Abstract
One half of a group of 20 patients with human papillomavirus type 16 (HPV16)-induced vulvar intraepithelial neoplasia grade 3 displayed a complete regression (CR) after therapeutic vaccination with HPV16 E6/E7 synthetic long peptides. Patients with relatively larger lesions generally did not display a CR. To investigate immune correlates of treatment failure, patients were grouped according to median lesion size at study entry, and HPV16-specific immunity was analyzed at different time points by complementary immunological assays. The group of patients with smaller lesions displayed stronger and broader vaccine-prompted HPV16-specific proliferative responses with higher IFNγ (P = 0.0003) and IL-5 (P < 0.0001) levels than patients with large lesions. Characteristically, this response was accompanied by a distinct peak in cytokine levels after the first vaccination. In contrast, the patient group with larger lesions mounted higher frequencies of HPV16-specific CD4+CD25+Foxp3+ T cells (P = 0.005) and displayed a lower HPV16-specific IFNγ/IL-10 ratio after vaccination (P < 0.01). No disparity in T memory immunity to control antigens was found, indicating that the differences in HPV-specific immunity did not reflect general immune failure. We observed a strong correlation between a defined set of vaccine-prompted specific immune responses and the clinical efficacy of therapeutic vaccination. Notably, a high ratio of HPV16-specific vaccine-prompted effector T cells to HPV16-specific CD4+CD25+Foxp3+ T cells was predictive of clinical success. Foxp3+ T cells have been associated previously with impaired immunity in malignancies. Here we demonstrate that the vaccine-prompted level of this population is associated with early treatment failure.
Keywords: human papilloma virus, immunomonitoring, therapeutic vaccine, regulatory T cells
Genital infection with human papillomavirus (HPV) is a common sexually transmitted disease (1–3). The high-risk HPV types (e.g., HPV16) are causally related to the development of anogenital lesions, including vulvar intraepithelial neoplasia (VIN), and their subsequent progression to invasive squamous cell carcinoma (4, 5). Notably, spontaneous regression of high-grade VIN (VIN3) occurs in <1.5% of patients, regardless of frequent biopsy of these lesions, and recurrence after surgical treatment is high (6).
Virus-specific IFNγ-producing CD4+ and CD8+ T cells are essential components in the immune control of chronic viral infection (7, 8). Healthy donors display relatively robust recall proliferative T-cell responses against early viral proteins E2, E6, and E7, characterized by CD4+ T cells producing IFNγ and IL-5 (9–11). In addition, the majority of subjects clearing HPV16 displayed an HPV16 E6-specific CD8+ cytotoxic T-lymphocyte (CTL) response (12, 13). These findings suggest that successful defense against HPV16 infection is associated with a systemic HPV-specific T-cell response. Notably, such IFNγ-associated T-cell responses are weak or absent in patients with cervical cancer (11, 14, 15), cervical intraepithelial neoplasia lesions (11, 13, 16, 17), and VIN3 (18–20).
We recently reported that half the patients with histologically confirmed HPV16+ VIN3 display a complete regression (CR) of their lesion after three or four vaccinations with an HPV16 E6/E7 synthetic long-peptide vaccine (HPV16-SLP). Patients with CR displayed a stronger vaccine-induced HPV16-specific immune response, as measured by IFNγ-ELISPOT and proliferation assay after the last vaccination, but it was unclear why vaccination did not lead to CR in all patients. However, these non- or partial responders (non-CR) had larger lesions on average (21). The availability of blood and tissue samples from these patients at multiple time points allowed an in-depth analysis of the HPV16 E6- and E7-specific immune response. Here, we show that, even after the first vaccination, vaccine-induced immune reactivity in patients with larger lesions is quantitatively and qualitatively different from that in patients with smaller lesions.
Results
HPV16-SLP Vaccine Induces Broad and Durable T-Cell Responses to HPV16 E6 and E7.
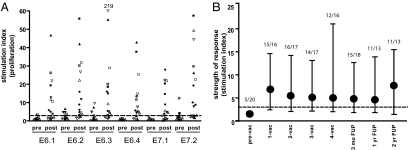
Peripheral blood mononuclear cells (PBMC) isolated before the first and after every vaccination were used to study the breadth and duration of the vaccine-induced HPV-specific T-cell response by measuring the proliferative capacity of these PBMC. In a number of cases, strong proliferative responses to a large number of peptide pools was detected, whereas in other patients the proliferative response was lower or reactive to fewer peptide pools (Fig. 1A). In total, 16 (80%) and 14 (70%) of the 20 vaccinated patients responded to HPV16 E6 and E7, respectively, after the last vaccination. In all but one patient, proliferation to both E6 and E7 was detected, when all time points were considered. Excellent immune memory was apparent from maintenance of the strength of the response in this group during 2-y follow-up (Fig. 1B). Vaccine-induced T-cell reactivity consisted of broad HPV16-specific CD4 T-cell responses, with reactivity, on average, to five of the six peptide pools tested (Table S1) and HPV16-specific CD8 T-cell responses against one to six peptide pools (mean, two pools; Table S2).
