Abstract
Here we present data concerning the pattern of dental development derived from the microcomputed tomography (microCT) study of a recently discovered immature hominin mandible with a mixed dentition recovered from the TD6 level of the Gran Dolina Lower Pleistocene cave site in Sierra de Atapuerca, northern Spain. These data confirm our previous results that nearly 1 million years ago at least one European hominin species had a fully modern pattern of dental development with a clear slowdown in the development of the molar field regarding the anterior dental field. Furthermore, using available information about enamel formation times and root extension rates in chimpanzees, early hominins, and modern humans, we have estimated that the formation time of the upper and lower first molars of individual 5 (H5) from TD6, which had just erupted at the time of the death of this individual, ranges between 5.3 and 6.6 y. Therefore, the eruption time of the first permanent molars (M1) in the TD6 hominins was within the range of variation of modern human populations. Because the time of M1 eruption in primates is a robust marker of life history, we suggest, as a working hypothesis, that these hominins had a prolonged childhood in the range of the variation of modern humans. If this hypothesis is true, it implies that the appearance in Homo of this important developmental biological feature and an associated increase in brain size preceded the development of the neocortical areas leading to the cognitive capabilities that are thought to be exclusive to Homo sapiens.
Keywords: Atapuerca, childhood, human evolution, life-history pattern
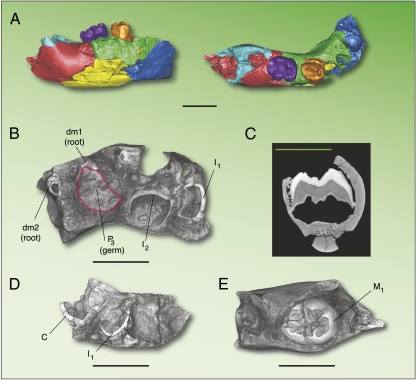
Fossils of infant and juvenile hominins are extremely rare, and most hominin fossil assemblages are dominated by adult specimens. Nonetheless, only subadult individuals, in whom teeth were still developing and erupting, provide the opportunity to study precisely the evolution of growth and development in the human lineage, and subadults are therefore important for reconstructing the life history of fossil species. Modern humans are characterized by a unique developmental trajectory, in which a prolonged childhood is associated with the growth of our relatively large brains (1–3). Although brain size has been estimated for many fossil hominins, and it is well known that the development and eruption of the first molar provide an accurate assessment of brain growth in mammals (4, 5), little is known about when the specific ontogenetic patterns accompanying this brain expansion first appeared in the human fossil record. In this context, we describe here the dental developmental sequence of a recently discovered immature mandible (ATD6-112) recovered in the 2006 field season from the upper layers of the TD6 lithostratigraphic unit, the so-called Aurora Archaeostratigraphic Set (6), at the Gran Dolina paleoanthropological site (Sierra de Atapuerca, Burgos, Spain). The mandible was strongly cemented, and the restoration process was completed in late 2008. The mandible was recovered in seven isolated pieces, which fit together precisely (Fig. 1), and represents individual number 11 of the TD6 hypodigm (H11). ATD6-112 is represented by the right part of the mandibular corpus from the symphysis to the crypt of the second permanent molar (M2), the crowns of the deciduous dm1 and dm2, and the germs of the permanent first and second incisors, the permanent canine, and the permanent third premolar (I1, I2, C, P3), and the first permanent molar (M1) in different stages of development (Table 1). We have also undertaken observations of the upper and lower first molars of another subadult individual from TD6 (individual H5) to address whether the TD6 hominins represent the earliest appearance of the unique and prolonged phase of childhood in the genus Homo.
Fig. 1.
Some microCT images of ATD6-112. (A) Virtual reconstruction of ATD6-112 in lateral view (Left) and in occlusal view from a careful digital fit of the seven fragments recovered in TD6; a portion of the crown of the dm1 and dm2 was missing during the excavation and it was difficult to find the precise position of these teeth in the specimen. The light blue in the distal portion of the specimen covers the M1 and allows us to see a small surface of this tooth, still far from early emergence (see also C). (B) Inferior view of ATD6-112 showing the developing stage of some permanent and deciduous teeth. (C) A virtual cross-section through the mesial pulpal horns of the M1 showing a minimum development of the root. (D) Visual aspect of the symphysis in superior view showing the germs of the permanent first lower incisor and canine. (E) Occlusal view of the M1 inside its cript. Scale bars: 1 cm.
Table 1.
