Abstract
Bacteroides is an abundant genus of bacteria of the human intestinal microbiota. Bacteroides species synthesize a large number of capsular polysaccharides (PS), a biological property not shared with closely related oral species, suggesting importance for intestinal survival. Bacteroides fragilis, for example, synthesizes eight capsular polysaccharides per strain, each of which phase varies via inversion of the promoters located upstream of seven of the eight polysaccharide biosynthesis operons. In a single cell, many of these polysaccharide loci promoters can be simultaneously oriented on for transcription of the downstream biosynthesis operons. Here, we demonstrate that despite the promoter orientations, concomitant transcription of multiple polysaccharide loci within a cell is inhibited. The proteins encoded by the second gene of each of these eight loci, collectively designated the UpxZ proteins, inhibit the synthesis of heterologous polysaccharides. These unique proteins interfere with the ability of UpxY proteins encoded by other polysaccharide loci to function in transcriptional antitermination of their respective operon. The eight UpxZs have different inhibitory spectra, thus establishing a hierarchical regulatory network for polysaccharide synthesis. Limitation of concurrent polysaccharide synthesis strongly suggests that these bacteria evolved this property as an evasion-type mechanism to avoid killing by polysaccharide-targeting factors in the ecosystem.
Keywords: microbiota, upxZ, upxY, antitermination
The human intestinal microbiota is home to hundreds of different species of bacteria collectively reaching densities of nearly a trillion microbes per gram. This bacterial consortium contributes significantly to human development and provides a multitude of health benefits (reviewed in ref. 1). Within the last several years, genome sequences of many of the most abundant species of this ecosystem have been completed. The National Institutes of Health Human Microbiome Project is furthering these analyses providing reference genomes of hundreds of additional strains. The next challenge will be to use these sequences to begin to answer the numerous biological questions regarding the properties that these bacteria evolved to survive in their ecosystem and to interact with the host and other microbial members.
Bacteroides is one of the most abundant genera of bacteria in the human colon. Comparisons of the genome sequences of different Bacteroides species allows for identification of genes and/or properties that are conserved among the intestinal species that are not conserved in closely related oral species. Conserved features within closely related species of one ecosystem but not another suggest importance for survival in that ecosystem. Intestinal Bacteroides species share the conserved property of synthesizing a large number of phase variable capsular polysaccharides (PS) (2). We have shown that the synthesis of one PS is necessary for Bacteroides fragilis to colonize its niche (3), but the biological importance of the synthesis of multiple phase variable PSs by Bacteroides species is unknown.
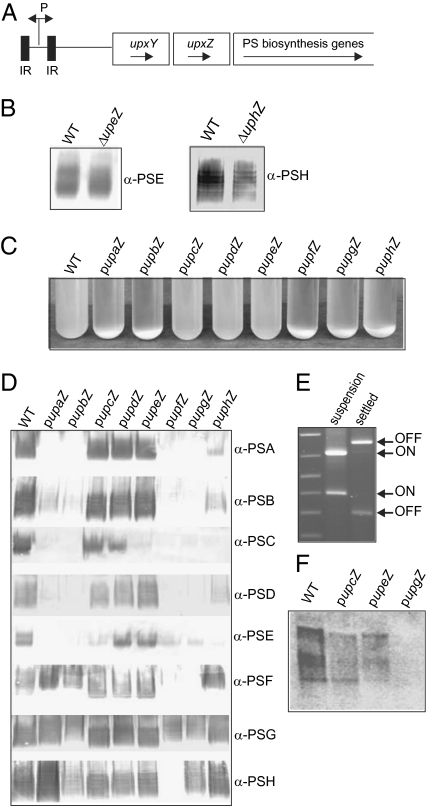
In B. fragilis, the eight capsular polysaccharide biosynthesis loci (designated PSA-PSH) are scattered throughout the B. fragilis genome and share a common genetic organization (Fig. 1A). Each locus is an operon of between 11 and 22 genes. For seven of the eight loci, the promoter regions undergo inversion between 19- and 25-bp inverted repeats (IRs) leading to phase variable synthesis of the PSs (4, 5). The first two genes of each locus encode products that are similar between loci and are designated the UpxY and UpxZ families, where x is a–h, depending on the locus (4).
