Abstract
The affinities of the Hippopotamidae are at the core of the phylogeny of Cetartiodactyla (even-toed mammals: cetaceans, ruminants, camels, suoids, and hippos). Molecular phylogenies support Cetacea as sister group of the Hippopotamidae, implying a long ghost lineage between the earliest cetaceans (∼53 Ma) and the earliest hippopotamids (∼16 Ma). Morphological studies have proposed two different sister taxa for hippopotamids: suoids (notably palaeochoerids) or anthracotheriids. Evaluating these phylogenetic hypotheses requires substantiating the poorly known early history of the Hippopotamidae. Here, we undertake an original morphological phylogenetic analysis including several “suiform” families and previously unexamined early Miocene taxa to test previous conflicting hypotheses. According to our results, Morotochoerus ugandensis and Kulutherium rusingensis, until now regarded as the sole African palaeochoerid and the sole African bunodont anthracotheriid, respectively, are unambiguously included within the Hippopotamidae. They are the earliest known hippopotamids and set the family fossil record back to the early Miocene (∼21 Ma). The analysis reveals that hippopotamids displayed an unsuspected taxonomic and body size diversity and remained restricted to Africa during most of their history, until the latest Miocene. Our results also confirm the deep nesting of Hippopotamidae within the paraphyletic Anthracotheriidae; this finding allows us to reconstruct the sequence of dental innovations that links advanced selenodont anthracotheriids to hippopotamids, previously a source of major disagreements on hippopotamid origins. The analysis demonstrates a close relationship between Eocene choeropotamids and anthracotheriids, a relationship that potentially fills the evolutionary gap between earliest hippopotamids and cetaceans implied by molecular analyses.
Keywords: Africa, dental pattern, Hippopotamoidea, paleobiogeography
The question of the immediate affinities of the Hippopotamidae is central to a major unresolved issue of mammalian evolution: the phylogeny of the Cetartiodactyla and the terrestrial origins of the Cetacea. For many years there had been two separate problems: the ancestry of whales and whether hippopotamuses were related to pigs or anthracotheres. The arrival of cladistics, molecular studies, and large-scale computer analyses led to the surprising assertion that whales were either the sister group of artiodactyls or nested within them. This brought together both the old problems and overshadowed the second. Nowadays molecular analyses of extant species suggest that Cetacea are the sister group of Hippopotamidae (e.g., refs. 1–8) whereas a close Hippopotamidae/Suoidea relationship is suggested by morphological data from fossil and extant species (e.g., ref. 9, figure 5; refs. 10 and 11). Use of enlarged data sets mainly drawn from molecular sources support a Cetacea + Hippopotamidae clade (Cetancodonta), but produce conflicting results for the placement of Suoidea (5, 12–14).
The consideration of fossil data has seemed to introduce more confusion, because the relationships between major cetartiodactyl clades (Cetancodonta, Ruminantia, Suoidea, and Tylopoda) remained unresolved in recent combined analyses of extinct and extant data (ref. 9, figure 2; refs. 15 and 16). However, these and other recent large-scale analyses aiming at clarifying cetartiodactyl basal relationships (10, 11, 17–20) included none or only a limited subset of fossil “suiforms” classically and more controversially interpreted as hippopotamid stem groups and, in all cases, no fossil hippopotamids. Failure to resolve the evolution that led to Hippopotamidae hinders our understanding of the common ancestry between extant hippopotamids and cetaceans. In turn, this creates difficulties in resolving the earlier branching of Ruminantia, Suoidea, and Tylopoda. A better understanding of the evolutionary history of Hippopotamidae is thus a critical step in clarifying the relationships within Cetartiodactyla and is addressed in this paper.
The origin of the Hippopotamidae has been a subject of inquiries for more than a century (see reviews in refs. 21–23), and identifying the stem group of these mammals is still a challenging issue for biologists (22, 23). A significant hippopotamid fossil record is thus far found only for the Hippopotaminae (large hippopotamids displaying a very derived morphology), which seem to appear abruptly in the late Miocene deposits of Africa, ∼7.5 Ma (24, 25). The identification of the Paleogene-early Neogene cetartiodactyl group from which hippopotamines are derived is thus challenging.
The only other members of Hippopotamidae are represented in subfamily Kenyapotaminae by the genus Kenyapotamus Pickford, 1983, known on the basis of mostly isolated dental remains from the middle and late Miocene of Kenya. This subfamily has a first appearance datum (FAD) at ∼16 Ma (26), which extends the time range of Hippopotamidae into the middle Miocene. To date, kenyapotamines have been recorded only in Africa, in various Kenyan localities (26–31) and elsewhere in Africa (Tunisia, ref. 32; Ethiopia, refs. 33 and 34). Their morphology and diversity are now better understood (22). Recent phylogenetic analyses suggested that the Kenyapotaminae are the sister taxon of the Hippopotaminae and that the Hippopotamidae are monophyletic as well as deeply nested within the extinct, paraphyletic Anthracotheriidae (22, 35). However, a morphological gap remains between these earliest known hippopotamids and their hypothesized antracotheriid stem group (22).
Despite this step forward, there is still no consensus on the earliest history of the hippopotamids. Phylogenies are divided between two mutually exclusive candidates for a hippopotamid stem group: Anthracotheriidae (e.g., ref. 16) and Suoidea (e.g., ref. 9, figure 5; and ref. 11). Among the latter, palaeochoerids also known as “Old World peccaries” (36), have been favored in some contributions (23, 29). The morphological gap between the earliest known hippopotamids and their potential sister taxon exists either because relevant remains are not yet documented in the known fossil record or because earlier hippopotamid remains have been collected, but not recognized as such.
In this study we addressed the challenging question of the immediate affinities of hippopotamids by reexamining Morotochoerus ugandensis Pickford, 1998 (36) (Fig. 1 A–D) and Kulutherium kenyensis Pickford, 2007 (Fig. 1F), two bunodont early Miocene eastern Africa suiforms known through partial dentitions. M. ugandensis (Moroto, Uganda, dated >20.6 Ma) (37) was initially referred to as a small anthracotheriid (38), later to the schizochoerine tayassuids (36), and finally assigned to the “Palaeochoeridae,” as their only African representative (ref. 39 and ref. 40, table 2). This makes this material crucial in testing previous hypotheses linking the Hippopotamidae to palaeochoerids. Surprisingly, it has never been discussed as such by proponents of a palaeochoerid origin for the Hippopotamidae. K. kenyensis is a large buno-selenodont cetartiodatyl reported from the Kulu Formation, Rusinga Island, Kenya (early Miocene, 17–15 Ma) (41). It was first identified as a hippopotamid (42) and later referred to as an anthracotheriid (40). Affinities of Kulutherium within Hippopotamoidea (clade including Hippopotamidae and Anthracotheriidae) (43) were never formally tested, although this taxon has been considered relevant to the question of hippopotamid origins on the basis of its dental morphology (42, 44, 45). Both taxa were reconsidered using the revised dental nomenclature proposed by Boisserie et al. (22), making possible relevant comparisons among suiform taxa. An in-depth description of available material of M. ugandensis and K. rusingensis is provided in SI Text S1). Both species were included in a phylogenetic analysis performed at the suiform level. This analysis builds upon a comparative study of tooth morphology and provides unique insights into hippopotamid phylogeny and diversity, as well as their time and place of origin.
