Abstract
Bone metastases are a substantial burden to men with advanced prostate cancer as they often cause pain and can cause fractures and spinal cord compression. Osteoblasts and osteoclasts are both pathologically activated in the setting of prostate cancer bone metastases. As osteoclast activation is associated with disease progression, skeletal complications and death, osteoclast-targeted therapies are a rational approach to disease management. Zoledronic acid is standard of care for castration-resistant prostate cancer with bone metastases as it reduces the risk for skeletal-related events. Additional trials are needed to better define the ideal dose, frequency and duration of zoledronic acid therapy. No bisphosphonate has yet been shown to prevent bone metastases or to benefit men with androgen-sensitive disease. Denosumab is an experimental osteoclast-targeted monoclonal antibody against receptor activator of nuclear factor-κB ligand. Two ongoing phase III trials are expected to define its efficacy in preventing bone metastases and disease-related skeletal events in men with prostate cancer. Androgen-deprivation therapy (ADT) for prostate cancer is associated with osteoporosis and fragility fractures. Several bisphosphonates have been shown to improve bone mineral density in men receiving ADT. Two recent phase III trials have shown that denosumab and toremifene reduce the incidence of fragility fractures in these men. The World Health Organization has developed a fracture risk assessment model (FRAX) for the general population to guide the selection of patients who may benefit from pharmacotherapy. In the absence of a prostate cancer-specific algorithm, we advocate the use of FRAX for men receiving ADT.
Keywords: bone metastases, osteoclast, bisphosphonate, zoledronic acid, skeletal-related events, denosumab
Normal bone physiology
The structural integrity of healthy bone is maintained by a process of perpetual remodeling. Bone formation by osteoblasts and bone resorption by osteoclasts are ongoing and balanced. Osteoclasts are bone-specific macrophages that arise from monocyte/macrophage progenitors. When activated, mature osteoclasts bind to bone and create a sealed resorption vacuole. They then resorb bone by acidifying the vacuole and secreting lytic enzymes such as tartrate-resistant acid phosphatase and cathepsin.1
Though many genes or loci are thought to be involved in osteoclast regulation, the receptor activator of nuclear factor-κB (RANK) signaling pathway is particularly important as it is involved in osteoclast regulation at multiple points of maturation. RANK ligand (RANKL) can bind to RANK on the surface of osteoclast precursors and cause them to differentiate to mature multinucleated osteoclasts. RANKL binding on the surface of mature osteoclasts leads to their survival and activation. These stimulatory effects of RANKL on RANK can be inhibited by the competitive inhibition of osteoprotegerin acting as a decoy receptor.
Pathophysiology of osteoblastic bone metastases
Metastatic prostate cancer causes a state of high bone turnover. Biopsies of tumor-involved bone in men with prostate cancer reveal activation of both osteoblasts and osteoclasts. Such activation leads to the erosion of trabecular bone by osteoclasts and the formation of sclerotic woven bone by osteoblasts. The woven bone generated by this process is radiographically dense but is structurally weak and associated with an increased risk of fracture.2
Two laboratory tests that are commonly elevated in men with bone metastases from prostate cancer are urinary N-telopeptide (NTx) and bone-specific alkaline phosphatase (BAP).3 NTx is associated with collagen breakdown by osteoclasts whereas BAP reflects bone formation by osteoblasts. Serum alkaline phosphatase is strongly correlated with BAP and can reasonably be used in place of the bone-specific isoform.4
NTx and BAP can each provide useful prognostic information and can used to monitor bone-targeted therapies. In patients with solid tumors metastatic to bone, high initial levels of NTx and BAP are correlated with progression of bone disease, skeletal-related events (SREs) and death. Therapeutic inhibition of osteoclasts or of RANKL suppresses NTx.5 Bone turnover markers are not routinely used to guide clinical practice but have been used to guide clinical trials involving bone-targeted drugs. For example, the RANKL inhibitor denosumab was recently shown to suppress NTx levels in most subjects with metastatic solid tumors and persistently elevated levels despite bisphosphonate therapy.6
Clinical manifestations of bone metastases
Pain is the most common symptom of bone metastases from prostate cancer. Fractures and spinal cord compression are also observed and can be debilitating. The vertebral bodies are the most common site of fracture, though pelvic, rib and long bone fractures are also seen. Disease-related fracture risk is particularly problematic given that androgen-deprivation therapy (ADT) is the first-line treatment for metastatic prostate cancer. ADT itself erodes bone mineral density (BMD),7–12 accelerates bone turnover10,12 and elevates fracture risk.13–15 Low serum calcium due to its consumption and deposition by osteoblasts is common but is usually asymptomatic.
Bisphosphonates
Bisphosphonates are the most commonly used class of bone-targeted drugs. Their central carbon and two phosphate groups make them structurally similar to inorganic phosphate, an essential component of normal bone.16 Bisphosphonates are easily incorporated into bone as their phosphate groups have a high affinity for calcium. Once localized to bone, they are inhibitory and even proapoptotic to the osteoclasts that encounter and ingest them.17
The composition of two organic side chains structurally distinguishes each bisphosphonate and accounts for differences in relative potency. One common laboratory surrogate for the potency of osteoclast inhibition is the activity of farnesyl pyrophosphate synthase, an enzyme in the mevalonate pathway that is essential in osteoclast function.18 Currently available bisphosphonates, listed from lowest potency to highest potency, are etidronate, clodronate, pamidronate, alendronate, ibandronate, risedronate and zoledronic acid.18
Bisphosphonates in metastatic bone disease
Two intravenous bisphosphonates have been Food and Drug Administration (FDA) approved for the treatment of cancer. Pamidronate gained approval in 1995 for the prevention of disease-related skeletal events in patients with bone disease from multiple myeloma or bone metastases from breast cancer. Zoledronic acid gained approval in 2002 for the prevention of disease-related skeletal complications in patients with myeloma bone disease and bone metastases from prostate, breast or lung cancer. The FDA approved zoledronic acid in the wake of three large phase III trials that collectively enrolled over 3000 patients.19–21 In men with metastatic castration-resistant prostate cancer (CRPC), zoledronic acid reduces SREs.21 Zoledronic acid has not been shown to benefit men without bone metastases or men with hormone-sensitive bone metastases.
