Abstract
AIM: To explore the oncological outcomes of unresectable lung metastases without extrapulmonary metastases in colorectal cancer.
METHODS: Patients with unresectable isolated lung metastases from colorectal cancer were prospectively collected in a single institution during a 5-year period. All patients received either the fluorouracil/leucovorin plus oxaliplatin, fluorouracil/leucovorin plus irinotecan or capecitabine plus oxaliplatin regimen as first-line treatment. The resectability after preoperative chemotherapy was evaluated. Patients’ outcome and predictive factors for overall survival were also investigated by univariate and multivariate analysis.
RESULTS: A total of 70 patients were included in the study. After standardized first-line chemotherapy, only 4 patients (5.7%) were converted to resectable disease. The median overall survival time in all patients was 19 mo (95% CI: 12.6-25.4), with a 2-year overall survival rate of 38.8%. No survival difference was found among different first-line chemotherapeutic regimens. Prognostic analysis demonstrated that only the first response assessment for first-line treatment was the independent factor for predicting overall survival. The median survival time in partial response, stable disease and progressive disease patients were 27 mo, 16 mo and 8 mo (P = 0.00001).
CONCLUSION: Pulmonary metastasectomy can only be performed in a small part of unresectable lung metastases patients after chemotherapy. Patients’ first response assessment is an important prognostic factor.
Keywords: Colorectal cancer, Lung, Metastases, Chemotherapy
INTRODUCTION
Colorectal cancer is the second leading cause of cancer death in the USA, and its frequency is increasing in China in recent years. In Shanghai, colorectal cancer has become the third most prevalent malignancy[1]. Approximately 50%-60% of patients diagnosed with colorectal cancer will develop metastases[2,3]. The liver and lungs are the most common sites of metastases for colorectal cancers. Nearly 50% of patients with colorectal cancer will develop liver metastases, while only approximately 10%-15% of patients will develop lung metastases ultimately[4,5]. Commonly, metastases from colorectal cancer occur first in the liver and then later appear systemically, because of venous drainage via the portal system. However, lung metastases without extrapulmonary metastasis, also called isolated lung metastases, have been reported in several studies[4,6,7]. Through the bypass route of venous or lymphatic drainage, isolated lung metastases were only found in 2%-8% of all colorectal cancers[4,6].
Since the majority of metastases from colorectal cancer are considered initially unresectable disease, neoadjuvant chemotherapy has been applied in potentially resectable liver metastases for years. Studies demonstrated that 10%-15% of initially unresectable liver metastases could be converted to resectable disease after preoperative chemotherapy. However, the experience in unresectable isolated lung metastasis was rare. Current opinions consider that the recommendation in liver metastases may be reasonable to apply to the treatment of lung metastasis[8], but the evidence is weak and the result of this application is unknown, especially for initially unresectable isolated lung metastases.
In this current study, we prospectively collected data from all patients with unresectable isolated lung metastases from colorectal cancer at the first recurrence in a single institution during a 5-year period. The resectability after chemotherapy, outcomes of the patients, results with different chemotherapeutic regimens, and predictive factors for overall survival were all studied in the current cohort.
MATERIALS AND METHODS
Between May 2003 and January 2008, a prospective study was performed to collect data from all the colorectal cancer patients who developed unresectable lung metastasis without extrapulmonary lesions as the first recurrence from the outpatient and inpatient services of Fudan University Shanghai Cancer Center. All the unresectable patients had multiple lung metastastic lesions in two or more than two lobes and had no possibility of radical resection. According to the institutional protocol, all patients who were diagnosed with recurrence received intensive oncological assessment, including chest computed tomography (CT) scans, abdominopelvic CT or magnetic resonance imaging scans and serum carcinoembryonic antigen (CEA). Positron emission tomography-CT scans were performed in some patients, and digital rectal examinations were performed in all patients whose primary tumor was located in rectum.
For viable patients, core biopsy or fine needle biopsy was performed to confirm the diagnosis. For patients whose cytological or histological diagnosis was unavailable, the diagnoses of lung metastases were discussed by the colorectal cancer multidisciplinary treatment (MDT) team, which included a specialized radiologist. When there was doubt about the case, close follow-up without chemotherapy and dynamic change in CT scans were observed to confirm the diagnosis. The resectability of lung metastases was discussed by the colorectal MDT team and a specialized thoracic surgeon according to image study.