Fig. 1.
Strong and broad vaccine-induced T-cell immunity was seen. (A) HPV16 E6/E7-specific proliferation of PBMC in blood samples drawn before and after vaccination as determined in the LST assay. Each symbol represents a particular patient; black and red symbols indicate CR. (B) The strength of vaccine-induced proliferative T-cell response in time (median + interquartile range). The numbers above each time point indicate the ratio of the number of patients displaying a positive proliferative response to the number of patients evaluated before vaccination (pre-vac), after one (1-vac), two (2-vac), three (3-vac), and four (4-vac) vaccinations, and at 3-mo, 1-y, and 2-y follow-up (FUP).
Vaccine-Induced HPV16-Specific T Cells Can Home to Vaccination Sites and to the Lesion.
A biopsy from the last vaccination site was obtained from all 20 patients. T cells could be cultured from 11 patients, comprising 5 patients with small lesions, 4 of whom were CR, and 6 patients with larger lesions, of whom 5 were non-CR. In nine of these patients, including all tested CR patients, T-helper type 1/T-helper type 2 (Th1/Th2) cytokine-producing HPV16-specific proliferative T cells were detected. In two patients (one CR and one non-CR), a predominant Th2 response was found; in another non-CR patient, only a Th2 response was found. Notably, in all four CR patients with a smaller lesion, the vaccine site was infiltrated by HPV16-specific IFNγ-producing T cells, as was the case in three of the five non-CR patients with a larger lesion.
A biopsy from the VIN lesion was obtained from 16 patients (including all CR patients) before vaccination and from all 20 patients at 3-mo follow-up. HPV16 E6-specific T cells were detected in the VIN lesion of one CR patient before vaccination, but not in 10 others from whom T cells could be cultured. In nine cases, T cells from the VIN biopsy were obtained after vaccination, but no HPV-specific response was detected in five clinical nonresponders (NR) or three CR patients at that time point. The latter finding was to be expected, because these epithelia showed normal histology. Interestingly, in one partial responder (PR) (patient ID-1; Tables S1 and S2), a mixed Th1/Th2 HPV16-specific response was detected in PBMC and in the T cells isolated from the last vaccine site, whereas a predominant HPV16-specific Th2 cell response was found in the VIN lesion (Fig. S1). Their presence may explain the lack of a complete clinical response. Semiquantitative analyses of the number of stromal or intraepithelial infiltrating immune cells in the section used for diagnosis revealed no overt differences in non-CR and CR patients before vaccination or within the patient groups before and after vaccination.
Peak in the HPV16-Specific Cytokine Production After the First Vaccination Is Associated with Lesion Size at Study Entry and Segregates Clinical CR from Non-CR Patients.
Because CR patients on average displayed a smaller lesion and a shorter history of disease than the non-CR (PR+NR) patients (21), we investigated whether lesion size or disease duration at study entry is associated with the immune response to vaccination. The patients were divided into two groups based on either the lesion size (median 9.5 cm2) or disease duration (median 30 mo).
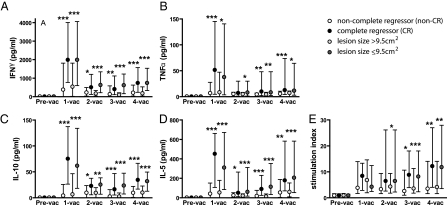
The immune response, especially with respect to proliferation-associated production of IFNγ and IL-5, and to a lesser extent TNFα and IL-10, was significantly higher in the group of patients with a smaller lesion than in the group with a larger lesion (Fig. 2). Vaccine-induced immunity peaked after the first vaccination, decreased after the second vaccination, and then gradually increased again. The cytokine levels produced by the PBMC samples from patients with small lesions always were higher than those of patients with larger lesions. Moreover, from the second vaccination and thereafter, the ratio between IFNγ and IL-10 was significantly higher at each time point in the group with smaller lesions (P < 0.01 at each time point) than in patients with larger lesions (Table S3). The IFNγ (Th1 cytokine)/IL-5 (Th2 cytokine) ratio differed only after the fourth vaccination, suggesting that the lower ratio of IFNγ to IL-10 (Th2 cytokine) in the group of patients with a larger lesion does not simply reflect a shift from a Th1 toward a Th2 response. No differences were observed with respect to strength of proliferation or the IFNγ/IL-10 ratio when patients were grouped according to the disease duration. This set of data suggests that the vaccine-induced HPV16-specific T-cell response is regulated differently in patients with larger lesions.
