Abstract
Smac mimetics target cancer cells in a TNFα-dependent manner, partly via proteasome degradation of cellular inhibitor of apoptosis 1 (cIAP1) and cIAP2. Degradation of cIAPs triggers the release of receptor interacting protein kinase (RIPK1) from TNF receptor I (TNFR1) to form a caspase-8 activating complex together with the adaptor protein Fas-associated death domain (FADD). We report here a means through which cancer cells mediate resistance to Smac mimetic/TNFα-induced apoptosis and corresponding strategies to overcome such resistance. These human cancer cell lines evades Smac mimetic-induced apoptosis by up-regulation of cIAP2, which although initially degraded, rebounds and is refractory to subsequent degradation. cIAP2 is induced by TNFα via NF-κB and modulation of the NF-κB signal renders otherwise resistant cells sensitive to Smac mimetics. In addition, other signaling pathways, including phosphatidyl inositol-3 kinase (PI3K), have the potential to concurrently regulate cIAP2. Using the PI3K inhibitor, LY294002, cIAP2 up-regulation was suppressed and resistance to Smac mimetics-induced apoptosis was also overcome.
Keywords: TNFα, NF-κB, PI3K, Caspase-8, PRIK1
The development of targeted therapeutics against specific pathways in cancer etiology has greatly expanded the repertoire of effective drugs against many cancer types to include the use of small molecule kinase inhibitors and monoclonal antibodies (1). However, significant obstacles to effective and long-lasting treatments remain due to variability of response to chemotherapeutics among patients as well as relapse and acquired resistance following successful initial treatment. The identification of mechanisms by which tumors are able to resist treatment and the development of specific strategies for overcoming resistance have the potential to broadly expand the effective usage of many chemotherapeutic agents. Hence, a key feature of the development of any new cancer therapeutic is the need to account for mechanisms of resistance. It must be assumed that tumors will either initially be resistant or develop resistance and that if it can be anticipated how that might occur then more effective initial therapies can be developed that mitigate the potential for relapse and expand the potential population of tumors that can be targeted.
One of the hallmark features of cancer is the ability to evade apoptosis, particularly by up-regulation of antiapoptotic genes such as certain members of the Bcl-2 family of proteins (2) and the inhibitor of apoptosis (IAP) family of proteins (3). IAPs, particularly cellular IAP1 (cIAP1), cIAP2, and X-linked IAP (XIAP), function to prevent cell death by preventing activation of caspase-8 or inhibiting the activity of caspases-9, -3, and -7, respectively (4–6). cIAP1 and cIAP2 possess an E3 ubiquitin ligase domain that promotes proteasome-dependent degradation of cIAP1 and cIAP2, as well as other targets (7, 8). IAP inhibition of apoptosis is relieved by second mitochondria-derived activator of caspases (Smac/Diablo) that, along with cytochrome c, is released from the mitochondria upon induction of the intrinsic apoptotic pathway in response to stimuli such as genotoxic stresses (9, 10).
Because IAPs have been shown to be up-regulated in many cancers and because the interaction of Smac with IAPs involves the four amino acid residues AVPI, an ideal point of attack is to introduce small molecules that mimic the AVPI sequence motif and relieve IAP inhibition without having to disrupt the mitochondria (4–6). Over the past several years many groups have designed and synthesized various versions of small molecule Smac mimetics with the hopes of sensitizing cancer cells to apoptosis (11–14). These reports have demonstrated that Smac mimetics are effective at inducing cell death in vitro and in vivo, both as a single agent and/or synergistically with other proapoptotic stimuli.
The primary mechanisms by which Smac mimetics induce cell death involves both direct binding and preventing XIAP from interacting with caspase-9, -3, and -7 as well as induced proteasome degradation of cIAP1 and cIAP2 (11–14). Degradation of cIAP1 and cIAP2 allows for the release of RIPK1 from TNFR1 and subsequent incorporation into a complex with caspase-8 and Fas-associated death domain (FADD) that is capable of promoting cell death and overriding the prosurvival effects of NF-κB signaling (15–17). This would imply that any cancer cell expressing TNF factor receptor 1 (TNFR1) should respond to Smac mimetic/TNFα treatment because TNFR1 expression is quite ubiquitous among various cancer cell types (18). However, most cell lines tested to date do not appear to be entirely responsive to Smac mimetics (15).
We report here the identification of a specific process by which some cancer cell lines are able to evade cell death induced by Smac mimetics and to translate that knowledge into specific strategies that expand the number of cell lines that can be made responsive.
Results
Resistant Cell Line NCI-H1299 Shows Elevated cIAP2 Levels Following Smac Mimetic/TNFα Treatment.