Patients with extrapulmonary metastasis or local recurrence were excluded from the current study. Patients, who refused to receive chemotherapy (3 cases) or sign the informed consent form (2 cases), were also excluded from the study. Besides concurrent chemotherapy, 4 patients received weekly administration of cetuximab 8, 8, 11 and 16 times, respectively, in the first line setting; they were excluded from the following outcome analysis because of the small number of cases. After screening, a total of 70 colorectal cancer patients who developed synchronous or metachronous unresectable isolated lung metastasis were included in the study.
Chemotherapeutic regimens and response assessment
According to the medical oncologists’ preference, either the fluorouracil/leucovorin plus oxaliplatin (FOLFOX), fluorouracil/leucovorin plus irinotecan (FOLFIRI) or capecitabine plus oxaliplatin (XELOX) regimen was used as first-line treatment for patients who never received chemotherapy or who had received adjuvant chemotherapy over 12 mo ago. For patients who received adjuvant chemotherapy within 12 mo, a different regimen from their original first-line treatment was used. Second-line or third-line treatment regimens were determined by considering the first-line regimen, adjuvant regimen and patients’ consent. The “stop-and-go” strategy using a modified FOLFOX6 regimen with maintenance capecitabine during the interval period was applied to 5 patients in our cohort, and they were considered to have received first-line treatment of FOLFOX in the following analysis.
Physical examination, chest CT scans, abdominopelvic ultrasound or CT scans, and serum CEA were used according to the institutional routine assessment for metastatic colorectal cancers. During chemotherapy, response assessment was performed after the first 3 (XELOX regimen) or 4 cycles (FOLFOX or FOLFIRI regimen) and then repeated every 2 to 3 mo. In patients who stopped treatment, similar oncological assessments were asked to be performed every 3 to 6 mo. The response to treatment was defined based on Response Assessment Criteria in Solid Tumors (RECIST)[9].
Statistical analysis
All surviving patients were followed up between January and March 2009 for the purpose of this project. Progression free survival (PFS) was calculated from the start of first-line treatment to the time when disease progression was determined by response assessment. In patients with metachronous metastasis, the time from resection of the primary lesion to occurrence of metastases in the lung was defined as time to metastasis in the current study. The prognostic value of the first response assessment, which was performed after the first 3 (XELOX regimen) or 4 cycles (FOLFOX or FOLFIRI regimen) of chemotherapy, was analyzed as an independent factor.
The distribution of PFS and overall survival (OS) was calculated by the Kaplan-Meier method, and a log-rank test was used to compare the differences among curves in univariate analyses. Cox regression was used in multivariate analyses, and hazard ratios were calculated including 95% confidence interval (CI). P < 0.05 was considered statistically significant.
RESULTS
Basic clinical characteristics
Of the 70 patients who had unresectable lung metastases, 19 patients (27.1%) had synchronous lung metastases with primary colorectal cancers, while the other 51 patients (72.9%) had metachronous metastatic disease. All the patients with synchronous metastases in our series underwent resection of primary colorectal cancer first, and chemotherapy started within 2 mo of the surgery. All 70 patients received first-line treatment with a regimen of FOLFOX, FOLFIRI or XELOX. The detailed clinicopathological features are summarized in Table 1. In 51 patients who had metachronous lung metastasis, the median time to metastasis was 17 mo (range: 3-67 mo). The cumulative proportions of time to metastasis within 1, 2 and 3 years were 29.4%, 70.6% and 84.3%, respectively.
Table 1.