Developmental stages of teeth preserved in ATD6-112
| Tooth | Description |
| dm1 | Crown and root complete. |
| dm2 | Crown complete. The apex of the mesial and distal roots are still open. Stage H1 of Liversidge and Molleson (53) |
| I1 | Crown complete. No evidences of root formation. |
| I2 | Crown complete. No evidences of root formation. |
| C | About 10 mm of the crown formed; on the basis of the morphology of the cervix, as well as on the data about the crown height of hominid 1 from TD6 (12.5 mm), we estimate that the crown of this canine is about Cr 3/4 + (80%). |
| P3 | About 5 mm of the crown complete: Cr ½. |
| M1 | Crown complete. Root length at protoconid is 0.48 mm; root length at metaconid is 0.13 mm. The tip of the cusps are approximately 3 mm from alveolar eruption. |
General consensus among paleoanthropologists suggests that Australopithecus, Paranthropus, and other Pliocene hominins, as well as early members of the genus Homo, had growth and developmental patterns with similarities to those of great apes (7–14), including a relatively rapid overall period of tooth formation and eruption and relatively small brains compared with modern humans and other later hominins. In contrast, evolution within the genus Homo has been characterized by an increase of the brain size and the duration of somatic development, which has profound consequences for life-history patterns (15). Available evidence clearly demonstrates differences in developmental timing between later members of genus Homo and early hominins/African apes, but it is unknown precisely when this shift in developmental timing and the concomitant changes in life history occurred. A recent developmental study (16) suggests that childhood is a relatively late acquisition in the evolution of the genus Homo; thus, earlier fossil hominins possessed shorter growth periods and therefore life-history patterns different from those of Homo sapiens.
Prolongation of somatic development in H. sapiens relative to earlier hominins involves the appearance of a distinct childhood phase, as well as a marked period of adolescence (2, 3). These uniquely human developmental stages are critically important because this is when the brain finishes its growth and reaches its maximum size and when children develop and refine the cognitive abilities and social skills necessary to face life challenges. Determining the presence and duration of a childhood phase in an extinct Homo species is thus of great importance for understanding Pleistocene hominin evolution and the evolutionary origins of the modern human developmental trajectory.
During the past three decades, the most common method used to address questions of hominin growth and development has been the study of the duration and the sequence of dental development (7, 8, 10, 11), and within the past 10 y several important studies documenting aspects of hominin dental development have been published (17–22). These studies offer data and several techniques useful for addressing several aspects of growth and development relevant to human evolution. Concerning the timing of dental developmental events, it is well known that in great apes the crown formation and gingival emergence of M1 are considerably advanced with regard to the permanent incisors, I1 and I2 (23–25). In H. sapiens, in contrast, the permanent incisors and first molars develop and erupt nearly synchronously (26). In an effort to test the ability to recognize ape and human patterns of dental development, Smith (27) found a high degree of discrimination when molar and incisor/canine fields were considered. In a broad sense, differences in time between the gingival eruption of the M1 in great apes and modern humans are precisely related to the appearance of a distinct childhood period (2).
The fossils from the TD6 level of the Gran Dolina paleoanthropological site (Sierra de Atapuerca, Burgos, Spain) have a geological age of between 0.80 and 0.96 million years ago (Mya) (28, 29) and have been assigned to the hominin taxon Homo antecessor (30). These hominins facilitate study of a spacio-temporal interval where the fossil record is particularly scarce, offering a rare opportunity to study the transition from an early hominin/ape-like dental developmental pattern to the modern human sequence of calcification and eruption. Previous studies of the hominins from TD6 concluded that they had a modern human-like pattern of dental development (31), but that study was based on interpretations from individuals that are too advanced in their dental development to precisely assess the relationship between the developing incisors and molars. The new juvenile mandible (ATD6-112), however, provides a chance to directly assess the development of different tooth classes in situ in the same individual, facilitating a precise description of their relative development. Moreover, nondestructive methods and research approaches for estimating the age at death of fossil hominins have been developed in recent years (16, 21, 22). These studies are an important step in the determination of the time and timing of dental development of extinct hominin species, which can be applied to the juvenile fossils from the TD6 H. antecessor hypodigm.
Results
Unfortunately, histological study of teeth is not possible because teeth are either included in their crypts or strongly cemented and adhered to the walls of the alveolus. However, the microcomputed tomography (microCT) images of the specimen allowed us to assess with great accuracy the stage of development of each tooth at the time of death of the individual.
To test whether the sequence of dental developmental of ATD6-112 is more similar to that of African great apes or to that of modern humans, we compared the relative timing of the attainment of developmental stages of the I1 and M1 in Pan troglodytes and H. sapiens to that observed in the TD6 fossils, and we made comparisons to the few fossil hominins available for study of comparable developmental stages.