Fig. 1.
UpxZs inhibit synthesis of subsets of polysaccharides. (A) Genetic organization of the polysaccharide biosynthesis operons demonstrating the invertible promoter (P) flanked by inverted repeats (■). upxY and upxZ are the first two genes of each operon, and are followed by 9–20 genes involved in polysaccharide synthesis. (B) Western blot analysis of synthesis of PSE and PSH from both wild type and the ΔupeZ and ΔuphZ mutants. (C) Overnight broth cultures showing the phenotypes of wild-type bacteria containing each of the upxZ genes in trans. Aggregated bacteria are visible at the bottom of many cultures. (D) Western blot analysis of the synthesis of each of the eight capsular polysaccharides from wild-type bacteria and wild type with each of the eight upxZ genes constitutively expressed from a plasmid. Cultures were mixed before analysis so that both aggregated and suspended bacteria were included. (E) Analysis of the orientations of the PSG promoter from bacteria in suspension or settled from the broth culture of the upgZ transconjugant. (F) Northern blot analysis of the PSA transcript from wild type and transconjugants containing various PSA inhibitory and noninhibitory upxZs.
The UpxYs are NusGSP (6) transcriptional antitermination factors that associate with RNA polymerase in the 5′ untranslated region between the downstream IR and the start of the respective upxY gene, thereby allowing read through of downstream transcription termination sites (7). Deletion of an upxY renders the organism unable to produce the PS synthesized by the locus in which it resides. The UpxZs are unique proteins with no orthologs other than similar proteins encoded by the PS biosynthesis loci of other Bacteroides species. The UpxZs also do not contain any conserved functional domains to suggest a possible role for this family of proteins in PS regulation. Therefore, the purpose of this study was to determine the role of the UpxZ proteins in regulation of the multiple PSs of Bacteroides species. The data reveal that the UpxZs inhibit heterologous PS synthesis and suggest the possible biological importance of the synthesis of multiple phase variable PS in the survival of the Bacteroides in the human colonic ecosystem.
Results
UpxZs Inhibit Synthesis of Heterologous PSs.
The upxZs are invariably preceded by an upxY; therefore, we first predicted that these two products may function together in transcriptional antitermination. To test this hypothesis, we deleted the upxZ of two loci, the PSE locus (upeZ) and the PSH locus (uphZ). Unlike the UpxY products, the UpxZs are not necessary for synthesis of their own PSs, as PSE and PSH are synthesized by the ΔupeZ and ΔuphZ mutants, respectively (Fig. 1B).
Next, the opposite experiment was performed where we analyzed the resulting phenotypes when each of the eight upxZs was individually cloned into an expression vector behind a constitutive promoter and placed in the wild-type background. These constructs result in overexpression of each UpxZ compared with their wild-type expression levels, as they are no longer phase variable and are synthesized from a multicopy number plasmid. The resulting phenotypes of these eight different transconjugants grown in liquid broth are shown in Fig. 1C. Overexpression of some UpxZs resulted in the aggregation or settling of a portion of the bacterial culture. This phenotype has been correlated with the acapsular state (3, 8). Western blot analysis of the synthesis of each of the eight capsular PSs from these transconjugants revealed that UpxZs inhibit the synthesis of subsets of heterologous PSs (Fig. 1D). Some UpxZs, such as UpfZ, UpgZ, and UphZ, have very broad inhibitory capabilities in that they inhibit the synthesis of most capsular PSs, and others, such as UpcZ, UpdZ, and UpeZ, have narrow or no obvious inhibitory capabilities. The Western blot results show that PSG is the only PS whose synthesis is not inhibited when upfZ is constitutively expressed. Therefore, we predicted that the settled bacteria present in the upfZ transconjugant culture would have the PSG promoter in the off orientation, whereas the bacteria growing in suspension would have the PSG promoter oriented on. Using PCR digestion, a quantitative method to determine orientations of invertible DNA regions (4, 9), we confirmed that the settled bacteria from this transconjugant have the PSG promoter oriented off (and are therefore acapsular due to UpfZ's ability to inhibit synthesis of all other PSs with an on-oriented promoter), whereas the majority of the bacteria growing in suspension have the PSG promoter oriented on (Fig. 1E), and are therefore encapsulated.