Fig. 2.
Stronger HPV16-specific cytokine production by patients with small VIN3 lesions was observed. Patients are grouped according to clinical outcome (CR or non-CR) and lesion size (smaller or larger than ≤9.5 cm2) at study entry: The strength (median + interquartile range) of cytokine production (A–D) and proliferation at indicated time points (E) is depicted. *0.01 < P < 0.05; **0.001 < P < 0.01; ***P < 0.001. (Exact P values are given in Tables S3 and S4.)
The association between lesion size and clinical outcome after vaccination suggests that similar differences may be detected when patients are divided on the basis of clinical outcome (CR versus non-CR). Notably, NR and PR patients were grouped together, because it is known that even residual VIN3 has a high risk of progression (6, 22). Indeed, after each vaccination the strength of the proliferative response as well as cytokine production was higher in the CR group than in the non-CR group, and these responses peaked after the first vaccination (Fig. 2). The observed differences in the IFNγ/IL-10 or IFNγ/IL-5 ratios when patients were grouped according to lesion size were less clear when CR and non-CR patients were compared, although the CR group displayed a somewhat higher IFNγ/IL-10 ratio after the third vaccination (P = 0.03) (Table S4).
Importantly, a potential bias in the overall status of the immune system could be ruled out, because patients grouped on the basis of either lesion size or clinical outcome displayed comparable immunity to memory response mix (MRM) and influenza virus M1 (Fig. S2) and displayed no differences in the preexisting HPV16 peptide-specific response. These data suggest that patients responding well to the first vaccine dose will develop a complete clinical response.
Ratio of HPV16-Specific CD4+CD25+Foxp3+ and Foxp3− T Cells Is Associated with Lesion Size and Disease Duration at Study Entry.
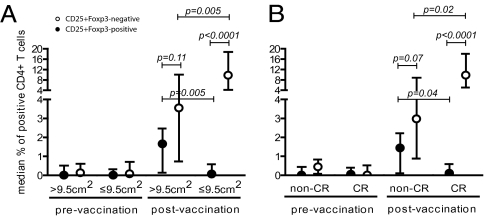
HVP16 E6- and E7-specific CD4+CD25+Foxp3+ regulatory T cells are detected in patients with HPV16-induced diseases (17, 23) and after vaccination with HPV16-SLP (24). Therefore, HPV16-specific CD4+CD25+Foxp3+ as well as Foxp3− T cells were analyzed in PBMC from the patients grouped according to lesion size at study entry or by clinical response at 12 mo. There was no difference before vaccination (Fig. 3). However, the vaccine-induced HPV-specific response in the group of patients with a larger lesion comprised relatively more CD4+CD25+Foxp3+ T cells than seen in patients with a smaller lesion (P = 0.005). Furthermore, not only was the strength of the HPV16E6/E7-specific CD4+CD25+ response in the group of patients with a small lesion higher than that of patients with a large lesion (P = 0.005); the response also was dominated by CD4+CD25+Foxp3− T cells (P < 0.0001). This CD4+CD25+Foxp3− T-cell predominance was not seen in patients with larger lesions (P = 0.11), indicating that in this group the responses of the Foxp3+ and Foxp3− CD4+CD25+ T cells were comparable in strength (Fig. 3A). Similarly, when patients were grouped according to their clinical response, the relative response of CD4+CD25+Foxp3− T cells was stronger in patients with a CR (P = 0.02), and the subset of CD4+CD25+Foxp3− T cells dominated the immune response (P < 0.0001; Fig. 3B) in CR patients but not in non-CR patients. Notably, when the patients were grouped according to the duration of the lesion at study entry, the HPV16-specific CD4+CD25+Foxp3− T-cell population dominated the response in both groups (≥30 mo, P = 0.02; <30 mo, P < 0.0001), but the frequency of CD4+CD25+Foxp3− T cells was significantly higher in patients with a relatively shorter disease history (P = 0.01). The overall percentages of CD4+CD25+Foxp3+ T cells in unstimulated PBMC were low before (median 0.19%, interquartile range 0.13–1.23) and after (median 0.32%, interquartile range 0.12–1.08) vaccination and did not differ between the patient groups. Similarly, analysis of the CD8+CD25+Foxp3+ HPV16-specific T cells revealed low frequencies before (median 0.08, interquartile range 0–0.8) and after (median 0.43, interquartile range 0.11–0.86) vaccination. No differences were observed when patients were grouped according to the clinical parameters. These results indicate that patients with larger VIN lesions display a shifted balance in vaccine-induced HPV-specific immunity, tending toward an HPV-specific CD4+CD25+Foxp3+ T-cell response.
