Abstract
Cytolytic effectors polarize toward target cells for effective killing and IFN-γ secretion. The spatiotemporal features of this polarization and their importance for cytolysis have not been resolved. In cytotoxic T cells and natural killer (NK) cells, transient polarization was consistently associated with effective killing. Polarization was regulated by Cdc42, a small Rho family GTPase universally critical for cytoskeletal dynamics. Transient accumulation of active Cdc42 at the cytolytic effector/target cell interface and focus of such accumulation on the interface center were closely related to cytolysis. Surprisingly, however, the intensity of Cdc42 activation was not. We interfered with Cdc42 activation in NK cells such that sustained polarization in long lasting nonkilling cell couples was selectively blocked. Thus the proportion of the NK cell population displaying transient polarization was increased. As a consequence, cytolytic responder frequency and IFN-γ production were enhanced upon such interference with Cdc42 activation. These data support the notion that transience in polarization is critical for cytolytic effector function, likely by preventing cytolytic effectors from becoming trapped in nonproductive target cell interactions.
Keywords: cytotoxic T cells, lymphocyte polarization, natural killer cells
Cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells are the central cytolytic effectors of the immune system. Each can also secrete IFN-γ to modulate adaptive immune function. To acquire effector capability, cytolytic effectors need to be primed. CTL priming occurs as the consequence of naive CD8 T cell interactions with antigen-presenting dendritic cells. NK cell priming requires cytokines generated predominantly by dendritic cells. However, the nature of the cytokines involved is less certain. IL-12 and IL-18 enable NK cells to secrete IFN-γ (1, 2) and are synergistic (3). IL-15 can promote NK cell expansion (4, 5). IL-15 needs transpresentation by the IL-15 receptor α chain (6, 7), which is induced on dendritic cells by engagement of Toll-like receptors (7). High concentrations of IL-2 effectively prime NK cells in vitro, as often used in clinical settings (8, 9). However, in vivo, IL-2 is dispensable for NK cell priming (5). To understand the role of the polarization of cytolytic effector in their function, the effect of priming conditions on NK cell polarization needs to be taken into account.
Target cell contact triggers the complex polarization of primed cytolytic effectors, recruitment of cytolytic granules to the interface (10, 11), cytoskeletal reorientation (12, 13), and receptor clustering (14, 15). Cytolytic effector polarization is likely functionally important, as key regulators of cytoskeletal dynamics, Vav and WASP, play critical roles (16–19). However, WASP and Vav are complex molecules with functions that may or may not require cytoskeletal regulation (20, 21). The Rho family GTPases Rac and Cdc42 are the central regulators of cytoskeletal dynamics in many cell types (22). Although it has been established that Rac is required for cell coupling and polarization of cytolytic granules (16), the role Cdc42 has not been addressed. Despite the established importance of cytolytic effector polarization, the spatiotemporal features of this polarization and their importance for cytolysis have been discussed (23) but not resolved.
Here we show that the transience of polarization of cytolytic effectors, i.e., rapid initial recruitment of actin to the cytolytic effector/target cell interface followed by actin release from the interface, was coupled to effective cytolytic effector function. Such transient actin interface accumulation was dependent on Cdc42. Initial rapid polarization permits effective signaling and secretion of cytolytic granules. Later reductions in Cdc42 activity and actin accumulation at the cytolytic effector/target cell interface likely allow dissociation of nonproductive cell couples. To test this notion, we interfered with Cdc42 activation (24, 25) such that initial polarization was preserved while sustained actin accumulation was blocked. This enhanced the frequency of cytolytic effectors involved in killing and secretion of IFN-γ. Thus, specific spatiotemporal features of cytolytic effector polarization, prominently its transience, were critical for cytolytic effector function.
Results
In Vitro Models of NK Cell Polarization.
Here we characterize features of cytolytic effector polarization and its regulation. Addressing different NK cell priming conditions is a practical necessity in establishing appropriate in vitro models of murine NK cell polarization. We therefore consistently compared multiple NK cell priming conditions; splenocytes were cultured in IL-12 plus IL-18 (“IL-12/18 NK cells”) or with LPS-activated dendritic cells transpresenting IL-15 (“IL-15 NK cells”). In addition, high doses of IL-2 with concomitant depletion of T cells are commonly used (“IL-2 NK cells”). To allow CTL activation by physiological T cell receptor (TCR) engagement, T cells from TCR transgenic P14 mice were used. We used susceptible targets: YAC-1 lymphoma cells for NK cells and EL4 thymoma cells for CTLs. In all of the different effector/target cell combinations killing is mediated by cytolytic granule release, independent of FasL (12, 26). Expression of the critical activating receptor NKG2D and of perforin was comparable (Fig. S1). Importantly, even under the strong activation conditions used, the fate of individual NK cell/target cell couples varied (12). Some cell couples rapidly (<5 min) proceeded to target cell lysis, some more slowly so, and approximately 50% of the cell couples persisted (>30 min) without over target cell damage. At the single-cell level, we thus can separately analyze polarization in lytic and nonkilling interactions to discover particular polarization features linked to cytolysis. Differences in the morphological changes in the target cells upon killing by different cytolytic effectors (SI Text) allow a separate assessment of lytic and nonkilling interactions only for IL-12/18 and IL-15 NK cells.
