Abstract
Synthetic analogues of naturally occurring triterpenoids; glycyrrhetinic acid, arjunolic acid and boswellic acids, by modification of A-ring with a cyano- and enone- functionalities, have been reported. A novel method of synthesis of α-cyanoenones from isoxazoles is reported. Bio-assays using primary mouse macrophages and tumor cell lines indicate potent anti-inflammatory and cytotoxic activities associated with cyanoenones of boswellic acid and glycyrrhetinic acid.
1. Introduction
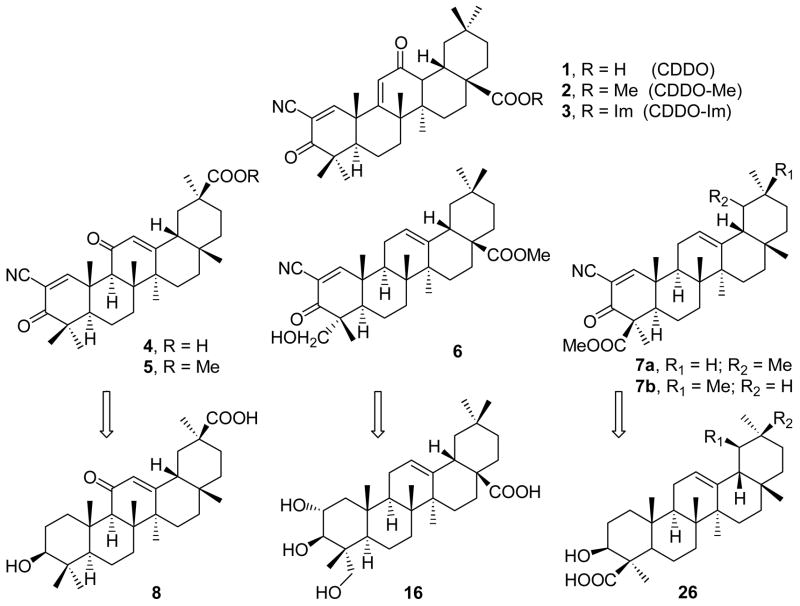
Since the first report1 of the synthesis of 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO) 1 and its methyl ester 2, there have been a number of studies displaying the multifarious biological activity of various analogues of this compound 1. Various derivatives of this compound possess significant biological activities. The CDDO-Im 3 triggers2 apoptosis in multiple myelome (MM) cells. Compounds 1 and 3 are also highly active3 in suppressing cellular proliferation of human leukemia and breast cancer cell lines. CDDO 1 and its methyl ester 2 exhibit4,5,6 high inhibitory activity against production of nitric oxide induced by interferon-γ (IFN-γ) in mouse macrophages (IC50 = 0.1 nM). CDDO 1 was also found7,8 to induce monocytic differentiation of human myeloid leukemia cells and adipogenic differentiation of mouse 3T3-L1 fibroblasts. The compound CDDO 1 was synthesized from an abundantly occurring triterpene, oleanolic acid. SAR studies6 conducted on CDDO have mainly addressed to the structural variations at C-2 of the A ring, the functionality in C-ring and the ester moiety. We now report the synthesis of the compounds, 5 from glycyrrhetinic acid 8, 6 from arjunolic acid 16, and 7 from boswellic acids 26 (Scheme 1), which are readily available from Indian medicinal plants. These cyano-enones 5, 6 and 7 have been screened for nitric oxide inhibitory and cytotoxic activities in in vitro bioassays.
Scheme 1.