Bisphosphonates in the treatment of CRPC
Zoledronic acid is standard of care for the prevention of SREs in men with CRPC and bone metastases. Though clodronate and pamidronate had each failed to reduce SREs in this setting, the Zometa 039 study established the efficacy of zoledronic acid (Table 1).
Table 1.
Notable randomized controlled trials using bisphosphonates for prostate cancer metastatic to bone
| Study | n | Study population | Arms | Outcome |
|---|---|---|---|---|
| Study 039 | 643 | Asymptomatic or minimally symptomatic, castration resistant |
4mg zoledronic acid vs placebo, every 3 weeks for 15 months |
Significant decrease in skeletal-related events, trend toward improved survival; established zoledronic acid as standard of care in this setting |
| Study 032/ INT 05 (combined) |
350 | Symptomatic, castration resistant |
90mg pamidronate vs placebo, every 3 weeks for 27 weeks |
No significant difference in pain, analgesic use or skeletal-related events |
| NCIC Pr06 | 209 | Symptomatic, castration resistant |
Mitoxantrone and prednisone ±1500mg IV clodronate, every 3 weeks until progression |
No significant difference in palliative response (pain scores or analgesic use) or in duration of response, symptomatic disease progression-free survival, overall survival or quality of life |
| MRC Pr05 | 311 | Androgen sensitive | 2080mg daily oral clodronate vs placebo, for 3 years maximum |
Trend toward improved symptomatic bone progression-free survival (P = 0.066); follow-up revealed an overall survival benefit (HR 0.77, 95% CI 0.60–0.98, P = 0.03) |
The Zometa 039 trial enrolled 643 men with CRPC and asymptomatic or minimally symptomatic bone metastases and randomized them to zoledronic acid (4 or 8mg every 3 weeks for 15 months) or placebo. All men were treated with ADT. Additional therapies were at the discretion of the treating physicians. The primary end point was the proportion of men who experienced at least one SRE during the trial. SREs were defined to include surgery or radiation to bone, spinal cord compression, pathological bone fractures and anticancer therapy to treat bone pain.21
The observation of nephrotoxicity led to two protocol modifications during the trial. Though no patient required dialysis, 14 of 643 experienced grade 3 increases in serum creatinine (7 in the 4-mg group, 5 in the 8/4-mg group and 2 in the placebo group). Because of this toxicity, the 8-mg cohort was dose reduced to 4mg and the infusion time for all subjects was lengthened from 5 to 15 min.
Subjects treated with 4mg zoledronic acid experienced significantly fewer SREs than the control subjects (33.2 vs 44.2%; P = 0.02) during the 15-month trial. The median time to first SRE was extended from 321 to 488 days (P = 0.009).22 The reduction in SREs within the 4-mg cohort remained significant at 24 months (38 vs 49%; P = 0.028). Median overall survival (OS) was nonsignificantly improved from 464 days in the placebo group to 546 days in the 4-mg group (P = 0.091).21
Pamidronate was not found to be effective when two similarly designed randomized, placebo-controlled pamidronate trials (protocol 032 and INT 05) were combined for analysis. These trials together enrolled 350 symptomatic subjects and each evaluated 90mg pamidronate given intravenously every 3 weeks for 27 weeks. When compared with placebo, pamidronate did not produce statistically significant improvements in analgesic use, self-reported pain score or the proportion of patients with an SRE. Laboratory evidence suggests that this disappointing efficacy may be at least partially attributable to inadequate osteoclast inhibition. Urinary NTx levels have been observed to fall 50% with pamidronate treatment but 70% in response to zoledronic acid.23,24
Sodium clodronate also failed to produce significant clinical benefit in this setting. In National Cancer Institute of Canada Pr06, 209 men with CRPC and symptomatic bone metastases were treated with mitoxantrone (12mgm−2 intravenously every 3 weeks) and prednisone (5mg twice daily) and were randomized to placebo or to clodronate (1500mg intravenously every 3 weeks). Most subjects (77%) had mild pain scores at enrollment. Clodronate did not produce a statistically significant improvement in the primary end point of the study, palliative response (46 vs 39%; P = 0.54). Secondary end points progression-free survival, OS and quality of life were also statistically unchanged.25
Bisphosphonates in the treatment of androgen-sensitive prostate cancer
The use of bisphosphonates for androgen-sensitive prostate cancer has been less well studied. Oral sodium clodronate was evaluated in combination with first-line ADT for metastatic prostate cancer in Medical Research Council Pr05 (MRC Pr05). This double-blind trial randomized 311 men to oral sodium clodronate (2080mg daily) or to placebo for a maximum of 3 years. The primary end point was the proportion of patients experiencing clinically appreciable skeletal events, known as symptomatic bone progression-free survival. OS was a secondary end point. After an initial 59 months of follow-up, clodronate had produced nonsignificant benefits in symptomatic bone progression-free survival (HR 0.79, 95% CI 0.61–1.02, P = 0.066) and in OS (HR 0.80, 95% CI 0.62–1.03, P = 0.082). The most prominent clodronate toxicity was gastrointestinal adverse events (HR 1.71, 95% CI 1.21–2.41; P = 0.002).26
Long-term follow-up from the MRC Pr05 study was presented in early 2009. After 258 deaths (93%) had occurred, a significant OS benefit was observed favoring the men receiving clodronate (8-year OS 22 vs 14%; HR for death 0.77, 95% CI 0.60–0.98, P = 0.03).27 This benefit was not seen in the similarly designed MRC Pr04 trial that evaluated the same sodium clodronate dose in men without metastases.
Early use of bisphosphonates in men with metastatic disease is under further study in CALGB/CTSU 90202. Subjects initiating ADT for prostate cancer metastatic to bone are randomized either to early zoledronic acid (within 3 months of initiation of ADT) or to standard zoledronic acid on diagnosis of castration resistance. The primary end point is proportion of subjects experiencing SREs. Given the encouraging results in MRC Pr05 with the comparatively weak bisphosphonate clodronate, data from CALGB/CTSU 90202 are eagerly anticipated. The trial was designed to accrue 680 patients.
