Abstract
Target of rapamycin (TOR) kinases are key regulators of cell growth, proliferation, and structure in eukaryotes, processes that are highly coordinated during the infectious cycle of eukaryotic pathogens. Database mining revealed three TOR kinases in the trypanosomatid parasite Leishmania major, as defined by homology to the phosphoinositide 3-kinase–related kinase (PIKK) family and a signature conserved FKBP12/rapamycin-binding domain. Consistent with the essential roles of TOR complexes in other organisms, we were unable to generate null TOR1 or TOR2 mutants in cultured L. major promastigotes. In contrast, tor3− null mutants were readily obtained; while exhibiting somewhat slower growth, tor3− maintained normal morphology, rapamycin sensitivity, and differentiation into the animal-infective metacyclic stage. Significantly, tor3− mutants were unable to survive or replicate in macrophages in vitro, or to induce pathology or establish infections in mice in vivo. The loss of virulence was associated with a defect in acidocalcisome formation, as this unique organelle was grossly altered in tor3- mutants and no longer accumulated polyphosphates. Correspondingly, tor3- mutants showed defects in osmoregulation and were sensitive to starvation for glucose but not amino acids, glucose being a limiting nutrient in the parasitophorous vacuole. Thus, in Leishmania, the TOR kinase family has expanded to encompass a unique role in AC function and biology, one that is essential for parasite survival in the mammalian infective stage. Given their important roles in cell survival and virulence, inhibition of TOR kinase function in trypanosomatids offers an attractive target for chemotherapy.
Keywords: chemotherapy, protozoan parasite, Trypanosomatidae, virulence, Kinetoplastida
Leishmaniasis is typically classified as a “neglected tropical disease,” with 12 million persons infected worldwide. This disease is caused by trypanosomatid protozoan parasites of the genus Leishmania, which are transmitted by Phlebotomine sand flies and cause a range of symptoms from self-healing skin lesions to lethal visceral ulcers. In the past 15 y, coinfections with the HIV have increased the burden of leishmaniasis in public health, especially in sub-Saharan Africa, where access to treatment still remains a challenge (1).
The infective cycle of Leishmania includes a series of developmental steps, as promastigotes within the midgut of the sand fly and as amastigotes within the phagolysosome of the mammalian host cell. In these different environments, the parasites must adapt to variable conditions of temperature, pH and nutrient availability (2–4). Understanding the molecular mechanisms used by Leishmania parasites to survive under these harsh conditions is fundamental to our understanding of the disease, and ultimately for its control.
Target of rapamycin (TOR) kinases are key master regulators in eukaryotes, linking environmental conditions such as nutrient availability and stimuli to protein synthesis and to the cell cycle machinery to coordinate cell growth and replication. In humans, abnormal regulation of the mammalian TOR (mTOR) pathway has been associated with several diseases, such as cancer and type 2 diabetes (5). Initially, TORs were identified by mutations that conferred resistance to a potent antifungal metabolite called rapamycin (6), which acts against many TOR kinases through formation of an inhibitory complex with the peptidyl-prolyl cis/trans isomerase FKBP12 (7). TOR kinases belong to the family of phosphoinositide 3-kinase–related kinases (PIKKs), a protein family playing roles in cellular response to various types of stresses (8, 9). PIKKs possess a C-terminal domain similar to the catalytic domain of PI3 and PI4 lipid kinases, but acting as Ser/Thr kinases. PIKKs exhibit several other conserved domains such as FAT and FATC domains flanking the kinase domain, and HEAT domains in the N terminus, which participate in protein–protein interactions (8, 9). One defining feature of TORs is the FKBP12/rapamycin-binding (FRB) domain, originally identified as the binding site for FKBP12/rapamycin complexes but since shown to mediate important TOR functions (10–12).
Whereas most organisms encode one or two TORs that act within complexes known as TORCs, we found three TOR kinase genes bearing the signature FRB domain in Leishmania major and other trypanosomatids. To investigate their role, we attempted to produce null mutants for each. Although TOR1 and TOR2 appear to be essential, consistent with their essential function in organisms including trypanosomes (13), we were able to generate null mutants of TOR3. Notably, tor3- grew and differentiated normally in culture but was highly attenuated in macrophage and mouse infection. These phenotypes were associated with a specific defect in the synthesis of acidocalcisomes (ACs), a unique organelle of protozoans playing an important role in energy metabolism.
Results
Identification of TOR Kinases in L. major.
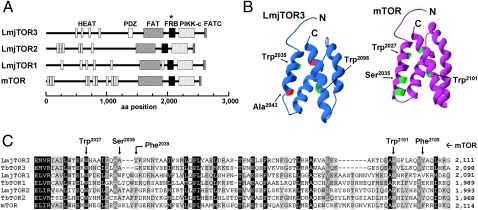
Database mining of the genome of L. major for TOR-related genes revealed four candidates (Fig. S1 and Table S1), showing similarity (43–50%) spanning ∼1,200 aa encompassing the C terminus, and including characteristic PIKK family domains, including HEAT, FAT, PI-kinase domain, and FATC. Only three bore the TOR signature FRB domain (14–16), and consistent with the nomenclature established recently in T. brucei (13), we termed them TOR1 (LmjF36.6320), TOR2 (LmjF34.4530), and TOR3 (LmjF34.3940; this is termed TOR-like1 in trypanosomes; Fig. 1A). Only TOR3 showed a PDZ motif, a protein-interaction domain often found in signaling complexes located at cell membranes (17) (Fig. 1A). The catalytic kinase domain is well conserved in all three L. major TOR kinases, including the residue corresponding to Asp2338 in mTOR (Fig. S2) required for kinase activity (18).
Fig. 1.
