Abstract
In a previous study, we demonstrated that β1,3-N-acetylglucosaminyltransferase 5 (B3gnt5) is a lactotriaosylceramide (Lc3Cer) synthase that synthesizes a precursor structure for lacto/neolacto-series glycosphingolipids (GSLs) in in vitro experiments. Here, we generated B3gnt5-deficient (B3gnt5−/−) mice to investigate the in vivo biological functions of lacto/neolacto-series GSLs. In biochemical analyses, lacto/neolacto-series GSLs were confirmed to be absent and no Lc3Cer synthase activity was detected in the tissues of these mice. These results demonstrate that β3GnT5 is the sole enzyme synthesizing Lc3Cer in vivo. Ganglioside GM1, known as a glycosphingolipid-enriched microdomain (GEM) marker, was found to be up-regulated in B3gnt5−/− B cells by flow cytometry and fluorescence microscopy. However, no difference in the amount of GM1 was observed by TLC-immunoblotting analysis. The GEM-stained puncta on the surface of B3gnt5−/− resting B cells were brighter and larger than those of WT cells. These results suggest that structural alteration of GEM occurs in B3gnt5−/− B cells. We next examined whether BCR signaling-related proteins, such as BCR, CD19, and the signaling molecule Lyn, had moved into or out of the GEM fraction. In B3gnt5−/− B cells, these molecules were enriched in the GEM fraction or adjacent fraction. Moreover, B3gnt5−/− B cells were more sensitive to the induction of intracellular phosphorylation signals on BCR stimulation and proliferated more vigorously than WT B cells. Together, these results suggest that lacto/neolacto-series GSLs play an important role in clustering of GEMs and tether-specific proteins, such as BCR, CD19, and related signaling molecules to the GEMs.
Keywords: β1,3-N-acetylglucosaminyltransferase; glycosyltransferase; polylactosamine; glycosphingolipid; B cell receptor
Almost all organisms possess lipids and proteins to which a broad range of carbohydrate chains are linked. Some carbohydrate structures are known to participate in vital processes, such as the molecules responsible for cell–cell, receptor–ligand, and carbohydrate–carbohydrate interactions. It is known that glycosphingolipids (GSLs) have an important role in biological functions. In mammals, GSLs can be classified into several major classes, such as globo-, isoglobo-, ganglio-, and lacto/neolacto-series GSLs and others (Fig. S1). Gangliosides that contain one or more sialic acids on their carbohydrate chains modulate the responsiveness of signaling molecules and cell surface receptors (1). Some functional carbohydrate antigens, such as blood group Lewis antigens and the HNK-1 antigen, are carried on lacto/neolacto-series GSLs. During the development of hematopoietic cells, expression of HNK-1 (CD57) and Lewis-related antigens on lacto/neolacto-series GSLs is spatially and temporally regulated; the expression of such GSLs is also dramatically changed during the immune response (2).
Membrane microdomains are cholesterol- and GSL-rich components of the plasma membrane, also known as glycosphingolipid-enriched microdomains (GEMs), glycosphingolipid-signaling domains, lipid rafts, detergent-resistant membrane structures (DRMs), or detergent-insoluble glycolipid-enriched complex (3–5). General/classic lipid rafts, referred to here as GEMs, serve as platforms for different mechanisms of cell signaling. GEMs contain other sphingolipids, cholesterol, GPI-anchored proteins, many receptor molecules, and selected signaling molecules. GSLs are major components of GEMs. GSLs in microdomains are involved in various biological functions, such as signal transduction, toxin and infection receptors for bacteria and viruses (6), cell adhesion (5, 7), and cell growth modulation (8). It is thought that the most probable function exerted by GSLs in microdomains is to concentrate proteins that play a role in transmembrane signaling events and their regulated activation (9). GEMs also contain many important molecules, including immunoreceptors, such as the B cell receptor (BCR), T cell receptor (TCR), CD4, and CD8 among others, as well as cellular signaling molecules (10–14). It is known that GPI-anchored glycoproteins, such as CD48, CD59, and others, are also localized within GEMs. Recent studies have also shown the importance of GEM formation in cell differentiation and the acquired immune response among other processes. In T cells, clustering of GEMs regulates sustained TCR signaling because this process maintains engagement of the TCR with costimulators, such as CD28 and GPI-anchored glycoproteins, leading to partitioning within GEMs and resulting in exclusion of large highly glycosylated proteins, such as CD43 and CD45 (15). For facilitating sustained TCR signal transduction, TCR localization within GEMs results in high local concentrations of TCR signal-related molecules, such as protein tyrosine kinases and signal transducers, and the exclusion of CD45 phosphatase activity, which negatively regulates TCR signaling.
β1,3-N-acetylglucosaminyltransferases (β3GnTs) transfer an N-acetylglucosamine (GlcNAc) from UDP-GlcNAc to Gal on the nonreducing end of the carbohydrate chain with a β1,3-linkage. At present, eight members of the human β3GnT family have been cloned and characterized (16). In a previous study (17), β3GnT5 was cloned as lactotriaosylceramide (Lc3Cer) synthase and characterized as a key enzyme for the biosynthesis of lacto/neolacto-series GSLs (17, 18). B3gnt2 (β3GnT2) mRNA is ubiquitously expressed in mouse tissues, and thus also in B cells, T cells, and other immune cells. The previous study demonstrated that B3gnt2−/− B and T cells, which lack polylactosamine (PLN) on the N-glycans of their glycoproteins, showed hyperactivation following BCR or TCR/CD28 stimulation (19). Thus, PLN chains on glycoproteins have an important role in determining thresholds for in vitro immunocyte activation. On the other hand, we and other groups have observed that the expression of B3gnt5 mRNA is limited mainly to B cells (Fig. S1). We are also interested in the functions of PLN chains not only on glycoproteins but on GSLs in B cells.
