Abstract
Purpose
Osteoporosis causes morbidity and mortality in men. The National Osteoporosis Foundation recommends fracture risk assessment with the online WHO/FRAX® tool. Although androgen deprivation therapy for prostate cancer increases fracture risk, there is limited information about which men require preventative drug therapy. We applied the WHO/FRAX tool to men treated with androgen deprivation therapy for prostate cancer.
Materials and Methods
Information was collected from a practice cohort of men treated with gonadotropin-releasing hormone agonists, and included age, height, weight, history of gonadotropin-releasing hormone agonist treatment, dual energy x-ray absorptiometry results, prior bone targeted therapy and clinical risk factors for fracture. Subjects were evaluated with the WHO/FRAX algorithm (http://www.shef.ac.uk/FRAX/).
Results
A total of 363 men treated with androgen deprivation therapy (median age 72 years) were evaluated. By the FRAX algorithm with clinical information (no dual energy x-ray absorptiometry data) the 3% hip fracture risk threshold for treatment was exceeded by 51.2% of the men (median risk 3.1%). When subjects were grouped by age the treatment threshold was reached by 3.3% of those younger than 70 years, 76.6% of those 70 to 79 years old and by 98.8% of those 80 years old or older. Using FRAX with bone mineral density data in the 93 patients who underwent bone mineral density testing the median 10-year hip fracture risk was 0.9% and the treatment threshold was exceeded by 15% of these subjects.
Conclusions
In this cohort of men receiving androgen deprivation therapy the prevalence of risk sufficient to necessitate drug therapy was high and was strongly influenced by age. The WHO/FRAX algorithm identifies a greater proportion of men for treatment than the traditional threshold of T score −2.5 or less.
Keywords: prostatic neoplasms; osteoporosis; fractures, bone; androgens
OSTEOPOROSIS is a substantial burden in men. Worldwide, men suffer from a third of all hip fractures1 and the mortality associated with a male hip fracture can be as high as 37.5%.2 The most common causes of acquired osteoporosis in men are hypogonadism, chronic glucocorticoid therapy and excessive alcohol intake.3
The long established WHO diagnostic criteria compare BMD measured at the spine, hip or forearm to the young normal mean BMD for the reference population. Normal is BMD within 1 standard deviation (T score −1 or greater), low bone mass is 1.0 to 2.5 standard deviations below (T score between −1.0 and −2.5) and osteoporosis is 2.5 or greater standard deviations below (T score −2.5 or less).4
Most fractures occur in men whose BMD measurements are not in the osteoporotic range.5 The WHO/FRAX tool (http://www.shef.ac.uk/FRAX/) is designed to improve fracture risk assessment by incorporating age, BMI and a group of clinical risk factors in addition to femoral neck BMD.6 Clinical risk factors include prior fragility fracture, family history of hip fracture, current tobacco smoking, chronic use of glucocorticoids, daily consumption of alcohol, rheumatoid arthritis and other conditions associated with secondary osteoporosis.7–13 The FRAX model is constructed from population based data and, therefore, is population specific.
The NOF guidelines recommend initiation of drug therapy to prevent fractures if 1 of several conditions is met (see Appendix).14 Drug therapy should be started if the T score is −2.5 or less, or if the patient has a history of hip or vertebral fracture. Additionally, drug therapy should be started for those who have low bone mass (T score between −1.0 and −2.5, osteopenia) and a 3% or greater 10-year risk for hip fracture, or 20% or greater 10-year risk of MOF by the United States adapted FRAX model.15 MOF is defined as a hip, shoulder, forearm or clinical spine fracture. These recommendations align well with a United States economic model that suggests that osteoporosis treatment is cost-effective when the 10-year probability of hip fracture is at least 3%.16
Androgen deprivation therapy for prostate cancer decreases bone mineral density17–20 and is associated with a greater risk of clinical fractures.21–23 Little is known about the application of WHO/FRAX in men receiving ADT for prostate cancer. In this study we assessed fracture risks with the FRAX algorithm in a cohort of men with prostate cancer treated with ADT. We compared the results to traditional BMD criteria. These findings may provide practical insight to identify patients with prostate cancer most likely to benefit from drug therapy to prevent fractures.