Cyano-enone derivatives of triterpenes
2. Results and Discussion
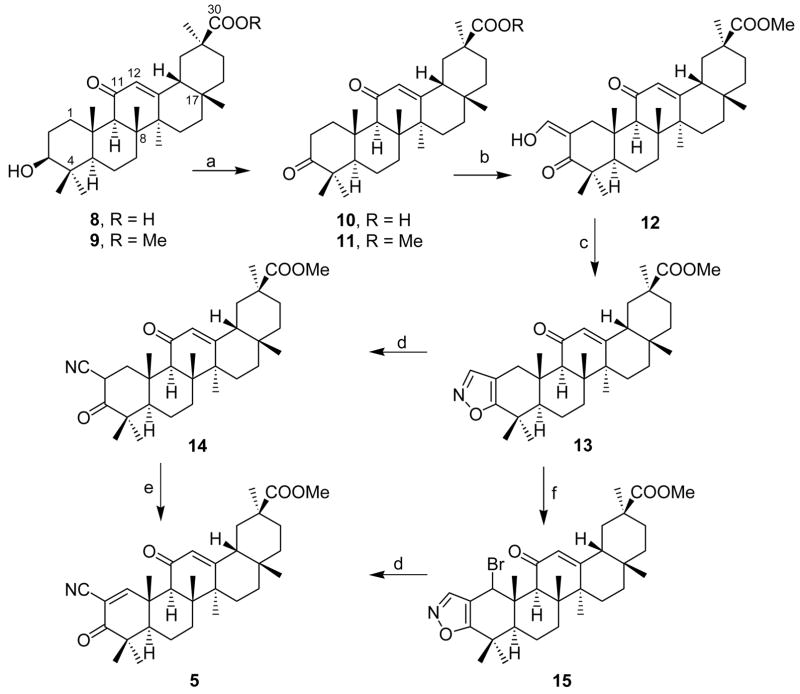
With a view to study the effect of changes in the position of the carboxylic moiety and increase in the polarity of the A ring, three naturally abundant triterpenoids from Indian medicinal plants were chosen as substrates for the synthesis of the CDDO analogues. Since glycyrrhetinic acid 8, isolated from liquorice root,9 already has the α,β-unsaturated system in the C ring, it was only necessary to introduce a cyano-enone functionality in the A ring of the triterpenoid. The formal synthesis of the cyano-ketone was carried out using the Johnson’s10 protocol via the isoxazole intermediate as depicted in Scheme 2. Glycyrrhetinic acid 8, derived from the acidic hydrolysis of glycyrrhizin, was esterified using diazomethane in quantitative yields. The ester 9 thus obtained was oxidized at 0 °C using the Jones Reagent to give the 3-keto derivative 11 in 84% yield. The compound was conversely prepared by reversing the oxidation and esterification procedures to initially give the keto acid 10 that was esterified to give the keto ester 11. The formylation of the keto ester using ethyl formate in the presence of an alkoxide base gave 2-formyl-3-keto compound 12, the precursor for the isoxazole in 80% yield. The isoxazole 13 was prepared11 in an acetate buffer as reported by Doorenbos in 82% yield to give the single desired isomer. This was quantitatively isomerized under basic conditions to the cyanoketone 14 that was dehydrogenated using DDQ in benzene to give the cyano enone ester 5 in 65% yield. An alternative protocol for the synthesis of the cyanoenone 5 was attempted via the allylic bromination-dehydrobromination. The isoxazole 13 was brominated using NBS under radical conditions and the crude product 15 gave the cyanoenone 5 when treated with a base in comparable yields.
Scheme 2.
Reagents: a) i) CH2N2/MeOH; ii) Jones oxidation b) NaOMe/HCOOEt; c) NH2OH/AcOH; AcONa/MeOH/C6H6; d) NaOMe/MeOH/Et2O; e) DDQ/C6H6; f) NBS/AIBN/CHCl3
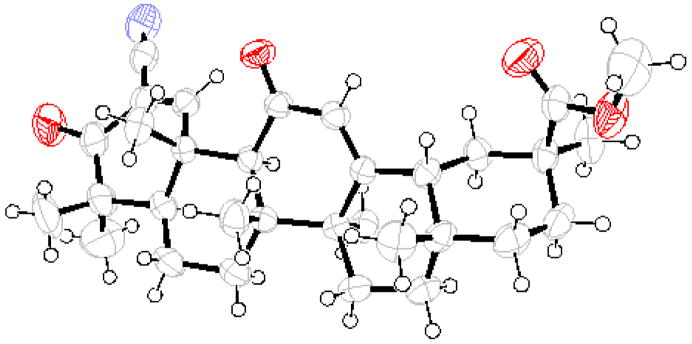
Spectroscopic data established the assigned structure which was further supported by x-ray crystal structure analysis (Figure 1). This also revealed that the stereochemistry12 of the starting material 8 had been retained in the product without any epimerization of the CD rings taking place under the basic conditions of the reactions.
Figure 1.
