Abstract
Adipose tissue controls body lipid and energy metabolism, as well as food intake, and abnormalities in adipose function play a central role in diseases such as obesity and type-2 diabetes. Adipocyte differentiation is controlled by a transcriptional cascade involving PPARγ and members of the C/EBP family of transcription factors. Here, we demonstrate that C/EBPα is targeted for degradation by the ubiquitin ligase Fbxw7 in a phosphorylation-dependent manner. Importantly, inactivation of Fbxw7 is sufficient to convert mouse preadipocytes into mature adipocytes in a manner dependent on C/EBPα. In addition, inactivation of Fbxw7 promotes adipocyte differentiation of human adult stem cells. Taken together, our results suggest that Fbxw7 is a negative regulator of adipogenesis by targeting C/EBPα for degradation. This notion is supported by the observation that the expression of Fbxw7 is down-regulated during adipocyte differentiation, resulting in the accumulation of proadipogenic proteins such as C/EBPα. Thus, Fbxw7 could be an important regulator of energy and lipid metabolism.
Keywords: adipogenesis, CCAAT/enhancer-binding protein
Adipose tissue is crucial for whole-body insulin sensitivity and energy homeostasis (1, 2). Excessive adipocyte cell size and/or number is a hallmark of obesity, a major risk factor for the development of type-2 diabetes, cardiovascular disease, and hypertension. White adipose tissue serves as a storage depot for excess energy in the form of fat. Adipocytes also secrete cytokines that regulate several physiological processes, including glucose metabolism, appetite and blood pressure. During adipocyte differentiation, fibroblast-like preadipocytes differentiate into lipid-loaded adipocytes, a highly insulin-sensitive cell type. The differentiation of preadipocytes is regulated by nutritional, hormonal, and growth factor signals. This process involves a cascade of transcription factors, with peroxisome proliferator-activated receptor gamma (PPARγ) and members of the CCAAT/enhancer-binding protein (C/EBP) family of proteins considered to be of key importance (3–7). However, other transcription factors, such as sterol regulatory element-binding protein 1 (SREBP1) and Kruppel-like factors, also play important roles during adipogenesis. Members of the C/EBP family of transcription factors regulate the proliferation and differentiation of a large number of cell types (8–10) and were among the first transcription factors to be implicated in adipocyte differentiation (11, 12). C/EBPβ and C/EBPδ are induced early during the differentiation process, whereas C/EBPα is induced at a later stage. C/EBPβ and C/EBPδ bind to the PPARγ promoter and enhance the expression of the PPARγ gene. PPARγ subsequently activates the expression of multiple genes involved in lipogenesis and adipogenesis. PPARγ also activates the C/EBPα gene. Interestingly, C/EBPα can bind to the PPARγ promoter and activate the expression of PPARγ, thereby creating a proadipogenic feed-forward loop (13).
Fbxw7 (also known as Fbw7 or human CDC4), is the substrate recognition component of a specific SCF ubiquitin ligase (14). Fbxw7 is a tumor suppressor (14) and targets cyclin E (15, 16), c-Myc (17, 18), Notch (19, 20), c-Jun (21), SREBP1 (22, 23), and the PPARγ coactivator PGC-1 (24) for degradation in a phosphorylation-dependent manner. Inactivation of Fbxw7 results in the accumulation of transcriptionally active SREBP1 and enhanced expression of SREBP1 target genes, most of which are involved in lipid metabolism (23). SREBP1c (also known as ADD1) plays an important role during adipocyte differentiation in vitro (25), suggesting that Fbxw7 could influence this process. Here, we demonstrate that Fbxw7 is a negative regulator of adipogenesis by targeting phosphorylated C/EBPα for proteasome-mediated degradation. Fbxw7 inhibits C/EBPα-dependent transcription and inactivation of Fbxw7 results in the accumulation of C/EBPα. Importantly, inactivation of Fbxw7 in mouse preadipocytes and adult human stem cells enhances their differentiation into mature adipocytes. Taken together, our results suggest that Fbxw7 is an important regulator of adipocyte differentiation.
Results
Fbxw7 Is a Negative Regulator of Adipogenesis.