MATERIALS AND METHODS
This cross-sectional study included men treated for prostate cancer with a GnRH agonist at an academic genitourinary medical oncology practice (Massachusetts General Hospital Cancer Center, Partners institutional review board approval). Enrollment took place during a 4-month period. Treatment and evaluation of the patients were at the discretion of the treating physicians. All men were encouraged to take calcium (1,200 mg or more daily) and vitamin D (800 to 1,000 IU daily) per NOF guidelines.
Information was collected on patient age, height, weight, timing and duration of GnRH agonist treatment, relevant Hologic QDR® 4500A densitometer DXA results, relevant prior bone targeted therapy, race, clinical status and presence or absence of clinical risk factors for fracture. DXA scans were obtained at the discretion of the treating physicians. Clinical risk factors for fracture include prior fragility fracture, parental history of hip fracture, current tobacco smoking, daily consumption of 3 or more units of alcohol, history of long-term use of oral glucocorticoids, rheumatoid arthritis and additional causes of secondary osteoporosis (organ transplantation, untreated thyroid disorders, type 1 diabetes, inflammatory bowel disease and prolonged immobility). Subjects were evaluated according to the WHO/FRAX fracture risk assessment tool. We considered ADT to be a form of secondary osteoporosis within the FRAX algorithm.
We described the cohort prevalence of an indication for drug therapy to prevent fracture according to the NOF guidelines and FRAX. All subjects were included in the analysis and reporting except 18 men receiving bisphosphonates who were excluded from BMD analyses. The primary end point was the percentage of subjects in the study cohort who reached NOF guideline treatment thresholds for fracture prevention pharmacotherapy based on FRAX. Secondary end points were the calculated 10-year risk of hip fracture and MOF, as well as BMD.
RESULTS
A total of 363 patients were evaluated (table 1). Median age was 72 years (range 48 to 98). The majority of subjects (89.3%) were white. Mean BMI was 29.6 kg/m2 and median ADT duration was 1.6 years (range 0 to 17). All men were considered at risk for secondary osteoporosis due to ADT. Daily consumption of at least 3 units of alcohol (11.6%) was the most prevalent additional clinical risk factor assessed in the FRAX algorithm. The prevalence of each of the other clinical risk factors was less than 10%. Bone metastases were present in 30.8% of the subjects. Fewer than 1 in 4 patients (22.3%) had received bisphosphonate treatment.
Table 1.
Patient demographics
| Mean ± SD pt age (range) | 72.0 ± 9.5 (48–98) | |
| No. race (%): | ||
| White | 324 | (89.3) |
| African-American | 20 | (5.5) |
| Asian | 7.0 | (1.9) |
| Hispanic | 12 | (3.3) |
| Mean ± SD kg/m2 BMI (range) | 29.6 ± 5.2 (18–63) | |
| No. individual clinical risk factors for fracture (%): | ||
| Secondary osteoporosis (ADT) | 363 | (100) |
| Daily consumption 3 or more drinks | 42 | (11.6) |
| Long-term use of glucocorticoids | 30 | (8.3) |
| Parental history of fracture | 27 | (7.4) |
| Ongoing tobacco use | 17 | (4.7) |
| Personal history of fracture | 9 | (2.5) |
| No. bone metastases (%) | 112 | (30.8) |
| No. bisphosphonate use (%) | 81 | (22.3) |
Using the FRAX algorithm with clinical information alone (no DXA data) the median 10-year hip fracture risk was 3.1% and 51.2% of subjects exceeded the 3% threshold for drug therapy (table 2). Median MOF risk was 12%. Hip fracture risk accounted for a higher proportion of patients reaching treatment thresholds than did MOF risk (51.2% vs 10.2%). In analyses that excluded ADT as a risk factor for secondary osteoporosis the median estimated 10-year hip fracture risk was 1.8% and 32.8% of subjects still exceeded the 3% threshold for pharmacotherapy.
Table 2.
