Abstract
Patients with ulcerative colitis (UC) experience unpredictable bouts of active inflammation and ulceration. Relatively little attention has been paid to the role of antiinflammatory mediators in the pathogenesis of UC, although rodent studies suggest an important role of prostaglandin (PG) D2 in the resolution of tissue injury and inflammation. The present study was performed to determine if colonic PGD2 synthesis was altered in patients in remission from UC and if expression of the key enzymes and receptors related to PGD2 was altered. During routine colon-cancer screening, colonic biopsies were obtained from healthy individuals, some of whom had been in remission from UC, without treatment, for >4 y. UC patients with active disease or in medically induced remission were also biopsied. Only patients with active UC exhibited elevated expression of several proinflammatory cytokines (TNFα and IFNγ) and colonic PGE2 synthesis. In contrast, colonic PGD2 synthesis was only elevated (∼3-fold) in the healthy individuals with a prior history of UC. This group also exhibited significantly elevated expression of DP1, the key receptor mediating the antiinflammatory actions of PGD2. Expression of the synthetic enzymes cyclooxygenase-1, cyclooxygenase-2, and hematopoietic PGD synthase was not altered in the healthy individuals with a prior history of UC. These results show a marked up-regulation of synthesis of an antiinflammatory prostanoid and expression of its receptor, specifically in individuals in long-term remission from UC. This is consistent with animal studies showing the importance of PGD2 in the induction and maintenance of remission from colitis.
Keywords: inflammation, inflammatory bowel disease, eicosanoid, colon cancer
Although the etiology of ulcerative colitis (UC) remains unknown, it is clear that interactions among a number of genetic, microbial, and environmental factors result in disregulation of the immune system (1). UC is characterized by unpredictable bouts of active disease and remission. Within a given cohort of UC patients, approximately one-half are in clinical remission at any one time (2). Acute inflammatory episodes compromise mucosal integrity and are characterized by the mucosal infiltration of mast cells, lymphocytes, macrophages, and activated neutrophils (1). These cells are recruited in response to release of a variety of proinflammatory mediators. Although they can contribute to an amplification of the inflammatory response, there is accumulating evidence that they can also release mediators that trigger the activation of various antiinflammatory and proresolution circuits (3–5).
Cyclooxygenase- (COX) and lipoxygenase-driven synthesis of lipid mediators has been the subject of much interest with regard to mechanisms of resolution (3–5), a process whereby inflammation is actively switched off and healing of tissue injury is promoted. During this process, mediators are released that can modulate cytokine and chemokine levels and regulate leukocyte and monocyte trafficking (4, 5). Inadequate production of proresolving mediators or the inability of these mediators to execute their antiinflammatory effects may exacerbate an inflammatory disorder and could represent an important stage in the progression from acute to chronic inflammation. To this end, the roles of prostaglandins (PGs) have received much attention because of their seemingly dichotomous nature. Whereas PGE2 has been linked with the promotion of edema formation and pain (6), PGD2 and its metabolite 15-deoxyΔ12,14 PGJ2 (15-PGJ2) exert significant antiinflammatory effects (7–9).
Rectal biopsies from patients with active UC have been shown to have elevated levels of PGE2, PGI2, and PGF2α (10, 11). In UC and experimental colitis, the predominant source of PGs seems to be COX-2 (12, 13). Several studies of experimental colitis suggest important roles of PGD2 in promoting the resolution of inflammation and long-term alterations in colonocyte and barrier function (7, 14, 15), but little is known about the roles of this eicosanoid in human colitis. In the present study, we have examined PGD2 levels in biopsies from UC patients, comparing them with those from healthy individuals who had no prior history of UC or those from healthy individuals who had experienced a prior bout of UC but had been in remission without medication for >4 y. We also examined transcript levels for the key synthetic enzymes responsible for PGD2 production and inactivation and expression of the receptors through which PGD2 exerts its effects. We observed a pronounced elevation of PGD2 synthesis and DP1 receptor expression only in healthy individuals with a prior history of UC. In these individuals, as has been observed in animal studies, the elevated mucosal PGD2 levels may contribute to the maintenance of colonic tissue homeostasis and possibly, also to an increased risk of colorectal cancer.