First, we estimated the age at death of H11 from TD6 assuming that his or her dental developmental rate was similar either to that of chimpanzees or to that of modern humans. We considered the following aspects: ATD6-112 exhibits the crown of the M1 fully formed and with some development of the root. Measurements of the M1 root in millimeters are the following: mesiobuccal, 0.48; mesiolingual, 1.14; distobuccal, 0.92; and distolingual, 1.58. The mean length is 1.03 mm. The time of crown formation of the M1 in P. troglodytes is between 2.18 and 2.64 y, but because it begins to form before birth the age at the end of crown formation of this tooth would be 2.01–2.58 y (32). In accordance with the data of Dean and Vesey (22), we have estimated that the rate of root extension in P. troglodytes during the first 1,030 μm of growth is 4.9 μm/d. Thus, this length of the root will be formed in 210 d (0.57 y). Therefore, using the M1, we estimate the age at death of the TD6 hominin to be between 2.58 and 3.15 y when his or her dental developmental rate was similar to that of chimpanzees. The duration of I1 crown formation in P. troglodytes is between 4.09 and 5.35 y (n = 4) (24). Because in the sample studied by Reid et al. (24) the I1 begins to form 0.15–0.46 y after birth, the estimated age at death of ATD6-112 using the I1 would be between 4.24 and 5.81 y. Thus, there is no overlapping in the attainment of equivalent stages of M1 and I1 development in chimpanzees and ATD6-112, and the hypothesis that ATD6-112 follows a Pan-like pattern of dental developmental sequence is unlikely (Table 2).
Table 2.
Age at death of individual 11 from Gran Dolina-TD6, considering that his or her dental development pattern was similar to that of chimpanzees or modern humans
| Time of the crown completion after birth | Time of root formation | Age at death | |
| P. troglodytes | |||
| M1 | 2.01–2.58 | 0.57 | 2.58–3.15 |
| I1 | 4.24–5.81 | 0.0 | 4.24–5.81 |
| H. sapiens | |||
| M1 | 3.10–3.30 | 0.68 | 3.78–3.98 |
| I1 | 3.50–3.90 | 0.0 | 3.50–3.90 |
All values are in years.
In H. sapiens, M1 begins to form just around the time of birth, and crown formation ends between 3.1 and 3.3 y (21). Following Dean and Vesey (22), we have estimated that the rate of root extension in H. sapiens during the first 1,030 μm of growth is 4.1 μm/d. Thus, this length of root will be formed in 251 d (0.68 y). Therefore, using the M1 we can estimate the age at death of the TD6 hominin to be between 3.78 and 3.98 y in the case that his or her dental development was similar to that of the modern humans. The duration of I1 crown formation in H. sapiens is between 3.4 and 3.8 y (21). Because the I1 begins to form about 0.15 y after birth, the age at death would have been between 3.5 and 3.9 y based on I1 development. The minimum difference in the attainment of equivalent stages of M1 and I1 development in modern humans and ATD6-112 is 0.01 y. The stage of development of the permanent canine and first premolar of ATD6-112 (Table 1) fits well within an age range of 3.5–3.9 y assuming that this specimen had a dental development time similar to modern humans (21, 33). With regard to relative dental developmental timing, ATD6-112 fits well within the modern human pattern, confirming our previous observations of other individuals from the TD6 hypodigm (31).
Regarding fossil hominins, it is known that delayed development of I1 relative to M1 (as in African apes) is characteristic of Australopithecus (27, 34). For example, the M1 of the Taung specimen (Australopithecus africanus) had just come into occlusion with a root length of about 5.0–6.0 mm (35), whereas Taung's lower permanent incisors are crown complete but lacking root development (35). Likewise, the australopith Sts 24 has the roots of M1 developed for more than half of their length, and this tooth is in functional occlusion, whereas only a minimal length of the root is formed in the I1 (26). CT images of the australopith infant from Dikika (DIK-1-1) also show M1 root growth in the absence of crown complete incisors (36). Taung, Sts 24, and DIK-1–1 therefore exhibit an ape-like M1/I1 developmental pattern, unlike that of modern humans and ATD6-112.
In contrast, and similar to the modern human pattern and that observed in ATD6-112, the completion of crown formation of the first permanent molar is nearly coincident with that of the first permanent incisor in Paranthropus (11). However, Paranthropus dental development exhibits approximately the same relatively rapid overall period of dental development as Australopithecus and African apes (11); the coincident M1/I1 calcification and eruption in Paranthropus is therefore interpreted as a homoplasy between Paranthropus and later Homo, rather than evidence of shared ancestry (25).
