Abstract
From bird flocks to fish schools, animal groups often seem to react to environmental perturbations as if of one mind. Most studies in collective animal behavior have aimed to understand how a globally ordered state may emerge from simple behavioral rules. Less effort has been devoted to understanding the origin of collective response, namely the way the group as a whole reacts to its environment. Yet, in the presence of strong predatory pressure on the group, collective response may yield a significant adaptive advantage. Here we suggest that collective response in animal groups may be achieved through scale-free behavioral correlations. By reconstructing the 3D position and velocity of individual birds in large flocks of starlings, we measured to what extent the velocity fluctuations of different birds are correlated to each other. We found that the range of such spatial correlation does not have a constant value, but it scales with the linear size of the flock. This result indicates that behavioral correlations are scale free: The change in the behavioral state of one animal affects and is affected by that of all other animals in the group, no matter how large the group is. Scale-free correlations provide each animal with an effective perception range much larger than the direct interindividual interaction range, thus enhancing global response to perturbations. Our results suggest that flocks behave as critical systems, poised to respond maximally to environmental perturbations.
Keywords: animal groups, collective behavior, flocking, self-organization, emergent behavior
Of all distinctive traits of collective animal behavior the most conspicuous is the emergence of global order, namely the fact that all individuals within the group synchronize to some extent their behavioral state (1–3). In many cases global ordering amounts to an alignment of the individual directions of motion, as in bird flocks, fish schools, mammal herds, and in some insect swarms (4–6). Yet, global ordering can affect also other behavioral states, as it happens with the synchronous flashing of tropical fireflies (7) or the synchronous clapping in human crowds (8).
The presence of order within an animal group is easy to detect. However, order may have radically different origins, and discovering what is the underlying coordination mechanism is not straightforward. Order can be the effect of a top–down centralized control mechanism (for example, due to the presence of one or more leaders), or it can be a bottom–up self-organized feature emerging from local behavioral rules (9). In reality, the lines are often blurred and hierarchical and distributed control may combine together (10). However, even in the two extreme cases, discriminating between the two types of global ordering is not trivial. In fact, the prominent difference between the centralized and the self-organized paradigm is not order, but response.
Collective response is the way a group as a whole reacts to its environment. It is often crucial for a group, or for subsets of it, to respond coherently to perturbations. For gregarious animals under strong predatory pressure, in particular, collective response is vital (2, 11, 12). The remarkable thing about a flock of birds is not merely the globally ordered motion of the group, but the way the flock dodges a falcon's attack. Collective response is the trademark of self-organized order as opposed to a centralized one. Consider a group where all individuals follow a leader, without interacting with one another. Such a system is strongly ordered, as everyone moves in the same direction. Yet, there is no passing of information from individual to individual and hence behavioral fluctuations are independent: The change of direction of one animal (different from the leader) has very little influence on that of other animals, due to the centralized nature of information transfer. As a consequence, collective response is very poor: Unless detected directly by the leader, an external perturbation does not elicit a global reaction by the group. Response, unlike order, is the real signature of self-organization.
In self-organized groups the efficiency of collective response depends on the way individual behavioral changes, typically forced by localized environmental perturbations, succeed in modifying the behavior of the whole group. This key process is ruled by behavioral correlations. Correlation is the expression of an indirect information transfer mediated by the direct interaction between the individuals: Two animals that are outside their range of direct interaction (be it visual, acoustic, hydrodynamic, or any other) may still be correlated if information is transferred from one to another through the intermediate interacting animals. The turn of one bird attacked by a predator has an influence not only over the neighbors directly interacting with it, but also over all birds that are correlated to it. Correlation measures how the behavioral changes of one animal influence those of other animals across the group. Behavioral correlations are therefore ultimately responsible for the group's ability to respond collectively to its environment. In the same way, correlations are likely to play a fundamental role in other kinds of collective decision-making processes where informed individuals (e.g., on food location or migration routes) can extend their influence over many other group members (10).
Of course, behavioral correlations are the product of interindividual interaction. Yet interaction and correlation are different things and they may have a different spatial (and sometimes temporal) span. Interaction is local in space and its range is typically quite short. A former study (13) shows that in bird flocks the interaction range is of the order of few individuals. On the other hand, the correlation length, namely the spatial span of the correlation, can be significantly larger than the interaction range, depending chiefly on the level of noise in the system. An elementary example is the game of telephone: A player whispers a phrase into her neighbor's ear. The neighbor passes on the message to the next player and so on. The direct interaction range is equal to one, whereas the correlation length, i.e., the number of individuals the phrase can travel before being corrupted, can be significantly larger than one, depending on how clearly the information is transmitted at each step.
Although the correlation length is typically larger than the interaction range, in most biological and physical cases it is significantly smaller than the size of the system. For example, in bacteria the correlation length was found to be much smaller than the size of the swarm (14, 15). In this case parts of the group that are separated by a distance larger than the correlation length are by definition independent from each other and therefore react independently to environmental perturbations (16). Hence, the finite scale of the correlation necessarily limits the collective response of the group.
However, in some cases the correlation length may be as large as the entire group, no matter the group's size. When this happens, we are in the presence of scale-free correlations (17, 18). The group cannot be divided into independent subparts, because the behavioral change of one individual influences and is influenced by the behavioral change of all other individuals in the group. Scale-free correlations imply that the group is, in a strict sense, different from and more than the sum of its parts (19). The effective perception range of each individual is as large as the entire group and it becomes possible to transfer undamped information to all animals, no matter their distance, making the group respond as one. Here, we provide experimental evidence that bird flocks exhibit scale-free correlations and we discuss under what conditions such correlations may arise in animal groups.