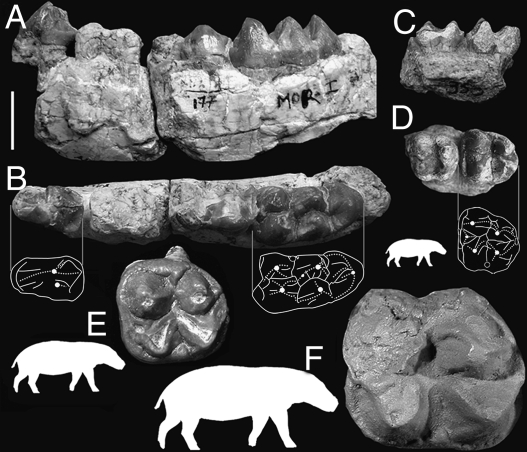
Fig. 1.
Dentition of Morotochoerus ugandensis: lingual (A) and occlusal (B) views of the right dentary (holotype: MOR 177–178) and lingual (C) and occlusal (D) views of the left maxillary fragment (MOR II BUMP 350), compared with the occlusal view of the right M1 of Kenyapotamus ternani (E, holotype: KNM-FT 3934, reversed) and of the left M1 of Kulutherium rusingense (F, holotype: R 773′49, cast at Natural History Museum, London). The white outlines suggest the respective body masses of the figured hippopotamids, estimated from m1 measurements (47). (Scale bar: 10 mm.)
Results
Phylogenetic Analysis: Consensus Topology.
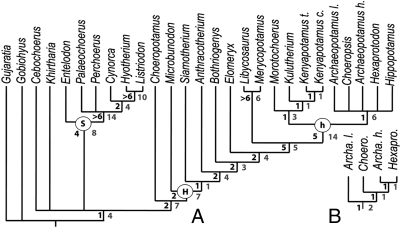
The strict consensus (Fig. 2A) of the 78 most-parsimonious trees [L = 228; consistency index (CI) = 0.40; retension index (RI) = 0.76] yielded by the cladistic analysis unambiguously rejected a suoid origin for the Hippopotamidae. Two major clades are supported: a robust clade Suina (i.e., entelodonts + suoids; clade S, Fig. 2A) and a clade gathering Choeropotamus, the paraphyletic anthracotheriids and the Hippopotamidae, in agreement with Hippopotamoidea sensu (43) (clade H, Figs. 2A and 3). The latter clade is defined by presence of a mesostyle on upper molars (311; RI = 1.00), connection between pre- and postcristae of upper molar labial cusps with cingular structure (321; RI = 1.00), paraconule of upper molars independent with its own crest pattern (281; RI = 0.75), postparacrista and premetacrista do not form a straight line (331; RI = 0.70), p4 with flat lingual wall and convex buccal wall (511; RI = 0.66), P4 labial orientation of the preparacrista (201; RI = 0.75), and protocone of the P4 crescentic (231; RI = 0.57). The tree basal relationships remain unresolved with a major polytomy involving the raoellid Khirtharia, Cebochoerus, the Suina, and the (Choeropotamus, Hippopotamoidea) clade. Khirtharia branches with (Choeropotamus, Hippopotamoidea) or at the base of a (Suina, (Choeropotamus, Hippopotamoidea)) clade. Cebochoerus displays the latter position or is grouped with Suina.
Fig. 2.
Phylogeny of hippopotamoids based on the consensus of 78 parsimonious trees (consensus tree length = 234; CI = 0.39; RI = 0.75). (A) whole data matrix; (B) relationships within Hippopotaminae when excluding Hippopotamus. H, Hippopotamoidea; h, Hippopotamidae; S, Suina. Shaded numbers indicate nonambiguous synapomorphies, and solid numbers indicate Bremer support. Abbreviations: Archaeopotamus h, A. harvardi; Archaeopotamus l., A. lothagamensis; Kenyapotamus c., K. coryndonae; Kenyapotamus t., K. ternani.
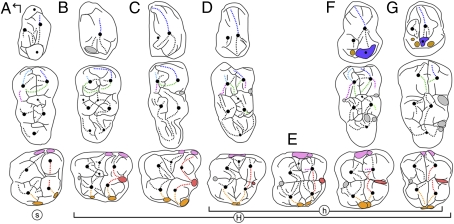
Fig. 3.
Sketches of p4, lower and upper molars (from top to bottom) of (A) Palaeochoerus, (B) Choeropotamus, (C) Bothriogenys, (D) Morotochoerus, (E) Kulutherium, (F) Kenyapotamus, and (G) Hexaprotodon. H, Hippopotamoidea; h, Hippopotamidae; s, Suoidea. Color correspondence: upper molars, red, postparacrista and premetacrista; pink, parastyle and mesiostyle; and orange, metastyles and distostyles; lower cheek teeth, dark green, postmetacristid and postprotocristid; light green, postectohypocristids; light blue, premetacristid; blue, preprotocristid; purple, postectoprotocristid and postectometacristid; violet, hypoconid; and brown, accessory conulids. The arrow indicates mesial (to top) and lingual (to left) directions.