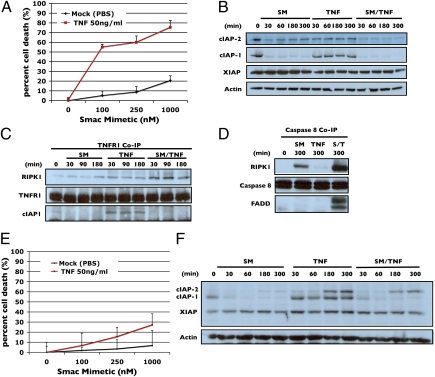
It has been demonstrated that certain non-small-cell lung cancer cell lines are responsive to low-dose (100 nM) Smac mimetic treatments in the presence of autocrine or exogenously added TNFα (15). Comparison of one such cell line, NCI-H2009, with a nonresponsive cell line, NCI-H1299, showed a marked difference in how these cell lines respond to Smac mimetic treatment. H2009 cells underwent apoptosis when treated with 100 nM Smac mimetic and 50 ng/mL TNFα and sensitivity was only modestly improved when Smac mimetic was increased to 1 μM (Fig. 1A). A time course of H2009 cells’ response to Smac mimetic, TNFα, and both in combination, showed that, as expected, both cIAP1 and cIAP2 were degraded and that neither cIAP1 nor cIAP2 were particularly sensitive to up-regulation by TNFα (Fig. 1B). As previously mentioned, one of the functions of cIAP1 and cIAP2 at TNFR1 is to promote NF-κB signaling by holding RIPK1 at the receptor (16, 17). Coimmunoprecipitation of TNFR1 showed that TNFα caused recruitment of cIAP1 and RIPK1 to the receptor and, that upon Smac mimetic cotreatment, cIAP1 was lost while RIPK1 recruitment was initially enhanced but was subsequently released from the receptor (Fig. 1C). The freed RIPK1 then entered into a caspase-8-FADD-containing complex that was required for cell death to occur (Fig. 1D).
Fig. 1.
cIAP2 respond differently to Smac mimetic and TNF in resistant cell H1299 as compared with a sensitive cell line H2009. (A) Dose–response of H2009 cells to increasing concentrations of Smac mimetic with and without 50 ng/mL TNF. (B) Western blot analysis of cIAP1, cIAP2, and XIAP during a time course of response of H2009 to 100 nM of Smac mimetic (SM), 50 ng/mL of TNFα, and Smac mimetic/TNFα from 0 to 300 min. The same amounts of Smac mimetic and TNFα were used in all of the following experiments. (C) TNFR1 coimmunoprecipitation of H2009 cells treated with Smac mimetic, TNFα, or both as in B, and the pellet were analyzed by Western blotting using antibodies against RIPK1, cIAP1, and cIAP2 as indicated. (D) Caspase-8 coimmunoprecipitation of H2009 treated with Smac mimetic, TNFα, and Smac mimetic/TNFα as in B and the pellet were analyzed by Western blotting using antibodies against RIPK1, caspase-8, and FADD as indicated. (E) Dose–response of H1299 cells to increasing concentrations of Smac mimetic with and without 50 ng/mL TNFα. (F) Western blot analysis of cIAP1, cIAP2, and XIAP during a time course of response of H1299 to Smac mimetic, TNFα, and Smac mimetic/TNF from 0 to 300 min. Percentage of cell death is based on cell titer glo (Promega) cell viability assay in 96-well plates. Graphical representations indicate the mean ± SD of at least three independent experiments.
In contrast, H1299 cells showed only modest sensitivity to Smac mimetics at higher concentrations (1 μM) in the presence of 50 ng/mL TNFα (Fig. 1E). Interestingly, cIAP2 was initially degraded, but after 3 h following Smac mimetic, TNFα or Smac mimetic/TNFα treatment, cIAP2 protein was up-regulated to higher levels (Fig. 1F and Fig. S1), indicating that the cIAP2 in these cells are highly sensitive to TNFα. Conversely, the expression of cIAP1 was only mildly responsive to TNFα and protein levels did not change following these treatments.
Loss of cIAP2 Restores Sensitivity of H1299 Cells to Low-Dose Smac Mimetic Treatment.