Results
We measured the 3D positions and velocities of individual birds within large flocks of starlings (Sturnus vulgaris) in the field (20). Data were taken at sunset over a major roosting site in Rome in the winter months of 2005–2007 (Movies S1–S4). Analyzed flocks ranged from 122 to 4,268 individuals (21–23), two orders of magnitude larger than any previously studied animal group in three dimensions. The degree of global ordering in a flock is measured by the so-called polarization Φ,
 |
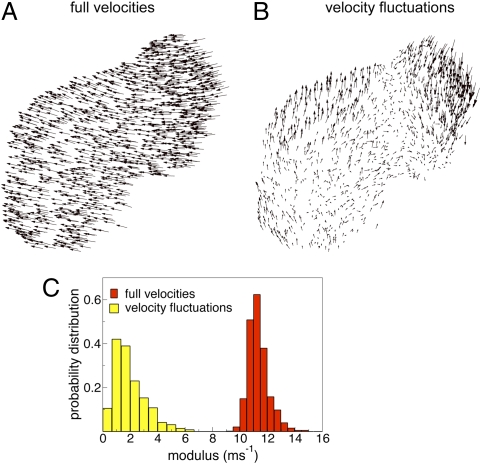
where 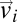 is the velocity of bird i and N is the total number of birds within the flock. The polarization is zero if the individual velocities are pointing in different directions, whereas it is close to one if most of them are nearly parallel. In fact, a nonzero value of Φ means that there is net motion of the center of mass. Polarization is therefore used as a standard measure of global order in the study of collective animal behavior (24, 25) (see also ref. 26 for a similar order parameter). In all analyzed flocks we found very high values of the polarization (Table S1). The average value over all 24 flocks is Φ = 0.96 ± 0.03 (SD), confirming the visual impression of strongly ordered birds’ velocities (see Fig. 1A for a 2D projection of the individual 3D velocities).
is the velocity of bird i and N is the total number of birds within the flock. The polarization is zero if the individual velocities are pointing in different directions, whereas it is close to one if most of them are nearly parallel. In fact, a nonzero value of Φ means that there is net motion of the center of mass. Polarization is therefore used as a standard measure of global order in the study of collective animal behavior (24, 25) (see also ref. 26 for a similar order parameter). In all analyzed flocks we found very high values of the polarization (Table S1). The average value over all 24 flocks is Φ = 0.96 ± 0.03 (SD), confirming the visual impression of strongly ordered birds’ velocities (see Fig. 1A for a 2D projection of the individual 3D velocities).
Fig. 1.
(A) 2D projection of the velocities of the individual birds within a starling flock at a fixed instant of time (flock 28-10; 1,246 birds, linear size L = 36.5 m). Vectors are scaled for clarity (see Dataset S1 for original data). The flock is strongly ordered and the velocities are all aligned. (B) 2D projection of the individual velocity fluctuations in the same flock at the same instant of time (vectors scaled for clarity). The velocity fluctuation is equal to the individual velocity minus the center of mass velocity, and therefore the spatial average of the fluctuations must be zero. Two large domains of strongly correlated birds are clearly visible. (C) Normalized probability distribution of the absolute value of the individual velocities and of the absolute value of the velocity fluctuations (same flock as in A and B). The velocity fluctuations are much smaller in modulus than the full velocities.
However, as we have stressed above, order tells us little about collective response. To learn something about response we must study how the fluctuations in the behavioral state (in this case the velocity) of one bird are correlated to those of another bird. Let us introduce for each bird i the fluctuation around the mean flock's velocity,
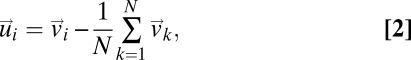 |
which is nothing else than the bird's velocity in the center of mass reference frame (assuming identical masses for all of the birds). The spatial mean of the velocity fluctuations is zero by construction,
 |
Relation [3] encodes the obvious fact that there cannot be overall net motion in the center of mass reference frame.
In Fig. 1C we report the probability distribution of the modulus of the full velocity (the speed) and of the modulus of the velocity fluctuations in a typical flock. The modulus of the fluctuations 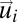 is on average much smaller than that of the velocities
is on average much smaller than that of the velocities 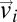 . This is expected, because the polarization is very large and thus the fluctuations around the mean are small. Yet, despite their small values, the velocity fluctuations contain a great deal of information, as is clear from an inspection of Fig. 1B. Even in such 2D projection of a 3D flock it is possible to detect the presence of two large domains where the fluctuations are nearly parallel to each other (see Fig. S1 for another flock). The existence of these domains is not a consequence of the fact that birds are all flying in the same direction, because the overall center of mass velocity has been subtracted in Eq. 2. Hence, what Fig. 1B shows is the presence of strong spatial correlations: The change of heading of a bird within one of these domains is highly correlated to that of all birds within the same domain. Previous studies on starling flocks suggest that each bird interacts on average with approximately seven neighbors (13). From Fig. 1B it is clear that the correlated domains contain much more than seven birds. Hence, the span of spatial correlation is significantly larger than the interaction range. To quantify the size of the domains we define in three dimensions the correlation function of the fluctuations,
. This is expected, because the polarization is very large and thus the fluctuations around the mean are small. Yet, despite their small values, the velocity fluctuations contain a great deal of information, as is clear from an inspection of Fig. 1B. Even in such 2D projection of a 3D flock it is possible to detect the presence of two large domains where the fluctuations are nearly parallel to each other (see Fig. S1 for another flock). The existence of these domains is not a consequence of the fact that birds are all flying in the same direction, because the overall center of mass velocity has been subtracted in Eq. 2. Hence, what Fig. 1B shows is the presence of strong spatial correlations: The change of heading of a bird within one of these domains is highly correlated to that of all birds within the same domain. Previous studies on starling flocks suggest that each bird interacts on average with approximately seven neighbors (13). From Fig. 1B it is clear that the correlated domains contain much more than seven birds. Hence, the span of spatial correlation is significantly larger than the interaction range. To quantify the size of the domains we define in three dimensions the correlation function of the fluctuations,
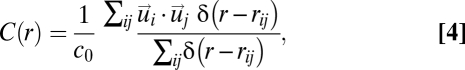 |
where 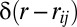 is a smoothed Dirac δ-function selecting pairs of birds at mutual distance
is a smoothed Dirac δ-function selecting pairs of birds at mutual distance 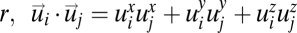 and c0 is a normalization factor (of dimension m2·s−2) such that C(r = 0) =1. The correlation function measures the average inner product of the velocity fluctuations of birds at distance r. A large value of C(r) implies that the fluctuations are nearly parallel and thus strongly correlated. Conversely, when the fluctuations are antiparallel, and therefore anticorrelated, the correlation function has a negative value. On the other hand, when the fluctuations are uncorrelated, pointing in random directions, the correlation function averages to zero.