Crystal structure of methyl 2-cyano-3,11-dioxo-30-norolean-1,12-dien-30-oate 5
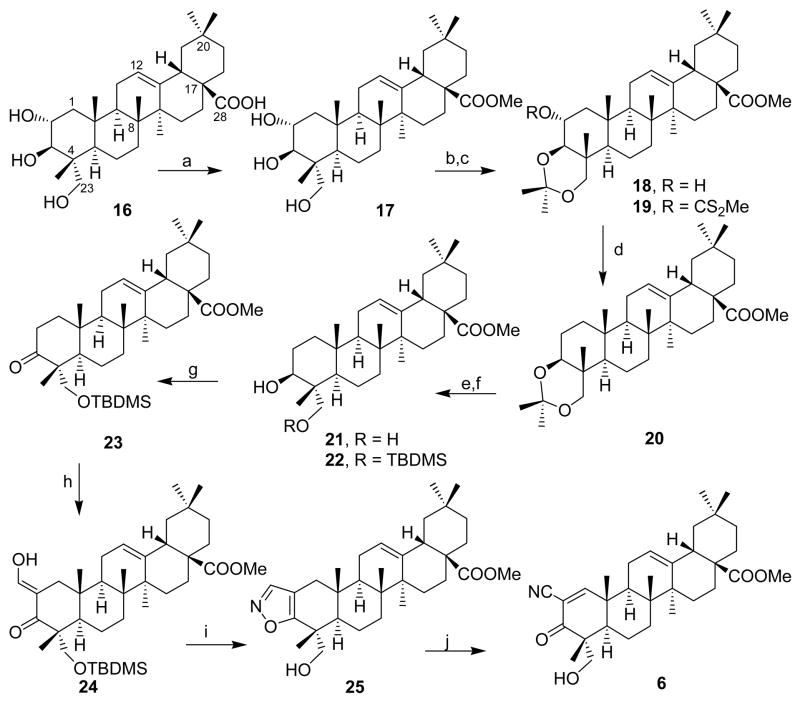
Arjunolic acid 16, isolated13 from Terminalia arjuna, while retaining an olean skeleton with a C-28 carboxylic acid has an additional hydroxyl group (C-23) in the A ring providing additional polar functionality. It was initially attempted to selectively activate the C-2 hydroxy and displace with a nitrile, however various attempts gave intractable mixtures. As an alternate protocol, the hydroxyl group at C-2 of methyl arjunolate 17, was removed following the Barton’s xanthate protocol. Initially, the two hydroxyl groups at C-3 and C-23 of 17 were protected as an acetonide 18 which readily formed a xanthate ester 19 with carbon disulfide. Reduction of the xanthate ester 19 with tributyltin hydride, followed by hydrolysis afforded the diol 21 in good yield. This compound was identical with methyl hederagenin, obtained by the esterification of hederagenin, a natural triterpene isolated14 from Sapindus emarginatus. Selective protection of the primary alcohol as its TBDMS ether 22, followed by the oxidation of the secondary alcohol at C-3 using PDC afforded the ketone 23. Following the Johnson’s protocol10, the isoxazole 25 was prepared which subsequently afforded the desired cyano-enone 6 in good yield as depicted in Scheme 3. The silyl protecting group was cleaved under the acidic conditions during the preparation of the isoxazole.
Scheme 3.
Reagents: a) CH2N2/Et2O; b) acetone, HCl; c) CS2/NaH, imidazole;d) TBTH, AIBN, PhH; e) Amberlite resin; f) t-BDMSi-Cl, imidazole, CH2Cl2; g) PCC/CH2Cl2; h) HCOOEt, NaOtAm; i) NH2OH.HCl/MeOH; j) (i) NaOMe; (ii) DDQ/C6H6.