Bisphosphonates for the prevention of bone metastases
Bisphosphonates have not been shown to prevent bone metastases due to prostate cancer in the two randomized controlled trials that have evaluated them for this purpose.
The MRC Pr04 trial enrolled 508 men with locally advanced (T2 to T4) prostate cancer and a negative bone scan. They were randomized to a maximum of 5 years of either daily oral clodronate (2080mg per day) or placebo. With a median of almost 10 years of follow-up, no benefit was seen. There was a nonsignificant trend toward more new bone metastases with clodronate (HR = 1.22; 95% CI 0.88–1.68). After 281 deaths (60%) in follow-up, no improvement in OS was seen (HR 1.12, 95% CI 0.89–1.42; P = 0.33).27 Clodronate caused only mild increases in lactate dehydrogenase levels and in gastrointestinal complaints.28 The negative results of MRC Pr04 showed that the relatively weak bisphosphonate was not beneficial in men without metastatic disease.
The more potent zoledronic acid was evaluated in the Zometa 704 trial. Men with progression to CRPC were enrolled and randomized to zoledronic acid (4mg intravenously every 4 weeks) or placebo. The primary objective was to lengthen the time to development of the first bone metastasis. The study was placed on hold after only partial accrual (398 of the planned 991) due to a low event rate. It was later terminated. Comparison of the partial cohorts revealed no significant difference in the time to first metastasis.
Despite the eventual termination of Zometa 704, its prospective design and regularly scheduled imaging did provide a rigorous evaluation of the natural history of PSA-recurrent disease. After 2 years, 33% of the 201 placebo-treated subjects had developed bone metastases. Median bone metastasis-free survival was 30 months. Metastasis-free survival and OS were independently predicted by low baseline PSA and by low PSA velocity.29
These two unsuccessful trials have shaped the design of subsequent trials. The low event rate among men with CRPC without bone metastases has led to higher target enrollment in an effort to achieve adequate statistical power. The Amgen 147 trial investigates metastasis prevention with denosumab and is designed to accrue 1400 subjects.
Bisphosphonates: safety
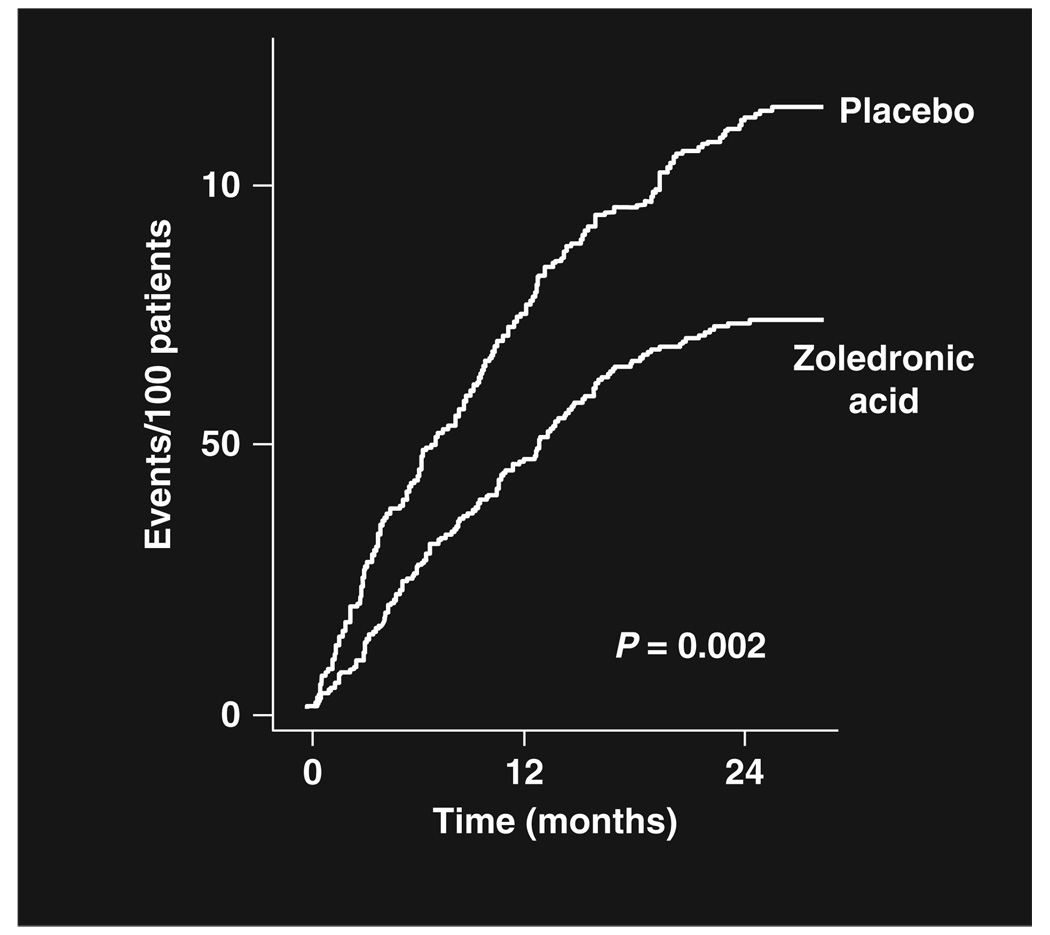
Zoledronic acid (4mg every 3–4 weeks) is now standard–of-care to reduce the risk for SREs for men with CRPC and bone metastases (Figure 1).30 Bisphosphonate treatment of men with androgen-sensitive disease is under investigation. Given that men with prostate cancer are so often treated with substantial courses of bisphosphonates, treatment-related toxicities are an important issue.
Figure 1.