It has been reported that disruption of the B3gnt5 gene leads to preimplantation lethality; therefore, no individuals homozygous for B3gnt5-null alleles are viable (20). However, we have succeeded in generating B3gnt5-deficient mice by a different approach, for example, by using a different ES cell clone and genomic construction (targeting vector). The B3gnt5−/− mice generated in this study were viable and fertile and showed normal growth, although they were B3gnt5-null in all tissues tested. Thus, this study on gene-disrupted mice lacks lacto/neolacto-series GSLs. We found that abnormal formation of GEMs was significantly increased in B3gnt5−/− mice. This observation prompted us to investigate whether the mice show immunological disorders of B cells and how lacto/neolacto-series GSLs are involved in BCR/CD19 signaling.
Results
Generation of B3gnt5−/− Mice.
Quantitative real-time RT-PCR analysis confirmed that B3gnt5 mRNA expression was present at a high level in immune organs and/or cells (Fig. S1). To analyze whether disorders of the immune system would occur in its absence, we generated B3gnt5-deficient mice.
To confirm the generation of B3gnt5-deficient mice, we analyzed expression of the B3gnt5 gene and lactotriaosylceramide (Lc3Cer)-synthesizing activity in the tissues of WT and B3gnt5−/− mice. Previously, we reported that the HL-60 cell line expressed abundant B3gnt5 transcripts, whereas the Jurkat cell line did not (17). In Fig. 1, tissue homogenates of WT mice showed apparent activity of Lc3Cer synthase in all organs except the cerebrum. This is consistent with a previous report by others in which the adult cerebral cortex was found to express little or no B3gnt5 transcript. In contrast, the adult cerebellum expresses B3gnt5 transcripts at a comparatively high level in Purkinje cells (18). Tissue homogenates of B3gnt5−/− mice exhibited no Lc3Cer-synthesizing activity. These results indicate that there is no other glycosyltransferase with Lc3Cer-synthesizing activity.
Fig. 1.
Confirmation of Lc3Cer synthase activity in B3gnt5−/− mice and MS analysis of the glycans derived from GSLs of mouse B cells. (A) Homogenates (100 μg of protein) of mouse tissues were used to assay Lc3Cer synthase activity. The products were separated on a high-performance twin-layer chromatography (HPTLC) plate with a solvent system of chloroform/methanol/0.2% CaCl2 (60:35:8 vol/vol/vol). The intensities of the radioactive bands were measured with a FLA3000 Image Analyzer (Fujifilm, Tokyo). HL-60, which has Lc3Cer synthase activity, and Jurkat, which does not, were used as positive and negative controls, respectively. Lc3Cer synthase assays were performed on two TLC plates, with positive and negative control on each plate. This figure was prepared from the results of two TLC plates to demonstrate Lc3Cer synthase activity in cerebellum, spleen, and colon between WT and B3gnt5−/− mice. (B) MS spectra of the glycans derived from GSLs of mouse B cells. Arrows indicate glycan signals that are absent from GSLs of B3gnt5−/− B cells. Arrowheads indicate glycan signals that are decreased in GSLs of B3gnt5−/− B cells. Table S1 summarizes the results of the assigned GSL-derived glycan structures of mouse B cells. Detailed methods for MS of glycans derived from GSLs are described in Fig. S2.
Glycan Structure of GSLs Extracted from Tissues of WT and B3gnt5-Deficient Mice.
We performed TLC-immunoblotting using anti-GlcNAc Ab (kindly provided by Minoru Suzuki, RIKEN, Saitama, Japan) on GSLs from the cerebellum. Orcinol staining revealed no differences in the patterns of GSLs (Fig. S2). This is probably attributable to the low content of lacto/neolacto-series GSLs as compared with gangliosides. However, the immunoblotting results shown in Fig. S2 clearly demonstrated that Lc3Cer (amino-CTH) was absent in the B3gnt5−/− cerebellum. On the other hand, we could not detect the signal for Lc3Cer on TLC (-immunoblotting) analysis of spleen, probably because the amount of Lc3Cer was too low in total GSLs. Orcinol staining again showed no difference between WT and B3gnt5−/− mice in acidic GSLs from spleen (Fig. S2). By immunostaining the acid fraction of GSLs treated with sialidase, at least three positive bands were detected in WT spleen using the 1B2-1B7 mAb (Fig. S2). These bands entirely disappeared in B3gnt5−/− mice. It is known that mAb 1B2-1B7 reacts with neolactotetraosylceramide (nLc4Cer), nLc6Cer, and other glycolipids having LacNAc units at the nonreducing end of their carbohydrate chains.
As seen from the results of MS analysis (Fig. 1 and Fig. S2), we confirmed the loss of elongated GSLs on Lc3Cer in B3gnt5−/− B cells. Signals of nos. 7 and 16 were absent in B3gnt5−/− mice, although they were present in WT B cells. No. 7 was identified as Galβ1-4GlcNAcβ1-3(Galβ1-4GlcNAcβ1-6)Galβ1-4Glc, which is one of the lacto/neolacto-series GSL structures, using the method described in SI Experimental Procedures and Results. The m/z (1,987.147) of no. 16 indicates three candidates that are possibly ganglio-series glycolipids or lacto/neolacto-series GSLs, as listed in Table S1. In in vitro recombinant β3GnT5, enzyme acts on ganglio-series GSLs, including GA1, GM1 (GM1a), and GD1b, as an acceptor substrate (18). Therefore, this is likely to be one of the lacto/neolacto-series GSLs having a Hex-Hex-N-acetylhexosamine (HexNAc)-[sialic acid (NeuGc)-Hex-HexNAc]Hex-Hex structure and/or one of the ganglio-series GSLs having a Hex-Hex-HexNAc-GM1a structure, because it was absent in the B cells of B3gnt5−/− mice. The signals of nos. 4, 14, and 17 (Fig. 1B and Fig. S2) significantly decreased in B3gnt5−/− B cells. These molecular masses correspond to both ganglio-series and lacto/neolacto-series GSLs, as listed in Table S1. A mixture of both ganglio-series and lacto/neolacto-series GSLs may be contained in these samples, and the lack of lacto/neolacto-series and/or minor ganglio-series GSLs in B3gnt5−/− B cells may reflect this.
