Abstract
Prostate cancer is the most commonly diagnosed noncutaneous cancer in men in the United States. Treatment of men with prostate cancer commonly involves surgical, radiation, or hormone therapy. Most men with prostate cancer live for many years after diagnosis and may never suffer morbidity or mortality attributable to prostate cancer. The short-term and long-term adverse consequences of therapy are, therefore, of great importance. Adverse effects of radical prostatectomy include immediate postoperative complications and long-term urinary and sexual complications. External beam or interstitial radiation therapy in men with localized prostate cancer may lead to urinary, gastrointestinal, and sexual complications. Improvements in surgical and radiation techniques have reduced the incidence of many of these complications. Hormone treatment typically consists of androgen deprivation therapy, and consequences of such therapy may include vasomotor flushing, anemia, and bone density loss. Numerous clinical trials have studied the role of bone antiresorptive therapy for prevention of bone density loss and fractures. Other long-term consequences of androgen deprivation therapy may include adverse body composition changes and increased risk of insulin resistance, diabetes, and cardiovascular disease. Ongoing and planned clinical trials will continue to address strategies to prevent treatment-related side effects and improve quality of life for men with prostate cancer.
OVERVIEW
Prostate cancer is diagnosed in over 186,000 men in the United States and accounts for more than 28,000 deaths annually.1 Numerous modalities are involved in treating men with this condition, and a multidisciplinary approach to treatment is often beneficial. For localized prostate cancer, the most common treatments are radical prostatectomy (RP) or whole prostate radiation therapy. In more advanced disease, medical therapy is often used, and the mainstay of treatment is androgen deprivation therapy (ADT). The widespread use of each of these approaches has increased the relevance and importance of characterizing and treating the potential complications of treatment. This article will focus on management of complications from surgery, radiation, or medical therapy of prostate cancer.
I. SURGICAL COMPLICATIONS IN THE TREATMENT OF PROSTATE CANCER
Introduction
RP is a standard definitive therapy for localized prostate cancer. Surgical techniques have continued to improve, and technology and surgical training have expanded the urologist's armamentarium in this arena. Options currently include open retropubic, laparoscopic (with or without robotic assistance), or perineal approaches. These procedures, in experienced hands, appear to yield equivalent oncologic outcomes.2–9 The ultimate decision as to which surgical approach should be used depends on several factors, including surgeon skill and comfort level with a particular approach; the patient's body habitus; comorbidities; previous surgical history; and, perhaps most importantly, the preoperative Gleason score and preoperative staging.
In the last 25 years, there have been considerable advances in operative and postoperative care, as well as significant modifications in the surgical technique itself. A better understanding of pelvic anatomy, including the vasculature and innervation surrounding the prostate, has led to a significant decrease in the mortality and relative morbidity for patients undergoing RP.10,11 Furthermore, improved surgical training and the establishment of centers specializing in minimally invasive surgery have increased the popularity of laparoscopic (or robotic-assisted laparoscopic) radical prostatectomy (LRP) for the treatment of localized prostate cancer. Minimally invasive techniques such as LRP have also led to a decreased length of stay, shorter convalescence, and improved cosmesis.
Regardless of the surgical approach used, there are complications inherent to the procedure itself. Complications inherent to LRP and laparoscopy itself are beyond the scope of this review and have been covered in detail elsewhere.12 Overall, complications can be divided into those that occur during and immediately after surgery (perioperative) and those that occur late after surgery (postoperative).
Perioperative Complications
Injury During Patient Positioning
Patient positioning during RP requires meticulous attention to detail in order to prevent serious injury. Potential complications solely related to prolonged extrinsic compression on tissues are not uncommon.13 During patient positioning, care should be taken to pad all pressure points, including the sites of the ulnar and common peroneal nerves. Care should be taken not to hyperextend the shoulders or hips, as this may cause brachial plexus or lumbar plexus injuries.14,15 The eyes should also be protected by the anesthesia team since corneal abrasions and conjunctivitis from corneal irritation by saliva have been reported in some series.14
Peripheral Neurological Injuries
The most frequent cause of neurological injuries during RP, occurring in 0.5% to 1.4% of cases,12 is direct damage to nerves or traction injuries from inadequate patient positioning (see previous section). Injury to the obturator nerve is a known complication during pelvic lymphadenectomy in both RP and LRP and may result in failure of leg adduction. Care must be taken to identify visually the obturator nerve before ligation and cutting of the inferior aspect of the node bundle. If the nerve is ligated but not cut, removal of the clip is all that is necessary, although most of these patients will be symptomatic from the crush injury. Transection of the obturator nerve during RP can be repaired by reapproximating the nerve sheath with sutures.16
Bowel Complications
Small and large bowel injuries (excluding rectal injuries) are rare during radical retropubic prostatectomy (RRP) and radical perineal prostatectomy (RPP) since these are “extraperitoneal” procedures. Bowel injuries leading to perforation during LRP, however, are more common (0.5% to 1.5%) and can occur during trocar placement, during instrument exchange, or during tissue dissection; bowel injury can also result from thermal injury due to electrocautery devices. In LRP, the lateral large bowel (sigmoid and cecum) is vulnerable to injury during placement of the lateral ports and when instruments are being changed. Thermal tissue damage from a harmonic scalpel or electrocautery device, as well as arcing of the monopolar current to adjacent organs, is a common etiology of bowel injury and accounts for more than half of all laparoscopic bowel injuries.17 The most important aspect regarding bowel injuries is that they need to be identified intraoperatively. Failure to recognize these injuries when they occur can have significant morbidity and mortality. In fact, in a large study of laparoscopic entry access injuries, Chandler et al showed that delayed recognition and diagnosis of these injuries, along with age greater than 59 years, were significant predictors of fatal outcomes.18 They also noted that unrecognized bowel injuries were significantly more likely to cause death than injuries to major retroperitoneal vessels. If bowel injury is suspected in a postoperative patient, abdominal pelvic computed tomography (CT) with oral contrast is a reliable method for diagnosis.19 Alternatively, a diagnostic laparoscopy can be performed with high diagnostic accuracy in 92% to 97% of cases.20,21
Rectal Injury
Injury to the rectum is a specific type of bowel injury that should be considered separately. Rectal injury during RP has been reported in 0.3% to 3.8% of cases.10,22 During RRP and RPP, most rectal injuries occur while transecting the rectourethralis muscle.23,24 In a series of 1,000 LRP, Guillonneau et al reported 13 rectal injuries, 10 of which occurred during dissection of the posterior surface of the prostate at the apex.23 These types of injuries can also occur from thermal or electrical injury to the rectum at any point during an LRP. The main point, as in other bowel injuries, is that these need to be recognized at the time they occur in the operating room in order to minimize morbidity. Once these injuries are diagnosed, the edges of the defect should be clearly identified and closed in 2 layers.25,26 Although there is no clear evidence for this, a diverting colostomy should be considered in cases of gross fecal spillage, previous radiation, a urethrovesical anastomosis under tension, or in a patient who is chronically treated with steroids.27,28 The consequences of a missed rectal injury or of inappropriate repair can have significant morbidity and are covered elsewhere.25,29
Bleeding
Some degree of bleeding can be expected for any surgical procedure, and at which point operative bleeding becomes a “complication” is subject to debate. Obviously, an unplanned or unrecognized injury to a blood vessel or an injury that requires any additional intervention beyond that planned during a procedure constitutes a complication. During LRP, inadvertent injury to vessels usually occurs during trocar placement, during pelvic lymphadenectomy, or during instrument exchange. Although these types of injuries are rare in contemporary series, ranging from 0% to 1%,30–33 recognition of vessel injury during surgery is very important. A theoretical advantage of minimally invasive surgery is decreased bleeding. A reliable description of bleeding as a complication may be blood transfusion rates. Both estimated blood loss (EBL) and transfusion rates vary widely in reported LRP series, with EBL ranging from 150 mL to 1100 mL and transfusion rates ranging from 0% to 31%.31,34–40 Despite these differences, the average EBL and average transfusion rates for LRP seem to be less than those of RRP.41 Furthermore, the EBL seems to decrease with the surgeon's experience.31
The 2 major sites of bleeding during RP are from the dorsal venous plexus and from the prostatic pedicles. Adequate ligation of the former and meticulous hemostasis during the pedicle dissection will obviate most serious hemorrhage. Unrecognized venous bleeding during LRP may lead to pelvic hematoma formation. Larger hematomas may lead to recurrent fevers, infection, voiding symptoms, urinary retention, pelvic pain, anastomotic disruption, and bladder neck contracture.42,43 In these instances, it may be appropriate to drain these hematomas either through a percutaneous approach or through a small infraumbilical incision. Although not technically a bleeding complication, lymphocele is a recognized complication of lymphadenectomy as part of RP and has been noted to occur in 0.1% to 1% of patients.44 If these lead to infection with symptoms such as pain or fever, percutaneous drainage is also a good treatment modality.
Ureteral Injury
Ureteral complications have been reported in 0% to 1% of patients undergoing LRP22,45 and are even less common during RRP and RPP. The ureter may be injured by thermal or electrical injury14 or by suture placement near or through the ureteral orifice at the time of urethrovesical anastomosis.
Ideally, ureteral injuries should be recognized at the time they occur. In these cases, the ureter can be repaired primarily over a ureteral stent, or a ureteroneocystostomy can be performed. Both of these techniques have been performed laparoscopically.46,47 Unrecognized ureteral orifice obstruction or damage will usually require anastomotic revision or temporary percutaneous nephrostomy tube placement. The clinical picture of an unrecognized ureteral injury depends on the extent of the injury and may include nonspecific signs and symptoms such as nausea, fever, or abdominal pain. If the ureter is inadvertently ligated, the corresponding kidney will be obstructed, leading to hydronephrosis, flank pain, decreased urine output, and a rise in the serum creatinine. If the ureter is divided or an injury leads to ureteral perforation, urinary ascites will ensue, resulting in abdominal pain, peritonitis, azotemia from peritoneal absorption of urine, or an increase in fluid output from the surgical drain. If a urine leak is suspected, the fluid from the drain may be analyzed for creatinine. A 2-fold or greater increase in the drain creatinine as compared with serum creatinine may indicate that a urine leak is present.
Bladder Injury
Bladder injuries are rare in RRP and RPP and occur almost exclusively in LRP during the dissection of the retrovesical space to gain access to the seminal vesicles or during dissection of the retropubic space during a transperitoneal approach. In their series of 567 patients, Guillonneau et al reported 9 bladder injuries, all of which occurred during dissection of the retropubic space.48 These were identified intraoperatively and repaired without sequelae. Bladder injury during retropubic dissection is a particular risk if the patient has undergone a previous laparoscopic prosthetic mesh inguinal herniorrhaphy.49
Thromboembolic Complications
Deep venous thrombosis and pulmonary embolus are infrequent but serious complications of RP. Perioperative prophylaxis with low-molecular weight heparin and/or pneumatic compression stockings, as well as early postoperative ambulation, have decreased the frequency of these complications even further. Early series of RRP reported the rate of these complications to be as high as 5%.50 Current LRP series report the frequency of these complications at 0% to 1%. In cases where thromboembolic events were reported, patients were usually confined to bed for prolonged periods of time, usually because of other complications (bleeding, peritonitis, anastomotic leak).31
Mortality
Mortality after RP is low and has been estimated at 0.5% in recent series.51 It seems likely that the implementation of routine prostate-specific antigen testing has led to prostate cancer diagnoses in younger and healthier men who would be less prone to having serious cardiovascular complications. Mortality rates seem to be directly correlated to patient classification by the American Society of Anesthesiologists system.52
Anastomotic Complications
Urine leak into the pelvis (or the peritoneum, depending on surgical approach) from the urethrovesical anastomosis can be observed as an early or late complication. Although it is seen in up to 13%12 of patients after LRP, it is less common in RRP and RPP. The best method to prevent a leak from the anastomosis is to ensure intravesical positioning of the bladder catheter and to test the anastomosis for watertightness with saline irrigation intraoperatively. If a leak is detected postoperatively, this should be managed by continuing the closed suction drain and bladder drainage until the leak closes.
