Abstract
We report here an efficient method for targeted mutagenesis of Arabidopsis genes through regulated expression of zinc finger nucleases (ZFNs)—enzymes engineered to create DNA double-strand breaks at specific target loci. ZFNs recognizing the Arabidopsis ADH1 and TT4 genes were made by Oligomerized Pool ENgineering (OPEN)—a publicly available, selection-based platform that yields high quality zinc finger arrays. The ADH1 and TT4 ZFNs were placed under control of an estrogen-inducible promoter and introduced into Arabidopsis plants by floral-dip transformation. Primary transgenic Arabidopsis seedlings induced to express the ADH1 or TT4 ZFNs exhibited somatic mutation frequencies of 7% or 16%, respectively. The induced mutations were typically insertions or deletions (1–142 bp) that were localized at the ZFN cleavage site and likely derived from imprecise repair of chromosome breaks by nonhomologous end-joining. Mutations were transmitted to the next generation for 69% of primary transgenics expressing the ADH1 ZFNs and 33% of transgenics expressing the TT4 ZFNs. Furthermore, ≈20% of the mutant-producing plants were homozygous for mutations at ADH1 or TT4, indicating that both alleles were disrupted. ADH1 and TT4 were chosen as targets for this study because of their selectable or screenable phenotypes (adh1, allyl alcohol resistance; tt4, lack of anthocyanins in the seed coat). However, the high frequency of observed ZFN-induced mutagenesis suggests that targeted mutations can readily be recovered by simply screening progeny of primary transgenic plants by PCR and DNA sequencing. Taken together, our results suggest that it should now be possible to obtain mutations in any Arabidopsis target gene regardless of its mutant phenotype.
Keywords: nonhomologous end-joining, gene knockout, alcohol dehydrogenase, chalcone synthase
In model organisms such as yeast and mice, targeted mutagenesis is a powerful tool for generating specific DNA sequence alterations that enable a greater understanding of gene function (1–3). Because of its potential for advancing basic plant science, an efficient method for targeted mutagenesis has been a long-sought-after goal of plant biology. Targeted mutagenesis also holds great promise for crop improvement. Precise methods for genome modification will be necessary if crop plants are to be fully harnessed to meet the burgeoning food, fiber, and fuel needs of an expanding world population.
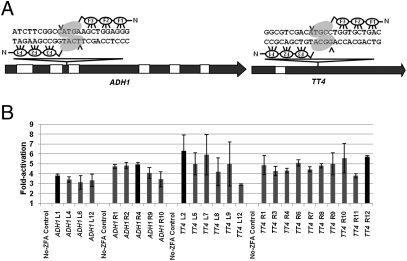
Engineered zinc finger nucleases (ZFNs) are powerful reagents for targeted genome modification (4). ZFNs function as dimers with each monomer composed of an engineered zinc finger array (ZFA), typically consisting of three or four fingers fused to a nonspecific cleavage domain of the FokI endonuclease (Fig. 1A). ZFAs can be engineered to bind diverse target DNA sequences within a genome of interest. Binding sites for the two ZFAs (each typically 18 or 24 bp in length) are separated by 5–7 bp, and this spacing allows the FokI monomers to dimerize and create a DNA double-strand break (DSB) in the spacer sequence between the half-sites.
Fig. 1.
OPEN ZFN target sites in the ADH1 and TT4 coding sequences. (A) The gene models of Arabidopsis ADH1 and TT4 loci are shown as block arrows. Black rectangles represent exons; white rectangles represent introns. The positions of OPEN ZFN target sites are indicated by triangles. Each engineered ZFA consists of three zinc fingers (F1, F2, F3), which together recognize a 9-bp target site. The two target sites are separated by 6-bp spacer sequences. Binding of the ZFAs to the target sequences enables the FokI nuclease monomers (indicated as gray ovals) to dimerize and cleave within the spacer. The sites of cleavage in the target sequences are indicated by black arrowheads. (B) ZFAs generated by OPEN were tested for their activity in bacterial two-hybrid (B2H) assays. The ZFAs were tested for their ability to bind target sequences in bacteria and activate transcription of a downstream lacZ reporter gene. Fold activation of each ZFA relative to its negative control, which does not express the ZFA, is plotted on the y axis. Multiple ZFAs generated by OPEN were tested for each of the ADH1 and TT4 left and right target sites. ZFAs with activities indicated by black bars were used in subsequent tests. Error bars denote SD; n = 3.
Chromosome breaks created by ZFNs are repaired predominantly by two DNA repair pathways—nonhomologous end-joining (NHEJ) and homologous recombination (HR) (5). Each pathway provides different opportunities for targeted mutagenesis. HR repairs chromosome breaks by copying information from homologous DNA templates; if a DNA template is delivered to cells along with the ZFN, sequence modifications in the template are introduced into the repaired chromosome to create the desired genetic variants. NHEJ also creates genetically useful sequence variation. Faithful repair by NHEJ restores DNA integrity by rejoining the broken chromosomes precisely; however, imprecise repair introduces small insertions/deletions at ZFN cleavage sites that frequently knock out gene function.
Arabidopsis thaliana is one of the premier models for plant biology, and an efficient targeted mutagenesis method would further enhance this plant's utility for experimental biology. Previous work has shown that ZFNs can generate mutations in Arabidopsis by NHEJ; for example, a well-characterized ZFN (QQR) was shown to create heritable mutations at an integrated, chromosomal reporter gene with a QQR recognition site (6). HR at a ZFN cleavage site in Arabidopsis was also reported (7). The HR experiments did not demonstrate alteration of an endogenous Arabidopsis gene, but also used a previously characterized ZFN and an integrated reporter gene with appropriate ZFN recognition sites. Further, the ZFNs used in this study (7) did not stimulate HR much above levels observed in the absence of a targeted chromosome break (i.e., 1 in 3,000 transformed plants) (8). Both studies are important because they demonstrate that ZFNs can be used to modify sequences in Arabidopsis by NHEJ and HR. They also identify the challenges in implementing efficient ZFN-induced mutagenesis in this species: (i) robust ZFN engineering methods are required that enable endogenous Arabidopsis sequences to be targeted, and (ii) efficient protocols are needed that allow recovery of germinal NHEJ- and HR-induced mutations at frequencies exceeding 10%, rates previously achieved with ZFNs in Drosophila (9), human cells (10), rat (11), zebrafish (12, 13), maize (14), and tobacco (15).