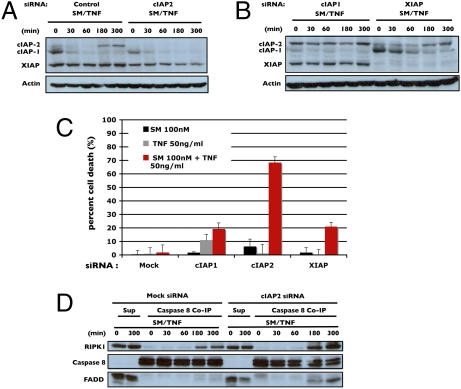
Given the dramatic up-regulation of cIAP2 following Smac mimetic treatment and that cell death was not enhanced with further addition of Smac mimetic following the initial treatment (Fig. S2), it might be possible that cIAP2 is the causative agent of the observed drug resistance. To determine whether this is the case, siRNAs targeting both cIAP1 and cIAP2 were used in H1299 cells before treatment with Smac mimetic/TNFα. Treatment with siRNA against cIAP2 was effective at preventing TNFα-induced cIAP2 accumulation, while having only moderate effects on the levels of cIAP1 and XIAP (Fig. 2A). Furthermore, knockdown of cIAP1 effectively blocked the initial degradation of cIAP2, supporting the role of cIAP1 as the E3 ubiquitin ligase of cIAP2 in the cell (19) (Fig. 2B). In addition, because cIAP1 is the E3 ligase for cIAP2, once it is gone there is nothing to promote future degradation of cIAP2 following its up-regulation either by Smac mimetic or TNFα. Thus, the very factor necessary for Smac mimetic action is paradoxically the very thing that causes resistance. siRNA knockdown of XIAP had no discernible effect on the behaviors of either cIAP1 or cIAP2 (Fig. 2B).
Fig. 2.
cIAP2 siRNA knockdown sensitizes resistant cells by preventing cIAP2 up-regulation during Smac mimetic/TNFα treatment. (A) Comparison of cIAP2 degradation in response to Smac mimetic/TNFα (SM/TNF) between mock-transfected versus cIAP2 siRNA-transfected H1299 cells. H1299 cells were treated as indicated and equal amounts of cell lysates were analyzed by Western blotting using antibodies against cIAP1, cIAP2, XIAP, and actin. (B) Time course in cIAP1 and XIAP siRNA knockdown H1299 cells treated as indicated and cell lysates were analyzed by Western blotting as in A. (C) Cell death of cIAP1, cIAP2, and XIAP siRNA treated H1299 cells in response to Smac mimetic, TNFα, or both. (D) Formation of the RIPK1–caspase-8–FADD complex as shown by caspase-8 coimmunoprecipitation in cIAP2 siRNA-treated cells in response to Smac mimetic/TNFα as compared with mock-transfected cells. Pellets from immunoprecipitation using an anti-caspase-8 antibody were analyzed by Western blotting with anti-RIPK1 and FADD antibodies as indicated. Percentage of cell death is based on cell titer glo (Promega) cell viability assay in 96-well plates. Graphical representations indicate the mean ± SD of at least three independent experiments.
The importance of cIAP2 in mediating Smac mimetic resistance was further supported by the conversion of a resistant cell line, H1299, to a sensitive one by siRNA knockdown of cIAP2 (Fig. 2C). The observed moderate improvement of sensitivity after cIAP1 and XIAP knockdown could probably be accounted for by the key role XIAP has in directly inhibiting caspase-3/7 and the fact that cIAP1 levels are typically much higher than cIAP2. Coimmunoprecipitation of caspase-8 in mock-transfected cells only weakly pulled down RIPK1, whereas under cIAP2-deficient conditions, robust formation of the RIPK1–caspase-8–FADD complex occurred (Fig. 2D), further demonstrating the role cIAP2 has in mediating resistance to Smac mimetics.
NEMO siRNA Sensitizes Resistant Cell Line H1299 by Blocking TNFα-Induced Up-Regulation of cIAP2.
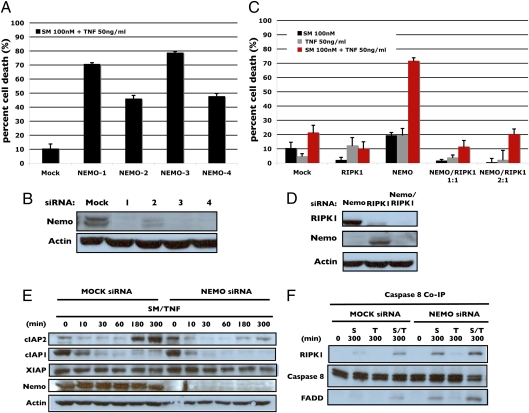
To ensure that the effect of cIAP2 was due to the NF-κB pathway, siRNAs targeting I kappa-B kinase-gamma (IKKγ/NEMO), the regulatory subunit of the IKK complex, was used to block NF-κB activation and subsequent cIAP2 induction following TNFα treatment. Testing of four independent oligos each was able to effectively knockdown NEMO and sensitize H1299 cells to Smac mimetic/TNFα (Fig. 3 A and B). In all subsequent knockdowns, oligo no. 3 was used. To verify cell death induced by NEMO knockdown plus Smac mimetic/TNFα was RIPK1 dependent, cells were cotransfected with siRNA targeting both RIPK1 and NEMO. When both proteins were knocked down simultaneously, cell death was blocked (Fig. 3 C and D). This form of cell death, therefore, matches that seen in Smac mimetic single agent sensitive cell lines, which are RIPK1 dependent (15–17).