Bladder neck contracture is usually a late complication of RP and has been reported in approximately 0.5% to 2% of LRP patients and 0.5% to 17.5% of RRP patients.53 Prior transurethral resection of the prostate, excessive intraoperative blood loss, and urinary extravasation at the anastomotic site seem to be risk factors for bladder neck contracture.54 Most anastomotic strictures require cold-knife incision and/or periodic dilation to maintain adequate urine flow.
Urinary and Sexual Function
Perhaps the 2 most feared complications of RP from a patient's perspective are urinary complications and impotence. One difficulty in assessing these complications is that there are no precise definitions of incontinence or impotence in the literature. Furthermore, centers have obtained their outcomes data by widely divergent methods, including questionnaires, telephone interviews, or surgeon assessment. Lastly, continence and potency rates are improved when the patient population is highly selected (younger and with fewer comorbidities).
Urinary incontinence can be a devastating complication following prostatectomy. Given the difficulty in measuring this clinical outcome, however, continence results in the literature vary widely. Most centers with a high-volume experience in this procedure report continence rates between 80% and 95% (Table 1).
TABLE 1.
Continence Rates in Contemporary Laparoscopic Radical Prostatectomy and Retropubic Prostatectomy Series
| Approach | Patients (n) | Continence Rate at 12 Months (%) | Reference |
|---|---|---|---|
| RRP | 500 | 92 | Lepor H, Kaci L, Xue X55 |
| RRP | 50 | 64 | Artibani W, Grosso G, Novara G, et al56 |
| LRP | 71 | 40 | Artibani W, Grosso G, Novara G, et al56 |
| LRP | 550 | 82 | Guillonneau B, Gupta R, El Fettouh H, et al23 |
| LRP | 235 | 90 | Salomon L, Anastasiadis AG, Katz R, et al57 |
| LRP | 500 | 84 | Rassweiler J, Schulze M, Teber D, et al45 |
| LRP | 300 | 90 | Stolzenburg JU, Truss MC, Bekos A, et al58 |
| LRP | 106 | 85 | Erdogru T, Teber D, Frede T, et al59 |
| LRP | 200 | 95 | Eden CG, King D, Kooiman GG, et al60 |
Abbreviations: RRP, radical retropubic prostatectomy; LRP, laparoscopic radical prostatectomy.
Several studies have examined the factors associated with improved postoperative continence.61–63 In multivariate analyses of pre- and postoperative factors, significant factors that improved chances of postoperative continence were younger age, preservation of both neurovascular bundles, absence of an anastomotic stricture, preservation of functional urethral length, eversion of the bladder neck, and a smaller prostate volume.61,64–68 If incontinence is present, pelvic floor exercise, as well as biofeedback, may be beneficial.69 Improvement in urinary continence may occur 1 or even 2 years following surgery. Therefore, invasive treatments for incontinence should be delayed for at least 1 year after prostatectomy.70 Once incontinence is noted to persist, several modalities, including cystoscopy, uroflow, and urodynamics, can be implemented to diagnose the cause and exclude potentially treatable etiologies. Final treatment decisions can then be based on findings from these studies. Surgical and conservative options for postprostatectomy incontinence are covered elsewhere.71,72
Before the development of an anatomic approach to RP, virtually all patients developed impotence following RP. The realization that impotence arose from damage to an anatomically distinct network of autonomic nerves to the corpora cavernosa led to modifications in surgical technique, with vastly improved potency outcomes.11 In 1991, Quinlan evaluated the recovery of sexual function in 600 consecutive men.73 Of the 503 patients who were potent preoperatively and followed up for a minimum of 18 months, 68% were potent postoperatively. Three factors were identified that correlated with return of sexual function: younger age, lower clinical and pathologic stage, and surgical technique (preservation or excision of the neurovascular bundle). Of note, patients who are immediately impotent after surgery may develop erections adequate for intercourse up to 24 to 48 months after surgery.74 Furthermore, with the use of phosphodiasterase inhibitors such as sildenafil citrate, up to 80% of men who had no erections after RP will eventually recover.75 In a questionnaire study, Walsh et al found that the recovery of sexual function occurred gradually after surgery, with 38% of patients potent at 3 months, 54% at 6 months, 73% at 12 months, and 86% at 18 months.76 The recovery of sexual function also correlated with the age of the patient at the time of surgery and was 100% in men aged 30 to 39 years, 88% in men aged 40 to 49 years, 90% in men aged 50 to 59 years, and 75% in men aged 60 to 67 years. These observations, they concluded, were secondary to the fact that in this era of prostate-specific antigen screening, more men are presenting at a younger age with organ-confined tumors that are amenable to nerve-sparing surgery.
Here we present the published data for both of these parameters in contemporary series of RRP and LRP.8,55–60,76–80 Table 1 shows urinary continence rates for RRP and LRP, defined as no need for any pads or “protection.” Table 2 shows the sexual potency rates. As can be seen, the rates of potency and continence are comparable for both techniques, especially when the mean patient age is considered. Essentially, postoperative results regarding continence and sexual potency are multifactorial and depend on preoperative function, coexisting disease, and social habits (tobacco and drug use).
TABLE 2.
Sexual Potency Rates in Contemporary Laparoscopic Radical Prostatectomy and Retropubic Prostatectomy Series
| Approach | Patients (n) | Follow Up (Months) | Mean Patient Age (Years) | Potency (%) | Definition | Nerve Sparing | Reference |
|---|---|---|---|---|---|---|---|
| LRP | 5,824 | 12 | 64 | 53 | N/A | Bilateral | Rassweiler J, Stolzenburg J, Sulser T, et al76 |
| LRP | 200 | 12 | 62 | 71 | Erections | N/A | Eden CG, King D, Kooiman GG, et al72 |
| LRP | 177 | 12 | N/A | 76 | Intercourse | Bilateral | Su LM, Link RE, Bhayani SB, et al80 |
| LRP | 26 | 12 | 63 | 65 | N/A | Bilateral | Roumeguere T, Bollens R, Vanden Bossche M, et al8 |
| RRP | 33 | 12 | 64 | 55 | N/A | Bilateral | Roumeguere T, Bollens R, Vanden Bossche M, et al8 |
| LRP | 143 | 12 | 64 | 88 | Erections | Bilateral | Katz R, Salomon L, Hoznek A, et al74 |
| RRP | 64 | 18 | 57 | 86 | Intercourse | Bilateral | Walsh PC61 |
| RRP | 1,291 | 18 | 63 | 44 | Intercourse | Bilateral | Stanford JL, Feng Z, Hamilton AS, et al78 |
Abbreviations: LRP, laparoscopic radical prostatectomy; RRP, radical retropubic prostatectomy; N/A, not available.
The fundamental principles of meticulous tissue handling and avoiding electrocautery during the neurovascular dissection hold true for both RRP and LRP and should minimize injury to these structures, hopefully resulting in optimal potency and continence rates.
II. COMPLICATIONS OF RADIATION THERAPY FOR PROSTATE CANCER
Introduction
Radiation administration is an effective means of prostate cancer treatment that provides cure rates comparable with those of RP. While treatment outcomes may be similar, radiation therapy possesses its own unique set of side effects. Additionally, the side-effect profiles differ depending on the method of radiation administration, namely external beam radiation therapy (EBRT) or interstitial brachytherapy (IB or radioactive seed implantation). While most published assessments of morbidity have used physician reports of significant side effects, more recent studies have focused on patient-driven indices of morbidity. Their analysis has been given greater power by the recent use of health-related quality-of-life (HRQOL) tools. No longer are these side effects viewed in abstraction, but rather they are viewed in the context of the individual whom they affect, painting a clearer picture of the nature and consequences of radiation-associated morbidity.
This section focuses on the side-effect profiles of EBRT and IB as separate entities and concludes with recent quality-of-life analyses relating to these modalities. Medical innovation continues to improve the overall quality and accuracy of radiation treatment. Mature data focuses its lens on therapies that have since been refined and improved. With this in mind, it is hoped that these advances will lessen the morbidity experienced by men treated today.
EBRT
EBRT for prostate cancer consists of the administration of ionizing radiation produced by a linear accelerator. The head of the accelerator rotates around the patient, allowing the radiation beam multiple angles of entry. The resultant effect is an intersection of multiple radiation beams at a specific site within the body. Planning of therapy begins with a CT scan that allows the physician to map out the prostate, seminal vesicles, and draining lymph nodes and to construct a 3D anatomic model. This map guides the planner in the best beam arrangement to provide adequate dose to the sites of disease while attempting to minimize dose to adjacent normal structures. Men with early-stage prostate cancer are treated to the entire prostate and the caudal portion of the seminal vesicles. Men with more advanced disease often receive radiation to the draining pelvic lymph nodes, at the discretion of the treating physician. Daily prostate position is usually ascertained by transabdominal ultrasound or by plain x-rays to identify implanted, inert, fiducial markers. Accounting for daily position allows for the use of tighter radiation fields and reduced normal tissue volumes within the high-dose area.
In order to adequately treat the prostate, adjacent normal structures are, by necessity, irradiated. The bladder neck (superior), penile bulb (inferior), and the anterior rectal wall (posterior) all receive significant doses of radiation (Figure 1). In addition, the prostatic urethra and neurovascular bundles lie within the treatment field. Finally, if pelvic nodes are irradiated, the bladder, rectum, sigmoid colon, and small bowel all receive additional doses. The anatomy of the male pelvis, therefore, dictates morbidity, namely a decline of sexual function, rectal urgency and bleeding, and obstructive and irritative voiding symptoms.
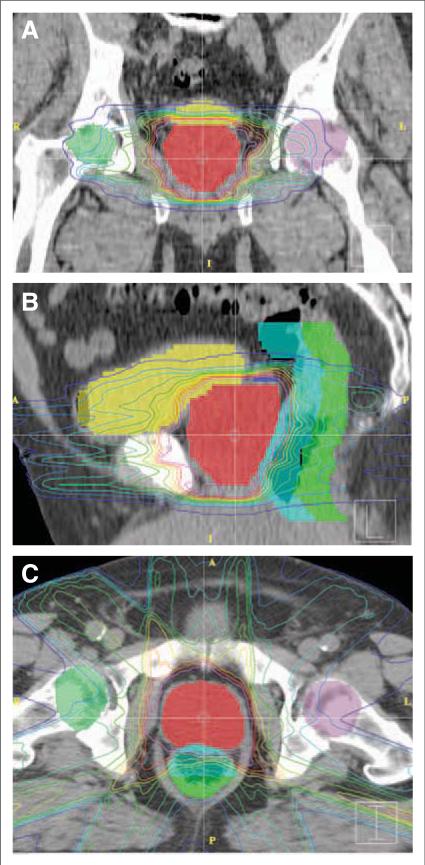
FIGURE 1.