UpxZs Function at the Transcriptional Level.
To determine whether the UpxZs inhibit PS synthesis by affecting transcription, the PSA transcript from transconjugants containing either upcZ or upeZ (neither of which inhibits PSA synthesis) or upfZ (which inhibits PSA synthesis) was analyzed by Northern blot. These results demonstrate that UpfZ, but not UpcZ nor UpeZ, prevents transcription of the PSA locus (Fig. 1F).
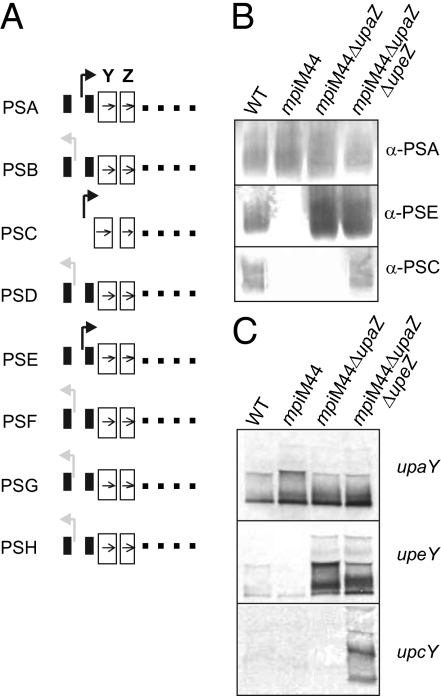
DNA inversion of the seven invertible PS promoters is mediated by the Mpi recombinase (10). Deletion of mpi results in mutants in which each of the invertible promoters is locked and does not invert. We previously analyzed several mpi mutants and found that in some cases, a PS locus promoter could be locked on without synthesis of that particular PS (10). One mutant, mpiM44, has the PSA and PSE promoters locked on and the five other invertible PS promoters locked off (Fig. 2A). On the basis of these genotypes, this mutant would be expected to express PSA, PSE, and PSC, as the PSC promoter does not invert and is always oriented on; however, this mutant only expresses PSA. The reported synthesis of PSF by this mutant (10) was found to be due to residual antibody to PSA in the α-PSF preparation. On the basis of the activity of the UpxZs, we predicted that constitutive synthesis of UpaZ in this mutant inhibits synthesis of PSC and PSE. To test this hypothesis, upaZ was deleted from mpiM44, resulting in a mutant that now synthesized both PSA and PSE (Fig. 2B). Further mutation by deletion of upeZ resulted in a mutant that expressed PSA, PSE, and PSC (Fig. 2B). Northern blot analysis confirmed that transcription of the PSE and PSC operons is inhibited in mpiM44 and restored when upaZ or both upaZ and upeZ are deleted (Fig. 2C). These data demonstrate that the lack of synthesis of PSs with locked on promoters in mpi mutants is due to constitutive expression of inhibitory UpxZs. These data also demonstrate that the synthesis of PSC is regulated by the phase variable expression of the UpxZs of other loci, providing a molecular rationale for its phase variation despite the absence of an invertible promoter.
Fig. 2.
Promoter orientation does not correlate with PS synthesis in B. fragilis mpi mutants due to UpxZ inhibition of heterologus PS loci transcription. (A) Schematic of the locked promoter orientations of mpiM44. PSA, PSE, and PSC promoters are locked on but the mutant expresses only PSA. (B) Western immunoblot analysis of PSA, PSE, and PSC synthesis in mpiM44, and the same mutant with successive deletions of upaZ and upeZ. (C) Northern blot analysis of PSA, PSE, and PSC transcript in mpiM44, mpiM44ΔupaZ, and mpiM44ΔupaZΔupeZ.