Results
Proinflammatory Cytokine Expression and Histology.
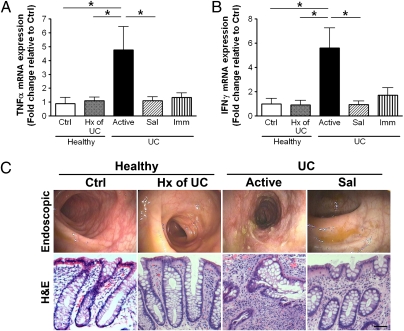
Expression of mRNA for the proinflammatory cytokines TNFα and IFNγ (Fig. 1 A and B) was significantly elevated in biopsies from patients with active disease. These findings were consistent with the level of macroscopic inflammation described at the time of colonoscopy (Fig. 1C Upper). Biopsies from patients with active UC (Fig. 1C Lower) exhibited inflammatory infiltrates and crypt distortion/atrophy, whereas biopsies from healthy subjects showed normal mucosal architecture.
Fig. 1.
Quantitative RT-PCR analysis of cytokine expression in human colon biopsies. (A) TNFα; (B) IFNγ. Biopsies were from healthy subjects without (Ctrl) or with a prior history (Hx) of UC, UC patients with active disease, or UC patients in remission induced by 5-aminosalicylic acid (Sal) or immunomodulators/biologics (Imm). Data were normalized to β-actin gene expression (n = 5–16; *P < 0.05). Representative endoscopic (C Upper) and histological (C Lower) images of colon mucosa from patients involved in this study (H&E staining). Superficial ulceration, granulocyte infiltration, and distorted/branching crypts are apparent in biopsies from patients with active disease, whereas those from the healthy subjects or those in remission appear normal. (Magnification bar: 100 μm.)
Colonic PG Synthesis.
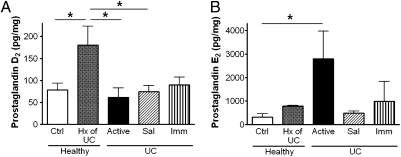
PGD2 levels were significantly elevated (∼3-fold) in healthy individuals who had been in treatment-free remission from UC for more than 4 y (Fig. 2A). In contrast, the production of PGE2 was elevated only in colonic biopsies from patients with active colitis (Fig. 2B).
Fig. 2.
Colonic mucosal prostaglandin D2 (A) and prostaglandin E2 (B) levels in biopsies from healthy subjects without (Ctrl) or with a prior history (Hx) of UC, UC patients with active disease, or UC patients in remission induced by 5-aminosalicylic acid (Sal) or immunomodulators/biologics (Imm). Prostaglandin D2 levels were significantly elevated in the samples from healthy subjects with a prior history (>4 y disease-free) of UC compared with healthy subjects or UC patients with active disease and those in remission induced by 5-aminosalicylic acid. Prostaglandin E2 levels were significantly elevated in biopsies from patients with active UC. Data are expressed as the mean ± SEM (n = 5–16; *P < 0.05).
COX-1 and COX-2 Expression.
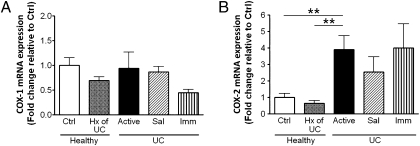
The expression of COX-1 mRNA did not differ among the treatment groups (Fig. 3A), whereas expression of COX-2 mRNA was increased in patients with active disease compared with healthy subjects (Fig. 3B).
Fig. 3.
Quantitative RT-PCR analysis of COX-1 (A) and COX-2 (B) gene expression in biopsies from healthy subjects without (Ctrl) or with a prior history (Hx) of UC, UC patients with active disease, or UC patients in remission induced by 5-aminosalicylic acid (Sal) or immunomodulators/biologics (Imm). Data were normalized against β-actin gene expression (mean ± SEM, n = 5–16; **P < 0.01).
Expression of PGD2 Synthetic Enzymes and Receptors.