Cumulative incidence of skeletal-related events.30 In combined analysis of two large prospective randomized controlled trials, the survival-adjusted cumulative incidence of skeletal-related events was significantly reduced by 35.3% relative to placebo with the use of 4mg zoledronic acid.20,21
The two most common bisphosphonate side effects are hypocalcemia and an acute phase reaction. Bisphosphonate-associated hypocalcemia is usually asymptomatic and can generally be prevented with calcium and vitamin D supplementation. The acute phase reaction is a self-limited flu-like syndrome that usually begins within 24 h of the infusion and may include fever, nausea and vomiting.31 The two most feared potential complications of bisphosphonate therapy are rare-but-morbid acute renal failure and osteonecrosis of the jaw (ONJ).
Bisphosphonate-associated renal toxicity consists of a spectrum from asymptomatic elevation of creatinine to dialysis dependence. Kidney biopsies in the wake of this toxicity reveal evidence of acute tubular necrosis with tubular cell degeneration, loss of brush border and apoptosis.32 In one series of cases resulting from zoledronic acid given as a 4mg dose over 15 min, mean baseline creatinine was 1.7mg per 100 ml (range 0.6–5.2) and average time to renal failure was 56 days (range 1–51 months).33 Early toxicity is a concern as one-fourth of these patients developed renal failure after a single dose.
Several recommendations within the zoledronic acid package insert are intended to minimize the risk of renal toxicity. The maximum single dose is 4 mg. All treatments should be infused over a minimum of 15 min. If a normal baseline creatinine rises ≥0.5mg per 100 ml or if an abnormal baseline creatinine rises ≥1.0mg per 100 ml, further doses should be held until the creatinine returns to within 10% of baseline. The zoledronic acid package insert also contains recommendations for initial doses in patients with stable baseline creatinine clearance <60 ml min−1.34
ONJ has emerged as prominent potential complication of bisphosphonate use since it was first described in 2003.35,36 Clinically, it is an area of nonhealing exposed or necrotic maxillofacial bone. Its incidence is difficult to estimate from retrospective data. ONJ appears to be rare with the use of oral bisphosphonates (for example, 0.7/ 100 000 person per year of exposure for alendronate according to Merck37), whereas it may be 0.8–12% with intravenous bisphosphonates.38 Retrospective data have identified dose, the use of zoledronic acid, duration of treatment and dental extractions as risk factors for ONJ.39 Though duration of treatment is relevant to ONJ risk, little is known about the most effective length of therapy for men with prostate cancer metastatic to bone. Completed clinical trials have treated subjects for a maximum of 24 months.
Several measures for the reduction of ONJ risk have been recommended by the American Association of Oral and Maxillofacial Surgeons.37 They recommend oral examination with extraction of nonrestorable teeth before bisphosphonate treatment. If extractions are performed, they recommend a 2- to 3-week wait before initiation of therapy. They recommend avoidance of invasive procedures when possible and discontinuation of the bisphosphonate for 3 months on either side of elective dental surgery. Finally, they provide guidance for the management of active ONJ. Early lesions can be managed with antimicrobial rinses such as chlorhexidine 0.12%. Antibiotics can be added for intermediate lesions. More advanced lesions can be managed with antibiotics and surgical debridement. Others advocate early intervention with surgical therapies such as laser treatment.40
Future directions in bone-targeted therapy for prostate cancer
Zoledronic acid is currently the only drug approved for the treatment of bone metastases from prostate cancer. Studies of other bone-targeted drugs are underway. The one drug that is in phase III study is denosumab, the monoclonal antibody that inhibits RANKL.
Denosumab: RANKL inhibitor in current clinical trials
RANKL is important to the regulation of osteoclast differentiation, survival and activation. RANKL inhibition is therefore a rational strategy for the treatment of osteoclast-mediated disease. Denosumab is a human monoclonal antibody that binds to and inhibits RANKL. It is in development for use in a variety of benign and malignant clinical settings.
Denosumab has shown efficacy for the management of postmenopausal bone loss41 and bone loss associated with adjuvant aromatase inhibition for breast cancer.42 Given subcutaneously to subjects with bone lesions due to myeloma and breast cancer, it was shown to have a half-life of up to 46 days and to suppress the bone turnover marker NTx through 84 days.43 It has been recently reported to be effective in reducing the risk for clinical fractures among men at elevated risk due to age and androgen deprivation.44 Ongoing phase III trials will evaluate its ability to prevent bone metastases in men with CRPC and to prevent SREs in men with CRPC and existing bone metastases. More than 3000 subjects are to be enrolled in these two clinical trials (Table 2).
Table 2.
Current randomized controlled trials of denosumab for prostate cancer
| Study/purpose | n | Study population | Arms | End point(s) | Outcome |
|---|---|---|---|---|---|
| Protocol 138: fracture prevention |
1468 | Current androgen- deprivation therapy; no metastases |
Denosumab vs placebo |
New vertebral fractures, bone mineral density |
Denosumab reduced 3-year incidence of new vertebral fractures by 62% |
| Protocol 147: metastasis prevention |
1400 | Castration resistant, high risk but no bone metastases |
Denosumab vs placebo |
Bone metastasis-free survival |
Ongoing |
| Protocol 103: prevention of skeletal complications |
1700 | Castration resistant, bone metastases |
Denosumab vs zoledronic acid |
Skeletal-related events | Ongoing |
Amgen protocol 147 examines the ability of denosumab to prevent bone metastases. The trial enrolls men with CRPC (rising PSA despite androgen deprivation) but no bone metastases and randomizes them to denosumab or placebo. Enrolled subjects are all at high risk to develop metastases given a PSA doubling time ≤10 months and/or PSA ≥8 ng per 100 ml. Bone metastasis-free survival is the primary end point. Target accrual is 1400 subjects.
Amgen protocol 103 compares denosumab to standard-of-care zoledronic acid for the prevention of SREs. The trial enrolls men with CRPC metastatic to bone and randomizes them to denosumab or to zoledronic acid. The primary end point is time to first SRE (pathological fracture, radiation to bone, surgery to bone or spinal cord compression). Target accrual is 1900 subjects. Analyses will be conducted to evaluate both noninferiority and superiority.
