1126/science.1082024. [DOI] [PubMed] [Google Scholar]
- 9.Siegel RM, Muppidi JR, Sarker M, Lobito A, Jen M, Martin D, et al. SPOTS: signaling protein oligomeric transduction structures are early mediators of death receptor-induced apoptosis at the plasma membrane. J Cell Biol. 2004;167:735–744. doi: 10.1083/jcb.200406101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan FK. Three is better than one: pre-ligand receptor assembly in the regulation of TNF receptor signaling. Cytokine. 2007;37:101–107. doi: 10.1016/j.cyto.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan KF, Siegel MR, Lenardo JM. Signaling by the TNF receptor superfamily and T cell homeostasis. Immunity. 2000;13:419–422. doi: 10.1016/s1074-7613(00)00041-8. [DOI] [PubMed] [Google Scholar]
- 12.Sigalov AB. Multichain immune recognition receptor signaling: different players, same game? Trends Immunol. 2004;25:583–589. doi: 10.1016/j.it.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Schamel WW, Arechaga I, Risueno RM, van Santen HM, Cabezas P, Risco C, et al. Coexistence of multivalent and monovalent TCRs explains high sensitivity and wide range of response. J Exp Med. 2005;202:493–503. doi: 10.1084/jem.20042155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berlanga O, Bori-Sanz T, James JR, Frampton J, Davis SJ, Tomlinson MG, et al. Glycoprotein VI oligomerization in cell lines and platelets. J Thromb Haemost. 2007;5:1026–1033. doi: 10.1111/j.1538-7836.2007.02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sigalov A. Multi-chain immune recognition receptors: spatial organization and signal transduction. Semin Immunol. 2005;17:51–64. doi: 10.1016/j.smim.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Sigalov AB. Signaling chain homooligomerization (SCHOOL) model. Adv Exp Med Biol. 2008;640:121–163. doi: 10.1007/978-0-387-09789-3_12. [DOI] [PubMed] [Google Scholar]
- 17.Sigalov A, Aivazian D, Stern L. Homooligomerization of the cytoplasmic domain of the T cell receptor zeta chain and of other proteins containing the immunoreceptor tyrosine-based activation motif. Biochemistry. 2004;43:2049–2061. doi: 10.1021/bi035900h. [DOI] [PubMed] [Google Scholar]
- 18.Sigalov AB, Zhuravleva AV, Orekhov VY. Binding of intrinsically disordered proteins is not necessarily accompanied by a structural transition to a folded form. Biochimie. 2007;89:419–421. doi: 10.1016/j.biochi.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loregian A, Palu G. Disruption of protein-protein interactions: towards new targets for chemotherapy. J Cell Physiol. 2005;204:750–762. doi: 10.1002/jcp.20356. [DOI] [PubMed] [Google Scholar]
- 20.Hershberger SJ, Lee SG, Chmielewski J. Scaffolds for blocking protein-protein interactions. Curr Top Med Chem. 2007;7:928–942. doi: 10.2174/156802607780906726. [DOI] [PubMed] [Google Scholar]
- 21.Sillerud LO, Larson RS. Design and structure of peptide and peptidomimetic antagonists of protein-protein interaction. Curr Protein Pept Sci. 2005;6:151–169. doi: 10.2174/1389203053545462. [DOI] [PubMed] [Google Scholar]
- 22.Norman PS. Immunotherapy: 1999–2004. J Allergy Clin Immunol. 2004;113:1013–1023. doi: 10.1016/j.jaci.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 23.Jackson SP, Schoenwaelder SM. Antiplatelet therapy: in search of the ‘magic bullet’. Nat Rev Drug Discov. 2003;2:775–789. doi: 10.1038/nrd1198. [DOI] [PubMed] [Google Scholar]