Transient Polarization of Cytolytic Effectors Is Coupled to Effective Killing.
Cytolytic effectors interacting with target cells polarize toward the cellular interface. To determine whether intensity of polarization, its duration, or localization within the interface are critical for effective killing, these elements of cytolytic effector polarization were quantified. First, the intensity of the interface accumulation of actin or signaling sensors at the population level is governed by two factors, the percentage of cytolytic effectors that show interface accumulation beyond a threshold and the amount of cellular actin or signaling sensor that is moved to the effector/target cell interface in the portion of the population that does shown accumulation. Therefore, to measure interface accumulation at the population level, the percentage of cell couples with interface accumulation was multiplied by the average fraction of actin or the signaling sensor that is translocated to the interface (25). This number thus gives the population-averaged percentage of cellular actin or signaling sensor that is moved to the effector/target cell interface and is used throughout the manuscript. Separate percentages of cell couples with interface accumulation of actin or signaling sensors is also provided. Second, we measured the transience of polarization by the decrease of interface accumulation following its initial peak at cell coupling. Third, spatial patterns of interface accumulation were measured (27).
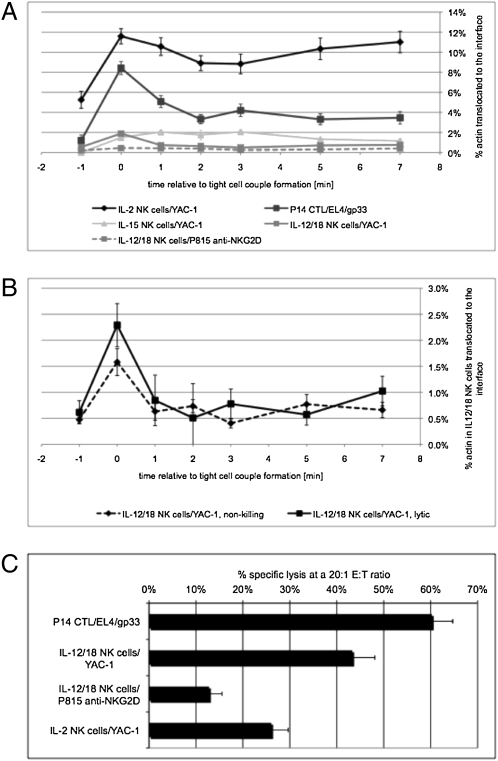
C57BL/6 IL-12/18 NK cells can effectively kill YAC-1 target cells (12). Actin interface accumulation in these cell couples was moderate and transient. Only 1.9 ± 0.2% of actin was moved to the interface at the time of tight cell couple formation (Fig. 1A). Within 1 min this percentage decreased significantly (P ≤ 0.01) to less than half (Fig. 1A and Fig. S2 A and B). Interestingly, interface actin accumulation was moderately more transient in lytic versus nonkilling interactions. In lytic interactions the average amount of actin moved to the interface decreased from 2.3 ± 0.4% at the time of tight cell coupling to 0.5% to 1.0% thereafter. This reduction was less in nonkilling interactions (1.6 ± 0.3% to 0.4–0.8%; Fig. 1B). Extending such analysis to NK cell priming with another innate cytokine, IL-15, we found similar phenotypes (Fig. 1A, SI Text, and Fig. S2 A–D) with a dramatic difference in transience between lytic and nonkilling interactions (Fig. S2C). Taken together, transient actin accumulation in NK cells primed with innate cytokines was closely related to efficient target cell lysis.
Fig. 1.
Transient accumulation of actin at the effector/target cell interface is related to effective target cell lysis (A). The percentage of actin-GFP translocated to the cytolytic effector/target cell interface is given with SEs as a function of time relative to tight cell couple formation for the following interactions: P14 T cells/EL4 target cells/10 μM gp33 agonist peptide, IL-2 or IL-15 NK cells/YAC-1 target cells, or IL-12/18 NK cells with YAC-1 or P815/α–NKG2D target cells, as indicated. To account for low numbers of cell couples with interface actin accumulation in IL-15 and IL-12/18 NK cells, time points 3 through 7 (also point 2 for P815/α-NKG2D targets) were averages of time points 2, 3, 4 to 6, 7, and 8, respectively. Differences relative to values at time 0 are significant (P ≤ 0.01) at all time points ≥1 min for P14 CTLs and IL-12/18 NK cells. On average 28 cell couples (range, 21–37) were analyzed per condition. For normalized data and percentage of cell couples with accumulation displayed separately, see Fig. S2 A and B. (B) For IL-12/18 NK cell/YAC-1 interactions, the data from A are displayed separately for nonkilling (broken line) and lytic (solid line) interactions. Differences relative to values at time 0 are significant (P ≤ 0.05) at all time points at or grearer than 1 min for lytic, but not nonkilling interactions. For comparable IL-15 NK cell data, see Fig. S2C. (C) The percent specific lysis with SEs at a 20:1 effector-to-target cell ratio from five to nine independent repetitions of chromium release killing assays per condition is given for cytolytic effector/target cell interactions defined in A as indicated. All pair-wise differences are significant at P < 0.05.