Sample Treatment Plan for External Beam Radiation Therapy to the Prostate. (A) Coronal, (B) sagittal, and (C) axial computed tomographic images of a representative plan for low-risk prostate cancer. Isodose lines depict radiation doses at various distances from the target tissue. Full dose is contained within the pink isodose line. Shading of organs of interest is as follows: prostate (red), bladder (yellow), anterior rectum (light blue), and posterior rectum (green). The close proximity of bladder and anterior rectum to the prostate leads to significant radiation doses to portions of these structures.
Urinary Function
The majority of the acute urinary dysfunction caused by EBRT relates to inflammation and mucosal loss at the bladder neck and within the prostate and prostatic urethra. Symptoms usually begin 2 to 3 weeks into therapy, when the mucosa first becomes denuded, and continue for several weeks until re-epithelialization is complete (Figure 2A). Both irritative and obstructive urinary symptoms may occur, with a tendency toward the latter in men with larger prostates. Pinkawa et al recently addressed acute urinary toxicity in 204 men treated to >70 Gy with external beam irradiation with special emphasis on pretreatment prostate volume.81 Side effects were measured at completion of treatment, as well as at 2 and 12 months afterward. One-third of men reported dysuria, and 37% reported obstructive symptoms at completion of treatment. By 2 months post-treatment, these symptoms had returned to baseline. Both dysuria and obstructive symptoms were more common in the large prostate group (>43 cm3). Obstructive and irritative urinary symptoms during and after radiation are often effectively managed with alpha blockers, such as tamsulosin or terazosin, or by anticholinergics.82 The latter must be used with caution in men with pre-existing benign prostatic hypertrophy because of a risk of acute urinary retention.
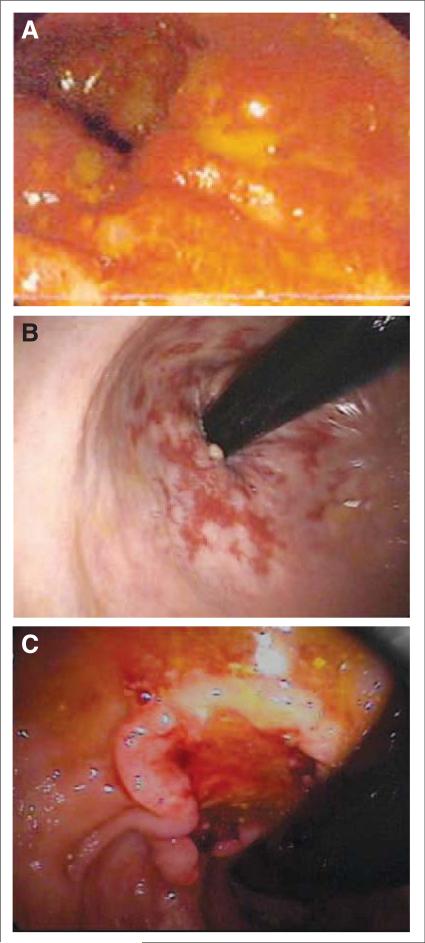
FIGURE 2.
Acute and Late Radiation Therapy Side Effects. (A) Acute inflammation and mucosal loss can occur in both the bladder and rectum (as pictured). Desquamation begins 2 to 3 weeks into treatment and may continue several weeks after treatment is complete. (B) Mucosal thickening and telangectasias in the anterior rectal wall following radiation therapy. These findings can be seen months to years after treatment in both the bladder and the rectum. (C) Secondary rectal cancer visualized on colonoscopy. Patients are at a slightly increased risk of rectal or bladder cancer >10 years after treatment.
Months or years after external radiation, late sequelae may develop. These are the result of changes in the small vessels of the irradiated tissues that lead to chronic hypoxia, mucosal thinning, and aberrant vessel development (telangiectasia) (Figure 2B). Patients may experience painless hematuria, a common though rarely serious event, or a syndrome similar to chronic interstitial cystitis, with frequency, dysuria, and even bladder contracture.83 The reported rates of urethral stricture are below 10% but are higher in men who have had prior transurethral resection.84
Gastrointestinal Side Effects
Irradiation of the anterior rectal wall is the cause of gastrointestinal sequelae in men with prostate cancer. During treatment, rectal urgency and tenesmus may occur. In later years, by similar mechanisms to the previously mentioned late bladder morbidity, men may experience rectal bleeding or, rarely, ulceration. Men receiving radiation to the pelvic lymph nodes have a larger volume of bowel irradiated and, consequently, an increased rate of side effects with a different profile. Small bowel issues such as cramping, diarrhea, and late adhesions are more likely to occur. Crook et al queried 192 patients treated to the prostate (+/−) iliac nodes a mean of 33 months after treatment completion.85 Two-thirds of these patients noted either no or mild gastrointestinal changes. The remaining third with moderate or severe dysfunction reported hematochezia (9%), rectal urgency (20%), or the need for transfusion or laser surgery (4%). The majority of mild to moderate symptoms are treated effectively with simple dietary changes, especially fiber, or by the use of hydrocortisone suppositories or foam. For the rare patient with persistent bleeding despite conservative medical management, the abnormal vessels can be treated electrosurgically by argon plasma coagulation via an endoscope to prevent further episodes. Late rectal complications peak in number and severity in the first 3 years, thereafter often resolving slowly.
Sexual Function
Radiation to both the neurovascular bundles and penile bulb lead to decreased potency in treated men compared with age-matched controls. There is thought to be a 3-fold pathogenic mechanism: penile arterial insufficiency, venous insufficiency at the level of the penile bulb with reduced blood trapping, and direct nerve damage within the neurovascular bundle. Patients who enter treatment with partial potency, diabetes, or who receive even a brief course of neoadjuvant or concomitant ADT are at markedly increased risk of treatment-induced impotence. Potosky et al followed 435 men treated with EBRT in 1994/1995. Of these men, 50.3% and 63.5% were unable to obtain erections sufficient for intercourse 2 and 5 years after treatment, respectively.86 Phosphodiesterase inhibitors have been shown to improve function in up to two-thirds of patients with erectile dysfunction after EBRT.87
Second Cancers
A rare but serious complication of irradiating normal tissues is the induction of second cancers. Brenner et al have looked at all patients with prostate cancer in the Surveillance, Epidemiology, and End Results (SEER) database treated by radiation between 1977 and 1993 and found a 1% additional absolute risk of cancer 10 or more years later.88 These were mainly bladder and rectal cancers (Figure 2C). While the risk is almost certainly lower with contemporary techniques, it will not be reduced to zero and remains a consideration when treating younger men.
EBRT Advances
In the 1970s and 1980s, the majority of external treatments were delivered using large fields and 2D-planning techniques treating significant volumes of normal tissue. In the 1990s, CT information improved the therapeutic ratio, and more recently, intensity-modulated radiation therapy (IMRT) has added an additional degree of precision. IMRT allows the physician to control the intensity of radiation produced by each individual beam, allowing for even greater conformality of dose to tumor. Several studies have shown a decreased rectal dose with IMRT compared with the 3D conformal technique. As expected, an associated decrease in chronic gastrointestinal morbidity was also noted.89
Proton-beam therapy has recently gained popularity as an EBRT modality. Based on their physical characteristics, protons also offer the potential advantage of reducing morbidity while providing equivalent levels of cure. Both IMRT and proton-beam therapy may reduce bladder and rectal morbidity but are unlikely to reduce the rates of erectile impotence since the neurovascular bundles running so close to the prostate remain within the high-dose volume. If these techniques are used to deliver increased radiation doses, the potency rates may even decline further.
IB
IB consists of the introduction of radioactive sources (“seeds”) into the prostate itself. These seeds emit ionizing radiation, providing high doses of radiation to the tumor and surrounding structures. Similar to EBRT, treatment of the prostate necessitates irradiation of the penile bulb, neurovascular bundles, anterior rectal wall, prostatic urethra, and bladder neck. Classically, low-dose–rate (LDR) emitters such as iodine and palladium have been used. Recently, temporary high-dose–rate (HDR) brachytherapy with iridium has seen increasing use.
Brachytherapy procedures begin with a volume study. A transrectal ultrasound probe is used to visualize and create a 3D map of the prostate. With this information, radioactive seed number, location, and activity can be calculated using a planning computer. Once a plan is in place, seeds are loaded into 18-gauge needles and inserted into the prostate using a perineal approach under general anesthesia. Transrectal ultrasound is used to visualize the prostate and determine correct needle placement during the procedure. Seeds can be placed homogenously throughout the prostate or can be concentrated in the prostate periphery. Peripheral loading prevents the extremely high central doses and damage to the prostatic urethra caused by a homogenous plan. Peripheral loading, however, increases the number of sources in close proximity to bowel and bladder, increasing radiation dose to these tissues. Due to these considerations, a modified peripheral loading technique in which peripheral dose can be decreased by placement of a limited number of centrally located sources has gained favor.
Urinary Function
Urinary issues are the most common side effects of seed implantation. A study of 693 consecutive patients at Memorial Sloan Kettering from 1992 to 1997 reported 37% Grade 1 urinary toxicity, 41% Grade 2 toxicity, and 2.2% Grade 3 toxicity within 60 days of seed placement, by the Radiation Therapy Oncology Group toxicity scale.90 There is a biphasic pattern to these effects. The first phase occurs within 24 hours of the procedure due to the immediate trauma of needle insertion, and the second begins 2 to 3 weeks after seed introduction when a radiation-induced inflammatory prostatitis becomes evident. Irritative and obstructive symptoms, namely frequency, urgency, dysuria, incomplete emptying, and weak stream, peak about 1 month after seed implantation and generally completely resolve by 1 year.91 The vast majority of these symptoms are mild in nature and can be treated effectively with alpha blockers.92 Preprocedure gland size and American Urological Association benign prostatic hypertrophy symptom score effectively predict the men at higher risk of significant urinary side effects.93 Less common side effects include hematuria and urethral stricture.
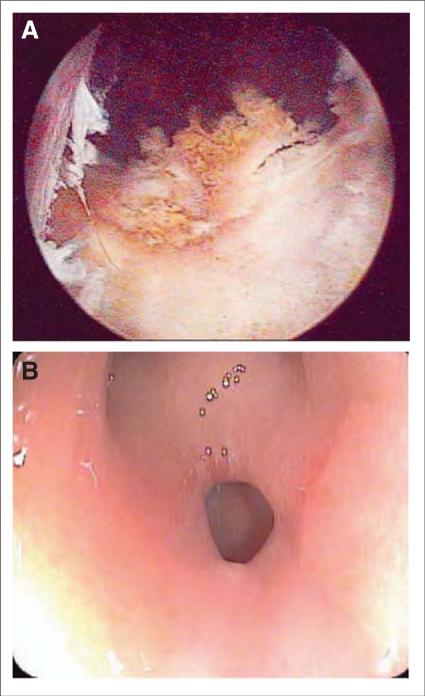
Urinary retention requiring catheterization can occur both directly following as well as 2 to 3 weeks postprocedure. The chance of retention is directly related to prostate gland size, with glands >60 cm3 conferring a markedly increased risk.94 A postoperative course of corticosteroids decreases the rate of urinary retention.90 It is of note that men undergoing a transurethral resection of the prostate either before or following brachytherapy have a significant risk of a relatively unique complication, superficial urethral necrosis (Figure 3A). The pathogenesis of this complication is unclear and usually leads to incontinence.
FIGURE 3.
Serious Complications of Brachytherapy. (A) Superficial urethral necrosis visualized on cystoscopy. Patients who undergo a transurethral resection of the prostate before treatment or to relieve treatment-induced retention are at increased risk. (B) Rectal fistula seen on colonoscopy. Combination external beam radiation therapy and interstitial brachytherapy; inflammatory bowel disease and severe vascular disease increase the risk of this rare complication.
