Abstract
Purpose
In a recently completed 3-year, randomized, double-blind study, denosumab, a fully human monoclonal antibody against receptor activator of nuclear factor κB ligand, significantly increased bone mineral density and decreased new vertebral fractures in men receiving androgen deprivation therapy for prostate cancer. We conducted subgroup analyses to evaluate the relationships between subject characteristics and the effects of denosumab on bone mineral density at multiple skeletal sites.
Materials and Methods
A total of 1,468 subjects were randomized 1:1 to receive 60 mg subcutaneous denosumab every 6 months or placebo for 36 months. In these analyses we evaluated the effects of denosumab on bone mineral density at the lumbar spine, total hip and distal 1/3 radius (substudy of 309 subjects) during 36 months in specific subgroups according to age, duration and type of prior androgen deprivation therapy, bone mineral density T score, weight, body mass index, bone turnover marker levels and prevalent vertebral fractures.
Results
After 36 months denosumab significantly increased bone mineral density of the lumbar spine, total hip and distal 1/3 radius by 7.9%, 5.7% and 6.9%, respectively, compared with placebo (p < 0.0001 for each comparison). Denosumab significantly increased bone mineral density to a degree similar to that observed in the overall analysis for every subgroup including older men as well as those with prevalent fractures, lower baseline bone mineral density, and higher serum C-telopeptide and tartrate-resistant alkaline phosphatase 5b. Mean increases in bone mineral density at each skeletal site were greatest for men with the highest levels of serum C-telopeptide and tartrate-resistant alkaline phosphatase 5b.
Conclusions
Denosumab significantly and consistently increased bone mineral density at all skeletal sites and in every subgroup, including men at greatest risk for bone loss and fractures.
Keywords: RANK ligand, denosumab, clinical trial, bone resorption, prostatic neoplasms
Androgen deprivation therapy with GnRH agonists or bilateral orchiectomy is well established for the treatment of advanced prostate cancer,1,2 and is also used in certain cases for the management of nonmetastatic prostate cancer when radical treatment cannot be administered or is insufficient.3 Early intervention with ADT improves disease-free survival and overall survival in men with locally advanced prostate cancer treated with radiation therapy4,5 and in men with lymph node positive prostate cancer treated with radical prostatectomy and pelvic lymphadenectomy.6 ADT increases bone turnover markers, decreases BMD and increases fracture risk.7–9 Fracture risk increases with longer exposure to ADT, and is associated with significant skeletal morbidity and mortality in men with prostate cancer.8,10–12
Denosumab is a fully human monoclonal antibody against RANKL, a key mediator of osteoclast formation, function and survival.13 In a 2-year, randomized, placebo controlled study of 252 women receiving adjuvant aromatase inhibitor therapy for nonmetastatic breast cancer, denosumab significantly increased BMD at all skeletal sites.14 In a recently reported 3-year, randomized, double-blind, placebo controlled study of 1,468 men receiving ADT for nonmetastatic prostate cancer, denosumab significantly increased BMD at the lumbar spine and other skeletal sites, and decreased new vertebral fractures.15 The study met its primary end point, which showed that at 24 months lumbar spine BMD increased by 6.7% in the denosumab arm compared with placebo (p < 0.0001). These BMD increases were significantly greater than placebo beginning 1 month after the initiation of therapy and persisted for the duration of the treatment period.
We conducted a prespecified subgroup analysis to evaluate the relationships between subject characteristics and the effects of denosumab on BMD at the lumbar spine, total hip and distal 1/3 radius at 36 months. The subgroups evaluated in this analysis included baseline characteristics that may influence the risk of clinical fractures in the general population16 and in men receiving ADT for prostate cancer8,17 such as age, prevalent vertebral fracture, BMI and BMD T score. Other characteristics were also evaluated including type of ADT and baseline serum levels of the bone resorption marker C-telopeptide (sCTx, a bone collagen breakdown product due to osteoclast action) and the osteoclast marker tartrate-resistant alkaline phosphatase 5b (TRAP-5b, an osteoclast specific enzyme).