C57BL/6 IL-2 NK cells can also kill YAC-1 target cells, albeit less efficiently. Surprisingly, interface actin accumulation was intense and sustained (Fig. 1A and Fig. S2E). At all time points after cell coupling, 8.5% to 11.5% of actin was moved to the interface, significantly higher (P < 0.005) than interface actin accumulation in all other cytolytic effectors (Fig. 1A). Interface actin accumulation was virtually identical at cell coupling and 7 min thereafter (Fig. 1A), establishing a lack of transience. The dynamics of MTOC localization and the delivery of the lytic hit also differed between IL-12/18 and IL-2 NK cells (SI Text, Fig. S2F, Movie S1, Movie S2, Movie S3, Movie S4, Movie S5, Movie S6, Movie S7, and Movie S8). Actin interface accumulation in the interaction of P14 CTLs with EL4 target cells in the presence of 10 μM gp33 peptide (12), a strong stimulus, was intense and transient. At the time of tight cell couple formation 8.4 ± 0.6% of the cellular actin was moved to the interface. However, within 2 min after tight cell coupling, the intensity of interface actin accumulation decreased significantly (P < 0.001) to half (Fig. 1A and Fig. S2A).
In a comparison of the different cytolytic effector populations, transience of polarization was also strongly related to target cell lysis. Using the same effector/target combinations as in the assessment of interface actin accumulation, P14 CTLs with strong and transient polarization yielded 60 ± 4.5% specific lysis (Fig. 1C). IL-12/18 NK cells with moderate and transient interface actin accumulation, similar to ex vivo human NK cells (28, 29), yielded moderately less specific lysis (43.5 ± 5%, P < 0.05; Fig. 1C). IL-2 NK cells, combining strong polarization with a lack of transience, yielded the least specific lysis (26 ± 3%, P < 0.02; Fig. 1C). Thus, both a comparison of lytic versus nonkilling IL-12/18 and IL-15 NK cell target cell couples and a comparison of different cytolytic effector populations established that, in response to susceptible targets, transience of polarization is closely related to cytolysis. Sustained effector polarization might trap individual cytolytic effectors in long-lasting, nonproductive cell couples, thus preventing lysis of the target and sequential interactions of the cytolytic effector with other targets. As a consequence, target cell killing at the population level is reduced.
Transient and Central Accumulation of Active Cdc42 at the Interface Between Cytolytic Effectors and Their Targets Is Related to Effective Killing.
Cdc42 is a well expressed (SI Text and Fig. S3A), critical regulator of cytoskeletal dynamics and cellular polarization. To address its role in cytolytic effector polarization, we investigated the intensity, transience, and patterns of active Cdc42 at the cytolytic effector/target cell interface using live cell imaging of a retrovirally expressed biosensor for active Cdc42 (25, 27). As the function of the closely related Rho GTPase family member Rac has previously been studied (16), we will address Rac only (SI Text and Figs. S3 and S4) to assess specificity of roles of Cdc42.
Accumulation of active Cdc42 at the effector/target cell interface in the interaction of B6 IL-12/18 NK cells with YAC-1 target cells (Fig. 2A) was moderate and transient with a mix of spatial patterns. Similar to the actin data (Table S1), only 1.6 ± 0.2% of the Cdc42 sensor was moved to the effector/target cell interface upon cell coupling (Fig. 3A and Fig. S3 B and C). Interface accumulation of active Cdc42 was strongly transient in lytic cell couples (Fig. 3B). Interface sensor accumulation decreased from 1.1 ± 0.4% of sensor moved to the interface at cell coupling to no accumulation at 5 min thereafter. In contrast, in nonkilling interactions, such accumulation was barely transient, remaining greater than 1.25%. These data extend the notion that transience in polarization is coupled to effective target cell lysis. Central, diffuse, and asymmetric patterns occurred in fairly even proportions (Fig. 3C).
Fig. 2.
