Abstract
BACKGROUND
A decrease in inflammation after cure of atrial arrhythmias suggests that such arrhythmias are pro-inflammatory, and lower inflammatory marker levels in the coronary sinus suggest that atrial arrhythmias result in the intracardiac appropriation of inflammatory cytokines.
OBJECTIVE
To investigate the effect of atrial fibrillation on inflammatory markers drawn from intra and extracardiac chambers.
METHODS
We performed a case control study of 167 AF patients and 207 controls. Blood from intra and extracardiac sites was obtained from a subset of patients undergoing curative AF ablation (n=46).
RESULTS
There were no significant differences in C-Reactive Protein (CRP) or interleukin-6 (IL-6) between those with and without a history of AF. Both were significantly higher when blood was drawn during AF compared to in sinus rhythm: median CRP 3.1 mg/dL (interquartile range [IQR] 1.0–6.0) versus 1.7 mg/dL (IQR 0.7 – 3.9, p=0.0005); median IL-6 2.3 ng/ml (IQR 1.5–3.9) versus 1.5 ng/ml (IQR 0.7–2.5; p=0.007). This finding persisted after adjusting for potential confounders. AF ablation patients in AF exhibited a positive median left atrial minus coronary sinus (LA-CS gradient) CRP (0.3 mg/dL , IQR −0.03–1.1), whereas those in sinus rhythm had a negative median LA-CS gradient CRP (−0.2, IQR −0.8-[−0.02], p=0.01); femoral artery minus femoral vein gradients in AF versus sinus rhythm failed to show any differences.
CONCLUSIONS
AF at the time of the blood draw, rather than a history of AF, was independently associated with inflammation. Differences in trans-cardiac gradients suggest that AF results in sequestration of inflammatory cytokines in the heart.
Keywords: Atrial fibrillation, CRP, C-Reactive Protein, IL-6, inflammation, coronary sinus, left atrium
Introduction
Although atrial fibrillation (AF) is the most common arrhythmia encountered in clinical practice, the etiology remains unknown.1 Left atrial biopsy specimens in individuals with lone AF demonstrate evidence of inflammation,2 and higher C-Reactive Protein (CRP) levels have been associated with AF,3 but the role of inflammation in the genesis, maintenance, or propagation of AF is poorly understood. Data suggesting that inflammation is causal of common AF include a prospective cohort wherein higher CRP levels predicted incident AF4 and a polymorphism in the promoter region of inteleukin-6 (IL-6) shown to be associated with higher IL-6 levels and a greater odds of AF.5 However, a reduction in inflammatory biomarker levels have also been observed after cardioversion of AF6 and curative ablation of atrial flutter,7 suggesting that atrial tachyarrhythmias may be responsible for or at least stimulate a systemic inflammatory process. The reasons for and consequences of such a pro-inflammatory response have not been explored.
While it is known that AF results in atrial remodeling,8 the process by which this occurs remains largely unknown. We previously demonstrated that patients in atrial flutter exhibited significantly lower levels of inflammatory markers in the coronary sinus than the femoral vein, while sinus rhythm patients showed no such difference.7 This may suggest that atrial arrhythmias result in a collection of inflammatory cytokines in the heart, a process that could contribute to adverse remodeling.9 To test the hypothesis that AF results in sequestration of inflammatory mediators in the left atrium, we sought to measure CRP and IL-6 levels in multiple vascular and intracardiac blood pools, correlating cytokine levels with the atrial rhythm at the time of the blood draw.
Methods
Patients
Consecutive consenting patients presenting to a single electrophysiology laboratory for curative AF ablation, AV nodal ablation, and cardioversion of AF were enrolled. Controls included consecutive consenting patients with no history of AF or atrial flutter presenting to the same electrophysiology laboratory for ablation of supraventricular tachycardia (SVT). Controls with no history of arrhythmias were also obtained from a convenience sample of health care personnel employed by the same medical center. Patients were excluded from either group if they were likely to have elevated serologic markers of inflammation independent of the association with their arrhythmia: patients with a history of a myocardial infarction or elevated troponin levels within the previous 3 months, major trauma, surgery, or ablation procedure within the previous 3 months, any chronic inflammatory disease (including chronic rheumatologic diseases requiring immunosuppressive agents), chronic infectious diseases requiring treatment, any active malignancy, any acute rheumatologic or infectious disease (including symptoms of a common upper respiratory tract infection), or any other condition that would be expected to cause a fever, elevated white blood cell count, or elevated erythrocyte sedimentation rate. Those who might have been unable to produce elevated serologic markers were also excluded: patients on immunosuppressive therapy (e.g., steroids), and those with leukopenia of any etiology. Patients with congenital heart disease (corrected or not) were also excluded.