The clinicopathological characteristics and survival time of all the unresectable pulmonary metastases patients
| Characteristics | Number (%) | Median OS (mo) | 95% CI | P value |
| Gender | ||||
| Male | 21 (30) | 22 | 9.0-35.0 | 0.487 |
| Female | 49 (70) | 19 | 10.8-27.1 | |
| Age (yr) | ||||
| ≤ 60 | 42 (60) | 24 | 19.5-28.5 | 0.093 |
| > 60 | 28 (40) | 13 | 7.8-13.2 | |
| Location of primary lesion | ||||
| Colon | 23 (32.9) | 22 | 6.8-37.2 | 0.957 |
| Rectum | 47 (67.1) | 19 | 10.7-27.2 | |
| Stage of primary lesion | ||||
| I | 4 (5.7) | 11 | 5.1-16.9 | 0.766 |
| II | 11 (15.7) | 27 | 14.1-39.9 | |
| III | 36 (51.4) | 18 | 8.6-27.4 | |
| IV | 19 (27.1) | 22 | 11.7-32.3 | |
| Unilateral or bilateral | ||||
| Unilateral | 3 (4.3) | Not reached | Not reached | 0.769 |
| Bilateral | 67 (95.7) | 19 | 12.6-25.4 | |
| Synchronous or metachronous | ||||
| Synchronous | 19 (27.1) | 22 | 11.5-32.5 | 0.693 |
| Metachronous | 51 (72.9) | 18 | 11.0-24.9 | |
| Regimen of first-line treatment | ||||
| FOLFOX | 32 (45.7) | 17 | 9.4-24.6 | 0.556 |
| XELOX | 12 (17.1) | 24 | 18.5-29.5 | |
| FOLFIRI | 26 (37.1) | 17 | 8.0-26.0 | |
| First response assessment | ||||
| PR | 25 (35.7) | 27 | 23.4-30.6 | 0.00001 |
| SD | 36 (51.4) | 16 | 8.3-23.7 | |
| PD | 9 (12.9) | 8 | 5.2-10.8 | |
FOLFOX: Fluorouracil/leucovorin plus oxaliplatin; XELOX: Capecitabine plus oxaliplatin; FOLFIRI: Fluorouracil/leucovorin plus irinotecan; PR: Partial response; SD: Stable disease; PD: Progressive disease.
Treatment outcomes
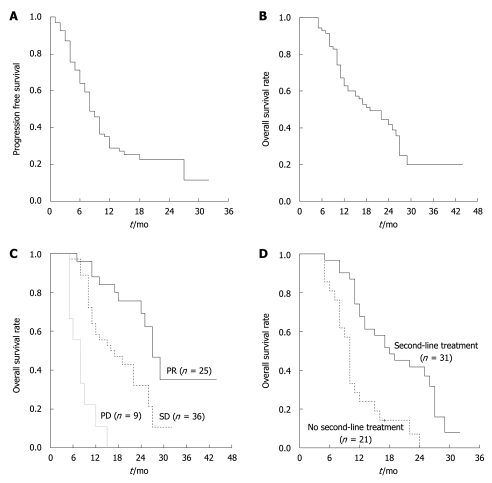
With a median follow-up time of 17 mo (range: 5-44 mo), 81.4% of patients (57 cases) had disease progression, and 26 patients were still alive (37.1%). The median PFS for first-line treatment was 8 mo (95% CI: 6.4-9.6 mo, range: 1-32 mo, Figure 1A). The median PFS for FOLFOX, XELOX and FOLFIRI regimens in the first-line setting were 10, 8 and 8 mo, respectively (P = 0.693). The median overall survival time was 19 mo (95% CI: 12.6-25.4 mo, range: 5-44 mo), with a 2 years OS rate of 38.8% (Figure 1B). At the patients’ first response assessment, none of the patients had complete response; partial response (PR), stable disease (SD) and progressive disease (PD) were observed in 35.7%, 51.4% and 12.9% of patients, respectively. Four patients (5.7%) in our cohort underwent pulmonary resection of metastases for curative intent after first-line chemotherapy. We analyzed the 4 patients whose pulmonary metastastic lesions converted to resection in more detail: all 4 patients had multiple lesions in bilateral lungs. Two patients received preoperative chemotherapy with a regimen of modified FOLFOX6, while the other two received FOLFIRI. All 4 patients had a PR after first-line chemotherapy. The decision to perform metastatectomy in the 4 patients was made on the basis of the disappearance of lesions in one side of lung and stillness of residual disease in the other side of lung during ongoing chemotherapy. One patient underwent thoracoscopic surgery and the other three underwent open surgery. Wedge resections were performed and postoperative chemotherapy was applied using the same regimen before resection in all 4 patients. However, 1 patient developed disseminated liver and pelvic metastases later and died of cancer 7 mo after surgery; 1 patient had lung metastasis recurrence in the place where lesions disappeared after first-line chemotherapy. The other two patients were still free of recurrence until the last follow-up.
Figure 1.
The median progression free survival and overall survival time. A: Progression free survival time of first-line chemotherapy; B: Overall survival rate for colorectal cancer patients with unresectable pulmonary metastases; C: Kaplan and Meier plots for median survival time according to chemotherapy efficacy; D: Kaplan and Meier plots for median survival time according to whether the second-line treatment was received. PR: Partial response; SD: Stable disease; PD: Progressive disease.