Accumulation of active Cdc42 at the effector/target cell interface. Representative interactions between cytolytic effectors (NK or CTL) transduced with a sensor for active Cdc42 and their target cells (T) are shown as follows: (A) IL-12/18 NK cell with YAC-1 target cell, nonkilling interaction (Movie S9 and Movie S6 with an alternate cell couple, Movie S10 at higher resolution); (B) IL-2 NK cell with YAC-1 target cell, two sequential lytic interactions [Movie S3 for the second cell couple (only cell couple formation is shown), Movie S11 at higher resolution]; and (C) P14 CTL with EL4 target cell/10 μM gp33 agonist peptide (Movie S12). Differential interference contrast images are shown in the top rows, with top-down, maximum projections of 3D Cdc42 sensor fluorescence data (in a rainbow false-color scale increasing from blue to red) in the bottom rows. Time relative to tight cell couple formation (in min) is indicated at the bottom.
Fig. 3.
Transient and central accumulation of active Cdc42 at the effector/target cell interface is related to effective target lysis (A). The percentage of sensor for active Cdc42 translocated to the cytolytic effector/target cell interface is given as a function of time relative to tight cell coupling with SEs for the following interactions: P14 T cells/EL4 target cells/10 μM gp33 agonist peptide, IL-2 NK cells/YAC-1 target cells, or IL-12/18 NK cells with YAC-1 or P815/α-NKG2D target cells, as indicated. To account for low numbers of cell couples with interface Cdc42 accumulation in IL-12/18 NK/ P815/α-NKG2D targets cell interactions, time points 2 through 7 were averages of time points 1, 2, 3 to 6, 7, and 8, respectively. On average 42 cell couples (range, 31–64) were analyzed per condition. For normalized data and percentage of cell couples with accumulation displayed separately, see Fig. S3 B and C. (B) For IL-12/18 NK cell/YAC-1 interactions, the data from A are displayed separately for nonkilling (broken line) and lytic (solid line) interactions. Comparable data for IL-12/18 NK cell/P815/α-NKG2D interactions are given in Fig. S3D. (C) Of all time points with interface accumulation from all cell couples analyzed for each condition, percent time points with interface accumulation of active Cdc42 in the indicated pattern is given with SEs for interactions defined in A. Differences in percentage of central accumulation of active Cdc42 between CTLs and both NK cell types are all significant at P < 0.001. On average 220 time points (range, 71–687) from an average of 42 cell couples (range, 31–64) were analyzed. (D) Average percentage of specific lysis from chromium release assays (Fig. 1C) is plotted against measures for the intensity of interface accumulation of active Cdc42 (percentage of sensor for active Cdc42 translocated to the interface at time 0; Fig. 3A), its transience (percentage of sensor for active Cdc42 translocated to the interface at time point 0 min relative to time 5; Fig. S3B), and its centrality (percentage of time points with central accumulation; Fig. 3C) for the following interactions: P14 T cells/EL4 target cells/10 μM gp33 agonist peptide (data points on the right), IL-2 (data points on left), or IL-12/18 NK cells (data points in middle) with YAC-1 target cells. Measures of Cdc42 accumulation are normalized to the most intense value. Linear regression analysis was performed and regression curves with correlation coefficients are given.
Contrasting the IL-12/18 NK cell data, accumulation of active Cdc42 at the effector/target cell interface in the interaction of B6 IL-2 NK cells with YAC-1 target cells (Fig. 2B) was intense and sustained with a mix of spatial patterns. Similar to the actin results (Table S1), the accumulation of active Cdc42 was higher in IL-2 NK cells than in any other cytolytic effector (P ≤ 0.01 at all time points ≥0; Fig. 3A), with 3.5% to 4.5% of the Cdc42 sensor being moved to the interface. Interface accumulation of the sensor of active Cdc42 did not decline over time (Fig. 3A and Fig. S3B). Central, diffuse, and asymmetric patterns occurred in fairly even proportions (Fig. 3C). Accumulation of active Cdc42 at the effector/target cell interface in the interaction of P14 CTLs with EL4 target cells in the presence of 10 μM gp33 peptide (Fig. 2C) was of low intensity, yet strongly transient and central. Only 0.6 ± 0.05% of the Cdc42 sensor was moved to the interface at the time of tight cell couple formation. Within 7 min, enhanced interface Cdc42 activity could no longer be detected (Fig. 3A and Fig. S3 B and C). Interface accumulation of active Cdc42 occurred preferentially at the center of the interface: 57 ± 4% of time points with accumulation showed a central pattern (Fig. 3C). The role of the weak and central nature of the accumulation of active Cdc42 at the CTL/target cell interface in the regulation of CTL interface actin dynamics is discussed in SI Text. Interestingly, limited and highly transient interface accumulation of active Cdc42 in CTLs was in stark contrast to the extensive Cdc42 accumulation of helper T cells (SI Text and Fig. S3E), consistent with a selective need of transient target cell interactions for sequential killing in cytolytic effector function.