The efficacy of denosumab is under investigation as described above. Though toxicities have been comparable to placebo, ongoing investigation and follow-up are needed to further define the risks of long-term therapy. ONJ and nephrotoxicity have not been observed in association with denosumab.
Prevention of osteoporosis and fractures during ADT
Fragility fractures are common among men and become increasingly common with advancing age. One in five men will experience an osteoporosis-related fracture in his lifetime.45 One-fourth of all hip fractures occur in men, a proportion that is climbing.46 Mortality after hip fracture is approximately 37.5% in men.47 The most common causes of acquired osteoporosis in men are hypogonadism, chronic glucocorticoid therapy and excessive alcohol intake.48 ADT for men with prostate cancer causes severe hypogonadism.
Lifelong ADT is common for men with metastatic prostate cancer. ADT (6 months to 3 years) is often used in conjunction with radiation therapy for the neoadjuvant/concomitant/adjuvant treatment of locally advanced and high-risk localized disease.49,50 Whether accomplished by a gonadotropin-releasing hormone agonist or by bilateral orchiectomies, ADT eliminates gonadal androgen production and drops serum testosterone to less than 50 ng per 100 ml. This hormonal environment is accompanied by a variety of metabolic changes including accelerated deterioration of BMD and an associated rise in the risk for fractures.13,14
Assessment of fracture risk in men receiving ADT is essential as it guides the identification of candidates for medical therapy. Though low BMD is one risk factor for clinical fracture, most fractures occur in men whose BMD measurements are not in the osteoporotic range.51 The addition of clinical factors has been shown to improve sensitivity of fracture risk assessment without losing specificity.52,53 For example, meta-analyses have shown that advancing age substantially increases fracture risk independent of measured BMD.54
As accurate estimates of fracture risks depend on more than BMD alone, Kanis et al.55 have developed a fracture risk assessment model (FRAX) to improve diagnostic accuracy. The online FRAX tool (http://www.shef.ac.uk/FRAX/) makes use of a group of clinical risk factors with or without femoral neck BMD measurement. Derived from a group of meta-analyses, these clinical risk factors include prior fragility fracture, family history of hip fracture, current tobacco smoking, chronic use of glucocorticoids, daily consumption of at least 3 units of alcohol, rheumatoid arthritis and other causes of secondary osteoporosis.56–63
Low BMD accounts for a portion of fracture risk. An important group of clinical trials have consistently shown that bisphosphonates improve BMD in men receiving ADT for prostate cancer. Alendronate,64 pamidronate,65,66 zoledronic acid67,68 and neridronate69 are among the agents that have improved BMD in this clinical setting. Selective estrogen receptor modulators are also effective as raloxifene and toremifene have been shown to improve BMD in men treated with ADT for prostate cancer.70,71
As BMD alone is an inadequate surrogate for fracture risk, fracture prevention is a more compelling end point for clinical trials. Two recently reported phase III fracture prevention trials for men receiving ADT for prostate cancer are therefore likely to shape practice. Denosumab and toremifene both have been shown to significantly reduce fracture risk in this clinical setting.
Amgen protocol 138 enrolled a total of 1468 men receiving ADT for prostate cancer. These men were at high risk for fracture due to age ≥70, history of osteoporotic fracture or low BMD. Subjects were randomized to 3 years of denosumab (60mg subcutaneously every 6 months) or placebo. They were followed for percent change in lumbar spine BMD at 24 months and for incident fractures. The primary end point was positive as BMD significantly increased at the lumbar spine (6.7%; 95% CI 6.2–7.1), total hip (4.8%; 95% CI 4.4–5.1) and 1/3 radius (5.5%; 95% CI 4.5–6.6) at 24 months.72 Most importantly, denosumab reduced the 3-year incidence of new vertebral fractures by 62% (P = 0.006) and fractures at any site by 28% (P = 0.10) and of multiple fractures at any site by 72% (P = 0.006).44 Adverse events were similar in the denosumab and placebo arms.
Another phase III fracture prevention trial enrolled men 50 years or older who were receiving ADT for prostate cancer and were at particularly high risk for fractures due to age ≥70 and/or low BMD. A total of 1389 subjects were randomized to 80-mg daily oral toremifene or placebo for 2 years. The primary objective of the trial was to show fewer new morphometric vertebral fractures in those treated with toremifene. Secondary end points were BMD at the hip and lumbar spine, breast pain, hot flashes and lipid profile. The primary objective was met as men treated with toremifene experienced significantly fewer new vertebral fractures than those assigned to placebo (2.5 vs 4.9%; relative risk 0.50, P<0.05 by modified intent to treat analysis).73 When compared with placebo, toremifene significantly increased BMD at the lumbar spine by 2% and at the hip by 1.6%. Toremifene also significantly decreased low-density lipoprotein, decreased triglycerides, increased high-density lipoprotein and decreased breast pain. In subjects who experienced ≥6 hot flashes daily, toremifene decreased the frequency of hot flashes.
Conclusion
Bone metastases can cause substantial morbidity and mortality among men with advanced prostate cancer. They commonly cause pain and can potentially cause fractures and spinal cord compression. Given these burdensome clinical manifestations, bone metastases represent a valid target for pharmacotherapy. The first class of drugs to be FDA approved for the treatment of bone metastases is bisphosphonates. Zoledronic acid is the current standard treatment to reduce the incidence of SREs in men with CRPC and bone metastases. Bisphosphonates are under investigation for the management of androgen-sensitive disease. Though generally well tolerated, bisphosphonates commonly cause a self-limited acute phase reaction and have the potential to cause ONJ and renal failure. Denosumab is a human monoclonal antibody against RANKL and is in phase III clinical trials for three distinct indications. First, it has been shown to reduce incidence of fracture among men with elevated risk due to ADT. Second, ongoing trials examine its ability to prevent bone metastases in CRPC and its ability to prevent SREs in CRPC with bone metastases.
Footnotes
Conflict of interest
Dr Saylor declares no conflict of interest. Dr Smith has served as a consultant to Amgen, Novartis, and GTX.