and c0 is a normalization factor (of dimension m2·s−2) such that C(r = 0) =1. The correlation function measures the average inner product of the velocity fluctuations of birds at distance r. A large value of C(r) implies that the fluctuations are nearly parallel and thus strongly correlated. Conversely, when the fluctuations are antiparallel, and therefore anticorrelated, the correlation function has a negative value. On the other hand, when the fluctuations are uncorrelated, pointing in random directions, the correlation function averages to zero.
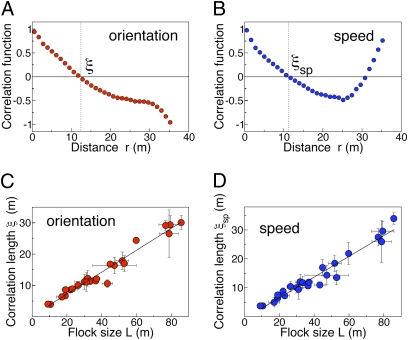
The typical form of C(r) in starling flocks is reported in Fig. 2A (for other flocks, see Fig. S2). At short distances the correlation is close to 1, and it decays with increasing r, becoming negative at large interindividual distances [for r larger than the flock's size C(r) is no longer defined]. Such behavior indicates that within a flock there is either strong correlation (short distance) or strong anticorrelation (large distance), whereas in no range of r the correlation function is consistently equal to zero, as one would expect in the case of absence of correlation. This phenomenon can be seen at a qualitative level in Fig. 1B: Each correlated domain has an anticorrelated domain with opposite fluctuations. Their mutual negative correlation must not be misunderstood for an absence of correlation: The latter case would imply a random distribution of orientations and therefore a correlation function equal to zero over a finite interval, at variance with the correlation functions we find. We note that the presence of correlated/anticorrelated domains pairs, and therefore the fact that the correlation function is positive and negative, is a trivial consequence of the fact that the spatial average of 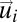 is zero (Eq. 3). However, what is highly not trivial is the fact that just two domains (the minimum number) span the entire system: indeed Eq. 3 can in principle be satisfied in many possible ways, for example forming many small domains pairs, instead of two large ones.
is zero (Eq. 3). However, what is highly not trivial is the fact that just two domains (the minimum number) span the entire system: indeed Eq. 3 can in principle be satisfied in many possible ways, for example forming many small domains pairs, instead of two large ones.
Fig. 2.
(A) The correlation function C(r) is the average inner product of the velocity fluctuations of pairs of birds at mutual distance r. This correlation function therefore measures to what extent the orientations of the velocity fluctuations are correlated. The function changes sign at r = ξ, which gives a good estimate of the average size of the correlated domains (flock 28-10). (B) The correlation function Csp(r), on the other hand, measures the correlations of the fluctuations of the modulus of the velocity, i.e., the speed. This correlation function measures to what extent the variations with respect to the mean of the birds’ speed are correlated to each other. The speed correlation function changes sign at a point r = ξsp, which gives the size of the speed-correlated domains (flock 28-10). Both correlation functions in A and B are normalized to give C(r = 0) = 1. (C) The orientation correlation length ξ is plotted as a function of the linear size L of the flocks. Each point corresponds to a specific flocking event and it is an average over several instants of time in that event. Error bars are SDs. The correlation length grows linearly with the size of the flock, ξ = aL, with a = 0.35 (Pearson's correlation test: n = 24, r = 0.98, P < 10−16), signaling the presence of scale-free correlations. (D) Also in the case of the correlation function of the speed, the correlation length ξsp grows linearly with the size of the flock, ξsp = aL, with a = 0.36 (Pearson's correlation test: n = 24, r = 0.97, P < 10−15). Error bars are SDs.
To explain the behavior of C(r) we introduce the correlation length ξ, which can be defined as the zero of the correlation function,
The value of ξ coincides with the average size of the correlated domains (Materials and Methods and Fig. S3). Indeed, the fact that the correlation function changes sign at 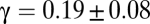 corresponds to the fact that when we increase r we pass from considering individuals in the same correlated domain to considering individuals in anticorrelated domains. What is the typical value of ξ? A former study showed that the interaction range has a constant value in units of birds, rather than in units of meters (13). Hence, one may naively expect that the correlation length also has a constant “topological” value (units of individuals), rather than a constant metric value. What we find is, however, completely different and somewhat surprising: We measured the correlation length in all analyzed flocks and found that ξ does not have a constant value, either in units of birds or in units of meters. Rather, the correlation length grows linearly with the size of the flock L (Fig. 2C). Accordingly, correlated domains in starling flocks are larger the larger the flock.
corresponds to the fact that when we increase r we pass from considering individuals in the same correlated domain to considering individuals in anticorrelated domains. What is the typical value of ξ? A former study showed that the interaction range has a constant value in units of birds, rather than in units of meters (13). Hence, one may naively expect that the correlation length also has a constant “topological” value (units of individuals), rather than a constant metric value. What we find is, however, completely different and somewhat surprising: We measured the correlation length in all analyzed flocks and found that ξ does not have a constant value, either in units of birds or in units of meters. Rather, the correlation length grows linearly with the size of the flock L (Fig. 2C). Accordingly, correlated domains in starling flocks are larger the larger the flock.