Gastrointestinal Side Effects
While far less common than urinary sequelae, rectal irritation, urgency, bleeding, and loose stool are all possible side effects of brachytherapy. Shah and Ennis evaluated postimplant rectal toxicity in a cohort of 135 patients, with a median follow up of 41 months. Diarrhea was noted in 17.5% of patients in the 6 months after treatment, although only 0.8% met Grade 2 criteria (by National Cancer Institute Common Toxicity grading). In addition, urgency, proctitis, incontinence, and bleeding were noted in 5% to 10% of patients. Late toxicity (more than 6 months postprocedure) consists of these same symptoms, although with decreased frequency. Radiation proctitis is directly related to the amount of rectum receiving significant radiation dose. Specifically, increased late toxicity was noted when greater than 25% of the rectum received greater than 25% of the prescribed dose.95 A recent study promotes injection of hyaluronic acid as a spacer between prostate and rectal wall to reduce rectal dose and hopefully mitigate side effects.96
Diarrhea can be effectively managed with antidiarrheal agents such as loperamide (Imodium), while proctitis and urgency are often effectively treated with dietary modification and/or psyllium (Metamucil) to add increased bulk. For those patients with significant rectal irritation, cramping, and tenesmus, steroid suppositories may provide relief.
While occurring in less than 1% of patients, formation of a rectal fistula is a serious complication (Figure 3B). Men who are at higher risk for this dreaded complication include those who receive combined EBRT and IB, those with vascular disease, those with poorly controlled diabetes, and those with inflammatory bowel disease. Postimplantation biopsy also increases this risk.
Sexual Function
Brachytherapy carries a risk of sexual dysfunction similar to EBRT. Merrick et al studied 128 potent men who received seed implants between 2001 and 2003. The actuarial rate of potency preservation was 51% at 3 years. The median time to loss of potency was 5.4 months after the procedure.97 Dose to the proximal crura and marginal potency pretreatment were statistically significant predictors of treatment-induced impotence. Potters et al noted a 5-year potency rate of 76% for those receiving seeds as monotherapy. The addition of EBRT or ADT markedly decreased potency in this study group.98 In this study, 62% of impotent patients were able to achieve erections capable of intercourse with the addition of sildenafil. Notably, this percentage was >80% when men receiving ADT were excluded.
HDR Brachytherapy
HDR IB has increased in use over the last several years. As with standard LDR brachytherapy, HDR brachytherapy is administered by placement of radioactive sources within the prostate using a perineal approach. In contrast to LDR, HDR brachytherapy is administered in 2 or more large fractions over a period of 24 to 48 hours and requires hospitalization during administration. HDR is traditionally used in combination with EBRT for men with advanced local disease.
Limited data are available comparing HDR brachytherapy with other radiotherapy options. A single study compared standard LDR brachytherapy with HDR monotherapy in 149 men with early-stage prostate cancer.99 In this study, HDR resulted in decreased dysuria (36% versus 67%), urinary urgency (54% versus 92%), and rectal pain (6% versus 20%). There was an increased risk of urethral stricture with HDR (8% versus 3%).
Quality of Life
Recent analysis of patient outcomes has shifted from simply a list of the prevalence and incidence of various side effects to a more “holistic” quality-of-life measurement. HRQOL measures the interplay between the sequelae of an intervention and the patient's expectations, tolerances, personal and professional relationships, satisfaction, and overall happiness.100
HRQOL is determined using standardized question sets directly administered to patients. Several validated disease-specific questionnaires have been generated for prostate cancer that address the specific influence of bowel, bladder, and sexual function on overall quality of life, including the Expanded Prostate Cancer Index Composite101 and the Functional Assessment of Cancer Therapy-Prostate instrument.99 Several recent studies have used these questionnaires to compare HRQOL following various treatment modalities.
Lee et al used the Functional Assessment of Cancer Therapy-Prostate instrument questionnaire to assess HRQOL at set intervals during the first year after radiotherapy treatment.102 A significant decrease in HRQOL was noted among IB patients at 1 and 3 months post-treatment. No significant differences compared with pretreatment were noted in either the EBRT group at any point or the IB group 1 year after therapy.
Clark et al looked at HRQOL in 130 patients who received EBRT and 16 patients who received brachytherapy 12 to 48 months after treatment completion.103 Significant urinary obstructive/irritative, bowel, and sexual dysfunction were noted compared with controls. There was not a significant increase in self-reported urinary incontinence compared with controls. These findings correlated with a statistically significant decrease in urinary control, sexual intimacy, and sexual confidence on quality-of-life analysis. Marital affection and masculine self-esteem were not lessened.
Wei et al measured HRQOL among 902 subjects treated with RP, EBRT (via a 3D conformal technique), or IB at the University of Michigan with a median follow up of 2.6 years.104 An additional 114 male volunteers served as age-matched controls. General HRQOL did not differ amongst any treatment group and age-matched controls. IB patients had worse sexual, bowel, and urinary irritative and obstructive quality of life than controls. EBRT patients had worse bowel and sexual quality of life than controls, but comparable urinary quality of life. Comparison of IB and EBRT revealed increased incontinence and slightly worse bowel quality of life in the IB cohort compared with EBRT.
Miller et al re-evaluated this patient cohort at a median of 6.2 years after treatment.105 3D conformal EBRT patients continued to have poorer bowel and sexual HRQOL than the control group. IB patients continued to have poorer bowel, urinary, and sexual HRQOL compared with controls. Notably, urinary irritative HRQOL and bowel HRQOL improved markedly in IB patients in the study interval, although both were still substantially worse than the control group.
Provocatively, urinary incontinence HRQOL, measured as frequent urinary dribbling or pad requirements, worsened in both the EBRT and IB groups during the study interval while remaining unchanged in the control population. Furthermore, there was a decreased sexual HRQOL in the EBRT group, although this was mirrored by a similar decline in the control group, suggesting an age-related rather than treatment-related phenomenon.
III. COMPLICATIONS OF HORMONAL TREATMENT OF PROSTATE CANCER
Introduction
The number of prostate cancer survivors in the United States is estimated at 2 million, and approximately one-third of these men are currently receiving ADT. In addition to a role in the adjuvant treatment of early, localized prostate cancer, ADT represents the primary treatment for recurrent prostate cancer. Once ADT is initiated for recurrent prostate cancer, therapy typically continues until the time of death, a period that may extend over many years. A singularly effective therapy in treating prostate cancer, ADT is increasingly recognized to have undesirable physiologic effects that may result in important long-term adverse consequences. Awareness of the potential adverse effects of ADT and the overall risk/benefit ratio is important in determining the suitability of treatment in individual patients.
ADT may be accomplished by either surgical or chemical castration. GnRH (gonadotropin-releasing hormone) agonists are the most commonly used agents for this purpose and effectively result in hypogonadism with reduction in serum testosterone levels of >95% and in estrogen levels of >80%. The adverse effects of ADT are due to hypogonadism and include vasomotor flushing, loss of libido, decreased bone density, increased fat mass, and decreased muscle mass.106 Additionally, long-term ADT is associated with an increased risk of diabetes and cardiovascular disease.107
Vasomotor Flushing
Most men treated with ADT will experience vasomotor flushing, or hot flashes, which may have substantial impact on quality of life in some men. Hot flashes are described as unpredictable episodes of intense warmth in the upper part of the body and face, accompanied at times by diaphoresis. A number of “natural remedies” have been touted to treat hot flashes, including acupuncture, black cohosh, flax seed, or soy products, but none is demonstrated to help in other than anecdotal instances.
Transdermal estrogen and megestrol acetate have been shown to reduce hot flashes, but with adverse consequences, including breast swelling and nipple tenderness with estrogens and weight gain with megestrol.108,109 Selective serotonin uptake inhibitors are effective in women with breast cancer and menopausal hot flashes; although no randomized trials have been carried out in men, venlafaxine and paroxetine appear to be occasionally effective in small studies of men with hot flashes.110,111
Anemia
Androgens promote erythropoiesis by increasing erythopoietin production and by direct activation of erythrocyte progenitors.112 GnRH agonists significantly decrease hemoglobin concentrations in men with prostate cancer.113–116 The median decrease in hemoglobin concentrations is about 1 g/dL but sufficient to cause anemia in most men.115,116 Treatment-related anemia is usually mild and not associated with symptoms. It is characteristically normochromic and normocytic. Erythropoietin increases hemoglobin concentrations in men receiving GnRH agonists for prostate cancer,115 although treatment for anemia in this setting is rarely necessary.
Fatigue
Fatigue is a common and important adverse effect of ADT. Approximately two-thirds of men report increases in fatigue after treatment with a GnRH agonist.117 Changes in body composition, particularly decreased lean body mass, may contribute to treatment-related fatigue. Anemia may also contribute to treatment-related fatigue.
Gynecomastia and Mastodynia
Gynecomastia is defined as benign proliferation of the glandular subareolar breast tissue. Mastodynia refers to breast/nipple tenderness. The incidence of gynecomastia and/or mastodynia varies with the type and duration of ADT. About 10% to 15% of men develop gynecomastia after bilateral orchiectomies or treatment with a GnRH agonist.118 In contrast, the majority of men receiving monotherapy with an antiandrogen develop gynecomastia.119 Prophylactic breast irradiation appears the most effective strategy to prevent or mitigate gynecomastia; treatment after development of gynecomastia may improve pain but does not improve enlargement.120,121 Tamoxifen is the most effective medical therapy for gynecomastia and/or mastodynia.122,123 Breast reduction surgery may benefit the occasional man with severe breast symptoms that are refractory to medical treatment.
Osteoporosis and Fractures
Several prospective studies have demonstrated that ADT results in immediate and sustained decrease in bone mineral density (BMD) in men with prostate cancer.124–129 The decrease in BMD continues at an average rate of 2% to 3% per year during treatment, and the risk of fracture and development of osteoporosis therefore appears to increase steadily with duration of therapy.
The mechanism of BMD decline may relate to an increase in bone turnover due to ADT. Bone turnover markers measured in serum and urine demonstrate increased activity of both osteoblasts and osteoclasts and reach a plateau after 6 months of ADT.125,127 Changes in sensitivity of bone to parathyroid hormone might also contribute to increased osteoclast activation and decreased BMD.130
Osteoporosis is common in men, with an estimated prevalence of more than 2 million men in the United States.131 Hypogonadism may be the most common cause of acquired osteoporosis in men and together with alcohol abuse and chronic glucocorticoid therapy accounts for one-half of all cases of male osteoporosis.132 The decreased BMD and increased risk of osteoporosis engendered by ADT in men with prostate cancer is now firmly linked to an increased fracture risk.133–135 In one claims-based analysis, for example, men with prostate cancer who were receiving GnRH agonists were 1.4 times as likely to develop fractures as men with prostate cancer who had not received GnRH agonists.133
Management of Treatment-related Osteoporosis
Daily supplementation of calcium and vitamin D is recommended by the National Institute of Health at doses of 1200 to 1500 milligrams/day and 400 IU/day, respectively. Supplementation with calcium and vitamin D modestly decreases fracture incidence in men and women over age 65 years.136 However, this is not sufficient to prevent bone loss in men treated with ADT.127
The increased risks of osteoporosis and fractures in men with prostate cancer receiving ADT have led to randomized controlled trials aimed at preventing or ameliorating these risks. Bisphosphonates, including pamidronate and zoledronic acid (ZA), are effective at preventing ADT-related BMD loss.127,129,137 In a study that treated 106 men receiving ADT for nonmetastatic prostate cancer with ZA versus placebo, ZA administered at 4 mg every 12 weeks significantly increased BMD in the hip and lumbar spine by 3.9% and 7.3%, respectively.129 In another randomized study, a single infusion of ZA resulted in significantly increased BMD in the hip and spine after 1 year.138 The differences in BMD between the men treated with ZA versus placebo were similar in the 2 studies, suggesting that annual infusion of ZA might be sufficient to prevent treatment-related loss of BMD.