Fig. 3.
Blocking NF-κB with a NEMO siRNA sensitizes resistant cell line H1299 to Smac mimetic and TNFα treatment. (A) Testing of four individual NEMO siRNA oligos for the ability to sensitize H1299 cells to Smac mimetic and TNFα. (B) Western blot analysis of efficiency of NEMO siRNA knockdown for each individual siRNA. (C) Determination of RIPK1 dependence in NEMO knockdown induced sensitivity. Cells were cotransfected with both NEMO and RIPK1 at both a 1:1 and 2:1 ratio and cell death was determined after the indicated treatments. (D) Western blot analysis of efficiency of double knockdown of RIPK1 and NEMO. (E) Time course comparison of indicated protein levels measured by Western blotting in mock-transfected and NEMO siRNA-transfected cells in response to Smac mimetic and TNFα. (F) Western blot analysis of pellets from caspases-8 immunoprecipitation using anti-RIPK1, caspase-8, and FADD from mock-transfected and NEMO siRNA-transfected cells treated for 300 min with Smac mimetic, TNFα, or both. Percentage of cell death is based on cell titer glo (Promega) cell viability assay in 96-well plates. Graphical representations indicate the mean ± SD of at least three independent experiments.
A time course of Smac mimetic/TNFα treatment revealed that cIAP2 induction became much weaker when the cells were treated with NEMO siRNA (Fig. 3E). In contrast, loss of NEMO did not overtly affect the level of cIAP1 or XIAP (Fig. 3E). Consistent with the cell death shown in Fig. 3C, the RIPK1–caspase-8–FADD complex was efficiently formed under NEMO-deficient condition (Fig. 3F).
Chemical Inhibition of NF-κB with BMS-345541 Sensitizes H1299 Cells to Smac Mimetic/TNFα Treatment.
To determine the feasibility of using chemical inhibitors of the NF-κB pathway to block up-regulation of cIAP2 in response to TNFα, it was decided to target IKK phosphorylation, which prevents phosphorylation of Iκ-Bα and its subsequent proteasomal degradation, using an IKK2/IKKβ-specific inhibitor, BMS-345541 (20). One of the issues with inhibiting NF-κB is the dependence cells have on it for maintaining proper levels of both prosurvival and antiapoptotic gene products, which when disrupted, results in severe sensitivity to proapoptotic stimuli. However, because cIAP2 up-regulation is such an acute response to TNFα, it might be possible to use sub threshold concentrations of the inhibitor and not aversely affect other key proteins involved in cell survival, such as c-FLIP.
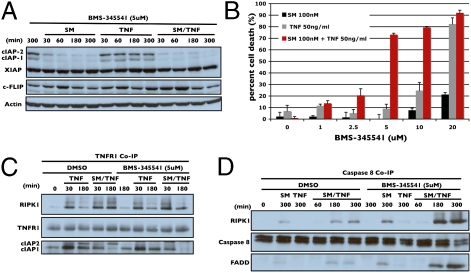
A time course of Smac mimetic/TNFα treatment showed that when cells were pretreated with 5 μM BMS-345541, the up-regulation of cIAP2, both basally following Smac mimetic treatment alone and induced by TNFα, were completely prevented (Fig. 4A). Pretreatment did not adversely affect cIAP1, XIAP, or c-FLIP (Fig. 4A). BMS-345541 synergized with Smac mimetic/TNFα in a dose-dependent manner to induce cell death with an optimal killing concentration of 5 μM (Fig. 4B). Only at higher concentrations did pretreatment with BMS-345541 induce sensitivity to TNFα alone, which is able to induce apoptosis through an alternative, RIPK1-independent caspase-8 activation pathway (Fig. 4B) (16). The differences between low- (5 μM) and high- (20 μM) dose IKK inhibition was demonstrated by its effects on degradation and phosphorylation IκBα (Fig. S3). The normal observed cellular response to Smac mimetic/TNFα is to cause an initial degradation of IκBα followed by phosphorylation of IκBα and its accumulation over time. BMS-345541 at low dose merely delays and attenuates both degradation and accumulation of phosphorylated IκBα, but does not completely shut off the signal, whereas higher doses completely block phosphorylation and act like cycloheximide by shutting off all NF-κB-mediated transcription.
Fig. 4.