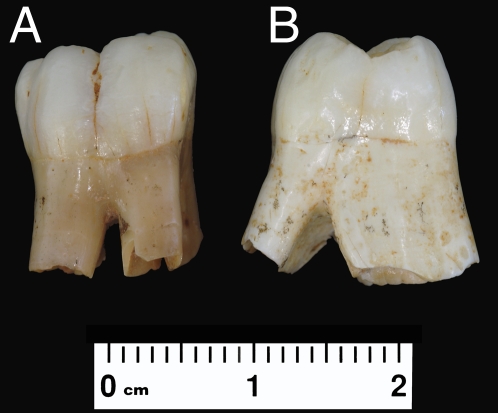
First molar development and gingival emergence are important biological events for determining the timing of neural and somatic development, which has become essential in reconstructing primate life-history scheduling (8, 22, 37). In this sense, we have the opportunity to estimate in an indirect way the time of formation and eruption of the M1 in the TD6 hominins. This evidence comes from individual H5, which is represented by a permanent upper right M1 (ATD6-103) and a permanent lower right M1 (ATD6-93) (Fig. 2). As discussed in Methods, both M1s exhibit minimal enamel attrition, indicating a very short duration of functional occlusion before death. The mesial root length of ATD6-93 is 9.0 mm long and the distal root length is 8.0 mm. The mesiobuccal part of the root is broken, but our estimates result in a length of ≈8.0 mm. The mesiolingual part of the root of ATD6-103, which is the best preserved, measures 9.2 mm in length. In modern humans, M1 eruption is completed when the root is between 7.7 and 10.0 mm long, whereas in P. troglodytes, gingival eruption occurs when the root is about 4.0 mm long (22). Concerning the root lengths of the lower M1s at the stage of immediate postgingival emergence in a sample of Pan specimens, Kelley, Dean, and Ross (38) obtained mean values of 4.83, 3.83, and 3.76 mm, respectively. These lengths are 4.17, 4.17, and 4.24 mm less than the values obtained in the lower M1 ATD6-93 from TD6, which are out of the range of variation obtained for these authors. Using the data of Dean and Vesey (22) for the root extension rates of the M1, the first 8 mm of the root length are formed in 3.2 y in modern humans and in 2.81 y in chimpanzees (Table 3). If we assume the most conservative hypothesis that the crown formation time of the M1 in the Gran Dolina hominins is similar to that of Australopithecus (2.5–2.7 y), then gingival emergence in these hominins occurred between 5.3 (2.5 + 2.8) and 5.5 (2.7 + 2.8) y. If we assume a crown time formation for the M1 of H. antecessor similar to that of modern humans (3.1–3.3 y), then gingival emergence would occur between 6.3 (3.1 + 3.2) and 6.5 (3.3 + 3.2) y (Table 4). Therefore, in every possible scenario, gingival emergence of the M1 in the TD6 hominins is attained at an age within the range of variation of modern humans (mean: 6.2; SD: 0.8) (39).
Fig. 2.
Teeth of individual 5 from TD6. (A) Lingual view of the permanent lower M1. (B) Mesial view of the permanent upper M1.
Table 3.
Estimations of the mean extension rates and times of formation of the root of M1
| H. sapiens | P. troglodytes | |||||
| Root length (μm) | MER* | Days | Years | MER | Days | Years |
| 0–1,000† | 4.1 | 243.9 | 0.67 | 4.9 | 201.3 | 0.55 |
| 1,000–2,000 | 5.7 | 175.4 | 0.48 | 6.4 | 156.2 | 0.43 |
| 2,000–8,000 | 8.0 | 750.0 | 2.05 | 9.0 | 667.0 | 1.83 |
| Total | 3.20 | 2.81 | ||||
Data were obtained from Dean and Vesey (2008).
*The estimations of the mean extension rates (MER) in H. sapiens and P. troglodytes were obtained in micrometers per day.
†The estimations of the time of formation of the root were obtained from different portions of the root.
Table 4.
Estimation of the time of gingival eruption of the upper and lower permanent first molars of H5 from Gran Dolina-TD6
| Australopithecus | H. sapiens | P. troglodytes | H. sapiens | |
| Range of crown formation time | 2.5–2.7 | 3.1–3.3 | ||
| Time of root formation | 2.8 | 3.2 | ||
| Range of total time of formation (crown + root) at gingival eruption* | 2.5 + 2.8 = 5.3 | 2.7 + 2.8 = 5.5 | 3.1 + 3.2 = 6.3 | 3.3 + 3.2 = 6.5 |
Because the maximum length of the root of these molars is 9.0–9.2 mm and the two molars exhibit a minimum functional occlusal wear, we assume that gingival eruption occurred when the root was about 8.0 mm in length. For this estimation, we used the range of crown formation time in Australopithecus and H. sapiens (see text), as well as the time of formation of the root of M1 that we obtained for P. troglodytes and H. sapiens (Table 3). All values are in years.
*Different combinations to obtain the minimum and maximum total time of formation of the permanent first molars from H5.
It is interesting that the mean root extension rate over 10 mm of root growth in the modern human sample used by Dean and Vesey (22) is about 7.0 μm/d, compared with 6.3 μm/d obtained over the entire root length (13.3 mm) of a Neanderthal M1 from La Chaise (20). Because in the modern human sample studied by Dean and Vesey (22) the root extension rate declines from 8.3 μm/d to a minimum of 5.5 μm/d between the value estimated at 6.0 mm of root length until the value estimated at 10.0 mm of root length, it seems that modern humans and Neanderthals probably do not differ significantly in this biological dental growth feature. We consider that the data for the root extension rates in modern humans better fit our estimations of the M1 root time formation in the TD6 hominins, given the more obvious phylogenetic proximity of these hominins to Neanderthals and modern humans than to chimpanzees. This would strengthen the hypothesis that the time of gingival emergence for the M1 in H. antecessor was probably within the time range of modern humans.