AF patients were categorized as having paroxysmal or persistent AF per the most recent AHA/ ACC/ ESC guidelines.1 All patients provided witnessed and written informed consent. The study was approved by the UCSF Committee on Human Research.
Markers of inflammation
The atrial rhythm during the blood draw was recorded. For AV nodal ablation patients, cardioversion patients and the controls with no arrhythmias, measurements of the inflammatory markers were performed on blood drawn from a peripheral vein. Blood was drawn prior to AV nodal ablation (or pacemaker placement) and/ or cardioversions in patients undergoing those procedures. For AF and SVT patients undergoing curative ablation, all patients had standard electrode catheters placed for electrophysiology study, including a luminal coronary sinus catheter, a His catheter, and an ablation catheter. After the sheaths and catheters had been placed but before any ablation was performed, a total of 40 cc of blood was obtained for study purposes. After discarding 10 cc of blood, blood was withdrawn from the right femoral venous sheath. For the last 46 AF ablation patients enrolled, 10 cc of blood was drawn from each of the following compartments immediately after left atrial access was obtained and prior to any ablations or administration of heparin: the left atrium, coronary sinus, femoral vein, and the femoral artery.
Of note, blood could not always be successfully withdrawn from the luminal coronary sinus catheter (typically attributed to an obstruction of the tip by the coronary sinus wall). In such circumstances, an attempt to initiate adequate blood flow was made by withdrawing the catheter, but the catheter was never withdrawn beyond the mid coronary sinus as visualized using the left anterior oblique fluoroscopic view.
All blood obtained was centrifuged and serum aliquoted and stored in a −80 degree Celsius freezer. Assays of the samples were batched together and run by a technician blinded to the patient’s diagnosis and rhythm. High-sensitivity C-Reactive Protein (CRP) was determined by ELISA (Alpha Diagnostic International, San Antonio, TX) and high-sensitivity interleukin-6 (IL-6) levels were determined by ELISA (R & D systems, Minneapolis, MN). The lower limit of detection for CRP was 0.00035 mg/L and the lower limit of detection for IL-6 was 0.447 pg/ml.
Left atrial volume measurements
All those undergoing curative AF ablation underwent contrast-enhanced 16 detector computed tomographic (CT) imaging studies obtained through the heart in the axial plane, with a slice thickness of 1mm to 1.25mm and no interval. A chest radiologist blinded to patient diagnosis analyzed the studies offline on a picture archiving communication system. By manually outlining the left atrium endocardial contour in one axial slice every 5 slices, the left atrial area was calculated. This area was then multiplied by the thickness of the 5 slices, and the products were added in order to calculate the volume of the left atrium.
Statistical analysis
Normally distributed continuous variables are expressed as means ± SD; continuous variables not normally distributed are presented as medians and interquartile ranges (IQR). Bivariate analyses of normally distributed continuous variables were assessed using t-tests and categorical variables were compared using the χ2 test. Consistent with previous studies, IL-65 and CRP3 were right-skewed. Bivariate analysis of these biomarkers across groups was therefore performed using the Wilcoxon rank sum test; bivariate analyses within groups (e.g., femoral vein versus coronary sinus levels) were performed using the Wilcoxon signed rank test. Kruskal–Wallis rank test was used to assess overall differences in markers across multiple categories (e.g., race). Multivariable analysis was performed with logistic regression analysis for dichotomous outcomes and linear regression analysis for continuous outcomes; covariates/ potential confounders were selected based on both important demographics (e.g., age and gender), face value (paroxysmal versus persistent AF when relevant) and those covariates significantly associated with both the predictors and outcomes of interest with p values <0.10. CRP and IL-6 were logged transformed to facilitate fitting the regression analyses. Two-tailed p values < 0.05 were considered statistically significant.
Results
Three hundred and seventy four patients were enrolled, including 167 patients with AF (105 undergoing curative AF ablation, 50 undergoing cardioversion, and 12 undergoing AV nodal ablation) and 207 controls without a history of AF or atrial flutter (151 SVT ablation patients and 56 controls with no arrhythmias). The baseline characteristics are shown in Table 1. AF patients were older, more likely to be male, white, have hypertension, a history of congestive heart failure, a larger body mass index (BMI), be on a statin, and be on a angiotensin converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB). Based on the criteria described in the Statistical Analysis section, the covariates selected as potential confounders for inclusion in multivariate models are listed in Table 2.
Table 1.