We next examined the relation between spatiotemporal features of Cdc42 interface accumulation and target cell lysis (Fig. 1C) at the population level. In comparing killing to intensity, transience, and centrality of interface activity of active Cdc42 (Fig. 3D), only transience and centrality showed a positive relation. This strongly suggests that, in response to susceptible targets, transience and centrality of Cdc42 activity are required for effective killing. These data mirror the actin data (Table S1), underscoring the importance of transience in polarization and consistent with a critical role of Cdc42 in the regulation of cytolytic effector polarization, as detailed later. The lack of a positive relation between the intensity of Cdc42 interface activity and killing is somewhat counterintuitive, as one would expect that more intense polarization yielded higher killing efficacy. We confirmed that effective polarization was indeed related to cytolysis over large differences in the efficiency of polarization by studying the response of IL-12/18 NK cells to less susceptible target cells, P815 target cells incubated with α-NKG2D (SI Text).
Cdc42 Is a Critical Regulator of Interface Actin Accumulation Preferentially in Nonkilling Interactions.
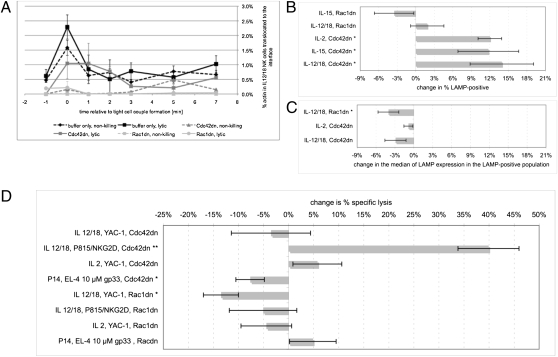
To address roles of Cdc42 in cytolytic effector function, we interfered with the activation of Cdc42, and Rac as a control, with well established dominant-negative mutants (Cdc42dn, Rac1dn), as applied short-term and quantitatively as protein transduction reagents at a concentration of 100 nM (24, 25). As expected (22), both Cdc42dn and Rac1dn blocked interface actin accumulation, by more than 70% in NK cells and a bit less in CTLs (Fig. S5 A–C). Interestingly, however, in lytic IL-12/18 NK cell/YAC-1 target cell interactions, 100 nM Cdc42 had no significant effect (Fig. 4A). The amount of actin moved to the interface at all times was only modestly reduced from a range of 0.5% to 2.3% to a range of 0.4% to 1.0% upon treatment with 100 nM Cdc42dn, not reaching significance at any time point. In contrast in nonkilling interactions, actin moved to the interface dropped from a range of 0.4% to 1.6% to a range of 0% to 0.4%. The selective lack of an effect of Cdc42dn in lytic interactions is consistent with the prior observation that Cdc42 activity ceases rapidly in such interactions, thus rendering interference with Cdc42 activation mute. Cdc42dn thus exerted its most prominent effect on nonkilling interactions, suggesting a dominant role of Cdc42 in the stabilization of such interactions. Rac data that establish specificity for Cdc42dn are given in the SI Text (Fig. 4A and Fig. S5).
Fig. 4.
Increasing transience in NK cell polarization with Cdc42dn yields enhanced target cell lysis (A). The percentage of actin-GFP translocated to the IL-12/18 NK cell/YAC-1 target cell interface is given with SEs upon treatment with 100 nM Cdc42dn or Rac1dn as a function of time relative to tight cell couple formation separately for nonkilling (broken line) and lytic (solid line) interactions, as indicated. Differences relative to buffer only are significant (P ≤ 0.05) at all time points at or greater than 0 min for treatment with Rac1dn and Cdc42dn in nonkilling interactions, but not for treatment with Cdc42dn in lytic interactions. On average 30 cell couples (range, 18–37) were analyzed per treatment condition. For data on all cytolytic effectors without distinction between lytic and nonkilling cell couples, see Fig. S5 A–C. (B–D) Changes in cytolysis upon treatment with 100 and 350 nM Cdc42dn or Rac1dn in the following interactions as indicated was analyzed: P14 T cells/EL4 target cells/10 μM gp33 agonist peptide, IL-2, IL-15, or IL-12/18 NK cells/YAC-1 (default) or P815/α-NKG2D target cells. (B and C) Percentage change with SEs in percent cytolytic effectors expressing CD107a (B) and in the median CD107a expression within this population (C) are given. Single and double asterisks denote significance (P < 0.05/P < 0.005) between Cdc42dn/Rac1dn–treated and buffer only samples. Three to six independent replicates of each assay were performed. Raw analysis and representative data are given as Table S2 and Fig. S6 A–C. (D) Percentage change in bulk killing as assessed by chromium release at a 20:1 effector-to-target cell ratio is given similar to B. Two to four independent replicates of each assay were performed. Raw analysis and representative data are given in Table S2 and Fig. S6 D–F.
Increasing Transience of NK Cell Polarization by Interference with Cdc42 Activation Enhances the Percentage of Responders in Target Cell Lysis and IFN-γ Secretion.