Discussion
H. antecessor is, at present, the earliest hominin known to possess the modern-human–like developmental pattern. In fact, the results that we have obtained using the microCT analysis of the ATD6-112 specimen provide direct evidence of dental developmental stages from the incisor and molar fields within the same juvenile H. antecessor individual and confirms our previous observations, based on less complete material, that the Gran Dolina-TD6 hominins, dating between 0.80 and 0.96 mya, exhibit an essentially modern human pattern of dental development.
Some authors suggest that a modern human pattern of dental development does not necessarily imply a time of dental development similar to that of modern humans (17). However, the concept of a dental development pattern implies a set of relative time values related to the onset and offset of the crown and root formation, as well as the time of gingival eruption of a given tooth with respect to other teeth of the same or other dental fields. In this sense, it is well known that the molar field is relatively independent of the incisor field in aspects such as the crown and root dimensions (40, 41), the crown morphology (42), and the time of development (27).
Concerning development, the most obvious difference between the early Pliocene hominins and later Homo is the slowdown in the overall development of the three molars relative to the development of the incisors (43). Although the direct observation of the perikymata is precluded by the firm adherence of the teeth to the walls of the alveolus, we have clear evidence that in the TD6 hominins a slowdown of the general processes of molar development had occurred (31). This implies that the somatic development of these hominins could have experienced a similar slowdown and that the whole process could be regulated by the growth of a large brain, the true pacemaker of development (4).
Furthermore, available information about crown formation times (17, 23, 24, 33, 35, 44), as well as data on rates of root growth in great apes and modern humans (22), allow us to make reliable estimations on the age at death in immature fossil individuals. Using this information, the range of time values for the eruption of the permanent first molars of the H5 from TD6 represents very robust data, which implies a prolonged childhood in the TD6 hominins. As Smith et al. (16) point out, eruption times and the attainment of developmental stages are “more robust indicators of life history” than crown formation times derived from histological analysis of incremental enamel features. Macchiarelli et al. (20 p 750) also write that “gingival emergence of the first permanent molar is a more reliable measure of relative dental development than are anterior tooth crown formation times.” We concur with the assessment of all these authors. On the basis of their criterion of the relative attainment of developmental stages, TD6 individuals H11 and H5 demonstrate that H. antecessor clearly exhibits a modern-human–like relative developmental sequence.
From a histological reconstruction of dental development, Dean et al. (17) estimated that the gingival emergence of the M1 of the Sangiran S7-37 specimen (45) occurred around 4.4 y, and around 7.6 for the P4 and M2, respectively. Dean and Smith (13) have obtained data from enamel and dentine microanatomy of some teeth of KNM-WT 15,000, and they concluded that the Nariokotome youth died when he was between 7.6 and 8.8 y old. Wear of the lower and upper second molars suggests that these teeth had been in functional occlusion for a short time. Therefore, it seems that gingival emergence of these molars in the Nariokotome youth occurred closer to 8 than to 12 y, as in modern humans. The specimen KNM-WT 15,000 is about 1.5 million years old (46). Thus, the TD6 hominins thus represent an important transition in the evolution of the genus Homo and are particularly advanced compared with early Pleistocene hominins such as KNM-WT 15,000 or S7-37 from Sangiran. Dean et al. (17 p 630) concluded that the emergence of a modern-human–like dental developmental pattern likely occurred “after the appearance of H. erectus, when both brain size and body size were well within the ranges known for modern humans.” A distinct and prolonged childhood is directly related to the increase of the brain volume in the genus Homo (3). On the basis of mean cranial capacity (826 cm3), Smith and Tompkins (47) estimate a mean age of 4.5 y for the emergence of M1 in H. erectus. Interestingly, the volume of the KNM-WT 15,000 specimen has been estimated as about 880 cm3 (48). Smith (49) and Bogin and Smith (2) suggested the hypothesis that an essentially human life cycle would appear once the threshold of 1,000 cm3 of cranial capacity is achieved or surpassed. It is therefore interesting to note that the brain volume of H. antecessor likely surpassed 1,000 cm3 (50). Therefore, it seems that our observations of the TD6 hominins support the hypothesis proposed by these authors.
The evidence presented here from the TD6 hypodigm concerning the relative stages of tooth development and eruption clearly documents that a European hominin species developed a childhood phase within the range of variation of that seen in H. sapiens during the late Early Pleistocene. These results push back the date of the earliest appearance of a prolonged childhood in hominins to more than 600 kya than previously thought (16). Therefore, the appearance of a prolonged childhood and an associated increase in brain size preceded the development of the neocortical areas leading to cognitive capabilities, such as language, which are thought to be exclusive to H. sapiens. In this context, Locke and Bogin's research (51) on the possible relationship between the appearance of language and the uniquely human stages of childhood and adolescence is relevant. These authors hypothesize that the process of linguistic evolution took a step forward about 2 mya with the elongation of childhood. Following the reasoning of Locke and Bogin (51), 1 mya speech may have been a matter of fact in populations like those of TD6. All these conclusions have important implications for understanding the evolution of hominin life-history patterns and open an intriguing debate about the possibility of finding the same, or perhaps different, developmental patterns in contemporaneous African and Asian hominin populations.