Clinical characteristics, including patients’ gender, age, location of primary lesion, stage of primary lesion, metachronous or synchronous lung metastasis, unilateral or bilateral lung metastases, first-line chemotherapeutic regimens and first response assessment, were analyzed in univariate analyses to find potential predictive factors for patients’ OS. The time to metastasis (continuous variable) in 51 patients with metachronous lung metastases was also studied using univariate Cox regression. Univariate analysis revealed that only the first response assessment was related to overall survival. Using Cox regression, multivariate analyses also demonstrated that first response assessment was the only independent factor for predicting OS. The risks of death within 2 years in SD and PD patients at their first response assessment were 3.5 times (95% CI: 1.4-8.7, P = 0.007) and 18.2 times (95% CI: 6.1-54.5, P = 2 × 10-8) higher than that of PR patients at the first response assessment. The median survival time in PR, SD and PD patients was 27 mo, 16 mo and 8 mo (P = 0.00001, Figure 1C).
Second-line treatment
None of the patients without disease progression received second-line treatment. A total of 52 patients (74.3%) had disease progression during first-line chemotherapy. Thirty-one patients (59.2% of all first-line PD patients) underwent second-line chemotherapy. Unlike the first-line regimens, the chemotherapeutic regimens in the second-line were varied. Three patients only had a single agent regimen with 5-FU/leucovorin or capecitabine; 8 patients received FOLFOX or XELOX regimen; 16 patients received FOLFIRI or XELIRI regimen; 4 patients received a combination of chemotherapy and cetuximab. In 52 first-line PD patients, the median survival time was 18 mo for those receiving any of the above second-line regimens, compared with 10 mo for patients without any further chemotherapy (P = 0.0004, Figure 1D) .
DISCUSSION
In the current study, we focused on a typical group of patients with isolated lung metastasis. Lung metastases in all of our patients were the first and the only site of metastasis after diagnosing the primary tumor in the colon or rectum. Isolated lung metastasis is considered uncommon in colorectal cancers, as the major venous drainage is through the portal vein system and metastases in lungs usually occur along with concomitant systemic metastases. Pihl et al[4] reported 8.6% of 1578 patients with colorectal cancer who had undergone curative resection developed lung metastases, and that 11.5% of lung metastases were found in patients with rectal cancer, compared with 3.5% in colon cancer. Tan et al[6] reported 56 cases (7.4%) of isolated lung metastasis in 754 patients with colorectal cancer, and 13.3% of lung metastases were found in patients with primary rectal cancer vs 6.3% with primary colon cancer (P = 0.011). In our series, we also observed more isolated lung metastases in patients with primary rectal cancer. This result may partly be attributed to the bypass route of spreading into systemic circulation via the hemorrhoidal veins. However, the precise mechanism for this phenomenon of skip metastasis bypassing the first venous or lymphatic draining route is unclear. Differences in tumor biology among various patients, tumor types, or even within a given tumor, may also be related to this result[10,11].
Most of the lung metastases from colorectal cancer were unresectable disease because of synchronous metastases in other organs or multiple unilateral or bilateral lesions. Although resection of synchronous liver and lung metastases was performed in selected patients in several studies, a favorable outcome was only observed in a study with a small number of patients with limited metastases in liver and lung[12-14]. Different from the situation in the liver, lung metastases are more prone to disseminating to the lungs bilaterally, making the resection rate lower than that of liver metastasis[15,16]. The use of CT scans in diagnosing multiple lesions and thoracoscopic resection in the management of metastatic disease were also questioned[17-19]. Most of the patients selected for pulmonary metastasectomy presented with a single lesion in lung, and patients with a single metastasis were proven to have a better outcome compared with patients with multiple metastases[20-22]. The effectiveness of pulmonary resection in patients with multiple lesions is still unclear, and no consensus has been obtained for the indication of resection concerning the number of metastases[8,22-25]. Multiple lesions in bilateral lungs were usually considered unresectable disease. In our study, after discussing the resectability of isolated lung metastases, all cases with unresectable metastases were treated with first line chemotherapy. We reported the possibility of converting to resection in patients with multiple bilateral lesions by preoperative chemotherapy for the first time. Four cases (5.7%) were converted to resectable disease after first line treatment. To our knowledge, this is the first study reporting the resectability of initially unresectable lung metastases after preoperative chemotherapy. The proportion of resection seems lower than that of inoperable liver metastases[26], suggesting a different strategy might be used when considering preoperative chemotherapy for initially unresectable lung metastases. However, there are still other reasons which may be responsible for the low rate of conversion to resection, including the undetermined surgical indication, doubtful nodules in other areas, multiple lobular lesions after chemotherapy, and patients’ consent.