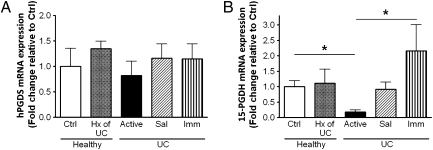
As shown in Fig. 4A, there were no significant changes in hematopoietic PGD synthase (hPGDS) among the groups (this enzyme is essential for the conversion of PGH2 to PGD2 in immune and inflammatory cells). In contrast, expression of the catabolic enzyme, 15-PGDH, was significantly down-regulated in patients with active disease compared with healthy subjects (Fig. 4B). Although this correlates well with an elevation in mucosal PGE2 levels, it does not explain the increased level of PGD2 present during long-term remission. This is an ongoing subject of interest in this lab, because we believe that PGD2 plays an important role in the initial maintenance of mucosal homeostasis. The actions of PGD2 are mediated through DP1 and DP2 receptors. We found that expression of DP1 receptor mRNA was significantly increased in healthy individuals with a prior history of UC (Fig. 5A), but there were no differences in DP2 receptor expression among the groups (Fig. 5B).
Fig. 4.
Quantitative RT-PCR analysis of the enzymes hPGDS (A) and 15-PGDH (B) in biopsies from healthy subjects without (Ctrl) or with a prior history (Hx) of UC, UC patients with active disease, or UC patients in remission induced by 5-aminosalicylic acid (Sal) or immunomodulators/biologics (Imm). Data were normalized against β-actin gene expression (mean ± SEM, n = 5–16; *P < 0.05).
Fig. 5.
Quantitative RT-PCR analysis of DP1 (A) and DP2 (B) receptor gene expression in biopsies from healthy subjects without (Ctrl) or with a prior history (Hx) of UC, UC patients with active disease, or UC patients in remission induced by 5-aminosalicylic acid (Sal) or immunomodulators/biologics (Imm). DP1-receptor gene expression was significantly elevated in the samples from healthy subjects with a prior history (>4 y disease-free) of UC. Data were normalized against β-actin gene expression (mean ± SEM, n = 5–16; *P < 0.05).
Immunohistochemistry.
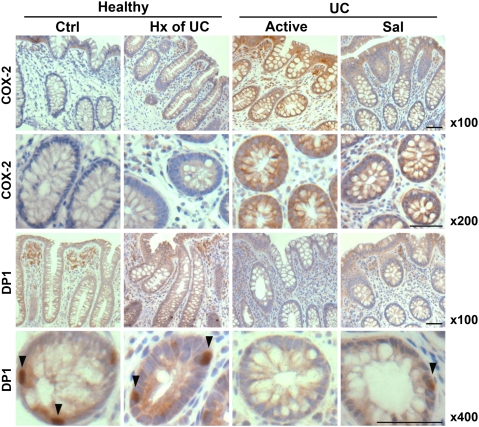
To verify the gene-expression changes of COX-2 and DP1 at the protein level, we used immunohistochemistry to assess their expression and localization in rectal biopsies. An up-regulation in COX-2 expression was evident in patients with active disease relative to biopsies from healthy subjects (± prior history of colitis) and UC patients with medically induced remission (Fig. 6). Staining for COX-2 expression was cytoplasmic and present in the apical and crypt epithelium. Immunostaining for the DP1 receptor revealed positive expression in the connective tissue of the lamina propria as well as in cells of the crypt epithelium and in biopsies from normal subjects and from those with active disease, with the lack of expression in the crypt epithelium shown in the transverse sections (Fig. 6 Bottom panel).
Fig. 6.
Expression of COX-2 and DP1 receptor in biopsies from healthy subjects without (Ctrl) or with a prior history (Hx) of UC, UC patients with active disease, or UC patients in remission induced by 5-aminosalicylic acid (Sal) or immunomodulators/biologics (Imm). Cytoplasmic COX-2 expression (Top two panels) was present in the apical and crypt epithelial cells and up-regulated in patients with active disease. Immunostaining for DP1 receptor (arrows) showed expression in the connective tissue of lamina propria and in crypt epithelial cells, depicted in the longitudinal and transverse sections, respectively (Bottom two panels). Reduced DP1 expression was observed in patients with active disease. Images are representative of each treatment group and were taken at 100× for longitudinal biopsy sections stained for COX-2 and DP1 and 200× and 400× for transverse biopsy sections stained for COX-2 and DP1, respectively. (Magnification bar: 100 μm.)