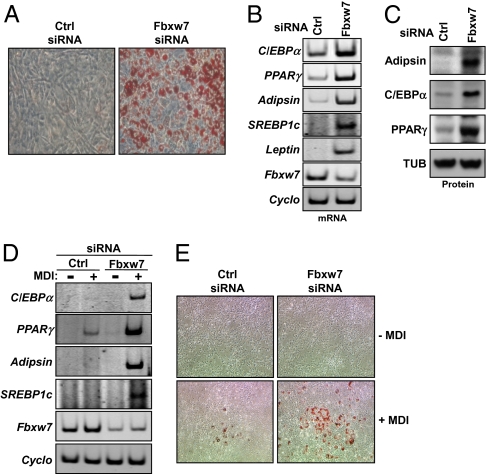
Adipocyte differentiation is controlled by members of the C/EBP, PPAR, and SREBP families of transcription factors. Fbxw7 targets SREBP1 and the PPARγ coactivator PGC-1 for ubiquitin-dependent degradation, suggesting that Fbxw7 could affect adipocyte differentiation. To test this hypothesis, Fbxw7 was knocked down in mouse 3T3-L1 preadipocytes. In response to a mixture of methylisobutylxanthine, dexamethasone, and insulin (MDI), these cells differentiate into adipocytes, resulting in the accumulation of large amounts of intracellular lipid droplets. A large fraction of the control cells differentiated in response to MDI treatment and we could not observe any increased differentiation in the Fbxw7-deficient cells. However, lipid droplets accumulated in a significant proportion of the Fbxw7-deficient 3T3-L1 cells in the absence of hormonal treatment (Fig. 1A). The accumulation of lipid droplets in the Fbxw7-deficient cells correlated with an enhanced expression of adipogenic markers, such as adipsin, SREBP1c, C/EBPα, PPARγ, and leptin (Figs. 1 B and C). Thus, inactivation of Fbxw7 is sufficient to promote adipocyte differentiation of 3T3-L1 cells in the absence of any hormonal signals. To test if Fbxw7 could affect the differentiation of other cells, Fbxw7 was inactivated in NIH/3T3 cells. These cells go through adipocyte differentiation in response to MDI treatment, although not as efficiently as 3T3-L1 preadipocytes. Inactivation of Fbxw7 in NIH/3T3 cells failed to induce adipocyte differentiation in the absence of MDI. However, the expression of adipogenic markers (Fig. 1D) and the accumulation of lipid droplets (Fig. 1E) in response to MDI were enhanced in the Fbxw7-deficient cells. Taken together, these results strongly suggest that Fbxw7 is a negative regulator of adipogenesis.
Fig. 1.
Fbxw7 is a negative regulator of adipogenesis. (A) 3T3-L1 preadipocyte cells were transfected with control or Fbxw7 siRNA. Ten days following transfection, cellular lipids were visualized with Oil Red O stain, and the cells were photographed. (B) 3T3-L1 preadipocyte cells were transfected as in A and the mRNA expression of the indicated genes was determined by RT-PCR analysis (Cyclo; cyclophilin). (C) 3T3-L1 preadipocyte cells were transfected as in A and the expression of the indicated proteins was determined by Western blotting (TUB; α-tubulin). (D) NIH/3T3 cells were transfected with control or Fbxw7 siRNA and treated with a mixture of methylisobutylxanthine, dexamethasone, and insulin (MDI). Ten days following the addition of MID, the mRNA levels of the indicated genes were determined by RT-PCR analysis. (E) NIH/3T3 cells were transfected and treated as in D. Ten days following the addition of MDI, cellular lipids were visualized with Oil Red O stain, and the cells were photographed.
C/EBPα Is a Substrate for Fbxw7.
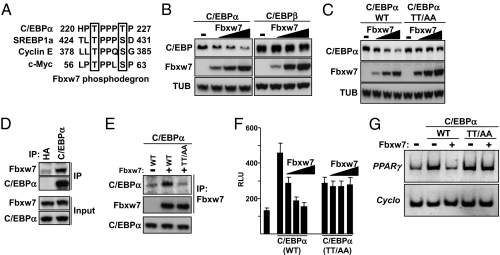
Fbxw7 binds to substrate proteins following the phosphorylation of specific Thr and/or Ser residues within sequences known as Fbxw7-dependent phosphodegrons. A visual inspection suggested that C/EBPα contains a potential Fbxw7 phosphodegron surrounding Thr222 and Thr226 (Fig. 2A). Both Thr222 and Thr226 are phosphorylated in liver and adipose tissue (26). A similar sequence is also found in C/EBPβ. However, C/EBPβ has a Ser residue in the position of Thr222 in C/EBPα (Fig. 2A), which should make it a weaker substrate for Fbxw7. To test if Fbxw7 could target C/EBPα or β for degradation, 3T3-L1 cells were transfected with C/EBPα or β in the absence or presence of increasing amounts of Fbxw7. As illustrated in Fig. 2B, C/EBPα was degraded in response to Fbxw7 expression, whereas C/EBPβ appeared resistant to Fbxw7-dependent degradation. In addition, Fbxw7 did not induce the degradation of PPARγ, a second key regulator of adipogenesis (Fig. S1). The degradation of C/EBPα was dependent on its potential Fbxw7 phosphodegron because mutation of Thr222 and Thr226 blocked the Fbxw7-dependent degradation of C/EBPα (Fig. 2C), suggesting that Fbxw7 recognizes phosphorylated C/EBPα. This notion was supported by our observation that Fbxw7 enhanced the ubiquitination of wild-type C/EBPα but not the nonphosphorylated mutant form of the protein (Fig. S2). Endogenous C/EBPα interacted with endogenous Fbxw7 in 3T3-L1 cells treated with the proteasome inhibitor MG132 (Fig. 2D). The interaction between C/EBPα and Fbxw7 was dependent on the integrity of Thr222 and Thr226 in C/EBPα (Fig. 2E), suggesting that the phosphorylation of these residues is important for the interaction between C/EBPα and Fbxw7. To determine if Fbxw7 could affect the transcriptional