Flow Cytometry and Immunoblot Analyses of PLN Using Lycopersicon esculentum Lectin.
Our previous study indicated that of the four members of the β3GnTs, β3GnT2 exhibits the strongest PLN synthetic ability in vitro and that β3GnT5 shows lower activity, ∼30% of that of β3GnT2 (17). To examine whether PLN on glycoproteins is affected in B3gnt5−/− mice, flow cytometry (FCM) analysis of splenocytes was performed using Lycopersicon esculentum (LEL) lectin. This lectin binds to PLN chains having at least three LacNAc repeats. By FCM, no difference between WT and B3gnt5−/− splenocytes and B cells in LEL staining at the cell surface was observed (Fig. S3). As seen in Fig. S3, blotting analysis using LEL showed strong signals for both WT and B3gnt5−/− splenocytes without any significant difference of intensity as compared with B3gnt2−/− splenocytes. This suggests that β3GnT5 is not involved in the expression of PLN chains on glycoproteins.
FCM Analysis of Hematocytes in B3gnt5-Deficient Mice.
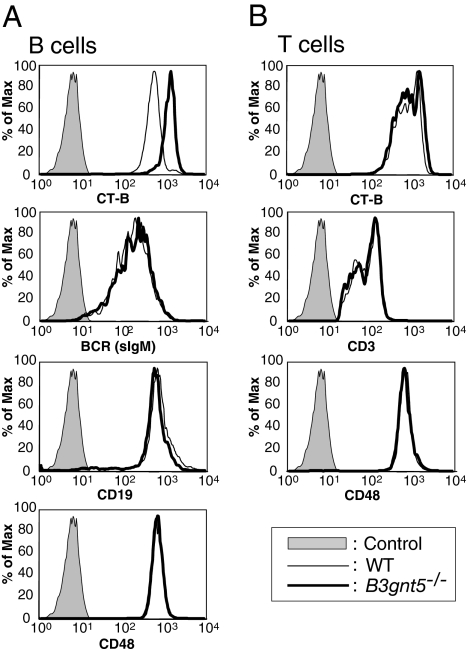
To investigate whether hematocytes manifest aberrant cell distribution or development in B3gnt5−/− mice, we analyzed the expression of a series of CD antigens on the cell surface of peripheral blood and splenic cells using FCM. The antigens investigated were the same as those described in a previous study (19). No significant disturbance in the ratios of cell populations, such as T cells, B cells, monocytes, or granulocytes, was observed. However, we documented a marginal increase in Ab levels in intact mouse serum; the levels of total IgG, IgG2a, and IgG2b were increased in B3gnt5−/− mice as compared with WT mice (Fig. S4). Lacto/neolacto-GSLs are known to be localized in GEMs on the cell surface. However, some antibodies that bind to lacto/neolacto-GSLs, such as the anti-LacNAc mAb 1B2-1B7, are known to cross-react with carbohydrate antigens on glycoproteins. Therefore, we investigated GSL antigens on the cell surface (i.e., GM1, GM3, Gb3, others) as GEM markers. In a series of FCM analysis, we were interested to observe that the intensity of GM1 staining with Ab or cholera toxin B subunit (CT-B) was significantly increased in the B cells of B3gnt5−/− mice as compared with WT mice (Fig. 2). GM1 levels were increased on splenic B cells (Fig. 2, gate: CD19+ cells) of B3gnt5−/− mice [mean fluorescence intensity (MFI) of ∼1,431] as compared with WT mice (MFI of ∼625).
Fig. 2.
Up-regulation of CT-B staining as a GEM marker but no alteration of surface protein expression on B cells. FCM analysis of GEMs and surface proteins on splenic B cells (A) and T cells (B). The expression on the splenocyte cell surface was analyzed by FCM using FITC-conjugated CT-B, anti-CD3ε, anti-BCR (sIgM), anti-CD48 Abs, and phycoerythrin (PE)-conjugated anti-CD19. Results are shown as histograms of fluorescence intensity. The shaded peak in each panel represents the negative control. Max, maximum.
BCR and CD19 antigens on the B cell surface were not different in WT and B3gnt5−/− mice. There was also no difference in the level of expression of these molecules in WT or B3gnt5−/− mice, as assessed by Western blotting (Fig. S5). Because it is known that many GPI-anchored proteins are localized in GEMs, we examined the expression profiles of other GPI-anchored proteins, such as CD48. However, we found no differences between WT and B3gnt5−/− mice in any expression profiles. In contrast to these findings with B cells, T cells did not manifest any differences in GM1 expression detected by CT-B. This may be interpreted in relation to their initially low expression of B3gnt5 transcripts.
Microscopic Analysis of GEMs in B Cells.