Methods
MicroCT Reconstruction and Measurement.
The seven fossil fragments constituting ATD6-112 were scanned individually with a Scanco microtomographic system (μCT 80; Scanco Medical) using identical settings for all scans: voltage: 70 kV; amperage: 140 mA; angular increment: 0.72°; and resultant isomeric voxel size: 36 μm3. Scans of individual fragments were rendered as surface models using the OsiriX software package, and these models were viewed using Mimics software, in which the individual pieces were digitally fit together to reconstruct the mandible's original morphology.
To produce virtual planes of section in which root lengths could be measured, the microCT scans of the fossil fragments containing individual teeth were examined using the OsiriX software package's 3D MPR tool. Using this tool, the microCT image stack was traversed and rotated simultaneously in multiple views until the best-fit plane calculated from four landmarks (the tips of two dentine horns and the tips of two pulpal horns) was located (Fig. 1). This process was carried out to acquire both the mesial and distal planes of section. From this plane, the length of the root was measured using OsiriX's straight-line measurement tool.
Observation of Dental Wear in H5.
To observe the extent of the dental wear on the teeth of individual H5, we used a binocular lens (×10). The permanent right M1 exhibits a minimal wear facet no more than 1 mm wide, affecting the lingual and internal slope of the protocone. This wear corresponds with facets 6 and 9 of the numerical system of Maier and Schneck (52). The mesial border of this cusp is also affected by minimal wear, which is practically invisible to the naked eye. The lower M1 exhibits minimal wear facets on the internal slope of the hypoconid (facet 6), the internal slope of the hypoconulid (facet 12), the internal slope of the protoconid (facet 1), and almost imperceptible wear (also invisible to the naked eye) along the cusp borders. This degree of wear suggests contact between the lower M1 and the upper dm2 and upper M1 for only a few weeks.
Root Time Formation.
To estimate the time of formation of the root in modern humans and P. troglodytes using the data of Dean and Vesey's tables 2 and 3 (22), we used the mean extension rates for the first 1,000 μm, for the length between 1,000 and 2,000 μm, and the length between 2,000 and 8,000 μm (Table 3).
Acknowledgments
We acknowledge all the members of the Atapuerca Research Team involved in the recovery and study of the archaeological, geological, and paleontological record of the Atapuerca sites, especially R. Blasco, J. Guiu, and J. Vilalta, who excavated the Gran Dolina cave site. We especially thank A. J. Olejniczak for his valuable help during the microCT scanning, virtual reconstruction, and analysis of the data. We also acknowledge P. Fernández-Colón and E. Lacasa from the Conservation and Restoration department of Centro Nacional de Investigación sobre la Evolución Humana for their assistance during the refitting of the ATD6-112 fragments. We also thank Chris Dean and the two reviewers for their useful comments and suggestions that much improved this work. This research was sponsored by the Ministerio de Ciencia e Innovación (Grant DGI CGL2006-1352-C03), Junta de Castilla y León (Grupo de Excelencia Project and field season), and Fundación Atapuerca (field season, staff support, and grants to L.P. and A.G.-R.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Bogin B. Patterns of Human Growth. Cambridge, UK: Cambridge University Press; 1988. p. 455. [Google Scholar]
- 2.Bogin B, Smith BH. Evolution of the human life cycle. Am J Hum Biol. 1996;8:703–716. doi: 10.1002/(SICI)1520-6300(1996)8:6<703::AID-AJHB2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 3.Bogin B. Evolutionary hypotheses for human childhood. Am J Phys Anthropol. 1997;104:63–89. [Google Scholar]
- 4.Smith BH. Dental development as a measure of life history in primates. Evolution. 1989;43:683–688. doi: 10.1111/j.1558-5646.1989.tb04266.x. [DOI] [PubMed] [Google Scholar]
- 5.Smith RJ, Gannon PJ, Smith BH. Ontogeny of australopithecines and early Homo: Evidence from cranial capacity and dental eruption. J Hum Evol. 1995;29:155–168. [Google Scholar]
- 6.Bermúdez de Castro JM, et al. A new early Pleistocene hominin mandible from Atapuerca-TD6, Spain. J Hum Evol. 2008;55:729–735. doi: 10.1016/j.jhevol.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Bromage TG, Dean MC. Re-evaluation of the age at death of immature fossil hominids. Nature. 1985;317:525–527. doi: 10.1038/317525a0. [DOI] [PubMed] [Google Scholar]