If transience in cytolytic effector polarization was required for efficacious function, then destabilization of nonkilling cell couples by impairing cytolytic effector polarization, as accomplished by Cdc42dn, should enhance cytolytic effector function. We therefore determined the effect of Cdc42dn on target cell killing and IFN-γ secretion for IL-12/18 NK cells, as summarized in relation to polarization features in Table S1.
As an indirect measure of killing activity at the single-cell level, we studied cell surface expression of CD107a (LAMP), a lysosomal protein that remains temporarily on the NK cell surface after lytic granule release (Fig. 4 B and C, Fig. S6 A–C, and Table S2). Upon treatment with Cdc42dn, the percentage of IL-12/18 NK cells with enhanced CD107a expression was increased by 14 ± 5% (P < 0.05; Fig. 4B and Table S2). In IL-15 and IL-2 NK cells, the frequency of NK cells with enhanced CD107a expression in response to YAC-1 contact was also increased by 12 ± 5% and 12 ± 2% (P < 0.05; Fig. 4B and Table S2). Treatment with Cdc42dn thus modestly enhanced the number of NK cells that degranulate. In addition, upon treatment with Cdc42dn, the percentage of IL-15 NK cells producing IFN-γ in response to YAC-1 target cells (Fig. S7 and Table S2) was significantly increased by 40 ± 16% (P < 0.05; Fig. S7A and Table S2). NK cell priming with IL-18 induces substantial IFN-γ secretion per se, preventing a determination of IFN-γ secretion in response to target cell contact. In the bulk lysis of YAC-1 target cells by IL-12/18 NK cells (Fig. 4D, Fig. S6 D–F, and Table S2), treatment of the IL-12/18 NK cells with Cdc42dn did not have a substantial effect (Fig. 4D). Given the high frequency of cell coupling under these conditions (Fig. S5D), the modest enhancement of the percentage of NK cells that degranulate (Fig. 4B) likely did not register as substantial change in bulk killing. Interestingly, however, in the lysis of P815 cells incubated with anti-NKG2D, a weaker stimulus as characterized in the supplementary text, killing by IL-12/18 NK cells was significantly enhanced (P = 0.001) upon treatment with Cdc42dn by 40 ± 6% (Fig. 4D) from 7.5% to 10% specific lysis at an effector-to-target ratio of 20:1 across multiple assays to 10% to 13.5% (Table S2). Thus, under limiting conditions, freeing IL-12/18 NK cells from nonproductive cell couples by enhancing their transience could affect bulk lysis.
Together these data establish an important role of transient polarization in effective NK cell function: selective destabilization of interface actin accumulation in nonproductive NK cell/target cell couples by Cdc42dn could consistently enhance responder frequencies in killing and cytokine secretion. Cdc42dn had no comparable effects on CTL function, neither with a strong stimulus (10 μM gp33 agonist peptide; Fig. 4D) nor with a limiting one (1 nM gp33; Table S2). In CTLs, cellular polarization was inherently more transient, thus alleviating a need for experimental destabilization of nonproductive cell couples. Establishing specificity, effects of Rac1dn differed (SI Text).
Discussion
Cytolytic effectors must polarize toward their target cells for effective function. Here we show that, in the physiological interaction of primary cytolytic effectors with susceptible target cells, spatiotemporal features of polarization, in particular transience, were critical. In the regulation of cytolytic effector polarization, Cdc42 promoted sustained actin dynamics, thus diminishing transience. This model refines our previous characterization of different mechanisms of polarization in adaptive versus innate cytolysis (12). CTLs, having undergone extensive control of their specificity in their initial priming with dendritic and helper T cells, can afford to polarize effectively and, as shown here, highly transiently for effective killing in sequential target cell interactions. In contrast, NK cells, because of more limited checks on their specificity during innate cytokine priming, have to polarize more tentatively. NK cell/target cell couples often proceed to target cell lysis more slowly. This delay should be beneficial in allowing time for the necessary, more sensitive distinction between self and nonself during the actual target cell contact. Yet, as shown here, the decreased transience comes at the price of reduced killing efficacy. NK cells are more easily trapped in long-lasting (>30 min) interactions, decreasing availability for sequential target cell interactions. Stable interface actin accumulation could impair killing by preventing repolarization toward secondary targets, as dynein motors associated with the peripheral actin ring are used to position the microtubule organizing center (MTOC) at the cytolytic effector/target cell interface (30). In addition, long lasting NK cell/target cell couples did not lead to target cell lysis, thus further diminishing killing at the population level. Here sustained strong and stable interface actin accumulation could impede the myosin-dependent transport of cytolytic granules through the actin cortex (31) or translocation of granules from the periphery of the interface to its center (32). As an important future challenge, signaling events that down-regulate the initially strong polarization in efficient cytolysis need to be identified. They could involve tyrosine phosphatases to terminate proximal signaling, GTPase-activating proteins to limit Rho GTPase activity, or actin severing proteins to depolymerize cortical actin.
Materials and Methods
Cells.