- 24.Kepley CL. New approaches to allergen immunotherapy. Curr Allergy Asthma Rep. 2006;6:427–433. doi: 10.1007/s11882-996-0017-4. [DOI] [PubMed] [Google Scholar]
- 25.Kraft S, Kinet JP. New developments in FcepsilonRI regulation, function and inhibition. Nat Rev Immunol. 2007;7:365–378. doi: 10.1038/nri2072. [DOI] [PubMed] [Google Scholar]
- 26.McNicol A, Israels SJ. Platelets and anti-platelet therapy. J Pharmacol Sci. 2003;93:381–396. doi: 10.1254/jphs.93.381. [DOI] [PubMed] [Google Scholar]
- 27.Molloy PE, Sewell AK, Jakobsen BK. Soluble T cell receptors: novel immunotherapies. Curr Opin Pharmacol. 2005;5:438–443. doi: 10.1016/j.coph.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Pons L, Burks W. Novel treatments for food allergy. Expert Opin Investig Drugs. 2005;14:829–834. doi: 10.1517/13543784.14.7.829. [DOI] [PubMed] [Google Scholar]
- 29.Chatenoud L, Bluestone JA. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat Rev Immunol. 2007;7:622–632. doi: 10.1038/nri2134. [DOI] [PubMed] [Google Scholar]
- 30.St Clair EW, Turka LA, Saxon A, Matthews JB, Sayegh MH, Eisenbarth GS, et al. New reagents on the horizon for immune tolerance. Annu Rev Med. 2007;58:329–346. doi: 10.1146/annurev.med.58.061705.145449. [DOI] [PubMed] [Google Scholar]
- 31.Hombach A, Heuser C, Abken H. The recombinant T cell receptor strategy: insights into structure and function of recombinant immunoreceptors on the way towards an optimal receptor design for cellular immunotherapy. Curr Gene Ther. 2002;2:211–226. doi: 10.2174/1566523024605573. [DOI] [PubMed] [Google Scholar]
- 32.Luzak B, Golanski J, Rozalski M, Bonclerand MA, Watala C. Inhibition of collagen-induced platelet reactivity by DGEA peptide. Acta Biochim Pol. 2003;50:1119–1128. [PubMed] [Google Scholar]
- 33.O’Herrin SM, Slansky JE, Tang Q, Markiewicz MA, Gajewski TF, Pardoll DM, et al. Antigen-specific blockade of T cells in vivo using dimeric MHC peptide. J Immunol. 2001;167:2555–2560. doi: 10.4049/jimmunol.167.5.2555. [DOI] [PubMed] [Google Scholar]
- 34.Andrasfalvy M, Peterfy H, Toth G, Matko J, Abramson J, Kerekes K, et al. The beta subunit of the type I Fcepsilon receptor is a target for peptides inhibiting IgE-mediated secretory response of mast cells. J Immunol. 2005;175:2801–2806. doi: 10.4049/jimmunol.175.5.2801. [DOI] [PubMed] [Google Scholar]
- 35.Cronin SJ, Penninger JM. From T-cell activation signals to signaling control of anti-cancer immunity. Immunol Rev. 2007;220:151–168. doi: 10.1111/j.1600-065X.2007.00570.x. [DOI] [PubMed] [Google Scholar]
- 36.Waldmann TA. Immune receptors: targets for therapy of leukemia/lymphoma, autoimmune diseases and for the prevention of allograft rejection. Annu Rev Immunol. 1992;10:675–704. doi: 10.1146/annurev.iy.10.040192.003331. [DOI] [PubMed] [Google Scholar]
- 37.Sigalov AB. Transmembrane interactions as immunotherapeutic targets: lessons from viral pathogenesis. Adv Exp Med Biol. 2007;601:335–344. doi: 10.1007/978-0-387-72005-0_36. [DOI] [PubMed] [Google Scholar]
- 38.Sigalov AB. Interaction between HIV gp41 fusion peptide and T cell receptor: putting the puzzle pieces back together. Faseb J. 2007;21:1633–1634. doi: 10.1096/fj.07-0603ltr. [DOI] [PubMed] [Google Scholar]