Three ongoing randomized controlled trials are assessing the impact of medical therapy with denosumab, toremifene, or ZA on fracture prevention:
Denosumab is a monoclonal antibody that blocks the activity of receptor activator of NF-κB ligand (RANKL), a protein that stimulates osteoclast activity. A Phase III trial of 1,468 men receiving ADT for nonmetastatic prostate cancer is assigning men to either denosumab or placebo administered every 6 months. The primary objectives are evaluation of fracture risk and BMD.
Toremifene is a selective estrogen-receptor modulator that has been shown to increase BMD and reduce vasomotor flushing in men receiving ADT. A Phase III trial of 1,392 men receiving ADT for nonmetastatic prostate cancer is assigning men to daily toremifene or placebo.139 The primary objectives are evaluation of fracture risk, BMD, lipid levels, and hot flashes.
As described previously, ZA is proven to prevent treatment-related BMD loss. Further, in hormone-refractory metastatic prostate cancer, ZA reduces the risk of bone complications, including fractures.140 A Phase III cooperative group study of 1,272 men with early-stage prostate cancer is assigning men treated with GnRH agonists in combination with radiation to either ZA or placebo every 3 months. The primary objectives are evaluation of fracture risk and BMD.
Currently, we recommend routine use of supplemental calcium and vitamin D in all men treated with ADT. Men undergoing long-term ADT for nonmetastatic prostate cancer should have assessment of fracture risk by history, laboratory testing, and measurement of BMD. For men at greatest fracture risk, selective use of drug therapy should be considered.
Obesity
ADT is accompanied by prompt and often marked changes in body composition.
Quantitative analysis of men beginning GnRH-agonist therapy has shown an increase in fat mass of 9.4% to 11% in 1 year, with a concurrent decrease in lean body mass of 2.7% to 3.8%.116,141 The increase in fat mass is observed in subcutaneous rather than intra-abdominal fat. Two other studies in men with nonmetastatic prostate cancer found an increase in mean fat mass of 8.5% or 4.3% within 3 months of starting ADT, suggesting that this effect may be important even for men treated with short courses of ADT.142,143 Because of the association between increased weight, fat mass, and insulin resistance, further studies have assessed the relationship between ADT and insulin resistance.
Lipid Alterations and Insulin Resistance
Treatment-related changes in body composition are accompanied by adverse metabolic effects. GnRH agonists increase serum total cholesterol, low-density lipoprotein cholesterol, and triglycerides.116,144 In a prospective 12-month study, for example, GnRH agonists increased serum total cholesterol, low-density lipoprotein cholesterol, and triglycerides by 9.0%, 7.3%, and 26.5%, respectively.142 GnRH agonists increase fasting plasma insulin level, a surrogate for insulin resistance.142,145 In a prospective study of non-diabetic men with prostate cancer initiating GnRH-agonist therapy, fasting plasma insulin levels increased by 26%, and the whole-body insulin sensitivity index decreased by 11%.146
The term “metabolic syndrome” refers to a clustering of specific cardiovascular disease risk factors whose pathophysiology appears related to insulin resistance.147 The National Cholesterol Education Program's Adult Treatment Panel (ATP III) and World Health Organization have defined the metabolic syndrome using distinct but related criteria. A recent cross-sectional study reported a higher prevalence of the metabolic syndrome (as defined by the ATP III) in 18 men receiving a GnRH agonist than in age-matched control groups of untreated men with prostate cancer and men without prostate cancer.148 Men receiving GnRH-agonist therapy had greater prevalence of increased abdominal girth, elevated triglycerides, and elevated fasting plasma glucose—consistent with results of prospective studies of GnRH-agonist treatment. In contrast with the metabolic syndrome, however, prospective studies have shown that GnRH agonists preferentially increase subcutaneous rather than visceral abdominal fat and increase rather than decrease high-density lipoprotein cholesterol.149 In addition, GnRH agonists increase serum adiponectin levels in men with prostate cancer, whereas the metabolic syndrome is associated with low adiponectin levels. Taken together, these observations suggest that GnRH agonists cause a pattern of metabolic changes that is distinct from the classically defined metabolic syndrome.
Cardiovascular Disease and Diabetes
Diabetes and cardiovascular disease are leading causes of death in men. The adverse treatment-related changes in weight, body composition, lipids, and insulin sensitivity raise the possibility that ADT may increase the risk of these medical conditions. To evaluate the relationship between ADT and risk for incident diabetes and cardiovascular disease, Keating and colleagues conducted a large population-based study using the SEER-Medicare database.107
This landmark study included 73,196 men diagnosed with local or locoregional prostate cancer between 1992 and 1999 with follow up through 2001. Of the total population, one-third of men were treated with ADT during the study period. The analysis was adjusted for both patient and tumor characteristics. After adjusting for a variety of covariates, ADT with a GnRH agonist was associated with a significantly greater risk of incident diabetes (adjusted hazard ratio 1.42, P <.001), coronary heart disease (adjusted hazard ratio 1.16, P <.001), and admission for myocardial infarction (adjusted hazard ratio 1.1142, P <.03). A subsequent study using SEER-Medicare data confirmed the link between ADT and incident cardiovascular disease.150
SUMMARY
Death rates from prostate cancer have steadily declined in recent years due to earlier diagnosis and treatment. The 5-year relative survival from prostate cancer is 98%.1 Improvements in cancer control, however, have been accompanied by a greater burden of treatment for prostate cancer survivors. Recognition of the potential adverse effects of surgery, radiation therapy, and hormone therapy and effective strategies to reduce treatment-related morbidity are necessary to decrease both death and suffering from prostate cancer.
Footnotes
To earn free CME credit for successfully completing the online quiz based on this article, go to http://CME.AmCancerSoc.org.
Disclosures: Dr. Zeitman receives an honorarium for serving as a speaker for Ismar Healthcare. Dr. Smith serves as a consultant for Amgen, Novartis Oncology, Merck, and GTx. No other potential conflict of interest relevant to this article was reported.
Contributor Information
M. Dror Michaelson, Assistant Professor of Medicine, Division of Hematology/Oncology, Massachusetts General Hospital, Boston, MA..
Shane E. Cotter, Resident, Harvard Radiation Oncology Program, Boston, MA..
Patricio C. Gargollo, Instructor in Surgery, Harvard Medical School, Department of Urology, Massachusetts General Hospital, Boston, MA..
Anthony L. Zietman, Professor of Radiation Oncology, Jenot and William Shipley, Harvard Medical School, Massachusetts General Hospital, Boston, MA..
Douglas M. Dahl, Associate in Urology, Massachusetts General Hospital; and Assistant Professor of Surgery (Urology), Harvard Medical School, Boston, MA..
Matthew R. Smith, Associate Professor of Medicine; and Director of Genitourinary Medical Oncology, Massachusetts General Hospital, Boston, MA..
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Catalona WJ, Ramos CG, Carvalhal GF. Contemporary results of anatomic radical prostatectomy. CA Cancer J Clin. 1999;49:282–296. doi: 10.3322/canjclin.49.5.282. [DOI] [PubMed] [Google Scholar]
- 3.Dahl DM, He W, Lazarus R, et al. Pathologic outcome of laparoscopic and open radical prostatectomy. Urology. 2006;68:1253–1256. doi: 10.1016/j.urology.2006.08.1054. [DOI] [PubMed] [Google Scholar]
- 4.Eden CG, Cahill D, Vass JA, et al. Laparoscopic radical prostatectomy: the initial UK series. BJU Int. 2002;90:876–882. doi: 10.1046/j.1464-410x.2002.03049.x. [DOI] [PubMed] [Google Scholar]
- 5.Han M, Partin AW, Pound CR, et al. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am. 2001;28:555–565. doi: 10.1016/s0094-0143(05)70163-4. [DOI] [PubMed] [Google Scholar]
- 6.Hull GW, Rabbani F, Abbas F, et al. Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J Urol. 2002;167:528–534. doi: 10.1016/S0022-5347(01)69079-7. [DOI] [PubMed] [Google Scholar]
- 7.Poulakis V, Ferakis N, Dillenburg W, et al. Laparoscopic radical prostatectomy using an extraperitoneal approach: Nordwest hospital technique and initial experience in 255 cases. J Endourol. 2006;20:45–53. doi: 10.1089/end.2006.20.45. [DOI] [PubMed] [Google Scholar]
- 8.Roumeguere T, Bollens R, Vanden Bossche M, et al. Radical prostatectomy: a prospective comparison of oncological and functional results between open and laparoscopic approaches. World J Urol. 2003;20:360–366. doi: 10.1007/s00345-002-0306-z. [DOI] [PubMed] [Google Scholar]
- 9.Salomon L, Levrel O, Anastasiadis AG, et al. Outcome and complications of radical prostatectomy in patients with PSA <10 ng/ml: comparison between the retropubic, perineal and laparoscopic approach. Prostate Cancer Prostatic Dis. 2002;5:285–290. doi: 10.1038/sj.pcan.4500605. [DOI] [PubMed] [Google Scholar]
- 10.Schraudenbach P, Bermejo CE. Management of the complications of radical prostatectomy. Curr Urol Rep. 2007;8:197–202. doi: 10.1007/s11934-007-0006-8. [DOI] [PubMed] [Google Scholar]
- 11.Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol. 1982;128:492–497. doi: 10.1016/s0022-5347(17)53012-8. [DOI] [PubMed] [Google Scholar]
- 12.Loughlin K, Gargollo P, Dahl DM, editors. Complications of Urologic Surgery and Practice: Diagnosis, Prevention, and Management. Informa Healthcare; New York, NY: 2007. Complications of laparoscopic radical prostatectomy. [Google Scholar]
- 13.Sterbis JR, Brassell SA, McLeod DG. Perioperative complications of radical retropubic prostatectomy. Clin Genitourin Cancer. 2005;4:160–166. doi: 10.3816/CGC.2005.n.027. [DOI] [PubMed] [Google Scholar]
- 14.Vallancien G, Cathelineau X, Baumert H, et al. Complications of transperitoneal laparoscopic surgery in urology: review of 1,311 procedures at a single center. J Urol. 2002;168:23–26. [PubMed] [Google Scholar]