Three conserved TOR kinases in the genome of L. major. (A) Domain organization of L. major TOR kinases. Star highlights the signature TOR kinase FRB domains. (B) Modeling of the L. major TOR3 FRB domain, developed using the Phyre remote homology modeling server (19). The structure of the modeled LmjTOR3 FRB is shown at the left, compared with the crystal structure of human mTOR at the right (20). Critical residues for rapamycin binding in mTOR FRB and conserved/mutated amino acids in the corresponding positions of LmjTOR3 FRB are highlighted. (C) Multiple sequence alignment of the FRB domain of the three TOR kinases of L. major, T. brucei, and human mTOR. Darker shading corresponds to a higher degree of conservation. Arrows point to conserved residues for rapamycin binding in mTOR (NP_004949).
The powerful protein structural homology and modeling system PHYRE (19) was used to identify and model the FRB domains of L. major TOR1/2/3 and TbTOR2 and TbTOR-like 1 (Fig. 1 B and C and Table S1), against the structure of the mTOR FRB domain (10, 20). The Trp residues corresponding to mTOR Trp2027 and Trp2101 required for kinase activation and FKBP12/rapamycin binding are conserved (Fig. 1 B and C) (6, 20–23). However, Trp2101 was replaced by Arg in TbTOR1, consistent with the finding that rapamcyin–FKBP complexes target TORC2 rather than TORC1 in the trypanosome bloodstream forms (13). All trypanosomatid TORs showed substitutions at residues corresponding to mTOR Ser2035 (Ala in TOR2s and TOR3/TbTOR-like 1, Trp in L. major and Leu in trypanosome TOR1s), which disrupt FKBP12/rapamycin binding to mTOR (21). Correspondingly, L. major promastigotes and the insect and bloodstream forms of trypanosomes are relatively insensitive to rapamycin (13, 24).
L. major TOR1 and TOR2 Are Likely Essential Genes, but tor3− Null Mutants Are Viable as Promastigotes.
The Leishmania genome is predominantly diploid, and two rounds of gene replacement are required to obtain null mutants (25). For both TOR1 and TOR2, heterozygotes were readily obtained, but we were unable to generate homozygous knockouts of either gene despite multiple well-controlled attempts (Fig. S3 A and B). These results suggest that L. major TOR1 and TOR2 may be essential.
In contrast, homozygous tor3− null mutants were readily obtained by two successive rounds of targeting (Figs. S3C and S4 A and B). An episomal expression construct bearing an N-terminal Myc tag (pXNG4SAT-MycTOR3) was introduced into both tor3− and WT (termed tor3−/+TOR3 and WT/+TOR3, respectively). Western blotting with anti-Myc antibodies revealed a band of molecular weight greater than 250 KDa in both lines, in agreement with the 295-KDa expected size of Myc-TOR3 (Fig. S4C).
TOR3 Affects Growth Rate but Not Cell Size of L. major Promastigotes.
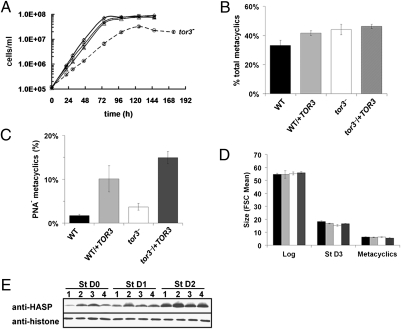
The tor3− mutant grew slower in culture, with a 10- to 11-h doubling time vs. 8 h for WT, reaching a stationary phase density of ∼25% that of WT (Fig. 2A). Both tor3−/+TOR3 and WT/+ TOR3 grew normally (Fig. 2A), showing that slower growth arose from loss of TOR3. All lines showed the same morphology and size in both log and stationary growth phases [55 ± 0.7 and 16.6 ± 1.2 arbitrary units (AU)], as assessed by forward light scatter by flow cytometry (26) (Fig. 2D).
Fig. 2.
L. major tor3− promastigotes have a slower-growth phenotype but do not show defective metacyclogenesis. (A) Growth of L. major promastigotes in vitro. WT (◇), WT/+MycTOR3 (□), tor3− (○), and tor3−/+MycTOR3 (Δ). Error bars show SE mean for two independent experiments. (B) Percentage of metacyclics in the total cell population on the second day of stationary phase (St D2) as determined by flow cytometry and gating for the smaller metacyclic vs. promastigote populations. Because tor3− grows more slowly and reaches stationary phase with a 1-d delay, St D2 occurs at 147 h for tor3− and 125 h for the other lines. Bars in Figs. 2–4 are shaded: WT, black; WT/+TOR3, gray; tor3−, white; and tor3−/+TOR3, darker gray. (C) Percentage of PNA- metacyclics, measured on St D2 as described above. (D) Size of promastigotes as measured by flow cytometry using the forward scatter (FSC) during logarithmic growth (Log), stationary phase (St D3), or of purified PNA− metacyclics (isolated on St D3). (E) Total cell lysates of promastigotes harvested upon entry or after 1 or 2 d in stationary phase (St D0,1,2) were analyzed by Western blotting with antisera to the metacyclic promastigote protein HASPB (28). Lanes are as follows: 1, WT; 2; WT/+TOR3; 3, tor3−; and 4, tor3−/+TOR3. Antihistone H2A antibodies were used as loading control.
Upon entry into stationary phase during in vitro culture, a significant fraction of Leishmania parasites differentiate into infective metacyclic forms, accompanied by changes in cell morphology, size, gene, and surface protein expression (2, 27, 28). First, the metacyclic marker protein HASPB was expressed similarly in WT, tor3−, tor3−/+TOR3, and WT/+ TOR3 (Fig. 2E). Second, the percentage of “small” metacyclics was assessed by flow cytometry and size gating; the percentage of small cells was similar, with the tor3-, tor3−/+ TOR3, and WT/+TOR3 lines somewhat increased relative to WT (33 ± 4% vs. 42–46 ± 1–3%; Fig. 2B). Third, a subset of metacyclic parasites fail to bind the lectin peanut agglutinin (PNA), which can be exploited to purify these parasites (27, 29). PNA− metacyclics again showed a similar size (6.4 ± 0.3 AU; Fig. 2D). Finally, tor3− parasites showed no reduction in levels of PNA− metacyclic formation (WT, 1.7 ± 0.3%, vs. tor3−, 3.6 ± 0.8% ; Fig. 2C). The tor3−/+ TOR3 and WT/+TOR3 cell lines showed an increase in the percentage of PNA− metacyclics (10.1 ± 3.0% and 14.9 ± 1.4%, respectively). This may reflect the properties of the episomal expression vector, which typically results in overexpression (30). These data establish that tor3− mutants are not defective in metacyclogenesis and are not altered in size.