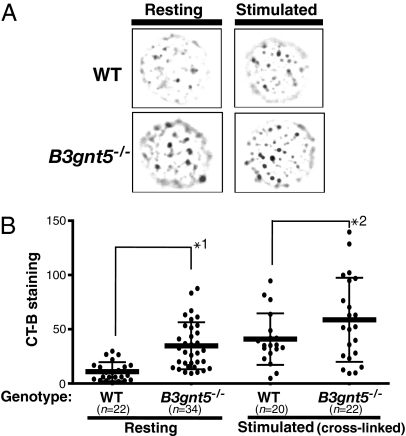
We observed GEM formation on the cell surface using cholera toxin as a probe, because it is known that CT-B binds to GM1. CT-B and various GPI-anchored proteins have been defined and identified previously as components of GEMs. CT-B staining of the cell surface showed a punctate pattern by fluorescence microscopy (Fig. 3). This pattern was identical to that reported in previous studies. In the present study, the puncta on the surface of B3gnt5−/− resting B cells were much brighter and larger and their number was significantly increased as compared with WT. These CT-B staining levels for the puncta of B3gnt5−/− resting cells were almost as high as for stimulated WT B cells (Fig. 3). Thus, the B cells of B3gnt5−/− mice may be easier to activate; furthermore, the staining levels of puncta were increased even more in stimulated B cells of B3gnt5−/− mice as compared with stimulated WT cells.
Fig. 3.
Fluorescence microscopy of GEMs on the cell surface. (A) Fluorescence microscopy showed a CT-B–induced punctate pattern indicating GEM structures on the B cell surface. Resting or stimulated isolated B cells were stained with FITC-conjugated CT-B. The results shown are representative of several independent experiments revealing markedly stronger staining of B3gnt5−/− than WT B cells. (B) Fluorescence intensity of positive signals for each CT-B–stained punctate region was analyzed by means of a BZ-Analyzer (KEYENCE). The total value of the positive signals on each cell surface was measured. Statistical analysis of the difference between WT and B3gnt5−/− B cells, with or without stimulation, was performed using one-way ANOVA and Tukey testing by means of PRISM4 software. Data are given as each cell type's mean (bold line) and SD. *1, P < 0.01; *2, P < 0.01.
Because GM1 staining on FCM was up-regulated, we examined six transcripts for glycosyltransferase genes involved in the synthesis of GM1 in resting B cells from WT and B3gnt5−/− mice. By the comparative PCR method using real-time PCR (Fig. S6), there were no significant differences between the B cells of WT and B3gnt5−/− mice in the levels of these six transcripts. No expression of B3gnt5 mRNA in resting B cells of B3gnt5−/− mice could be confirmed. In addition, the pattern of MS spectra indicated no marked change in the amount of GM1, with the exception of the decreased peak intensity related to lacto/neolacto-series GSLs. (Fig. 1 and Fig. S2). However, MS analysis and orcinol staining of GSLs on TLC (Fig. S6) are, in general, poorly quantitative. Therefore, the total amount of GM1 in B cells was determined by TLC-immunoblotting using HRP-conjugated CT-B. Total amounts of GM1 did not differ between WT and B3gnt5−/− mice (Fig. S6).
Distribution Analysis of GEM Proteins.
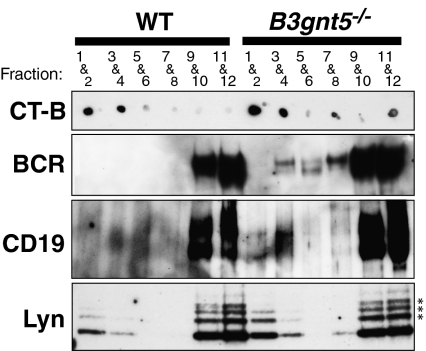
As reported in the previous studies, we observed in this study that BCR, CD19, and Lyn of B cells were colocalized in low-density membrane fraction GEMs (Fig. 4, designated fractions 1–4) prepared in medium containing 1% Triton X-100 and separated by sucrose density gradient centrifugation. We analyzed the behavior of BCR transduction-related proteins in GEMs after stimulation with anti-BCR Ab and anti-CD19 Ab cross-linking (Fig. 4). Western blotting showed that the BCR and CD19 antigens from B cells of B3gnt5−/− mice were markedly increased in the GEM fraction, as compared with WT mice. In addition, larger amounts of Lyn kinases were located in the GEM fraction in B3gnt5−/− B cells. On the other hand, we determined that not only cross-linking of BCR together with CD19 but cross-linking of CD19 alone resulted in abnormalities in the GEM structure. This suggests that GEM-related molecules, such as BCR, CD19, and Lyn, show increased motility into GEM (fractions 1–4; Fig. S7). These results indicated that B3gnt5−/− B cells might be in a state in which they could be more easily activated by external stimuli as compared with WT B cells. We therefore propose that GEM abnormalities are present in B3gnt5−/− B cells.
Fig. 4.
Distribution of BCR, CD19, and Lyn in GEMs separated into fractions by sucrose density gradient centrifugation. B cells stimulated with anti-BCR and anti-CD19 Abs were lysed with 1% Triton X-100 solubilization buffer. After sucrose gradient centrifugation, fractions were collected from the top of gradient. Then, every second fraction was combined starting from the top fraction. GM1, a GEM marker, was detected using HRP-conjugated CT-B as shown. After fractionation, immunoprecipitation of BCR and CD19 was performed using anti-BCR and anti-CD19 Abs, respectively. BCR (sIgM) and CD19 proteins in B3gnt5−/− B cells were up-regulated in the GEM fraction (fractions 1–4). Lyn proteins were also up-regulated in the GEM fraction of B3gnt5−/− B cells. The results shown are representative of several independent experiments. *Nonspecific bands.
Phosphorylation Signals Under BCR Stimulation Are Increased in B Cells of B3gnt5-Deficient Mice.
Many kinases have been reported to play important roles in signal transduction following BCR stimulation. To analyze the alteration of BCR downstream signals after anti-BCR cross-linking, we examined phosphorylation patterns in B cells of WT and B3gnt5−/− mice. Western blotting with the Ab 4G10, specific for phosphotyrosine, yielded multiple bands (Fig. 5). Immunoblotting with Ab 4G10 showed that tyrosine phosphorylation of multiple proteins in B3gnt5−/− B cells was dramatically increased as compared with WT mice.
Fig. 5.