activity of C/EBPα, 3T3-L1 cells were transfected with a C/EBP-responsive promoter-reporter construct and C/EBPα, either wild-type or mutant, in the absence or presence of Fbxw7. Fbxw7 attenuated the transcriptional activity of wild-type C/EBPα, whereas the nonphosphorylated mutant was unaffected (Fig. 2F), suggesting that Fbxw7 is a negative regulator of C/EBPα function. Fbxw7 also attenuated the expression of the endogenous C/EBPα target gene PPARγ in 3T3-L1 preadipocytes transfected with wild-type C/EBPα, whereas it was unable to affect the activity of the nonphosphorylated mutant under the same conditions (Fig. 2G). These results were explained by the fact that Fbxw7 reduced the levels of wild-type C/EBPα in the transfected cells but failed to affect the levels of the nonphosphorylated mutant (Fig. S3). Taken together, our results suggest that Fbxw7 is a negative regulator of C/EBPα function by targeting it to phosphorylation-dependent degradation.
Fig. 2.
C/EBPα is a substrate for Fbxw7. (A) The sequence surrounding Thr222 and Thr226 in human C/EBPα is aligned to the Fbxw7 phosphodegrons of c-Myc, cyclin E and SREBP1a. (B) 3T3-L1 cells were transfected with C/EBPα or C/EBPβ in the presence of increasing amounts of Fbxw7α. The levels of C/EBPα, C/EBPβ, Fbxw7α and α-tubulin were determined by Western blotting. (C) 3T3-L1 cells were transfected with C/EBPα, either wild-type or the T222A/T226A mutant (TT/AA), in the absence or presence of Flag-Fbxw7α. The levels of C/EBPα, Fbxw7α, and α-tubulin in cell lysates were determined by Western blotting. (D) Confluent 3T3-L1 cells were treated with MG132 (25 μM) for 5 h. Total cell lysates were immunoprecipitated with anti-HA or anti-C/EBPα antibodies. Immunoprecipitated (IP) C/EBPα and Fbxw7α and the levels of C/EBPα and Fbxw7 in cell lysates (Input) were determined by Western blotting. (E) HEK293T cells were transfected with dominant-negative cullin 1 and C/EBPα, either wild-type or the T222A/T226A mutant (TT/AA), in the absence or presence of Flag-Fbxw7α. Cell lysates were immunoprecipitated with anti-Flag antibodies. Immunoprecipitated C/EBPα and Fbxw7α and the levels of C/EBPα in cell lysates were determined by Western blotting. (F) 3T3-L1 cells were tranfected with a C/EBP-responsive promoter-reporter construct in the absence or presence of C/EBPα, either wild-type or the T222A/T226A mutant (TT/AA), and increasing amounts of Fbxw7α. Thirty-six hours after transfection, the luciferase activity was measured. (G) 3T3-L1 preadipocyte cells were transfected with C/EBPα, either wild-type or the T222A/T226A mutant (TT/AA), in the absence or presence of Fbxw7α. Forty-eight hours after transfection, the mRNA expression of PPARγ and cyclophilin was determined by RT-PCR analysis.
Endogenous Fbxw7 Regulates Endogenous C/EBPα.
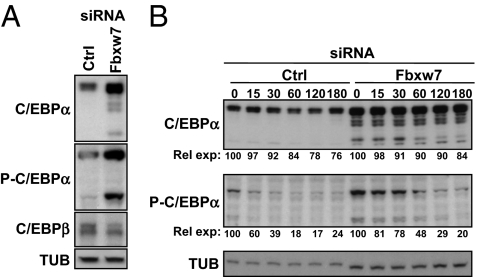
Our data suggest that Fbxw7 targets C/EBPα for degradation following phosphorylation of Thr222 and Thr226. This hypothesis is supported by our observation that C/EBPα protein accumulates in 3T3-L1 preadipocytes in response to siRNA-mediated inactivation of Fbxw7 (Fig. 1C). However, the expression of the C/EBPα gene is induced during adipocyte differentiation. It was, therefore, possible that C/EBPα accumulated in these cells as a result of the differentiation process, rather than as a result of reduced protein degradation. To clarify this issue, the levels of C/EBPα were determined in HepG2 cells transfected with Fbxw7 siRNA. There are two forms of C/EBPα, p42 and p30, and the levels of both proteins were elevated following inactivation of Fbxw7 (Fig. 3A). Interestingly, the C/EBPα protein that accumulated in response to Fbxw7 inactivation was highly phosphorylated on Thr222 and Thr226, supporting our hypothesis that C/EBPα molecules phosphorylated on these two residues are degraded by Fbxw7 under normal conditions. Interestingly, we were unable to detect any changes in the expression of C/EBPβ in response to Fbxw7 inactivation, supporting the notion that Fbxw7 does not target this protein. To confirm that the increased levels of C/EBPα observed in response to Fbxw7 inactivation resulted from decreased proteolysis, we measured the half-life of C/EBPα in HepG2 cells treated with control or Fbxw7 siRNA. Although there was only limited turn-over of total C/EBPα during the time frame of the experiment, the degradation of C/EBPα was reduced in Fbxw7-deficient cells (Fig. 3B). Importantly, the turnover of phosphorylated C/EBPα was very rapid in control cells, and inactivation of Fbxw7 significantly delayed the turnover of this form of the protein, confirming that Fbxw7 targets phophorylated C/EBPα for degradation.