A correlation length that is proportional to the system size implies that correlations are scale free. Let us briefly recall how this works. In general, we can write the leading contribution to the correlation function as
 |
(17, 18), where g(x) is a dimensionless scaling function. As we have seen, we find that the correlation length grows with the flock's size L; this result can be formalized as
where b is a generic scaling factor. Substituting Eq. 7 into the general relation [6] yields
By choosing b = 1/r, we finally obtain the following form for the correlation function in starling flocks,
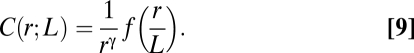 |
Eq. 9 explains the meaning of the expression “scale free”: The correlation between birds does not have any characteristic length scale apart from the trivial one fixed by the size of the flock, L. The correlation length ξ defined above is not an intrinsic length scale, for it is proportional to L. The scaling function f(r/L) in Eq. 9 embodies the effect of the flock's finite size on the correlation function. To get rid of such an effect and find the asymptotic correlation function C∞(r), we simply ask what the correlation is between two birds at distance r within a very large flock; to answer this question we perform the limit L→∞ in Eq. 9 and get
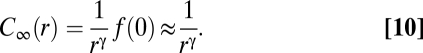 |
Eqs. 9 and 10 make the main point of our work: The empirical observation that the correlation length is proportional to L (Fig. 2C and Eq. 7) implies that correlations in starling flocks are scale free and that the asymptotic correlation function is a power law.
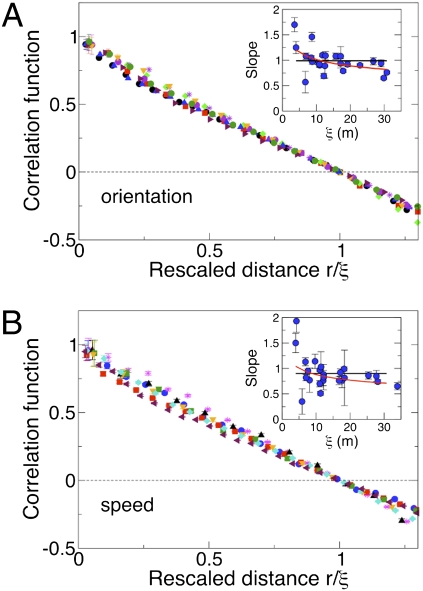
What is the value of γ? The sharpest way to work out the value of this exponent is to calculate the derivative of the finite size correlation function with respect to the rescaled variable x = r/ξ. According to Eq. 6, when we evaluate this derivative at the zero of the correlation function, i.e., at x = 1, we obtain
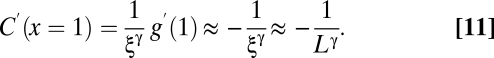 |
Hence, the rescaled correlation function at its zero should flatten (lower derivative) in larger flocks. In Fig. 3A we plot several correlation functions vs. the rescaled variable x = r/ξ: Up to experimental error the curves seem to collapse quite well one onto the other, with no clear evidence of a flattening of the derivative for larger flocks, indicating that γ in Eq. 11 has a very small value. In the Inset of Fig. 3A we report for all of the analyzed flocks the absolute value of the derivative in x = 1 vs. ξ. What we observe is indeed a very weak decrease of the derivative with increasing correlation length. The best fit to Eq. 11 gives a very small exponent [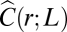 , with reduced chi square (RCS) = 0.045], but the data are equally compatible with a logarithmic decay (RCS = 0.040) and even with a constant value of the derivative, equivalent to γ = 0 or no decay (RCS = 0.059). The data barely span one order of magnitude, so it would be unwise to commit to any of these fits. However, what the data positively demonstrate is that the value of γ is very low indeed.
, with reduced chi square (RCS) = 0.045], but the data are equally compatible with a logarithmic decay (RCS = 0.040) and even with a constant value of the derivative, equivalent to γ = 0 or no decay (RCS = 0.059). The data barely span one order of magnitude, so it would be unwise to commit to any of these fits. However, what the data positively demonstrate is that the value of γ is very low indeed.
Fig. 3.
(A) The correlation functions of several flocks are plotted vs. the rescaled variable x = r/ξ. (Inset) The modulus of the derivative of the correlation function with respect to the rescaled variable x, evaluated at x = 1, plotted vs. the correlation length ξ for all flocking events. The derivative is almost constant with ξ, indicating that the exponent γ in the scale-free asymptotic correlation is very close to zero. The black and red lines represent the best fits to, respectively, a constant and a logarithm (see text). (B) Same as in A for the speed correlation.
This result is rather startling. In a non-scale-free system, the asymptotic correlation between two individuals drops to zero when their distance gets larger than ξ. On the contrary, in a scale-free system the asymptotic correlation is never zero, but it nevertheless decays, albeit as a power law, 1/rγ. However, if γ is barely different from zero, as seems to be the case in starling flocks, then the asymptotic correlation (i.e., the correlation within infinitely large flocks) practically does not decay with the distance. From Eq. 8 we see that an almost zero value of γ implies that two birds 1 m apart in a 10-m-wide flock are as strongly correlated as two birds 10 m apart in a 100-m-wide flock. Behavioral correlations in starling flocks are therefore not simply scale free, but in fact are unusually long ranged.