Analysis of phosphorylation status and enhanced response to BCR cross-linking in resting B cells. (A) Resting B cells from spleen were stimulated by cross-linking anti-BCR Ab for the indicated time. The same volume of lysates was added to each lane. Phosphorylated proteins were analyzed by immunoblots using anti-phospho-Tyr mAb (4G10). B3gnt5−/− B cells exhibited significantly up-regulated phosphorylation signals. (B) Resting B cells from B3gnt5−/− mice show enhanced responses to BCR-mediated stimulation. Resting B cells were cultured with the indicated dose of F(ab′)2 anti-IgM. Proliferative responses are given as [3H]-thymidine (TdR) incorporation for the final 6 h of the 42-h culture period. Each assay was performed in triplicate, and all data are representative of three experiments. Open circles indicate WT mice, and closed triangles indicate B3gnt5−/− mice. (C) Response to TI-II antigen. Mice were immunized i.p. with 25 μg of TNP-Ficoll in PBS at day 0. Sera were collected sequentially from the eye socket and tested at a 1:100 dilution. The levels of TNP-specific IgM Ab in sera were determined by ELISA. Representative data were obtained for five (WT) and six (B3gnt5−/−) mice, respectively. Open circles indicate WT mice, and closed circles indicate B3gnt5−/− mice. Statistical analysis of the difference between WT and B3gnt5−/− B cells with days after stimulation was performed by two-way ANOVA using PRISM4 software. The levels of IgM in serum are presented as mean (symbols) ± SE. *P < 0.05.
Enhanced Response of Stimulated B3gnt5-Deficient B Cells.
BCR and CD19 are major immune receptors and costimulatory molecules, respectively, for B cell activation (21). Because the behavior of these molecules in GEMs was different between WT and B3gnt5−/− B cells, we examined the impact of lacto/neolacto-GSL deficiency on B cell proliferation. Resting B cells were stimulated with anti-BCR (anti-IgM) and assessed for proliferation after 2 d. A greater proliferation of B3gnt5−/− than WT splenic B cells was observed with low concentrations of anti-BCR (Fig. 5). These results indicate that the lack of PLN in GSLs of B cells lowers the cellular threshold for proliferation triggered by BCR stimulation. In addition, we examined the in vivo response to the T-independent type II antigen [TI-II antigen, trinitrophenol (TNP)-Ficoll]. The specific Ab response to TNP was slightly up-regulated in B3gnt5−/− mice (Fig. 5C). The levels of IgM production were up-regulated at an early stage of the response in B3gnt5−/− mice as compared with WT mice. However, 14 d after immunization, differences between WT and B3gnt5−/− mice were no longer apparent in the levels of TNP-specific Ab produced.
Discussion
The results of previous studies had strongly indicated that β3GnT5 was the most feasible candidate for the Lc3Cer synthase (17, 18). We had also determined that mouse B cells expressed abundant B3gnt5 transcripts and possessed Lc3Cer synthase activity. To approach the question of the significance of lacto/neolacto-series GSLs in biological function, we generated B3gnt5−/− mice. In the present study, we confirmed that Lc3Cer synthase activity was undetectable in any of the tissue homogenates of B3gnt5−/− mice examined. These results indicate that Lc3Cer synthase is the sole enzyme responsible for the extension of PLN chains on lacto/neolacto-series GSLs.
Lacto/neolacto-series GSLs contain sialic acid or sulfate, resulting in sialylparagloboside, sulfoglucuronylglycolipid, and other similar derivatives. Mouse lymphocytes contain globo-series GSLs (Gb3 and Gb4) and ganglio-series GSL (GM3) (22), but it was not known whether they possessed neolacto-series GSLs. There is a report that human B cells contain nLc4Cer (paragloboside) (23). In the present study, we demonstrated that three major GSL bands, which were detected by the 1B2-1B7 mAb after sialidase treatment, were absent from the splenocytes of B3gnt5−/− mice. This was confirmed by MS showing the absence of branching structures elongated on Lc3Cer.
We investigated whether lacto/neolacto-series GSLs have an important role in biological functions, particularly in the immune system. The results indicated that B3gnt5−/− mice showed an enhanced response to BCR/CD19 stimuli. Recent studies have shown the importance of appropriate GEM formation for cell activation. Incorporation of a number of receptors, including the BCR, TCR, and GM1, into GEMs constitutes an important step in receptor function. After stimulation with various different agents, receptors and signaling molecules are incorporated into GEMs, effectively accumulate, and interact to result in signal transduction from the site of aggregation (i.e., GEMs). In T cells, aggregation of GEMs by cross-linking with CT-B, analogous to TCR cross-linking, induces signaling events and the respective signaling molecules are phosphorylated (24). We observed higher levels of CT-B staining (Fig. 2) and higher levels of GEM aggregation (Fig. 3) in resting B3gnt5−/− B cells, which could be the reason for their enhanced response to BCR stimulation. Because there were no differences in the total amount of GM1 and BCR-related molecules (e.g., BCR, CD19, Lyn), we hypothesize that some abnormalities or alterations of GEM microstructure might have occurred. We found that there was no increased expression of genes relating to the biosynthesis of GM1. This finding indicates that GM1 synthesis was not up-regulated at the level of gene expression. In addition, on the basis of the results of MS glycan analysis, as shown in Fig. 1 and Fig. S2, lacto/neolacto-series GSLs are present as a minor component of total GSLs. On the other hand, GM1 is the major component of total GSLs in mouse B cells. In fact, the pattern of MS spectra indicated no marked change in the amount of GM1, except for the decreased peak intensity related to lacto/neolacto-series GSLs. Nonetheless, the data raise the possibility that acceptor substrate (i.e., lactosylceramide) is shifted from the lacto/neolacto-series GSL synthetic pathway to the GM1 synthetic pathway. However, lacto/neolacto-series GSLs are normally only minor components in total GSLs, and it is difficult to accept that they are the reason for the increase in GM1. MS analysis or orcinol staining on TLC is, in general, poorly quantitative. Therefore, we performed TLC-immunoblotting as a more quantitative mode of analysis of GM1 GSL (Fig. S6). The results demonstrated that the total amount of GM1 did not differ between WT and B3gnt5−/− mice.