Fig. 3.
Endogenous Fbxw7 regulates endogenous C/EBPα. (A) HepG2 cells were transfected with control or Fbxw7 siRNA. Forty-eight hours after transfection, total cell extracts were prepared from the tranfected cells and analyzed by SDS/PAGE. The expression of C/EBPα, C/EBPβ, and α-tubulin and the phosphorylation of C/EBPα (P-C/EBPα) was determined by Western blotting. (B) HepG2 cells were transfected with control or Fbxw7 siRNA. Forty-eight hours after transfection, cells were treated for the indicated times with cyclohexamide and total cell extracts were prepared from the transfected cells and analyzed by SDS/PAGE. The expression of C/EBPα and α-tubulin and the phosphorylation of C/EBPα (P-C/EBPα) were determined by Western blotting. The levels of total and phosphorylated C/EBPα are indicated. The levels of total and phosphorylated C/EBPα in the absence of cyclohexamide was set as 100.
Fbxw7 Regulates Adipocyte Differentiation of Human Adult Stem Cells.
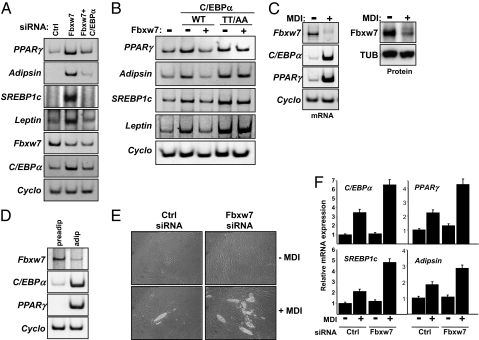
Our data suggest that Fbxw7 functions as a negative regulator of adipogenesis by targeting phosphorylated C/EBPα for degradation. To test this hypothesis, 3T3-L1 preadipocytes were transfected with Fbxw7 siRNA in the absence or presence of C/EBPα siRNA. As expected, inactivation of Fbxw7 resulted in increased expression of adipogenic markers and the accumulation of lipid droplets. Inactivation of C/EBPα in the Fbxw7-deficient cells attenuated the expression of adipocyte markers (Fig. 4A) and blocked the accumulation of lipid droplets (Fig. S4), suggesting that the differentiation observed in response to Fbxw7 inactivation is dependent on C/EBPα. Inactivation of C/EBPα alone did not affect the expression of adipocyte markers under these conditions (Fig. S5). These results suggest that Fbxw7 acts at, or before, the level of C/EBPα to regulate adipocyte differentiation. To clarify this issue further, 3T3-L1 preadipocytes were transfected with C/EBPα, either wild-type or the T222A/T226A mutant, in the absence or presence of Fbxw7 and monitored for adipocyte differentiation. Because of the rather low transfection efficiency of postconfluent 3T3-L1 cells, only a few individual mature adipocytes could be observed in response to C/EBPα expression. However, ectopic expression of wild-type C/EBPα resulted in the expression of adipocyte markers and this was inhibited by the simultaneous expression of Fbxw7 (Fig. 4B). Expression of the nonphosphorylated version of C/EBPα also resulted in the enhanced expression of adipocyte markers but this process was resistant to Fbxw7-dependent repression. Taken together, these results clearly suggest that Fbxw7 regulates adipogenesis at the level of C/EBPα degradation.
Fig. 4.