- 39.Sigalov AB. More on: glycoprotein VI oligomerization: a novel concept of platelet inhibition. J Thromb Haemost. 2007;5:2310–2312. doi: 10.1111/j.1538-7836.2007.02714.x. [DOI] [PubMed] [Google Scholar]
- 40.Sigalov AB. SCHOOL model and new targeting strategies. Adv Exp Med Biol. 2008;640:268–311. doi: 10.1007/978-0-387-09789-3_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sigalov AB. Novel mechanistic concept of platelet inhibition. Expert Opin Ther Targets. 2008;12:677–692. doi: 10.1517/14728222.12.6.677. [DOI] [PubMed] [Google Scholar]
- 42.Sigalov AB. Novel mechanistic insights into viral modulation of immune receptor signaling. PLoS Pathog. 2009;5:1000404. doi: 10.1371/journal.ppat.1000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sigalov AB. Protein intrinsic disorder and oligomericity in cell signaling. Mol Biosyst. 2010;6:451–461. doi: 10.1039/b916030m. [DOI] [PubMed] [Google Scholar]
- 44.Bennasroune A, Fickova M, Gardin A, Dirrig-Grosch S, Aunis D, Cremel G, et al. Transmembrane peptides as inhibitors of ErbB receptor signaling. Mol Biol Cell. 2004;15:3464–3474. doi: 10.1091/mbc.E03-10-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bennasroune A, Gardin A, Auzan C, Clauser E, Dirrig-Grosch S, Meira M, et al. Inhibition by transmembrane peptides of chimeric insulin receptors. Cell Mol Life Sci. 2005;62:2124–2131. doi: 10.1007/s00018-005-5226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hebert TE, Moffett S, Morello JP, Loisel TP, Bichet DG, Barret C, et al. A peptide derived from a beta2-adrenergic receptor transmembrane domain inhibits both receptor dimerization and activation. J Biol Chem. 1996;271:16384–16392. doi: 10.1074/jbc.271.27.16384. [DOI] [PubMed] [Google Scholar]
- 47.George SR, Lee SP, Varghese G, Zeman PR, Seeman P, Ng GY, et al. A transmembrane domain-derived peptide inhibits D1 dopamine receptor function without affecting receptor oligomerization. J Biol Chem. 1998;273:30244–30248. doi: 10.1074/jbc.273.46.30244. [DOI] [PubMed] [Google Scholar]
- 48.Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell. 2006;125:1137–1149. doi: 10.1016/j.cell.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 49.Jiang G, Hunter T. Receptor signaling: when dimerization is not enough. Curr Biol. 1999;9:568–571. doi: 10.1016/s0960-9822(99)80357-1. [DOI] [PubMed] [Google Scholar]
- 50.Syed RS, Reid SW, Li C, Cheetham JC, Aoki KH, Liu B, et al. Efficiency of signalling through cytokine receptors depends critically on receptor orientation. Nature. 1998;395:511–516. doi: 10.1038/26773. [DOI] [PubMed] [Google Scholar]
- 51.Livnah O, Johnson DL, Stura EA, Farrell FX, Barbone FP, You Y, et al. An antagonist peptide-EPO receptor complex suggests that receptor dimerization is not sufficient for activation. Nat Struct Biol. 1998;5:993–1004. doi: 10.1038/2965. [DOI] [PubMed] [Google Scholar]
- 52.Ballinger MD, Wells JA. Will any dimer do? Nat Struct Biol. 1998;5:938–940. doi: 10.1038/2911. [DOI] [PubMed] [Google Scholar]
- 53.Gay NJ, Gangloff M, Weber AN. Toll-like receptors as molecular switches. Nat Rev Immunol. 2006;6:693–698. doi: 10.1038/nri1916. [DOI] [PubMed] [Google Scholar]