References
- 1.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 2.Clarke NW, McClure J, George NJ. Osteoblast function and osteomalacia in metastatic prostate cancer. Eur Urol. 1993;24:286–290. doi: 10.1159/000474311. [DOI] [PubMed] [Google Scholar]
- 3.Demers LM, Costa L, Lipton A. Biochemical markers and skeletal metastases. Cancer. 2000;88(12 Suppl):2919–2926. doi: 10.1002/1097-0142(20000615)88:12+<2919::aid-cncr7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Cook RJ, Coleman R, Brown J, Lipton A, Major P, Hei YJ, et al. Markers of bone metabolism and survival in men with hormone-refractory metastatic prostate cancer. Clin Cancer Res. 2006;12(11 Pt 1):3361–3367. doi: 10.1158/1078-0432.CCR-06-0269. [DOI] [PubMed] [Google Scholar]
- 5.Bekker PJ, Holloway DL, Rasmussen AS, Murphy R, Martin SW, Leese PT, et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res. 2004;19:1059–1066. doi: 10.1359/JBMR.040305. [DOI] [PubMed] [Google Scholar]
- 6.Fizazi K, Lipton A, Mariette X, Body JJ, Rahim Y, Gralow JR, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27:1564–1571. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- 7.Berruti A, Dogliotti L, Terrone C, Cerutti S, Isaia G, Tarabuzzi R, et al. Changes in bone mineral density, lean body mass and fat content as measured by dual energy X-ray absorptiometry in patients with prostate cancer without apparent bone metastases given androgen deprivation therapy. J Urol. 2002;167:2361–2367. discussion 7. [PubMed] [Google Scholar]
- 8.Daniell HW, Dunn SR, Ferguson DW, Lomas G, Niazi Z, Stratte PT. Progressive osteoporosis during androgen deprivation therapy for prostate cancer. J Urol. 2000;163:181–186. [PubMed] [Google Scholar]
- 9.Diamond T, Campbell J, Bryant C, Lynch W. The effect of combined androgen blockade on bone turnover and bone mineral densities in men treated for prostate carcinoma: longitudinal evaluation and response to intermittent cyclic etidronate therapy. Cancer. 1998;83:1561–1566. [PubMed] [Google Scholar]
- 10.Maillefert JF, Sibilia J, Michel F, Saussine C, Javier RM, Tavernier C. Bone mineral density in men treated with synthetic gonadotropin-releasing hormone agonists for prostatic carcinoma. J Urol. 1999;161:1219–1222. [PubMed] [Google Scholar]
- 11.Smith MR, Eastham J, Gleason DM, Shasha D, Tchekmedyian S, Zinner N. Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J Urol. 2003;169:2008–2012. doi: 10.1097/01.ju.0000063820.94994.95. [DOI] [PubMed] [Google Scholar]
- 12.Smith MR, McGovern FJ, Zietman AL, Fallon MA, Hayden DL, Schoenfeld DA, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001;345:948–955. doi: 10.1056/NEJMoa010845. [DOI] [PubMed] [Google Scholar]
- 13.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 14.Smith MR, Lee WC, Brandman J, Wang Q, Botteman M, Pashos CL. Gonadotropin-releasing hormone agonists and fracture risk: a claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol. 2005;23:7897–7903. doi: 10.1200/JCO.2004.00.6908. [DOI] [PubMed] [Google Scholar]
- 15.Smith MR, Boyce SP, Moyneur E, Duh MS, Raut MK, Brandman J. Risk of clinical fractures after gonadotropin-releasing hormone agonist therapy for prostate cancer. J Urol. 2006;175:136–139. doi: 10.1016/S0022-5347(05)00033-9. discussion 9. [DOI] [PubMed] [Google Scholar]
- 16.Santini D, Vespasiani Gentilucci U, Vincenzi B, Picardi A, Vasaturo F, La Cesa A, et al. The antineoplastic role of bisphosphonates: from basic research to clinical evidence. Ann Oncol. 2003;14:1468–1476. doi: 10.1093/annonc/mdg401. [DOI] [PubMed] [Google Scholar]
- 17.Fisher JE, Rogers MJ, Halasy JM, Luckman SP, Hughes DE, Masarachia PJ, et al. Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro. Proc Natl Acad Sci USA. 1999;96:133–138. doi: 10.1073/pnas.96.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell RG, Xia Z, Dunford JE, Oppermann U, Kwaasi A, Hulley PA, et al. Bisphosphonates: an update on mechanisms of action and how these relate to clinical efficacy. Ann NY Acad Sci. 2007;1117:209–257. doi: 10.1196/annals.1402.089. [DOI] [PubMed] [Google Scholar]
- 19.Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J, et al. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trial. Cancer J. 2001;7:377–387. [PubMed] [Google Scholar]
- 20.Rosen LS, Gordon D, Tchekmedyian S, Yanagihara R, Hirsh V, Krzakowski M, et al. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial—the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J Clin Oncol. 2003;21:3150–3157. doi: 10.1200/JCO.2003.04.105. [DOI] [PubMed] [Google Scholar]
- 21.Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 22.Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96:879–882. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 23.Small EJ, Smith MR, Seaman JJ, Petrone S, Kowalski MO. Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancer. J Clin Oncol. 2003;21:4277–4284. doi: 10.1200/JCO.2003.05.147. [DOI] [PubMed] [Google Scholar]
- 24.Lipton A, Cook R, Saad F, Major P, Garnero P, Terpos E, et al. Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumors and elevated bone resorption receiving zoledronic acid. Cancer. 2008;113:193–201. doi: 10.1002/cncr.23529. [DOI] [PubMed] [Google Scholar]
- 25.Ernst DS, Tannock IF, Winquist EW, Venner PM, Reyno L, Moore MJ, et al. Randomized, double-blind, controlled trial of mitoxantrone/prednisone and clodronate versus mitoxantrone/prednisone and placebo in patients with hormone-refractory prostate cancer and pain. J Clin Oncol. 2003;21:3335–3342. doi: 10.1200/JCO.2003.03.042. [DOI] [PubMed] [Google Scholar]