Infectivity of L. major tor3− to Mice Is Highly Attenuated.
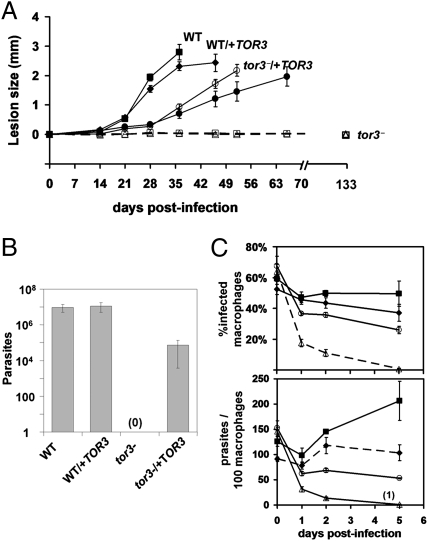
In footpad inoculations of susceptible BALB/c mice, metacyclic or stationary phase WT parasites induced progressive lesion pathology in a dose-dependent manner. Although heterozygous TOR3 replacements resembled WT, several independent tor3− lines failed to induce any lesion pathology even when inoculated at high levels (107 stationary cells) more than 1 y postinfection. In contrast, 10 independent tor3−/+TOR3 lines showed pathology similar to that of WT. We followed lesion pathology in Balb/C mice quantitatively after inoculation with lower numbers of parasites (106 stationary cells or 105 metacyclics); again, tor3− mutants failed to show pathology, which was restored by complementation with episomal expression of TOR3 (Fig. 3A and Fig. S5). As often seen in Leishmania virulence studies, complementation with the episomal vector was imperfect, with a 2- to 3-wk delay in lesion formation relative to WT. Nonetheless, the substantive restoration compared with the complete attenuation of tor3− argues that the effect on virulence is specific.
Fig. 3.
L. major tor3− mutant has highly attenuated virulence during the amastigote stage. (A) Increase in footpad thickness after inoculation of 105 purified PNA− metacyclics into Balb/C mice; WT (■), WT/+TOR3 (◆), two clonal lines of tor3− (□, △) and tor3−/+TOR3 (○,●). SEs are shown (n = 4). (B) Parasitemia was measured by limiting dilution assay at day 21 postinfection in an experiment similar to that shown in (A), except that the inoculums was 106 stationary phase cells/foot. SEs are shown (n = 4). (C) Macrophage infections. WT (■), WT/+TOR3 (◆), tor3− (△) and tor3−/+TOR3 (○). Three independent experiments were performed, with triplicate samples within each; data show results of one representative experiment. Bars indicate SEs for triplicate wells.
Several studies were undertaken to establish whether parasites persisted in the absence of pathology. After inoculation of 106 stationary phase cells, parasites were recovered from only 1/16 total tor3− infected mice after 19–25 wk (580 ± 170 parasites). In a second experiment, mice were examined at 21 d postinfection; WT and WT/+ TOR3 infections yielded comparable numbers of parasites (∼107 /foot), whereas no tor3− parasites were recovered (Fig. 3B). Partial rescue was again seen with tor3−/+ TOR3 (∼105/foot). Increased survival of tor3− was seen with higher inocula. Although lesions were never observed with 107 stationary phase parasites (16 mice), 7/8 mice showed small lesions (0.08–0. 6 mm) when inoculated with 108 parasites. These were accompanied by a correspondingly low level of parasitemia (0–1,000 with the 107 inocula, to 104–105 with the 108; Fig. S6). Thus, under some conditions, limited persistence of tor3− occurred. However, preliminary studies of recovered persistent parasites did not reveal any evidence of secondary changes leading to elevated virulence.
tor3− Is Unable to Survive in Macrophages.
Although tor3− parasites showed normal invasion of peritoneal exudate macrophages, these parasites were rapidly eliminated afterward, decreasing 80% within the first 2 d and nearly eliminated after 5 d (Fig. 3C). Restoration of TOR3 in turn rescued parasite survival, albeit to levels lower than seen in WT. Interestingly, the WT/+TOR3 line also showed somewhat reduced macrophage survival (Fig. 3C), again pointing to effects associated with TOR3 overexpression from episomal vectors. These data argue that TOR3 is required for establishment and survival of L. major in macrophage infections.
tor3− Shows an ACs Defect.
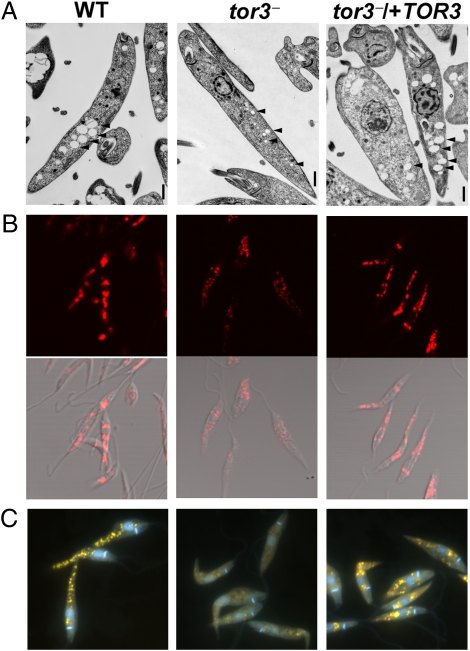
Although tor3− promastigotes appeared morphologically normal, transmission EM showed alterations in ACs, which appeared to be 2-fold smaller (0.6 vs. 0.3 μM; Fig. 4A and Fig. S7). ACs are acidic organelles that store calcium and other divalent cations, as well as pyrophosphate and polyphosphates (Poly-P) (31). ACs were visualized by reactivity with antisera to the marker T. brucei VP1, a vacuolar proton pyrophosphatase (32). This confirmed the smaller AC size and increased numbers, which suggested some AC fragmentation in tor3− (Fig. 4B). Both phenotypes were reversed by restoration of TOR3 (Fig. 4 A and B). DAPI staining was used to visualize Poly-P accumulation, which upon binding exhibits strong fluorescence emission at 530 nm (33, 34). DAPI staining was greatly reduced in the tor3− mutant, which again was reversed upon restoration of TOR3 (Fig. 4C). These data establish that tor3− is defective in ACs formation and Poly-P accumulation therein.