In our study, the median PFS for first-line treatment and OS was similar to other studies in stage IV colorectal cancer, which combined all metastatic disease together[27,28]. Different chemotherapeutic regimens in the first-line setting, including FOLFOX, FOLFIRI and XELOX, resulted in a similar PFS and response rate. The National Comprehensive Cancer Network (NCCN) guidelines recommended that the practice in liver metastases can be applied to lung metastases[29]. However, few data were focused on this group of patients with unresectable isolated lung metastases. Our data confirmed the similar oncological results in the management of isolated lung metastases. Although the second-line chemotherapy regimens varied in progressed patients, we confirmed the benefit of continuing chemotherapy for patients with isolated lung metastases who progressed after first-line treatment. However, which regimen would be better and whether a monoclonal antibody should be used were unknown according to our study.
During first-line chemotherapy, the first response assessment is usually performed after the second month of treatment in most clinical trials of metastatic colorectal cancer. In our cohort, multivariate analysis demonstrated that the first response assessment was the independent factor predicting patients’ overall survival. The risk of death within 2 years was found to be 3.5 times higher in SD patients than that in PR patients. This result raised doubts about whether we should continue first-line treatment in SD patients. In our study, the response assessment was performed according to RECIST guidelines. Patients assessed to have SD included patients whose targeted lesions showed less than 20% increase to less than 30% decrease in the sum of the longest diameter. Changing of chemotherapeutic regimens might be reasonable in selected SD patients, such as patients with increased lesions. For patients with metachronous lung metastases, the time to relapse was considered a risk factor predicting survival in several studies[30], but most other studies did not find the time to relapse a significant risk factor[31-33]. In 906 patients who relapsed after curative treatment of colorectal cancer, Kobayashi et al[16] found that the time to relapse was only related to the survival of liver metastases and local recurrence, but no survival difference was found in patients with lung metastases.
Novel treatment approaches have been introduced into the local treatment of unresectable lung metastases. Radiofrequency ablation was studied for patients with unresectable lung metastases in several studies. Marginal survival benefit and improvement in quality of life were observed in selected cases[34-36], and it is only used as an adjunct to resection or as an alternative for unresectable lesions. Transpulmonary chemoembolization is another new technique used for the local treatment of inoperable lung metastases. Preliminary results found this technique was a well-tolerated procedure for palliative treatment, but the benefit of survival was still unknown[37-39].
In conclusion, isolated lung metastases were relatively rare in metastatic colorectal cancer. The outcomes of chemotherapy in unresectable lung metastases patients were similar to the results of liver metastases, and first response assessment was an important risk factor to predict survival. Pulmonary metastasectomy can only be performed in a small group of patients with initially unresectable lung metastases after preoperative chemotherapy. Further studies are needed to validate our results and evaluate the rate of resectability after chemotherapy.
COMMENTS
Background
Approximately 50%-60% of colorectal cancer patients will develop metastases. Nearly half of patients with colorectal cancer will develop liver metastases, while only approximately 10%-15% of patients will develop lung metastases ultimately. Studies demonstrated that 10%-15% of initially unresectable liver metastases could be converted to resectable disease after preoperative chemotherapy. However, there was little experience in unresectable isolated lung metastasis.
Research frontiers
Current opinions consider that it may be reasonable to apply the recommendations for treatment of liver metastases to the treatment of lung metastasis, but the evidence is weak and the result of this application is unknown, especially for initially unresectable isolated lung metastases.
Innovations and breakthroughs
This study prospectively collected data from all patients with unresectable isolated lung metastases from colorectal cancer as the first recurrence in a single institution during a 5-year period. The outcomes of chemotherapy were similar to the results of liver metastases, and first response assessment was an important risk factor to predict survival. Furthermore, only 5.7% of patients were converted to resectable disease after preoperative chemotherapy, which was obviously lower than the conversion rate in liver metastasis. It is the first study reporting the resectability of initially unresectable lung metastases after preoperative chemotherapy.
Applications
The results of this study suggested that a different strategy might be used when considering the preoperative chemotherapy for initially unresectable lung metastases.
Peer review
This is an interesting and novel paper looking at isolated lung metastases in colorectal cancer.