UpaZ Increases Transcriptional Termination Within the 5′ Untranslated Regions of the PSE Locus.
On the basis of the preceding results that conclusively demonstrated that UpaZ inhibits the transcription of the PSE locus, we studied this inhibitory interaction. To determine whether the UpxZs are repressors that inhibit transcription initiation, we used xylE transcriptional fusion clones. We used three different PSE promoter–xylE transcriptional fusions previously constructed to analyze transcription of the PSE locus in an upeY deletion mutant (7). All three constructs contained DNA from just inside the upstream inverted repeat so that the PSE promoter is locked on and extended to various regions downstream of the promoter (Fig. 3A). These constructs were individually placed in trans in mpiM44 (constitutively expressing UpaZ with PSE synthesis inhibited) or mpiM44ΔupaZ (expressing PSE), and xylE transcript was analyzed by Northern blot. The results demonstrate that UpaZ does not inhibit initiation of transcription of the PSE locus, as xylE is transcribed when clones 8 and 10 are placed in the mpiM44 and mpiM44ΔupaZ backgrounds. Rather, UpaZ inhibits transcription of the PSE locus further downstream in the region between clones 10 and 11 (Fig. 3B). Using these same clones, we previously showed that the PSE locus transcript also terminates between clones 10 and 11 in the upeY deletion mutant (7), indicating that the transcriptional effect of expression of UpaZ is similar to that when UpeY is absent. These data suggest that UpaZ affects the ability of UpeY to perform its transcriptional antitermination function.
Fig. 3.
XylE transcriptional fusion analyses of the inhibitory action of UpaZ on PSE locus transcription. (A) Schematic of the PSE regions cloned into the promoterless xylE reporter plasmid. Each clone was amplified with the same forward primer (F) located just inside the upstream IR and contains the promoter in the on orientation and extending 44 bp (clone 8), 135 bp (clone 10), and 229 bp (clone 11) downstream of the transcriptional start site. (B) Northern blot analysis of xylE transcription when each clone is placed in the mpiM44 or mpiM44ΔupaZ background.
UpaZ Interferes with the Ability of UpeY to Mediate Transcriptional Antitermination.
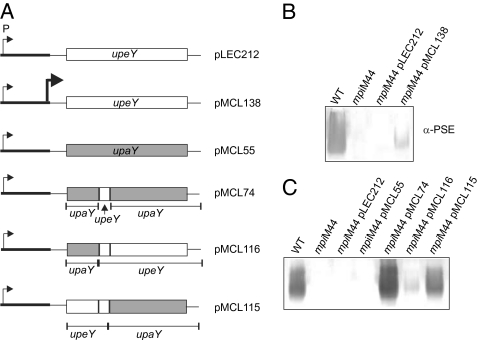
If UpaZ inhibits at the level of UpeY, we reasoned it might be possible to overcome its inhibitory effect by overexpressing UpeY in mpiM44. Two different upeY expression constructs were made, one in which upeY is transcribed from a moderately strong plasmid-borne promoter located 245 bp from the start of upeY (pLEC212) and another where a strong promoter was inserted into this construct just 51 bp upstream of upeY (pMCL138) (Fig. 4A). Western blot analysis of PSE synthesis when these two constructs were added in trans to mpiM44 demonstrated that the UpeY produced from pLEC212 is insufficient to overcome the UpaZ inhibitory properties; however, the increased expression of UpeY from plasmid pMCL138 resulted in a detectable amount of PSE synthesis (Fig. 4B). These data further suggest that UpaZ inhibits at the level of UpeY.
Fig. 4.