Low-dose chemical inhibition of IKK2 BMS-345541 sensitizes H1299 cells to Smac mimetic and TNFα treatment by preventing up-regulation of cIAP2. (A) Time course response of H1299 cells pretreated with 5 μM BMS-345541. These cells were then treated with Smac mimetic, TNFα, or both and the indicated protein levels were analyzed by Western blotting. (B) Dose–response of H1299 cells pretreated with increasing concentrations of BMS-345541 as indicated for 1 h. Cells were then treated with Smac mimetic, TNFα, or both overnight and cell death was measured and plotted. (C) H1299 cells pretreated either with DMSO or BMS-345541 as indicated. These cells were then treated with Smac mimetic, TNFα, or both for the times indicated. TNFR1 was then immunoprecipitated and the elutes were analyzed by Western blotting for recruitment of RIPK1, cIAP1, and cIAP2 to the receptor. (D) Caspase-8 coimmunoprecipitation comparing DMSO and BMS-345541 pretreated cells that were subsequently treated with Smac mimetic, TNFα, or both for the times indicated. Elutes were analyzed by Western blot for formation of the RIPK1–caspase-8–FADD complex. Percentage of cell death is based on cell titer glo (Promega) cell viability assay in 96-well plates. Graphical representations indicate the mean ± SD of at least three independent experiments.
To ensure that the mechanism of resistance observed for H1299 applies to a wider range of cells, further screening of other resistant cell lines was conducted. Among six additional cell lines tested, half displayed cIAP2 expression in response to Smac mimetic and TNFα and the up-regulation of cIAP2 was suppressed when cotreated with BMS-345541 (Fig. S4). These cell lines, including human non-small-cell lung cancer HCC827 and HCC1355 as well as human gastric cancer cell MKN1, were also sensitized to undergo apoptosis when cotreated with BMS-345541, Smac mimetic, and TNFα, indicating that cIAP2-mediated resistance might be fairly common and that subthreshold inhibition of NF-κB is effective at synergizing with Smac mimetic and TNFα to promote cell death in otherwise resistant cells (Fig. S4).
Because one of the functionalities of cIAP1 and cIAP2 is to hold RIPK1 at TNFR1, loss of cIAP2 should effect how RIPK1 comes off the receptor and its incorporation into the caspase-8-FADD-containing complex. TNFR1 coimmunoprecipitation showed that upon TNFα treatment, both cIAP1 and cIAP2 were recruited to the receptor and that in the presence of Smac mimetic, cIAP2 appeared to be able to compensate for the loss of cIAP1 (Fig. 4C). When the IKK inhibitor was added, cIAP2 was not associated with the receptor (Fig. 4C) and RIPK1 was able to come off the receptor more easily to enter into the caspase-8-FADD complex as seen by casapse-8 coimmunoprecipitation (Fig. 4D).
Inhibition of Alternative Pathways Regulating cIAP2 Sensitizes Resistant Cells to Smac Mimetic/TNFα.
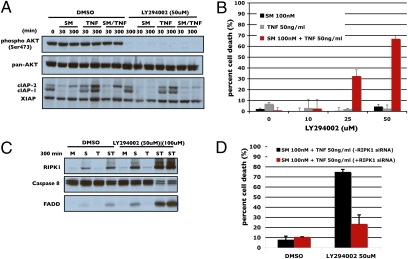
Inhibiting NF-κB does not appear to affect baseline levels of cIAP2 (Fig. 4A), indicating that other signaling pathways operate to maintain the initial state of cIAP2. H1299 cell have been documented to display constitutive protein kinase B (AKT) activation (Fig. 5A Upper). To test whether inhibition of AKT might affect cIAP2 levels, the phosphoinositide-3 kinase (PI-3K) inhibitor, LY294002, was used to inhibit AKT phosphorylation (Fig. 5A). Inhibition effected cIAP2 levels in a similar fashion as BMS-345541 did, blocking basal cIAP2 return and limiting TNFα-induced cIAP2 up-regulation (Fig. 5A Lower). Pretreatment with LY294002 synergized with Smac mimetic/TNFα in a dose-dependent manner with optimal concentration at 50 μM, although such a treatment had no effect when cotreated with TNFα or Smac mimetic alone (Fig. 5B). LY294002 cotreatment also promoted significant formation of the RIPK1-caspase 8-FADD complex (Fig. 5C). The cell death effect of LY294002 cotreatment seen here was RIPK1 dependent, because under conditions where RIPK1 was knocked down, LY294002 lost its ability to synergize with Smac mimetic/TNFα (Fig. 5D).
Fig. 5.
Inhibition of PI-3K renders H1299 cells sensitive to Smac mimetic. (A) Effects of inhibition of AKT. H1299 cells were pretreated with 50 μM LY294002 or DMSO for 1 h and then treated with Smac mimetic, TNFα, or both for the indicated times and then analyzed by Western blot for AKT phosphorylation and levels of cIAP1, cIAP2, and XIAP as indicated. (B) Dose-dependent response of H1299 cells to pretreatment with increasing concentrations of LY294002 followed by overnight treatment with Smac mimetic, TNFα, or both. (C) Caspase-8 coimmunoprecipitation comparing DMSO and LY294002 pretreated cells followed by treatment with Smac mimetic, TNFα, or both for 300 min. Elutes were analyzed by Western blot for formation of the RIPK1–caspase-8–FADD complex. (D) Evaluation of RIPK1 dependence on LY294002 induced sensitivity. RIPK1 knockdown cells were pretreated with LY294002 followed by treatment with Smac mimetic, TNFα, or both. Percentage of cell death is based on cell titer glo (Promega) cell viability assay in 96-well plates. Graphical representations indicate the mean ± SD of at least three independent experiments.