In this study, we report a highly efficient method for targeted mutagenesis of Arabidopsis genes by imprecise repair of ZFN-induced chromosome breaks by NHEJ. ZFNs that recognize the Arabidopsis ADH1 and TT4 genes were generated by using a publicly available platform (Oligomerized Pool ENgineering, OPEN) that is sufficiently robust to target most Arabidopsis genes (16, 17). To make our approach more accessible, we developed a web-based genome browser that identifies potential OPEN ZFN target sites in the Arabidopsis genome. Primary transgenic Arabidopsis plants expressing OPEN-derived ZFNs early in development generated loss-of-function mutations in the next generation at frequencies ranging from 33 to 69% for TT4 and ADH1, respectively. The efficiency of the mutagenesis indicates that OPEN ZFNs can be routinely and reliably used for targeted genetic modification of Arabidopsis.
Results
Engineering ZFAs That Recognize Endogenous Arabidopsis Genes.
We used OPEN (17) to generate ZFAs for potential target sites in two Arabidopsis genes: ALCOHOL DEHYDROGENASE1 (ADH1; AT1G77120) and TRANSPARENT TESTA4 (TT4; AT5G13930) (Fig. 1A). The web-based program, ZiFiT (18) was used to identify OPEN target sites within approximately the first one-third of the ORF to increase the likelihood that ZFN-induced mutations would result in loss of gene function. Four OPEN selections (one for each half-site of the two full ZFN target sites) were performed, and 12 resulting ZFAs for each half-site were tested for activity in a bacterial two-hybrid assay (17). Between four and nine ZFAs per half-site activated the expression of a lacZ reporter gene ≈3-fold or more over negative controls (Fig. 1B and Table S1). Previous studies have shown that activities >3-fold reliably identify ZFAs with a high probability of functioning as ZFNs in eukaryotic cells (16). A single ZFA with the highest activity for each half-site was chosen for subsequent tests.
Testing OPEN ZFNs in Yeast and Arabidopsis Protoplasts.
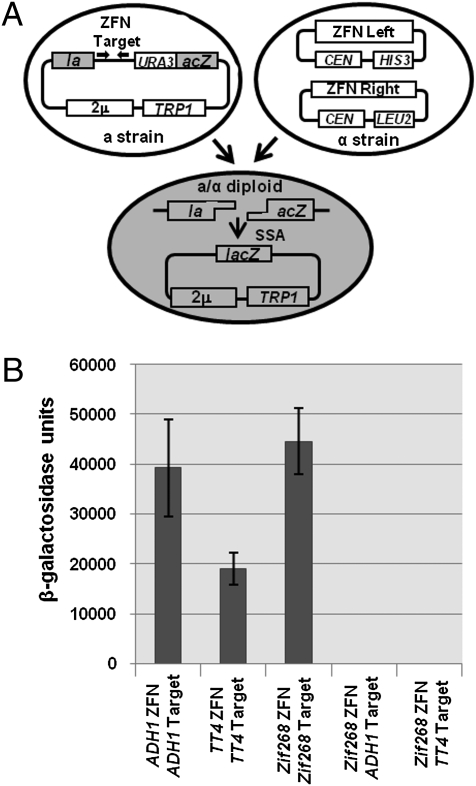
The ADH1 and TT4 ZFAs were fused to FokI and tested in a yeast-based assay for their ability to function as ZFNs. This assay uses a target plasmid and two ZFN expression plasmids that are brought together in the same cell by mating (Fig. 2A) (15). The target plasmid has a lacZ reporter gene with a 125-bp duplication of coding sequence. The duplication flanks a URA3 gene and a target site recognized by a given ZFN. A ZFN-induced DSB at the target site is repaired through single-strand annealing between the duplicated sequences, creating a functional lacZ gene and resulting in loss of URA3. Using this assay, the ZFAs targeting the ADH1 and TT4 genes were found to function effectively as ZFNs (Fig. 2B). LacZ activity resulting from cleavage by the ADH1 ZFN pair and subsequent recombination-based repair was comparable with activity observed for a control ZFN engineered from the well-characterized ZFA, Zif268 (19). Activity of the TT4 ZFN pair was ≈2-fold less than that of the Zif268 ZFN.
Fig. 2.
ZFN activity in yeast. (A) Schematic of the yeast-based ZFN activity assay. ZFN expression plasmids are introduced into a yeast strain of α mating-type; the reporter plasmid is transformed into a yeast strain of a mating type. Mating of the two strains brings together the ZFNs and the reporter plasmid into a diploid cell. This results in cleavage of the ZFN target sequence and restoration of the lacZ gene through single strand annealing (SSA). (B) Results of the yeast assay performed with the ADH1 and TT4 ZFNs. A ZFN derived from the well-characterized ZFA, Zif268, is included as the positive control. Negative controls are the ADH1 and TT4 target sites combined with the Zif268 ZFN. Error bars denote SD; n = 3.