Fig. 4.
L. major tor3− promastigotes show alterations in ACs structure and polyphosphate content. (A) Transmission EM reveal much smaller ACs (arrows) in tor3− compared with WT or tor3−/+TOR3 parasites. (Scale bars, 1 μm.) (B) Immunofluorescence with anti-TbVP1 antibody. (Upper) Results. (Lower) superimposition of results on DIC image. (C) Polyphosphate staining. DAPI staining of paraformaldehyde-fixed promastigotes in late stationary phase to detect polyphosphates (yellow fluoresecence) in the ACs.
TOR3 Is Involved in Environmental Stress Sensing.
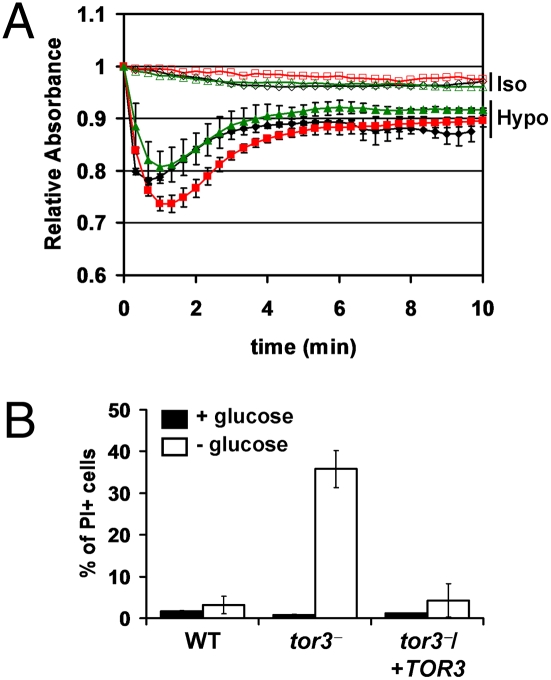
Both TOR kinases and ACs have been implicated in sensing environmental signals including nutrients and osmotic stress (2–4, 35). tor3− was exposed to hypo-osmotic stress, and changes in cell volume were monitored by light scattering, in which a decrease in absorbance corresponds to an increase in volume (36). As expected, in WT and tor3−/+TOR3 promastigotes, the volume quickly increased, peaking after 1 min and recovering after 3 min (Fig. 5A). However, tor3− parasites showed a more pronounced increase in volume and required 5 min to stabilize.
Fig. 5.
Lack of TOR3 in L. major promastigotes show alterations in response to hyposmotic stress and elevated sensitivity to glucose starvation. Promastigotes were subjected to isotonic (open symbols) or hypo-osmotic (filled symbols) conditions, and the absorbance monitored over time as described in Materials and Methods. (A) WT (♢, ◆), tor3− ( ,
,  ), and tor3−/+TOR3 (
), and tor3−/+TOR3 ( ,
,  ). Volume recovery was measured by light scattering. Result shown is for one of two similar experiments, each containing duplicate samples. (B) Glucose tolerance. Parasites were grown in RPMI media with glucose (closed bars) or without glucose (open bars). After 4 d, viability was assessed by uptake of propidium iodide using flow cytometry (PI+ indicates dead cells). Error bars are SDs of two independent experiments.
). Volume recovery was measured by light scattering. Result shown is for one of two similar experiments, each containing duplicate samples. (B) Glucose tolerance. Parasites were grown in RPMI media with glucose (closed bars) or without glucose (open bars). After 4 d, viability was assessed by uptake of propidium iodide using flow cytometry (PI+ indicates dead cells). Error bars are SDs of two independent experiments.
Next, tor3− promastigotes were tested for their sensitivity to starvation for serum, amino acids, or glucose. In contrast to TOR mutants in other species, tor3- showed no alterations in sensitivity to amino acids and serum starvation. However, tor3− promastigotes were highly sensitive to glucose starvation; after 4 d in culture medium lacking glucose ≈35% of the mutant parasites were dead, in contrast to only 2% for WT or tor3−/+TOR3− cells (Fig. 5B). Thus the absence of TOR3 confers sensitivity to glucose but not amino acid or serum starvation.
Discussion
TOR kinases are evolutionarily conserved in eukaryotes, where they play a major role in sensing and integrating nutrient/energy status and mitogens in the environment to promote coordinated cell growth and replication (reviewed in refs. 15, 37, 38). Although most organisms encode only 1 or 2 TOR kinases, in L. major we identified at least three members of the TOR kinase family. L. major TOR1 and TOR2 are orthologous to trypanosome TbTOR1 and TbTOR2, which carry out typical TOR kinase functions such as growth control, size, and macroautophagy (TbTOR1), or cell polarization, endocytosis, and cytokinesis (TbTOR2) (13, 39, 40). Inducible RNAi knockdowns suggested TbTOR1 and TbTOR2 were essential, and similarly we were unable to generate null mutants in TOR1 or TOR2 in Leishmania major. In the future, it may be possible to carry out further tests of TOR1 and TOR2 function in Leishmania, by generating hypomorphic or conditional mutations using a unique protein-based regulatory system (24), or by exploiting the possibility of RNAi technology in the distantly related species L. braziliensis (41).