Fbxw7 regulates adipocyte differentiation of human adult stem cells. (A) 3T3-L1 preadipocyte cells were transfected with control, Fbxw7 or Fbxw7 plus C/EBPα siRNA. Ten days following transfection, the mRNA expression of the indicated genes was determined by RT-PCR analysis. (B) 3T3-L1 preadipocyte cells were transfected with C/EBPα, either wild-type or the T222A/T226A mutant (TT/AA), in the absence or presence of Fbxw7α. Ten days after transfection, the mRNA expression of the indicated genes was determined by RT-PCR analysis. (C) 3T3-L1 preadipocytes were left untreated or allowed to differentiate to mature adipocytes. The expression of Fbxw7, C/EBPα, PPARγ and cyclophilin was determined by RT-PCR analysis (Left). 3T3-L1 preadipocytes were treated as above and total cell extracts were prepared. Fbxw7 was immunoprecipitated and the levels of Fbxw7 in the immunoprecipitates and α-tubulin in cell lysates were determined by Western blotting (Right). (D) The expression of Fbxw7, C/EBPα, PPARγ, and cyclophilin in total RNA from preadipocytes (preadip) and adipocytes (adip) obtained from human donors was analyzed by RT-PCR analysis. (E) Adipose-derived human adult stem cells were transfected with control or Fbxw7 siRNA and left untreated or treated with a mixture of methylisobutylxanthine, dexamethasone, and insulin (MDI). Ten days after the addition of MDI, the cells were photographed. (F) Adipose-derived human adult stem cells were transfected and treated as in (E). Ten days following the addition of MDI, the mRNA expression of the indicated genes was determined by RT-PCR analysis and correlated to the expression of cyclophilin in each sample. The expression of each gene in control cells in the absence of MDI was set as 1.
Our data suggest that Fbxw7 is a negative regulator of adipogenesis. It was, therefore, important to determine if the expression of Fbxw7 was regulated during adipocyte differentiation. As illustrated in Fig. 4C, the expression of the endogenous Fbxw7 gene was reduced in fully differentiated 3T3-L1 cells as compared to undifferentiated preadipocytes. Importantly, this down-regulation resulted in reduced levels of Fbxw7 protein in the differentiated cells. Similar results were obtained when the expression of Fbxw7 was analyzed in preadipocytes and mature adipocytes from human donors (Fig. 4D). The expression of Fbxw7 mRNA and protein was down-regulated at an early time-point during the differentiation of 3T3-L1 cells, preceding the peak in C/EBPα expression (Fig. S6). These results are in agreement with the hypothesis that Fbxw7-dependent regulation of C/EBPα could influence adipocyte differentiation. Taken together, these results suggest that the expression of Fbxw7 is down-regulated during adipogenesis to allow the accumulation of proadipogenic Fbxw7 target proteins such as C/EBPα and SREBP1c.
It has been suggested that the differentiation of established preadipocyte cell lines such as 3T3-L1 differ from that seen in vivo or in primary preadipocytes. To determine if Fbxw7 controlled adipocyte differentiation in a more physiological setting, we used adult stem cells isolated from human s.c. adipose tissue. These cells are pluripotent and can differentiate along adipogenic, chondrogenic, or osteogenic lineages depending on hormonal cues. siRNA-mediated inactivation of Fbxw7 in these cells did not result in increased expression of adipocyte markers or accumulation of lipid droplets in the absence of hormonal stimulation (Figs. 4 E and F). However, in response to MDI treatment, a larger proportion of the Fbxw7-deficient stem cells accumulated intracellular lipid droplets compared to control transfected cells (Fig. 4E). The accumulation of lipid droplets in the Fbxw7-deficient stem cells correlated with an enhanced expression of adipocyte markers (Fig. 4F). As in 3T3-L1 cells and human adipocytes, the expression of Fbxw7 was down-regulated in human adult stem cells during adipocyte differentiation (Fig. S7). Thus, Fbxw7 could be an important regulator of adipogenesis also in humans by controlling the levels of proadipogenic proteins such as C/EBPα.
Discussion
Our data suggest that the ubiquitin ligase Fbxw7 targets C/EBPα for degradation following phosphorylation of Thr222 and Thr226. C/EBPα plays an important role during adipocyte differentiation by regulating the expression of several proadipogenic factors. Consequently, we found that inactivation of Fbxw7 in the well-established preadipocyte cell line 3T3-L1 resulted in the accumulation of C/EBPα, enhanced expression of adipogenic markers, and enhanced accumulation of intracellular lipid droplets. Interestingly, inactivation of Fbxw7 was sufficient to activate the differentiation of these preadipocytes in the absence of any external proadipogenic signals. Inactivation of Fbxw7 also enhanced the adipogenic differentiation of mouse NIH/3T3 cells and adult human stem cells in the presence of proadipogenic signals. Interestingly, the expression of the Fbxw7 gene was down-regulated during adipocyte differentiation. Down-regulation of Fbxw7 could potentially enhance the accumulation of proadipogenic Fbxw7 substrates such as C/EBPα and SREBP1c, thereby promoting adipocyte differentiation (Fig. 5). Thus, Fbxw7 could play an important gatekeeper function during adipocyte differentiation, thereby influencing lipid and energy metabolism. The expression of Fbxw7 in adipocytes could, therefore, be a potential target for the treatment of obesity and type-2 diabetes.