Fig. 3.
More CD4+CD25+Foxp3+ T-regulatory cells were seen in patients with large lesions. The median + interquartile range percentage of CD4+CD25+Foxp3− and CD4+CD25+Foxp3+ T cells is depicted for patients grouped according to (A) lesion size at study entry and (B) clinical response.
Discussion
The study of a cohort of 20 HPV16+ VIN3 patients, 47% of whom showed a CR after vaccination with an HPV16E6/E7 synthetic long-peptide vaccine (21), allowed us to observe that vaccine efficacy can be correlated with a set of complementary immune parameters, rather than with one assay. Our previous study showed that the size of the lesion at study entry was associated with the clinical response (21), and this finding prompted us to examine HPV16-specific immunity in relation to lesion size. This finding turned out to be of key importance, because patients with smaller lesions displayed much stronger effector T-cell responses even after the first vaccination, whereas the group of patients with larger lesions displayed a weaker effector response and a stronger HPV16-specific regulatory immune response, demonstrated by a significantly lower IFNγ/IL-10 ratio after the second vaccination and a significantly higher percentage of HPV16-specific CD4+CD25+Foxp3+ T cells after vaccination. Although the first set of observations can explain the success of therapeutic vaccination, the latter observations may indicate a shift in the type of the vaccine-prompted HPV16-specific T-cell response so that it fails to attack the lesions effectively.
An HPV16-induced high-grade lesion is caused by chronic viral infection and can be regarded as a tumor in situ. Adoptive T-cell therapy studies in chronic viral infections and cancer indicate that a well-coordinated response of IFNγ-producing CD4+ T cells and CD8+ CTL is needed for effective immunity (25, 26). Our analysis of HPV16-specific T-cell reactivity in the group of CR patients underlines these insights and reveals that vaccination can be an approach to treat chronic viral infections or cancer, provided that robust and durable T-cell responses are induced. Previously, we demonstrated that healthy individuals display broad IFNγ-producing HPV16-specific proliferative T-cell responses, as witnessed by successful clearance of HPV (9–11). Similarly, in the group of CR patients, vaccination resulted in broad and IFNγ-associated HPV-specific T–cell responses. Notably, HPV-specific immunity is weak or absent in subjects with HPV-induced neoplasia of the cervix or vulva (9–11, 20) and consists of HPV16-specific regulatory T cells (17, 23). Here, unsuccessfully treated VIN3 patients also displayed a lower response and harbored more HPV-specific CD4+CD25+Foxp3+ T cells. The increased numbers of the latter T cells after vaccination may reflect either the expansion of an undetectable preexisting T-cell repertoire or the activation of naïve T cells. These Foxp3+ cells likely comprise HPV-specific suppressor cells, because similar populations of CD4+CD25high T cells stably expressing (≥7 d) Foxp3 after stimulation with cytomegalovirus or tetanus toxoid were suppressive in vitro (27). Moreover, we also documented that suppressive T cells in cervical cancer patients had the CD4+CD25highFoxp3+ phenotype (23) and that, as in many malignancies, their presence impaired tumor immunity as reflected in worse patient survival (28). In contrast to the antigen-specific stimulation used here, strong mitogenic agents were reported to induce Foxp3 expression in nonsuppressive T cells (29, 30). The tumor-specific immune response, the local environment, and immune escape mechanisms in HPV-induced cancers are similar to those in other immunogenic cancer types, and it is likely that the association of treatment failure and vaccine-enhanced Foxp3+ cells we describe can extend to immunotherapy with other tumor antigens.
The data are reminiscent of those made in the A20HA mouse tumor model in which a growing tumor is associated with expansion of HA-specific CD4+ T cells, whereas during tumor progression the HA-specific T-cell response is blunted and comprises activated HA-specific CD4+ suppressive T cells (31). Both HPV16-specific CD4+ effector and regulatory T cells are present in patients with HPV16-induced high-grade lesions or cancer (17, 23). By analogy a larger HPV-induced VIN may affect the nature of the HPV-specific responding T-cell population (17). Moreover, in A20HA tumor-bearing mice, HA-specific CD4+ T cells were less responsive to HA-specific vaccination. This reduced response was not a reflection of global immunosuppression, because these mice still mounted strong virus-specific T-cell responses (32), but was caused by the coactivation of HA-specific CD4+ regulatory T cells (31). Also, in our study, patients with larger VIN lesions displayed normal responses to pathogens but had more vaccine-prompted HPV16-specific regulatory T-cell and blunted HPV16-specific effector T-cell responses.