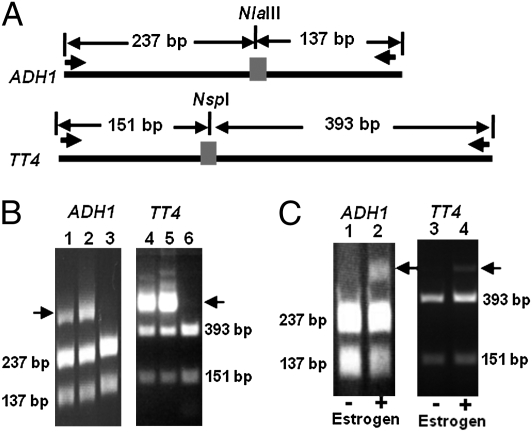
ZFN pairs were next tested for their ability to recognize and cleave native chromosomal sites in Arabidopsis protoplasts. ZFNs were cloned into expression vectors containing the 35S promoter, and the respective pairs of ADH1 and TT4 ZFNs were introduced into Arabidopsis protoplasts by PEG-mediated transformation (20). Cleavage activity was assessed at 24 or 48 h after transformation by the presence of target site mutations introduced through imprecise repair by NHEJ. To detect such mutations, we adopted a previously described PCR assay that preferentially amplifies mutated DNA sequences (6). In this assay, genomic DNAs extracted from transformed protoplasts are first digested with restriction enzymes (NlaIII for ADH1 and NspI for TT4), which occur within the ZFN cleavage site (Fig. 3A). Because the restriction sites are destroyed by most NHEJ-induced mutations, the mutated sequences are resistant to restriction digestion and are amplified preferentially in a subsequent round of PCR with primers flanking the target sites. When PCR products are further digested by the same restriction enzymes and subjected to gel electrophoresis, uncleaved sequences with mutations can be distinguished from cleaved wild-type sequences. Using this assay, mutated alleles were observed in DNA samples derived from cells transformed with either ADH1 or TT4 ZFN pairs (Fig. 3B).
Fig. 3.
Detection of ZFN-induced mutations in Arabidopsis protoplasts and somatic cells. (A) Schematic of the strategy used to amplify and identify mutations at the ZFN target sites (gray rectangle). Restriction enzyme sites coincident with the ZFN cleavage sites are NlaIII for ADH1 and NspI for TT4. Short black arrows indicate the positions of PCR primers relative to the target sites. (B) Restriction endonuclease assay to detect ZFN-induced mutations in Arabidopsis protoplasts. Mutations introduced by NHEJ are resistant to restriction enzyme digestion due to loss of restriction sites and result in uncleaved PCR products (indicated by arrows). Genomic DNA was cleaved with restriction enzymes before PCR amplification to enrich for ZFN-induced mutations. Lanes: 1, protoplast sample transformed with ADH1 ZFN after 24 h incubation; 2, protoplast sample transformed with ADH1 ZFN after 48 h of incubation; 3, control protoplast sample without transformation; 4, protoplast sample transformed with TT4 ZFN after 24 h of incubation; 5, protoplast sample transformed with TT4 ZFN after 48 h of incubation; 6, control protoplast sample without transformation; (C) Restriction endonuclease assay to detect ZFN-induced mutations in Arabidopsis seedlings after estrogen induction. DNA fragments lacking restriction sites (arrows) are detected in experimental samples with estrogen induction but not in control samples without estrogen. In this case, enrichment by prior restriction digestion of genomic DNA was not performed. Lanes: 1, ADH1 ZFN transgenic T1 plants without estrogen induction; 2, ADH1 ZFN transgenic T1 plants with estrogen induction; 3, TT4 ZFN transgenic T1 plants without estrogen induction; 4, TT4 ZFN transgenic T1 plants with estrogen induction.
ZFN-induced mutations in the uncleaved PCR products were verified and characterized by cloning and DNA sequencing. Forty-eight clones lacking NlaIII or NspI sites (24 clones each) were sequenced from samples transformed with either the ADH1 or TT4 ZFNs. DNA sequences of all clones contained mutations at cognate target sites. Eighteen of 24 clones from the ADH1 ZFN treatment and 16 of 24 clones from the TT4 treatment showed distinct mutations, indicating that they were independent events. Mutations included both insertions (from 1 to 58 bp) and deletions (from 2 to 53 bp). All insertions >1 bp consisted of DNA sequences that are also present in the ZFN expression vectors (Fig. S1); extrachromosomal plasmid DNA was observed to serve as filler DNA in the repair of DNA DSBs in tobacco cells (21). In conclusion, ZFN pairs generated by OPEN for Arabidopsis ADH1 and TT4 loci induce mutations at target sites in their native chromosomal context.
High Frequency ZFN-Induced Mutagenesis in Arabidopsis Somatic Tissues.
The ability of OPEN ZFNs to induce mutations was next tested in planta. ADH1 and TT4 ZFN pairs were introduced into wild-type Arabidopsis plants by the Agrobacterium floral dip method (22). An estrogen-inducible expression system was used to regulate production of the ZFNs (23, 24). After floral dip transformation, seeds (T0) were collected and germinated on Murashige–Skoog (MS) plates that contained hygromycin and 17β-estradiol. Ten days after germination, the primary transgenic plants (T1) were resistant to hygromycin, and the expression of ZFNs in these T1 plants should have been induced by the 17β-estradiol. Mutations at ADH1 and TT4 target sites were assessed by limited-cycle PCR using DNA prepared from pooled seedlings (in this case with no prior restriction digestion). The PCRs were digested with NlaIII or NspI, and the presence of undigested PCR products suggested that both the ADH1 and TT4 ZFNs induced mutations in the seedlings (Fig. 3C). No undigested PCR products were observed in uninduced plants. To confirm the mutagenesis and to estimate mutagenesis frequencies, the PCR products from induced transgenic plants were cloned and sequenced. In the T1 seedlings with the ADH1 ZFN, 16% (14/90) of the clones contained distinct mutations at the target site, whereas in the induced TT4 ZFN T1 seedlings, 7% (7/95) of the clones showed distinct target site mutations (Table 1). The identified mutations included insertions (1–2 bp), deletions (3–142 bp), and one case of a single nucleotide substitution derived from the TT4 ZFN (Fig. S2). Taken together, these results indicate that expression of OPEN ZFNs for both ADH1 and TT4 genes efficiently creates mutations at their target sites in Arabidopsis somatic tissues.
Table 1.