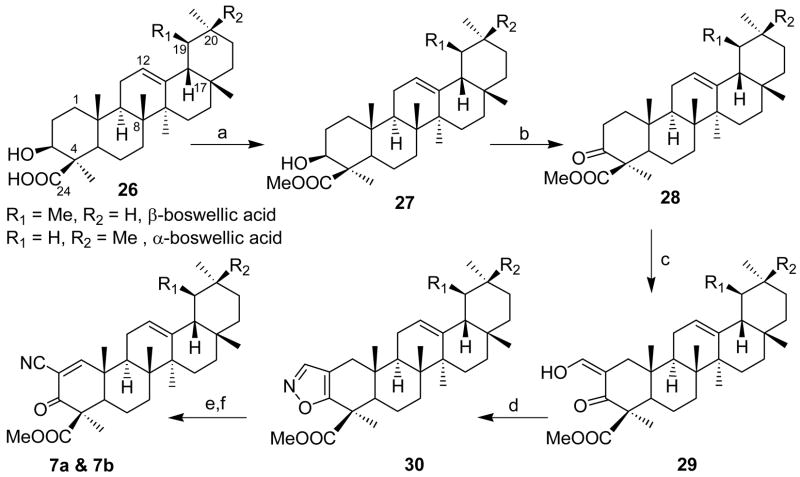
Boswellic acids, isolated15 from the gum of Boswellia serrata, were obtained as a mixture of α and β isomers in the ratio of (9:1), belonging to the olean and ursane skeletons and differing only in the position of the methyl groups in the ring-E of the triterpene moiety. Both α and β isomers however retain a carboxylic moiety as a pendant at C-4 in the A ring of the triterpenoid. The synthesis of the cyano-enone derivative of boswellic acid was depicted in Scheme 4. The free acids 26 were esterified to furnish 27 in order to ensure safe oxidation of the C-3 hydroxyl group to the keto group 28. Formylation of 28 afforded the compound 29. Since this compound decomposed on standing, the crude compound was directly employed in the subsequent steps without purification. Thus, reaction of 29 with hydroxylamine hydrochloride in refluxing methanol gave the isoxazole 30. Bromination-dehydrobromination protocol was employed to afford the desired mixture of cyanoenones 7 of boswellic acids in a (9:1) ratio as indicated from its nmr spectrum. Further work on the purification of 7 is in progress.
Scheme 4.
Reagents: a) CH2N2/MeOH; b) PDC/CH2Cl2; c) HCOOEt/NaOMe; d) NH2OH/HOAc e) NBS/AIBN/CHCl3; f) NaOMe/CH3OH
3. Bioassays
In a previous study Suh et al.7 reported that 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO) shows potent anti-inflammatory, anti-proliferative, apoptotic and differentiating activities. Subsequently its methyl and imidazolide analogues have been found to be more potent in these activities than CDDO.3,8,16,17 Here we have screened 5, 6 and 7 for their ability to inhibit nitric oxide production (Table 1) induced by IFN-γ in primary mouse macrophages and for cytotoxicity using MTT assay (Table 2).
Table 1.
Inhibition of IFN-γ induced Nitric Oxide production by modified triterpenoids
| Triterpenoids | EC50 |
|---|---|
| Boswellic acids (26a and 26b) | No effect till 20 μM |
| Methyl 3-oxoboswellate (28) | No effect till 20 μM |
| Isoxazole from methyl boswellate (30) | No effect till 20 μM |
| Cyano-enone from methyl boswellate (7) | 0.074 μM |
| Glycyrrhetinic acid (8) | >20 μM |
| Isoxazole from methyl glycyrrhetinate (13) | >20 μM |
| Cyano-ketone from methyl glycyrrhetinate (14) | 14.5 μM |
| Cyano-enone from methyl glycyrrhetinate (5) | 0.173 μM |
| Arjunolic acid (16) | >20 μM |
| Cyano-ketone of methyl arjunolate | 7.5 μM |
| Isoxazole of methyl arjunolate (25) | 8.7 μM |
| Cyano-enone from methyl arjunolate (6) | 1.6 μM |
Table 2.
Cytotoxic actitivities of 5, 6 and 7 (EC50 values) on different tumor cell lines as determined by MTT assay.