MATERIALS AND METHODS
Subjects
The randomized, placebo controlled trial included men with histologically confirmed prostate cancer who were receiving ADT with an expected duration of on-study treatment of 12 or more months.15 Men were 70 years old or older, or if younger than 70 years were required to have a low baseline BMD (T score at the lumbar spine, total hip or femoral neck less than −1.0) or a history of an osteoporotic fracture. All subjects had an Eastern Cooperative Oncology Group performance status of 0, 1 or 2. Key exclusion criteria were concurrent antineoplastic therapy or radiotherapy and prostate specific antigen greater than 5 ng/ml after receiving ADT for more than 1 month. Subjects were also excluded from study if they had a BMD T score less than −4.0 at lumbar spine, total hip or femoral neck, or were currently receiving treatment for osteoporosis.
Study Design
This was a 3-year, randomized, double-blind, placebo controlled, phase 3 study. Subjects were randomly assigned to receive subcutaneous injections of 60 mg denosumab or matching placebo every 6 months. Randomization was stratified by duration of prior ADT (6 or less vs greater than 6 months) and age (younger than 70 vs 70 years old or older). All subjects were instructed to take calcium (1 gm daily) and vitamin D (400 or more IU daily). Subjects who enrolled with a baseline level of 25-hydroxyvitamin D level between 12 and 20 ng/ml were instructed to receive at least 800 IU vitamin D daily. Institutional review boards at each center approved the protocol. All subjects provided written informed consent before participating.
BMD and Bone Turnover Marker Assessment
BMD was measured by dual energy x-ray absorptiometry at baseline, and at months 1, 3, 6, 12, 24 and 36 using Hologic (Hologic Inc., Bedford, Massachusetts) or Lunar (General Electric Lunar Corp., Madison, Wisconsin) densitometers. All BMD results were assessed in blinded fashion by a central reader (Synarc, San Francisco, California). sCTx was measured using the Serum Cross-Laps® ELISA kit. Serum TRAP-5b was measured using the BoneTRAP® assay kit.
Subgroup Analyses
Analyses of percentage change from baseline in BMD at the lumbar spine, total hip and distal 1/3 radius (subgroup of 309 subjects) at 24 and 36 months were performed in several subgroups in both treatment arms including age (younger than 70 vs 70 years old or older), baseline BMI (less than 26 vs 26 or greater kg/m2), prevalent vertebral fracture at baseline (yes vs no), baseline BMD T score (−1.0 or less vs greater than −1.0), duration of prior ADT (6 or less vs greater than 6 months), type of prior ADT (GnRH agonist vs bilateral orchiectomy), baseline sCTx tertiles (less than 0.475 vs 0.475 or greater to less than 0.764 vs 0.764 μg/l or greater) and baseline TRAP-5b tertiles (less than 4.219 vs 4.219 or greater to less than 5.561 vs 5.561 U/l or greater). For the subgroup of prior ADT type, if subjects had previously undergone surgical (bilateral orchiectomy) and chemical (GnRH agonist) castration, then they were assigned to the bilateral orchiectomy subgroup. Subjects in the GnRH agonist subgroup were required to have been treated with chemical castration only.
Subgroup analyses were prespecified at 36 months. Multiplicity adjustment was not used for these analyses. Subgroup analyses included all randomized subjects who had observations for the relevant BMD end point at baseline and at least once at or before month 36. The data were imputed using the last observation carried forward method. The treatment by baseline subgroup interaction was tested based on an ANCOVA model adjusting for treatment, stratification variables, baseline BMD value, densitometer type and baseline BMD value by densitometer type interaction. If p <0.05 then the treatment by subgroup interaction was significant.
RESULTS
Study Population
Baseline demographic and clinical characteristics of the study subjects are shown in the table, and are well balanced between the treatment arms (734 subjects in each treatment arm).15 Mean age (range) of the study population was 75 (48 to 97) years. Mean BMD T scores were −0.36 at the lumbar spine and −0.87 at the total hip. Overall 83% of subjects were 70 years old or older and 76% had received ADT for greater than 6 months. Baseline demographics and clinical characteristics of the distal 1/3 radius sub-study population (309) were similar to those of the overall population (data not shown).