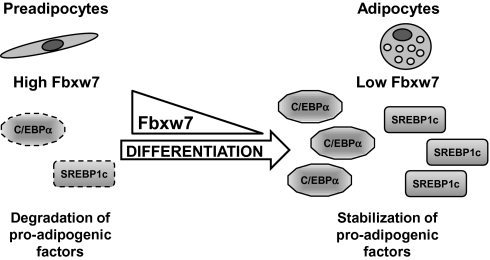
Fig. 5.
Fbxw7 is a negative regulator of adipocyte differentiation. High expression of the ubiquitin ligase Fbxw7 in preadipocytes promotes the phosphorylation-dependent degradation of proadipogenic transcription factor such as C/EBPα and SREBP1c, thereby attenuating adipocyte differentiation. The expression of Fbxw7 is down-regulated early during adipogenesis, leading to the stabilization of C/EBPα and SREBP1c, which in turn results in enhanced expression of proadipogenic target genes and enhanced adipogenesis.
The Fbxw7-dependent degradation of C/EBPα was dependent on the phosphorylation of Thr222 and Thr226. These residues are found within a sequence that resembles the Fbxw7-dependent phosphodegron of many other Fbxw7 substrates, including SREBP1, c-Myc, and cyclin E. Both these residues are phosphorylated in vivo (26). It was initially suggested that both residues were phosphorylated by GSK-3 in an insulin-regulated manner (27). However, this conclusion has been questioned in recent reports (26, 28). Pedersen et al. (26) found that both residues were phosphorylated in mouse liver and adipose tissue, but they were unable to detect any changes in the phosphorylation in response to insulin signaling. However, they did observe a dramatic increase in the phosphorylation of both residues in liver in response to refeeding and glucose stimulation. Whether this was the result of enhanced phosphorylation of C/EBPα or stabilization of the phosphorylated form of the protein remains to be determined. Also, the kinase(s) responsible for the phosphorylation of these residues remain to be identified.
Not much is known about the functional role of the phosphorylation of Thr222/226 in C/EBPα. However, a T222A/T226A mutant of C/EBPα was found to be more efficient than the wild-type protein to induce neutrophil differentiation, suggesting that phosphorylation of these residues could play a negative role during this process (29). Recently, knock-in mutagenesis was used to replace endogenous C/EBPα with a T222A/T226A/S210A triple mutant form of the protein (26). The mutant mice had no apparent physiological abnormalities. However, the expression of genes involved in gluconeogenesis, including G6Pc and PEPCK, were increased in the livers of the mutant mice. Interestingly, the mutant mice displayed decreased levels of liver glycogen, minor increases in serum insulin and glucose, and developed glucose intolerance. Based on their results, the authors speculated that phosphorylation of Thr222 and Thr226 could play a negative role in these processes by restricting the activity of C/EBPα on the G6Pc and PEPCK promoters. In agreement with these two reports, we also find that phosphorylation of Thr222/226 has a negative effect on C/EBPα function by promoting its Fbxw7-dependent degradation. The phosphodegron of SREBP1, another transcription factor targeted by Fbxw7, is highly phosphorylated when the protein is associated with target promoters, and Fbxw7-dependent degradation of this form of the protein is an efficient way to inactivate SREBP1-dependent transcription (30). It is possible that C/EBPα molecules phosphorylated on Thr222/226 represent a specialized form of this transcription factor, and it will be interesting to further analyze the link between the activity, phosphorylation, and degradation of C/EBPα, especially because SREBP1 and C/EBPα interact and coregulate a number of genes in vivo (26).
We found that the expression of the Fbxw7 gene was down-regulated during adipocyte differentiation of mouse preadipocytes, human adipocytes and adipose-derived human stem cells. Down-regulation of Fbxw7 expression during adipogenesis would allow the accumulation of proadipogenic Fbxw7 substrates (Fig. 5). We propose that Fbxw7 blocks adipocyte differentiation by targeting C/EBPα for degradation. However, Fbxw7 also targets SREBP1c and the PPARγ coactivator PGC-1 for degradation, and it is possible that the accumulation of C/EBPα, SREBP1c, and PGC-1 synergistically promotes adipocyte differentiation in response to Fbxw7 inactivation (Fig. 5). It will be very important to identify the signals and factors regulating the expression of Fbxw7 during adipogenesis, especially because the expression of Fbxw7 in adipocytes could be a potential target for the treatment of obesity and type-2 diabetes. It will also be interesting to analyze the role of Fbxw7 in other processes regulated by C/EBPα.
Materials and Methods
Cell Culture and Adipocyte Differentiation.