| No. | Cell line | Description | EC50 (nM) of the compounds | ||
|---|---|---|---|---|---|
| 7 | 5 | 6 | |||
| 1 | A549 | Lung carcinoma | 599 | 7598 | 7936 |
| 2 | A431 | Skin carcinoma | 505 | 4271 | 1697 |
| 3 | HL60 | Leukemia | 108 | 925 | 548 |
| 4 | MCF-7 | Breast carcinoma | 522 | 6213 | 2249 |
| 5 | T47D | Breast carcinoma | 327 | 5541 | 3907 |
| 6 | HT1080 | Acetabulum fibro sarcoma | 393 | 7747 | 3029 |
As shown in table 1, 7 shows highest activity (EC50 74.14 nM) for the inhibition of Nitric Oxide followed by 5 (EC50=172. 8 nM). In contrast 6, showed lower potency (EC50 1.6 μM). All others (intermediates) showed activities at > 5 μM. The ability of the modified triterpinenes to inhibit growth has been demonstrated previously. Hence, we tested the growth inhibitory/cytotoxic activities of modified triterpenoids derived from boswellic acid (7), glycyrrhetinic acid (5) and arjunolic acid (6) using MTT assay. As shown in table 2, compound 7 shows a potent activity with an EC50 of 0.108 μM on HL60 leukemia cells. The cytotoxic activity of 5 and 6 on HL60 was much less at EC50 of 0.925μM and 0.548 μM respectively. In other cell lines the EC50 ranged from 0.3 to 0.6μM for 7 and 4.2 to 7.8 μM for 5 and 1.7–8.0 μM for 6 (Table-2). These results suggest that 7 has a very potent anti inflammatory and growth inhibitory activities among the three modified triterpenoids. Although, cyano-enone modification results in more potent activities of the triterpenoids, not all the compounds are comparable. This is obvious when we compare CDDO7 and 5–7 of this study. Recently, modified betulinic acid was reported18 to have a very potent activity as compared to CDDO. Taken together, modified triterpenoids are a good source of lead molecules for drug development targeting inflammation and cancer.
Experimental
General methods: Melting points were recorded on a Buchi B-540 apparatus and are uncorrected. Infrared spectra were recorded on JASCO FTIR 410. Proton (1H NMR) and carbon magnetic resonance (13C NMR) spectra were generally recorded on a JEOL JNM-LA 300 spectrometer. Mass spectra measurements were carried out on a JEOL JMS DX 303 spectrometer. Elemental analyses were carried out on a Carlo Erba Element Analyzer 1106 at the department of organic chemistry, Indian Institute of Science. Analytical thin-layer chromatography (TLC) was performed on (10 x 5 cm) glass plates coated with Acme silica gel G or GF254 (containing 13% calcium sulfate as binder). Visualization of the spots on TLC plates was achieved either by exposure to iodine vapor or UV light or by spraying sulfuric acid and heating the plates at 120°C. Column chromatography was performed by using Acme silica gel (100–200 mesh) or neutral alumina. All solvents were freshly distilled over CaH2 or Na/benzophenone as appropriate. X-ray data were collected at 293K on a SMART CCD–BRUKER diffractometer with graphite monochromated MoKα radiation (γ =0.7107Å). Structure was solved by direct methods (SIR92). Refinement was by full-matrix least-squares procedures on F2 using SHELXL-97. The non-hydrogen atoms were refined anisotropically whereas hydrogen atoms were refined isotropically.
Glycyrrhetinic acid, (3β-hydroxy-11-oxo-30-norolean-12-en-30-oic acid) 89
Mp. 295°C; νmax(neat)/cm−1 3440, 2945, 1705, 1664, 1457, 1386 and 867; 1H NMR (300 MHz; CDCl3): δ 5.72 (1H, s), 3.21 (1H, dd, J 9.0 and 6.8 Hz), 2.80 (1H, d, J 13.5 Hz), 2.32 (1H, s), 2.21–0.66 (42H, m); 13C NMR (75 MHz; CDCl3): δ 199.6, 181.0, 168.5, 128.4, 100.4, 85.7, 78.6, 61.7, 55.0, 48.0, 45.3, 43.6, 43.0, 40.9, 39.0, 37.6, 37.0, 32.7, 31.8, 30.8, 28.5, 28.3, 28.0, 27.3, 26.5, 23.4, 18.6, 17.4, 16.2 and 15.5; m/z (DI) 470 (M+), 303, 262, 175 and 135 (100).