We identified a third TOR kinaseTOR3, based on the clear presence of an FRB domain (Fig. 1). This important TOR domain was originally identified as the binding site of FKBP12/rapamycin complexes to some but not all TORs, and since has been shown to mediate important TOR functions independent of this, including binding to phosphatidic acid (10–12). Trypanosomatid TORs show some diversity in FRB structure (Fig. 1C) and function relative to other species, as evidenced by the binding of TbFKB12 to TbTOR2 but not TbTOR1 or TOR-like 1 (13).
TOR3: A TOR Kinase with “Nonclassical” Function in Leishmania Virulence.
Notably, the role of TOR3 in L. major is distinct from that of other TORs. tor3− Null mutants were readily obtained, and in culture appeared remarkably normal, growing only somewhat more slowly than WT, and retaining the ability to differentiate to the infective metacyclic stage in vitro, without affecting cell size or shape (Fig. 2). However, the virulence of the tor3− mutant in susceptible Balb/C mice model was dramatically attenuated, and tor3− was unable to survive or replicate in macrophages cultured in vitro (Fig. 3). Only after inoculation of massive numbers of parasites could persistence or occasional lesion pathology occur. Importantly, all phenotypes could be reversed, fully or partially, upon restoration of TOR3 expression.
TOR3 and AC Function.
Unexpectedly, tor3- showed strong alterations in ACs. ACs are ancient organelles, typically associated with storage of cations such as calcium and zinc, and polyphosphates of various chain lengths (31). ACs play important roles in calcium homeostasis, intracellular pH, osmoregulation, and phosphate metabolism, and through the high-energy phosphoanhydride Poly-P bonds, as a crucial source of energy (31, 42, 43). EM and immunofluorescence microscopy showed that the tor3− mutant had altered ACs, with a fragmented appearance consisting of numerous smaller vesicles, most clearly evident when visualized with a specific AC membrane marker (Fig. 4 and Fig. S7). tor3− ACs showed functional alterations as well, as evident by a severe defect in Poly-P accumulation (Fig. 4C). Consistent with a role for ACs and Poly-P in adaptation to extreme conditions and energy metabolism, tor3− was highly sensitive to glucose starvation (Fig. 5B), and showed defects in responding to osmotic shock (Fig. 5A). Thus, in every parameter studied, the AC in the tor3− was both structurally and functionally compromised. Again emphasizing its functional divergence from other TORs, tor3− was insensitive to serum or amino acid starvation.
How might TOR3 control AC function? TOR kinases generally occur in complexes (37), and TOR3 may also occur in a complex, which in trypanosomes differs from those formed by both TOR1 and TOR2 (13). Although the basis of TOR kinase specificity is not well understood, cellular localization and membrane localization are likely to be important, and TOR3 bears a PDZ domain often implicated in protein–protein interactions and membrane targeting (13, 44–46) (Fig. 1A). The strong effects of TOR3 on AC function raised the possibility that TOR3 may be localized to this organelle, and preliminary immunoblotting data suggest the MycTOR3 protein localizes to particulate fractions. However we have been unable to visualize TOR3 by fluorescence or immune EM microscopy, despite the use of a variety of methods and antisera. The identification of the components of a putative TOR3 complex as well as its specific substrates are the obvious next steps toward a deeper understanding of the pathways involved in the control of Leishmania virulence by this protein kinase.
ACs and/or Poly-Ps have been shown to play important roles in adaptation to changing environmental conditions, including starvation and osmotic stress, which are encountered by Leishmania and other pathogens in the interactions with mammalian hosts (reviewed in refs. 31, 42, 43).
Alterations in both ACs and poly-P levels have been associated with reduced virulence in trypanosomatids (32–34, 43, 47–52), and the importance of AC during the infectious cycle is also supported by quantitative studies of AC abundance, which show their numbers to increase 3- to 6-fold in amastigotes (53). The inability of L. major tor3− parasites to survive in the macrophages or mice may reflect their increased sensitivity to glucose starvation (Fig. 5B), as the phagolysosome is thought to be a compartment poor in hexoses (54, 55), and deregulation of other important AC functions in the tor3− mutants may similarly account for their poor survival in the mammalian host. The similarity in the phenotypes seen in studies of AC protein mutants with those of tor3− leads us to propose that the tor3− phenotype(s) may arise solely from their affect on AC. In the future, studies of tor3− will help to elucidate the dynamics involved in the origin and maintenance of the structure of the ACs.
Altogether, our data show that L. major TOR3 has remarkably little phenotype during growth in vitro, but is required for survival and disease pathology in the mammalian host. In addition to shedding light on the role of the AC and TOR kinases in virulence, these studies have several practical implications. First, the limited persistence of tor3− under some conditions suggests some potential in live vaccination studies. Second, TOR3 inhibition may serve as an attractive target for chemotherapy. Recent work has led to the development of drugs that target the mTOR pathway (56–59). The strong divergence of trypanosomatid TORs generally and the function of TOR3s specifically suggest that there may similarly be an opportunity for the design of selective antiparasite TOR agents in the future.
Materials and Methods
Leishmania Culture and Isolation of Metacyclics.
L. major Friedlin clone V1 (MHOM/IL/81/Friedlin) and LV39 clone 5 (Rho/SU/59/P) were grown in M199-based medium supplemented with 10% FBS, 62.5 μM adenine, and 2 μg/mL biopterin (60). Cell density was determined by using a model Z1 Coulter counter (logarithmic phase) or hemocytometer (stationary phase). Metacyclics were isolated by negative selection with PNA (29) or by Ficoll gradient centrifugation (27).
In starvation studies, promastigotes were grown in M199 medium until mid log phase (∼8 × 106 cells/mL), washed in incomplete RPMI lacking serum, amino acids, and glucose, and then split into flasks with different RPMI compositions. Complete RPMI (Gibco-BRL) medium was supplemented with 30 mM Hepes, pH 7.4, 62.5 μM adenine, 2 μg/mL biopterin, 5 μg/mL hemin, and 1% (vol/vol) heat-inactivated FCS (61).