Discussion
Previous animal studies have documented an important role of PGD2 in reducing infiltration of leukocytes into the inflamed colon (7) and promoting resolution of inflammation and healing of damaged tissue (8, 9, 14). In rats, colonic PGD2 synthesis remained elevated for several weeks after resolution of colonic injury that had been induced by trinitrobenzene sulfonic acid (14, 15). The elevated colonic PGD2 synthesis, along with elevated expression of DP1 receptors, mediated some of the long-term consequences of colitis in rats, including elevated epithelial proliferation and an increased susceptibility to colon cancer (14, 15). In the present study, we examined the synthesis of PGD2 in biopsies of colon from patients with UC (active or in medically induced remission) and did not observe any significant changes relative to biopsies from healthy controls. However, PGD2 synthesis and expression of DP1 receptors were significantly elevated in biopsies from a group of healthy individuals who had previously had UC. These individuals were indistinguishable, from a clinical perspective, from other healthy controls having colonoscopy performed as screening for colon cancer, except that they had, at least 4 y previously, experienced at least one bout of UC. These data, therefore, suggest that the same long-term increase in colonic PGD2 synthesis that had been observed in rats after resolution of colitis (14, 15) also persists in humans long after resolution of colitis. As in rats, the prolonged elevation of PGD2 synthesis in humans may have beneficial (antiinflammatory) and/or detrimental (increased predisposition to colon cancer) effects.
Although studies of inflammation have historically focused on the mediators that initiate and amplify the inflammatory process, in recent years, there has been a growing recognition of the importance of several chemical mediators in regulating the timely resolution of such reactions (3–5, 16). Mediators such as lipoxins, resolvins, annexin A1, and certain prostanoids serve as important stop signals, limiting leukocyte infiltration while coordinating the efflux of inflammatory cells (e.g., macrophages) from affected tissues (3–5, 16). Interference with the resolution process can result in a progression from acute to chronic inflammation and impaired healing of tissue injury. In the context of colitis, it is noteworthy that lipoxin analogs have been reported to accelerate resolution in rodent models (17, 18), whereas expression of annexin A1 has been shown to be elevated in human UC (19) and contribute significantly to mucosal healing in rodent models of gastrointestinal injury (20).
As mentioned above, a role for PGD2 in the resolution of experimental colitis has been shown in several studies (6, 14, 15). This prostanoid has also been shown to be a critical mediator of the resolution of inflammation in experimental pleurisy (8). In that study, PGD2 derived from COX-2 was shown to be responsible for the reduction of leukocyte numbers in the inflamed pleural cavity. Inhibition of PGD2 synthesis with a COX-2 inhibitor delayed resolution, whereas exogenous PGD2 or its key metabolite (15-PGJ2) reversed the effect of the COX-2 inhibitor. The effects of PGD2 are mediated through the activation of two G protein-coupled receptors, DP1 (involved in the modulation of both innate and adaptive immune responses) (21) and DP2 (involved in the promotion of allergic inflammation) (22). PGD2 preferentially binds to DP1 (23), and its activation is largely thought to be responsible for the antiinflammatory effects of PGD2. In the present study, in addition to an increase in mucosal PGD2 synthesis, the biopsies from healthy individuals with a prior history of UC exhibited elevated expression of DP1 receptors but not of DP2 receptors. The elevated expression of DP1 is consistent with an antiinflammatory role of PGD2 in the mucosa. As reported in models of self-resolving inflammation (9) and experimental colitis (7), the use of selective inhibitors of DP1 has been shown to abrogate the protective effects of PGD2, resulting in an increase in inflammatory-cell infiltration and an imbalance in pro- and antiinflammatory cytokines. However, some of the long-term detrimental effects of PGD2 after resolution of colitis in rats, which included enhancement of epithelial proliferation and increased barrier permeability, could be reversed by treatment with a DP1 receptor antagonist (14). Likewise, the increased susceptibility to chemically induced colon cancer in rats that had recovered from a bout of colitis was reversed by treatment with a DP1 receptor antagonist. This latter detrimental effect of PGD2 may be caused, in part, by an enhancement of epithelial proliferation induced by its metabolite, 15-PGJ2, which has been shown to activate peroxisome proliferator-activated receptor γ (24). Some of the antiinflammatory properties attributed to PGD2 may similarly be attributed to actions of this metabolite, which has been shown to exert potent antiinflammatory effects in animal models (8).