UpeY variants overcome UpaZ-mediated inhibition of PSE synthesis in mpiM44. (A) Schematics of the upeY and upaY wild-type and hybrid constructs added in trans to mpiM44. pLEC212 contains wild-type upeY, including its immediate 27-bp upstream region, cloned into expression vector pFD340. Transcription is driven by a vector-borne promoter (thin arrow) located 245 bp upstream of upeY. pMCL138 is the same as pLEC212 except that a strong promoter (thick arrow) is situated 51 bp upstream of upeY. pMLC55 contains wild-type upaY, including its 38-bp immediate upstream region, cloned into expression vector pFD340. pMCL74 is the same as pMCL55 except that a 51-bp region of upaY (corresponding to amino acids 62–78) was replaced with a 51-bp region of upeY (also corresponding to amino acids 62–78). This small substitution confers UpeY activity to this hybrid (7). Both pMCL116 and pMCL115 are additional hybrids of upeY and upaY and both include the 51-bp region of upeY sufficient to confer UpeY activity to UpaY. In addition, pMCL116 contains 38-bp upstream upaY through the first 183 bp of upaY with the remainder of the gene upeY, whereas pMCL115 contains 27-bp upstream upeY through the first 234 nt of upeY with the remainder of the hybrid gene derived from upaY. (B) Western blot analysis of PSE synthesis from whole cell lysates demonstrating that overexpression of UpeY overcomes inhibition of PSE synthesis in mpiM44. (C) Western blot analysis of PSE synthesis from whole cell lysates demonstrating that upaY–upeY hybrids restore PSE synthesis to mpiM44.
We previously constructed plasmid pMCL74 that contains a hybrid upaY–upeY gene that is largely upaY except for a 51-bp segment (corresponding to amino acids 62–78) that was changed to that of upeY (Fig. 4A). This small segment of upeY is sufficient to confer UpeY activity to the hybrid protein in that it is able to restore PSE synthesis to an upeY deletion mutant (7). When pMCL74 was placed in the mpiM44 background, PSE was expressed (Fig. 4C). Transcription of this hybrid gene is being driven from the same plasmid-borne promoter as pLEC212, from which native upeY did not restore PSE synthesis to mpiM44. We created two additional upaY–upeY hybrid constructs, each containing the 51-bp DNA region corresponding to the 17-aa region of UpeY necessary for its ability to function in PSE locus transcriptional antitermination. The hybrid gene of pMCL115 has the 5′ end identical to upeY and the 3′ end identical to that of upaY, whereas in pMCL116 the hybrid is reversed (Fig. 4A). When pMCL115 was placed in trans in mpiM44, the inhibitory effect of UpaZ on PSE synthesis was overcome, whereas the UpaY–UpeY hybrid encoded by pMCL116 restored only marginal PSE synthesis when present in mpiM44. pMCL115 contains not only the 5′ region of upeY, but also the native upeY ribosome binding site (rbs). Therefore, UpaZ does not inhibit PSE synthesis by blocking the upeY rbs, thereby preventing UpeY translation. Rather, the hybrid protein data demonstrate that modification of amino acids 79–172 of UpeY to those of the UpaY alters UpaZ's ability to prevent transcriptional antitermination of the PSE locus. These data suggest that UpaZ likely interacts with native UpeY, thereby preventing it from functioning in transcriptional antitermination.
Discussion
Collectively, these data demonstrate that the UpxZs inhibit transcription of PS loci indirectly by interfering with the ability of subsets of heterologous UpxYs to function in transcriptional antitermination. These data provide a molecular rationale for why this organism evolved to produce eight distinct and specific UpxYs rather than one factor such as RfaH, which functions as a transcriptional antitermination factor of several operons in Escherichia coli (11). This mechanism of limiting production of multiple PSs by synthesizing inhibitors that affect locus-specific antitermination factors, with a hierarchical mode of regulation, circumvents the need to coordinate PS promoter orientations for synthesis of single PSs.