Calculated fracture risks
| FRAX fracture risk from clinical risk factors including ADT as secondary osteoporosis: |
||
| Median % hip fracture risk (range) | 3.1 | (0.2–26) |
| No. 3% or greater 10-yr hip fracture risk (%) | 186 | (51.2) |
| Median % MOF risk (range) | 12 | (2.2–33) |
| No. 20% or greater 10-y MOF risk (%) | 37 | (10.2) |
| FRAX fracture risk from clinical risk factors excluding ADT as secondary osteoporosis: |
||
| Median % hip fracture risk (range) | 1.8 | (0.1–16) |
| No. 3% or greater 10-yr hip fracture risk (%) | 119 | (32.8) |
| Median % MOF risk (range) | 8.4 | (1.6–25) |
| No. 20% or greater 10-yr MOF risk (%) | 4 | (1.1) |
| FRAX fracture risk calculated using measured femoral neck BMD (in 93 pts): |
||
| Median T score (range) | −1.0 | (−3.4–2.2) |
| Median gm/cc BMD (range) | 0.81 | (0.47–1.23) |
| Median % hip fracture risk with DXA (range) | 0.9 | (0–15) |
| Median % hip fracture risk without DXA (range) | 2.4 | (0.2–26) |
| Median % MOF risk with DXA (range) | 7.0 | (2.5–25) |
| Median % MOF risk without DXA (range) | 11 | (3.0–33) |
| No. 3% or greater 10-yr hip fracture risk + T score less than −1 (%) |
14 | (15) |
| No. 3% or greater 10-yr hip fracture risk without DXA (%) |
41 | (44.1) |
| No. T score −2.5 or less (%) | 6 | (6.5) |
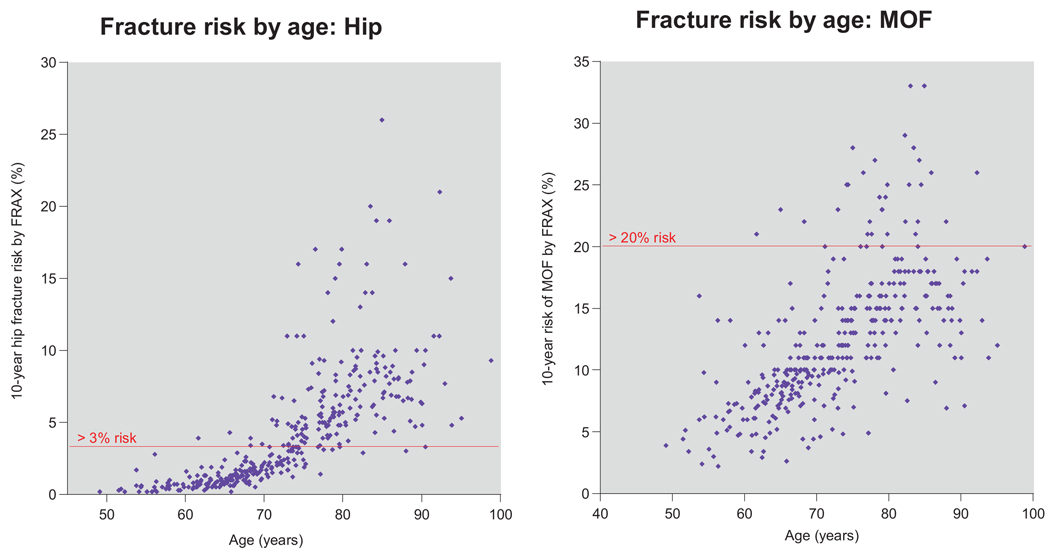
Figure 1 shows individual estimates of hip fracture and MOF risk by age. None of the 32 subjects younger than 60 years and only 5 of the 119 age 60 to 69 reached the hip fracture treatment threshold. In contrast the hip fracture treatment threshold was exceeded by 76.6% (98 of 128) of subjects 70 to 79 years old and by 98.8% (83 of 84) of those 80 years old or older.
Figure 1.
Scatter plots for fracture risk by age. Risks are calculated with FRAX using clinical factors without DXA information.
Among the 93 subjects who underwent BMD assessment by Hologic DXA scan, median BMD was 0.81 gm/cm2 (range 0.47 to 1.23) and median femoral neck T score was −1.0 (range −3.4 to 2.2). Median ADT duration was only slightly longer in this subset than in the entire cohort (1.8 vs 1.6 years). Using the FRAX algorithm with BMD data the median 10-year hip fracture risk was 0.9% and the hip fracture treatment threshold was exceeded by 15% (14 of 93) of subjects. In contrast the median 10-year hip fracture risk by FRAX without BMD data was 2.4% and 41 of 93 subjects (44.1%) exceeded the 3% risk threshold.