To better understand the significance of scale-free correlations it is useful to see what happens in the non-scale-free case. To this aim we use synthetic data (Materials and Methods). In each flock we substitute the actual velocity fluctuations with a set of synthetic random vectors correlated according to the following asymptotic correlation function:
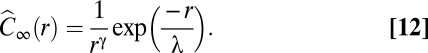 |
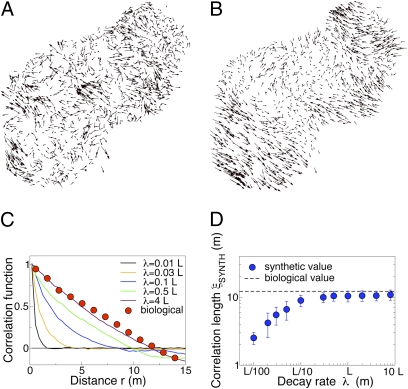
We use the hat to distinguish this synthetic correlation function from the biological one. In contrast with Eq. 10, the synthetic correlation function [12] is clearly not scale free, as the decay rate λ (which we can arbitrarily tune) fixes a spatial scale, and the correlation is exponentially suppressed for r > λ. Hence, the finite size correlation function 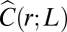 , calculated according to definition [4], does not obey the scale-free relation [9]. When λ is small, domains are also small (Fig. 4A) and
, calculated according to definition [4], does not obey the scale-free relation [9]. When λ is small, domains are also small (Fig. 4A) and 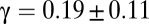 is consistently equal to zero beyond distances of order λ (Fig. 4C). This means that portions of the flock separated by a distance larger than λ are uncorrelated and behave independently. The correlation length ξ is a constant, approximately equal to λ, and it does not scale with L. As we increase λ, the size of the synthetic domains grows and the correlation function becomes more and more long ranged (Fig. 4C), but nothing qualitative changes as long as λ < L.
is consistently equal to zero beyond distances of order λ (Fig. 4C). This means that portions of the flock separated by a distance larger than λ are uncorrelated and behave independently. The correlation length ξ is a constant, approximately equal to λ, and it does not scale with L. As we increase λ, the size of the synthetic domains grows and the correlation function becomes more and more long ranged (Fig. 4C), but nothing qualitative changes as long as λ < L.
Fig. 4.
Random synthetic velocities. In each flock we replace the actual birds’ velocity fluctuations with a set of synthetic random vectors correlated over a length λ that we can arbitrarily tune (see text). The synthetic fluctuations are located at the same positions as birds in a real flock (we used flock 28-10, the same as in Fig.1). (A) Synthetic fluctuations in the non-scale-free case, λ = 0.05L. The domains are quite small and have a size comparable to λ. (B) Synthetic fluctuations for λ = 4L. In this scale-free limit the domains are very similar to the actual biological ones displayed in Fig.1B. (C) Synthetic correlation functions 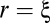 for various values of the decay length λ. By increasing λ the synthetic correlation function becomes more and more long ranged and it finally becomes very close to the actual biological one in the scale-free regime λ > L. (D) Synthetic correlation length ξSYNTH, as a function of the decay length λ (each point is an average over 50 synthetic samples; errors bars are SDs). As long as λ is smaller than the size of the flock L, ξSYNTH grows following λ. However, in the scale-free regime, λ > L, ξSYNTH saturates to a value very close to the actual biological one.
for various values of the decay length λ. By increasing λ the synthetic correlation function becomes more and more long ranged and it finally becomes very close to the actual biological one in the scale-free regime λ > L. (D) Synthetic correlation length ξSYNTH, as a function of the decay length λ (each point is an average over 50 synthetic samples; errors bars are SDs). As long as λ is smaller than the size of the flock L, ξSYNTH grows following λ. However, in the scale-free regime, λ > L, ξSYNTH saturates to a value very close to the actual biological one.
On the other hand, if the decay rate λ is larger than the size L of the flock, then all possible values of the interindividual distance r are much smaller than λ, and therefore the exponential in Eq. 12 is always well approximated by 1. In this case the asymptotic correlation function of the synthetic data decays as a scale-free power law,
 |
exactly as in the case of real flocks, Eq. 10. We therefore expect that in the scale-free limit (Eq. 13) the synthetic finite-size correlation function must become equal to that of real flocks, provided that we choose a value of γ that is small enough. This is exactly what we find: The synthetic correlation function (Fig. 4C) and the synthetic domain size and correlation length (Fig. 4 B and D) become barely distinguishable from their biological counterparts when the scale-free form (Eq. 15) holds, i.e., in the regime λ > L.
So far we have studied the correlations of the orientation of the velocity (Eq. 4). However, when we compute the correlation function of the speed (i.e., the modulus of the velocity—see Materials and Methods for details), we find an identical linear scaling with L of the corresponding correlation length (Fig. 2 B and D). Hence, speed correlations are scale free, exactly as orientation correlations. Moreover, the analysis of γ (Fig. 3B and Inset in Fig. 3B) gives a very small value for this exponent, exactly as for the orientation (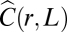 , RCS = 0.10; logarithmic decay, RCS = 0.068; constant, no decay, RCS = 0.097). Therefore, speed fluctuations also are very long ranged, almost not decaying with the distance.
, RCS = 0.10; logarithmic decay, RCS = 0.068; constant, no decay, RCS = 0.097). Therefore, speed fluctuations also are very long ranged, almost not decaying with the distance.
The speed is a stiffer mode than the orientation, as it is more costly for a bird to change its speed (accelerate/decelerate) than its heading. Hence, the fact that both orientation and speed are scale-free correlated means that birds are able to transfer across the flock their whole dynamical state. In flocking, any external perturbation, and in particular predation, is likely to directly cause a change of velocity (direction, modulus, or both) of a small subset of birds that first detect the perturbation (Movie S4). Such localized change must transmit to the whole flock to produce a collective response. We do not focus here on the timescale for this to happen, but on the very possibility for the information to reach the whole group, irrespective of the time needed to do this. In a group with finite correlation length ξ the fluctuation of the dynamical state gets damped beyond ξ. On the contrary, in a flock where correlations in both speed and orientation are scale free, and where the power-law exponent γ is very small, information can reach the whole group without damping. Therefore, scale-free correlations are the key to collective response in bird flocks.