The phase variable expression of UpxZs creates a very dynamic regulatory network within a given cell. If a promoter switches from the off to on orientation, transcription is initiated, and based on the PS region and the other PS loci with on-oriented promoters, its UpxZ is either able to shut down transcription of other PS loci or heterologous UpxZs are able to inhibit its synthesis. As B. fragilis must be encapsulated to colonize (3), PSC serves as the default or fail-safe PS that is synthesized when all other PS promoters are oriented off, as the PSC locus has a constitutive promoter but its synthesis is inhibited by all heterologous UpxZs.
We initially hypothesized that phase variable synthesis of eight distinct PSs by B. fragilis could potentially create 256 different surface PS combinations, depending on whether all of the promoters are oriented off, on, or any combination in between, with the assumption that an on-oriented promoter results in synthesis of that PS (4). Coexpression of surface PSs has been observed from in vitro grown bacteria (4) likely reflecting a phenotypic lag between the genetic events that halt synthesis of one PS and the loss of the enzymatic machinery and respective PS from the surface. However, this study clearly demonstrates that this organism evolved to limit simultaneous synthesis of multiple PSs. This process allows a single strain of B. fragilis within an intestinal ecosystem to produce cells expressing one of each of the eight distinct surface PSs.
Surface PSs are often the targets of phage adhesions (12–14), phage glycosidases (15), and host immune molecules that are deleterious to bacteria. The ability to create diverse bacterial cells each synthesizing different single surface PSs would allow the organism to evade attack from phage and potentially other factors of the intestinal milieu that target specific PSs. As families of UpxZ proteins are encoded by the genomes of many abundant intestinal Bacteroides species including B. thetaiotaomicron, B. uniformis, B. intestinalis, B. eggerthii, B. cellulosilyticus, and B. stercoris, it is likely that this PS regulatory network is more broadly applicable to this genus of human intestinal symbionts.
Methods
The sequences of the primers used in this study are listed in Table S1.
Bacterial Growth Conditions.
B. fragilis NCTC9343 was the parental strain of all mutants. B. fragilis was grown anaerobically in basal medium or on brain–heart infusion plates supplemented with hemin (50 μg mL−1) and menadione (0.5 μg mL−1) (BHIS), with gentamicin (200 μg mL−1) and erythromycin (5 μg mL−1) added where appropriate. Escherichia coli DH5α containing recombinant plasmids was grown on L broth or on L agar plates containing ampicillin (100 μg mL−1) and/or kanamycin (50 μg mL−1).
Western Immunoblot Analysis.
Bacteria were boiled in LDS sample buffer and electrophoresed using NuPAGE 4–12% gradient polyacrylamide gels with MES buffer (Invitrogen) and transferred to PVDF. Polysaccharide synthesis was tested with rabbit antisera specific to each polysaccharide (4) that were subsequently probed with alkaline phosphatase-labeled anti-rabbit IgG secondary antibody (Pierce) and developed with BCIP/NBT substrate (KPL).
Northern Blot Analysis.
Northern blots were performed using total RNA extracted from midlog phase cultures (OD600nm ∼0.8) using an RNeasy mini kit, RNAprotect, and on-column digestion via the RNase-free DNase set (all from Qiagen). Twenty-microgram samples of total RNA in formaldehyde loading buffer were electrophoresed using 1% agarose gels cast with Northern Max denaturing buffer and containing formaldehyde at 120 V with Mops gel running buffer. The RNA was transferred to Bright Star-Plus membrane with Northern Max transfer buffer. All electrophoresis and Northern blot materials were purchased from Ambion. Probe labeling and signal detection were performed with the ECL direct nucleic acid labeling and detection system (Pharmacia).
Cloning of Each of the upxZ Genes for Constitutive Expression.
Each upxZ gene was amplified with primers listed in Table S1, cloned into the BamHI site of the pFD340 expression vector (16), and mobilized into B. fragilis. pMCL140 was created by cloning 102 bp of the PSH promoter region into the BamHI site of pFD340.
Quantitative Analysis of the Orientation of the PSG Locus Promoter.