Vaccine-prompted HPV16-specific T-cell immunity was characterized by a peak after the first vaccination, followed by a steep decline after the second vaccination and a gradual increase in reactivity after subsequent vaccinations. Should multiple vaccinations therefore be avoided because they may reduce the desired immune response? Our data indicate that this approach is not called for, because immunity was detectable long after vaccination and particularly because the observation was made in the group of CR patients. Therefore, the decline in T-cell reactivity as measured by sampling circulating lymphocytes from the blood probably reflects the migration of vaccine-activated HPV16-specific T cells from the blood as part of the constant migration of activated effector T cells to extralymphatic tissue and bone marrow (33). The subsequent gradual increase may reflect a phase in which HPV16-specific T cells entering and exiting the blood stream are in equilibrium, their number rising after each vaccination.
The treatment of HPV-induced high-grade vulvar lesions with a local inflammation-inducing agent such as imiquimod has resulted in the shrinkage of VIN lesions (34). If the failure of the HPV16-SLP vaccine to induce the full regression of HPV16-induced lesion is simply the result of the size of the lesion, treatment of the lesion with imiquimod before or simultaneously with vaccination may increase the likelihood of successful treatment (20, 34), especially because reduction of the lesion size may alleviate immunosuppression (35). Similarly, one can envisage that the success rate of vaccine strategies in cancer patients may increase when the tumor size is reduced.
In conclusion, our comparison of vaccine-prompted immune responses in patients who were or were not treated successfully revealed that therapeutic vaccination can be a truly effective therapy for the treatment of well-established premalignant lesions when it results in strong and broad effector T-cell responses, but it also shows that during the premalignant phase of disease the larger lesions already have deregulated the specific immune response to such an extent that the current strategy of immunotherapy for those patients needs further adjustment. These results clearly impact on immunotherapy trials in patients with cancer, suggesting that for these patients multimodality approaches should be sought.
Materials and Methods
Patients, Study Design, and Vaccination.
In a one-arm phase II trial, women with histologically confirmed HPV16+ VIN3 lesions were vaccinated s.c. three or four times and followed up to 2 y after the last vaccination. The HPV16-SLP vaccine represents the entire sequence of the E6 and E7 proteins of HPV16 (24, 36). An objective CR was defined as no visible or histologically demonstrable VIN lesion after treatment. For our analyses, patients with either a partial response (at least 50% reduction in lesion size) or no response were defined as non-CR. The study was approved by the Medical Ethics Committee of the Leiden University Medical Center and has been reported recently (21).
Antigens.
The antigens used for immunomonitoring have been reported previously (10, 17, 21, 24, 37). Briefly, four peptide pools for HPV16 E6 (pools E6.1–E6.4) and two pools for E7, each consisting of four peptides 22 aa long and overlapping by 12 aa, were used to screen for CD4+ T-cell responses. CD8+ T-cell responses were analyzed by 15 peptide pools for HPV16 E6 and nine pools for E7, each containing 10-aa peptides (overlap, 9 aa). As positive control , four pools of 30-mer influenza matrix 1 protein of A/PR/8/34 (M1) peptides (overlap, 15 aa) and/or MRM (0.75/mL Limus Flocculentius tetanus toxoid , 5 μg/mL Mycobacterium tuberculosis ,and 0.015% Candida) were used.
Proliferative Capacity of HPV16-Specific T Cells by Lymphocyte Stimulation Test.
Antigen-specific T-cell proliferation was determined in blood drawn before each vaccination and 2 wk after the last vaccination by a previously described short-time proliferation assay, the lymphocyte stimulation test (LST) (9–11, 21, 24). Briefly, freshly isolated PBMC were incubated in eight replicate wells in medium with 10% autologous serum in the presence of the indicated antigens. On day 6, supernatant was harvested for cytokine analysis, and the cells were pulsed with [3H]thymidine overnight. The mean plus 3 SD of the eight control-medium wells was used as cutoff value. The stimulation index was calculated by dividing the mean of tested wells by the mean of the control medium. A positive proliferative response was defined as a stimulation index of 3 or higher and by counts above the cutoff value in six or more of the eight wells (11, 21, 24). A vaccine-induced response was defined as an increase in the stimulation index of 3-fold or more over the baseline sample (21, 24).
Cytokine Polarization Analysis.