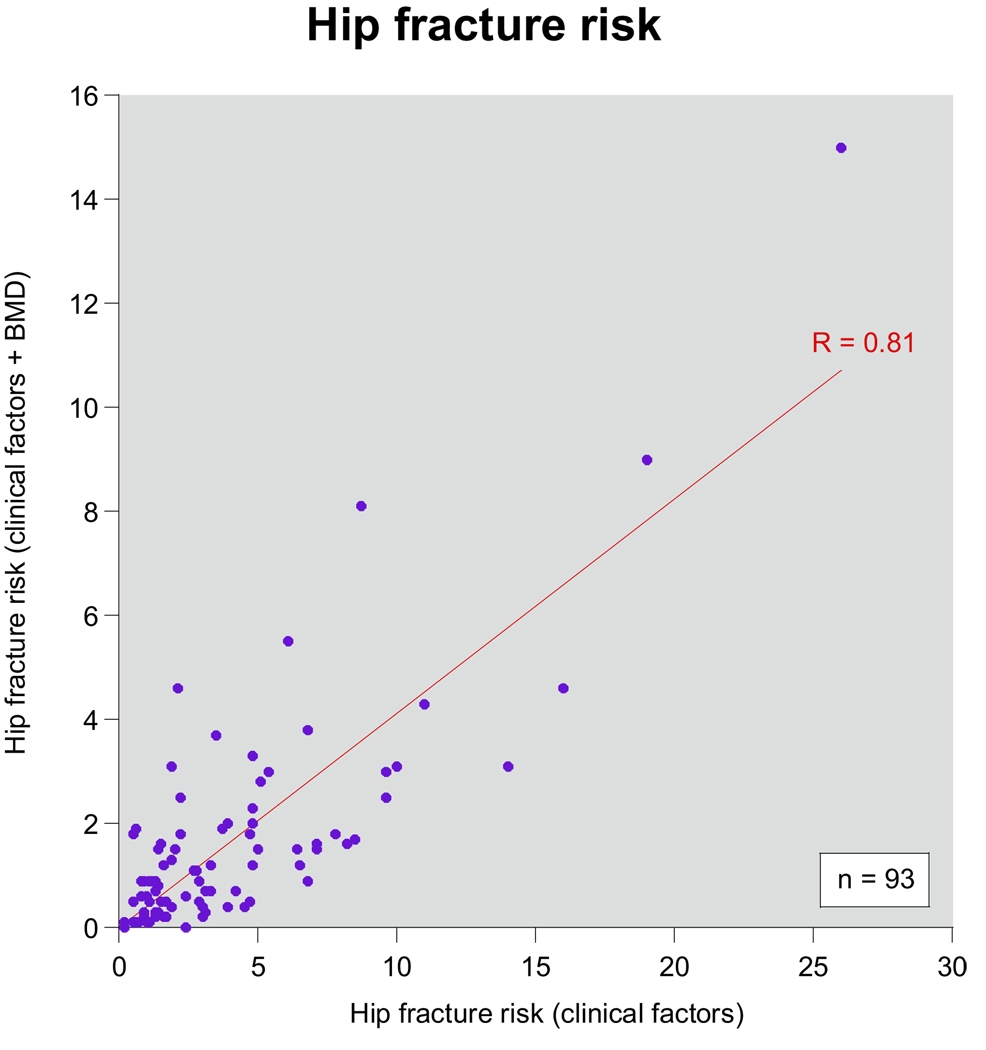
Figure 2 is a scatter plot with the 10-year hip fracture risk assessments by FRAX with (y-axis) and without (x-axis) BMD data. The fracture estimates with and without BMD data were highly correlated (r = 0.81, p < 0.0001), although the estimated fracture risk rates were consistently lower when BMD data were included in the FRAX algorithm. Of the 6 subjects with a T score less than −2.5, 2 did not meet the treatment threshold by FRAX. Of the 14 subjects who exceeded the treatment threshold by FRAX only 4 had T scores less than −2.5.