Footnotes
Supported by The Grant from Shanghai Science and Technology Committee, No. 09411967000
Peer reviewer: Francis Seow-Choen, MBBS, FRCSEd, FAMS, Professor, Seow-Choen Colorectal Centre, Mt Elizabeth Medical Centre, Singapore, 3 Mt Elizabeth Medical Centre #09-10, Singapore 228510, Singapore
S- Editor Wang YR L- Editor O'Neill M E- Editor Lin YP
References
- 1.Shanghai Municipal Center for Disease Control and Prevention. Shanghai Cancer Report 2007. Shanghai: SCDC; 2007. [Google Scholar]
- 2.Van Cutsem E, Nordlinger B, Adam R, Köhne CH, Pozzo C, Poston G, Ychou M, Rougier P. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer. 2006;42:2212–2221. doi: 10.1016/j.ejca.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Yoo PS, Lopez-Soler RI, Longo WE, Cha CH. Liver resection for metastatic colorectal cancer in the age of neoadjuvant chemotherapy and bevacizumab. Clin Colorectal Cancer. 2006;6:202–207. doi: 10.3816/CCC.2006.n.036. [DOI] [PubMed] [Google Scholar]
- 4.Pihl E, Hughes ES, McDermott FT, Johnson WR, Katrivessis H. Lung recurrence after curative surgery for colorectal cancer. Dis Colon Rectum. 1987;30:417–419. doi: 10.1007/BF02556487. [DOI] [PubMed] [Google Scholar]
- 5.Foster JH. Treatment of metastatic disease of the liver: a skeptic's view. Semin Liver Dis. 1984;4:170–179. doi: 10.1055/s-2008-1040656. [DOI] [PubMed] [Google Scholar]
- 6.Tan KK, Lopes Gde L Jr, Sim R. How uncommon are isolated lung metastases in colorectal cancer? A review from database of 754 patients over 4 years. J Gastrointest Surg. 2009;13:642–648. doi: 10.1007/s11605-008-0757-7. [DOI] [PubMed] [Google Scholar]
- 7.Scheele J, Altendorf-Hofmann A, Stangl R, Gall FP. Pulmonary resection for metastatic colon and upper rectum cancer. Is it useful? Dis Colon Rectum. 1990;33:745–752. doi: 10.1007/BF02052319. [DOI] [PubMed] [Google Scholar]
- 8.Headrick JR, Miller DL, Nagorney DM, Allen MS, Deschamps C, Trastek VF, Pairolero PC. Surgical treatment of hepatic and pulmonary metastases from colon cancer. Ann Thorac Surg. 2001;71:975–979; discussion 979-980. doi: 10.1016/s0003-4975(00)02522-4. [DOI] [PubMed] [Google Scholar]
- 9.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 10.Merrie AE, Phillips LV, Yun K, McCall JL. Skip metastases in colon cancer: assessment by lymph node mapping using molecular detection. Surgery. 2001;129:684–691. doi: 10.1067/msy.2001.113887. [DOI] [PubMed] [Google Scholar]
- 11.Tang R, Wang JY, Chen JS, Chang-Chien CR, Tang S, Lin SE, You YT, Hsu KC, Ho YS, Fan HA. Survival impact of lymph node metastasis in TNM stage III carcinoma of the colon and rectum. J Am Coll Surg. 1995;180:705–712. [PubMed] [Google Scholar]
- 12.Miller G, Biernacki P, Kemeny NE, Gonen M, Downey R, Jarnagin WR, D'Angelica M, Fong Y, Blumgart LH, DeMatteo RP. Outcomes after resection of synchronous or metachronous hepatic and pulmonary colorectal metastases. J Am Coll Surg. 2007;205:231–238. doi: 10.1016/j.jamcollsurg.2007.04.039. [DOI] [PubMed] [Google Scholar]
- 13.Nagakura S, Shirai Y, Yamato Y, Yokoyama N, Suda T, Hatakeyama K. Simultaneous detection of colorectal carcinoma liver and lung metastases does not warrant resection. J Am Coll Surg. 2001;193:153–160. doi: 10.1016/s1072-7515(01)00970-x. [DOI] [PubMed] [Google Scholar]