3,11-Dioxo-30-norolean-12-en-30-oic acid 10
Jones reagent (prepared from 18.24 g of CrO3) was added drop wise to a mechanically stirred suspension of glycyrrhetinic acid 7 (9.21 g, 19.6 mmol) in acetone (400 mL) at 0°C over a period of 30 min till the brown color persisted. The mixture was stirred for further 30 min. Propan-2-ol (5 mL) was added, reaction mixture filtered, and the residue was thoroughly washed with dichloromethane. The solution was concentrated to 100 mL and was diluted with dichloromethane (400 mL). The organic layer was washed with water (2 x 200 mL), brine and dried over sodium sulfate. The organic layer was concentrated under vacuum and the residual solid was recrystallized from methanol/dichloromethane to give the pure ketone 10 (8.43 g, 92%) as a colorless crystalline solid. mp 308–310 °C (from dichloromethane/methanol); (Found: C, 76.7; H, 9.5. C30H44O4 requires C, 76.9; H, 9.4%); νmax(KBr)/cm−1 3311, 2965, 1726, 1682, 1644, 1455 and 1386; 1H NMR (300 MHz; CDCl3): δ 5.75 (1H, s), 2.97 (1H, m), 2.64 (1H, m), 2.41 (1H, s), 2.41–0.87 (40H, m); 13C NMR (75 MHz; CDCl3): δ 217.3, 199.7, 181.2, 169.8, 128.4, 76.6, 61.0, 55.4, 48.2, 47.8, 45.3, 43.8, 43.3, 40.9, 39.7, 37.7, 36.7, 34.2, 32.1, 31.9, 30.9, 28.6, 28.4, 26.5, 26.3, 23.3, 21.4, 18.8, 18.5 and 15.6.
Methyl 3β-hydroxy-11-oxo-30-norolean-12-en-30-oate 9
A solution of ethereal diazomethane (in excess) was added to a suspension of glycyrrhetinic acid 7 (9.4 g, 20 mmol) in methanol (150 mL) at 0°C. The reaction mixture was allowed to stand overnight. The excess of diazomethane was quenched with acetic acid (6 drops) and the mixture was concentrated under vacuum. The solid was recrystallized from methanol/dichloromethane mixture to give colorless crystals 9 (9.6 g, 99%). mp 254–256°C (from dichloromethane/methanol); (Found: C, 76.65; H, 10.1. C31H48O4 requires C, 76.85; H, 9.9%); νmax(neat)/cm−1 3357, 2946, 1722, 1656 and 1467; 1H NMR (300 MHz; CDCl3): δ 5.63 (1H, s), 3.69 (3H, s), 3.20 (1H, m), 2.80 (1H, d, J 13.5 Hz), 2.30 (1H, s) and 2.15–0.65 (41H, m); 13C NMR (75 MHz; CDCl3): δ 199.2, 176.2, 168.2, 128.5, 78.5, 61.7, 54.9, 51.5, 48.2, 45.2, 43.9, 43.0, 41.1, 39.1 (2 C), 37.7, 37.0, 32.8, 31.7, 31.0, 28.5, 28.2, 28.1, 27.3, 26.4, 23.4, 18.6, 17.4, 16.3 and 15.6; m/z (DI) 484 (M+), 317, 276, 175 and 135 (100%).
Methyl 3,11-dioxo-30-norolean-12-en-30-oate 11
Following the procedure for the synthesis of 10 from 8 described earlier, the oxidation of the methyl ester 9 (9.21 g, 19 mmol) gave 11 as amorphous solid (8.80 g, 95%) [This compound was found to be identical to that obtained by diazomethane esterification of the acid 10]. mp 248–250 ºC (from dichloromethane/methanol); (Found: C, 77.6; H, 9.8. C31H46O4 requires C, 77.2; H, 9.5%); νmax(neat)/cm−1 2942, 1725, 1704, 1655, 1625, 1463 and 1386; 1H NMR (300 MHz; CDCl3): δ 5.68 (1H, s), 3.69 (3H, s), 2.97 (1H, m), 2.62 (1H, m), 2.40 (1H, s) and 2.37–0.83 (39H, m); 13C NMR (75 MHz; CDCl3): δ 215.6, 198.4, 176.1, 168.7, 128.3, 60.9, 55.3, 51.5, 48.2, 47.5, 45.1, 43.8, 43.2, 41.1, 39.6, 37.7, 36.6, 33.9, 32.1, 31.7, 31.0, 28.5, 28.2, 26.5, 26.4, 26.3, 23.3, 21.3, 18.7, 18.5 and 15.5; m/z (DI) 482 (M+), 317, 276 and 135(100%).