- 26.Dearnaley DP, Sydes MR, Mason MD, Stott M, Powell CS, Robinson AC, et al. A double-blind, placebo-controlled, randomized trial of oral sodium clodronate for metastatic prostate cancer (MRC PR05 Trial) J Natl Cancer Inst. 2003;95:1300–1311. doi: 10.1093/jnci/djg038. [DOI] [PubMed] [Google Scholar]
- 27.Dearnaley D, Mason MD, Parmar M, Sanders K, Sydes MR.Survival benefit with oral sodium clodronate in metastatic but not localised prostate cancer: long-term results of MRC PR04 & PR05. In: Genitourinary Cancers Symposium 2009American Society of Clinical Oncology [Google Scholar]
- 28.Mason MD, Sydes MR, Glaholm J, Langley RE, Huddart RA, Sokal M, et al. Oral sodium clodronate for nonmetastatic prostate cancer—results of a randomized double-blind placebo-controlled trial: Medical Research Council PR04 (ISRCTN61384873) J Natl Cancer Inst. 2007;99:765–776. doi: 10.1093/jnci/djk178. [DOI] [PubMed] [Google Scholar]
- 29.Smith MR, Kabbinavar F, Saad F, Hussain A, Gittelman MC, Bilhartz DL, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23:2918–2925. doi: 10.1200/JCO.2005.01.529. [DOI] [PubMed] [Google Scholar]
- 30.Major PP, Cook RJ, Chen BL, Zheng M. Survival-adjusted multiple-event analysis for the evaluation of treatment effects of zoledronic acid in patients with bone metastases from solid tumors. Support Cancer Ther. 2005;2:234–240. doi: 10.3816/SCT.2005.n.017. [DOI] [PubMed] [Google Scholar]
- 31.Zojer N, Keck AV, Pecherstorfer M. Comparative tolerability of drug therapies for hypercalcaemia of malignancy. Drug Saf. 1999;21:389–406. doi: 10.2165/00002018-199921050-00004. [DOI] [PubMed] [Google Scholar]
- 32.Markowitz GS, Fine PL, Stack JI, Kunis CL, Radhakrishnan J, Palecki W, et al. Toxic acute tubular necrosis following treatment with zoledronate (Zometa) Kidney Int. 2003;64:281–289. doi: 10.1046/j.1523-1755.2003.00071.x. [DOI] [PubMed] [Google Scholar]
- 33.Chang JT, Green L, Beitz J. Renal failure with the use of zoledronic acid. N Engl J Med. 2003;349:1676–1679. doi: 10.1056/NEJM200310233491721. discussion 9. [DOI] [PubMed] [Google Scholar]
- 34.Novartis. Zometa full prescribing information. 2008 Package insert. [Google Scholar]
- 35.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–1117. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 36.Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004;62:527–534. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Advisory Task Force on Bisphosphonate-Related Osteonecrosis of the Jaws, American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2007;65:369–376. doi: 10.1016/j.joms.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Wang EP, Kaban LB, Strewler GJ, Raje N, Troulis MJ. Incidence of osteonecrosis of the jaw in patients with multiple myeloma and breast or prostate cancer on intravenous bisphosphonate therapy. J Oral Maxillofac Surg. 2007;65:1328–1331. doi: 10.1016/j.joms.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Hoff AO, Toth BB, Altundag K, Johnson MM, Warneke CL, Hu M, et al. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res. 2008;23:826–836. doi: 10.1359/JBMR.080205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vescovi P, Manfredi M, Merigo E, Meleti M. Early surgical approach preferable to medical therapy for bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2008;66:831–832. doi: 10.1016/j.joms.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 41.McClung MR, Lewiecki EM, Cohen SB, Bolognese MA, Woodson GC, Moffett AH, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354:821–831. doi: 10.1056/NEJMoa044459. [DOI] [PubMed] [Google Scholar]
- 42.Ellis GK, Bone HG, Chlebowski R, Paul D, Spadafora S, Smith J, et al. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol. 2008;26:4875–4882. doi: 10.1200/JCO.2008.16.3832. [DOI] [PubMed] [Google Scholar]
- 43.Body JJ, Facon T, Coleman RE, Lipton A, Geurs F, Fan M, et al. A study of the biological receptor activator of nuclear factor-kappaB ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clin Cancer Res. 2006;12:1221–1228. doi: 10.1158/1078-0432.CCR-05-1933. [DOI] [PubMed] [Google Scholar]
- 44.Saad F, Smith MR, Egerdie B, Tammela TL, Feldman RA, Heracek J, et al. Denosumab for prevention of fractures in men receiving androgen deprivation therapy (ADT) for prostate cancer (PC); ASCO Annual Meeting; Orlando. 2009. [Google Scholar]
- 45.US Department of Health and Human Services. Bone health and osteoporosis: a report of the surgeon general. Rockville, MD: US Department of Health and Human Services, Office of Surgeon General; 2004. [Google Scholar]
- 46.Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int. 1997;7:407–413. doi: 10.1007/pl00004148. [DOI] [PubMed] [Google Scholar]
- 47.Jiang HX, Majumdar SR, Dick DA, Moreau M, Raso J, Otto DD, et al. Development and initial validation of a risk score for predicting in-hospital and 1-year mortality in patients with hip fractures. J Bone Miner Res. 2005;20:494–500. doi: 10.1359/JBMR.041133. [DOI] [PubMed] [Google Scholar]
- 48.Bilezikian JP. Osteoporosis in men. J Clin Endocrinol Metab. 1999;84:3431–3434. doi: 10.1210/jcem.84.10.6060. [DOI] [PubMed] [Google Scholar]
- 49.Bolla M, Collette L, Blank L, Warde P, Dubois JB, Mirimanoff RO, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360:103–106. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 50.D’Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA. 2004;292:821–827. doi: 10.1001/jama.292.7.821. [DOI] [PubMed] [Google Scholar]