CTLs were generated from P14 TCR transgenic mice by priming with APC plus peptide (12). IL-12/18 NK cells were generated by culturing murine B6 splenocytes with 1 ng/mL IL-12 and 100 ng/mL IL-18 (12). For the generation of IL-15 NK cells, first, mitomycin C–treated splenocytes were treated with 10 ng/mL LPS to induce expression of the IL-15 receptor α-chain. One day later, 3 × 106 B6 splenocytes were added in the presence of 20 ng/mL IL-15 (R & D Systems). For the generation of IL-2 NK cells, T cell–depleted B6 splenocytes were cultured with 500 U/mL IL-2. Before use, IL-2 NK cells were sorted again for absence of CD3. Moloney murine leukemia virus–based retroviral transduction was performed 1 d after culture initiation (12). All functional assays were executed on d 5 or 6 of the cultures. As target cells, EL-4 thymoma cells incubated with gp33 agonist peptide (for CTLs), YAC-1 lymphomas, and P815 cells incubated with 5 μg/mL anti-NKG2D (for NK cells) were used. All experiments involving vertebrate animals were approved by the University of Texas Southwestern Medical Center Institutional Animal Care and Use Committee.
Protein Transduction.
Protein transduction versions of Cdc42dn and Rac1dn, their purification, and their functionality in primary lymphocytes are established (24, 25). Cytolytic effectors were incubated with the protein transduction reagents for 30 min at 37 °C at the indicated concentrations before the functional assays (25). To account for the slow loss [τ of approximately 4 h (24)] of protein transduction reagents in the long-term IFN-γ assays, the initial incubation concentration was doubled (e.g., 200 nM reagents for the 100 nM condition). As in the functional assays significant differences between 100 and 350 nM data were not found, pooled data for 100 and 350 nM Cdc42dn are presented.
Imaging.
Imaging methods are extensively published (12, 24, 25, 27) and repeated in detail in the SI Text.
Cytolysis, IFN-γ Production, NKG2D, and Perforin Expression.
Chromium-release bulk killing assays were executed as established (12). As numbers of IL-15 NK cells were low, chromium-release assays could not be executed. CD107a expression on NK cells was determined in NK cell–target cell couples by FACS. Target cells were identified by labeling with carboxyfluorescein succinimidyl ester. The staining was as in the work of Andzelm et al. (11). For the reliable detection of changes in CD107a cell surface expression, strong NK cell stimuli were required, as provided by YAC-1 target cells. Of note, CD107a expression is an indirect measure of killing activity that is not necessarily correlated with target cell lysis. IFN-γ production in cytolytic effector–target cell couples was determined by intracellular cytokine staining (33). For FACS staining for NKG2D, a phycoerythrin-conjugated antibody from eBioscience (CX5) was used. For Western blotting for perforin, 250,000 live cells were sorted and lysates were blotted with the antiperforin antibody from Cell Signaling Technologies.
Supplementary Material
Acknowledgments
We thank Tracy Diaz, Thanh Tran, and Kara Chan for experimental contributions. This work was supported by the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.L.D. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0913422107/-/DCSupplemental.
References
- 1.Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev. 2008;223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- 2.Watford WT, Moriguchi M, Morinobu A, O'Shea JJ. The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 2003;14:361–368. doi: 10.1016/s1359-6101(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 3.Agaugué S, Marcenaro E, Ferranti B, Moretta L, Moretta A. Human natural killer cells exposed to IL-2, IL-12, IL-18, or IL-4 differently modulate priming of naive T cells by monocyte-derived dendritic cells. Blood. 2008;112:1776–1783. doi: 10.1182/blood-2008-02-135871. [DOI] [PubMed] [Google Scholar]
- 4.Ferlazzo G, et al. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci USA. 2004;101:16606–16611. doi: 10.1073/pnas.0407522101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vosshenrich CA, et al. Roles for common cytokine receptor gamma-chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. J Immunol. 2005;174:1213–1221. doi: 10.4049/jimmunol.174.3.1213. [DOI] [PubMed] [Google Scholar]
- 6.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 7.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan CJ, Andrews DM, Smyth MJ. Can NK cells be a therapeutic target in human cancer? Eur J Immunol. 2008;38:2964–2968. doi: 10.1002/eji.200838764. [DOI] [PubMed] [Google Scholar]