The supernatants isolated on day 6 of the proliferation assay were subjected to a Th1/Th2 inflammation cytometric bead array (CBA) kit (BD Biosciences) as described previously. The cutoff value was 20 pg/mL, except for IFNγ, for which it was 100 pg/mL (21, 24). Antigen-specific cytokine production was positive when above the cutoff value and at least twice that of the control medium (11, 21, 24). A vaccine-induced response was defined as a 3-fold or greater increase over the baseline sample (21, 24).
Culture and Analysis of Tissue-Infiltrated Lymphocytes.
Infiltrating lymphocytes from a 3-mm biopsy of the VIN lesion before vaccination, from the last vaccination site 2 wk after the last vaccination, and from the VIN lesion 3 mo after the last vaccination were expanded as described previously (38, 39). When sufficient cells were obtained, their HPV16-specific proliferative capacity was tested in triplicate in a 3-d proliferation assay. Adherent autologous monocytes, activated with GM-CSF and loaded with 5 μg/mL of the indicated peptide pools or 10 μg/mL recombinant proteins, served as antigen-presenting cells. After 48 h, supernatant was harvested and stored at −20 °C for cytokine analysis by Th1/Th2 CBA.
Identification of T-Cell Type by Intracellular IFNγ and IL-5 Cytokine Staining.
PBMC isolated before the first vaccination and 2 wk after the last vaccination were presensitized for 10 d with a pool of all peptides of HPV16 E6, HPV16 E7, or influenza A/PR/8/34 M1 before stimulation with the indicated antigens, loaded on autologous monocytes, stained intracellularly for IFNγ, and analyzed by flow cytometry (21, 24). A positive response was defined as a 2-fold or greater increase in the percentage of IFNγ-producing CD4+ or CD8+ T cells as compared with the control medium. A vaccine-induced reaction was defined as a 3-fold or greater increase in the percentage of antigen-specific IFNγ-producing T cells compared with the baseline sample (21, 24, 40).
Detection of HPV16-Specific CD4+CD25+Foxp3+ T Cells.
HPV16-specific CD4+CD25+Foxp3+ T cells were detected as described previously (24). Briefly, PBMC stimulated with HPV16 E6 or E7 peptides (5 μg/mL) and rested for 10 d were stained for CD4, CD25, and Foxp3. As a control, PBMC were cultured without antigen (control medium). Antigen-induced up-regulation of Foxp3 or CD25 was defined a 2-fold or greater increase in the percentages of CD25+ and/or Foxp3+ cells as compared with control medium. A vaccine-induced increase was defined as a 3-fold or greater increase in the percentage compared with the baseline sample for the same condition.
Statistical Analysis.
Differences between the median cytokine responses in pre- and postvaccination PBMC samples against every individual peptide pool of HPV16 E6 and E7 were determined by the nonparametric Mann–Whitney test. Comparisons of the strength of the different types of immune responses were made by the nonparametric Mann–Whitney test using GraphPad InStat Software to analyze the differences between the patients with VIN3 lesions larger or smaller at study entry than the median lesion size of 9.5 cm2, between CR and non-CR patients 12 mo after the last vaccination, and between patients who had disease duration shorter or longer than the median of 30 mo (21). For each different type of immune assay, the strength was defined as the median specific spot count (ELISPOT), stimulation index (LST), or amount of cytokine production (CBA) obtained for all six different peptide pools per patient, of all patients in one group. All reported P values are two-sided and have not been adjusted for multiple comparisons. P < 0.05 was considered to indicate statistical significance.
Supplementary Material
Acknowledgments
We thank the patients who participated in the study and their referring physicians, Dr. M. I. E. van Poelgeest and Dr. M. van den Hende, for clinical help. W. E. Benckhuijsen and colleagues synthesized the peptides used for immunomonitoring. This study was supported by Grants RUL 2007-3848 form the Dutch Cancer Society (to S.H.v.d.B. and G.G.K.), LSHC-CT-2006-518234 from the European Union Integrated Project Cancer Immunotherapy, and LSHB-CT-2004-512074 from the European Union Network of Excellence DC-THERA and by ISA Pharmaceuticals B.V.
Footnotes
Conflict of interest statement: This study has been conducted by the Leiden University Medical Center (LUMC), which holds a patent on the use of synthetic long peptides as vaccine (US 7.202.034). C.J.M.M. and S.H.v.d.B. are named as inventors on this patent. The LUMC does not share the financial benefit from this patent with its employees. C.J. M. M. has been employed part-time (75%) since January 20, 2008, by ISA Pharmaceuticals, which exploits this long-peptide vaccine patent, and has been granted options on ISA Pharmaceuticals stock.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006500107/-/DCSupplemental.