- 15.Wagner JR, Russo P. Urologic complications of major pelvic surgery. Semin Surg Oncol. 2000;18:216–228. doi: 10.1002/(sici)1098-2388(200004/05)18:3<216::aid-ssu5>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 16.Hu JC, Nelson RA, Wilson TG, et al. Perioperative complications of laparoscopic and robotic assisted laparoscopic radical prostatectomy. J Urol. 2006;175:541–546. doi: 10.1016/S0022-5347(05)00156-4. [DOI] [PubMed] [Google Scholar]
- 17.Bishoff JT, Allaf ME, Kirkels W, et al. Laparoscopic bowel injury: incidence and clinical presentation. J Urol. 1999;161:887–890. doi: 10.1016/s0022-5347(01)61797-x. [DOI] [PubMed] [Google Scholar]
- 18.Chandler JG, Corson SL, Way LW. Three spectra of laparoscopic entry access injuries. J Am Coll Surg. 2001;192:478–490. doi: 10.1016/s1072-7515(01)00820-1. [DOI] [PubMed] [Google Scholar]
- 19.Cadeddu JA, Regan F, Kavoussi LR, Moore RG. The role of computerized tomography in the evaluation of complications after laparoscopic urological surgery. J Urol. 1997;158:1349–1352. [PubMed] [Google Scholar]
- 20.Bauer JJ, Schulam PG, Kaufman HS, et al. Laparoscopy for the acute abdomen in the postoperative urologic patient. Urology. 1998;51:917–919. doi: 10.1016/s0090-4295(98)00027-2. [DOI] [PubMed] [Google Scholar]
- 21.Burova RA, Tronin RIu, Nesterenko IuA, Grinberg AA. Laparoscopy in the differential diagnosis of acute abdomen [in Russian]. Khirurgiia (Mosk) 1994;3:16–20. [PubMed] [Google Scholar]
- 22.Trabulsi EJ, Guillonneau B. Laparoscopic radical prostatectomy. J Urol. 2005;173:1072–1079. doi: 10.1097/01.ju.0000154970.63147.90. [DOI] [PubMed] [Google Scholar]
- 23.Guillonneau B, Gupta R, El Fettouh H, et al. Laparoscopic [correction of laproscopic] management of rectal injury during laparoscopic [correction of laproscopic] radical prostatectomy. J Urol. 2003;169:1694–1696. doi: 10.1097/01.ju.0000059860.00022.07. [DOI] [PubMed] [Google Scholar]
- 24.Lassen PM, Kearse WS., Jr Rectal injuries during radical perineal prostatectomy. Urology. 1995;45:266–269. doi: 10.1016/0090-4295(95)80016-6. [DOI] [PubMed] [Google Scholar]
- 25.Katz R, Borkowski T, Hoznek A, et al. Operative management of rectal injuries during laparoscopic radical prostatectomy. Urology. 2003;62:310–313. doi: 10.1016/s0090-4295(03)00326-1. [DOI] [PubMed] [Google Scholar]
- 26.Pisters LL, Wajsman Z. A simple test for the detection of intraoperative rectal injury in major urological pelvic surgery. J Urol. 1992;148:354. doi: 10.1016/s0022-5347(17)36594-1. [DOI] [PubMed] [Google Scholar]
- 27.Borland RN, Walsh PC. The management of rectal injury during radical retropubic prostatectomy. J Urol. 1992;147:905–907. doi: 10.1016/s0022-5347(17)37418-9. [DOI] [PubMed] [Google Scholar]
- 28.Häggman M, Brändstedt S, Norlen BJ. Rectal perforation after retropubic radical prostatectomy: occurrence and management. Eur Urol. 1996;29:337–340. doi: 10.1159/000473772. [DOI] [PubMed] [Google Scholar]
- 29.Dafnis G, Wang YH, Borck L. Transsphincteric repair of rectourethral fistulas following laparoscopic radical prostatectomy. Int J Urol. 2004;11:1047–1049. doi: 10.1111/j.1442-2042.2004.00928.x. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalgo ML, Pavlovich CP, Trock BJ, et al. Classification and trends of perioperative morbidities following laparoscopic radical prostatectomy. J Urol. 2005;174:135–139. doi: 10.1097/01.ju.0000161607.04334.26. [DOI] [PubMed] [Google Scholar]
- 31.Guillonneau B, Cathelineau X, Doublet JD, et al. Laparoscopic radical prostatectomy: assessment after 550 procedures. Crit Rev Oncol Hematol. 2002;43:123–133. doi: 10.1016/s1040-8428(02)00024-0. [DOI] [PubMed] [Google Scholar]
- 32.Rozet F, Galiano M, Cathelineau X, et al. Extraperitoneal laparoscopic radical prostatectomy: a prospective evaluation of 600 cases. J Urol. 2005;174:908–911. doi: 10.1097/01.ju.0000169260.42845.c9. [DOI] [PubMed] [Google Scholar]
- 33.Stolzenburg JU, Rabenalt R, Do M, et al. Categorisation of complications of endoscopic extraperitoneal and laparoscopic transperitoneal radical prostatectomy. World J Urol. 2006;24:88–93. doi: 10.1007/s00345-005-0036-0. [DOI] [PubMed] [Google Scholar]
- 34.Bollens R, Vanden Bossche M, Roumeguere T, et al. Extraperitoneal laparoscopic radical prostatectomy. Results after 50 cases. Eur Urol. 2001;40:65–69. doi: 10.1159/000049750. [DOI] [PubMed] [Google Scholar]
- 35.Dahl DM, L'Esperance JO, Trainer AF, et al. Laparoscopic radical prostatectomy: initial 70 cases at a U.S. university medical center. Urology. 2002;60:859–863. doi: 10.1016/s0090-4295(02)01953-2. [DOI] [PubMed] [Google Scholar]
- 36.Hoznek A, Salomon L, Olsson LE, et al. Laparoscopic radical prostatectomy. The Créteil experience. Eur Urol. 2001;40:38–45. doi: 10.1159/000049747. [DOI] [PubMed] [Google Scholar]
- 37.Raboy A, Ferzli G, Albert P. Initial experience with extraperitoneal endoscopic radical retropubic prostatectomy. Urology. 1997;50:849–853. doi: 10.1016/S0090-4295(97)00485-8. [DOI] [PubMed] [Google Scholar]
- 38.Rassweiler J, Sentker L, Seemann O, et al. Laparoscopic radical prostatectomy with the Heilbronn technique: an analysis of the first 180 cases. J Urol. 2001;166:2101–2108. [PubMed] [Google Scholar]
- 39.Schuessler WW, Schulam PG, Clayman RV, Kavoussi LR. Laparoscopic radical prostatectomy: initial short-term experience. Urology. 1997;50:854–857. doi: 10.1016/S0090-4295(97)00543-8. [DOI] [PubMed] [Google Scholar]
- 40.Türk I, Deger S, Winkelmann B, et al. Laparoscopic radical prostatectomy. Technical aspects and experience with 125 cases. Eur Urol. 2001;40:46–52. doi: 10.1159/000049748. [DOI] [PubMed] [Google Scholar]
- 41.Salomon L, Sèbe P, De la Taille A, et al. Open versus laparoscopic radical prostatectomy: part I. BJU Int. 2004;94:238–243. doi: 10.1111/j.1464-410X.2004.04950.x. [DOI] [PubMed] [Google Scholar]
- 42.Davidson PJ, van den Ouden D, Schroeder FH. Radical prostatectomy: prospective assessment of mortality and morbidity. Eur Urol. 1996;29:168–173. [PubMed] [Google Scholar]
- 43.Hedican SP, Walsh PC. Postoperative bleeding following radical retropubic prostatectomy. J Urol. 1994;152:1181–1183. doi: 10.1016/s0022-5347(17)32534-x. [DOI] [PubMed] [Google Scholar]
- 44.Lang GS, Ruckle HC, Hadley HR, et al. One hundred consecutive laparoscopic pelvic lymph node dissections: comparing complications of the first 50 cases to the second 50 cases. Urology. 1994;44:221–225. doi: 10.1016/s0090-4295(94)80135-5. [DOI] [PubMed] [Google Scholar]
- 45.Rassweiler J, Schulze M, Teber D, et al. Laparoscopic radical prostatectomy with the Heilbronn technique: oncological results in the first 500 patients. J Urol. 2005;173:761–764. doi: 10.1097/01.ju.0000153486.94741.e5. [DOI] [PubMed] [Google Scholar]
- 46.Modi P, Goel R, Dodiya S. Laparoscopic ureteroneocystostomy for distal ureteral injuries. Urology. 2005;66:751–753. doi: 10.1016/j.urology.2005.04.048. [DOI] [PubMed] [Google Scholar]
- 47.Reddy PK, Evans RM. Laparoscopic ureteroneocystostomy. J Urol. 1994;152:2057–2059. doi: 10.1016/s0022-5347(17)32306-6. [DOI] [PubMed] [Google Scholar]
- 48.Guillonneau B, Rozet F, Cathelineau X, et al. Perioperative complications of laparoscopic radical prostatectomy: the Montsouris 3-year experience. J Urol. 2002;167:51–56. [PubMed] [Google Scholar]
- 49.Brown JA, Dahl DM. Transperitoneal laparoscopic radical prostatectomy in patients after laparoscopic prosthetic mesh inguinal herniorrhaphy. Urology. 2004;63:380–382. doi: 10.1016/j.urology.2003.09.073. [DOI] [PubMed] [Google Scholar]
- 50.Lieskovsky G, Skinner DG, Weisenburger T. Pelvic lymphadenectomy in the management of carcinoma of the prostate. J Urol. 1980;124:635–638. doi: 10.1016/s0022-5347(17)55592-5. [DOI] [PubMed] [Google Scholar]
- 51.Alibhai SM, Leach M, Tomlinson G, et al. Rethinking 30-day mortality risk after radical prostatectomy. Urology. 2006;68:1057–1060. doi: 10.1016/j.urology.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 52.Dillioglugil O, Leibman BD, Leibman NS, et al. Risk factors for complications and morbidity after radical retropubic prostatectomy. J Urol. 1997;157:1760–1767. [PubMed] [Google Scholar]
- 53.Geary ES, Dendinger TE, Freiha FS, Stamey TA. Incontinence and vesical neck strictures following radical retropubic prostatectomy. Urology. 1995;45:1000–1006. doi: 10.1016/s0090-4295(99)80121-6. [DOI] [PubMed] [Google Scholar]
- 54.Surya BV, Provet J, Johanson KE, Brown J. Anastomotic strictures following radical prostate-ctomy: risk factors and management. J Urol. 1990;143:755–758. doi: 10.1016/s0022-5347(17)40082-6. [DOI] [PubMed] [Google Scholar]
- 55.Lepor H, Kaci L, Xue X. Continence following radical retropubic prostatectomy using self-reporting instruments. J Urol. 2004;171:1212–1215. doi: 10.1097/01.ju.0000110631.81774.9c. [DOI] [PubMed] [Google Scholar]
- 56.Artibani W, Grosso G, Novara G, et al. Is laparoscopic radical prostatectomy better than traditional retropubic radical prostatectomy? An analysis of peri-operative morbidity in two contemporary series in Italy. Eur Urol. 2003;44:401–406. doi: 10.1016/s0302-2838(03)00315-4. [DOI] [PubMed] [Google Scholar]
- 57.Salomon L, Anastasiadis AG, Katz R, et al. Urinary continence and erectile function: a prospective evaluation of functional results after radical laparoscopic prostatectomy. Eur Urol. 2002;42:338–343. doi: 10.1016/s0302-2838(02)00360-3. [DOI] [PubMed] [Google Scholar]
- 58.Stolzenburg JU, Truss MC, Bekos A, et al. Does the extraperitoneal laparoscopic approach improve the outcome of radical prostatectomy? Curr Urol Rep. 2004;5:115–122. doi: 10.1007/s11934-004-0023-9. [DOI] [PubMed] [Google Scholar]
- 59.Erdogru T, Teber D, Frede T, et al. Comparison of transperitoneal and extraperitoneal laparoscopic radical prostatectomy using match-pair analysis. Eur Urol. 2004;46:312–319. doi: 10.1016/j.eururo.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 60.Eden CG, King D, Kooiman GG, et al. Transperitoneal or extraperitoneal laparoscopic radical prostatectomy: does the approach matter? J Urol. 2004;172:2218–2223. doi: 10.1097/01.ju.0000144640.26182.41. [DOI] [PubMed] [Google Scholar]
- 61.Konety BR, Sadetsky N, Carroll PR. Recovery of urinary continence following radical prostatectomy: the impact of prostate volume—analysis of data from the CaPSURE Database. J Urol. 2007;177:1423–1425. doi: 10.1016/j.juro.2006.11.089. [DOI] [PubMed] [Google Scholar]