- 54.Neiditch MB, Federle MJ, Pompeani AJ, Kelly RC, Swem DL, Jeffrey PD, et al. Ligand-induced asymmetry in histidine sensor kinase complex regulates quorum sensing. Cell. 2006;126:1095–1108. doi: 10.1016/j.cell.2006.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lofts FJ, Hurst HC, Sternberg MJ, Gullick WJ. Specific short transmembrane sequences can inhibit transformation by the mutant neu growth factor receptor in vitro and in vivo. Oncogene. 1993;8:2813–2820. [PubMed] [Google Scholar]
- 56.Call ME, Pyrdol J, Wiedmann M, Wucherpfennig KW. The organizing principle in the formation of the T cell receptor-CD3 complex. Cell. 2002;111:967–979. doi: 10.1016/s0092-8674(02)01194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manolios N, Bonifacino JS, Klausner RD. Transmembrane helical interactions and the assembly of the T cell receptor complex. Science. 1990;249:274–277. doi: 10.1126/science.2142801. [DOI] [PubMed] [Google Scholar]
- 58.Kim WM, Sigalov AB. Viral pathogenesis, modulation of immune receptor signaling and treatment. Adv Exp Med Biol. 2008;640:325–349. doi: 10.1007/978-0-387-09789-3_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manolios N, Collier S, Taylor J, Pollard J, Harrison LC, Bender V. T-cell antigen receptor transmembrane peptides modulate T-cell function and T cell-mediated disease. Nat Med. 1997;3:84–88. doi: 10.1038/nm0197-84. [DOI] [PubMed] [Google Scholar]
- 60.Gollner GP, Muller G, Alt R, Knop J, Enk AH. Therapeutic application of T cell receptor mimic peptides or cDNA in the treatment of T cell-mediated skin diseases. Gene Ther. 2000;7:1000–1004. doi: 10.1038/sj.gt.3301183. [DOI] [PubMed] [Google Scholar]
- 61.Amon MA, Ali M, Bender V, Chan YN, Toth I, Manolios N. Lipidation and glycosylation of a T cell antigen receptor (TCR) transmembrane hydrophobic peptide dramatically enhances in vitro and in vivo function. Biochim Biophys Acta. 2006;1763:879–888. doi: 10.1016/j.bbamcr.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 62.Collier S, Bolte A, Manolios N. Discrepancy in CD3-transmembrane peptide activity between in vitro and in vivo T-cell inhibition. Scand J Immunol. 2006;64:388–391. doi: 10.1111/j.1365-3083.2006.01806.x. [DOI] [PubMed] [Google Scholar]
- 63.Ali M, De Planque MRR, Huynh NT, Manolios N, Separovic F. Biophysical studies of a transmembrane peptide derived from the T cell antigen receptor. Letters in Peptide Science. 2002;8:227–233. [Google Scholar]
- 64.Wang XM, Djordjevic JT, Kurosaka N, Schibeci S, Lee L, Williamson P, et al. T-cell antigen receptor peptides inhibit signal transduction within the membrane bilayer. Clin Immunol. 2002;105:199–207. doi: 10.1006/clim.2002.5270. [DOI] [PubMed] [Google Scholar]
- 65.Huynh NT, Ffrench RA, Boadle RA, Manolios N. Transmembrane T-cell receptor peptides inhibit B- and natural killer-cell function. Immunology. 2003;108:458–464. doi: 10.1046/j.1365-2567.2003.01614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bender V, Ali M, Amon M, Diefenbach E, Manolios N. T cell antigen receptor peptide-lipid membrane interactions using surface plasmon resonance. J Biol Chem. 2004;279:54002–54007. doi: 10.1074/jbc.M403909200. [DOI] [PubMed] [Google Scholar]
- 67.Gerber D, Quintana FJ, Bloch I, Cohen IR, Shai Y. D-enantiomer peptide of the TCRalpha transmembrane domain inhibits T-cell activation in vitro and in vivo. Faseb J. 2005;19:1190–1192. doi: 10.1096/fj.04-3498fje. [DOI] [PubMed] [Google Scholar]