Discussion
Significant spatial correlations have already been observed in bacteria swarms (14, 15). In ref. 15 it was found that for large enough densities of the bacterial swarm the correlation length becomes several body-lengths long. However, in bacteria the correlation function decays exponentially and the correlation length remains much shorter than the swarm size: Correlation, as well as interaction, is short ranged. What we find in starling flocks is different: The correlation function is a scale-free power law and the correlation length scales with the group's size; hence, interaction is short ranged, but correlation is long ranged. If a correlation length larger than the interaction range is likely to be a common trait of self-organized groups, scale-free correlations seem to be the landmark of a qualitatively different kind of collective animal behavior, characterized by a superior level of collective response.
Under what conditions do scale-free correlations appear? And what do scale-free correlations teach us about the interindividual coordination mechanism? First, there is no need to postulate the existence of complicated coordination mechanisms to explain scale-free correlations: Simple behavioral rules based on imitation, such as those used in most numerical models (24, 25, 27), are compatible with scale-free correlations. Indeed there are several statistical models based on simple alignment rules that develop scale-free correlations under certain circumstances (17). The key point is not the rule, but the noise. Given a reasonable behavioral rule (for example, align your velocity to that of your neighbors), correlation strongly depends on the level of noise in implementing such a rule. In a thermal system noise is due to the temperature, whereas in animal groups it is introduced by the inevitable individual error in obeying to any behavioral rule (see, however, ref. 28). Ordinarily, in self-organized systems the lower the noise is, the longer the range of the correlation. In this context order and correlation have a common origin: They are both large when the noise level in the system is low. Hence, it may be expected that bird flocks, which as we have seen are highly ordered, also exhibit strong correlations. In this case order, correlation, and response would all be a consequence of the capability of flocking birds to obey a certain set of behavioral rules allowing very little tolerance, irrespective of the level of environmental perturbation the flock may undergo.
However, the relationship between noise and correlation may be more complex than that just described. In some cases, correlation (and hence response) reaches a maximum at a specific level of the noise. If noise is lowered below such a critical level, order continues to grow, whereas correlation of the fluctuations actually decreases. This behavior is what happens when a critical point is present. A classic example is ferromagnetism: Below the critical temperature the global magnetization grows, but the local fluctuations around the global magnetization become less correlated. In this case order and correlation are decoupled: Increasing the degree of order in the system (by lowering the noise below the critical point) makes the behavioral state of the individuals more stable, but also less sensitive to neighboring behavioral changes. Such higher behavioral inertia depresses, instead of enhancing, the correlation and the global response of the group. Too much noise, on the other hand, equally destroys correlation, so that the system must contain just the right amount of noise to produce a maximum response. For this reason, only at the critical point are correlations scale free. In most physical systems criticality is obtained by tuning some external parameter regulating the noise (such as the temperature) to its critical value. In the case of flocks, however, the critical value of the noise, i.e., of the random deviation from the coordination rules, may be evolutionary hardwired into birds’ behavior.
Discriminating between the two scenarios above (very low noise vs. criticality) is difficult. We know too little about the actual interindividual coordination mechanisms to conclude anything for sure. If scale-free correlations of a “soft” degree of freedom such as the orientation may be expected also off a critical point, the fact that a “stiff” mode such as the speed is scale-free correlated seems, however, to indicate that some kind of criticality might in fact be present in starling flocks. Indeed scale-free correlations of a stiff degree of freedom are difficult to obtain by simply decreasing the noise in the system. Too low a noise level in a hard-to-change behavioral mode, as speed is, can cause an excessive behavioral inertia, which in turn depresses correlation and global response. For this reason criticality is perhaps a more likely scenario for our results. A comparison with physical systems, where much is known on the relationship between correlations and criticality, is significant in this respect. In physics whenever a continuous symmetry is spontaneously broken, giving rise to global ordering, it is possible to prove that fluctuations transverse to the order parameter are scale free (Goldstone's theorem) (29). An example is given by continuous spin models with alignment interactions, where individual spins on a lattice can point in any direction in space. Here, the system orders at low temperature, giving rise to a global magnetic momentum, somewhat similarly to individual velocities orienting in a common direction in flocks. Fluctuations transverse to the global magnetization are soft modes and exhibit power-law decay in space. In this case, scale-free correlations are not a symptom of criticality, but a consequence of the spontaneous breaking of a continuous symmetry, the rotational one. Not all fluctuations are, however, scale free in this context: For the modulus of the spins (i.e., the analog of speed), in particular, correlations are short ranged (30). In flocks, on the contrary, as we have shown, we do find scale-free correlations also of the speed fluctuations. There is no obvious way to explain such correlations by using symmetry arguments, such as Goldstone's theorem. This result is quite important, as it shows that in flocks all dynamical modes are scale-free correlated, not only those connected to the rotational broken symmetry. It really seems that flocks are critical in some fundamental way.
Whatever the origin of the scale-free behavior is, the very low value of the exponent γ that we find, i.e., the fact that the correlation is almost not decaying with the distance, is by far the most surprising and exotic feature of bird flocks. How starlings achieve such a strong correlation remains a mystery to us.
Criticality is not uncommon in biological systems made up of many interacting components (SI Text). Being critical is a way for the system to be always ready to optimally respond to an external perturbation, such as a predator attack as in the case of flocks. Our empirical results, together with further study on the role of criticality in animal groups, may contribute to move the fascinating “collective mind” metaphor (31, 32) to a more quantitative level.
Materials and Methods
Empirical Observations.
Data were taken from the roof of Palazzo Massimo, Museo Nazionale Romano, in the city center of Rome, in front of one of the major roosting sites used by starlings during winter. Birds spend the day feeding in the countryside and come back to the roost in the evening, ∼1 h before sunset. Before settling on the trees for the night, starlings gather in flocks of various sizes and perform what is called “aerial display,” namely an apparently purposeless dance where flocks move and swirl in a remarkable way. By using stereometric digital photogrammetry and computer vision techniques we reconstructed the individual 3D positions and 3D velocities in 24 flocking events. A flocking event is a series of consecutive shots of a flock at a rate of 10 frames/s. Analyzed flocks had different numbers of birds (from 122 to 4,268 individuals) and different linear sizes (from 9.1 to 85.7 m). The details of the 3D reconstruction of the positions can be found in refs. 22 and 23. A tracking algorithm (SI Text) has been used to reconstruct the 3D velocities.