Concerning the brighter CT-B staining, we speculate that the alteration of GEM microstructure may cause the “unmasking” of GM1. Another possibility is that the alteration of GEM microstructure may affect the accessibility of GM1 molecules for CT-B. The clustering effect of GM1, caused by the ease of patching of GEM, may enhance the staining of CT-B. By immunoblotting, we observed slightly increased CT-B staining in separated GEM fractions. We interpret this as the result of the biochemical isolation of GM1 in clustered (patched) GEMs, as shown in Fig. 3. It is known that the microstructure of GEMs reflects their biochemical characteristics and that GM1 in clustered GEMs differs from monomolecular GM1. In general, lectin-like proteins, which can bind to glycans, exhibit stronger affinity to multivalent ligands than to monovalent ligands. CT-B is also known to form pentamer structures (25). It is possible that the clustered GEMs might be easily (and strongly) stained with CT-B in Figs. 2 and 3. However, we have not obtained enough evidence to explain the changes in GEM microstructure at this time. Although GEMs have been studied by a large number of groups, relatively little is known about their biochemical characteristics. Further study is therefore required to identify the detailed biological functions of ganglioside and GEMs.
One important issue in GEM function is their aggregation. There is now a consensus that GEMs are too small to be observed by light microscopy before stimulation (26). It is also reported that GEM formation is reversible, so that they exist only as transiently stabilized structures. GEMs can coalesce on clustering of their components, such as the BCR in B cells, with ligands, antibodies, and/or proteins having lectin activity. The small individual GEMs cluster into larger, visible, and functional units; such clustering facilitates efficient interaction of GEM-associated proteins. In the case of GPI-anchored proteins, such as CD59, dynamic and transient recruitment of GM1 to CD59 clusters was observed by single-molecule tracking and taken to suggest the involvement of GEMs in signal transduction (27). In this case, to form GEMs, clustering of such receptor molecules causes gathering of cholesterol and GSLs, such as GM1, around themselves. On the other hand, cross-linking of GM1 induces patching on the cell surface, resulting in signal transduction. Disruption of GEMs inhibits cell activation (13). Thus, both the association of receptor complexes and the platform of clustered GEMs are important for signaling via the GEMs. B3gnt5−/− resting B cells showed enlarged GM1 patches on their surface, resulting in increased BCR clustering. We observed no significant differences regarding the ratio of leukocyte populations in blood between WT and B3gnt5−/− mice. However, the data at 0 min in Fig. 5 showed that phosphorylation signals were slightly up-regulated in intact B3gnt5−/− B cells, and we found that Igs in serum were slightly increased in B3gnt5−/− mice (Fig. S4). We interpret these data as implying that the clustering of GEMs containing GM1 occurred more readily in B3gnt5−/− B cells than in WT B cells. The in vivo response of B cells to TI-II antigen (TNP) was more rapid and was slightly up-regulated in B3gnt5−/− B cells as compared with WT B cells (Fig. 5C). This result indicated that B3gnt5−/− B cells could be activated more easily than WT B cells.
We speculated that the up-regulation of GM1 clustering caused by the absence of PLN chains from GSLs depends on structural alterations at the cell surface, such as changes in lattice structures. These structures, consisting of galectin-carbohydrate cross-linked on the cell surface, regulate signal transduction through receptor segregation and turnover, and they also modulate cellular interaction (28). Their formation on the cell surface depends on galectin binding to LacNAc (Gal) residues on glycoproteins and GSLs (28, 29). However, the formation of lattice structures is proposed to occur only via glycoprotein–glycoprotein interactions. On the other hand, Galectin-1 can bind to GM1 and 1B2–1B7-reactive GSL-containing PLN chains (30). These interactions mainly result in inhibitory effects on the diffusion of receptor molecules and small GEMs. The decreased lattice structures in B3gnt5−/− B cells may be attributable to lectin-mediated interactions of GSLs-GSLs, and/or glycoproteins-GSLs, and may cause the rapid aggregation of GEMs. In B3gnt5−/− resting B cells, the small GEMs can easily aggregate into larger units when receptor complexes are formed. Thus, B3gnt5−/− B cells also exhibit up-regulated phosphorylation signals and hyperproliferation, as shown in this study. Galetin-1 negatively regulates B cell proliferation and Tyr-phosphorylation on BCR stimulation (31). Marked up-regulation of galectin-1 (Lgals1) and galectin-3 (Lgals3) gene expression has been demonstrated in anergic B cells (32). These data suggest that interactions of galectins and glycans may also play a role in the regulation of immune tolerance. Dissecting the detailed mechanisms responsible for enhanced GEM formation in B3gnt5−/− B cells is an attractive subject for future study.
In addition, we observed that the behavior of BCR and BCR-related molecules was different between WT and B3gnt5−/− B cells in the GEM fraction. As seen in Fig. 4 and Fig. S7, BCR and the signaling molecule Lyn are more abundant in the GEMs of B3gnt5−/− B cells than in those of WT B cells, and thus seem to be up-regulated on translocation of these molecules into GEMs. In Fig. 4, fractions 1–4 are observed as CT-B–positive bands in both WT and B3gnt5−/− B cells. We observed that the expression pattern of BCR, CD19, and Lyn corresponds to the GEM fraction, which suggests the incorporation of these molecules into GEMs. We thought that these molecules move into the GEM fractions. There is a large amount of these molecules in GEMs of B3gnt5−/− B cells stimulated with anti-CD19 Ab alone (Fig. S7). This observation also indicates that GEM-related molecules, such as BCR, CD19, and Lyn, seem to be translocated into GEMs. Alternatively, it is clear that a large amount of BCR molecules exists in the GEM fraction in B3gnt5−/− B cells as compared with WT B cells. This result reflects the state of increased ease of activation in B3gnt5−/− B cells as compared with WT B cells.