- 68.Enk AH, Knop J. T cell receptor mimic peptides and their potential application in T-cell-mediated disease. Int Arch Allergy Immunol. 2000;123:275–281. doi: 10.1159/000053639. [DOI] [PubMed] [Google Scholar]
- 69.Manolios N, Huynh NT, Collier S. Peptides in the treatment of inflammatory skin disease. Australas J Dermatol. 2002;43:226–227. doi: 10.1046/j.1440-0960.2002.00603.x. [DOI] [PubMed] [Google Scholar]
- 70.Ali M, Amon M, Bender V, Manolios N. Hydrophobic transmembrane-peptide lipid conjugations enhance membrane binding and functional activity in T-cells. Bioconjug Chem. 2005;16:1556–1563. doi: 10.1021/bc050127j. [DOI] [PubMed] [Google Scholar]
- 71.Wang XM, Djordjevic JT, Bender V, Manolios N. T cell antigen receptor (TCR) transmembrane peptides colocalize with TCR, not lipid rafts, in surface membranes. Cell Immunol. 2002;215:12–19. doi: 10.1016/s0008-8749(02)00002-3. [DOI] [PubMed] [Google Scholar]
- 72.Kurosaka N, Bolte A, Ali M, Manolios N. T-cell antigen receptor assembly and cell surface expression is not affected by treatment with T-cell antigen receptor-alpha chain transmembrane Peptide. Protein Pept Lett. 2007;14:299–303. doi: 10.2174/092986607780090865. [DOI] [PubMed] [Google Scholar]
- 73.Vandebona H, Ali M, Amon M, Bender V, Manolios N. Immunoreceptor transmembrane peptides and their effect on natural killer (NK) cell cytotoxicity. Protein Pept Lett. 2006;13:1017–1024. doi: 10.2174/092986606778777452. [DOI] [PubMed] [Google Scholar]
- 74.Luton F, Buferne M, Legendre V, Chauvet E, Boyer C, Schmitt-Verhulst AM. Role of CD3gamma and CD3delta cytoplasmic domains in cytolytic T lymphocyte functions and TCR/CD3 downmodulation. J Immunol. 1997;158:4162–4170. [PubMed] [Google Scholar]
- 75.Roifman CM. CD3delta immunodeficiency. Curr Opin Allergy Clin Immunol. 2004;4:479–484. doi: 10.1097/00130832-200412000-00002. [DOI] [PubMed] [Google Scholar]
- 76.Jung SM, Moroi M. Platelet glycoprotein VI. Adv Exp Med Biol. 2008;640:53–63. doi: 10.1007/978-0-387-09789-3_5. [DOI] [PubMed] [Google Scholar]
- 77.Scaldaferri F, Fiocchi C. Inflammatory bowel disease: progress and current concepts of etiopathogenesis. J Dig Dis. 2007;8:171–178. doi: 10.1111/j.1751-2980.2007.00310.x. [DOI] [PubMed] [Google Scholar]
- 78.Allez M, Tieng V, Nakazawa A, Treton X, Pacault V, Dulphy N, et al. CD4+NKG2D+ T cells in Crohn’s disease mediate inflammatory and cytotoxic responses through MICA interactions. Gastroenterology. 2007;132:2346–2358. doi: 10.1053/j.gastro.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 79.Ito Y, Kanai T, Totsuka T, Okamoto R, Tsuchiya K, Nemoto Y, et al. Blockade of NKG2D signaling prevents the development of murine CD4+ T cell-mediated colitis. Am J Physiol Gastrointest Liver Physiol. 2008;294:199–207. doi: 10.1152/ajpgi.00286.2007. [DOI] [PubMed] [Google Scholar]
- 80.Kjellev S, Haase C, Lundsgaard D, Urso B, Tornehave D, Markholst H. Inhibition of NKG2D receptor function by antibody therapy attenuates transfer-induced colitis in SCID mice. Eur J Immunol. 2007;37:1397–1406. doi: 10.1002/eji.200636473. [DOI] [PubMed] [Google Scholar]