The clade Hippopotamidae is strongly supported (Bremer index 5, clade h, Figs. 2A and 3). This clade splits into two basal clades: (i) a clade uniting Morotochoerus, Kulutherium, and Kenyapotamus species, with Morotochoerus as a first offshoot; and (ii) a clade corresponding to the Hippopotaminae, uniting the extant Choeropsis (Liberian hippo) and Hippopotamus as well as the extinct Archaeopotamus and Hexaprotodon. Traits unambiguously characterizing the node Hippopotamidae notably include six nonhomoplastic synapomorphies: lower incisor crown circular in transverse section (31; RI = 1.00), the presence of a deep indentation of the cervix of the lower incisor crown (42; RI = 1.00), postmetacristid of the lower molars orientated toward the center of the tooth (721; RI = 1.00), fused roots on the P1 (111; RI = 0.66), presence of an entoconid on p3 (511; RI = 0.66), and p4 with concave lingual and labial walls (522; RI = 0.91). Eight other character states that unambiguously define the clade Hippopotamidae also occur convergently in other cetartiodactyls of the analysis, such as a postmetacrista-distal cingulum connection on the distal face of their upper molars and not on the disto-labial corner (441; RI = 0.87), also observed in Cebochoerus. Two homoplastic characters are inferred to be reversions: lower incisor crown with no lingual fossid (50 RI = 0.83), a character state also observed in primitive cetartiodactyls such as Gujaratia pakistanensis, which exhibit a very simple incisor crown pattern, and P4 with a preparacrista mesially oriented (200; RI = 0.75). Four characters are convergently observed in suoids: parastyle of P4 not located in a labial position (190; RI = 0.81), preectostyle of the upper molars not individualized and most probably located in a mesial position (351; RI = 0.83), presence of an hypoconid on p2–3 (501; RI = 0.57, also observed in Cebochoerus), and presence of a posthypofossid (770; RI = 0.54, strongly homoplastic, also observed in some anthracotheriids, Cebochoerus and Gobiohyus).
The extended kenyapotamine clade is unambiguously supported by the presence of a postectohypocristid (741; RI = 1.00), the presence of a mesoconulid (731; RI = 0.70; this accessory cusp may occasionally occur in Hippopotaminae; cf. ref. 46, figures 8 and 10B), and a labial cingulid limited to the transversal valley (630; RI = 0.70). However, those synapomorphies concern the lower molars and cannot be controlled for Kulutherium, which is represented only by upper cheek teeth. Six other characters ambiguously support this node, among which two can be controlled in both Kenyapotamus and Kulutherium: the presence of an identifiable paraconule (271; RI = 0.80, ACCTRAN) lacking a distal extension (301; RI = 0.57, DELTRAN).
The clade Hippopotaminae is defined by six synapomorphies: relatively hypsodont upper molars (H > 70; 471; RI = 1.00), p2–3 with complex distal cristid (482; RI = 0.80), complex upper premolars, with an independent protocone on P3 (150; RI = 0.66), a complex paracone of P4 (222; RI = 0.66), postectoentocristid (801; RI = 0.72), and an absence of ectoentocristid on lower molars (780; RI = 0.50). This clade is supported by five supplemental ambiguous synapomorphies (DELTRAN optimization) including a postprotocristid more mesial than postmetacristid (680; RI = 0.80), a lack of paraconule (270, RI = 0.80), a deep indentation of cervix at middle of labial side of P3 (131; RI = 0.75, data missing for Archaeopotamus lothagamensis), and roots of one lobe unfused in lower molars (610; RI = 0.75). This clade is unresolved because the highly polymorphic dentition of Hippopotamus prevents a secure assessment in the absence of cranio-mandibular characters. Removing this taxon led to a resolution (Fig. 2B) in agreement with some previous phylogenies (for discussion, see ref. 22).
Implications for Hippopotamid Systematics and Diversity.
Our phylogenetic hypothesis supports the contention that both Morotochoerus and Kulutherium are hippopotamids. In the case of Morotochoerus, this relationship rejects previously claimed palaeochoerid affinities (36), implying that palaeochoerids were strictly restricted to Eurasia. In the case of Kulutherium, our analysis supports the initial attribution by Coryndon (42), and it must be noted that its identification as an anthracotheriid (40) is not irrelevant in the framework of a close relation between Hippopotamidae and anthracotheriids.
The nesting of these taxa with Kenyapotamus in the sister group of the Hippopotaminae suggests that they should be included within Kenyapotaminae. The subfamily diversity would thus rise from at least two species (22) to at least four. However, the (Morotochoerus, Kulutherium, Kenyapotamus) clade and its internal relationships are as yet weakly supported (Bremer index = 1) and both Morotochoerus and Kulutherium are still poorly known. For these reasons, we prefer to refer them to cf. Kenyapotaminae until further material enlightens these relationships. In any case, this grouping implies a considerable increase of body size range for early hippopotamids. Indeed, the body mass of both Kenyapotamus species is estimated at ∼170–220 kg (based on m1 measurements) (47), similar to that of Choeropsis liberiensis as an ecomorph. The body mass of M. ugandensis is significantly less, with an estimate of ∼30 kg, using the same method. The Hippopotamidae thus groups “peccary-sized” taxa (Morotochoerus, Fig. 1 A–D) with “hippo-size” taxa (including Kulutherium, Fig. 1F). This large body size range, comparable to that of anthracotheriids, reveals an unexpected past ecological diversity for hippopotamids.
Discussion
Emergence of a Unique Dental Pattern in Cetartiodactyla.
A peculiar cheek tooth pattern distinguishes the Hippopotamidae from all other extant and extinct ungulates and over the years it has been acknowledged through a diversity of terms (e.g., subbunodont, bunoselenodont, trefoliate, bunodont/bilophodont). It has further puzzled many anatomists attempting to classify the family (for a review, see ref. 23). Bunodonty and selenodonty are classically viewed as primitive and derived tooth patterns, respectively. It is for this reason that a differentiation of the bunodont Hippopotamidae from the most selenodont subfamily of anthracotheriids, the Bothriodontinae (48, 49), has not been supported by many contributions (e.g., ref. 43). This hypothesis would indeed imply relatively complex changes in the cheek tooth pattern, as discussed by Boisserie and Lihoreau (44) and Boisserie et al. (22). The inclusion of Morotochoerus and Kulutherium within the Hippopotamidae is particularly important in this regard, because it allows for a description of the nature and timing of these changes from ∼21 million years onward. Two main steps can be identified.
In the first step, it is hypothesized that the upper molar labial cristae migrated toward the median axis of the teeth (Fig. 3 D and E, cristae indicated in red on upper molars). Morotochoerus and Kulutherium (21 Ma to 17–15 Ma) already exhibit this lingual shift of postparacrista and premetacrista, such that the cristae are more aligned compared with the labially curving cristae in bothriodontines (Fig. 3C). Earliest representatives of Kenyapotamus (∼16 Ma) present a somewhat more derived condition (ref. 30: figure 5), and the inward migration of the crista is complete in the latest forms of Kenyapotamus (10.5–8.5 Ma, Fig. 3F) and in the Hippopotaminae (∼7.5 Ma; Fig. 3G). The migration of labial styles is accompanied by the migration of labial crests, with the para- and metastyles shifting toward the mesio- and distostyles respectively, until they are juxtaposed (Fig. 3 D–G, pink and orange areas on upper molars). In parallel to this migration, the lower molar anthracotheriid pattern was modified by a shift of the postmeta- and postprotocristids toward the transverse valley of the crown. This shift was accompanied by a lengthening of the postmetacristid, the latter being disconnected from the postprotocristid (Fig. 3 D–G, dark green cristids on lower molars).