Figure 2.
Correlation between FRAX calculations with and without BMD. Hip fracture risks were calculated with FRAX using clinical factors without DXA BMD information (x-axis) and again with DXA information (y-axis). Analysis was performed on subset of patients who had been evaluated with DXA scan and had not been treated with bisphosphonate (93). Correlation coefficient was 0.81 (p <0.0001).
DISCUSSION
The WHO/FRAX calculator is a convenient online tool to estimate fracture risk for individual patients. We applied this algorithm to a cohort of 363 men treated with GnRH agonists for prostate cancer and found that a high proportion of subjects reached treatment thresholds by NOF guidelines (51.2%). Use of the FRAX algorithm with clinical data identifies a larger proportion of patients who meet NOF defined treatment thresholds than would be identified by traditional BMD criteria.
Meta-analyses have shown that advancing age increases fracture risk beyond that predicted by age related loss of BMD.24 Although typical changes in BMD would predict a 4-fold increase in fracture risk from ages 50 to 90 years, fracture risk actually increases 30-fold. Estimated fracture rates using FRAX calculations in our cohort reflect the strong influence of older age on risk for clinical fracture. When clinical factors were used without BMD, FRAX estimated that 76.6% of men in their 70s and virtually all men 80 years old or older exceeded the NOF recommended risk threshold for drug therapy.
ADT is associated with an increased risk of fractures. 21,23 In an analysis of more than 50,000 men treated with ADT there was a statistically significant relationship between fracture risk and number of doses of GnRH agonist even when the analysis was adjusted for patient and tumor characteristics. The incidence of fracture 5 years beyond prostate cancer diagnosis was 19.4% among those who received ADT and 12.6% among those who did not.21 This hazard ratio of 1.54 is in line with the ADT attributable risk by FRAX within our cohort.
In another study of men with prostate cancer Adler et al found limited concordance between FRAX and T score treatment thresholds.25 Their cohort was older (average age 77 vs 72), was majority African-American (58% vs 5%), had a larger proportion of current smokers (15% vs 5%) and did not include men with bony metastases. They found a lower mean femoral neck T score (−1.4 vs −0.9) and a higher percentage of men with T scores −2.5 or less (33% vs 6.5%), although for the latter they used T scores at all sites rather than just at the femoral neck. In contrast to our cohort their study population had a higher percentage of subjects who met the treatment threshold by T score (33%) than by FRAX calculation (17%). Similar to our cohort their study population had a higher percentage of patients that met the treatment threshold by FRAX when the calculation was performed without BMD.
The epidemiological data on which FRAX is based are from the general population rather than men receiving ADT. There are several reasons that FRAX calculated risk may underestimate fracture risk in men treated for prostate cancer. Although ADT is a potent cause of secondary osteoporosis, FRAX considers all causes of secondary osteoporosis to be equivalent. In addition, the relative risk of fracture increases with increasing duration of ADT, a phenomenon not captured by binary responses within FRAX.21 FRAX also does not consider the potential for the additive impact of more than 1 form of secondary osteoporosis. Finally FRAX considers greater BMI to be protective against fractures. GnRH agonists increase BMI and fat mass but decrease lean muscle mass, a phenotype known as sarcopenic obesity.17,26 As some data support a strong relationship between lean mass and BMD, sarcopenia may be detrimental beyond that which is captured by FRAX.27 ADT related sarcopenic obesity may also contribute to frailty and fall related fracture risk.
We did not consider bone metastases for the purposes of this analysis. Zoledronic acid is standard of care for the prevention of skeletal related events in men with castration resistant prostate cancer metastatic to bone.28–30 Therefore, some subjects within the cohort are treated with zoledronic acid independent of osteoporotic fracture risk.
Our subjects were drawn from a single institution academic practice. Different results may be observed in patients in other practice settings. As risk calculations for the United States adapted FRAX model are race specific, our observations within a largely white cohort may differ from those of practices in the United States with a different racial profile or from practices outside of the United States. Additionally, the subset of men evaluated with DXA may not be broadly representative because those men were motivated and able to pursue an additional test. The proportion of men meeting treatment thresholds was more modest in that group (1 of 39 men younger than 70 years, 7 of 37 men in their 70s, 7 of 16 men 80 years old or older).