Baseline variables
| No. Placebo (%) | No. Denosumab (%) | |||
|---|---|---|---|---|
| Age: | ||||
| Younger than 70 | 124 | (17) | 125 | (17) |
| 70 or Older | 610 | (83) | 609 | (83) |
| BMI (kg/m2): | ||||
| Less than 26 | 250 | (34) | 223 | (30) |
| 26 or Greater | 483 | (66) | 510 | (70) |
| Unknown | 1 | (less than 1) | 1 | (less than 1) |
| Prevalent vertebral fracture: | ||||
| Yes | 174 | (24) | 155 | (21) |
| No | 504 | (69) | 531 | (72) |
| Not readable or missing | 56 | (8) | 48 | (7) |
| Lumbar spine BMD T score: | ||||
| −1.0 or Less | 321 | (44) | 276 | (38) |
| Greater than −1.0 | 408 | (56) | 451 | (61) |
| Unknown | 5 | (less than 1) | 7 | (1) |
| Total hip BMD T score: | ||||
| −1.0 or Less | 359 | (49) | 339 | (46) |
| Greater than −1.0 | 359 | (49) | 373 | (51) |
| Unknown | 16 | (2) | 22 | (3) |
| Distal 1/3 radius BMD T score (substudy):* | ||||
| −1.0 or Less | 125 | (84) | 128 | (80) |
| Greater than −1.0 | 10 | (7) | 16 | (10) |
| Unknown | 13 | (9) | 17 | (11) |
| Mos prior ADT: | ||||
| 0–6 | 175 | (24) | 175 | (24) |
| Greater than 6 | 559 | (76) | 559 | (76) |
| Type of prior ADT: | ||||
| GnRH agonist | 671 | (91) | 655 | (89) |
| Bilat orchiectomy | 61 | (8) | 77 | (10) |
| Unknown | 2 | (less than 1) | 2 | (less than 1) |
| sCTx levels (μg/l): | ||||
| Less than 0.475 | 231 | (31) | 221 | (30) |
| 0.475–Less than 0.764 | 236 | (32) | 229 | (31) |
| 0.764 or Greater | 216 | (29) | 240 | (33) |
| Not available | 51 | (7) | 44 | (6) |
| TRAP-5b levels (U/l): | ||||
| Less than 4.219 | 228 | (31) | 225 | (31) |
| 4.219–Less than 5.561 | 230 | (31) | 236 | (32) |
| 5.561 or Greater | 223 | (30) | 231 | (31) |
| Not available | 53 | (7) | 42 | (6) |
In 148 placebo and 161 denosumab.
Subgroup Analyses
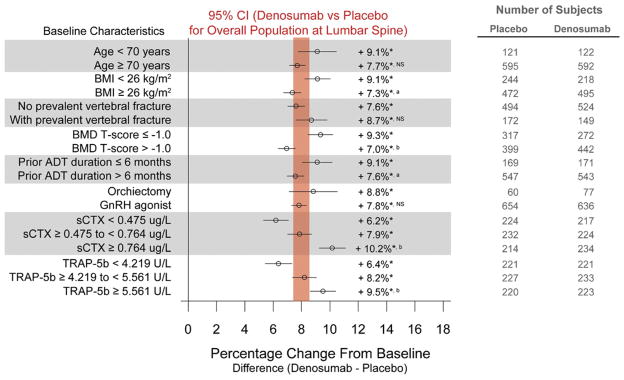
Overall denosumab significantly increased BMD at all measured skeletal anatomical sites.15 The magnitude of the BMD increases at 36 months was 7.9%, 5.7% and 6.9% at the lumbar spine, total hip and distal 1/3 radius, respectively (p < 0.0001 for each comparison with placebo) (figs. 1 to 3).
Figure 1.
Forest plots of treatment effect of denosumab at 36 months in percentage change from baseline (±95% CI) in BMD at the lumbar spine. Results are presented as least squares means. Vertical bar represents 95% CI for percentage difference in lumbar spine BMD between denosumab and placebo for overall population at 36 months. Asterisk indicates p < 0.0001 vs placebo. a indicates p < 0.05 between subgroups. b indicates p < 0.0001 between subgroups. NS, not significant between subgroups.