Consistent with previous studies (25, 26), we observed that PGE2 synthesis and the expression of several proinflammatory cytokines (TNFα and IFNγ) were elevated in biopsies from patients with active disease but not in those who were in remission. Somewhat surprisingly, we did not detect significant changes in mucosal expression of COX-2, which is the major source of PG synthesis in inflamed mucosal tissue (12, 13). Of course, this does not rule out the possibility of increased COX-2 activity, as opposed to expression, or the possibility of elevated activity of phospholipase A2, which can liberate the precursor of PG synthesis (arachidonic acid) from membrane phospholipids. Increased expression of group II phospholipase A2 has been reported in UC (27). Like most eicosanoids, PGE2 is rapidly metabolized in the local milieu. The key enzyme responsible for the inactivation of PGE2 (and other PGs) is NAD+-dependent 15-hydroxyprostaglandin dehydrogenase (15-PGDH). We observed a marked decrease in expression of this enzyme in the patients with active UC, which may have contributed to the higher mucosal levels of PGE2 in that group.
In summary, this study has documented an increase in colonic mucosal synthesis of a proresolution mediator, PGD2, and expression of the receptor that mediates its antiinflammatory effects (DP1) specifically in a group of healthy individuals with a prior history of UC. It is possible that the elevated PGD2 synthesis contributes to the maintenance of remission in these individuals. These results are consistent with studies of rodents in which prolonged elevations in PGD2 synthesis were observed after resolution of colitis. The animal studies further showed that elevated PGD2 synthesis contributed not only to resolution of inflammation but also to long-term alterations in epithelial function, some of which may have contributed to an increased susceptibility to colon cancer. It remains to be determined if the elevated PGD2 synthesis observed in healthy individuals who had been in remission from UC similarly contributes to the known increase in incidence of colonic cancer in this group of patients.
Methods
Patients and Tissue Samples.
Colonic mucosal biopsy samples were obtained during diagnostic colonoscopy of patients from two broad groups: healthy individuals who underwent colonoscopy for routine colon-cancer screening and individuals with UC. Each of these groups had subgroups. In the case of the healthy individuals, some had no history of UC (control group; n = 12 females and 4 males; mean age = 51 ± 9 y), whereas others had been diagnosed previously with UC but had not experienced any bout of disease or required any medication for UC for at least 4 y (prior history of UC group; n = 6 females; mean age = 47 ± 11 y). The patients with UC were subdivided into three groups: those who had active disease (n = 5 males and 3 females; mean age = 43 ± 16 y) and those who were in clinical and endoscopic remission while on maintenance therapy with either oral/topical 5-aminosalicylic acid (n = 4 males and 5 females; age range = 44 ± 13 y) or immunosuppressive/biologic therapy (n = 4 males and 1 female; mean age = 40 ± 11 y). Details regarding patient characteristics, such as gender, age, and clinical activity, were obtained from medical records. Mucosal biopsies were taken from the left colon in close proximity to biopsies used for assessment of histopathology. Samples intended for quantitative PCR or PGD2 measurement were stored at −80 °C until ready for use. Samples intended for PGE2 measurement were kept on ice (4 °C) before processing, whereas those used for immunohistochemical analysis were fixed in 10% neutral-buffered formalin. The measurements of PGD2 and PGE2 were performed as described previously (14, 28).
This study was approved by the Ethics Committee at the University of Calgary. Each patient gave their written consent before participation in this study, and all experiments were conducted according to the principles expressed in the Declaration of Helsinki.
Quantitative PCR.