- 51.Seeman E, Bianchi G, Khosla S, Kanis JA, Orwoll E. Bone fragility in men—where are we? Osteoporos Int. 2006;17:1577–1583. doi: 10.1007/s00198-006-0160-8. [DOI] [PubMed] [Google Scholar]
- 52.De Laet C, Oden A, Johansson H, Johnell O, Jonsson B, Kanis JA. The impact of the use of multiple risk indicators for fracture on case-finding strategies: a mathematical approach. Osteoporos Int. 2005;16:313–318. doi: 10.1007/s00198-004-1689-z. [DOI] [PubMed] [Google Scholar]
- 53.Kanis JA, Johnell O, Oden A, De Laet C, Jonsson B, Dawson A. Ten-year risk of osteoporotic fracture and the effect of risk factors on screening strategies. Bone. 2002;30:251–258. doi: 10.1016/s8756-3282(01)00653-6. [DOI] [PubMed] [Google Scholar]
- 54.Kanis JA, Borgstrom F, De Laet C, Johansson H, Johnell O, Jonsson B, et al. Assessment of fracture risk. Osteoporos Int. 2005;16:581–589. doi: 10.1007/s00198-004-1780-5. [DOI] [PubMed] [Google Scholar]
- 55.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19:385–397. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Laet C, Kanis JA, Oden A, Johanson H, Johnell O, Delmas P, et al. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int. 2005;16:1330–1338. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 57.Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20:1185–1194. doi: 10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- 58.Kanis JA, Johansson H, Johnell O, Oden A, De Laet C, Eisman JA, et al. Alcohol intake as a risk factor for fracture. Osteoporos Int. 2005;16:737–742. doi: 10.1007/s00198-004-1734-y. [DOI] [PubMed] [Google Scholar]
- 59.Kanis JA, Johansson H, Oden A, De Laet C, Johnell O, Eisman JA, et al. A meta-analysis of milk intake and fracture risk: low utility for case finding. Osteoporos Int. 2005;16:799–804. doi: 10.1007/s00198-004-1755-6. [DOI] [PubMed] [Google Scholar]
- 60.Kanis JA, Johansson H, Oden A, Johnell O, De Laet C, Eisman JA, et al. A family history of fracture and fracture risk: a meta-analysis. Bone. 2004;35:1029–1037. doi: 10.1016/j.bone.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 61.Kanis JA, Johansson H, Oden A, Johnell O, de Laet C, Melton IL, et al. A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res. 2004;19:893–899. doi: 10.1359/JBMR.040134. [DOI] [PubMed] [Google Scholar]
- 62.Kanis JA, Johnell O, De Laet C, Johansson H, Oden A, Delmas P, et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone. 2004;35:375–382. doi: 10.1016/j.bone.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 63.Kanis JA, Johnell O, Oden A, Johansson H, De Laet C, Eisman JA, et al. Smoking and fracture risk: a meta-analysis. Osteoporos Int. 2005;16:155–162. doi: 10.1007/s00198-004-1640-3. [DOI] [PubMed] [Google Scholar]
- 64.Greenspan SL, Nelson JB, Trump DL, Resnick NM. Effect of once-weekly oral alendronate on bone loss in men receiving androgen deprivation therapy for prostate cancer: a randomized trial. Ann Intern Med. 2007;146:416–424. doi: 10.7326/0003-4819-146-6-200703200-00006. [DOI] [PubMed] [Google Scholar]
- 65.Smith MR, McGovern FJ, Zietman AL, Fallon MA, Hayden DL, Schoenfeld DA, et al. Pamidronate to prevent bone loss in men receiving gonadotropin releasing hormone agonist therapy for prostate cancer. N Engl J Med. 2001;345:948–955. doi: 10.1056/NEJMoa010845. [DOI] [PubMed] [Google Scholar]
- 66.Diamond TH, Winters J, Smith A, De Souza P, Kersley JH, Lynch WJ, et al. The antiosteoporotic efficacy of intravenous pamidronate in men with prostate carcinoma receiving combined androgen blockade: a double blind, randomized, placebo-controlled crossover study. Cancer. 2001;92:1444–1450. doi: 10.1002/1097-0142(20010915)92:6<1444::aid-cncr1468>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 67.Smith MR, Eastham J, Gleason D, Shasha D, Tchekmedyian S, Zinner N. Randomized controlled trial of zoledronic acid to prevent bone loss in men undergoing androgen deprivation therapy for nonmetastatic prostate cancer. J Urol. 2003;169:2008–2012. doi: 10.1097/01.ju.0000063820.94994.95. [DOI] [PubMed] [Google Scholar]
- 68.Michaelson MD, Kaufman DS, Lee H, McGovern FJ, Kantoff PW, Fallon MA, et al. Randomized controlled trial of annual zoledronic acid to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer. J Clin Oncol. 2007;25:1038–1042. doi: 10.1200/JCO.2006.07.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morabito N, Gaudio A, Lasco A, Catalano A, Atteritano M, Trifiletti A, et al. Neridronate prevents bone loss in patients receiving androgen deprivation therapy for prostate cancer. J Bone Miner Res. 2004;19:1766–1770. doi: 10.1359/JBMR.040813. [DOI] [PubMed] [Google Scholar]
- 70.Smith MR, Fallon MA, Lee H, Finkelstein JS. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89:3841–3846. doi: 10.1210/jc.2003-032058. [DOI] [PubMed] [Google Scholar]
- 71.Steiner MS, Patterson A, Israeli R, Barnette KG, Boger R, Price D. Toremifene citrate versus placebo for treatment of bone loss and other complications of androgen deprivation therapy in patients with prostate cancer. Proc ASCO. 2004 (abstract 4597) [Google Scholar]
- 72.Smith MR, Ellis GK, Saad F, Tammela TL, Bone HG, Egerdie B, et al. Effect of denosumab on bone mineral density (BMD) in women with breast cancer (BC) and men with prostate cancer (PC) undergoing hormone ablation therapy; ASCO Annual Meeting; Orlando. 2009. [Google Scholar]
- 73.Smith MR, Morton RA, Malkowicz B, Wallace H, Rodriguez D, Hancock M, et al. A phase III randomized controlled trial of toremifene to prevent fractures and other adverse effects of androgen deprivation therapy in men with prostate cancer; AACR Annual Meeting; San Diego, CA. 2009. [Google Scholar]