Frequencies of ZFN-induced mutations at ADH1 and TT4
| ZFN | Somatic mutation frequency, % | Frequency of mutant-producing T1 plants | Percent frame shift/in-frame mutant alleles, % |
| ADH1 ZFN | 16 | 69% (11/16) | 73/27 |
| TT4 ZFN | 7 | 33% (10/30) | 88/12 |
Cleavage of ZFNs at noncognate sites can generate unwanted background mutations (15, 25). To look for evidence of off-target cleavage, we examined DNA sequences in the Arabidopsis genome that were most similar to the target sites of the ADH1 and TT4 ZFNs. The sequence most similar to the ADH1 target site has one mismatch in the left half-site and two mismatches in the right half-site, whereas the sequence most similar to the TT4 target site has one mismatch in each half-site (Table S2). PCR amplification was carried out on the DNA samples used above to detect somatic mutations. No evidence of ZFN-induced mutations was detected by sequencing 96 clones derived from PCR products that span the potential off-target sequences. The observed preference of OPEN ZFNs for their intended targets indicates that these reagents have a high degree of specificity in vivo.
Efficient Germinal Transmission of ZFN-Induced Mutations.
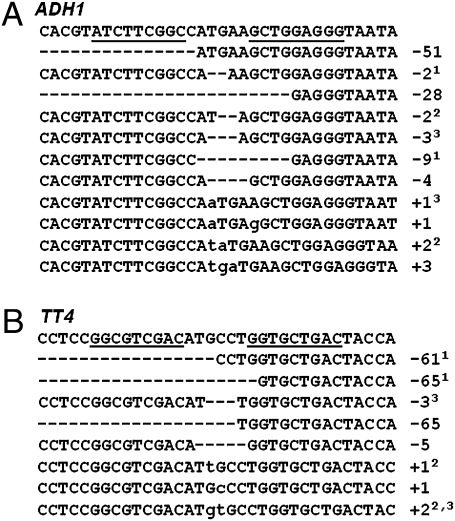
Mutations occurring early in development in the L2 cells of the shoot apical meristem are likely to be transmitted to the next generation (26). To determine whether ZFN-induced mutations at ADH1 could be transmitted germinally, 16 T1 plants exposed to estrogen during germination were grown to maturity in soil, and seeds were collected from individual lines. Fifty seeds from each line were then subjected to allyl alcohol treatment. A functional ADH1 gene product converts allyl alcohol to highly toxic acrylaldehyde, so wild-type ADH1 seeds will die after treatment, whereas mutant adh1 seeds will survive (Fig. S3A) (27). As shown in Table 1, 69% (11 of 16) of the induced T1 plants give rise to adh1 mutants. Nine of the 11 plants segregated mutant and wild-type phenotypes in their progeny (Table 1). Two plants (18%) produced exclusively adh1 mutants, indicating that both ADH1 alleles were disrupted (i.e., biallelic modification). The mutations likely occurred early in development and gave rise to homozygous mutant floral tissue. Sequence of the ADH1 target site in all mutant plants uncovered 11 distinct mutations, all of which were insertions/deletions at the ZFN recognition site (Fig. 4A). Furthermore, three mutant plants, including the plant with exclusively adh1 progeny, gave rise to distinct mutation types (Fig. 4A), providing molecular evidence that independent mutations can occur in both alleles of the ADH1 gene.
Fig. 4.
Sequences of germinally transmitted mutations induced by ZFNs. For each target gene, the wild-type sequence is shown at the top with the ZFN recognition sites underlined. The recovered mutant alleles are shown below the wild-type sequence. Deletions are indicated by dashed lines, and insertions are indicated by lowercase letters. (A) Germinal mutations at ADH1. The numbers in superscript (i.e., 1, 2 and 3) identify mutations derived from individual T1 plants. In many cases, a given plant produced multiple independent mutations. For example, plant 1 produced exclusively adh1 double knock-out (i.e., biallelic) mutant progeny, and the two distinct mutant alleles are indicated by the superscript 1. (B) Germinal mutations at TT4. The numbers in superscript identify T1 plants that contain multiple independent mutations at both alleles of TT4.
The germinal transmission frequency of ZFN-induced mutations at TT4 was determined by scoring progeny derived from 30 estrogen-exposed T1 transgenic plants. Disruption of TT4 results in colorless seed coats (28), but because the seed coat is derived from maternal tissues, only homozygous mutant flowers, in which both alleles sustained an independent mutation, give rise to colorless seeds (Fig. S3B). We observed that seven of 30 T1 plants produced colorless seeds (≈20–30 mutant seeds of 1,000), indicating that those plants contained clones of cells with both TT4 alleles disrupted. Biallelic disruption was corroborated by PCR amplification and DNA sequencing of the ZFN cleavage site in the T2 plants derived from the homozygous mutant seeds (Fig. 4B). In addition, as shown in Fig. 4B, three of seven mutant-producing T1 plants contained independent mutations in both TT4 alleles. Because monoallelic gene disruption cannot be scored by the seed coat color phenotype, T2 progeny derived from the remaining T1 plants were subjected to a PCR screen. PCR amplification was carried out on genomic DNA made from 20 pooled seedlings derived from each line. Digestion with NspI identified an additional three T1 plants that produced heritable tt4 mutant DNA sequences. In agreement with the molecular analyses, we also observed that the T2 progeny of these three T1 plants segregated tt4 homozygous mutants, which lack purple anthocyanin pigmentation in the cotyledon and hypocotyl (29). The mutations in these T2 plants were verified by DNA sequencing (Fig. 4B). Taken together, the germinal mutagenesis frequency for TT4 was estimated to be 33% (10/30; Table 1).
Web-Based Tool To Identify ZFN Target Sites in the Arabidopsis Genome.