Using bacterial DNA from the settled and suspended bacteria of the upfZ transconjugant as templates, PCR was performed using primers (Table S1) that flank the PSG locus invertible promoter region. The 1059-bp PCR products were digested with DraI, which cleaves asymmetrically between the inverted repeats yielding fragments of 666 bp and 393 bp when the promoter is in the on orientation and fragments of 760 bp and 299 bp when the promoter is in the off orientation.
Creation of ΔupaZ, ΔupeZ, and ΔuphZ Deletion Mutants.
Deletions of the upaZ, upeZ, and uphZ genes were constructed such that 405 bp of 474 bp, 370 bp of 483 bp, and 400 bp of 486 bp, respectively, were removed by allelic replacement. DNA segments upstream and downstream of each region to be deleted were PCR amplified and cloned by three-way ligation into the Bacteroides conjugal suicide vectors pJST55 (17) or pNJR6 (18). The resulting plasmids were conjugally transferred into wild-type B. fragilis and/or Δmpim44, and cointegrates were selected on the basis of Emr encoded by the vectors. The cointegrate strains were passaged, plated on nonselective medium, and replica plated on medium containing erythromycin. Ems colonies were screened by PCR to select colonies that had acquired the mutant genotype.
PSE Promoter–xylE Transcriptional Analysis Experiments.
PSE promoter–xylE transcriptional fusion clones were created as described (7). The plasmids were mobilized into both ΔmpiM44 and ΔmpiM44ΔupaZ backgrounds and xylE transcription was examined by Northern blot.
Construction of Hybrid Genes.
The 5′ upeY end of pMCL115 including the rbs was PCR amplified with primers “upeY forward” and “upeY reverse” (Table S1). The 3′ upaY end of pMCL115 was amplified with primers “upaY forward” and “upaY reverse.” These PCR products were digested with BamHI and ligated by three-way ligation into pFD340. The BamHI site internal to the hybrid gene was removed using the QuikChange XL site-directed mutagenesis kit (Stratagene) using primers “BamHI deletion forward” and “BamHI deletion reverse.” The 5′ upaY end of pMCL116 including the rbs along with the 51-bp region of upeY was PCR amplified with primers “upaY forward2” and “hybrid reverse” using pMCL74 as template. The 3′ upeY end of pMCL116 was amplified with primers “upeY forward2” and “upeY reverse2.” These PCR products were digested with BamHI and ligated by three-way ligation into pFD340. The BamHI site internal to the hybrid gene was removed using primers “BamHI deletion forward2” and “BamHI deletion reverse2.” Each hybrid gene and its flanking region was sequenced.
Supplementary Material
Acknowledgments
We thank M. J. Coyne, A. Nichols, C. M. Krinos, and C. M. Fletcher for assistance and discussions. This work was funded by National Institutes of Health/National Institute of Allergy and Infectious Diseases Grant AI044193.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1005039107/-/DCSupplemental.