Baseline characteristics of those with and without atrial fibrillation
| Atrial Fibrillation n=167 |
No Atrial Fibrillation n=207 |
p value | |
|---|---|---|---|
| Age | 58 ± 11 | 45 ± 15 | <0.0001 |
| Male | 132 (79%) | 94 (45%) | <0.001 |
| Race | |||
| White | 135 (81%) | 132 (64%) | |
| Black | 2 (1%) | 7 (3%) | |
| Asian | 19 (11%) | 40 (19%) | |
| Latino | 6 (4%) | 19 (9%) | |
| Other | 5 (3%) | 9 (4%) | 0.007 |
| Hypertension | 53 (32%) | 38 (18%) | 0.003 |
| Type 2 Diabetes | 12 (7%) | 17 (8%) | 0.71 |
| Congestive Heart Failure | 10 (6%) | 2 (1%) | 0.006 |
| Coronary Artery Disease | 14 (8%) | 8 (4%) | 0.065 |
|
Left Ventricular Ejection Fraction (%)* |
59 ± 10 | 60 ± 8 | 0.34 |
|
Body Mass Index (kg/m2) |
28 ± 5 | 27 ± 6 | 0.019 |
| Statin Therapy | 46 (28%) | 33 (16%) | 0.006 |
| ACE Inhibitor or ARB | 28 (17%) | 15 (7%) | 0.004 |
ACE denotes angiotensin converting enzyme; ARB denotes angiotensin receptor blocker
A uniform measurement was available in 138 subjects (80 with atrial fibrillation, 58 without atrial fibrillation)
Table 2.
Covariates included in the multivariate models
| History of Atrial Fibrillation versus No History of Atrial Fibrillation |
Paroxysmal versus Persistent Atrial Fibrillation |
Atrial Fibrillation versus Sinus Rhythm During the Blood Draw |
Atrial Fibrillation versus Sinus Rhythm within the Curative Atrial Fibrillation Ablation Group |
|
|---|---|---|---|---|
|
Potential Confounders added to the Multivariate Model |
Not applicable (no multivariate model was assessed given negative bivariate findings) |
Age gender race hypertension CHF statin use ACEI/ARB use |
Age gender race hypertension CHF statin use ACEI/ ARB use AF type |
Atrial volume AF type |
Criteria for selection of covariates is described in the Statistical Analysis section
CHF denotes congestive heart failure
ACEI/ ARB denotes angiotensin converting enzyme inhibitor or angiotensin receptor blocker
AF type denotes paroxysmal versus persistent atrial fibrillation
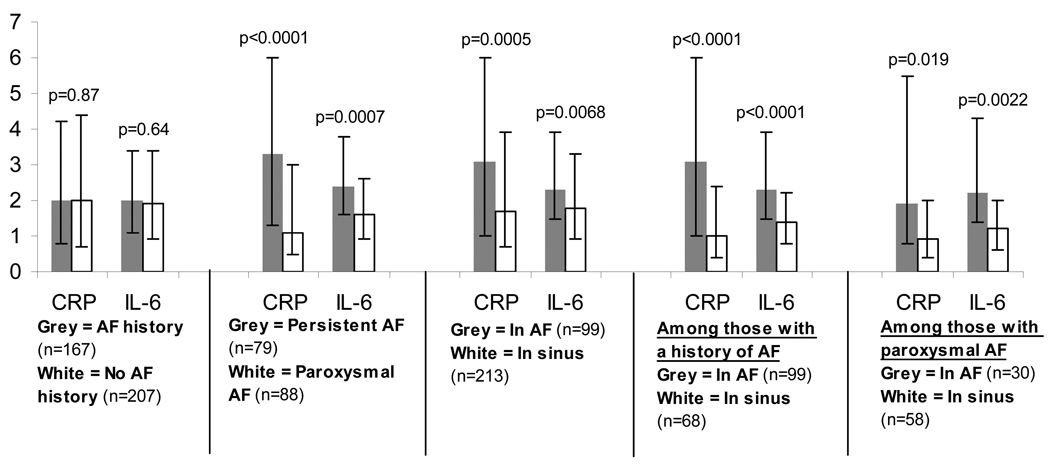
There were no differences in peripheral venous (peripheral or femoral vein) CRP and IL-6 levels between those with and without a history of AF (Figure 1). However, those with persistent AF had significantly elevated CRP and IL-6 levels compared to those with paroxysmal AF (Figure 1). In addition, those in AF at the time of the blood draw had significantly higher CRP and IL-6 levels than those in sinus rhythm, whether analyzing all participants, only participants with a history of AF, or only those participants with paroxysmal AF (Figure 1). In addition, there were no meaningful differences in CRP or IL-6 when comparing patients with a history of AF in sinus rhythm to the control group with no history of arrhythmias.
Figure 1.