Discussion
Smac mimetics have the potential to be a powerful tool in cancer treatment given the strong potentiation of TNFα or tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced cell death to cancer cells. However, a significant number of cell lines tested do not respond very well to treatment. The identification of key mechanisms of resistance and strategies for overcoming them should help to expand and identify appropriate patient populations to target, as well as appropriate combinatorial therapies.
One of the paradoxes of Smac mimetic-induced cell death is the reliance on TNFα signaling. Smac mimetics normally do not impair normal NF-κB signaling but are able to exploit the signal to induce the formation of a RIPK1-dependent death-inducing complex (16). Because the normal survival signal generated by TNFα is not impaired, cells’ NF-κB pathway, which is highly responsive to TNFα, renders resistance to Smac mimetics. Specifically, cIAP2, a NF-κB target gene, is quickly up-regulated and binds to TNFR1 at higher than basal levels within a few hours and can assume the role of cIAP1, as well as its own, in preventing further release of RIPK1 from the receptor. At this time point, if the treated cells had not accumulated enough RIPK1-FADD-caspase-8 complexes to commit to apoptosis, they were no longer able to do so despite continuous presence of Smac mimetics. The newly generated cIAP2 is no longer subject to Smac mimetic-induced degradation because its ubiquitin E3 ligase, cIAP1, is already all degraded during the earlier time of treatment. When IKK inhibitors are used, even at a lower dose that does not completely shut down NF-κB pathway, cIAP2 up-regulation is attenuated, allowing for continuous RIPK1 release from the receptor and eventual formation of sufficient amounts of the death-inducing complex to tip the balance between death and survival.
Additionally, cIAP2 protein levels can be regulated by alternative signaling pathways either in parallel or in addition to NF-κB signaling. It has been reported that cIAP2 and XIAP are up-regulated in a ras-dependent manner through receptor tyrosine kinase activation (21), as well as through other signaling pathways including phosphoinositide-3 kinase (22, 23), protein kinase C delta (PKCδ) (24), and cAMP (25). In H1299 cells, chemical inhibitors of PI3K blocking Akt phosphorylation had the effect of preventing TNFα-mediated up-regulation of cIAP2. This presents the possibility of being able to bypass NF-κB inhibition to induce sensitivity and eliminate the inherent difficulty in trying to inhibit NF-κB.
Of curiosity is the difference between resistant and sensitive cell lines and why some resistant cell lines have such a strong propensity to up-regulate cIAP2 whereas sensitive cells seem to be less responsive to signals that up-regulate cIAP2. One possibility is that resistant cells also seem to have defects in other signaling pathways, such as PI3K activation, and that these additional alterations promote a greater sensitivity to signals that up-regulate cIAP2. Further study into defects in different signaling pathways between sensitive and resistant cells might give more insight into why they respond differently to treatments and provide rational designs for combinational therapies.
A key limitation of any potential cancer therapeutic is how to deal with variability of patient response and the acquisition of resistance to the compound by the tumor. Identifying potential mechanism of initial and acquired resistance before treatment begins, via potential biomarkers such as cIAP2 expression, would allow for more targeted and more effective treatment regimens incorporating Smac mimetic with already establish inhibitors of specific signaling pathways.
Materials and Methods
Reagents.
Smac mimetic was synthesized as previously described (11). The compound was diluted to 100 μM stocks. Antibodies used are: Caspase-8 (Cell Signaling, 9746), caspase-8 (Santa Cruz, sc-6136) TNFR1 (Abcam, ab19139), TNFR1 (Santa Cruz, sc-7895), FADD (Santa Cruz, sc-56093), RIPK1 (BD Pharmingen, 551041), cIAP2 (BD Pharmingen, 552782), cIAP1 (R&D, AF8181), pan IAP (R&D, MAB3400), c-FLIP (Alexis, ALX-804-428), NEMO (Santa Cruz, sc-8256), phospho AKT (ser473) (Cell Signaling, 4058), AKT (Cell Signaling, 9272), phospho Ik-B (Cell Signaling, 9246), and Ik-B (Cell Signaling, 9242). Chemicals used are: DMSO (Sigma, D8418), BMS-345541 (Sigma, B9939), and LY294002 (Sigma, L9908).
Cell Culture.