References
- 1.Leser TD, Mølbak L. Better living through microbial action: The benefits of the mammalian gastrointestinal microbiota on the host. Environ Microbiol. 2009;11:2194–2206. doi: 10.1111/j.1462-2920.2009.01941.x. [DOI] [PubMed] [Google Scholar]
- 2.Coyne MJ, Comstock LE. Niche-specific features of the intestinal bacteroidales. J Bacteriol. 2008;190:736–742. doi: 10.1128/JB.01559-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coyne MJ, Chatzidaki-Livanis M, Paoletti LC, Comstock LE. Role of glycan synthesis in colonization of the mammalian gut by the bacterial symbiont Bacteroides fragilis. Proc Natl Acad Sci USA. 2008;105:13099–13104. doi: 10.1073/pnas.0804220105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krinos CM, et al. Extensive surface diversity of a commensal microorganism by multiple DNA inversions. Nature. 2001;414:555–558. doi: 10.1038/35107092. [DOI] [PubMed] [Google Scholar]
- 5.Patrick S, et al. Multiple inverted DNA repeats of Bacteroides fragilis that control polysaccharide antigenic variation are similar to the hin region inverted repeats of Salmonella typhimurium. Microbiology. 2003;149:915–924. doi: 10.1099/mic.0.26166-0. [DOI] [PubMed] [Google Scholar]
- 6.Belogurov GA, Mooney RA, Svetlov V, Landick R, Artsimovitch I. Functional specialization of transcription elongation factors. EMBO J. 2009;28:112–122. doi: 10.1038/emboj.2008.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatzidaki-Livanis M, Coyne MJ, Comstock LE. A family of transcriptional antitermination factors necessary for synthesis of the capsular polysaccharides of Bacteroides fragilis. J Bacteriol. 2009;191:7288–7295. doi: 10.1128/JB.00500-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu CH, Lee SM, Vanlare JM, Kasper DL, Mazmanian SK. Regulation of surface architecture by symbiotic bacteria mediates host colonization. Proc Natl Acad Sci USA. 2008;105:3951–3956. doi: 10.1073/pnas.0709266105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim JK, et al. In vivo phase variation of Escherichia coli type 1 fimbrial genes in women with urinary tract infection. Infect Immun. 1998;66:3303–3310. doi: 10.1128/iai.66.7.3303-3310.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coyne MJ, Weinacht KG, Krinos CM, Comstock LE. Mpi recombinase globally modulates the surface architecture of a human commensal bacterium. Proc Natl Acad Sci USA. 2003;100:10446–10451. doi: 10.1073/pnas.1832655100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey MJ, Hughes C, Koronakis V. RfaH and the ops element, components of a novel system controlling bacterial transcription elongation. Mol Microbiol. 1997;26:845–851. doi: 10.1046/j.1365-2958.1997.6432014.x. [DOI] [PubMed] [Google Scholar]
- 12.Nesper J, Kapfhammer D, Klose KE, Merkert H, Reidl J. Characterization of vibrio cholerae O1 antigen as the bacteriophage K139 receptor and identification of IS1004 insertions aborting O1 antigen biosynthesis. J Bacteriol. 2000;182:5097–5104. doi: 10.1128/jb.182.18.5097-5104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dupont K, Janzen T, Vogensen FK, Josephsen J, Stuer-Lauridsen B. Identification of Lactococcus lactis genes required for bacteriophage adsorption. Appl Environ Microbiol. 2004;70:5825–5832. doi: 10.1128/AEM.70.10.5825-5832.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stummeyer K, et al. Evolution of bacteriophages infecting encapsulated bacteria: Lessons from Escherichia coli K1-specific phages. Mol Microbiol. 2006;60:1123–1135. doi: 10.1111/j.1365-2958.2006.05173.x. [DOI] [PubMed] [Google Scholar]
- 15.Lindberg AA. Bacterial surface carbohydrates and bacteriophage adsorption. In: Sutherland I, editor. Surface Carbohydrates of the Prokaryotic Cell. New York: Academic; 1977. pp. 289–356. [Google Scholar]
- 16.Smith CJ, Rogers MB, McKee ML. Heterologous gene expression in Bacteroides fragilis. Plasmid. 1992;27:141–154. doi: 10.1016/0147-619x(92)90014-2. [DOI] [PubMed] [Google Scholar]
- 17.Thompson JS, Malamy MH. Sequencing the gene for an imipenem-cefoxitin-hydrolyzing enzyme (CfiA) from Bacteroides fragilis TAL2480 reveals strong similarity between CfiA and Bacillus cereus beta-lactamase II. J Bacteriol. 1990;172:2584–2593. doi: 10.1128/jb.172.5.2584-2593.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens AM, Shoemaker NB, Salyers AA. The region of a Bacteroides conjugal chromosomal tetracycline resistance element which is responsible for production of plasmidlike forms from unlinked chromosomal DNA might also be involved in transfer of the element. J Bacteriol. 1990;172:4271–4279. doi: 10.1128/jb.172.8.4271-4279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.