- 14.Mineo TC, Ambrogi V, Tonini G, Bollero P, Roselli M, Mineo D, Nofroni I. Longterm results after resection of simultaneous and sequential lung and liver metastases from colorectal carcinoma. J Am Coll Surg. 2003;197:386–391. doi: 10.1016/S1072-7515(03)00387-9. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi H, Mochizuki H, Sugihara K, Morita T, Kotake K, Teramoto T, Kameoka S, Saito Y, Takahashi K, Hase K, et al. Characteristics of recurrence and surveillance tools after curative resection for colorectal cancer: a multicenter study. Surgery. 2007;141:67–75. doi: 10.1016/j.surg.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi H, Mochizuki H, Morita T, Kotake K, Teramoto T, Kameoka S, Saito Y, Takahashi K, Hase K, Ohya M, et al. Timing of relapse and outcome after curative resection for colorectal cancer: a Japanese multicenter study. Dig Surg. 2009;26:249–255. doi: 10.1159/000226868. [DOI] [PubMed] [Google Scholar]
- 17.McCormack PM, Ginsberg KB, Bains MS, Burt ME, Martini N, Rusch VW, Ginsberg RJ. Accuracy of lung imaging in metastases with implications for the role of thoracoscopy. Ann Thorac Surg. 1993;56:863–865; discussion 865-866. doi: 10.1016/0003-4975(93)90344-h. [DOI] [PubMed] [Google Scholar]
- 18.Nakajima J, Murakawa T, Fukami T, Sano A, Sugiura M, Takamoto S. Is finger palpation at operation indispensable for pulmonary metastasectomy in colorectal cancer? Ann Thorac Surg. 2007;84:1680–1684. doi: 10.1016/j.athoracsur.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Parsons AM, Detterbeck FC, Parker LA. Accuracy of helical CT in the detection of pulmonary metastases: is intraoperative palpation still necessary? Ann Thorac Surg. 2004;78:1910–1916; discussion 1916-1918. doi: 10.1016/j.athoracsur.2004.05.065. [DOI] [PubMed] [Google Scholar]
- 20.Kanemitsu Y, Kato T, Hirai T, Yasui K. Preoperative probability model for predicting overall survival after resection of pulmonary metastases from colorectal cancer. Br J Surg. 2004;91:112–120. doi: 10.1002/bjs.4370. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe I, Arai T, Ono M, Sugito M, Kawashima K, Ito M, Nagai K, Saito N. Prognostic factors in resection of pulmonary metastasis from colorectal cancer. Br J Surg. 2003;90:1436–1440. doi: 10.1002/bjs.4331. [DOI] [PubMed] [Google Scholar]
- 22.Welter S, Jacobs J, Krbek T, Poettgen C, Stamatis G. Prognostic impact of lymph node involvement in pulmonary metastases from colorectal cancer. Eur J Cardiothorac Surg. 2007;31:167–172. doi: 10.1016/j.ejcts.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Yano T, Fukuyama Y, Yokoyama H, Tanaka Y, Miyagi J, Kuninaka S, Asoh H, Ichinose Y. Failure in resection of multiple pulmonary metastases from colorectal cancer. J Am Coll Surg. 1997;185:120–122. doi: 10.1016/s1072-7515(97)00032-x. [DOI] [PubMed] [Google Scholar]
- 24.McCormack PM, Burt ME, Bains MS, Martini N, Rusch VW, Ginsberg RJ. Lung resection for colorectal metastases. 10-year results. Arch Surg. 1992;127:1403–1406. doi: 10.1001/archsurg.1992.01420120037006. [DOI] [PubMed] [Google Scholar]
- 25.Onaitis MW, Petersen RP, Haney JC, Saltz L, Park B, Flores R, Rizk N, Bains MS, Dycoco J, D'Amico TA, et al. Prognostic factors for recurrence after pulmonary resection of colorectal cancer metastases. Ann Thorac Surg. 2009;87:1684–1688. doi: 10.1016/j.athoracsur.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 26.Adam R, Avisar E, Ariche A, Giachetti S, Azoulay D, Castaing D, Kunstlinger F, Levi F, Bismuth F. Five-year survival following hepatic resection after neoadjuvant therapy for nonresectable colorectal. Ann Surg Oncol. 2001;8:347–353. doi: 10.1007/s10434-001-0347-3. [DOI] [PubMed] [Google Scholar]