- 81.Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol. 2000;164:4991–4995. doi: 10.4049/jimmunol.164.10.4991. [DOI] [PubMed] [Google Scholar]
- 82.Klesney-Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006;7:1266–1273. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- 83.Murakami Y, Akahoshi T, Aoki N, Toyomoto M, Miyasaka N, Kohsaka H. Intervention of an inflammation amplifier, triggering receptor expressed on myeloid cells 1, for treatment of autoimmune arthritis. Arthritis Rheum. 2009;60:1615–1623. doi: 10.1002/art.24554. [DOI] [PubMed] [Google Scholar]
- 84.Quintana FJ, Gerber D, Kent SC, Cohen IR, Shai Y. HIV-1 fusion peptide targets the TCR and inhibits antigen-specific T cell activation. J Clin Invest. 2005;115:2149–2158. doi: 10.1172/JCI23956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bloch I, Quintana FJ, Gerber D, Cohen T, Cohen IR, Shai Y. T-Cell inactivation and immunosuppressive activity induced by HIV gp41 via novel interacting motif. Faseb J. 2007;21:393–401. doi: 10.1096/fj.06-7061com. [DOI] [PubMed] [Google Scholar]
- 86.Bosch ML, Earl PL, Fargnoli K, Picciafuoco S, Giombini F, Wong-Staal F, et al. Identification of the fusion peptide of primate immunodeficiency viruses. Science. 1989;244:694–697. doi: 10.1126/science.2541505. [DOI] [PubMed] [Google Scholar]
- 87.Gallaher WR. Detection of a fusion peptide sequence in the transmembrane protein of human immunodeficiency virus. Cell. 1987;50:327–328. doi: 10.1016/0092-8674(87)90485-5. [DOI] [PubMed] [Google Scholar]
- 88.Kurosaka N, Ali M, Byth K, Manolios N. The mode of anti-arthritic peptide delivery impacts on the severity and outcome of adjuvant induced arthritis. APLAR J Rheumatol. 2007;10:198–203. [Google Scholar]
- 89.Manolios N, Ali M, Amon M, Bender V. Therapeutic application of transmembrane T and natural killer cell receptor peptides. Adv Exp Med Biol. 2008;640:208–219. doi: 10.1007/978-0-387-09789-3_16. [DOI] [PubMed] [Google Scholar]
- 90.Cianciolo GJ, Copeland TD, Oroszlan S, Snyderman R. Inhibition of lymphocyte proliferation by a synthetic peptide homologous to retroviral envelope proteins. Science. 1985;230:453–455. doi: 10.1126/science.2996136. [DOI] [PubMed] [Google Scholar]
- 91.Ruegg CL, Monell CR, Strand M. Identification, using synthetic peptides, of the minimum amino acid sequence from the retroviral transmembrane protein p15E required for inhibition of lymphoproliferation and its similarity to gp21 of human T-lymphotropic virus types I and II. J Virol. 1989;63:3250–3256. doi: 10.1128/jvi.63.8.3250-3256.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ruegg CL, Monell CR, Strand M. Inhibition of lymphoproliferation by a synthetic peptide with sequence identity to gp41 of human immunodeficiency virus type 1. J Virol. 1989;63:3257–3260. doi: 10.1128/jvi.63.8.3257-3260.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yaddanapudi K, Palacios G, Towner JS, Chen I, Sariol CA, Nichol ST, et al. Implication of a retrovirus-like glycoprotein peptide in the immunopathogenesis of Ebola and Marburg viruses. Faseb J. 2006;20:2519–2530. doi: 10.1096/fj.06-6151com. [DOI] [PubMed] [Google Scholar]
- 94.Arnon TI, Achdout H, Levi O, Markel G, Saleh N, Katz G, et al. Inhibition of the NKp30 activating receptor by pp65 of human cytomegalovirus. Nat Immunol. 2005;6:515–523. doi: 10.1038/ni1190. [DOI] [PubMed] [Google Scholar]