References
- 1.Weaver BA. Epidemiology and natural history of genital human papillomavirus infection. J Am Osteopath Assoc. 2006;106(3, Suppl 1):S2–S8. [PubMed] [Google Scholar]
- 2.Wheeler CM. Natural history of human papillomavirus infections, cytologic and histologic abnormalities, and cancer. Obstet Gynecol Clin North Am. 2008;35:519–536, vii. doi: 10.1016/j.ogc.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Bosch FX, et al. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008;26(Suppl 10):K1–K16. doi: 10.1016/j.vaccine.2008.05.064. [DOI] [PubMed] [Google Scholar]
- 4.Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walboomers JM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 6.van Seters M, van Beurden M, de Craen AJ. Is the assumed natural history of vulvar intraepithelial neoplasia III based on enough evidence? A systematic review of 3322 published patients. Gynecol Oncol. 2005;97:645–651. doi: 10.1016/j.ygyno.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zajac AJ, Murali-Krishna K, Blattman JN, Ahmed R. Therapeutic vaccination against chronic viral infection: The importance of cooperation between CD4+ and CD8+ T cells. Curr Opin Immunol. 1998;10:444–449. doi: 10.1016/s0952-7915(98)80119-2. [DOI] [PubMed] [Google Scholar]
- 9.de Jong A, et al. Frequent detection of human papillomavirus 16 E2-specific T-helper immunity in healthy subjects. Cancer Res. 2002;62:472–479. [PubMed] [Google Scholar]
- 10.Welters MJ, et al. Frequent display of human papillomavirus type 16 E6-specific memory t-Helper cells in the healthy population as witness of previous viral encounter. Cancer Res. 2003;63:636–641. [PubMed] [Google Scholar]
- 11.de Jong A, et al. Human papillomavirus type 16-positive cervical cancer is associated with impaired CD4+ T-cell immunity against early antigens E2 and E6. Cancer Res. 2004;64:5449–5455. doi: 10.1158/0008-5472.CAN-04-0831. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa M, et al. Cytotoxic T lymphocyte responses to E6 and E7 proteins of human papillomavirus type 16: Relationship to cervical intraepithelial neoplasia. J Infect Dis. 1997;175:927–931. doi: 10.1086/513992. [DOI] [PubMed] [Google Scholar]
- 13.Nakagawa M, et al. Persistence of human papillomavirus type 16 infection is associated with lack of cytotoxic T lymphocyte response to the E6 antigens. J Infect Dis. 2000;182:595–598. doi: 10.1086/315706. [DOI] [PubMed] [Google Scholar]
- 14.Bontkes HJ, et al. Human papillomavirus type 16 E6/E7-specific cytotoxic T lymphocytes in women with cervical neoplasia. Int J Cancer. 2000;88:92–98. [PubMed] [Google Scholar]
- 15.Youde SJ, et al. Use of fluorogenic histocompatibility leukocyte antigen-A*0201/HPV 16 E7 peptide complexes to isolate rare human cytotoxic T-lymphocyte-recognizing endogenous human papillomavirus antigens. Cancer Res. 2000;60:365–371. [PubMed] [Google Scholar]
- 16.Nimako M, Fiander AN, Wilkinson GW, Borysiewicz LK, Man S. Human papillomavirus-specific cytotoxic T lymphocytes in patients with cervical intraepithelial neoplasia grade III. Cancer Res. 1997;57:4855–4861. [PubMed] [Google Scholar]
- 17.de Vos van Steenwijk PJ, et al. Surgery followed by persistence of high-grade squamous intraepithelial lesions is associated with the induction of a dysfunctional HPV16-specific T-cell response. Clin Cancer Res. 2008;14:7188–7195. doi: 10.1158/1078-0432.CCR-08-0994. [DOI] [PubMed] [Google Scholar]
- 18.Smyth LJ, et al. Immunological responses in women with human papillomavirus type 16 (HPV-16)-associated anogenital intraepithelial neoplasia induced by heterologous prime-boost HPV-16 oncogene vaccination. Clin Cancer Res. 2004;10:2954–2961. doi: 10.1158/1078-0432.ccr-03-0703. [DOI] [PubMed] [Google Scholar]
- 19.Davidson EJ, et al. Immunological and clinical responses in women with vulval intraepithelial neoplasia vaccinated with a vaccinia virus encoding human papillomavirus 16/18 oncoproteins. Cancer Res. 2003;63:6032–6041. [PubMed] [Google Scholar]
- 20.van Poelgeest MI, et al. Detection of human papillomavirus (HPV) 16-specific CD4+ T-cell immunity in patients with persistent HPV16-induced vulvar intraepithelial neoplasia in relation to clinical impact of imiquimod treatment. Clin Cancer Res. 2005;11:5273–5280. doi: 10.1158/1078-0432.CCR-05-0616. [DOI] [PubMed] [Google Scholar]