To aid plant biologists in performing ZFN-mediated mutagenesis of Arabidopsis, ZiFiT was used to identify all potential OPEN ZFN target sites in the Arabidopsis genome (18). In total, 381,497 ZFN target sites were identified in 76% (30,193 of 39,640) of Arabidopsis gene models (TAIR9 release). Of these sites, 171,409 sites are located in protein coding regions (mean of ≈4.3 sites per gene model). A web-based genome browser, ZFNGenome, was implemented to display OPEN ZFN sites along with gene annotations (Fig. S4). ZFNGenome is freely available and can be accessed at http://bindr.gdcb.iastate.edu:8888/ZFNGenome/Arabidopsis/.
Discussion
We report here a highly efficient method for targeted mutagenesis of Arabidopsis genes. ZFNs were used to create chromosome breaks at specific, endogenous loci, and the cleaved chromosomes were repaired imprecisely by nonhomologous end-joining to yield small DNA insertions or deletions that disrupted gene function. Previous reports on the use of ZFNs for targeted mutagenesis in Arabidopsis used ZFNs derived from ZFAs that do not recognize endogenous Arabidopsis sequences (6, 7). Rather, reporter constructs with the appropriate ZFN recognition sites were first introduced into the Arabidopsis genome, and these integrated reporters were then used to measure targeted mutagenesis with the corresponding ZFN. We believe the key to making endogenous sequences readily amenable to ZFN-induced modification was the development of the OPEN platform for ZFN engineering (16). To date, OPEN has been used to engineer ZFNs that recognize 16 different target sequences in the human (16, 30), zebrafish (31), tobacco (15), and now Arabidopsis genomes. Further, as its name implies, OPEN is an open-source platform, and we anticipate that its continued practice will lead to improvements that streamline this approach to ZFN engineering, thereby making it even more accessible to plant biologists.
To aid Arabidopsis researchers in using OPEN, we developed a genome browser (ZFNGenome) that displays all possible OPEN ZFN target sites along with gene model annotations. The density of ZFN sites in a target sequence is determined by the reagents available for OPEN selections, which enable engineering of ZFAs composed of all GNN and some TNN subsites (16). Even with available reagents, OPEN ZFN sites are present in more than three-quarters of the Arabidopsis coding sequences, and the average gene has more than four different OPEN ZFN sites. As additional reagents for OPEN selections are developed, the number of sites that can be targeted will grow considerably. For example, availability of reagents for ANN subsites would enable 130 OPEN ZFNs to be generated per kb of DNA (formula for calculation in ref. 16).
In addition to the use of OPEN, a second key to the success of our targeted mutagenesis strategy was our approach to limit ZFN toxicity. Toxicity has been reported in human cells, zebrafish, and Drosophila and is likely due to activity of ZFNs at off-target sites (12, 13, 31–33). Reducing toxicity in these systems was achieved by increasing ZFN specificity (e.g., by using OPEN) and/or by modifying the FokI nuclease domain to make it function as an obligate heterodimer (16, 34, 35). This latter approach ensures that DNA is cleaved only when ZFNs for the left and right half-sites are positioned at the appropriate target sequence on the chromosome. Limited expression of ZFNs either by transient DNA delivery, injected RNA, or inducible expression systems has also been suggested as a strategy to reduce undesired toxic effects (32, 33, 36).
Toxicity was of particular concern in our targeted mutagenesis strategy, because in contrast to most mutagenesis approaches with ZFNs, we created transgenic Arabidopsis plants that have the ZFN expression construct stably integrated. Transgenic plants were also created in the first report of the use of a ZFN in Arabidopsis (6), and transgenic Drosophila were generated in initial reports of targeted mutagenesis in this species (9). In both the initial Drosophila and Arabidopsis studies, heat shock-inducible expression systems were used to control ZFN expression (6, 33). A second, recent report of a ZFN stably integrated into the Arabidopsis genome did not describe toxicity when the ZFN was expressed constitutively (7); however, in our experiments, we did not recover Arabidopsis transformants with certain ZFNs, suggesting that their expression was deleterious. To minimize toxicity, we used the obligate heterodimeric form of the FokI nuclease in all in planta experiments (34). Further, we chose an estrogen-inducible system (23) to regulate expression because (i) relative to heat shock, estrogen-induced expression is more tightly controlled with no background expression observed (37); (ii) induction with 17β-estradiol is rapid (i.e., within 30 min) and expression reaches levels 8-fold higher than those attained with the constitutive 35S promoter (23); and (iii) 17β-estradiol does not affect Arabidopsis, whereas heat pulses can be stressful to young seedlings and flowers (38). These features allowed us to induce ZFN expression at early developmental stages (i.e., during seed germination) and then turn off expression to reduce potential toxicity associated with prolonged exposure of the Arabidopsis genome to ZFNs.
The estrogen-inducible system proved highly effective, allowing us to recover mutations in seeds generated from 33 to 69% of the primary transgenic plants. Using a heat shock promoter to drive expression of the QQR ZFN, Lloyd et al. (6) reported mutations in progeny of 10% of the heat-induced T1 plants. We initially tested a similar protocol of heat-induction by using the ADH1 and TT4 ZFNs under control of an Arabidopsis heat shock promoter; however, somatic ZFN-induced mutations could be detected only if they were enriched before PCR by first cleaving the genomic DNA with the restriction enzyme that recognizes the spacer sequence between the two ZFN half-sites. Furthermore, no germinal events were recovered in the progeny of >200 T1 plants that were exposed to the heat shock regime. Although we cannot explain why our attempt with heat induction failed, we believe the very high burst of ZFN expression afforded by estrogen induction was critical to success. The resulting efficiency of mutagenesis made it possible to look at small populations of T1 plants (<30) and, therefore, it should be possible to recover mutations in genes without observable phenotypes by simply screening progeny of primary transgenic plants by PCR and DNA sequencing.
Although all T1 plants generated in this study by using OPEN ZFN constructs looked normal and healthy, there is still a possibility of off-target cleavage, which has been reported and could create unwanted secondary mutations (15, 25). Off-target cleavage is consistent with our observation that stable transgenics could not be recovered with some constitutively expressed ZFNs. In this study, examination of DNA sequences in the Arabidopsis genome most similar to the target sites of ADH1 and TT4 ZFNs provided no evidence of off-target cleavage. Although we cannot rule out off-target activity in such a small survey, any unlinked off-target mutations that do arise with the use of ZFNs can be removed by a simple backcross, which is standard practice in other mutagenesis protocols with Arabidopsis.