Correlation Function.
The correlation function C(r) defined in Eq. 4 is calculated by averaging the inner (or scalar) product of the velocity fluctuations of all pairs of birds with mutual distance in the interval (r, r + dr), where dr sets the discrete scale of C(r). The smoothed Dirac δ in Eq. 4 must be interpreted in this sense. The correlation function is normalized in such a way to give C(r = 0) = 1. The integral over r between 0 and L (size of the flock) of the numerator of Eq. 4 is 0 due to Eq. 3,
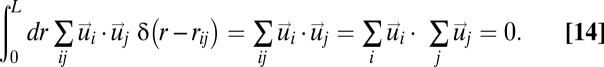 |
As a consequence, the numerator in Eq. 4 must have a 0 in the interval [0:L], and therefore the same holds for the whole function C(r). Because of this condition we can define the correlation length ξ as in Eq. 5. The correlation function of the velocity modulus, the speed, is defined as
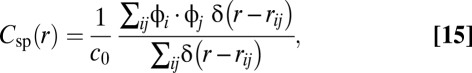 |
where the δ-function has the same meaning as explained above, c0 is a normalization factor such that the correlation is one in zero, and where
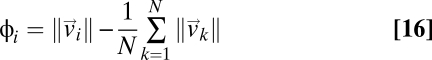 |
is the (scalar) fluctuation of the speed with respect to the global mean. The same arguments used for the velocity fluctuations hold also for the speed fluctuations, so that Csp(r) must have a zero, ξsp.
Size of the Domains.
The correlation length ξ provides a good estimate of the size of the correlated domains. To check this point we computed the size of the domains in an alternative way, by diagonalizing the covariance matrix 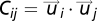 . The N-dimensional eigenvector wmax relative to the maximum eigenvalue of this matrix can be used to identify the direction of maximal mutual alignment of the fluctuations, i.e., the average orientation of the largest correlated domain. Defining this eigenvector is useful, because if bird i belongs to the correlated domain, then the i component of the eigenvector wmax is significantly different from zero. This is the rigorous way to identify the birds belonging to a correlated domain. Once the domain is defined, we calculate the domain's size using the median of the mutual distances of the birds belonging to it. In Fig. S2 we report the domain's size thus calculated as a function of the correlation length ξ. The clear linear correlation, with angular coefficient very close to 1, shows that ξ is indeed a good estimate of the domain's size.
. The N-dimensional eigenvector wmax relative to the maximum eigenvalue of this matrix can be used to identify the direction of maximal mutual alignment of the fluctuations, i.e., the average orientation of the largest correlated domain. Defining this eigenvector is useful, because if bird i belongs to the correlated domain, then the i component of the eigenvector wmax is significantly different from zero. This is the rigorous way to identify the birds belonging to a correlated domain. Once the domain is defined, we calculate the domain's size using the median of the mutual distances of the birds belonging to it. In Fig. S2 we report the domain's size thus calculated as a function of the correlation length ξ. The clear linear correlation, with angular coefficient very close to 1, shows that ξ is indeed a good estimate of the domain's size.
Synthetic Random Velocities.
At each instant of time a flock is characterized by a set of 3D coordinates (the birds positions  ) and of 3D vectors (the fluctuations
) and of 3D vectors (the fluctuations  around the mean velocity). Given a flock, we keep the actual 3D positions, but replace the 3D fluctuations with a set of random vectors
around the mean velocity). Given a flock, we keep the actual 3D positions, but replace the 3D fluctuations with a set of random vectors 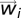 (synthetic fluctuations), drawn with a distribution whose covariance matrix is given by
(synthetic fluctuations), drawn with a distribution whose covariance matrix is given by
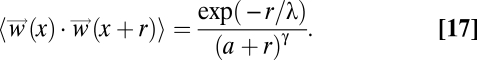 |
The length λ sets the decay rate of the synthetic correlation, whereas the factor a simply makes the correlation nonsingular in r = 0. When λ >> L, the exponential is always unity, and the correlation becomes a power law with exponent γ. Not any power makes a power law scale free, though. In three dimensions the power law is actually scale free only for γ < 3, whereas if γ > 3 the correlation length does not scale linearly with L and the correlation is effectively short ranged. As we have seen in the main text, to have a good agreement with the biological data we need to use a very small value of this exponent. Practically speaking, any value γ < 1 gives synthetic results compatible with the biological ones, within the experimental error.
Supplementary Material
Acknowledgments
We acknowledge the help of N. Cabibbo and A. Orlandi in the early stages of this work and of the whole STARFLAG-Consiglio Nazionale delle Ricerche experimental team during the first data-taking season. We are also indebted to W. Bialek, R. Bon, E. Branchini, C. Castellano, G. Cavagna, F. Cecconi, M. Cencini, I. D. Couzin, A. Gabrielli, D. Grunbaum, P. S. Krishnaprasad, J. Lorenzana, M. Magnasco, and G. Theraulaz for several helpful discussions. A. Cavagna and I.G. are particularly grateful to T. S. Grigera for some key remarks on information transfer and to J. Gautrais for a critical reading of the manuscript. We thank R. Paris and M. Petrecca for granting the access to Palazzo Massimo, Museo Nazionale Romano, which was indispensable for the data taking. This work was partially financed by a grant from the European Commission under the STARFLAG project.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1005766107/-/DCSupplemental.