All tissue culture media and antibiotics were obtained from Invitrogen and Sigma. HEK293T, HepG2, 3T3-L1, and NIH/3T3 cells were from ATCC. Adipose-derived adult stem cells from human donors were from Zen-Bio. Differentiation of 2-day postconfluent 3T3-L1 and adult stem cells was initiated with 0.5 mM methylisobutylxanthine, 1 μM dexamethasone, and 1 μg/mL insulin in DMEM supplemented with 10% FBS. After 96 h, the culture medium was replaced with DMEM supplemented with 10% FBS and 1 μg/mL insulin for an additional 48 h, and the cells were then fed every other day with DMEM containing 10% FBS. Standard methods were employed to stain intracellular lipid droplets with Oil-red O. Stained cells were photographed using a digital camera.
Reagents and Antibodies.
Anti-Flag antibody (M5) and standard chemicals were from Sigma. C/EBPα, C/EBPβ, adipsin, phospho-C/EBPα (Thr222/Thr226), and PPARγ antibodies were from Cell Signaling. Fbxw7 antibodies were from Bethyl Laboratories.
Plasmids, siRNA, and Transfections.
The expression vectors for C/EBPα and C/EBPβ were from Addgene (Addgene plasmids 12550 and 12558). Expression vectors for Fbxw7 have been described (23). Point mutants were generated by site-directed mutagenesis (QuikChange, Stratagene). The C/EBP-responsive promoter-reporter construct p15-4xSBR2 was from Addgene (Addgene plasmid 15709). Transient transfections were performed using the MBS transfection kit (Stratagene). 3T3-L1 cells were transfected with GeneJuice (Novagen). The Fbxw7 siRNA was from Invitrogen and the C/EBPα siRNA was from Dharmacon.
Immunoprecipitations and Immunoblotting.
Cells were lysed in buffer A [50 mM hepes (pH 7.2), 150 mM NaCl, 1 mM EDTA, 20 mM NaF, 2 mM sodium orthovanadate, 10 mM β-glycerophosphate, 1% (wt/vol) Triton X-100, 10% (wt/vol) glycerol, 1 mM PMSF, 10 mM sodium butyrate, and 1% aprotinin, and cleared by centrifugation. Cell lysates and immunoprecipitates were resolved by SDS/PAGE and transferred to nitrocellulose membranes (Millipore). To ensure that equal amounts of protein were loaded in each well, the levels of α-tubulin in the samples were estimated by Western blotting.
Luciferase and β-Galactosidase Assays.
Cells were transiently transfected with the p15-4xSBR2 promoter-reporter gene in the absence or presence of expression vectors for C/EBPα, either wild-type or the indicated mutants. After 36 h, luciferase activities were determined in duplicate samples as described by the manufacturer (Promega). The pCH110 vector encoding the β-galactosidase gene under the control of the SV40 promoter (Amersham Biosciences) was used as an internal control for transfection efficiency. Luciferase values (relative light units) were calculated by dividing the luciferase activity by the β-galactosidase activity. The data represent the average ± SD of three independent experiments performed in duplicates.
RT-PCR Assays.
RNA was extracted with TRIzol reagent (Invitrogen). Total RNA was subjected to RT with oligo dT, followed by PCR with target-specific primers. The PCR reactions, using Invitrogen High Fidelity DNA polymerase, were optimized for the individual target genes. The PCR programs and primer sequences for the individual target genes are available on request. Total RNA from preadipocytes and mature adipocytes from human donors was obtained from Zen-Bio.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Ludwig Institute for Cancer Research Ltd. J.E. is the recipient of a Science Foundation Ireland Stokes Professorship Award (07/SK/B1242b).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0913367107/-/DCSupplemental.