The second step was marked by the acquisition of a distinctive trifoliate pattern for the molar cusps, characterizing the earliest Hippopotaminae (late Miocene, ∼7.5 Ma) and all later hippopotamids (Fig. 3G). This distinctive pattern corresponds to crest reduction in the four major cusps/cuspids, associated with the deepening and widening of lingual and labial fossae/fossids already present in anthracotheriids. The hippopotamine simplified lower molar structure is characterized by the loss of the ecto- and postectoentocristids and the premetacristid (Fig. 3G, light blue; for this particular cristid, see discussion in ref. 22). The postectocristids of the mesial cusps are also reduced (postectoprotocristid) or totally lacking (postectometacritid; Fig. 3G, cristids in purple on lower molars). The overall simplification of the molar pattern seen in hippopotamids is accompanied by an increase in the complexity of the premolar pattern in kenyapotamines and hippopotamines, including the isolation of a hypoconid (Fig. 3 F and G, violet cuspid) and an increasing number of accessory conulids on p4 (Fig. 3 F and G, brown cuspids), as well as a more complicated paracone structure on P4 (22).
In 1935, Colbert presented two alternative hypotheses of hippopotamid dental evolution from putative ancestors (bothriodontines or suoids) (48), a hypothesis summarized by Thenius (50). Our contribution offers a fossil-documented resolution of Colbert's case (48), with significant bearing on current views of hippopotamid origins. At a larger scale, it also documents the rooting time and place of the family.
Anchoring the Hippopotamidae into the Biogeographical History of Africa.
Before this work, the earliest known hippopotamid was Kenyapotamus ternani dated ∼16 Ma (early middle Miocene, ref. 26). The identification of Morotochoerus as the earliest hippopotamid implies moving back the FAD of the family to the earliest Miocene (Fig. 4), at >20.6 Ma (40Ar/39Ar on capping basalts, ref. 37). It further indicates that hippopotamid history was strictly African from the onset of the Miocene until their first dispersal toward Eurasia at 6.2 Ma (51).
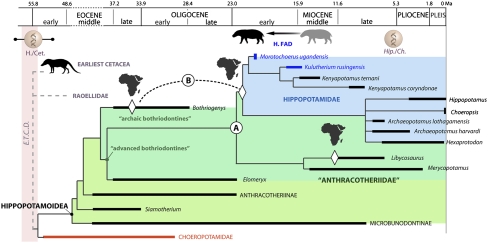
Fig. 4.
Temporal distribution, divergence estimates, and suggested phylogenetic relationships within cetartiodactyls classically related to the question of hippopotamid origin. The putative position of Cetacea is based on work by Boisserie et al. (35); that of Raoellidae is based on work by Thewissen et al. (11). E.T.C.D., estimated time for cetancodont differentiation; H./Cet., molecular divergence estimates between extant hippopotamids and cetaceans (data from refs. 1, 59, and 77); Hip./Ch., molecular divergence estimates between extant Hippopotamus and Choeropsis (data from ref. 59); H. FAD, first appearance datum for the Hippopotamidae based on this work (on the right, previous FAD); A, scenario for an emergence of the Hippopotamidae from a stock of Asian advanced bothriodontines; B, scenario for an emergence of the Hippopotamidae from a stock of African archaic bothriodontines (see text for discussion). White diamond symbols indicate dispersals to Africa.
Alternate paleobiogeographical scenarios of hippopotamid emergence relying on different phylogenetic grounds have been recently proposed. A first one suggested that a common ancestor exclusive to Libycosaurus and Hippopotamidae dispersed from Asia to Africa after 15 Ma (44). This scenario was discarded with the firm integration of the earliest kenyapotamines (∼16 Ma) within Hippopotamidae (22), a conclusion also supported by the present results. A second scenario proposes that Hippopotamidae emerged from Asian latest bothriodontines (Fig. 4A) (44). In this scenario, the forerunners of Hippopotamidae and of African advanced bothriodontines (Sivameryx, Afromeryx) would have dispersed alongside one another from Asia to Africa during the first main faunal interchange between Eurasia and Africa at 18 ± 1 Ma (proboscidean datum event sensu ref. 52), i.e., later than the age of Morotochoerus. Despite this discrepancy, this scenario remains congruent with our phylogenetic results (see also ref. 22), because it implies that advanced bothriodontines (Merycopotamus and Libycosaurus) should figure within the sister group of Hippopotamidae. Furthermore, the co-occurrence at Moroto of taxa of Eurasian origin, such as the ruminant cetartiodactyl Walangania africanus and the cricetodontid rodent Notocricetodon (39, 53), implies strong land connections occurred between Afro-Arabia and Asia earlier than 20.6 Ma. This statement was recently substantiated by the coeval occurrence of proboscideans (with deinotheriid Prodeinotherium and gomphotheriid elephantoids) of African origin along with bothriodontine anthracotheres in the earliest Miocene faunas of the Bugti Hills, Pakistan (54, 55). This result indicates that some anthracotheriid exchanges may have preceded the main faunal interchange by several million years. In fact, it could correspond to the early proboscidean datum event proposed by Tassy (56), which is compatible with the FAD of ∼21 Ma we propose for the Hippopotamidae.
A third paleobiogeographical scenario (Fig. 4B), elaborated by Boisserie et al. (22) on the basis of dental evidence, suggested that the Hippopotamidae could have derived from African archaic bothriodontines. These bothriodontines colonized Africa as early as the late Eocene (57). The hippopotamids would thus have a long, strictly African evolutionary history. The recognition of Morotochoerus as the earliest hippopotamid is temporally congruent with this view, but our cladistic analysis indicates that Hippopotamidae are closer to advanced and crown bothriodontines than to archaic bothriodontines (represented by Bothriogenys). Furthermore, it must be noted that the fossil record of the earliest hippopotamids remains scarce—even augmented by our results—and that late Oligocene bothriodontines are even scarcer (22). Fossil record gaps can alter phylogenetic reconstructions—there is no better illustration of this than the long overlooked relationship between cetaceans and hippopotamids. In addition, it has already been demonstrated that parallel adaptations between most derived hippopotamines and crown bothriodontines can impact the constituency of the anthracotheriid sister group of Hippopotamidae (35). These alternative paleobiogeographical scenarios are fully testable by future discoveries of Oligocene anthracotheriids in Africa as well as in Eurasia. In the meantime, both scenarios are compatible with molecular phylogenies of extant taxa, whereas this is not the case for any hypothesis rooting the Hippopotamidae into the Suina. Our data are therefore relevant to helping to reconstruct the evolutionary history of Cetancodonta.