References
- 1.Parrish JK, Hammer WM, editors. Animal Groups in Three Dimensions. Cambridge, UK: Cambridge Univ Press; 1997. [Google Scholar]
- 2.Krause J, Ruxton GD. Living in Groups. Oxford: Oxford Univ Press; 2002. [Google Scholar]
- 3.Couzin ID, Krause J. Self-organization and collective behaviour in vertebrates. Adv Study Behav. 2003;32:1–75. [Google Scholar]
- 4.Okubo A. Dynamical aspects of animal grouping: Swarms, schools, flocks, and herds. Adv Biophys. 1986;22:1–94. doi: 10.1016/0065-227x(86)90003-1. [DOI] [PubMed] [Google Scholar]
- 5.Emlen JT. Flocking behaviour in birds. Auk. 1952;69:160–170. [Google Scholar]
- 6.Buhl J, et al. From disorder to order in marching locusts. Science. 2006;312:1402–1406. doi: 10.1126/science.1125142. [DOI] [PubMed] [Google Scholar]
- 7.Buck J, Buck E. Mechanism of rhythmic synchronous flashing of fireflies. Fireflies of Southeast Asia may use anticipatory time-measuring in synchronizing their flashing. Science. 1968;159:1319–1327. doi: 10.1126/science.159.3821.1319. [DOI] [PubMed] [Google Scholar]
- 8.Néda Z, Ravasz E, Brechet Y, Vicsek T, Barabási A-L. The sound of many hands clapping. Nature. 2000;403:849–850. doi: 10.1038/35002660. [DOI] [PubMed] [Google Scholar]
- 9.Camazine S, et al. Self-Organization in Biological Systems. Princeton: Princeton Studies in Complexity (Princeton Univ Press; 2001. [Google Scholar]
- 10.Couzin ID, Krause J, Franks NR, Levin SA. Effective leadership and decision-making in animal groups on the move. Nature. 2005;433:513–516. doi: 10.1038/nature03236. [DOI] [PubMed] [Google Scholar]
- 11.Cresswell W. Flocking is an effective anti-predation strategy in redshanks, Tringa totanus. Anim Behav. 1994;47:433–442. [Google Scholar]
- 12.Roth TC, II, Lima SL, Vetter WE. Determinants of predation risk in small wintering birds: The hawk's perspective. Behav Ecol Sociobiol. 2006;60:195–204. [Google Scholar]
- 13.Ballerini M, et al. Interaction ruling animal collective behavior depends on topological rather than metric distance: Evidence from a field study. Proc Natl Acad Sci USA. 2008;105:1232–1237. doi: 10.1073/pnas.0711437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dombrowski C, Cisneros L, Chatkaew S, Goldstein RE, Kessler JO. Self-concentration and large-scale coherence in bacterial dynamics. Phys Rev Lett. 2004;98:098103. doi: 10.1103/PhysRevLett.93.098103. [DOI] [PubMed] [Google Scholar]
- 15.Sokolov A, Aranson IS, Kessler JO, Goldstein RE. Concentration dependence of the collective dynamics of swimming bacteria. Phys Rev Lett. 2007;98:158102. doi: 10.1103/PhysRevLett.98.158102. [DOI] [PubMed] [Google Scholar]
- 16.Simon H. The architecture of complexity. Proc Am Philos Soc. 1962;106:467–482. [Google Scholar]
- 17.Wilson KG. Problems in physics with many scales of length. Sci Am. 1979;241:158–179. [Google Scholar]
- 18.Vicsek T. Fluctuations and Scaling in Biology. Oxford: Oxford Univ Press; 2001. [Google Scholar]
- 19.Anderson PW. More is different. Science. 1972;177:393–396. doi: 10.1126/science.177.4047.393. [DOI] [PubMed] [Google Scholar]
- 20.Feare C. The Starling. Oxford: Oxford Univ Press; 1984. [Google Scholar]
- 21.Ballerini M, et al. Empirical investigation of starling flocks: A benchmark in collective animal behaviour. Anim Behav. 2008;76:201–215. [Google Scholar]
- 22.Cavagna A, et al. The STARFLAG handbook on collective animal behaviour: 1. Empirical methods. Anim Behav. 2008;76:217–236. [Google Scholar]
- 23.Cavagna A, et al. The STARFLAG handbook on collective animal behaviour: 2. Three-dimensional analysis. Anim Behav. 2008;76:237–248. [Google Scholar]
- 24.Vicsek T, Czirók A, Ben-Jacob E, Cohen I, Shochet O. Novel type of phase transition in a system of self-driven particles. Phys Rev Lett. 1995;75:1226–1229. doi: 10.1103/PhysRevLett.75.1226. [DOI] [PubMed] [Google Scholar]
- 25.Grégoire G, Chaté H. Onset of collective and cohesive motion. Phys Rev Lett. 2004;92:025702. doi: 10.1103/PhysRevLett.92.025702. [DOI] [PubMed] [Google Scholar]
- 26.Cisneros LH, Cortez R, Dombrowski C, Goldstein RE, Kessler JO. Fluid dynamics of self-propelled microorganisms, from individuals to concentrated populations. Exp Fluids. 2007;43:737–753. [Google Scholar]
- 27.Toner J, Tu Y, Ramaswamy S. Hydrodynamics and phases of flocks. Ann Phys. 2005;318:170–244. [Google Scholar]
- 28.Yates CA, et al. Inherent noise can facilitate coherence in collective swarm motion. Proc Natl Acad Sci USA. 2009;106:5464–5469. doi: 10.1073/pnas.0811195106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldstone J. Field theories with “superconductor” solutions. Nuovo Cimento C. 1961;19:154–164. [Google Scholar]
- 30.Ryder LH. Quantum Field Theory. Cambridge, UK: Cambridge Univ Press; 1985. [Google Scholar]
- 31.Couzin ID. Collective minds. Nature. 2007;445:715. doi: 10.1038/445715a. [DOI] [PubMed] [Google Scholar]
- 32.Couzin ID. Collective cognition in animal groups. Trends Cogn Sci. 2009;13:36–43. doi: 10.1016/j.tics.2008.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.