The spectrum of mutations generated with ZFNs through imprecise nonhomologous end-joining offers much opportunity for genetic analysis. The independent alleles generated by ZFNs are not limited to null mutants that cause frame shifts in the coding sequences, but some are alleles with in-frame insertions/deletions (i.e., the size of the insertion/deletion is a multiple of 3). For instance, three adh1 alleles and one tt4 allele identified in this study showed in-frame deletions or insertions (Fig. 4). Such a wide spectrum of allelic variants will provide valuable opportunities to better understand gene function.
The Arabidopsis community is fortunate to have large collections of mutants generated by T-DNA. However, in one of the largest T-DNA mutant collections generated at the Salk Institute (39), ≈20% of genes do not have T-DNA insertions and many T-DNA insertions do not provide null phenotypes. Furthermore, 17% of the genes in Arabidopsis exist as tandem arrays, so even when two T-DNA lines exist with insertions in each member of a tandem array, it is very difficult to recover double mutants by genetic recombination, because the two genes are so tightly linked (40). ZFNs could make this fraction of the genome amenable to genetic analysis. For example, a recent report (41) suggests it is possible to use ZFNs to create large deletions of genomic DNA at high efficiencies. In summary, we believe targeted mutagenesis with ZFNs is now practical in Arabidopsis and is a valuable addition to the suite of genetic tools available for this important plant model.
Materials and Methods
OPEN Selections and ZFN Expression Vectors.
OPEN selections and the bacterial two-hybrid assay were conducted as described (Fig. S5) (16, 17). The resulting ZFAs were PCR amplified from phagemids (primers OK.1677 and OK.1678 (31); Table S3), digested with XbaI and BamHI, and cloned into yeast expression vectors pCP3 and pCP4, which have HIS3 and LEU2 marker genes, respectively. Each vector also encodes a homodimeric FokI nuclease domain with the SV40 nuclear localization signal (NLS); expression is driven by the yeast TEF1 promoter. The ADH1 and TT4 ZFAs for the left half-sites were cloned into pCP3 (generating pFZ9 and pFZ11, respectively); ZFAs for the right half-sites were cloned into pCP4 (generating pFZ10 and pFZ12, respectively). The plant expression vectors, pFZ14 and pFZ15, were used for the protoplast assays and encode left and right obligate heterodimeric FokI domains (34) with the SV40 NLS; expression is driven by the 35S promoter. ZFAs for the left half-sites were cloned into pFZ14 (generating pJH1, ADH1; pJH9, TT4); ZFAs for right half-sites were cloned into pFZ15 (generating pJH2, ADH1; pJH10, TT4).
An estrogen-inducible T-DNA expression vector, pMDC7 (23, 24), was used to generate transgenic Arabidopsis plants. As a Gateway-compatible destination vector, pMDC7 can recombine with an entry vector that contains ZFAs fused with FokI nuclease. The ADH1 and TT4 ZFN entry vectors encode the left and right ZFNs. Between the ZFN coding sequences is an in-frame 18-amino acid ribosome skipping signal (T2A), which allows translation of multiple ORFs from a single transcript (42). The final ADH1 and TT4 ZFN expression vectors (pFZ62 and pFZ63, respectively) were made by recombining the entry vectors with pMDC7 (Fig. S6).
Testing ZFNs in Yeast and Plants.
The yeast assay and the assay for testing ZFN activity in protoplasts were carried out as described (15, 20). To generate stably transformed Arabidopsis lines, the T-DNA expression vectors pFZ62 and pFZ63 were transformed into Agrobacterium strain GV3101 (43). Floral-dip transformation was conducted by using ecotype Columbia (22). Transformed seeds were plated onto solid Murashige and Skoog (MS) medium containing 25 mg/L hygromycin to select for transformants and 10 μM 17β-estadiol to induce expression of ZFNs. The seedlings were grown under a regime of 16 h light/8 h dark at 21 °C in a growth chamber. To evaluate somatic mutations, genomic DNA was prepared from 10 7-day-old T1 seedlings. High fidelity PCR was then performed to amplify target sites (primers oFZ1–oFZ4; Table S3). PCR products were TOPO cloned (Invitrogen), and 96 clones were randomly selected for DNA sequencing.
To evaluate germinal mutations, hygromycin resistant seedlings (T1) were transferred to soil at 10 days and grown to maturity. To screen for adh1 mutants, seeds were collected from 16 transgenic plants carrying the ADH1 ZFN constructs. Approximately 50 seeds from each T1 plant were treated with allyl alcohol (27) and grown on MS medium plates. Homozygous adh1 mutants typically survive allyl alcohol treatment, and genomic DNA from eight surviving sibling plants were pooled and subjected to high fidelity PCR (primers, oFZ1 and oFZ2; Table S3). PCR products were cloned and sequenced as described above.
The tt4 mutants were identified by screening 30 T1 transgenic plants carrying TT4 ZFN constructs to identify those that produced seeds with colorless seed coats. For those plants producing putative tt4 mutants, DNA was prepared from eight mutant seedlings and genotyped by using high fidelity PCR (primers, oFZ3 and oFZ4; Table S3). PCR products were cloned and sequenced as described above. For plants that did not show mutant phenotypes, DNA was prepared from ≈20 seedlings, and the ZFN cleavage site was amplified by PCR. PCR products were subjected to NspI digestion; mutant sequences can be readily identified as being resistant to restriction enzyme digestion. The undigested DNA molecules were TOPO cloned and sequenced.