- 27.Chibaudel B, Maindrault-Goebel F, Lledo G, Mineur L, André T, Bennamoun M, Mabro M, Artru P, Carola E, Flesch M, et al. Can chemotherapy be discontinued in unresectable metastatic colorectal cancer? The GERCOR OPTIMOX2 Study. J Clin Oncol. 2009;27:5727–5733. doi: 10.1200/JCO.2009.23.4344. [DOI] [PubMed] [Google Scholar]
- 28.Golfinopoulos V, Salanti G, Pavlidis N, Ioannidis JP. Survival and disease-progression benefits with treatment regimens for advanced colorectal cancer: a meta-analysis. Lancet Oncol. 2007;8:898–911. doi: 10.1016/S1470-2045(07)70281-4. [DOI] [PubMed] [Google Scholar]
- 29.NCCN Clinical Practice Guidlines in Oncology. V2 2009. Accessed Apr 28. Shanghai: SCDC; 2009. Available from: http://www.nccn.org/professionals/physician_gls/PDF/colon.pdf. [Google Scholar]
- 30.Rena O, Casadio C, Viano F, Cristofori R, Ruffini E, Filosso PL, Maggi G. Pulmonary resection for metastases from colorectal cancer: factors influencing prognosis. Twenty-year experience. Eur J Cardiothorac Surg. 2002;21:906–912. doi: 10.1016/s1010-7940(02)00088-x. [DOI] [PubMed] [Google Scholar]
- 31.Saito Y, Omiya H, Kohno K, Kobayashi T, Itoi K, Teramachi M, Sasaki M, Suzuki H, Takao H, Nakade M. Pulmonary metastasectomy for 165 patients with colorectal carcinoma: A prognostic assessment. J Thorac Cardiovasc Surg. 2002;124:1007–1013. doi: 10.1067/mtc.2002.125165. [DOI] [PubMed] [Google Scholar]
- 32.Sakamoto T, Tsubota N, Iwanaga K, Yuki T, Matsuoka H, Yoshimura M. Pulmonary resection for metastases from colorectal cancer. Chest. 2001;119:1069–1072. doi: 10.1378/chest.119.4.1069. [DOI] [PubMed] [Google Scholar]
- 33.Shiono S, Ishii G, Nagai K, Yoshida J, Nishimura M, Murata Y, Tsuta K, Nishiwaki Y, Kodama T, Ochiai A. Histopathologic prognostic factors in resected colorectal lung metastases. Ann Thorac Surg. 2005;79:278–282; discussion 283. doi: 10.1016/j.athoracsur.2004.06.096. [DOI] [PubMed] [Google Scholar]
- 34.Suh RD, Wallace AB, Sheehan RE, Heinze SB, Goldin JG. Unresectable pulmonary malignancies: CT-guided percutaneous radiofrequency ablation--preliminary results. Radiology. 2003;229:821–829. doi: 10.1148/radiol.2293021756. [DOI] [PubMed] [Google Scholar]
- 35.Shah SA, Haddad R, Al-Sukhni W, Kim RD, Greig PD, Grant DR, Taylor BR, Langer B, Gallinger S, Wei AC. Surgical resection of hepatic and pulmonary metastases from colorectal carcinoma. J Am Coll Surg. 2006;202:468–475. doi: 10.1016/j.jamcollsurg.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Yan TD, King J, Sjarif A, Glenn D, Steinke K, Morris DL. Percutaneous radiofrequency ablation of pulmonary metastases from colorectal carcinoma: prognostic determinants for survival. Ann Surg Oncol. 2006;13:1529–1537. doi: 10.1245/s10434-006-9101-1. [DOI] [PubMed] [Google Scholar]
- 37.Vogl TJ, Lehnert T, Zangos S, Eichler K, Hammerstingl R, Korkusuz H, Lindemayr S. Transpulmonary chemoembolization (TPCE) as a treatment for unresectable lung metastases. Eur Radiol. 2008;18:2449–2455. doi: 10.1007/s00330-008-1056-0. [DOI] [PubMed] [Google Scholar]
- 38.Vogl TJ, Wetter A, Lindemayr S, Zangos S. Treatment of unresectable lung metastases with transpulmonary chemoembolization: preliminary experience. Radiology. 2005;234:917–922. doi: 10.1148/radiol.2343032091. [DOI] [PubMed] [Google Scholar]
- 39.Lindemayr S, Lehnert T, Korkusuz H, Hammerstingl R, Vogl TJ. Transpulmonary chemoembolization: a novel approach for the treatment of unresectable lung tumors. Tech Vasc Interv Radiol. 2007;10:114–119. doi: 10.1053/j.tvir.2007.09.010. [DOI] [PubMed] [Google Scholar]