Total RNA from colonic biopsies was extracted using the RNeasy Mini Kit (Qiagen) according to manufacturer's instructions. For gene-expression studies, two-step quantitative PCR was used, as described previously (29). Bioinformatically validated high-efficiency primer assays for human TNFα (NM_000594), IFNγ (NM_000619), COX-1 (NM_000962), COX-2 (NM_000963), DP1 (NM_000953), DP2 (NM_004778), hPGDS (NM_014485), 15-PGDH (NM_000860), and β-actin (NM_001101) were obtained from Qiagen. All data were analyzed using cycle threshold values obtained from Realplex software (Eppendorf), and amplification and relative quantification of gene products were determined by normalizing target genes against the housekeeping gene β-actin.
Histology and Immunohistochemistry.
Biopsies from four patients from each group were used for histological and immunohistochemical examination. For histology, 5-μm-thick serial sections were processed by routine methods and stained with H&E. For immunohistochemistry, the sections were deparaffinized in xylene, rehydrated in graded concentrations of ethanol, and then incubated in 3% H2O2 for 15 min to block endogenous peroxidase activity. The antigen was exposed by steaming the sections for 30 min in 10 mM trisodium citrate buffer (pH 6.0)/0.05% Triton X-100. Sections were incubated with either polyclonal anti–COX-2 (1:500 dilution; Cayman Chemical) or monoclonal anti-DP1 (1:1,000 dilution; Cayman Chemical) antibodies overnight at 4 °C. The bound antibody was visualized by avidin-biotin-peroxidase detection using the Vectastain Elite ABC kit (Vector Laboratories), according to the manufacturer's instructions. 3–3′ diaminobenzidine (DAB; Vector Laboratories) was used as the chromagen.
Statistical Analysis.
Data are presented as mean ± SEM. Comparisons among groups of data were made using a one-way ANOVA followed by the Kruskal–Wallis test. An associated probability (P value of less than 5%) was considered significant.
Acknowledgments
We thank Ida Rabbani-Nejad of the Intestinal Tissue Bank at the University of Calgary for her assistance with these studies. This research was supported by a grant-in-aid from the Crohn's and Colitis Foundation of Canada (to J.G.P.F. and J.L.W.) and a grant from the Canadian Institutes of Health Research (to J.L.W).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 2.Langholz E, Munkholm P, Davidsen M, Nielsen OH, Binder V. Changes in extent of ulcerative colitis: A study on the course and prognostic factors. Scand J Gastroenterol. 1996;31:260–266. doi: 10.3109/00365529609004876. [DOI] [PubMed] [Google Scholar]
- 3.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: Signals in resolution. Nat Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 4.Serhan CN, et al. Resolution of inflammation: State of the art, definitions and terms. FASEB J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spite M, et al. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murakami M, Kudo I. Prostaglandin E synthase: A novel drug target for inflammation and cancer. Curr Pharm Des. 2006;12:943–954. doi: 10.2174/138161206776055912. [DOI] [PubMed] [Google Scholar]
- 7.Ajuebor MN, Singh A, Wallace JL. Cyclooxygenase-2-derived prostaglandin D(2) is an early anti-inflammatory signal in experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G238–G244. doi: 10.1152/ajpgi.2000.279.1.G238. [DOI] [PubMed] [Google Scholar]
- 8.Gilroy DW, et al. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 9.Rajakariar R, et al. Hematopoietic prostaglandin D2 synthase controls the onset and resolution of acute inflammation through PGD2 and 15-deoxyDelta12 14 PGJ2. Proc Natl Acad Sci USA. 2007;104:20979–20984. doi: 10.1073/pnas.0707394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris DW, Smith PR, Swan CH. Determination of prostaglandin synthetase activity in rectal biopsy material and its significance in colonic disease. Gut. 1978;19:875–877. doi: 10.1136/gut.19.10.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharon P, Ligumsky M, Rachmilewitz D, Zor U. Role of prostaglandins in ulcerative colitis. Enhanced production during active disease and inhibition by sulfasalazine. Gastroenterology. 1978;75:638–640. [PubMed] [Google Scholar]