The WHO/FRAX tool appears to provide improved guidance relative to traditional criteria based on BMD alone. NCCN guidelines recommend screening and treatment for osteoporosis in men with prostate cancer according to the NOF guidelines for the general population. 28 NCCN recommendations specify that FRAX should be used and that ADT should be considered secondary osteoporosis in the algorithm. Other pertinent NOF recommendations include calcium (1,200 mg or greater daily) and vitamin D (800 to 1,000 IU daily) supplementation, regular weight-bearing and muscle strengthening exercise, tobacco cessation and avoidance of excessive alcohol intake.
CONCLUSIONS
Men treated with ADT for prostate cancer are particularly vulnerable to clinical fractures. Thoughtful selection of appropriate patients for drug therapy to reduce fracture risk is essential. The use of FRAX to quantify fracture risk is a rational strategy as it incorporates clinical risk factors that add to the discriminatory power of BMD. The use of FRAX is recommended by NOF and NCCN guidelines. We informed the clinical management of prostate cancer survivors by using the WHO/FRAX risk assessment tool to characterize fracture risk in a cohort of men receiving ADT. The prevalence of risk sufficient to necessitate drug therapy was high and was strongly influenced by age. Since FRAX was not designed specifically for men receiving ADT, important clinical factors unique to men receiving ADT may not be accounted for and the risk of fracture may be underestimated. Further work is needed to refine risk assessment in this vulnerable population.
Abbreviations and Acronyms
- ADT
androgen deprivation therapy
- BMD
bone mineral density
- BMI
body mass index
- DXA
dual energy x-ray absorptiometry
- GnRH
gonadotropin-releasing hormone
- MOF
major osteoporotic fracture
- NCCN
National Comprehensive Cancer Network
- NOF
National Osteoporosis Foundation
APPENDIX
| Relevant National Osteoporosis Foundation (NOF) recommendations |
|---|
| Screening |
NOF recommends testing of bone mineral density for:
|
| Treatment |
NOF recommends calcium (1,200 mg or greater daily) and vitamin D (800–1,000 IU daily) for all and consideration for treatment if age 50 or older and any of the following:
|
Adapted from National Osteoporosis Foundation’s Clinician’s Guide to Prevention and Treatment of Osteoporosis.14
Footnotes
Recipient of a National Institutes of Health K24 Midcareer Investigator Award (5K24CA121990-02) and grants from the Prostate Cancer Foundation.
Study was received institutional review board approval.
REFERENCES
- 1.Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int. 1997;7:407. doi: 10.1007/pl00004148. [DOI] [PubMed] [Google Scholar]
- 2.Jiang HX, Majumdar SR, Dick DA, et al. Development and initial validation of a risk score for predicting in-hospital and 1-year mortality in patients with hip fractures. J Bone Miner Res. 2005;20:494. doi: 10.1359/JBMR.041133. [DOI] [PubMed] [Google Scholar]
- 3.Bilezikian JP. Osteoporosis in men. J Clin Endocrinol Metab. 1999;84:3431. doi: 10.1210/jcem.84.10.6060. [DOI] [PubMed] [Google Scholar]
- 4.Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1. [PubMed]
- 5.Seeman E, Bianchi G, Khosla S, et al. Bone fragility in men–where are we? Osteoporos Int. 2006;17:1577. doi: 10.1007/s00198-006-0160-8. [DOI] [PubMed] [Google Scholar]
- 6.Kanis JA, Johnell O, Oden A, et al. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19:385. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Laet C, Kanis JA, Oden A, et al. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int. 2005;16:1330. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 8.Johnell O, Kanis JA, Oden A, et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20:1185. doi: 10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- 9.Kanis JA, Johansson H, Johnell O, et al. Alcohol intake as a risk factor for fracture. Osteoporos Int. 2005;16:737. doi: 10.1007/s00198-004-1734-y. [DOI] [PubMed] [Google Scholar]