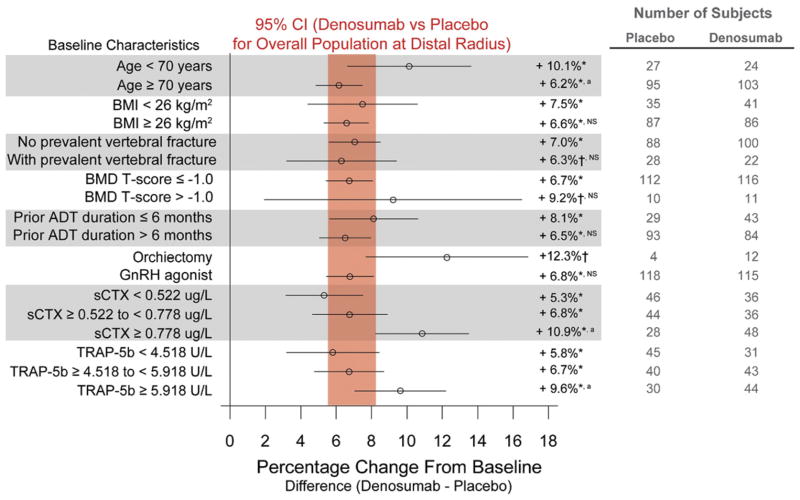
Figure 3.
Forest plots of treatment effect of denosumab at 36 months in percentage change from baseline (±95% CI) in BMD at distal 1/3 radius. Results are presented as least squares means. Vertical bar represents 95% CI for percentage difference in distal 1/3 radius BMD between denosumab and placebo for overall population at 36 months. Asterisk indicates p < 0.0001 vs placebo. Dagger indicates p ≤ 0.01 vs placebo. a indicates p < 0.05 between subgroups. NS, not significant between subgroups.
Compared with placebo denosumab significantly increased BMD at all skeletal sites from baseline to month 36 across all subgroups. Examples include age (9.1% for subjects younger than 70 years vs 7.7% for those 70 years old or older), prevalent vertebral fracture (7.6% for subjects without vs 8.7% for those with prevalent vertebral fracture) and type of ADT (8.8% for subjects who received bilateral orchiectomy vs 7.8% for those who received GnRH agonist) at the lumbar spine (fig. 1). Mean increases in lumbar spine BMD were more pronounced for men with lower BMI, lower BMD T scores, shorter ADT duration and higher levels of bone turnover markers (sCTx and TRAP-5b).
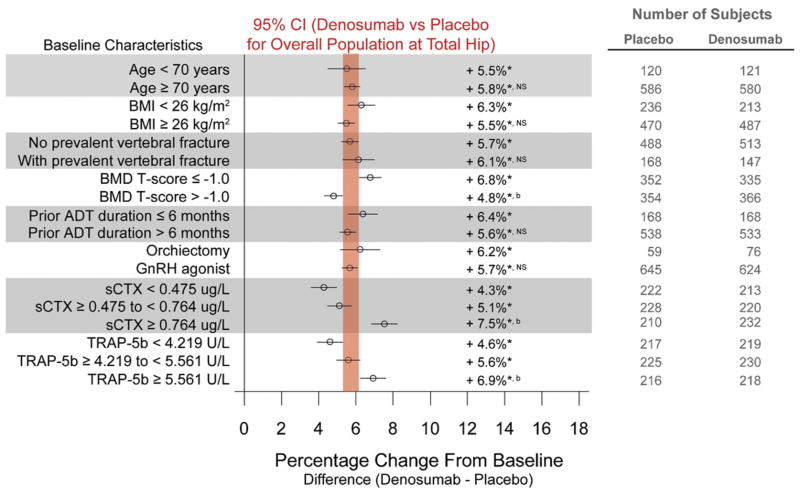
Similarly, compared with placebo, increases in BMD at the total hip were consistent across all subgroups including age (5.5% for subjects younger than 70 years vs 5.8% for those 70 years old or older), prevalent vertebral fracture (5.7% for subjects without vs 6.1% for those with prevalent vertebral fracture) and type of ADT (6.2% for subjects who received bilateral orchiectomy vs 5.7% for those who received GnRH agonist) (fig. 2). Mean increases in total hip BMD were greater for men with lower BMD T scores, and higher levels of sCTx and TRAP-5b.
Figure 2.
Forest plots of treatment effect of denosumab at 36 months in percentage change from baseline (±95% CI) in BMD at total hip. Results are presented as least squares means. Vertical bar represents 95% CI for percentage difference in total hip BMD between denosumab and placebo for overall population at 36 months. Asterisk indicates p < 0.0001 vs placebo. a indicates p < 0.05 between subgroups. b indicates p < 0.0001 between subgroups. NS, not significant between subgroups.