Sequences similar to the target sites of ADH1 and TT4 ZFNs were identifed by using a web-based pattern search tool (44). Two sequences (one for each target site) most similar to the target sites were chosen for subsequent genotyping. The region encompassing the candidate cleavage site was PCR amplified (primers oFZ5–oFZ8; Table S3), and the products were TOPO cloned. DNA sequences were obtained from 96 independent clones from each site.
ZFNGenome.
Arabidopsis chromosomal contigs were scanned for potential OPEN target sites by using a variation of the ZiFiT algorithm (18). Potential target sites were limited to those for which ZFNs can be engineered by using currently available OPEN reagents (17). Only protein coding regions were searched by using gene models from TAIR9 release (45). Spacers between the two half-sites of 5–7 nts were considered. Sites that contain potential dam or dcm methylation sites were excluded, as were sites without a GNN subsite, because previous studies have shown that successful OPEN sites have at least one GNN (17). A web browser (ZFNGenome) was implemented to display all potential OPEN sites along with gene annotations. The browser used a Berkeley DB backend (46) linked to a Generic Genome Browser (GBrowse) frontend via open source adaptors from BioPerl (47). ZFN target sites were included along with default features, such as “loci” and “gene models.” Each ZFN target site is hyperlinked to ZiFDB and ZiFiT (18, 48).
Supplementary Material
Acknowledgments
We thank David Wright for assistance in ZFN design, Yi Hou for help with the Arabidopsis protoplast assay, and Kenichi Tsuda for help with plasmid construction. The National Science Foundation supported work carried out by F.Z., J.P.H., M.C., and D.F.V. (MCB 0209818) and D.R. and D.D. (DBI 0923827). M.L.M. and J.K.J. are supported by National Institutes of Health Grants R01 GM069906, R24 GM078369, and R21 RR024189 and the Massachusetts General Hospital Pathology Service.
Footnotes
The authors declare no conflict of interest.
See commentary on page 11657.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0914991107/-/DCSupplemental.
References
- 1.Doetschman T, et al. Targetted correction of a mutant HPRT gene in mouse embryonic stem cells. Nature. 1987;330:576–578. doi: 10.1038/330576a0. [DOI] [PubMed] [Google Scholar]
- 2.Rothstein RJ. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- 3.Thomas KR, Capecchi MR. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987;51:503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- 4.Cathomen T, Joung JK. Zinc-finger nucleases: The next generation emerges. Mol Ther. 2008;16:1200–1207. doi: 10.1038/mt.2008.114. [DOI] [PubMed] [Google Scholar]
- 5.Wyman C, Kanaar R. DNA double-strand break repair: All's well that ends well. Annu Rev Genet. 2006;40:363–383. doi: 10.1146/annurev.genet.40.110405.090451. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd A, Plaisier CL, Carroll D, Drews GN. Targeted mutagenesis using zinc-finger nucleases in Arabidopsis. Proc Natl Acad Sci USA. 2005;102:2232–2237. doi: 10.1073/pnas.0409339102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Pater S, Neuteboom LW, Pinas JE, Hooykaas PJ, van der Zaal BJ. ZFN-induced mutagenesis and gene-targeting in Arabidopsis through Agrobacterium-mediated floral dip transformation. Plant Biotechnol J. 2009;7:821–835. doi: 10.1111/j.1467-7652.2009.00446.x. [DOI] [PubMed] [Google Scholar]
- 8.Britt AB, May GD. Re-engineering plant gene targeting. Trends Plant Sci. 2003;8:90–95. doi: 10.1016/S1360-1385(03)00002-5. [DOI] [PubMed] [Google Scholar]
- 9.Bibikova M, Beumer K, Trautman JK, Carroll D. Enhancing gene targeting with designed zinc finger nucleases. Science. 2003;300:764. doi: 10.1126/science.1079512. [DOI] [PubMed] [Google Scholar]
- 10.Urnov FD, et al. Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature. 2005;435:646–651. doi: 10.1038/nature03556. [DOI] [PubMed] [Google Scholar]
- 11.Geurts AM, et al. Knockout rats via embryo microinjection of zinc-finger nucleases. Science. 2009;325:433. doi: 10.1126/science.1172447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doyon Y, et al. Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat Biotechnol. 2008;26:702–708. doi: 10.1038/nbt1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meng X, Noyes MB, Zhu LJ, Lawson ND, Wolfe SA. Targeted gene inactivation in zebrafish using engineered zinc-finger nucleases. Nat Biotechnol. 2008;26:695–701. doi: 10.1038/nbt1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shukla VK, et al. Precise genome modification in the crop species Zea mays using zinc-finger nucleases. Nature. 2009;459:437–441. doi: 10.1038/nature07992. [DOI] [PubMed] [Google Scholar]
- 15.Townsend JA, et al. High-frequency modification of plant genes using engineered zinc-finger nucleases. Nature. 2009;459:442–445. doi: 10.1038/nature07845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maeder ML, et al. Rapid “open-source” engineering of customized zinc-finger nucleases for highly efficient gene modification. Mol Cell. 2008;31:294–301. doi: 10.1016/j.molcel.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maeder ML, Thibodeau-Beganny S, Sander JD, Voytas DF, Joung JK. Oligomerized pool engineering (OPEN): an 'open-source' protocol for making customized zinc-finger arrays. Nat Protoc. 2009;4:1471–1501. doi: 10.1038/nprot.2009.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sander JD, Zaback P, Joung JK, Voytas DF, Dobbs D. Zinc Finger Targeter (ZiFiT): An engineered zinc finger/target site design tool. Nucleic Acids Res. 2007;35:W599–W605. doi: 10.1093/nar/gkm349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pavletich NP, Pabo CO. Zinc finger-DNA recognition: Crystal structure of a Zif268-DNA complex at 2.1 A. Science. 1991;252:809–817. doi: 10.1126/science.2028256. [DOI] [PubMed] [Google Scholar]