Median levels of C-Reactive Protein (CRP) in mg/dL and interleukin-6 (IL-6) in ng/ml compared between those with and without an AF history, paroxysmal versus chronic atrial fibrillation, in atrial fibrillation versus in sinus rhythm at the time of the blood draw, in atrial fibrillation versus in sinus rhythm at the time of the blood draw only among those with a history of atrial fibrillation, and in atrial fibrillation versus in sinus rhythm at the time of the blood draw only among those with a history of paroxysmal atrial fibrillation. Y-error bars denote interquartile ranges.
Although those with persistent AF continued to have both higher CRP and IL-6 levels than those with paroxysmal AF after adjusting for age, gender, race, hypertension, congestive heart failure, statin and ACE inhibitor or ARB use, neither CRP nor IL-6 remained higher in the persistent versus the paroxysmal group after adjusting for the rhythm present during the blood draw. In contrast, after adjusting for the same potential confounders as well as persistent versus paroxysmal AF, the presence of AF at the time of the blood draw remained significantly associated with both higher CRP levels (odds ratio [OR] for log CRP 1.79, 95% confidence interval [CI] 1.20–2.66, p=0.004) and higher IL-6 levels (OR for IL-6 1.69, 95% CI 1.11–2.58, p=0.015). Given the log transformation, this means that a doubling of CRP after adjustment (including adjusting for persistent versus paroxysmal AF) was associated with an approximate 50% greater odds of being in AF and that a doubling of IL-6 was associated with an approximate 40% greater odds of being in AF.
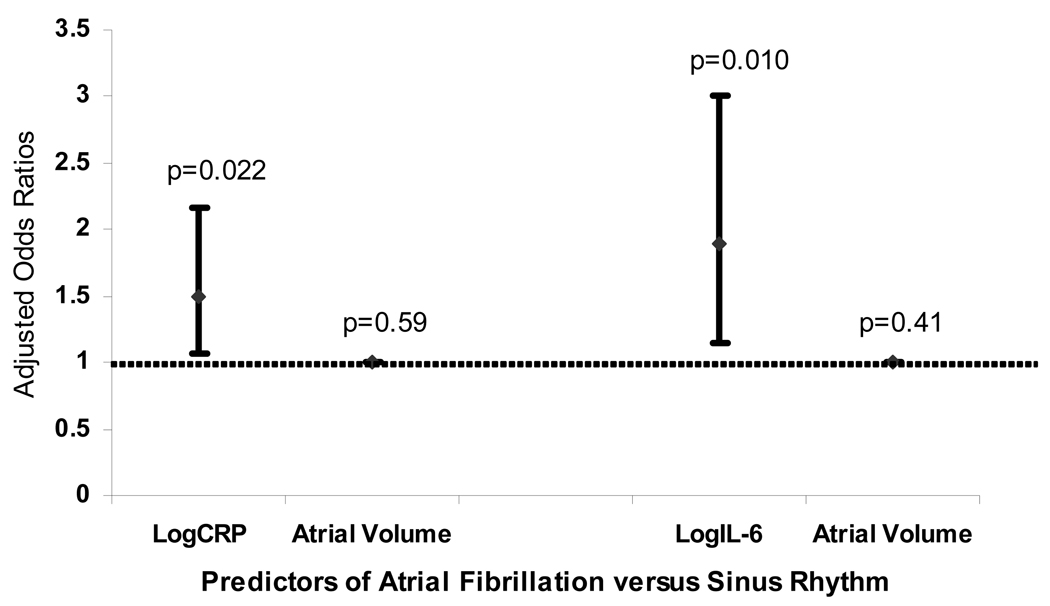
Left atrial volume was measured in all curative AF ablation patients (n=105) a median 1 day (IQR 1–3) prior to the blood draw. Those in AF at the time of the blood draw (n=46) had a larger left atrial volume than those in sinus rhythm at the time of the blood draw (n=59): 130 ± 38 ml versus 111 ± 34 ml, respectively (p=0.005). These two groups otherwise did not differ by demographics or medical histories. After adjusting for left atrial volume and AF type (paroxysmal versus persistent), both CRP and IL-6 remained independently associated with the presence of atrial fibrillation versus sinus rhythm at the time of the blood draw (Figure 2).
Figure 2.
Adjusted odds ratios of logCRP and logIL-6 each adjusted for left atrial volume and a history of paroxysmal versus persistent atrial fibrillation as predictors of the presence of atrial fibrillation versus sinus rhythm at the time of the blood draw among 105 consecutive patients presenting for curative atrial fibrillation ablation. The odds ratios for left atrial volume as a predictor of the presence of atrial fibrillation versus sinus rhythm after adjustment for a history of paroxysmal versus persistent atrial fibrillation and either logCRP or logIL-6 are also shown. Y error bars denote 95% confidence intervals.