Cell lines HCC1299, H460, H2009, HCC827, and HCC15 were cultured in HyQ RPMI-1640 medium (HyClone) supplemented with 5% FBS (HyClone) and 100 U/mL penicillin/streptomycin (Gibco). All of the cell lines have been DNA fingerprinted for provenance using the PowerPlex 1.2 kit (Promega) and confirmed to be the same as the DNA fingerprint library maintained by either ATCC or the Minna/Gazdar lab.
Cell Survival Assay.
Cells were plated onto 96-well assay plates [white with clear bottom (3610), Corning Costar] at different cell densities, depending on cell type, in 100 μL media per well. Cells were allowed to grow to near confluence and treated with Smac mimetic or vehicle (H20) by adding 100 μL media with compound 3, and diluted to two times the desired final concentration, to each well. Cells were incubated overnight and assayed the following day using the Cell Titer-Glo Luminescent Cell Viability assay (Promega). Cells were allowed to equilibrate to room temperature at which time 25 μL of a 1:1 mixture of Cell Titer-Glo reagent and 1% Triton X-100 PBS was added. Cells were placed on rocking shaker for 5 min and incubated for an additional 5 min on the bench top. Luminescent measurements were done on a Tecan SPECTRAFluor Plus 96-well plate reader. For assays measuring toxicity effects, all values were normalized to the mock-treated or mock-transfected conditions to account for variability in the cytotoxicity of transfecting siRNA into cells and for possible cytotoxic effects that knockdown of the particular gene used might have. All values are represented graphically as mean ± SD for three independent samples.
Western Blot Analysis of Time Course Treatments and of siRNA Knockdown Efficiency.
Cells were plated onto 6-well cell culture dishes (Corning Costar) at differing cell densities, depending on the application, in 2 ml media. For all time courses, cells were plated to near confluence and allowed to attach overnight. Cells were treated to a final concentration of 100 nM Smac mimetic or 50 ng/mL TNF at each time point. For cells treated with z-vad fmk (Sigma), BMS-34554 (Sigma), or DMSO, compounds were added 1 h before Smac mimetic or TNFα treatment. For siRNA knockdown efficiency, H1299 cells were plated in 2 ml antibiotic-free media at a density of 2 × 104 cells per well and allowed to attach overnight. siRNAs were then transfected as described below. Cells were lysed in lysis buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.5 mM DDT and Complete protease inhibitor (Roche) and were then incubated on ice for 20 min and spun down at 10,000 RPM for 10 min. The soluble fraction was kept and protein concentration determined by Bradford assay. Protein concentrations were normalized to 50 μg total and SDS/PAGE was done followed by Western blotting of target antibody.
siRNA Transfection.
siRNA transfections were done in both 6-well and 96-well dish formats. For 6-well dishes, the day before transfection, cells were plated at a density of 2 × 104 cells per well in antibiotic-free media. The next day, Lipofectamine RNAiMax was used to transfect cell, as per manufacture's protocol. Briefly, 3 μL Lipofectamine RNAiMax was combined with 120 pmol (6 μL of a 20 μM stock) siRNA in a volume of 250 μL Opti-mem media (Gibco) and incubated for 20 min; the complexes of Lipofectamine RNAiMax and siRNAs were then added directly to each well and the cells were incubated until nearly confluent, ≈48–72 h later depending on growth conditions. For 96-well dishes, the day before transfection cells were plated at a density of 1 × 103 cells per well in antibiotic-free media. Lipofectamine RNAiMax was used, as above, by mixing 0.3 μL Lipofectamine 2000 and 12 pmol (0.6 μL of a 20 μM stock) siRNA in a total volume of 20 μL. All siRNAs were purchased from Dharmacon. siRNA oligos targeting RIPK1, cIAP1, cIAP2, and XIAP that were used in the current studies have been previously validated (21, 22). The target sequences are: cIAP1 (5-UUCGUACAUUUCUCUCUUA-3), cIAP2 (5-AAUGDAGAGUCAUCAAUUA-3), and XIAP (5-CCAGAAUGGUCAGUAACAAA-3). For NEMO, a set of four oligos were purchased from Dharmacon and tested. The sequences of these oligos are: no. 1 (5-AACAGGAGGUGAUCGAUAAUU-3), no. 2 (5-GAAGCGGCAUGUCGAGGUCUU-3), no. 3 (5-GAAUGCAGCUGGAAGAUCUUU-3), and no. 4 (5-GGAAGAGCCAACUGUGUGAUU-3).
Caspase-8 and TNFR1 Antibody Immunoprecipitation.
Cells were grown on 15-cm plates, treated as indicated, and harvested in ×5 volume lysis buffer (as previously described). Cells were left on ice for 20 min and centrifuged at 20,000 g for 20 min. Twenty microliters (bead volume) protein G agarose beads (GE Healthcare), 2 μg of caspase 8 antibody (Santa Cruz, SC-6136) or 3 μg TNFR1 antibody (Santa Cruz, sc-7895) were mixed with 2 mg of cell lysates (2 mg/mL) and incubated overnight at 4 Co. The following day, beads were washed four times with 500 mM NaCl lysis buffer and protein was eluted off the beads using ×2 SDS loading buffer and boiled for 5 min. Samples were analyzed by SDS/PAGE followed by Western blot.