Cell volume was measured by forward-scatter using a FACSCalibur cytometer (Beckton-Dickinson), and analyszed used CellQuest software. Samples were incubated with propidium iodide (0.5μg/mL) in M199 for 5 min at room temperature to assess viability. Methodology for assays of AC-related phenotype in the tor3− mutant, such as EM, immunofluorescence, and DAPI staining are detailed in SI Text. Regulatory volume decrease response was measured by light scatter (36). Briefly, 108 late log-phase promastigotes were washed twice in isotonic Iso-Cl buffer (20 mM Hepes, pH 7.4, 11 mM glucose, 1 mM CaCl2, 0.8 mM MgSO4, 137 mM NaCl, 4 mM KCl, 1.5 mM K2HPO4, and 8.5 mM Na2HPO4), and resuspended in 550 μL Iso-Cl, after which 250-μL aliquots were added to cuvettetes containing an equal volumes of Iso-Cl or water (hypotonic stress). Absorbance at 550 nm was measured using a Beckman DU640 spectrophotometer at 10-s intervals for 10 min.
Targeted Gene Replacement of L. major TORs.
TOR kinase domains were identified in searches of the Pfam database (62), and the FRB domain model was viewed using the Swiss-PdbViewer (63). We used gene replacement by homologous recombination to generate knockout lines of each of the L. major TOR kinases. Details on the replacement targeting cassettes, primers, and procedures used are provided in SI Text and Table S2.
Infectivity Studies.
L. major FV1 promastigotes (stationary phase or purified metacyclics) were injected s.c. into the left hind footpads of 6- to 8-wk-old female BALB/C mice (Charles River Laboratories). Unless otherwise stated, parasites were harvest after 3 d in the stationary phase. Lesion sizes were measured using by Vernier calipers, and parasite numbers were enumerated by limiting dilution assay (64). In vitro infection of peritoneal macrophages was performed as described previously (65, 66), using metacyclics isolated from a Ficoll density gradient (27).
Supplementary Material
Acknowledgments
We thank W. Beatty and L. Gu (Imaging Facility of the Department of Molecular Microbiology, Washington University School of Medicine) for EM imaging; R. Docampo (University of Georgia) for anti-TbVP1 antisera; A. Barquilla and M. Navarro (University of Granada) for antisera; S. Schenkman (Universidade Federal do Estado de São Paulo) for suggesting the acidocalcisome defect; D. A. Scott for protocols; D. F. Smith (Hull York Medical School) for anti-HASPB antisera; I. L. K. Wong (Hong Kong Polytechnic University, Hong Kong SAR) for anti-H2A antibodies; and all of these individuals and the members of our laboratory for helpful discussions. L.M.d.S. was partially funded by the Washington University Infectious Diseases Scholar Program. This work was supported by National Institutes of Health Grant AI29646.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1004599107/-/DCSupplemental.
References
- 1.Alvar J, et al. The relationship between leishmaniasis and AIDS: The second 10 years. Clin Microbiol Rev. 2008;21:334–359. doi: 10.1128/CMR.00061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bates PA, Rogers ME. New insights into the developmental biology and transmission mechanisms of Leishmania. Curr Mol Med. 2004;4:601–609. doi: 10.2174/1566524043360285. [DOI] [PubMed] [Google Scholar]
- 3.Kamhawi S. Phlebotomine sand flies and Leishmania parasites: Friends or foes? Trends Parasitol. 2006;22:439–445. doi: 10.1016/j.pt.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 4.Naderer T, McConville MJ. The Leishmania-macrophage interaction: A metabolic perspective. Cell Microbiol. 2008;10:301–308. doi: 10.1111/j.1462-5822.2007.01096.x. [DOI] [PubMed] [Google Scholar]
- 5.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- 7.Sabers CJ, et al. Isolation of a protein target of the FKBP12-rapamycin complex in mammalian cells. J Biol Chem. 1995;270:815–822. doi: 10.1074/jbc.270.2.815. [DOI] [PubMed] [Google Scholar]
- 8.Abraham RT. PI 3-kinase related kinases: ‘Big’ players in stress-induced signaling pathways. DNA Repair (Amst) 2004;3:883–887. doi: 10.1016/j.dnarep.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Bakkenist CJ, Kastan MB. Initiating cellular stress responses. Cell. 2004;118:9–17. doi: 10.1016/j.cell.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 10.Veverka V, et al. Structural characterization of the interaction of mTOR with phosphatidic acid and a novel class of inhibitor: Compelling evidence for a central role of the FRB domain in small molecule-mediated regulation of mTOR. Oncogene. 2008;27:585–595. doi: 10.1038/sj.onc.1210693. [DOI] [PubMed] [Google Scholar]
- 11.Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science. 2001;294:1942–1945. doi: 10.1126/science.1066015. [DOI] [PubMed] [Google Scholar]
- 12.Foster DA. Regulation of mTOR by phosphatidic acid? Cancer Res. 2007;67:1–4. doi: 10.1158/0008-5472.CAN-06-3016. [DOI] [PubMed] [Google Scholar]
- 13.Barquilla A, Crespo JL, Navarro M. Rapamycin inhibits trypanosome cell growth by preventing TOR complex 2 formation. Proc Natl Acad Sci USA. 2008;105:14579–14584. doi: 10.1073/pnas.0802668105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosotti R, Isacchi A, Sonnhammer EL. FAT: A novel domain in PIK-related kinases. Trends Biochem Sci. 2000;25:225–227. doi: 10.1016/s0968-0004(00)01563-2. [DOI] [PubMed] [Google Scholar]