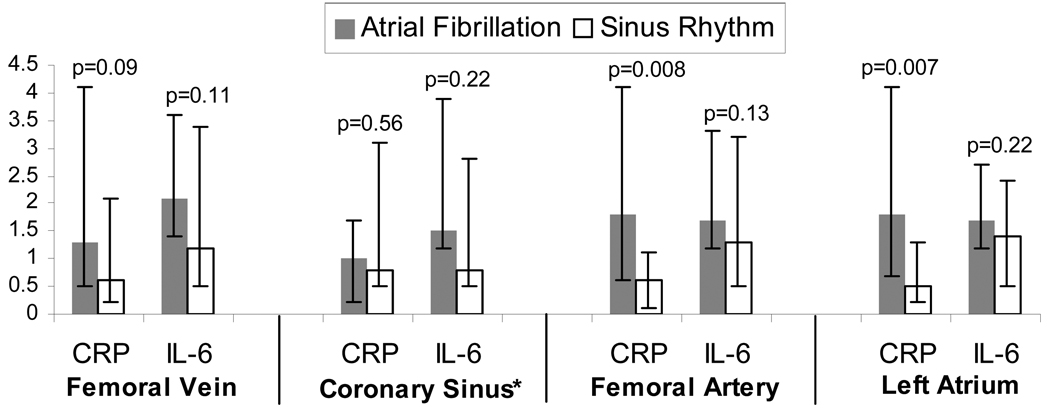
Of the 46 AF ablation patients who had cytokine measurements performed in the femoral vein, femoral artery, coronary sinus, and left atrium, 20 were in AF at the time of the blood draw and the remainder were in sinus rhythm. The AF ablation demographics and past medical histories did not differ between those in AF or sinus rhythm at the time of the blood draw; specifically, there were no significant differences regarding age, gender, race, BMI, or proportions with hypertension, heart failure (n=0), coronary artery disease, statin use, and ACE inhibitor or ARB use (all p values>0.1). Due to difficulty withdrawing blood from the luminal coronary sinus catheter in some patients, coronary sinus blood was available in 10 of the patients in AF and 12 of the patients in normal sinus rhythm.
Inflammatory biomarker concentration differences between blood pools are shown in Figure 3. CRP differences in arterial levels (either femoral artery or left atrial) between those in and out of AF were more pronounced than differences in venous levels.
Figure 3.
Median levels of C-Reactive Protein (CRP) in mg/dL and interleukin-6 (IL-6) in ng/ml compared between atrial fibrillation ablation patients in atrial fibrillation (n=20) versus in sinus rhythm (n=26) at the time of the blood draw assessed from the femoral vein, coronary sinus, femoral artery, and left atrium. Y error bars denote interquartile ranges.
*Coronary sinus levels were available in 10 patients in atrial fibrillation and 12 patients in sinus rhythm.
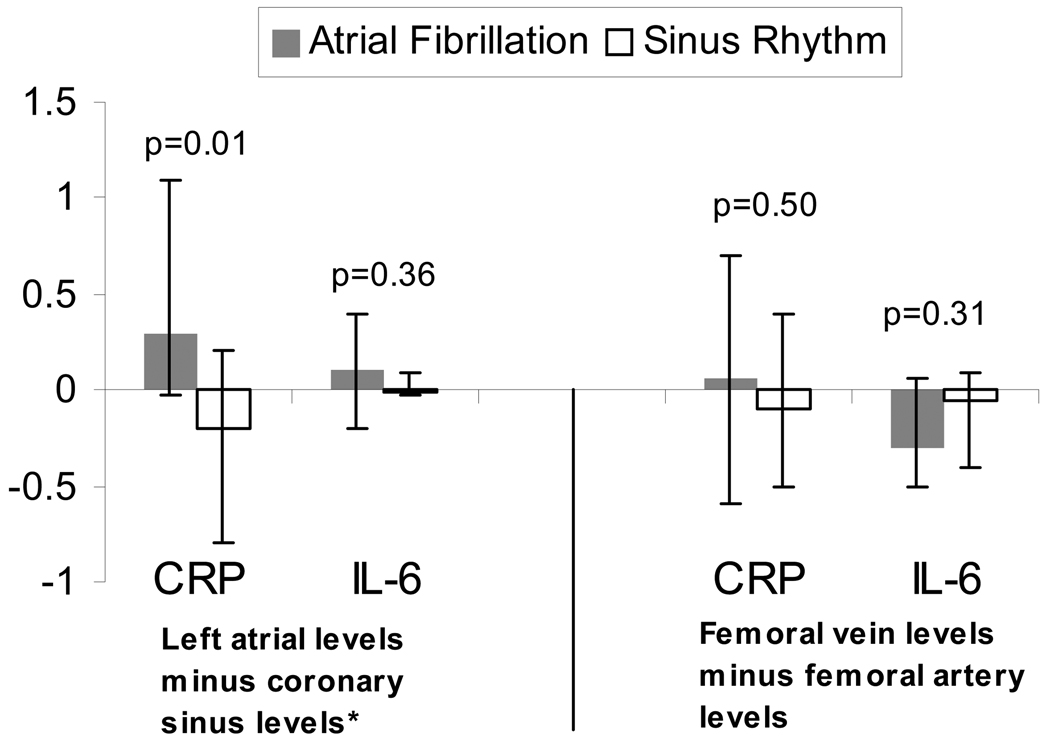
In order to determine the trans-cardiac gradient, the coronary sinus blood levels were subtracted from the left atrial blood levels; the trans-femoral gradient (femoral vein levels subtracted from the femoral artery levels) was used as a control. The median trans-cardiac CRP gradient was positive in those in AF (more CRP in the LA than in the coronary sinus), whereas it was negative in those in sinus rhythm (less CRP in the LA than the coronary sinus), a significant difference (p=0.01, Figure 4). The difference in trans-femoral gradients between those in AF versus sinus rhythm was small and did not reach statistical significance (p=0.50, Figure 4).