- 8.Smith BH. Dental development in Australopithecus and early Homo. Nature. 1986;323:327–330. [Google Scholar]
- 9.Dean MC. The dental developmental status of six East African juvenile hominids. J Hum Evol. 1987;16:197–213. [Google Scholar]
- 10.Conroy GC, Vannier MW. Dental development of the Taung skull from computerized tomography. Nature. 1987;329:625–627. doi: 10.1038/329625a0. [DOI] [PubMed] [Google Scholar]
- 11.Beynon AD, Dean MC. Distinct dental development patterns in early fossil hominids. Nature. 1988;335:509–514. doi: 10.1038/335509a0. [DOI] [PubMed] [Google Scholar]
- 12.Christopher Dean M. Tooth microstructure tracks the pace of human life-history evolution. Proc Biol Sci. 2006;273:2799–2808. doi: 10.1098/rspb.2006.3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dean MC, Smith BH. In: The First Humans: Origin and Early Evolution of the Genus Homo. Grine FE, Fleagle JG, Leakey RE, editors. New York: Springer; 2009. pp. 101–120. [Google Scholar]
- 14.Dean MC, Lucas VS. Dental and skeletal growth in early fossil hominins. Ann Hum Biol. 2009;36:545–561. doi: 10.1080/03014460902956725. [DOI] [PubMed] [Google Scholar]
- 15.Aiello L, Dean MC. An Introduction to Human Evolutionary Anatomy. London: Academic Press; 1990. [Google Scholar]
- 16.Smith TM, et al. Earliest evidence of modern human life history in North African early Homo sapiens. Proc Natl Acad Sci USA. 2007;104:6128–6133. doi: 10.1073/pnas.0700747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dean C, et al. Growth processes in teeth distinguish modern humans from Homo erectus and earlier hominins. Nature. 2001;414:628–631. doi: 10.1038/414628a. [DOI] [PubMed] [Google Scholar]
- 18.Guatelli-Steinberg D, Reid DJ, Bishop TA, Larsen CS. Anterior tooth growth periods in Neandertals were comparable to those of modern humans. Proc Natl Acad Sci USA. 2005;102:14197–14202. doi: 10.1073/pnas.0503108102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lacruz RS, Ramirez Rozzi F, Bromage TG. Dental enamel hypoplasia, age at death, and weaning in the Taung child. S Afr J Sci. 2005;101:567–569. [Google Scholar]
- 20.Macchiarelli R, et al. How Neanderthal molar teeth grew. Nature. 2006;444:748–751. doi: 10.1038/nature05314. [DOI] [PubMed] [Google Scholar]
- 21.Reid DJ, Dean MC. Variation in modern human enamel formation times. J Hum Evol. 2006;50:329–346. doi: 10.1016/j.jhevol.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Dean MC, Vesey P. Preliminary observations on increasing root length during the eruptive phase of tooth development in modern humans and great apes. J Hum Evol. 2008;54:258–271. doi: 10.1016/j.jhevol.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 23.Kuykendall KL. Dental development in chimpanzees (Pan troglodytes): The timing of tooth calcification stages. Am J Phys Anthropol. 1996;99:135–157. doi: 10.1002/(SICI)1096-8644(199601)99:1<135::AID-AJPA8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 24.Reid DJ, Schwartz GT, Dean C, Chandrasekera MS. A histological reconstruction of dental development in the common chimpanzee, Pan troglodytes. J Hum Evol. 1998;35:427–448. doi: 10.1006/jhev.1998.0248. [DOI] [PubMed] [Google Scholar]
- 25.Robson SL, Wood B. Hominin life history: Reconstruction and evolution. J Anat. 2008;212:394–425. doi: 10.1111/j.1469-7580.2008.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conroy GC, Vannier MW. Dental development in South African Australopithecines. Part I: Problems of pattern and chronology. Am J Phys Anthropol. 1991;86:121–136. [Google Scholar]
- 27.Smith BH. Patterns of dental development in Homo, Australopithecus, Pan, and Gorilla. Am J Phys Anthropol. 1994;94:307–325. doi: 10.1002/ajpa.1330940303. [DOI] [PubMed] [Google Scholar]
- 28.Falguères C, et al. Earliest humans in Europe: The age of TD6 Gran Dolina, Atapuerca fossils, Spain. J Hum Evol. 1999;37:343–352. doi: 10.1006/jhev.1999.0326. [DOI] [PubMed] [Google Scholar]
- 29.Berger GW, et al. Luminescence chronology of cave sediments at the Atapuerca paleoanthropological site, Spain. J Hum Evol. 2008;55:300–311. doi: 10.1016/j.jhevol.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Bermúdez de Castro JM, et al. A hominid from the lower Pleistocene of Atapuerca, Spain: Possible ancestor to Neandertals and modern humans. Science. 1997;276:1392–1395. doi: 10.1126/science.276.5317.1392. [DOI] [PubMed] [Google Scholar]