- 10.Kanis JA, Johansson H, Oden A, et al. A family history of fracture and fracture risk: a meta-analysis. Bone. 2004;35:1029. doi: 10.1016/j.bone.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Kanis JA, Johansson H, Oden A, et al. A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res. 2004;19:893. doi: 10.1359/JBMR.040134. [DOI] [PubMed] [Google Scholar]
- 12.Kanis JA, Johnell O, De Laet C, et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone. 2004;35:375. doi: 10.1016/j.bone.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Kanis JA, Johnell O, Oden A, et al. Smoking and fracture risk: a meta-analysis. Osteoporos Int. 2005;16:155. doi: 10.1007/s00198-004-1640-3. [DOI] [PubMed] [Google Scholar]
- 14.National Osteoporosis Foundation: Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, D. C.: BoneSource; 2008. pp. 1–37. [Google Scholar]
- 15.Watts NB, Lewiecki EM, Miller PD, et al. National Osteoporosis Foundation 2008 Clinician’s Guide to Prevention and Treatment of Osteoporosis and the World Health Organization Fracture Risk Assessment Tool (FRAX): what they mean to the bone densitometrist and bone technologist. J Clin Densitom. 2008;11:473. doi: 10.1016/j.jocd.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Dawson-Hughes B, Tosteson AN, Melton LJ, 3rd, et al. Implications of absolute fracture risk assessment for osteoporosis practice guidelines in the USA. Osteoporos Int. 2008;19:449. doi: 10.1007/s00198-008-0559-5. [DOI] [PubMed] [Google Scholar]
- 17.Berruti A, Dogliotti L, Terrone C, et al. Changes in bone mineral density, lean body mass and fat content as measured by dual energy x-ray absorptiometry in patients with prostate cancer without apparent bone metastases given androgen deprivation therapy. J Urol. 2002;167:2361. [PubMed] [Google Scholar]
- 18.Daniell HW, Dunn SR, Ferguson DW, et al. Progressive osteoporosis during androgen deprivation therapy for prostate cancer. J Urol. 2000;163:181. [PubMed] [Google Scholar]
- 19.Maillefert JF, Sibilia J, Michel F, et al. Bone mineral density in men treated with synthetic gonadotropin-releasing hormone agonists for prostatic carcinoma. J Urol. 1999;161:1219. [PubMed] [Google Scholar]
- 20.Smith MR, McGovern FJ, Zietman AL, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001;345:948. doi: 10.1056/NEJMoa010845. [DOI] [PubMed] [Google Scholar]
- 21.Shahinian VB, Kuo YF, Freeman JL, et al. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 22.Smith MR, Boyce SP, Moyneur E, et al. Risk of clinical fractures after gonadotropin-releasing hormone agonist therapy for prostate cancer. J Urol. 2006;175:136. doi: 10.1016/S0022-5347(05)00033-9. [DOI] [PubMed] [Google Scholar]
- 23.Smith MR, Lee WC, Brandman J, et al. Gonadotropin-releasing hormone agonists and fracture risk: a claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol. 2005;23:7897. doi: 10.1200/JCO.2004.00.6908. [DOI] [PubMed] [Google Scholar]
- 24.Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporos Int. 2005;16:581. doi: 10.1007/s00198-004-1780-5. [DOI] [PubMed] [Google Scholar]
- 25.Adler RA, Hastings FW, Petkov VI. Treatment thresholds for osteoporosis in men on androgen deprivation therapy: T-score versus FRAX. Osteoporos Int. 2009 doi: 10.1007/s00198-009-0984-0. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 26.Smith MR, Finkelstein JS, McGovern FJ, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87:599. doi: 10.1210/jcem.87.2.8299. [DOI] [PubMed] [Google Scholar]
- 27.Douchi T, Kuwahata R, Matsuo T, et al. Relative contribution of lean and fat mass component to bone mineral density in males. J Bone Miner Metab. 2003;21:17. doi: 10.1007/s007740300003. [DOI] [PubMed] [Google Scholar]
- 28.NCCN: National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: Prostate Cancer. vol. V. 2009. p. 2. 2009. [Google Scholar]
- 29.Rosen LS, Gordon D, Tchekmedyian S, et al. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial–the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J Clin Oncol. 2003;21:3150. doi: 10.1200/JCO.2003.04.105. [DOI] [PubMed] [Google Scholar]
- 30.Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]