Figure 4.
The trans-cardiac (left atrial minus coronary sinus) and trans-femoral (femoral artery minus femoral vein) gradients in median C-Reactive Protein (CRP) and interleukin-6 (IL-6) levels in those in atrial fibrillation (n=20) versus sinus rhythm (n=26) at the time of the blood draw. Y error bars denote interquartile ranges.
* Coronary sinus levels were available in 10 patients in atrial fibrillation and 12 patients in sinus rhythm.
Although none of the IL-6 comparisons were statistically significant, the trends were all consistent with the findings involving CRP. Of interest, whereas the median trans-cardiac IL-6 gradient was positive in those with AF (more IL-6 in the left atrium than the coronary sinus) and negative in those with sinus rhythm, the median trans-femoral gradient was negative (more IL-6 in the vein than the artery) regardless of AF status (Figure 4).
Discussion
Major Study Findings
We showed that the presence of AF at the time of the blood draw determined an elevation of CRP and IL-6, even after restricting to only those with paroxysmal AF and after adjusting for confounders (including paroxysmal versus persistent AF status). Of interest, despite the fact that the AF group as a whole more often exhibited co-morbidities expected to be associated with inflammation (older and more cardiovascular disease), neither CRP nor IL-6 was higher in those with a history of AF (including those in AF and sinus rhythm at the time of the blood draw) compared to controls.
In a subset of patients undergoing AF ablation, we found that the difference between left atrial and coronary sinus CRP levels was significantly greater (with a median positive number) in those in AF at the time of the blood draw than the same difference in CRP in those in sinus rhythm during the blood draw (a median negative number). This means that more CRP is present in the left atrium than is leaving the heart in those in AF, whereas more CRP is leaving the heart than is present in the left atrium during sinus rhythm (even in patients with a history of AF). Although not statistically significant, IL-6 levels trended in the same direction. These data, suggesting that AF results in sequestration of inflammatory cytokines in the heart, are consistent with our previous finding that both CRP and IL-6 levels were significantly lower in the coronary sinus than peripheral veins in patients in atrial flutter at the time of the blood draw.7
Although inflammation has been associated with AF, the cause and effect nature of the relationship is just beginning to be elucidated. While CRP may predict the onset of AF,4 the marker also significantly decreases after cardioversion of AF6 and curative ablation of atrial flutter.7 A previous study restricted to lone AF patients in China suggested that the rhythm at the time of blood draw determined an elevated CRP.10 How AF may contribute to inflammation and whether pro-inflammatory effects of the arrhythmia have any consequence remain unknown. We previously showed that a higher left ventricular end-diastolic pressure was independently associated with a higher CRP level,11 but the signaling pathways involved have not been determined.
One previous study examined inflammatory markers in several intra and extra-cardiac compartments (including the left and right upper pulmonary veins) failed to demonstrate any difference between those with and without a history of AF, but the rhythm present at the time of the blood draw was not taken into account and gradients across compartments (such as what was done in this study) were not reported.12 We found a significant trans-cardiac gradient, with a surplus of CRP left in the heart, in patients in AF and the opposite gradient in those in sinus rhythm. Examination of the trans-femoral gradient (femoral artery to femoral vein) failed to demonstrate the same pattern, regardless of the atrial rhythm. This suggests that AF may involve a collection of inflammatory cytokines in the heart, presumably in the left atrium.