- 20.Hoshaw JP, Unger-Wallace E, Zhang F, Voytas DF. A transient assay for monitoring zinc-finger nuclease activity at endogenous plant gene targets. Methods Mol Biol. 2010 doi: 10.1007/978-1-60761-753-2_19. in press. [DOI] [PubMed] [Google Scholar]
- 21.Gorbunova V, Levy AA. Non-homologous DNA end joining in plant cells is associated with deletions and filler DNA insertions. Nucleic Acids Res. 1997;25:4650–4657. doi: 10.1093/nar/25.22.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clough SJ, Bent AF. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 23.Zuo J, Niu QW, Chua NH. Technical advance: An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J. 2000;24:265–273. doi: 10.1046/j.1365-313x.2000.00868.x. [DOI] [PubMed] [Google Scholar]
- 24.Curtis MD, Grossniklaus U. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 2003;133:462–469. doi: 10.1104/pp.103.027979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez EE, et al. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat Biotechnol. 2008;26:808–816. doi: 10.1038/nbt1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irish VF, Jenik PD. Cell lineage, cell signaling and the control of plant morphogenesis. Curr Opin Genet Dev. 2001;11:424–430. doi: 10.1016/s0959-437x(00)00213-6. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs M, Dolferus R, Van den Bossche D. Isolation and biochemical analysis of ethyl methanesulfonate-induced alcohol dehydrogenase null mutants of arabidopsis thaliana (L.) Heynh. Biochem Genet. 1988;26:105–122. doi: 10.1007/BF00555492. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Ou-Lee TM, Raba R, Amundson RG, Last RL. Arabidopsis flavonoid mutants are hypersensitive to UV-B irradiation. Plant Cell. 1993;5:171–179. doi: 10.1105/tpc.5.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shirley BW, et al. Analysis of Arabidopsis mutants deficient in flavonoid biosynthesis. Plant J. 1995;8:659–671. doi: 10.1046/j.1365-313x.1995.08050659.x. [DOI] [PubMed] [Google Scholar]
- 30.Zou J, et al. Gene targeting of a disease-related gene in human induced pluripotent stem and embryonic stem cells. Cell Stem Cell. 2009;5:97–110. doi: 10.1016/j.stem.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foley JE, et al. Rapid mutation of endogenous zebrafish genes using zinc finger nucleases made by Oligomerized Pool ENgineering (OPEN) PLoS One. 2009;4:e4348. doi: 10.1371/journal.pone.0004348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pruett-Miller SM, Reading DW, Porter SN, Porteus MH. Attenuation of zinc finger nuclease toxicity by small-molecule regulation of protein levels. PLoS Genet. 2009;5:e1000376. doi: 10.1371/journal.pgen.1000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bibikova M, Golic M, Golic KG, Carroll D. Targeted chromosomal cleavage and mutagenesis in Drosophila using zinc-finger nucleases. Genetics. 2002;161:1169–1175. doi: 10.1093/genetics/161.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller JC, et al. An improved zinc-finger nuclease architecture for highly specific genome editing. Nat Biotechnol. 2007;25:778–785. doi: 10.1038/nbt1319. [DOI] [PubMed] [Google Scholar]
- 35.Szczepek M, et al. Structure-based redesign of the dimerization interface reduces the toxicity of zinc-finger nucleases. Nat Biotechnol. 2007;25:786–793. doi: 10.1038/nbt1317. [DOI] [PubMed] [Google Scholar]
- 36.Beumer KJ, et al. Efficient gene targeting in Drosophila by direct embryo injection with zinc-finger nucleases. Proc Natl Acad Sci USA. 2008;105:19821–19826. doi: 10.1073/pnas.0810475105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masclaux F, Charpenteau M, Takahashi T, Pont-Lezica R, Galaud JP. Gene silencing using a heat-inducible RNAi system in Arabidopsis. Biochem Biophys Res Commun. 2004;321:364–369. doi: 10.1016/j.bbrc.2004.06.154. [DOI] [PubMed] [Google Scholar]
- 38.Kim SY, Hong CB, Lee I. Heat shock stress causes stage-specific male sterility in Arabidopsis thaliana. J Plant Res. 2001;114:301–307. [Google Scholar]
- 39.Alonso JM, et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science. 2003;301:653–657. doi: 10.1126/science.1086391. [DOI] [PubMed] [Google Scholar]
- 40.Jander G, Barth C. Tandem gene arrays: A challenge for functional genomics. Trends Plant Sci. 2007;12:203–210. doi: 10.1016/j.tplants.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 41.Lee HJ, Kim E, Kim JS. Targeted chromosomal deletions in human cells using zinc finger nucleases. Genome Res. 2010;20:81–89. doi: 10.1101/gr.099747.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halpin C, Cooke SE, Barakate A, El Amrani A, Ryan MD. Self-processing 2A-polyproteins–a system for co-ordinate expression of multiple proteins in transgenic plants. Plant J. 1999;17:453–459. doi: 10.1046/j.1365-313x.1999.00394.x. [DOI] [PubMed] [Google Scholar]
- 43.Koncz C, Schell J. The promoter of the TL-DNA gene 5 controls the tissue-specific expression of chimeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet. 1986;204:383–396. [Google Scholar]
- 44.Dong Q, et al. Comparative plant genomics resources at PlantGDB. Plant Physiol. 2005;139:610–618. doi: 10.1104/pp.104.059212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swarbreck D, et al. The Arabidopsis Information Resource (TAIR): Gene structure and function annotation. Nucleic Acids Res. 2008;36:D1009–D1014. doi: 10.1093/nar/gkm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olson M, Bostic K, Seltzer M, Berkeley D. Proceedings of the annual conference on USENIX Annual Technical Conference. 1999. [Google Scholar]
- 47.Stajich JE, et al. The Bioperl toolkit: Perl modules for the life sciences. Genome Res. 2002;12:1611–1618. doi: 10.1101/gr.361602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fu F, et al. Zinc Finger Database (ZiFDB): A repository for information on C2H2 zinc fingers and engineered zinc-finger arrays. Nucleic Acids Res. 2009;37:D279–D283. doi: 10.1093/nar/gkn606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.