- 21.Kenter GG, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 22.Kaufman RH. Intraepithelial neoplasia of the vulva. Gynecol Oncol. 1995;56:8–21. doi: 10.1006/gyno.1995.1003. [DOI] [PubMed] [Google Scholar]
- 23.van der Burg SH, et al. Association of cervical cancer with the presence of CD4+ regulatory T cells specific for human papillomavirus antigens. Proc Natl Acad Sci USA. 2007;104:12087–12092. doi: 10.1073/pnas.0704672104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Welters MJ, et al. Induction of tumor-specific CD4+ and CD8+ T-cell immunity in cervical cancer patients by an HPV16 E6 and E7 long-peptide vaccine. Clin Cancer Res. 2008;14:178–187. doi: 10.1158/1078-0432.CCR-07-1880. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: A clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riddell SR, Greenberg PD. Principles for adoptive T cell therapy of human viral diseases. Annu Rev Immunol. 1995;13:545–586. doi: 10.1146/annurev.iy.13.040195.002553. [DOI] [PubMed] [Google Scholar]
- 27.Pillai V, Ortega SB, Wang CK, Karandikar NJ. Transient regulatory T-cells: A state attained by all activated human T-cells. Clin Immunol. 2007;123:18–29. doi: 10.1016/j.clim.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jordanova ES, et al. Human leukocyte antigen class I, MHC class I chain-related molecule A, and CD8+/regulatory T-cell ratio: Which variable determines survival of cervical cancer patients? Clin Cancer Res. 2008;14:2028–2035. doi: 10.1158/1078-0432.CCR-07-4554. [DOI] [PubMed] [Google Scholar]
- 29.Valencic E, Piscianz E, Tommasini A, Granzotto M. T cells stimulated in vitro have a suppressive function but do not contain only regulatory T cells. Clin Exp Immunol. 2007;150:561–566. doi: 10.1111/j.1365-2249.2007.03502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kmieciak M, et al. Human T cells express CD25 and Foxp3 upon activation and exhibit effector/memory phenotypes without any regulatory/suppressor function. J Transl Med. 2009;7:89–96. doi: 10.1186/1479-5876-7-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou G, Drake CG, Levitsky HI. Amplification of tumor-specific regulatory T cells following therapeutic cancer vaccines. Blood. 2006;107:628–636. doi: 10.1182/blood-2005-07-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staveley-O'Carroll K, et al. Induction of antigen-specific T cell anergy: An early event in the course of tumor progression. Proc Natl Acad Sci USA. 1998;95:1178–1183. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Rosa F, Pabst R. The bone marrow: A nest for migratory memory T cells. Trends Immunol. 2005;26:360–366. doi: 10.1016/j.it.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 34.van Seters M, et al. Treatment of vulvar intraepithelial neoplasia with topical imiquimod. N Engl J Med. 2008;358:1465–1473. doi: 10.1056/NEJMoa072685. [DOI] [PubMed] [Google Scholar]
- 35.Danna EA, et al. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res. 2004;64:2205–2211. doi: 10.1158/0008-5472.can-03-2646. [DOI] [PubMed] [Google Scholar]
- 36.Kenter GG, et al. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin Cancer Res. 2008;14:169–177. doi: 10.1158/1078-0432.CCR-07-1881. [DOI] [PubMed] [Google Scholar]
- 37.van der Burg SH, et al. Identification of a conserved universal Th epitope in HIV-1 reverse transcriptase that is processed and presented to HIV-specific CD4+ T cells by at least four unrelated HLA-DR molecules. J Immunol. 1999;162:152–160. [PubMed] [Google Scholar]
- 38.Piersma SJ, et al. Human papilloma virus specific T cells infiltrating cervical cancer and draining lymph nodes show remarkably frequent use of HLA-DQ and -DP as a restriction element. Int J Cancer. 2008;122:486–494. doi: 10.1002/ijc.23162. [DOI] [PubMed] [Google Scholar]
- 39.van den Hende M, et al. Skin reactions to human papillomavirus (HPV) 16 specific antigens intradermally injected in healthy subjects and patients with cervical neoplasia. Int J Cancer. 2008;123:146–152. doi: 10.1002/ijc.23502. [DOI] [PubMed] [Google Scholar]
- 40.de Jong A, et al. Rapid enrichment of human papillomavirus (HPV)-specific polyclonal T cell populations for adoptive immunotherapy of cervical cancer. Int J Cancer. 2005;114:274–282. doi: 10.1002/ijc.20721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.