- 62.Manassero F, Traversi C, Ales V, et al. Contribution of early intensive prolonged pelvic floor exercises on urinary continence recovery after bladder neck-sparing radical prostatectomy: results of a prospective controlled randomized trial. Neurourol Urodyn. 2007;26:985–989. doi: 10.1002/nau.20442. [DOI] [PubMed] [Google Scholar]
- 63.Nisen H, Perttilä I, Ranta-Knuuttila T, et al. Laparoscopic radical prostatectomy: surgical, oncological and functional outcomes. Scand J Urol Nephrol. 2008;42:29–34. doi: 10.1080/00365590701561879. [DOI] [PubMed] [Google Scholar]
- 64.Huland H. Radical prostatectomy: options and issues. Eur Urol. 2001;39(suppl):3–9. doi: 10.1159/000052543. [DOI] [PubMed] [Google Scholar]
- 65.Kundu SD, Roehl KA, Eggener SE, et al. Potency, continence and complications in 3,477 consecutive radical retropubic prostatectomies. J Urol. 2004;172:2227–2231. doi: 10.1097/01.ju.0000145222.94455.73. [DOI] [PubMed] [Google Scholar]
- 66.Steiner MS. Current results and patient selection for nerve-sparing radical retropubic prostatectomy. Semin Urol Oncol. 1995;13:204–214. [PubMed] [Google Scholar]
- 67.Walsh PC. Radical prostatectomy for localized prostate cancer provides durable cancer control with excellent quality of life: a structured debate. J Urol. 2000;163:1802–1807. [PubMed] [Google Scholar]
- 68.Walsh PC, Marschke PL. Intussusception of the reconstructed bladder neck leads to earlier continence after radical prostatectomy. Urology. 2002;59:934–938. doi: 10.1016/s0090-4295(02)01596-0. [DOI] [PubMed] [Google Scholar]
- 69.Van Kampen M, De Weerdt W, Van Poppel H, et al. Effect of pelvic-floor re-education on duration and degree of incontinence after radical prostatectomy: a randomised controlled trial. Lancet. 2000;355:98–102. doi: 10.1016/S0140-6736(99)03473-X. [DOI] [PubMed] [Google Scholar]
- 70.Eastham JA, Kattan MW, Rogers E, et al. Risk factors for urinary incontinence after radical prostatectomy. J Urol. 1996;156:1707–1713. [PubMed] [Google Scholar]
- 71.Hunter KF, Glazener CM, Moore KN. Conservative management for postprostatectomy urinary incontinence. Cochrane Database Syst Rev. 2007;2:CD001843. doi: 10.1002/14651858.CD001843.pub3. [DOI] [PubMed] [Google Scholar]
- 72.Klingler HC, Marberger M. Incontinence after radical prostatectomy: surgical treatment options. Curr Opin Urol. 2006;16:60–64. doi: 10.1097/01.mou.0000193381.93608.dc. [DOI] [PubMed] [Google Scholar]
- 73.Quinlan DM, Epstein JI, Carter BS, Walsh PC. Sexual function following radical prostatectomy: influence of preservation of neurovascular bundles. J Urol. 1991;145:998–1002. doi: 10.1016/s0022-5347(17)38512-9. [DOI] [PubMed] [Google Scholar]
- 74.Walsh PC. Nerve grafts are rarely necessary and are unlikely to improve sexual function in men undergoing anatomic radical prostatectomy. Urology. 2001;57:1020–1024. doi: 10.1016/s0090-4295(01)00987-6. [DOI] [PubMed] [Google Scholar]
- 75.Walsh PC. The discovery of the cavernous nerves and development of nerve sparing radical retropubic prostatectomy. J Urol. 2007;177:1632–1635. doi: 10.1016/j.juro.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 76.Walsh PC, Marschke P, Ricker D, Burnett AL. Patient-reported urinary continence and sexual function after anatomic radical prostatectomy. Urology. 2000;55:58–61. doi: 10.1016/s0090-4295(99)00397-0. [DOI] [PubMed] [Google Scholar]
- 77.Katz R, Salomon L, Hoznek A, et al. Patient reported sexual function following laparoscopic radical prostatectomy. J Urol. 2002;168:2078–2082. doi: 10.1016/S0022-5347(05)64300-5. [DOI] [PubMed] [Google Scholar]
- 78.Rassweiler J, Stolzenburg J, Sulser T, et al. Laparoscopic radical prostatectomy—the experience of the German Laparoscopic Working Group. Eur Urol. 2006;49:113–119. doi: 10.1016/j.eururo.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 79.Stanford JL, Feng Z, Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA. 2000;283:354–360. doi: 10.1001/jama.283.3.354. [DOI] [PubMed] [Google Scholar]
- 80.Su LM, Link RE, Bhayani SB, et al. Nerve-sparing laparoscopic radical prostatectomy: replicating the open surgical technique. Urology. 2004;64:123–127. doi: 10.1016/j.urology.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 81.Pinkawa M, Fischedick K, Asadpour B, et al. Toxicity profile with a large prostate volume after external beam radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:83–9. doi: 10.1016/j.ijrobp.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 82.Prosnitz RG, Schneider L, Manola J, et al. Tamsulosin palliates radiation-induced urethritis in patients with prostate cancer: results of a pilot study. Int J Radiat Oncol Biol Phys. 1999;45:563–566. doi: 10.1016/s0360-3016(99)00246-1. [DOI] [PubMed] [Google Scholar]
- 83.Widmark A, Fransson P, Tavelin B. Self-assessment questionnaire for evaluating urinary and intestinal late side effects after pelvic radiotherapy in patients with prostate cancer compared with an age-matched control population. Cancer. 1994;74:2520–2532. doi: 10.1002/1097-0142(19941101)74:9<2520::aid-cncr2820740921>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 84.Sandhu AS, Zelefsky MJ, Lee HJ, et al. Long-term urinary toxicity after 3-dimensional conformal radiotherapy for prostate cancer in patients with prior history of transurethral resection. Int J Radiat Oncol Biol Phys. 2000;48:643–647. doi: 10.1016/s0360-3016(00)00714-8. [DOI] [PubMed] [Google Scholar]
- 85.Crook J, Esche B, Futter N. Effect of pelvic radiotherapy for prostate cancer on bowel, bladder, and sexual function: the patient's perspective. Urology. 1996;47:387–394. doi: 10.1016/S0090-4295(99)80458-0. [DOI] [PubMed] [Google Scholar]
- 86.Potosky AL, Davis WW, Hoffman RM, et al. Five-year outcomes after prostatectomy or radiotherapy for prostate cancer: the prostate cancer outcomes study. J Natl Cancer Inst. 2004;96:1358–1367. doi: 10.1093/jnci/djh259. [DOI] [PubMed] [Google Scholar]
- 87.Incrocci L, Slagter C, Slob AK, Hop WC. A randomized, double-blind, placebo-controlled, cross-over study to assess the efficacy of tadalafil (Cialis) in the treatment of erectile dysfunction following three-dimensional conformal external-beam radiotherapy for prostatic carcinoma. Int J Radiat Oncol Biol Phys. 2006;66:439–444. doi: 10.1016/j.ijrobp.2006.04.047. [DOI] [PubMed] [Google Scholar]
- 88.Brenner DJ, Curtis RE, Hall EJ, Ron E. Second malignancies in prostate carcinoma patients after radiotherapy compared with surgery. Cancer. 2000;88:398–406. doi: 10.1002/(sici)1097-0142(20000115)88:2<398::aid-cncr22>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 89.Jani AB, Su A, Correa D, Gratzle J. Comparison of late gastrointestinal and genitourinary toxicity of prostate cancer patients undergoing intensity-modulated versus conventional radiotherapy using localized fields. Prostate Cancer Prostatic Dis. 2007;10:82–86. doi: 10.1038/sj.pcan.4500910. [DOI] [PubMed] [Google Scholar]
- 90.Sacco DE, Daller M, Grocela JA, et al. Corticosteroid use after prostate brachytherapy reduces the risk of acute urinary retention. BJU Int. 2003;91:345–349. doi: 10.1046/j.1464-410x.2003.04082.x. [DOI] [PubMed] [Google Scholar]
- 91.Cesaretti JA, Stone NN, Stock RG. Urinary symptom flare following I-125 prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2003;56:1085–1092. doi: 10.1016/s0360-3016(03)00210-4. [DOI] [PubMed] [Google Scholar]
- 92.Elshaikh MA, Ulchaker JC, Reddy CA, et al. Prophylactic tamsulosin (Flomax) in patients undergoing prostate 125I brachytherapy for prostate carcinoma: final report of a double-blind placebo-controlled randomized study. Int J Radiat Oncol Biol Phys. 2005;62:164–169. doi: 10.1016/j.ijrobp.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 93.Gelblum DY, Potters L, Ashley R, et al. Urinary morbidity following ultrasound-guided transperineal prostate seed implantation. Int J Radiat Oncol Biol Phys. 1999;45:59–67. doi: 10.1016/s0360-3016(99)00176-5. [DOI] [PubMed] [Google Scholar]
- 94.Landis DM, Schultz D, Cormack R, et al. Acute urinary retention after magnetic resonance image-guided prostate brachytherapy with and without neoadjuvant external beam radiotherapy. Urology. 2005;65:750–754. doi: 10.1016/j.urology.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 95.Shah JN, Ennis RD. Rectal toxicity profile after transperineal interstitial permanent prostate brachytherapy: use of a comprehensive toxicity scoring system and identification of rectal dosimetric toxicity predictors. Int J Radiat Oncol Biol Phys. 2006;64:817–824. doi: 10.1016/j.ijrobp.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 96.Prada PJ, Fernández J, Martinez AA, et al. Transperineal injection of hyaluronic acid in anterior perirectal fat to decrease rectal toxicity from radiation delivered with intensity modulated brachytherapy or EBRT for prostate cancer patients. Int J Radiat Oncol Biol Phys. 2007;69:95–102. doi: 10.1016/j.ijrobp.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 97.Merrick GS, Butler WM, Wallner KE, et al. Erectile function after prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2005;62:437–447. doi: 10.1016/j.ijrobp.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 98.Potters L, Torre T, Fearn PA, et al. Potency after permanent prostate brachytherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2001;50:1235–1242. doi: 10.1016/s0360-3016(01)01578-4. [DOI] [PubMed] [Google Scholar]
- 99.Esper P, Mo F, Chodak G, et al. Measuring quality of life in men with prostate cancer using the Functional Assessment of Cancer Therapy-Prostate instrument. Urology. 1997;50:920–928. doi: 10.1016/S0090-4295(97)00459-7. [DOI] [PubMed] [Google Scholar]
- 100.Quek ML, Penson DF. Quality of life in patients with localized prostate cancer. Urol Oncol. 2005;23:208–215. doi: 10.1016/j.urolonc.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 101.Wei JT, Dunn RL, Litwin MS, et al. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 102.Lee WR, Hall MC, McQuellon RP, et al. A prospective quality-of-life study in men with clinically localized prostate carcinoma treated with radical prostatectomy, external beam radiotherapy, or interstitial brachytherapy. Int J Radiat Oncol Biol Phys. 2001;51:614–623. doi: 10.1016/s0360-3016(01)01707-2. [DOI] [PubMed] [Google Scholar]