- 9.Smyth MJ, et al. NKG2D recognition and perforin effector function mediate effective cytokine immunotherapy of cancer. J Exp Med. 2004;200:1325–1335. doi: 10.1084/jem.20041522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryceson YT, March ME, Barber DF, Ljunggren HG, Long EO. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J Exp Med. 2005;202:1001–1012. doi: 10.1084/jem.20051143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andzelm MM, Chen X, Krzewski K, Orange JS, Strominger JL. Myosin IIA is required for cytolytic granule exocytosis in human NK cells. J Exp Med. 2007;204:2285–2291. doi: 10.1084/jem.20071143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wülfing C, Purtic B, Klem J, Schatzle JD. Stepwise cytoskeletal polarization as a series of checkpoints in innate but not adaptive cytolytic killing. Proc Natl Acad Sci USA. 2003;100:7767–7772. doi: 10.1073/pnas.1336920100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banerjee PP, et al. Cdc42-interacting protein-4 functionally links actin and microtubule networks at the cytolytic NK cell immunological synapse. J Exp Med. 2007;204:2305–2320. doi: 10.1084/jem.20061893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Potter TA, Grebe K, Freiberg B, Kupfer A. Formation of supramolecular activation clusters on fresh ex vivo CD8+ T cells after engagement of the T cell antigen receptor and CD8 by antigen-presenting cells. Proc Natl Acad Sci USA. 2001;98:12624–12629. doi: 10.1073/pnas.221458898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis DM, et al. The human natural killer cell immune synapse. Proc Natl Acad Sci USA. 1999;96:15062–15067. doi: 10.1073/pnas.96.26.15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Billadeau DD, et al. The Vav-Rac1 pathway in cytotoxic lymphocytes regulates the generation of cell-mediated killing. J Exp Med. 1998;188:549–559. doi: 10.1084/jem.188.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colucci F, et al. Functional dichotomy in natural killer cell signaling: Vav1-dependent and -independent mechanisms. J Exp Med. 2001;193:1413–1424. doi: 10.1084/jem.193.12.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cella M, et al. Differential requirements for Vav proteins in DAP10- and ITAM-mediated NK cell cytotoxicity. J Exp Med. 2004;200:817–823. doi: 10.1084/jem.20031847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orange JS, et al. Wiskott-Aldrich syndrome protein is required for NK cell cytotoxicity and colocalizes with actin to NK cell-activating immunologic synapses. Proc Natl Acad Sci USA. 2002;99:11351–11356. doi: 10.1073/pnas.162376099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cannon JL, Burkhardt JK. Differential roles for Wiskott-Aldrich syndrome protein in immune synapse formation and IL-2 production. J Immunol. 2004;173:1658–1662. doi: 10.4049/jimmunol.173.3.1658. [DOI] [PubMed] [Google Scholar]
- 21.Silvin C, Belisle B, Abo A. A role for Wiskott-Aldrich syndrome protein in T-cell receptor-mediated transcriptional activation independent of actin polymerization. J Biol Chem. 2001;276:21450–21457. doi: 10.1074/jbc.M010729200. [DOI] [PubMed] [Google Scholar]
- 22.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 23.Davis DM. Mechanisms and functions for the duration of intercellular contacts made by lymphocytes. Nat Rev Immunol. 2009;9:543–555. doi: 10.1038/nri2602. [DOI] [PubMed] [Google Scholar]
- 24.Tskvitaria-Fuller I, Mistry N, Sun S, Wülfing C. Protein transduction as a means of effective manipulation of Cdc42 activity in primary T cells. J Immunol Methods. 2007;319:64–78. doi: 10.1016/j.jim.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tskvitaria-Fuller I, et al. Specific patterns of Cdc42 activity are related to distinct elements of T cell polarization. J Immunol. 2006;177:1708–1720. doi: 10.4049/jimmunol.177.3.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Austin Taylor M, Bennett M, Kumar V, Schatzle JD. Functional defects of NK cells treated with chloroquine mimic the lytic defects observed in perforin-deficient mice. J Immunol. 2000;165:5048–5053. doi: 10.4049/jimmunol.165.9.5048. [DOI] [PubMed] [Google Scholar]
- 27.Singleton KL, et al. Spatiotemporal patterning during T cell activation is highly diverse. Sci Signal. 2009;2:ra15. doi: 10.1126/scisignal.2000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orange JS, et al. The mature activating natural killer cell immunologic synapse is formed in distinct stages. Proc Natl Acad Sci USA. 2003;100:14151–14156. doi: 10.1073/pnas.1835830100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol. 2008;8:713–725. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Combs J, et al. Recruitment of dynein to the Jurkat immunological synapse. Proc Natl Acad Sci USA. 2006;103:14883–14888. doi: 10.1073/pnas.0600914103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanborn KB, et al. Myosin IIA associates with NK cell lytic granules to enable their interaction with F-actin and function at the immunological synapse. J Immunol. 2009;182:6969–6984. doi: 10.4049/jimmunol.0804337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beal AM, et al. Kinetics of early T cell receptor signaling regulate the pathway of lytic granule delivery to the secretory domain. Immunity. 2009;31:632–642. doi: 10.1016/j.immuni.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purtic B, Pitcher LA, van Oers NS, Wülfing C. T cell receptor (TCR) clustering in the immunological synapse integrates TCR and costimulatory signaling in selected T cells. Proc Natl Acad Sci USA. 2005;102:2904–2909. doi: 10.1073/pnas.0406867102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.