Methyl 2-hydroxymethylene-3,11-dioxo-30-norolean-12-en-30-oate 12
To an ice-cold suspension of sodium methoxide (440 mg, 8 mmol) in dry benzene (20 mL) was added a solution of ketone 11 (1.9 g, 4 mmol) and ethyl formate (634 mg, 8 m mol) over a period of 10 min under an atmosphere of nitrogen. The resulting mixture was allowed to stand overnight at room temperature. Ice water (20 mL) was added and the two layers separated. The organic layer was extracted with 20% sodium hydroxide solution (3 × 20 mL). The combined aqueous layer was acidified with 2N hydrochloric acid and extracted with ethyl acetate (3 x 20 mL). The ethyl acetate layer was washed with water, brine, dried over sodium sulfate and concentrated under vacuum. Recyrstallization of the solid from dichloromethane/methanol afforded the pure colourless formyl derivative 12 (1.60 g, 78%). mp 232–234 °C (from dichloromethane/methanol); (Found: C, 75.1; H, 9.15. C32H46O4 requires C, 75.3; H, 9.0%); νmax(KBr)/cm−1 3438, 2934, 1732, 1653, 1618, 1457 and 1386; 1H NMR (300 MHz; CDCl3): δ 14.84 (1H, d, J 3.0 Hz), 8.62 (1H, d, J 3.0 Hz), 5.71 (1H, s), 3.71 (3H, s), 3.46 (1H, d, J 14.1 Hz), 2.40 (1H, s) and 2.15–0.84 (38H, m); 13C NMR (75 MHz, CDCl3): δ 198.5, 189.2, 188.2, 176.1, 169.1, 128.4, 105.5, 59.5, 52.2, 51.5, 48.1, 44.8, 43.8, 43.1, 41.2, 39.9, 39.6, 37.7, 36.1, 31.7, 31.6, 31.0, 28.5, 28.4, 28.1, 26.4, 26.3, 23.2, 20.8, 18.7, 18.2 and 14.6; m/z (DI) 510 (M+), 317, 276, 135 and 83 (100%).
Isoxazole derivative of the methyl ester of glycyrrhetinic acid 13
The formyl derivative 12 (0.80 g, 1.6 mmol) in benzene (6 mL) was mixed with a solution of hydroxylamine hydrochloride (134 mg, 1.92 mmol), sodium acetate (312 mg, 1.92 mmol) in acetic acid (1 mL) and water (1 mL). The mixture was homogenized by the addition of methanol (2 mL) and refluxed for a period of 5h. The solvents were removed under reduced pressure and the residue was diluted with water (15 mL) and extracted with ethyl acetate (3 x 15 mL). The combined organic layer was washed with brine, dried over sodium sulfate and filtered. The solvent was removed under vacuum and the resulting solid was recrystallized from dichloromethane/methanol to give a colourless solid 13 (0.64 g, 80%). mp 279–280°C (from dichloromethane/methanol); (Found: C, 75.7; H, 9.05; N, 2.4. C32H45NO4 requires C, 75.7; H, 8.9; N, 2.7%); νmax(neat)/cm−1 2972, 1730, 1658, 1620, 1460, 1385 and 870; 1H NMR (300 MHz; CDCl3): δ 7.97 (1H, s), 5.72 (1H, s), 3.71 (3H, s), 3.65 (1H, d, J 15.1 Hz), 2.50 (1H, s) and 2.16–0.84 (38H, m); 13C NMR (75 MHz; CDCl3): δ 198.4, 176.2, 171.7, 169.0, 149.8, 128.5, 108.8, 59.9, 53.2, 51.5, 48.1, 45.1, 43.8, 43.2, 41.2, 38.3, 37.7, 35.9, 34.6, 31.8 (2 C), 31.0, 28.8, 28.5, 28.2, 26.5, 26.4, 23.2, 21.5, 18.2, 18.0 and 15.6; m/z (DI) 507(M+), 135 and 83 (100%).