- 15.Crespo JL, Hall MN. Elucidating TOR signaling and rapamycin action: Lessons from Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2002;66:579–591. doi: 10.1128/MMBR.66.4.579-591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Proud CG. The multifaceted role of mTOR in cellular stress responses. DNA Repair (Amst) 2004;3:927–934. doi: 10.1016/j.dnarep.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Fanning AS, Anderson JM. Protein modules as organizers of membrane structure. Curr Opin Cell Biol. 1999;11:432–439. doi: 10.1016/S0955-0674(99)80062-3. [DOI] [PubMed] [Google Scholar]
- 18.Brunn GJ, et al. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J. 1996;15:5256–5267. [PMC free article] [PubMed] [Google Scholar]
- 19.Kelley LA, Sternberg MJ. Protein structure prediction on the Web: A case study using the Phyre server. Nat Protoc. 2009;4:363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- 20.Choi J, Chen J, Schreiber SL, Clardy J. Structure of the FKBP12-rapamycin complex interacting with the binding domain of human FRAP. Science. 1996;273:239–242. doi: 10.1126/science.273.5272.239. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Zheng XF, Brown EJ, Schreiber SL. Identification of an 11-kDa FKBP12-rapamycin-binding domain within the 289-kDa FKBP12-rapamycin-associated protein and characterization of a critical serine residue. Proc Natl Acad Sci USA. 1995;92:4947–4951. doi: 10.1073/pnas.92.11.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lorenz MC, Heitman J. TOR mutations confer rapamycin resistance by preventing interaction with FKBP12-rapamycin. J Biol Chem. 1995;270:27531–27537. doi: 10.1074/jbc.270.46.27531. [DOI] [PubMed] [Google Scholar]
- 23.Vilella-Bach M, Nuzzi P, Fang Y, Chen J. The FKBP12-rapamycin-binding domain is required for FKBP12-rapamycin-associated protein kinase activity and G1 progression. J Biol Chem. 1999;274:4266–4272. doi: 10.1074/jbc.274.7.4266. [DOI] [PubMed] [Google Scholar]
- 24.Madeira da Silva L, Owens KL, Murta SM, Beverley SM. Regulated expression of the Leishmania major surface virulence factor lipophosphoglycan using conditionally destabilized fusion proteins. Proc Natl Acad Sci USA. 2009;106:7583–7588. doi: 10.1073/pnas.0901698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cruz A, Coburn CM, Beverley SM. Double targeted gene replacement for creating null mutants. Proc Natl Acad Sci USA. 1991;88:7170–7174. doi: 10.1073/pnas.88.16.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saraiva EM, et al. Flow cytometric assessment of Leishmania spp metacyclic differentiation: Validation by morphological features and specific markers. Exp Parasitol. 2005;110:39–47. doi: 10.1016/j.exppara.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Späth GF, Beverley SM. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp Parasitol. 2001;99:97–103. doi: 10.1006/expr.2001.4656. [DOI] [PubMed] [Google Scholar]
- 28.McKean PG, Denny PW, Knuepfer E, Keen JK, Smith DF. Phenotypic changes associated with deletion and overexpression of a stage-regulated gene family in Leishmania. Cell Microbiol. 2001;3:511–523. doi: 10.1046/j.1462-5822.2001.00135.x. [DOI] [PubMed] [Google Scholar]
- 29.Sacks DL, Perkins PV. Identification of an infective stage of Leishmania promastigotes. Science. 1984;223:1417–1419. doi: 10.1126/science.6701528. [DOI] [PubMed] [Google Scholar]
- 30.Murta SM, Vickers TJ, Scott DA, Beverley SM. Methylene tetrahydrofolate dehydrogenase/cyclohydrolase and the synthesis of 10-CHO-THF are essential in Leishmania major. Mol Microbiol. 2009;71:1386–1401. doi: 10.1111/j.1365-2958.2009.06610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Docampo R, de Souza W, Miranda K, Rohloff P, Moreno SN. Acidocalcisomes - conserved from bacteria to man. Nat Rev Microbiol. 2005;3:251–261. doi: 10.1038/nrmicro1097. [DOI] [PubMed] [Google Scholar]
- 32.Lemercier G, et al. A vacuolar-type H+-pyrophosphatase governs maintenance of functional acidocalcisomes and growth of the insect and mammalian forms of Trypanosoma brucei. J Biol Chem. 2002;277:37369–37376. doi: 10.1074/jbc.M204744200. [DOI] [PubMed] [Google Scholar]
- 33.Fang J, et al. Overexpression of a Zn2+-sensitive soluble exopolyphosphatase from Trypanosoma cruzi depletes polyphosphate and affects osmoregulation. J Biol Chem. 2007;282:32501–32510. doi: 10.1074/jbc.M704841200. [DOI] [PubMed] [Google Scholar]
- 34.Luo S, Ruiz FA, Moreno SN. The acidocalcisome Ca2+-ATPase (TgA1) of Toxoplasma gondii is required for polyphosphate storage, intracellular calcium homeostasis and virulence. Mol Microbiol. 2005;55:1034–1045. doi: 10.1111/j.1365-2958.2004.04464.x. [DOI] [PubMed] [Google Scholar]
- 35.Leslie G, Barrett M, Burchmore R. Leishmania mexicana: Promastigotes migrate through osmotic gradients. Exp Parasitol. 2002;102:117–120. doi: 10.1016/s0014-4894(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 36.Schoijet AC, et al. A Trypanosoma cruzi phosphatidylinositol 3-kinase (TcVps34) is involved in osmoregulation and receptor-mediated endocytosis. J Biol Chem. 2008;283:31541–31550. doi: 10.1074/jbc.M801367200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 38.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 39.Barquilla A, Navarro M. Trypanosome TOR as a major regulator of cell growth and autophagy. Autophagy. 2009;5:256–258. doi: 10.4161/auto.5.2.7591. [DOI] [PubMed] [Google Scholar]
- 40.Barquilla A, Navarro M. Trypanosome TOR complex 2 functions in cytokinesis. Cell Cycle. 2009;8:697–699. doi: 10.4161/cc.8.5.7808. [DOI] [PubMed] [Google Scholar]