- 12.McCartney SA, Mitchell JA, Fairclough PD, Farthing MJ, Warner TD. Selective COX-2 inhibitors and human inflammatory bowel disease. Aliment Pharmacol Ther. 1999;13:1115–1117. doi: 10.1046/j.1365-2036.1999.00585.x. [DOI] [PubMed] [Google Scholar]
- 13.Reuter BK, Asfaha S, Buret A, Sharkey KA, Wallace JL. Exacerbation of inflammation-associated colonic injury in rat through inhibition of cyclooxygenase-2. J Clin Invest. 1996;98:2076–2085. doi: 10.1172/JCI119013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zamuner SR, Warrier N, Buret AG, MacNaughton WK, Wallace JL. Cyclooxygenase 2 mediates post-inflammatory colonic secretory and barrier dysfunction. Gut. 2003;52:1714–1720. doi: 10.1136/gut.52.12.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zamuner SR, Bak AW, Devchand PR, Wallace JL. Predisposition to colorectal cancer in rats with resolved colitis: Role of cyclooxygenase-2-derived prostaglandin d2. Am J Pathol. 2005;167:1293–1300. doi: 10.1016/S0002-9440(10)61216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perretti M, et al. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nat Med. 2002;8:1296–1302. doi: 10.1038/nm786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arita M, et al. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci USA. 2005;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiorucci S, et al. A beta-oxidation-resistant lipoxin A4 analog treats hapten-induced colitis by attenuating inflammation and immune dysfunction. Proc Natl Acad Sci USA. 2004;101:15736–15741. doi: 10.1073/pnas.0404722101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vergnolle N, et al. Annexin 1 is secreted in situ during ulcerative colitis in humans. Inflamm Bowel Dis. 2004;10:584–592. doi: 10.1097/00054725-200409000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Martin GR, Perretti M, Flower RJ, Wallace JL. Annexin-1 modulates repair of gastric mucosal injury. Am J Physiol Gastrointest Liver Physiol. 2008;294:G764–G769. doi: 10.1152/ajpgi.00531.2007. [DOI] [PubMed] [Google Scholar]
- 21.Hammad H, et al. Activation of the D prostanoid 1 receptor suppresses asthma by modulation of lung dendritic cell function and induction of regulatory T cells. J Exp Med. 2007;204:357–367. doi: 10.1084/jem.20061196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujitani Y, et al. Pronounced eosinophilic lung inflammation and Th2 cytokine release in human lipocalin-type prostaglandin D synthase transgenic mice. J Immunol. 2002;168:443–449. doi: 10.4049/jimmunol.168.1.443. [DOI] [PubMed] [Google Scholar]
- 23.Kim N, Luster AD. Regulation of immune cells by eicosanoid receptors. ScientificWorldJournal. 2007;7:1307–1328. doi: 10.1100/tsw.2007.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clay CE, et al. 15-deoxy-Δ(12,14)PGJ(2) induces diverse biological responses via PPARgamma activation in cancer cells. Prostaglandins Other Lipid Mediat. 2000;62:23–32. doi: 10.1016/s0090-6980(00)00073-3. [DOI] [PubMed] [Google Scholar]
- 25.MacDonald TT, Hutchings P, Choy MY, Murch S, Cooke A. Tumour necrosis factor-alpha and interferon-gamma production measured at the single cell level in normal and inflamed human intestine. Clin Exp Immunol. 1990;81:301–305. doi: 10.1111/j.1365-2249.1990.tb03334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahida YR, Wu K, Jewell DP. Enhanced production of interleukin 1-β by mononuclear cells isolated from mucosa with active ulcerative colitis of Crohn's disease. Gut. 1989;30:835–838. doi: 10.1136/gut.30.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minami T, Tojo H, Shinomura Y, Matsuzawa Y, Okamoto M. Increased group II phospholipase A2 in colonic mucosa of patients with Crohn's disease and ulcerative colitis. Gut. 1994;35:1593–1598. doi: 10.1136/gut.35.11.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fiorucci S, et al. Cyclooxygenase-2-derived lipoxin A4 increases gastric resistance to aspirin-induced damage. Gastroenterology. 2002;123:1598–1606. doi: 10.1053/gast.2002.36558. [DOI] [PubMed] [Google Scholar]
- 29.Chávez-Piña AE, et al. Lack of effects of acemetacin on signalling pathways for leukocyte adherence may explain its gastrointestinal safety. Br J Pharmacol. 2008;155:857–864. doi: 10.1038/bjp.2008.316. [DOI] [PMC free article] [PubMed] [Google Scholar]