Supplementary Material
Acknowledgments
We thank Dr. Steve McKnight for his encouragement and support; Drs. Adi Gazdar and Luc Girard for cell line information, and the National Cancer Institute (NCI) Lung Cancer SPORE P5070907 for support. This work is also supported by a program project grant from the NCI (PO1 CA 95471).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1005667107/-/DCSupplemental.
References
- 1.Sawyers CL. Rational therapeutic intervention in cancer: Kinases as drug targets. Curr Opin Genet Dev. 2002;12:111–115. doi: 10.1016/s0959-437x(01)00273-8. [DOI] [PubMed] [Google Scholar]
- 2.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nachmias B, Ashhab Y, Ben-Yehuda D. The inhibitor of apoptosis protein family (IAPs): An emerging therapeutic target in cancer. Semin Cancer Biol. 2004;14:231–243. doi: 10.1016/j.semcancer.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Liu Z, et al. Structural basis for binding of Smac/DIABLO to the XIAP BIR3 domain. Nature. 2000;408:1004–1008. doi: 10.1038/35050006. [DOI] [PubMed] [Google Scholar]
- 5.Srinivasula SM, et al. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature. 2001;410:112–116. doi: 10.1038/35065125. [DOI] [PubMed] [Google Scholar]
- 6.Wu G, et al. Structural basis of IAP recognition by Smac/DIABLO. Nature. 2000;408:1008–1012. doi: 10.1038/35050012. [DOI] [PubMed] [Google Scholar]
- 7.Morizane Y, Honda R, Fukami K, Yasuda H. X-linked inhibitor of apoptosis functions as ubiquitin ligase toward mature caspase-9 and cytosolic Smac/DIABLO. J Biochem. 2005;137:125–132. doi: 10.1093/jb/mvi029. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki Y, Nakabayashi Y, Takahashi R. Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc Natl Acad Sci USA. 2001;98:8662–8667. doi: 10.1073/pnas.161506698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 10.Verhagen AM, et al. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102:43–53. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 11.Li L, et al. A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science. 2004;305:1471–1474. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 12.Vince JE, et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 13.Varfolomeev E, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 14.Lu J, et al. SM-164: A novel, bivalent Smac mimetic that induces apoptosis and tumor regression by concurrent removal of the blockade of cIAP-1/2 and XIAP. Cancer Res. 2008;68:9384–9393. doi: 10.1158/0008-5472.CAN-08-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen SL, et al. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12:445–456. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 17.Bertrand MJ, et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 19.Conze DB, et al. Posttranscriptional downregulation of c-IAP2 by the ubiquitin protein ligase c-IAP1 in vivo. Mol Cell Biol. 2005;25:3348–3356. doi: 10.1128/MCB.25.8.3348-3356.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burke JR, et al. BMS-345541 is a highly selective inhibitor of I kappa B kinase that binds at an allosteric site of the enzyme and blocks NF-kappa B-dependent transcription in mice. J Biol Chem. 2003;278:1450–1456. doi: 10.1074/jbc.M209677200. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z, et al. ras Oncogene triggers up-regulation of cIAP2 and XIAP in intestinal epithelial cells: Epidermal growth factor receptor-dependent and -independent mechanisms of ras-induced transformation. J Biol Chem. 2005;280:37383–37392. doi: 10.1074/jbc.M503724200. [DOI] [PubMed] [Google Scholar]
- 22.Seol DW. Up-regulation of IAPs by PI-3K: A cell survival signal-mediated anti-apoptotic mechanism. Biochem Biophys Res Commun. 2008;377:508–511. doi: 10.1016/j.bbrc.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 23.Terragni J, et al. Phosphatidylinositol 3-kinase signaling in proliferating cells maintains an anti-apoptotic transcriptional program mediated by inhibition of FOXO and non-canonical activation of NFkappaB transcription factors. BMC Cell Biol. 2008;9:6. doi: 10.1186/1471-2121-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Q, Wang X, Evers BM. Induction of cIAP-2 in human colon cancer cells through PKC delta/NF-kappa B. J Biol Chem. 2003;278:51091–51099. doi: 10.1074/jbc.M306541200. [DOI] [PubMed] [Google Scholar]
- 25.Nishihara H, Kizaka-Kondoh S, Insel PA, Eckmann L. Inhibition of apoptosis in normal and transformed intestinal epithelial cells by cAMP through induction of inhibitor of apoptosis protein (IAP)-2. Proc Natl Acad Sci USA. 2003;100:8921–8926. doi: 10.1073/pnas.1533221100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.