Although CRP is often considered to be a simple marker of inflammation, the protein itself is pro-inflammatory and may cause cellular damage.9, 13–17 Similarly, IL-6, which, although not statistically significant, trended in the same direction (a median surplus in the left atrium in AF and a median surplus in the coronary sinus in sinus rhythm), is also pro-inflammatory and has been shown to be associated with left atrial remodeling.5, 9, 18–21 It is thought that “AF begets AF”, i.e. , the longer an individual remains in AF, the more difficult it is to convert to and maintain sinus rhythm.1 Many studies from clinical experience and animal models of AF have confirmed the importance of both structural and electrical modeling in this arrhythmia.22, 23 Our results implicate the participation of inflammation as a modulating factor in AF arrhythmogenesis. It may be that inflammation begets AF, and AF begets inflammation, leading to a continuing spiral. Importantly, the origin of increased inflammation in AF remains unknown. Presumably, the arrhythmia stimulates this process, but the pathways responsible have yet to be elucidated. Consistent with our previous findings related to coronary sinus levels in atrial flutter,7 our intra-cardiac measurements suggest that the elevated CRP and IL-6 levels do not originate in the heart.
Study Limitations
This study has several limitations. First, although the finding that the rhythm present at the time of blood draw was associated with elevated inflammatory markers supports the notion that the rhythm causes the inflammation, it is not definitive proof. In fact, there are ample data from animal models,24 post-operative AF25 and common AF4, 5 that inflammation is likely a cause of atrial arrhythmias—as above, inflammation may be both a cause and effect. Second, the number of patients in whom left atrial and coronary sinus blood was obtained is relatively small. However, while this will reduce power (and may therefore explain a lack of statistically significant findings regarding IL-6), this should not result in false positive associations. In short, a small sample or lack of power would not explain the positive findings regarding trans-cardiac CRP gradients. In fact, the statistically significant findings, despite the small numbers, likely accentuate the positive results. The observed differences do not absolutely prove that an intracardiac sequestration of these markers is taking place; however, our findings are consistent with our a priori hypothesis (based on previous studies) and would seem to be the most likely explanation for the measured trans-cardiac gradients. Third, many of the AF patient characteristics were different than the controls; however, while these differences would likely increase differences in inflammatory markers (independent of the arrhythmia), no differences between those with and without a history of AF were seen. As the significant differences in inflammatory markers persisted when restricted to groups that were more similar (such as within AF groups) and after multivariate adjustment, it does not appear that baseline differences between those with and without a history of AF were responsible for our positive findings.
Conclusions
CRP and IL-6 are significantly elevated during AF, but not necessarily in patients with a history of AF. During AF, there appears to be intracardiac sequestration of inflammatory cytokines, potentially pointing to an important mechanism by which atrial remodeling occurs.
Acknowledgments
Funding Sources: This work was made possible by grant number KL2 RR024130 (G.M.M.) from the National Center for Research Resources (NCRR), a component of the NIH, Bethesda, MD; and the American Heart Association Western States Affiliate Beginning Grant-in-Aid Award (G. M. M.), Menlo Park, CA.
Abbreviations
- AF
atrial fibrillation
- ARB
angiotensin receptor blocker
- BMI
body mass index
- CI
confidence interval
- CT
computed tomographic
- CS
coronary sinus
- CRP
C-Reactive Protein
- IL-6
interleukin-6
- IQR
interquartile range
- LA
left atrium
- SVT
supraventricular tachycardia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author disclosures/ potential conflicts of interest: None
Conflict of Interest Disclosures: none
References
- 1.Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation) J Am Coll Cardiol. 2006;48:854–906. doi: 10.1016/j.jacc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–1184. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 3.Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–2891. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 4.Aviles RJ, Martin DO, Apperson-Hansen C, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 5.Marcus GM, Whooley MA, Glidden DV, Pawlikowska L, Zaroff JG, Olgin JE. Interleukin-6 and atrial fibrillation in patients with coronary artery disease: data from the Heart and Soul Study. Am Heart J. 2008;155:303–309. doi: 10.1016/j.ahj.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kallergis EM, Manios EG, Kanoupakis EM, et al. The role of the post-cardioversion time course of hs-CRP levels in clarifying the relationship between inflammation and persistence of atrial fibrillation. Heart. 2008;94:200–204. doi: 10.1136/hrt.2006.108688. [DOI] [PubMed] [Google Scholar]