- 95.Sullivan BM, Coscoy L. Downregulation of the T-cell receptor complex and impairment of T-cell activation by human herpesvirus 6 u24 protein. J Virol. 2008;82:602–608. doi: 10.1128/JVI.01571-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Min CK, Bang SY, Cho BA, Choi YH, Yang JS, Lee SH, et al. Role of amphipathic helix of a herpesviral protein in membrane deformation and T cell receptor downregulation. PLoS Pathog. 2008;4:1000209. doi: 10.1371/journal.ppat.1000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Park J, Lee BS, Choi JK, Means RE, Choe J, Jung JU. Herpesviral protein targets a cellular WD repeat endosomal protein to downregulate T lymphocyte receptor expression. Immunity. 2002;17:221–233. doi: 10.1016/s1074-7613(02)00368-0. [DOI] [PubMed] [Google Scholar]
- 98.Kikuta H, Lu H, Tomizawa K, Matsumoto S. Enhancement of human herpesvirus 6 replication in adult human lymphocytes by monoclonal antibody to CD3. J Infect Dis. 1990;161:1085–1087. doi: 10.1093/infdis/161.6.1085. [DOI] [PubMed] [Google Scholar]
- 99.Roffman E, Frenkel N. Replication of human herpesvirus-6 in thymocytes activated by anti-CD3 antibody. J Infect Dis. 1991;164:617–618. doi: 10.1093/infdis/164.3.617. [DOI] [PubMed] [Google Scholar]
- 100.van Praag RM, Prins JM, Roos MT, Schellekens PT, Ten Berge IJ, Yong SL, et al. OKT3 and IL-2 treatment for purging of the latent HIV-1 reservoir in vivo results in selective long-lasting CD4+ T cell depletion. J Clin Immunol. 2001;21:218–226. doi: 10.1023/a:1011091300321. [DOI] [PubMed] [Google Scholar]
- 101.Melnyk RA, Partridge AW, Yip J, Wu Y, Goto NK, Deber CM. Polar residue tagging of transmembrane peptides. Biopolymers. 2003;71:675–685. doi: 10.1002/bip.10595. [DOI] [PubMed] [Google Scholar]
- 102.Smith SO, Smith C, Shekar S, Peersen O, Ziliox M, Aimoto S. Transmembrane interactions in the activation of the Neu receptor tyrosine kinase. Biochemistry. 2002;41:9321–9332. doi: 10.1021/bi012117l. [DOI] [PubMed] [Google Scholar]
- 103.Cunningham F, Deber CM. Optimizing synthesis and expression of transmembrane peptides and proteins. Methods. 2007;41:370–380. doi: 10.1016/j.ymeth.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 104.Yin H, Slusky JS, Berger BW, Walters RS, Vilaire G, Litvinov RI, et al. Computational design of peptides that target transmembrane helices. Science. 2007;315:1817–1822. doi: 10.1126/science.1136782. [DOI] [PubMed] [Google Scholar]
- 105.Wimley WC, White SH. Designing transmembrane alpha-helices that insert spontaneously. Biochemistry. 2000;39:4432–4442. doi: 10.1021/bi992746j. [DOI] [PubMed] [Google Scholar]
- 106.Edwards RJ, Moran N, Devocelle M, Kiernan A, Meade G, Signac W, et al. Bioinformatic discovery of novel bioactive peptides. Nat Chem Biol. 2007;3:108–112. doi: 10.1038/nchembio854. [DOI] [PubMed] [Google Scholar]
- 107.Ashish Wimley WC. Visual detection of specific, native interactions between soluble and microbead-tethered alpha-helices from membrane proteins. Biochemistry. 2001;40:13753–13759. doi: 10.1021/bi011449n. [DOI] [PubMed] [Google Scholar]
- 108.Killian JA. Synthetic peptides as models for intrinsic membrane proteins. FEBS Lett. 2003;555:134–138. doi: 10.1016/s0014-5793(03)01154-2. [DOI] [PubMed] [Google Scholar]