- 31.Bermúdez de Castro JM, et al. A modern human pattern of dental development in lower pleistocene hominids from Atapuerca-TD6 (Spain) Proc Natl Acad Sci USA. 1999;96:4210–4213. doi: 10.1073/pnas.96.7.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith TM, Reid DJ, Dean MC, Olejniczak AJ, Martin LB. Molar development in common chimpanzees (Pan troglodytes) J Hum Evol. 2007;52:201–216. doi: 10.1016/j.jhevol.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Reid DJ, Guatelli-Steinberg D, Walton P. Variation in modern human premolar enamel formation times: Implications for Neandertals. J Hum Evol. 2008;54:225–235. doi: 10.1016/j.jhevol.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 34.Moggi-Cecchi J, Tobias PV, Beynon AD. The mixed dentition and associated skull fragments of a juvenile fossil hominid from Sterkfontein, South Africa. Am J Phys Anthropol. 1998;106:425–465. doi: 10.1002/(SICI)1096-8644(199808)106:4<425::AID-AJPA2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 35.Lacruz RS, Rozzi FR, Bromage TG. Variation in enamel development of South African fossil hominids. J Hum Evol. 2006;51:580–590. doi: 10.1016/j.jhevol.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Alemseged Z, et al. A juvenile early hominin skeleton from Dikika, Ethiopia. Nature. 2006;443:296–301. doi: 10.1038/nature05047. [DOI] [PubMed] [Google Scholar]
- 37.Dean MC. A radiographic and histological study of modern human lower first permanent molar root growth during the supraosseous eruptive phase. J Hum Evol. 2007;53:635–646. doi: 10.1016/j.jhevol.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Kelley J, Dean C, Ross S. Root growth during molar eruption in extant great apes. Front Oral Biol. 2009;13:128–133. doi: 10.1159/000242404. [DOI] [PubMed] [Google Scholar]
- 39.Gleiser I, Hunt EE., Jr. The permanent mandibular first molar: Its calcification, eruption and decay. Am J Phys Anthropol. 1955;13:253–283. doi: 10.1002/ajpa.1330130206. [DOI] [PubMed] [Google Scholar]
- 40.Bermúdez de Castro JM. The Atapuerca dental remains. New evidence (1987–1991 excavations) and interpretations. J Hum Evol. 1993;24:339–371. [Google Scholar]
- 41.Bermúdez de Castro JM, Rosas A, Nicolás ME. Dental remains from Atapuerca-TD6 (Gran Dolina site, Burgos, Spain) J Hum Evol. 1999;37:523–566. doi: 10.1006/jhev.1999.0323. [DOI] [PubMed] [Google Scholar]
- 42.Martinón-Torres M, et al. Dental evidence on the hominin dispersals during the Pleistocene. Proc Natl Acad Sci USA. 2007;104:13279–13282. doi: 10.1073/pnas.0706152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mann A, Lampl M, Monge J. Patterns of ontogeny in human evolution: Evidence from dental development. Am J Phys Anthropol. 1990;33:111–150. [Google Scholar]
- 44.Dean MC, Reid DJ. Perikymata spacing and distribution on hominid anterior teeth. Am J Phys Anthropol. 2001;116:209–215. doi: 10.1002/ajpa.1116. [DOI] [PubMed] [Google Scholar]
- 45.Grine FE, Franzen JL. Fossil hominid teeth form the Sangiran Dome (Java, Indonesia) Courier Forschungistitut Senkenberg. 1994;171:75–103. [Google Scholar]
- 46.Brown FH, McDougall I. In: The Nariokotome Homo erectus Skeleton. Walker A, Leakey RE, editors. Cambridge, MA: Harvard University Press; 1993. pp. 9–20. [Google Scholar]
- 47.Smith BH, Tompkins RL. Toward a life history of the Homindae. Annu Rev Anthropol. 1995;24:257–279. [Google Scholar]
- 48.Smith BH. Dental development and the evolution of life history in Hominidae. Am J Phys Anthropol. 1991;86:157–174. [Google Scholar]
- 49.Begun D, Walker A. In: The Nariokotome Homo erectus Skeleton. Walker A, Leakey RE, editors. Cambridge, MA: Harvard University Press; 1993. pp. 326–357. [Google Scholar]
- 50.Carbonell E, et al. Lower Pleistocene hominids and artifacts from Atapuerca-TD6 (Spain) Science. 1995;269:826–830. doi: 10.1126/science.7638598. [DOI] [PubMed] [Google Scholar]
- 51.Locke JL, Bogin B. Language and life history: A new perspective on the development and evolution of human language. Behav Brain Sci. 2006;29:259–280, discussion 280–325. doi: 10.1017/s0140525x0600906x. [DOI] [PubMed] [Google Scholar]
- 52.Maier W, Schneck G. Construction of morphologic examinations of the dentition of hominoid primates. Z Morphol Anthropol. 1981;72:127–169. [PubMed] [Google Scholar]
- 53.Liversidge HM, Molleson T. Variation in crown and root formation and eruption of human deciduous teeth. Am J Phys Anthropol. 2004;123:172–180. doi: 10.1002/ajpa.10318. [DOI] [PubMed] [Google Scholar]