BMD at the distal 1/3 radius was evaluated in a subgroup of 309 subjects (fig. 3). Mean BMD increases at the distal 1/3 radius were more marked for younger men as well as for those with higher levels of sCTx and TRAP-5b. Notably the increases in BMD at each anatomical skeletal site increased in a stepwise manner according to sCTx tertile. Likewise BMD increases at each skeletal site progressively increased according to TRAP-5b tertile.
Safety
Overall the rates of adverse events (87% denosumab, 87% placebo), serious adverse events (35% denosumab, 31% placebo) and death (6% denosumab, 6% placebo) were balanced between the treatment groups.15
DISCUSSION
Denosumab decreased new vertebral fractures and increased BMD in men receiving ADT for nonmetastatic prostate cancer.15 In these analyses we demonstrate that denosumab increased BMD in a consistent and reproducible manner at all skeletal sites and in all subgroups. In general the greatest increases in BMD vs placebo were observed in subjects with the highest levels of bone turnover markers at baseline and in those with features associated with high bone turnover, including older age, low BMD and prevalent fractures.
In the general population of adult males advanced age, low BMI, low BMD and prior history of fractures are associated with a greater risk of subsequent fractures.16 ADT is also associated with bone loss and increased risk of fracture. BMD generally decreases with initial ADT and continues to decrease with long-term treatment.11,18 It has been reported that the decrease in BMD is greatest in the first year of initial therapy.18 In this study denosumab significantly increased BMD in all subgroups including older men as well as those with lower BMI, lower BMD and prevalent vertebral fractures. Denosumab increased BMD at all skeletal sites regardless of the duration of prior ADT at study entry. With denosumab mean increases in lumbar spine BMD were greater for men who had less than 6 months of ADT than for those with a longer ADT duration at baseline. The significant treatment effects in these subgroups suggest that denosumab can increase BMD in men at greatest risk for fracture and during the high bone turnover state that accompanies initial ADT.
The study included men with a low baseline BMD (T score at the lumbar spine, total hip or femoral neck less than −1.0), history of an osteoporotic fracture, or age 70 years or older. With denosumab the increases in BMD at all skeletal sites was similar or greater in men younger than 70 years than in older men. As all men younger than 70 years had a low baseline BMD or prior osteoporotic fracture, the treatment effect in younger men is consistent with the observed effect of denosumab in men with high bone turnover.
RANKL is essential for osteoclast formation, function and survival.13 Expression of RANKL is increased in high bone turnover states. Denosumab significantly increased BMD at all skeletal sites in subjects in each tertile of sCTx and TRAP-5b. However, mean increases in BMD were greatest for subjects with the highest levels of sCTx and TRAP-5b at baseline. These observations underscore the pivotal role of RANKL in regulating osteoclast activity and highlight the effectiveness of RANKL inhibition in suppressing bone turnover, even in conditions of rapid bone loss.
Denosumab significantly increased the BMD of the distal radius, a site of predominantly cortical bone, in the overall population and in all subgroups. In contrast, bisphosphonates did not increase the BMD of the distal radius in men receiving ADT19 or in other settings including postmenopausal osteoporosis.20 The observed effects of denosumab on cortical bone may translate into improved bone quality and strength. Consistent with the significant increases in BMD in the distal radius, men who were assigned to denosumab had fewer fractures at the radius than those who were assigned to placebo (2 vs 10 fractures, hazard ratio 0.19, 95% CI 0.04–0.87, p = 0.02).
CONCLUSIONS
We previously demonstrated that denosumab significantly decreased new vertebral fractures, and increased BMD of the lumbar spine, total hip and distal radius in men receiving ADT for prostate cancer. In the present study we showed that denosumab significantly increased BMD for every subgroup including older men and those with lower baseline BMD, higher levels of sCTx and TRAP-5b, and prevalent vertebral fractures. The consistency of these results supports the continued investigation of 60 mg denosumab twice yearly for the prevention and treatment of ADT induced bone loss and fractures.
Acknowledgments
Dr. Ting Chang from Amgen Inc. provided writing support.
Supported by Amgen Inc., Thousand Oaks, California.