Calibrating Cetartiodactyl Evolution and the Origin of Hippopotamoidea.
The minimum age for the Cetacea–Hippopotamidae divergence based on the oldest cetacean fossil (Himalayacetus subathuensis Bajpai and Gingerich, 1998) coincides with the earliest Eocene (52.4 Ma according to ref. 58), which is also consistent with molecular divergence time estimates (e.g., refs. 59 and 60). The Choeropsis–Hippopotamus molecular divergence suggested a differentiation of crown hippopotamids at 5.7 Ma (59), thus implying a >40-million-year-old potential ghost lineage for hippopotamids in the framework of an exclusive sister group relationship between Hippopotamidae and Cetacea. The fossil record demonstrates that this molecular divergence estimate is pertinent only at the level of the Hippopotaminae. Indeed, extant hippopotamids are two relictual species of a much more diversified group with a much deeper history (22). Considering our reassignment of Morotochoerus, the hippopotamid fossil record initiated as early as the earliest Miocene, reducing the putative “ghost lineage” by more than one-third of its duration. The nesting of the Hippopotamidae within anthracotheriids further fills the evolutionary history of the sister group of Cetacea. Still, the earliest hippopotamoid (Siamotherium from the late middle Eocene of Asia) (49) postdates the earliest archaeocete by 15 Ma. Filling this remaining gap first requires clarifying the closest affinities of the earliest anthracotheriids.
Different hypotheses, reflecting the poorly understood basal relationships of Cetartiodactyla, have been proposed for the origin of anthracotheriids. Eocene Asian Helohyidae (61–65) and Diacodexeidae (66, 67) were suggested as stem groups. However, recent phylogenetic analysis did not support close relationships between those taxa and anthracotheriids (e.g., refs. 10, 15, 22, and 35). Alternative sister taxa to the Hippopotamoidea were recently suggested, notably archaeocetes (35), cebochoerids (22), or larger clades including cebochoerids, raoellids, cetaceans, and hippopotamids (e.g., ref. 15). The Raoellidae (Eocene, Asia) have also been suggested to be related to anthracotheriids (68), but to our knowledge, no formal phylogenetic analysis supported this hypothesis or included a suitable taxa sample to test this relationship. Additional confusion was recently introduced with results supporting a polyphyletic Anthracotheriidae (9), markedly at odds with the paleontological literature (49). Our results offer another hypothesis for hippopotamoid origins by suggesting close affinities with the middle Eocene European Choeropotamus (Choeropotamidae) based on molar and premolar morphology (Fig. 3). This hypothesis is congruent with older hypotheses (e.g., ref. 69), but disagrees with most recent ones (43, 70, 71). Choeropotamidae occur far back into the earliest Eocene of Europe, ∼54 Ma (Cuisitherium) (71), and are thus roughly contemporary with the first archaeocete known in the Indian subcontinent deposits. This hypothesis needs to be further investigated with review of additional evidence, notably the craniomandibular morphology. If confirmed, the basal history of the Hippopotamoidea would be filled in, reaching probably very close in time to the hippopotamid–cetacean last common ancestor.
The Anthracotheriidae have been considered to originate in eastern Asia during the Eocene (64, 66, 67, 72). In fact, several Eocene artiodactyl taxa from this very area still have problematic affinities, such as Asian helohyids (Pakkokuhyus and Gobiohyus) (73, 74). These Asian taxa might also be closely related to European choeropotamids. Choeropotamids have often been ignored in “total evidence” and other phylogenetic analyses of Cetartiodactyla. This is also the case for most endemic European Paleogene cetartiodactyls, often considered as phylogenetic dead ends. Nevertheless, new data from India suggest Europe–Asia exchanges during the early Eocene (75), and phylogenetic affinities of these European taxa are most probably to be found in the Asian early Eocene cetartiodactyl fauna. The choeropotamid/hippopotamoid relationship proposed here highlights the critical need for more completely including the diversity of Paleogene taxa into phylogenetic analyses of the Cetartiodactyla. In particular, the resolution of cetancodont relationships now depends on integrating these and other overlooked basal cetartiodactyls.
Materials and Methods
The data matrix includes 84 dental characters controlled for 26 taxa (the character list and details on the included taxa are provided in SI Text S1). The taxa included were chosen to address the questions of relationships within Hippopotamidae and the immediate affinities of Hippopotamidae with other artiodactyls. The ingroup includes extant (two species) and extinct (seven species) hippopotamids. Thirteen other taxa were selected by reference to the different hypotheses formulated for the hippopotamid stem group: anthracotheriids (35, 43, 48), suoids (29, 48), and cebochoerids (69). They were chosen to span the morphological, temporal, and geographical diversity of each of these groups. Together with these taxa, we included putative anthracotheriid stem groups (Gobiohyus, Khirtharia, and Choeropotamus) as well as Entelodon, a nonsuoid representative of the Suina (following ref. 76), to strengthen the validity of our results regarding hippopotamid affinities and to enhance character polarity among clades. Entelodon was preferred among other entelodonts for being a particularly well-known genus with a relatively archaic dentition.
The matrix was treated under the assumption of the minimal model of unweighted parsimony, using PAUP*(version 4.0_10), with a heuristic search (branchswapping Tree-Bisection-Reconnection, 1,000 replications with random taxa addition, 100 trees held per replicate). All characters are parsimony informative and were treated as unordered. Multistate taxa were treated both as uncertain and as polymorphic. The polymorph option has no effect on the number and topology of the parsimonious trees retrieved; the results presented here correspond to the “uncertain option.” Bremer indexes were computed manually. The analysis was rooted with G. pakistanensis, which was chosen as representative of the most primitive cetartiodactyls. The data matrix (nexus) is available in SI Text S2.