References
- 1.Attie AD, Scherer PE. Adipocyte metabolism and obesity. J Lipid Res. 2009;50(Suppl):S395–S399. doi: 10.1194/jlr.R800057-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lefterova MI, Lazar MA. New developments in adipogenesis. Trends Endocrinol Metab. 2009;20:107–114. doi: 10.1016/j.tem.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 5.Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol. 2000;16:145–171. doi: 10.1146/annurev.cellbio.16.1.145. [DOI] [PubMed] [Google Scholar]
- 6.Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14:1293–1307. [PubMed] [Google Scholar]
- 7.MacDougald OA, Lane MD. Transcriptional regulation of gene expression during adipocyte differentiation. Annu Rev Biochem. 1995;64:345–373. doi: 10.1146/annurev.bi.64.070195.002021. [DOI] [PubMed] [Google Scholar]
- 8.Johnson PF. Molecular stop signs: Regulation of cell-cycle arrest by C/EBP transcription factors. J Cell Sci. 2005;118:2545–2555. doi: 10.1242/jcs.02459. [DOI] [PubMed] [Google Scholar]
- 9.Nerlov C. The C/EBP family of transcription factors: A paradigm for interaction between gene expression and proliferation control. Trends Cell Biol. 2007;17:318–324. doi: 10.1016/j.tcb.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Schuster MB, Porse BT. C/EBPalpha: A tumour suppressor in multiple tissues? Biochim Biophys Acta. 2006;1766:88–103. doi: 10.1016/j.bbcan.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Darlington GJ, Ross SE, MacDougald OA. The role of C/EBP genes in adipocyte differentiation. J Biol Chem. 1998;273:30057–30060. doi: 10.1074/jbc.273.46.30057. [DOI] [PubMed] [Google Scholar]
- 12.Lane MD, Tang QQ, Jiang MS. Role of the CCAAT enhancer binding proteins (C/EBPs) in adipocyte differentiation. Biochem Biophys Res Commun. 1999;266:677–683. doi: 10.1006/bbrc.1999.1885. [DOI] [PubMed] [Google Scholar]
- 13.Rosen ED, et al. C/EBPalpha induces adipogenesis through PPARgamma: A unified pathway. Genes Dev. 2002;16:22–26. doi: 10.1101/gad.948702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Welcker M, Clurman BE. FBW7 ubiquitin ligase: A tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer. 2008;8:83–93. doi: 10.1038/nrc2290. [DOI] [PubMed] [Google Scholar]
- 15.Koepp DM, et al. Phosphorylation-dependent ubiquitination of cyclin E by the SCFFbw7 ubiquitin ligase. Science. 2001;294:173–177. doi: 10.1126/science.1065203. [DOI] [PubMed] [Google Scholar]
- 16.Strohmaier H, et al. Human F-box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line. Nature. 2001;413:316–322. doi: 10.1038/35095076. [DOI] [PubMed] [Google Scholar]
- 17.Welcker M, et al. The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc Natl Acad Sci USA. 2004;101:9085–9090. doi: 10.1073/pnas.0402770101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yada M, et al. Phosphorylation-dependent degradation of c-Myc is mediated by the F-box protein Fbw7. EMBO J. 2004;23:2116–2125. doi: 10.1038/sj.emboj.7600217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tetzlaff MT, et al. Defective cardiovascular development and elevated cyclin E and Notch proteins in mice lacking the Fbw7 F-box protein. Proc Natl Acad Sci USA. 2004;101:3338–3345. doi: 10.1073/pnas.0307875101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsunematsu R, et al. Mouse Fbw7/Sel-10/Cdc4 is required for notch degradation during vascular development. J Biol Chem. 2004;279:9417–9423. doi: 10.1074/jbc.M312337200. [DOI] [PubMed] [Google Scholar]
- 21.Wei W, Jin J, Schlisio S, Harper JW, Kaelin WG., Jr The v-Jun point mutation allows c-Jun to escape GSK3-dependent recognition and destruction by the Fbw7 ubiquitin ligase. Cancer Cell. 2005;8:25–33. doi: 10.1016/j.ccr.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Bengoechea-Alonso MT, Ericsson J. A phosphorylation cascade controls the degradation of active SREBP1. J Biol Chem. 2009;284:5885–5895. doi: 10.1074/jbc.M807906200. [DOI] [PubMed] [Google Scholar]
- 23.Sundqvist A, et al. Control of lipid metabolism by phosphorylation-dependent degradation of the SREBP family of transcription factors by SCF(Fbw7) Cell Metab. 2005;1:379–391. doi: 10.1016/j.cmet.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Olson BL, et al. SCFCdc4 acts antagonistically to the PGC-1alpha transcriptional coactivator by targeting it for ubiquitin-mediated proteolysis. Genes Dev. 2008;22:252–264. doi: 10.1101/gad.1624208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JB, Spiegelman BM. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev. 1996;10:1096–1107. doi: 10.1101/gad.10.9.1096. [DOI] [PubMed] [Google Scholar]
- 26.Pedersen TA, et al. Distinct C/EBPalpha motifs regulate lipogenic and gluconeogenic gene expression in vivo. EMBO J. 2007;26:1081–1093. doi: 10.1038/sj.emboj.7601563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross SE, Erickson RL, Hemati N, MacDougald OA. Glycogen synthase kinase 3 is an insulin-regulated C/EBPalpha kinase. Mol Cell Biol. 1999;19:8433–8441. doi: 10.1128/mcb.19.12.8433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu HK, et al. Functional characterisation of the regulation of CAAT enhancer binding protein alpha by GSK-3 phosphorylation of Threonines 222/226. BMC Mol Biol. 2006;7:14. doi: 10.1186/1471-2199-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buitenhuis M, et al. Protein kinase B (c-akt) regulates hematopoietic lineage choice decisions during myelopoiesis. Blood. 2008;111:112–121. doi: 10.1182/blood-2006-07-037572. [DOI] [PubMed] [Google Scholar]
- 30.Punga T, Bengoechea-Alonso MT, Ericsson J. Phosphorylation and ubiquitination of the transcription factor sterol regulatory element-binding protein-1 in response to DNA binding. J Biol Chem. 2006;281:25278–25286. doi: 10.1074/jbc.M604983200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.