- 103.Clark JA, Inui TS, Silliman RA, et al. Patients’ perceptions of quality of life after treatment for early prostate cancer. J Clin Oncol. 2003;21:3777–3784. doi: 10.1200/JCO.2003.02.115. [DOI] [PubMed] [Google Scholar]
- 104.Wei JT, Dunn RL, Sandler HM, et al. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol. 2002;20:557–566. doi: 10.1200/JCO.2002.20.2.557. [DOI] [PubMed] [Google Scholar]
- 105.Miller DC, Sanda MG, Dunn RL, et al. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005;23:2772–2780. doi: 10.1200/JCO.2005.07.116. [DOI] [PubMed] [Google Scholar]
- 106.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–244. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 107.Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 108.Gerber GS, Zagaja GP, Ray PS, Rukstalis DB. Transdermal estrogen in the treatment of hot flushes in men with prostate cancer. Urology. 2000;55:97–101. doi: 10.1016/s0090-4295(99)00370-2. [DOI] [PubMed] [Google Scholar]
- 109.Loprinzi CL, Michalak JC, Quella SK, et al. Megestrol acetate for the prevention of hot flashes. N Engl J Med. 1994;331:347–352. doi: 10.1056/NEJM199408113310602. [DOI] [PubMed] [Google Scholar]
- 110.Loprinzi CL, Barton DL, Carpenter LA, et al. Pilot evaluation of paroxetine for treating hot flashes in men. Mayo Clin Proc. 2004;79:1247–1251. doi: 10.4065/79.10.1247. [DOI] [PubMed] [Google Scholar]
- 111.Quella SK, Loprinzi CL, Sloan J, et al. Pilot evaluation of venlafaxine for the treatment of hot flashes in men undergoing androgen ablation therapy for prostate cancer. J Urol. 1999;162:98–102. doi: 10.1097/00005392-199907000-00024. [DOI] [PubMed] [Google Scholar]
- 112.Shahidi NT. Androgens and erythropoiesis. N Engl J Med. 1973;289:72–80. doi: 10.1056/NEJM197307122890205. [DOI] [PubMed] [Google Scholar]
- 113.Leuprolide versus diethylstilbestrol for metastatic prostate cancer. The Leuprolide Study Group. N Engl J Med. 1984;311:1281–1286. doi: 10.1056/NEJM198411153112004. [DOI] [PubMed] [Google Scholar]
- 114.Crawford ED, Blumenstein BA, Goodman PJ, et al. Leuprolide with and without flutamide in advanced prostate cancer. Cancer. 1990;66:1039–1044. doi: 10.1002/cncr.1990.66.s5.1039. [DOI] [PubMed] [Google Scholar]
- 115.Strum SB, McDermed JE, Scholz MC, et al. Anaemia associated with androgen deprivation in patients with prostate cancer receiving combined hormone blockade. Br J Urol. 1997;79:933–941. doi: 10.1046/j.1464-410x.1997.00234.x. [DOI] [PubMed] [Google Scholar]
- 116.Smith MR, Finkelstein JS, McGovern FJ, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87:599–603. doi: 10.1210/jcem.87.2.8299. [DOI] [PubMed] [Google Scholar]
- 117.Stone P, Hardy J, Huddart R, et al. Fatigue in patients with prostate cancer receiving hormone therapy. Eur J Cancer. 2000;36:1134–1141. doi: 10.1016/s0959-8049(00)00084-8. [DOI] [PubMed] [Google Scholar]
- 118.Thompson CA, Shanafelt TD, Loprinzi CL. Andropause: symptom management for prostate cancer patients treated with hormonal ablation. Oncologist. 2003;8:474–487. doi: 10.1634/theoncologist.8-5-474. [DOI] [PubMed] [Google Scholar]
- 119.See WA, Wirth MP, McLeod DG, et al. Bicalutamide as immediate therapy either alone or as adjuvant to standard care of patients with localized or locally advanced prostate cancer: first analysis of the early prostate cancer program. J Urol. 2002;168:429–435. [PubMed] [Google Scholar]
- 120.Gagnon JD, Moss WT, Stevens KR. Pre-estrogen breast irradiation for patients with carcinoma of the prostate: a critical review. J Urol. 1979;121:182–184. doi: 10.1016/s0022-5347(17)56713-0. [DOI] [PubMed] [Google Scholar]
- 121.Widmark A, Fosså SD, Lundmo P, et al. Does prophylactic breast irradiation prevent antiandrogen-induced gynecomastia? Evaluation of 253 patients in the randomized Scandinavian trial SPCG-7/SFUO-3. Urology. 2003;61:145–151. doi: 10.1016/s0090-4295(02)02107-6. [DOI] [PubMed] [Google Scholar]
- 122.Boccardo F, Rubagotti A, Battaglia M, et al. Evaluation of tamoxifen and anastrozole in the prevention of gynecomastia and breast pain induced by bicalutamide monotherapy of prostate cancer. J Clin Oncol. 2005;23:808–815. doi: 10.1200/JCO.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 123.Saltzstein D, Sieber P, Morris T, Gallo J. Prevention and management of bicalutamide-induced gynecomastia and breast pain: randomized endocrinologic and clinical studies with tamoxifen and anastrozole. Prostate Cancer Prostatic Dis. 2005;8:75–83. doi: 10.1038/sj.pcan.4500782. [DOI] [PubMed] [Google Scholar]
- 124.Diamond T, Campbell J, Bryant C, Lynch W. The effect of combined androgen blockade on bone turnover and bone mineral densities in men treated for prostate carcinoma: longitudinal evaluation and response to intermittent cyclic etidronate therapy. Cancer. 1998;83:1561–1566. [PubMed] [Google Scholar]
- 125.Maillefert JF, Sibilia J, Michel F, et al. Bone mineral density in men treated with synthetic gonadotropin-releasing hormone agonists for prostatic carcinoma. J Urol. 1999;161:1219–1222. [PubMed] [Google Scholar]
- 126.Daniell HW, Dunn SR, Ferguson DW, et al. Progressive osteoporosis during androgen deprivation therapy for prostate cancer. J Urol. 2000;163:181–186. [PubMed] [Google Scholar]
- 127.Smith MR, McGovern FJ, Zietman AL, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001;345:948–955. doi: 10.1056/NEJMoa010845. [DOI] [PubMed] [Google Scholar]
- 128.Berruti A, Dogliotti L, Terrone C, et al. Changes in bone mineral density, lean body mass and fat content as measured by dual energy x-ray absorptiometry in patients with prostate cancer without apparent bone metastases given androgen deprivation therapy. J Urol. 2002;167:2361–2367. [PubMed] [Google Scholar]
- 129.Smith MR, Eastham J, Gleason DM, et al. Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J Urol. 2003;169:2008–2012. doi: 10.1097/01.ju.0000063820.94994.95. [DOI] [PubMed] [Google Scholar]
- 130.Leder BZ, Smith MR, Fallon MA, et al. Effects of gonadal steroid suppression on skeletal sensitivity to parathyroid hormone in men. J Clin Endocrinol Metab. 2001;86:511–516. doi: 10.1210/jcem.86.2.7177. [DOI] [PubMed] [Google Scholar]
- 131.National Osteoporosis Foundation [March 12, 2008];Osteoporosis in Men. Available at: http://www.nof.org/men/strategies_men.htm.
- 132.Bilezikian JP. Osteoporosis in men. J Clin Endocrinol Metab. 1999;84:3431–3434. doi: 10.1210/jcem.84.10.6060. [DOI] [PubMed] [Google Scholar]
- 133.Smith MR, Lee WC, Brandman J, et al. Gonadotropin-releasing hormone agonists and fracture risk: a claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol. 2005;23:7897–7903. doi: 10.1200/JCO.2004.00.6908. [DOI] [PubMed] [Google Scholar]
- 134.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 135.Smith MR, Boyce SP, Moyneur E, et al. Risk of clinical fractures after gonadotropin-releasing hormone agonist therapy for prostate cancer. J Urol. 2006;175:136–139. doi: 10.1016/S0022-5347(05)00033-9. [DOI] [PubMed] [Google Scholar]
- 136.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337:670–676. doi: 10.1056/NEJM199709043371003. [DOI] [PubMed] [Google Scholar]
- 137.Diamond TH, Winters J, Smith A, et al. The antiosteoporotic efficacy of intravenous pamidronate in men with prostate carcinoma receiving combined androgen blockade: a double blind, randomized, placebo-controlled crossover study. Cancer. 2001;92:1444–1450. doi: 10.1002/1097-0142(20010915)92:6<1444::aid-cncr1468>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 138.Michaelson MD, Kaufman DS, Lee H, et al. Randomized controlled trial of annual zoledronic acid to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer. J Clin Oncol. 2007;25:1038–1042. doi: 10.1200/JCO.2006.07.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Taneja SS, Smith MR, Dalton JT, et al. Toremifene—a promising therapy for the prevention of prostate cancer and complications of androgen deprivation therapy. Expert Opin Investig Drugs. 2006;15:293–305. doi: 10.1517/13543784.15.3.293. [DOI] [PubMed] [Google Scholar]
- 140.Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 141.Smith MR. Changes in fat and lean body mass during androgen-deprivation therapy for prostate cancer. Urology. 2004;63:742–745. doi: 10.1016/j.urology.2003.10.063. [DOI] [PubMed] [Google Scholar]
- 142.Smith JC, Bennett S, Evans LM, et al. The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J Clin Endocrinol Metab. 2001;86:4261–4267. doi: 10.1210/jcem.86.9.7851. [DOI] [PubMed] [Google Scholar]
- 143.Cook RJ, Coleman R, Brown J, et al. Markers of bone metabolism and survival in men with hormone-refractory metastatic prostate cancer. Clin Cancer Res. 2006;12:3361–3367. doi: 10.1158/1078-0432.CCR-06-0269. [DOI] [PubMed] [Google Scholar]
- 144.Eri LM, Urdal P, Bechensteen AG. Effects of the luteinizing hormone-releasing hormone agonist leuprolide on lipoproteins, fibrinogen and plasminogen activator inhibitor in patients with benign prostatic hyperplasia. J Urol. 1995;154:100–104. [PubMed] [Google Scholar]
- 145.Dockery F, Bulpitt CJ, Agarwal S, et al. Testosterone suppression in men with prostate cancer leads to an increase in arterial stiffness and hyperinsulinaemia. Clin Sci (Lond) 2003;104:195–201. doi: 10.1042/CS20020209. [DOI] [PubMed] [Google Scholar]
- 146.Smith MR, Lee H, Nathan DM. Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab. 2006;91:1305–1308. doi: 10.1210/jc.2005-2507. [DOI] [PubMed] [Google Scholar]
- 147.Reaven GM. The insulin resistance syndrome: definition and dietary approaches to treatment. Annu Rev Nutr. 2005;25:391–406. doi: 10.1146/annurev.nutr.24.012003.132155. [DOI] [PubMed] [Google Scholar]
- 148.Braga-Basaria M, Dobs AS, Muller DC, et al. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol. 2006;24:3979–3983. doi: 10.1200/JCO.2006.05.9741. [DOI] [PubMed] [Google Scholar]
- 149.Smith MR, Lee H, McGovern F, et al. Metabolic changes during gonadotropin-releasing hormone agonist therapy for prostate cancer: differences from the classic metabolic syndrome. Cancer. 2008;112:2188–2194. doi: 10.1002/cncr.23440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Saigal CS, Gore JL, Krupski TL, et al. Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer. 2007;110:1493–1500. doi: 10.1002/cncr.22933. [DOI] [PubMed] [Google Scholar]