Supplementary Material
Acknowledgments
We gratefully thank the heads and people of other institutions who graciously provided access to their collections (see SI Text S1) L.M. thanks the Uganda National Council for Science and Technology and the Uganda National Museum for permission to conduct research in Uganda. We thank M. Pickford for providing us with casts of published Morotochoerus specimens. We also thank S. Cote, S. Ducrocq, J. Hooker, and P.-O. Antoine for valuable help. This work was supported by the Agence Nationale pour la Recherche and European Research Council program Palasiafrica (ANR-08-JCJC-0017-01) (to M.O. and F.L.); and field work was supported by grants (to L.M.) from the Louis S. B. Leakey Foundation, the National Science Foundation (0456589), and the Wenner-Gren Foundation. J.-R.B. is also indebted to the Foundation Fyssen, the Omo Group Research Expedition, and the Mission Paléoanthropologique Franco-Tchadienne.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1001373107/-/DCSupplemental.
References
- 1.Arnason U, Gullberg A, Gretarsdottir S, Ursing B, Janke A. The mitochondrial genome of the sperm whale and a new molecular reference for estimating eutherian divergence dates. J Mol Evol. 2000;50:569–578. doi: 10.1007/s002390010060. [DOI] [PubMed] [Google Scholar]
- 2.Gatesy J. More DNA support for a Cetacea/Hippopotamidae clade: The blood-clotting protein gene gamma-fibrinogen. Mol Biol Evol. 1997;14:537–543. doi: 10.1093/oxfordjournals.molbev.a025790. [DOI] [PubMed] [Google Scholar]
- 3.Gatesy J. Molecular evidence for the phylogenetic affinities of Cetacea. In: Thewissen JGM, editor. The Emergence of Whales. New York: Plenum; 1998. pp. 63–111. [Google Scholar]
- 4.Irwin DM, Arnason U. Cytochrome b gene of marine mammals: Phylogeny and evolution. J Mamm Evol. 1994;2:37–55. [Google Scholar]
- 5.Marcot JD. Molecular phylogeny of terrestrial artiodactyls: Conflicts and resolution. In: Prothero DR, Foss SE, editors. The Evolution of Artiodactyls. Baltimore: The Johns Hopkins Univ Press; 2007. pp. 4–18. [Google Scholar]
- 6.Matthee CA, Burzlaff JD, Taylor JF, Davis SK. Mining the mammalian genome for artiodactyl systematics. Syst Biol. 2001;50:367–390. [PubMed] [Google Scholar]
- 7.Nikaido M, Rooney AP, Okada N. Phylogenetic relationships among cetartiodactyls based on insertions of short and long interpersed elements: Hippopotamuses are the closest extant relatives of whales. Proc Natl Acad Sci USA. 1999;96:10261–10266. doi: 10.1073/pnas.96.18.10261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waddell PJ, Okada N, Hasegawa M. Towards resolving the interordinal relationships of placental mammals. Syst Biol. 1999;48:1–5. [PubMed] [Google Scholar]
- 9.Spaulding M, O'Leary MA, Gatesy J. Relationships of Cetacea (Artiodactyla) among mammals: Increased taxon sampling alters interpretations of key fossils and character evolution. PLoS ONE. 2009;4:e7062. doi: 10.1371/journal.pone.0007062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Theodor JM, Foss SE. Deciduous dentitions of Eocene cebochoerid artiodactyls and cetartiodactyl relationships. J Mamm Evol. 2005;12:161–181. [Google Scholar]
- 11.Thewissen JGM, Cooper LN, Clementz MT, Bajpai S, Tiwari BN. Whales originated from aquatic artiodactyls in the Eocene epoch of India. Nature. 2007;450:1190–1194. doi: 10.1038/nature06343. [DOI] [PubMed] [Google Scholar]
- 12.Agnarsson I, May-Collado LJ. The phylogeny of Cetartiodactyla: The importance of dense taxon sampling, missing data, and the remarkable promise of cytochrome b to provide reliable species-level phylogenies. Mol Phylogenet Evol. 2008;48:964–985. doi: 10.1016/j.ympev.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 13.Gatesy J, Matthee C, DeSalle R, Hayashi C. Resolution of a supertree/supermatrix paradox. Syst Biol. 2002;51:652–664. doi: 10.1080/10635150290102311. [DOI] [PubMed] [Google Scholar]
- 14.Price SA, Bininda-Emonds ORP, Gittleman JL. A complete phylogeny of the whales, dolphins and even-toed hoofed mammals (Cetartiodactyla) Biol Rev Camb Philos Soc. 2005;80:445–473. doi: 10.1017/s1464793105006743. [DOI] [PubMed] [Google Scholar]
- 15.Geisler JH, Theodor JM, Uhen MD, Foss SE. Phylogenetic relationships of cetaceans to terrestrial artiodactyls. In: Prothero DR, Foss SE, editors. The Evolution of Artiodactyls. Baltimore: The Johns Hopkins Univ Press; 2007. pp. 19–31. [Google Scholar]
- 16.O'Leary MA, Gatesy J. Impact of increased character sampling on the phylogeny of Cetartiodactyla (Mammalia): Combined analysis including fossils. Cladistics. 2008;24:397–442. doi: 10.1111/j.1096-0031.2007.00187.x. [DOI] [PubMed] [Google Scholar]
- 17.O'Leary MA. The phylogenetic position of cetaceans: Further combined data analyses, comparisons with the stratigraphic record and a discussion of character optimization. Am Zool. 2001;41:487–506. [Google Scholar]
- 18.Geisler JH, Uhen MD. Morphological support for a close relationship between hippos and whales. J Vertebr Paleontol. 2003;23:991–996. [Google Scholar]
- 19.Geisler JH, Uhen MD. Phylogenetic relationships of extinct cetartiodactyls: Results of simultaneous analyses of molecular, morphological, and stratigraphic data. J Mamm Evol. 2005;12:145–160. [Google Scholar]
- 20.Geisler JH, Theodor JM. Hippopotamus and whale phylogeny. Nature. 2009;458:E1–E4. doi: 10.1038/nature07776. discussion E5. [DOI] [PubMed] [Google Scholar]