We also think that there is no possibility that expression of BCR, CD19, or Lyn was up-regulated in B cell lysates. As shown in Fig. 2, there is no difference in the cell surface expression of these molecules between WT and B3gnt5−/− B cells, as quantified by FCM analysis. In addition, Western blotting data on their presence in B cell lysates (Fig. S5) showed that the amount of these molecules did not differ between WT and B3gnt5−/− mice. However, Fig. 4 shows that the amount of BCR and/or CD19 protein in non-GEM fractions might be slightly increased. Before isolation of the GEM fraction, the lysates were separated by brief centrifugation at maximum speed to exclude nuclei and some cytoskeleton proteins, including actin. It has been reported that BCR molecules associate with certain cytoskeletal proteins; for example, cytoskeleton components, such as actin, associate with GEMs or GEM proteins, such as BCR. Actin polymerization regulates the strength of BCR stimulation (33, 34). Therefore, during this procedure, it is possible that the interaction between BCR molecules and the cytoskeleton might be affected by any alteration of GEM structure. However, we have no data to explain this difference directly at this time. In this case, it is difficult to be sure that total BCR staining is equivalent in WT and B3gnt5−/− B cells. In this study, we focused attention on the difference in the behavior of these molecules around the center or adjacent fractions of GEMs between WT and B3gnt5−/− mice.
There are at least two possible molecular mechanisms that could be responsible for the phenomenon observed in B3gnt5−/− B cells. First, BCR–CD19 complexes are transferred efficiently into GEMs, because many more clustered GEMs are formed in B3gnt5−/− B cells than in WT B cells. Second, PLN on GSLs may control the behavior of glycoprotein entry into or exclusion from GEMs. To test this hypotheses, we would need to elucidate further which molecular interactions are altered by GSL-(poly)lactosamine deficiency. The results presented here suggest that PLN on GSLs is a putative immune regulatory factor. PLN chains on GSLs may have an important role in suppression of excessive responses in the immune system in the same manner as PLN on N-glycans of glycoprotein. The deletion of a specific glycosyltransferase gene in knockout mice indicates that some glycosyltransferases are essential for immune system integrity. In the present study, we revealed that lacto/neolacto-series GSLs might also have significant effects on biological function in the immune system.
Experimental Procedures
Detailed information on the experimental procedures of this study, including generating B3gnt5-deficient mice, extraction of GSLs, glycan analysis, FCM analysis, fluorescence microscopy analysis, fractionation of GEMs, immunoblotting, cell proliferation assays, T-independent Ab production, and others, is provided in SI Experimental Procedures and Results. Materials, including the Abs and probes used, and glycosphingolipid structures are also described in SI Experimental Procedures and Results.
Supplementary Material
Acknowledgments
We thank Dr. Hiroyasu Ishida of Tsukuba University and Ms. Mihou Fushimi of the National Institute of Advanced Industrial Science and Technology for excellent assistance. This work was supported by the New Energy and Industrial Technology Development Organization (NEDO).
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AB045278).
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0914298107/-/DCSupplemental.
References
- 1.Prinetti A, Loberto N, Chigorno V, Sonnino S. Glycosphingolipid behaviour in complex membranes. Biochim Biophys Acta. 2009;1788:184–193. doi: 10.1016/j.bbamem.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Yohe HC, Coleman DL, Ryan JL. Ganglioside alterations in stimulated murine macrophages. Biochim Biophys Acta. 1985;818:81–86. doi: 10.1016/0005-2736(85)90141-5. [DOI] [PubMed] [Google Scholar]
- 3.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 4.Brown DA, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- 5.Hakomori S. Glycosynapses: Microdomains controlling carbohydrate-dependent cell adhesion and signaling. An Acad Bras Cienc. 2004;76:553–572. doi: 10.1590/s0001-37652004000300010. [DOI] [PubMed] [Google Scholar]
- 6.Smith DC, Lord JM, Roberts LM, Johannes L. Glycosphingolipids as toxin receptors. Semin Cell Dev Biol. 2004;15:397–408. doi: 10.1016/j.semcdb.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Hakomori S. Carbohydrate-to-carbohydrate interaction, through glycosynapse, as a basis of cell recognition and membrane organization. Glycoconj J. 2004;21:125–137. doi: 10.1023/B:GLYC.0000044844.95878.cf. [DOI] [PubMed] [Google Scholar]
- 8.Nishio M, Tajima O, Furukawa K, Urano T, Furukawa K. Over-expression of GM1 enhances cell proliferation with epidermal growth factor without affecting the receptor localization in the microdomain in PC12 cells. Int J Oncol. 2005;26:191–199. [PubMed] [Google Scholar]
- 9.Anderson RG, Jacobson K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 2002;296:1821–1825. doi: 10.1126/science.1068886. [DOI] [PubMed] [Google Scholar]
- 10.Petrie RJ, Schnetkamp PP, Patel KD, Awasthi-Kalia M, Deans JP. Transient translocation of the B cell receptor and Src homology 2 domain-containing inositol phosphatase to lipid rafts: evidence toward a role in calcium regulation. J Immunol. 2000;165:1220–1227. doi: 10.4049/jimmunol.165.3.1220. [DOI] [PubMed] [Google Scholar]