- 41.Peacock CS, et al. Comparative genomic analysis of three Leishmania species that cause diverse human disease. Nat Genet. 2007;39:839–847. doi: 10.1038/ng2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown MR, Kornberg A. Inorganic polyphosphate in the origin and survival of species. Proc Natl Acad Sci USA. 2004;101:16085–16087. doi: 10.1073/pnas.0406909101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rao NN, Gómez-García MR, Kornberg A. Inorganic polyphosphate: Essential for growth and survival. Annu Rev Biochem. 2009;78:605–647. doi: 10.1146/annurev.biochem.77.083007.093039. [DOI] [PubMed] [Google Scholar]
- 44.Ponting CP, Phillips C, Davies KE, Blake DJ. PDZ domains: Targeting signalling molecules to sub-membranous sites. Bioessays. 1997;19:469–479. doi: 10.1002/bies.950190606. [DOI] [PubMed] [Google Scholar]
- 45.Sturgill TW, et al. TOR1 and TOR2 have distinct locations in live cells. Eukaryot Cell. 2008;7:1819–1830. doi: 10.1128/EC.00088-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Díaz-Troya S, Florencio FJ, Crespo JL. Target of rapamycin and LST8 proteins associate with membranes from the endoplasmic reticulum in the unicellular green alga Chlamydomonas reinhardtii. Eukaryot Cell. 2008;7:212–222. doi: 10.1128/EC.00361-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Besteiro S, Tonn D, Tetley L, Coombs GH, Mottram JC. The AP3 adaptor is involved in the transport of membrane proteins to acidocalcisomes of Leishmania. J Cell Sci. 2008;121:561–570. doi: 10.1242/jcs.022574. [DOI] [PubMed] [Google Scholar]
- 48.Espiau B, et al. A soluble pyrophosphatase, a key enzyme for polyphosphate metabolism in Leishmania. J Biol Chem. 2006;281:1516–1523. doi: 10.1074/jbc.M506947200. [DOI] [PubMed] [Google Scholar]
- 49.Fang J, Rohloff P, Miranda K, Docampo R. Ablation of a small transmembrane protein of Trypanosoma brucei (TbVTC1) involved in the synthesis of polyphosphate alters acidocalcisome biogenesis and function, and leads to a cytokinesis defect. Biochem J. 2007;407:161–170. doi: 10.1042/BJ20070612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lemercier G, et al. A pyrophosphatase regulating polyphosphate metabolism in acidocalcisomes is essential for Trypanosoma brucei virulence in mice. J Biol Chem. 2004;279:3420–3425. doi: 10.1074/jbc.M309974200. [DOI] [PubMed] [Google Scholar]
- 51.Rodrigues CO, Ruiz FA, Vieira M, Hill JE, Docampo R. An acidocalcisomal exopolyphosphatase from Leishmania major with high affinity for short chain polyphosphate. J Biol Chem. 2002;277:50899–50906. doi: 10.1074/jbc.M208940200. [DOI] [PubMed] [Google Scholar]
- 52.Ruiz FA, Rodrigues CO, Docampo R. Rapid changes in polyphosphate content within acidocalcisomes in response to cell growth, differentiation, and environmental stress in Trypanosoma cruzi. J Biol Chem. 2001;276:26114–26121. doi: 10.1074/jbc.M102402200. [DOI] [PubMed] [Google Scholar]
- 53.Zhang K, et al. Leishmania salvage and remodelling of host sphingolipids in amastigote survival and acidocalcisome biogenesis. Mol Microbiol. 2005;55:1566–1578. doi: 10.1111/j.1365-2958.2005.04493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naderer T, et al. Virulence of Leishmania major in macrophages and mice requires the gluconeogenic enzyme fructose-1,6-bisphosphatase. Proc Natl Acad Sci USA. 2006;103:5502–5507. doi: 10.1073/pnas.0509196103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodríguez-Contreras D, Landfear SM. Metabolic changes in glucose transporter-deficient Leishmania mexicana and parasite virulence. J Biol Chem. 2006;281:20068–20076. doi: 10.1074/jbc.M603265200. [DOI] [PubMed] [Google Scholar]
- 56.Bjornsti MA, Houghton PJ. The TOR pathway: A target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 57.Brachmann S, Fritsch C, Maira SM, García-Echeverría C. PI3K and mTOR inhibitors: A new generation of targeted anticancer agents. Curr Opin Cell Biol. 2009;21:194–198. doi: 10.1016/j.ceb.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 58.Feldman ME, et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009 doi: 10.1371/journal.pbio.1000038. 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guertin DA, Sabatini DM. The pharmacology of mTOR inhibition. Sci Signal. 2009 doi: 10.1126/scisignal.267pe24. 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- 60.Kapler GM, Coburn CM, Beverley SM. Stable transfection of the human parasite Leishmania major delineates a 30-kilobase region sufficient for extrachromosomal replication and expression. Mol Cell Biol. 1990;10:1084–1094. doi: 10.1128/mcb.10.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vickers TJ, et al. Biochemical and genetic analysis of methylenetetrahydrofolate reductase in Leishmania metabolism and virulence. J Biol Chem. 2006;281:38150–38158. doi: 10.1074/jbc.M608387200. [DOI] [PubMed] [Google Scholar]
- 62.Finn RD, et al. The Pfam protein families database. Nucleic Acids Res. 2008;36(Database issue):D281–D288. doi: 10.1093/nar/gkm960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: An environment for comparative protein modeling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 64.Titus RG, Marchand M, Boon T, Louis JA. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985;7:545–555. doi: 10.1111/j.1365-3024.1985.tb00098.x. [DOI] [PubMed] [Google Scholar]
- 65.Racoosin EL, Beverley SM. Leishmania major: Promastigotes induce expression of a subset of chemokine genes in murine macrophages. Exp Parasitol. 1997;85:283–295. doi: 10.1006/expr.1996.4139. [DOI] [PubMed] [Google Scholar]
- 66.Späth GF, et al. Lipophosphoglycan is a virulence factor distinct from related glycoconjugates in the protozoan parasite Leishmania major. Proc Natl Acad Sci USA. 2000;97:9258–9263. doi: 10.1073/pnas.160257897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.