- 21.Boisserie J-R, Lihoreau F, Brunet M. Origins of Hippopotamidae (Mammalia, Cetartiodactyla): Towards resolution. Zool Scr. 2005;34:119–143. [Google Scholar]
- 22.Boisserie J-R, et al. Morphology and phylogenetic relationships of the earliest known hippopotamids (Cetartiodactyla, Hippopotamidae, Kenyapotaminae) Zool J Linn Soc. 2010;158:325–366. [Google Scholar]
- 23.Pickford M. The myth of the hippo-like anthracothere: The eternal problem of homology and convergence. Rev Esp Paleontol. 2008;23:31–90. [Google Scholar]
- 24.Boisserie J-R. The phylogeny and taxonomy of Hippopotamidae (Mammalia: Artiodactyla): A review based on morphology and cladistic analysis. Zool J Linn Soc. 2005;143:1–26. [Google Scholar]
- 25.Weston EM. Fossil Hippopotamidae from Lothagam. In: Harris JM, Leakey MG, editors. Lothagam. The Dawn of Humanity in Eastern Africa. New York: Columbia Univ Press; 2003. pp. 380–410. [Google Scholar]
- 26.Behrensmeyer AK, Deino AL, Hill A, Kingston JD, Saunders JJ. Geology and geochronology of the middle Miocene Kipsaramon site complex, Muruyur Beds, Tugen Hills, Kenya. J Hum Evol. 2002;42:11–38. doi: 10.1006/jhev.2001.0519. [DOI] [PubMed] [Google Scholar]
- 27.Nakaya H, Pickford M, Nakano Y, Ishida H. The late Miocene large mammal fauna from the Namurungule Formation, Samburu Hills, northern Kenya. Afr Study Monogr. 1984;(Suppl 2):87–131. [Google Scholar]
- 28.Nakaya H, Pickford M, Yasui K, Nakano Y. Additional large mammalian fauna from the Namurungule Formation, Samburu Hills, northern Kenya. Afr Study Monogr. 1987;(Suppl 5):47–98. [Google Scholar]
- 29.Pickford M. On the origins of Hippopotamidae together with descriptions of two species, a new genus and a new subfamily from the Miocene of Kenya. Geobios. 1983;16:193–217. [Google Scholar]
- 30.Pickford M. Suidae and Hippopotamidae from the middle Miocene of Kipsaraman, Kenya and other sites in East Africa. Paleontol Res. 2007;11:85–105. [Google Scholar]
- 31.Tsujikawa H. The updated late Miocene large mammal fauna from Samburu Hills, northern Kenya. Afr Study Monogr. 2005;(Suppl 32):1–50. [Google Scholar]
- 32.Pickford M. Découverte de Kenyapotamus en Tunisie. Ann Paleontol. 1990;76:277–283. [Google Scholar]
- 33.Geraads D, Alemseged Z, Bellon H. The late Miocene mammalian fauna of Chorora, Awash Basin, Ethiopia: Systematics, biochronology, and the 40K-40Ar ages of the associated volcanics. Tertiary Res. 2002;21:113–122. [Google Scholar]
- 34.Suwa G, Kono RT, Katoh S, Asfaw B, Beyene Y. A new species of great ape from the late Miocene epoch in Ethiopia. Nature. 2007;448:921–924. doi: 10.1038/nature06113. [DOI] [PubMed] [Google Scholar]
- 35.Boisserie J-R, Lihoreau F, Brunet M. The position of Hippopotamidae within Cetartiodactyla. Proc Natl Acad Sci USA. 2005;102:1537–1541. doi: 10.1073/pnas.0409518102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pickford M. A new genus of Tayassuidae (Mammalia) from the Middle Miocene of Uganda and Kenya. Ann Paleontol. 1998;84:275–285. [Google Scholar]
- 37.Gebo DL, et al. A hominoid genus from the early Miocene of Uganda. Science. 1997;276:401–404. doi: 10.1126/science.276.5311.401. [DOI] [PubMed] [Google Scholar]
- 38.Pickford M, Senut B, Hadoto D, Musisi J, Kariira C. New discoveries in the Miocene sites of Moroto (eastern Uganda): Biostratigraphical and paleoecological implications (Translated from French) C R Acad Sci. 1986;302:681–686. [Google Scholar]
- 39.Pickford M, Mein P. Early Middle Miocene mammals from Moroto II, Uganda. Beitr. Paläont. 2006;30:361–386. [Google Scholar]
- 40.Pickford M. A new suiform (Artiodactyla, Mammalia) from the Early Miocene of East Africa. C R Palevol. 2007;6:221–229. [Google Scholar]
- 41.Peppe DJ, et al. Stratigraphic interpretation of the Kulu Formation (Early Miocene, Rusinga Island, Kenya) and its implications for primate evolution. J Hum Evol. 2009;56:447–461. doi: 10.1016/j.jhevol.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 42.Coryndon SC. Hippopotamidae. In: Maglio VJ, Cooke HBS, editors. Evolution of African Mammals. Cambridge, MA: Harvard Univ Press; 1978. pp. 483–495. [Google Scholar]
- 43.Gentry AW, Hooker JJ. The phylogeny of the Artiodactyla. In: Benton MJ, editor. The Phylogeny and Classification of the Tetrapods, Volume 2: Mammals (Systematics Association Special Volume) 35B. Clarendon, Oxford; 1988. pp. 235–272. [Google Scholar]
- 44.Boisserie J-R, Lihoreau F. Emergence of Hippopotamidae: New scenarios. C R Palevol. 2006;5:749–756. [Google Scholar]
- 45.van der Made J. Superfamily Hippopotamoidea. In: Rössner GE, Heissig K, editors. The Miocene Land Mammals of Europe. München: Verlag Dr Friedrich Pfeil; 1999. pp. 203–208. [Google Scholar]
- 46.Gaziry AW. Hexaprotodon sahabiensis (Artiodactyla, Mammalia): A new hippopotamus from Libya. In: Boaz NT, El-Arnauti A, Gaziry AW, Heinzelin JD, Dechant Boaz D, editors. Neogene Paleontology and Geology of Sahabi. New York: Liss; 1987. pp. 303–315. [Google Scholar]
- 47.Damuth J, MacFadden BJ. Problems in estimating body masses of archaic ungulates using dental measurements. In: Damuth J, MacFadden B, editors. Body Size in Mammalian Paleobiology: Estimation and Biological Implications. Cambridge, UK: Cambridge Univ Press; 1990. pp. 229–253. [Google Scholar]
- 48.Colbert EH. The phylogeny of the Indian Suidae and the origin of the Hippopotamidae. Am Mus Novit. 1935;799:1–24. [Google Scholar]
- 49.Lihoreau F, Ducrocq S. Family Anthracotheriidae. In: Prothero DR, Foss SE, editors. The Evolution of Artiodactyls. Baltimore: The Johns Hopkins Univ Press; 2007. pp. 89–105. [Google Scholar]