Abbreviations and Acronyms
- ADT
androgen deprivation therapy
- BMD
bone mineral density
- BMI
body mass index
- GnRH
gonadotropin-releasing hormone
- RANKL
receptor activator of nuclear factor κB ligand
- sCTx
serum C-telopeptide
- TRAP-5b
tartrate-resistant alkaline phosphatase 5b
Footnotes
Clinical Trial Registration NCT00089674 (www.clinicaltrials.gov).
References
- 1.Loblaw DA, Virgo KS, Nam R, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2007;25:1596. doi: 10.1200/JCO.2006.10.1949. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich A, Aus G, Bolla M, et al. EAU guidelines on prostate cancer. Eur Urol. 2008;53:68. doi: 10.1016/j.eururo.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 4.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360:103. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 5.Bolla M. Adjuvant hormonal treatment with radiotherapy for locally advanced prostate cancer. Eur Urol. 1999;35:23. [PubMed] [Google Scholar]
- 6.Messing EM, Manola J, Sarosdy M, et al. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med. 1999;341:1781. doi: 10.1056/NEJM199912093412401. [DOI] [PubMed] [Google Scholar]
- 7.Michaelson MD, Marujo RM, Smith MR. Contribution of androgen deprivation therapy to elevated osteoclast activity in men with metastatic prostate cancer. Clin Cancer Res. 2004;10:2705. doi: 10.1158/1078-0432.ccr-03-0735. [DOI] [PubMed] [Google Scholar]
- 8.Shahinian VB, Kuo YF, Freeman JL, et al. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 9.Smith MR, McGovern FJ, Zietman AL, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001;345:948. doi: 10.1056/NEJMoa010845. [DOI] [PubMed] [Google Scholar]
- 10.Krupski TL, Smith MR, Lee WC, et al. Natural history of bone complications in men with prostate carcinoma initiating androgen deprivation therapy. Cancer. 2004;101:541. doi: 10.1002/cncr.20388. [DOI] [PubMed] [Google Scholar]
- 11.Lee H, McGovern K, Finkelstein JS, et al. Changes in bone mineral density and body composition during initial and long-term gonadotropin-releasing hormone agonist treatment for prostate carcinoma. Cancer. 2005;104:1633. doi: 10.1002/cncr.21381. [DOI] [PubMed] [Google Scholar]
- 12.Oefelein MG, Ricchiuti V, Conrad W, et al. Skeletal fractures negatively correlate with overall survival in men with prostate cancer. J Urol. 2002;168:1005. doi: 10.1016/S0022-5347(05)64561-2. [DOI] [PubMed] [Google Scholar]
- 13.Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 14.Ellis GK, Bone HG, Chlebowski R, et al. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol. 2008;26:4875. doi: 10.1200/JCO.2008.16.3832. [DOI] [PubMed] [Google Scholar]
- 15.Smith MR, Egerdie B, Hernández Toriz N, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanis JA. Diagnosis of osteoporosis and assessment of fracture risk. Lancet. 2002;359:1929. doi: 10.1016/S0140-6736(02)08761-5. [DOI] [PubMed] [Google Scholar]
- 17.Smith MR, Lee WC, Brandman J, et al. Gonadotropin-releasing hormone agonists and fracture risk: a claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol. 2005;23:7897. doi: 10.1200/JCO.2004.00.6908. [DOI] [PubMed] [Google Scholar]
- 18.Greenspan SL, Coates P, Sereika SM, et al. Bone loss after initiation of androgen deprivation therapy in patients with prostate cancer. J Clin Endocrinol Metab. 2005;90:6410. doi: 10.1210/jc.2005-0183. [DOI] [PubMed] [Google Scholar]
- 19.Greenspan SL, Nelson JB, Trump DL, et al. Effect of once-weekly oral alendronate on bone loss in men receiving androgen deprivation therapy for prostate cancer: a randomized trial. Ann Intern Med. 2007;146:416. doi: 10.7326/0003-4819-146-6-200703200-00006. [DOI] [PubMed] [Google Scholar]
- 20.Hosking D, Chilvers CE, Christiansen C, et al. Prevention of bone loss with alendronate in post-menopausal women under 60 years of age. Early Postmenopausal Intervention Cohort Study Group. N Engl J Med. 1998;338:485. doi: 10.1056/NEJM199802193380801. [DOI] [PubMed] [Google Scholar]