- 7.Marcus GM, Smith LM, Glidden DV, et al. Markers of inflammation before and after curative ablation of atrial flutter. Heart Rhythm. 2008;5:215–221. doi: 10.1016/j.hrthm.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dittrich HC, Pearce LA, Asinger RW, et al. Left atrial diameter in nonvalvular atrial fibrillation: An echocardiographic study. Stroke Prevention in Atrial Fibrillation Investigators. Am Heart J. 1999;137:494–499. doi: 10.1016/s0002-8703(99)70498-9. [DOI] [PubMed] [Google Scholar]
- 9.Kourliouros A, Savelieva I, Kiotsekoglou A, Jahangiri M, Camm J. Current concepts in the pathogenesis of atrial fibrillation. Am Heart J. 2009;157:243–252. doi: 10.1016/j.ahj.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Yao SY, Chu JM, Chen KP, et al. Inflammation in lone atrial fibrillation. Clin Cardiol. 2009;32:94–98. doi: 10.1002/clc.20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah SJ, Marcus GM, Gerber IL, et al. High-sensitivity C-reactive protein and parameters of left ventricular dysfunction. J Card Fail. 2006;12:61–65. doi: 10.1016/j.cardfail.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Liuba I, Ahlmroth H, Jonasson L, et al. Source of inflammatory markers in patients with atrial fibrillation. Europace. 2008;10:848–853. doi: 10.1093/europace/eun111. [DOI] [PubMed] [Google Scholar]
- 13.Singh U, Devaraj S, Jialal I. C-reactive protein stimulates myeloperoxidase release from polymorphonuclear cells and monocytes: implications for acute coronary syndromes. Clin Chem. 2009;55:361–364. doi: 10.1373/clinchem.2008.109207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagaoka T, Kuo L, Ren Y, Yoshida A, Hein TW. C-reactive protein inhibits endothelium-dependent nitric oxide-mediated dilation of retinal arterioles via enhanced superoxide production. Invest Ophthalmol Vis Sci. 2008;49:2053–2060. doi: 10.1167/iovs.07-1387. [DOI] [PubMed] [Google Scholar]
- 15.Liuzzo G, Santamaria M, Biasucci LM, et al. Persistent activation of nuclear factor kappa-B signaling pathway in patients with unstable angina and elevated levels of C-reactive protein evidence for a direct proinflammatory effect of azide and lipopolysaccharide-free C-reactive protein on human monocytes via nuclear factor kappa-B activation. J Am Coll Cardiol. 2007;49:185–194. doi: 10.1016/j.jacc.2006.07.071. [DOI] [PubMed] [Google Scholar]
- 16.Singh U, Devaraj S, Dasu MR, Ciobanu D, Reusch J, Jialal I. C-reactive protein decreases interleukin-10 secretion in activated human monocyte-derived macrophages via inhibition of cyclic AMP production. Arterioscler Thromb Vasc Biol. 2006;26:2469–2475. doi: 10.1161/01.ATV.0000241572.05292.fb. [DOI] [PubMed] [Google Scholar]
- 17.Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102:2165–2168. doi: 10.1161/01.cir.102.18.2165. [DOI] [PubMed] [Google Scholar]
- 18.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94:1543–1553. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- 19.Birks EJ, Burton PB, Owen V, et al. Elevated tumor necrosis factor-alpha and interleukin-6 in myocardium and serum of malfunctioning donor hearts. Circulation. 2000;102:III352–III358. doi: 10.1161/01.cir.102.suppl_3.iii-352. [DOI] [PubMed] [Google Scholar]
- 20.Janssen SP, Gayan-Ramirez G, Van den Bergh A, et al. Interleukin-6 causes myocardial failure and skeletal muscle atrophy in rats. Circulation. 2005;111:996–1005. doi: 10.1161/01.CIR.0000156469.96135.0D. [DOI] [PubMed] [Google Scholar]
- 21.Psychari SN, Apostolou TS, Sinos L, Hamodraka E, Liakos G, Kremastinos DT. Relation of elevated C-reactive protein and interleukin-6 levels to left atrial size and duration of episodes in patients with atrial fibrillation. Am J Cardiol. 2005;95:764–767. doi: 10.1016/j.amjcard.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 22.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 23.Corradi D, Callegari S, Maestri R, Benussi S, Alfieri O. Structural remodeling in atrial fibrillation. Nat Clin Pract Cardiovasc Med. 2008;5:782–796. doi: 10.1038/ncpcardio1370. [DOI] [PubMed] [Google Scholar]
- 24.Page PL, Plumb VJ, Okumura K, Waldo AL. A new animal model of atrial flutter. J Am Coll Cardiol. 1986;8:872–879. doi: 10.1016/s0735-1097(86)80429-6. [DOI] [PubMed] [Google Scholar]
- 25.Gaudino M, Andreotti F, Zamparelli R, et al. The −174G/C interleukin-6 polymorphism influences postoperative interleukin-6 levels and postoperative atrial fibrillation. Is atrial fibrillation an inflammatory complication? Circulation. 2003;108 Suppl 1:II195–II199. doi: 10.1161/01.cir.0000087441.48566.0d. [DOI] [PubMed] [Google Scholar]