- 50.Thenius E. About some problems of the evolution of mammals (Translated from German) J Zool Syst Evol Res. 1969;7:157–179. [Google Scholar]
- 51.Agustí J, Garcés M, Krijgsman W. Evidence for African–Iberian exchanges during the Messinian in the Spanish mammalian record. Palaeogeogr Palaeoclimatol Palaeoecol. 2006;238:5–14. [Google Scholar]
- 52.Madden CT, Van Couvering JA. The Proboscidean datum event: Early Miocene migrations from Africa. Geol Soc Am Abstracts with Programs. 1976;8:992–993. [Google Scholar]
- 53.MacLatchy L, Cote S, Orliac M, Sanders W, Winkler A. The faunal age of Moroto I and II, Uganda. J Vertebr Paleontol. 2008;28(Suppl 3):110A. [Google Scholar]
- 54.Antoine P-O, et al. Mammalian Neogene biostratigraphy of the Sulaiman Province, Pakistan. In: Wang X, Fortelius M, Flynn LJ, editors. Asian Mammal Biostratigraphy. New York: Columbia Univ Press; [Google Scholar]
- 55.Métais G, et al. Lithofacies, depositional environments, regional biostratigraphy and age of the Chitarwata Formation in the Bugti Hills, Balochistan, Pakistan. J Asian Earth Sci. 2009;34:154–167. [Google Scholar]
- 56.Tassy P. The “Proboscidean Datum Event”: How many proboscideans and how many events? In: Lindsay EH, Fahlbusch V, Mein P, editors. European Neogene Mammal Chronology. New York: Plenum; 1990. pp. 237–252. [Google Scholar]
- 57.Ducrocq S. The anthracotheriid genus Bothriogenys (Mammalia, Artiodactyla) in Africa and Asia during the Paleogene: Phylogenetical and paleobiogeographical relationships. Stuttg Beitr Naturk ser B. 1997;250:1–43. [Google Scholar]
- 58.Benton MJ, Donoghue PCJ, Asher RJ. Calibrating and constraining molecular clocks. In: Hedges SB, Kumar S, editors. The Timetree of Life. Oxford: Oxford Univ Press; 2009. pp. 35–86. [Google Scholar]
- 59.Montgelard C, Catzeflis FM, Douzery E. Phylogenetic relationships of artiodactyls and cetaceans as deduced from the comparison of cytochrome b and 12S rRNA mitochondrial sequences. Mol Biol Evol. 1997;14:550–559. doi: 10.1093/oxfordjournals.molbev.a025792. [DOI] [PubMed] [Google Scholar]
- 60.Murphy WJ, Pringle TH, Crider TA, Springer MS, Miller W. Using genomic data to unravel the root of the placental mammal phylogeny. Genome Res. 2007;17:413–421. doi: 10.1101/gr.5918807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coombs WP, Jr, Coombs MC. The origin of anthracotheres. N Jb Geol Paläont Mh. 1977;10:584–599. [Google Scholar]
- 62.Ducrocq S, Chaimanee Y, Suteethorn V, Jaeger J-J. First discovery of Helohyidae (Artiodactyla, Mammalia) in the Late Eocene of Thaïland: A possible transitional form for Anthracotheriidae. C R Acad Sci Paris ser II. 1997;325:367–372. [Google Scholar]
- 63.Matthew WD, Granger W. New mammals from the Irdin Manha Eocene of Mongolia. Am Mus Novit. 1925;198:1–10. [Google Scholar]
- 64.Pilgrim GE. The Artiodactyla of the Eocene of Burma. Mem Geol Surv India. 1928;13:1–39. [Google Scholar]
- 65.Pilgrim GE. Middle Eocene mammals from North-West India. Proc Zool Soc Lond. 1940;110:127–152. [Google Scholar]
- 66.Ducrocq S. Les anthracothères paléogènes de Thaïlande: paléogéographie et phylogénie. C R Acad Sci Paris ser II. 1994;318:549–554. [Google Scholar]
- 67.Ducrocq S. The late Eocene Anthracotheriidae (Mammalia, Artiodactyla) from Thailand. Palaeontogr Abt A. 1999;252:93–140. [Google Scholar]
- 68.Sahni A, et al. Vertebrates from the Subathu Formation and comments on the biogeography of Indian subcontinent during the early Paleogene. Bull Soc Geol Fr. 1981;23:689–695. [Google Scholar]
- 69.Pearson HS. On the skulls of early Tertiary Suidae, together with an account of the otic region in some other primitive Artiodactyla. Philos Trans R Soc Lond. 1927;215:389–460. [Google Scholar]
- 70.Hooker JJ, Thomas KM. A new species of Amphirhagatherium (Choeropotamidae, Artiodactyla, Mammalia) from the Late Eocene Headon Hill formation of Southern England and phylogeny of endemic European ‘Anthracotherioids’. Palaeontology. 2001;44:827–853. [Google Scholar]
- 71.Sudre J, Lecomte G. Relationships and systematic position of Cuisitherium Sudre et al., 1983, the most derived of the early Eocene artiodactyls of Europe (Translated from French) Geodiversitas. 2000;22(3):415–432. [Google Scholar]
- 72.Suteethorn V, Buffetaut E, Helmcke-Ingavat R, Jaeger J-J, Jongkanjanasoontorn Y. Oldest known Tertiary mammals from south east Asia: Middle Eocene primate and anthracotheres from Thailand. N Jb Geol Paläont Mh. 1988;9:563–570. [Google Scholar]
- 73.Foss SE. Family Helohyidae. In: Prothero DR, Foss SE, editors. The Evolution of Artiodactyls. Baltimore: The Johns Hopkins Univ Press; 2007. pp. 85–88. [Google Scholar]
- 74.Tsubamoto T, et al. The Anthracotheriidae (Mammalia; Artiodactyla) from the Eocene Pondaung Formation (Myanmar) and comments on some other anthracotheres from the Eocene of Asia. Paleontol Res. 2002;6:363–384. [Google Scholar]
- 75.Rose KD, et al. First tillodont from India: Additional evidence for an early Eocene faunal connection between Europe and India? Acta Palaeontol Pol. 2009;54:351–355. [Google Scholar]
- 76.Foss SE. Family Entelodontidae. In: Prothero DR, Foss SE, editors. The Evolution of Artiodactyls. Baltimore: The Johns Hopkins Univ Press; 2007. pp. 120–129. [Google Scholar]
- 77.Bininda-Emonds ORP, et al. The delayed rise of present-day mammals. Nature. 2007;446:507–512. doi: 10.1038/nature05634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.