- 11.Razzaq TM, et al. Regulation of T-cell receptor signalling by membrane microdomains. Immunology. 2004;113:413–426. doi: 10.1111/j.1365-2567.2004.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viola A, Schroeder S, Sakakibara Y, Lanzavecchia A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 1999;283:680–682. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- 13.Xavier R, Brennan T, Li Q, McCormack C, Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723–732. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- 14.Yuyama K, Sekino-Suzuki N, Sanai Y, Kasahara K. Lipid rafts in cellular signaling and disease. Trends Glycosci Glycotechnol. 2003;15:139–151. [Google Scholar]
- 15.Miceli MC, et al. Co-stimulation and counter-stimulation: Lipid raft clustering controls TCR signaling and functional outcomes. Semin Immunol. 2001;13:115–128. doi: 10.1006/smim.2000.0303. [DOI] [PubMed] [Google Scholar]
- 16.Togayachi A, Sato T, Narimatsu H. Comprehensive enzymatic characterization of glycosyltransferases with a β3GT or β4GT motif. Methods Enzymol. 2006;416:91–102. doi: 10.1016/S0076-6879(06)16006-1. [DOI] [PubMed] [Google Scholar]
- 17.Togayachi A, et al. Molecular cloning and characterization of UDP-GlcNAc:lactosylceramide β1,3-N-acetylglucosaminyltransferase (β3Gn-T5), an essential enzyme for the expression of HNK-1 and Lewis X epitopes on glycolipids. J Biol Chem. 2001;276:22032–22040. doi: 10.1074/jbc.M011369200. [DOI] [PubMed] [Google Scholar]
- 18.Henion TR, Zhou D, Wolfer DP, Jungalwala FB, Hennet T. Cloning of a mouse beta 1,3 N-acetylglucosaminyltransferase GlcNAc(beta 1,3)Gal(beta 1,4)Glc-ceramide synthase gene encoding the key regulator of lacto-series glycolipid biosynthesis. J Biol Chem. 2001;276:30261–30269. doi: 10.1074/jbc.M102979200. [DOI] [PubMed] [Google Scholar]
- 19.Togayachi A, et al. Polylactosamine on glycoproteins influences basal levels of lymphocyte and macrophage activation. Proc Natl Acad Sci USA. 2007;104:15829–15834. doi: 10.1073/pnas.0707426104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biellmann F, Hülsmeier AJ, Zhou D, Cinelli P, Hennet T. The Lc3-synthase gene B3gnt5 is essential to pre-implantation development of the murine embryo. BMC Dev Biol. 2008;8:109. doi: 10.1186/1471-213X-8-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsubata T. Co-receptors on B lymphocytes. Curr Opin Immunol. 1999;11:249–255. doi: 10.1016/s0952-7915(99)80041-7. [DOI] [PubMed] [Google Scholar]
- 22.Kovacic N, Müthing J, Marusic A. Immunohistological and flow cytometric analysis of glycosphingolipid expression in mouse lymphoid tissues. J Histochem Cytochem. 2000;48:1677–1690. doi: 10.1177/002215540004801211. [DOI] [PubMed] [Google Scholar]
- 23.Schwarting GA. Quantitative analysis of neutral glycosphingolipids from human lymphocyte subpopulations. Biochem J. 1980;189:407–412. doi: 10.1042/bj1890407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janes PW, Ley SC, Magee AI. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J Cell Biol. 1999;147:447–461. doi: 10.1083/jcb.147.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruddock LW, et al. A pH-dependent conformational change in the B-subunit pentamer of Escherichia coli heat-labile enterotoxin: Structural basis and possible functional role for a conserved feature of the AB5 toxin family. Biochemistry. 1996;35:16069–16076. doi: 10.1021/bi961865l. [DOI] [PubMed] [Google Scholar]
- 26.Pralle A, Keller P, Florin EL, Simons K, Hörber JK. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J Cell Biol. 2000;148:997–1008. doi: 10.1083/jcb.148.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki KG, Fujiwara TK, Edidin M, Kusumi A. Dynamic recruitment of phospholipase C gamma at transiently immobilized GPI-anchored receptor clusters induces IP3-Ca2+ signaling: single-molecule tracking study 2. J Cell Biol. 2007;177:731–742. doi: 10.1083/jcb.200609175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabinovich GA, Toscano MA, Jackson SS, Vasta GR. Functions of cell surface galectin-glycoprotein lattices. Curr Opin Struct Biol. 2007;17:513–520. doi: 10.1016/j.sbi.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garner OB, Baum LG. Galectin-glycan lattices regulate cell-surface glycoprotein organization and signalling. Biochem Soc Trans. 2008;36:1472–1477. doi: 10.1042/BST0361472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elola MT, Chiesa ME, Alberti AF, Mordoh J, Fink NE. Galectin-1 receptors in different cell types. J Biomed Sci. 2005;12:13–29. doi: 10.1007/s11373-004-8169-5. [DOI] [PubMed] [Google Scholar]
- 31.Yu X, Siegel R, Roeder RG. Interaction of the B cell-specific transcriptional coactivator OCA-B and galectin-1 and a possible role in regulating BCR-mediated B cell proliferation. J Biol Chem. 2006;281:15505–15516. doi: 10.1074/jbc.M509041200. [DOI] [PubMed] [Google Scholar]
- 32.Clark AG, et al. Multifunctional regulators of cell growth are differentially expressed in anergic murine B cells. Mol Immunol. 2007;44:1274–1285. doi: 10.1016/j.molimm.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 33.Brown BK, Song W. The actin cytoskeleton is required for the trafficking of the B cell antigen receptor to the late endosomes. Traffic. 2001;2:414–427. doi: 10.1034/j.1600-0854.2001.002006414.x. [DOI] [PubMed] [Google Scholar]
- 34.Hao S, August A. Actin depolymerization transduces the strength of B cell receptor stimulation. Mol Biol Cell. 2005;16:2275–2284. doi: 10.1091/mbc.E04-10-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.