Methyl 2-cyano-3,11-dioxo-30-norolean-1,12-dien-30-oate 5
The isoxazole 13 (270 mg, 0.5 mmol) in diethyl ether (5 mL) was added to a stirred solution of sodium (60 mg, 2.6 mmol) in dry methanol (3 mL) under an atmosphere of argon at 0°C. It was allowed to stir for 1 h. Cold water (5 mL) was added to the reaction mixture and the organic layer was washed thoroughly with 5% potassium hydroxide solution (3 x 10 mL). The combined alkaline extracts were acidified and extracted with ethyl acetate (3 x 15 mL). The organic layer was washed with brine, dried over sodium sulfate and concentrated under reduced pressure. The crude residue 14 (248 mg, 91%) was directly used in the next step without purification. νmax (KBr)/cm−1 2963, 2204, 1726, 1657, 1461, 1388 and 801; 1H NMR (300 MHz; CDCl3): δ 5.70, (1H, s), 3.70 (3H, s), 2.40 (1H, s) and 2.34–0.83 (40H, m); m/z (DI) 507(M+), 310, 276, 135 (100). A mixture of the crude cyano-ketone 14 (63 mg, 0.12 mmol) and DDQ (33 mg, 0.14 mmol) in benzene (3 mL) was refluxed for 8 h. After cooling the reaction mixture to ambient temperature, acetone was added till it became homogenous and the clear solution was filtered through a pad of alumina. The filtrate was concentrated and the solid residue was recrystallized from dichloromethane/methanol to give the pure cyano-enone 5 (40 mg, 65%). mp 237–239 °C (from dichloromethane/methanol); (Found: C, 76.1; H, 8.5; N, 2.8, C32H43NO4 requires C, 76.0; H, 8.6; N, 2.8%); νmax(neat)/cm−1 2951, 2232, 1727, 1685, 1655, 1610, 1457, 1387, 802 and 736; 1H NMR (300 MHz; CDCl3): δ 8.49 (1H, s), 5.78 (1H, s), 3.71 (3H, s), 2.70 (1H, s) and 2.20–0.84 (37H, m); 13C NMR (75 MHz; CDCl3): δ 197.4, 196.8, 176.2, 171.9, 170.9, 127.8, 114.5, 113.3, 54.2, 51.7, 51.6, 48.3, 45.5, 44.9, 43.8, 43.4, 41.1, 39.5, 37.6, 31.7, 31.6, 31.0, 28.5, 28.1, 27.4, 26.4, 26.2, 23.3, 21.4, 19.4, 18.8 and 18.0; m/z (DI) 83 (100%).
Crystal Data
Compound 5: C32H43O4N, MW = 505, colorless crystal, Crystal system: monoclinic, space group: P2(1), cell parameters: a = 7.282 (4) Å, b = 12.262 (8) Å, c = 16.173 (10) Å, β = 94.57° (1), V = 1440.00 (6) Å3, Z = 2, Dc = 1.162 g.cm−3, F(000)=544.0, μ=0.08 mm−1. Total number of l.s. Parameters = 342, R1 = 0.0494 for 4676 Fo > 4σ (Fo) and 0.0655 for all 5812 data. WR2=0.1230, GOF = 1.019, Restrained GOF = 1.024 for all data. Crystallographic data (without structure factor) have deposited with the Cambridge Crystallographic Data Center and the depository number is CCDC 213948.
Synthesis of 5 via allylic bromination–dehydrobromination
A mixture of the isoxazole 13 (270 mg, 0.5 mmol), NBS (98 mg, 0.55 mmol), AIBN (catalytic amount) in chloroform (5 mL) was allowed to reflux under the influence of tungsten lamp for 6 h. The solution was cooled, and the precipitated succinimide was filtered Removal of the solvent afforded the crude bromocompound 15 which was added to an ice-cold solution of sodium (46 mg, 2 mmol) in methanol (2 mL). The reaction mixture was stirred at room temperature for one hour. The solvent was removed under vacuum and the residue was diluted with water (10 mL). The aqueous layer was repeatedly extracted with ether (3 x 15 mL). The combined organic layer was dried over sodium sulfate and concentrated under vacuum. The crude solid was purified by column chromatography to give the cyano-enone 5 (63%). Mp. 237–239°C.
Arjunolic acid 1613
Arjunolic acid was isolated13 from the acetone extract of the heartwood of Terminalia arjuna Bedd. It was crystallized from acetone, mp 320–325 °C; νmax(KBr)/cm−1 3544, 3466, 3389, 2931, 1708, and 1639; 1H NMR (300 MHz; py-d5): δ 5.48 (1H, br t), 4.25–4.23 (3H, s), 3.74 (1H, d, J 10.2 Hz), 3.30 (1H, d, J 10.2 Hz), 2.34 (1H, d, J 9.6 Hz), 2.18–0.92 (41H, m); m/z (DI) 248 (M+), 203 (100), 191, 173, 133.
Methyl arjunolate 17
Ethereal diazomethane was added to an ice cooled suspension of arjunolic acid 16 (9.8 g, 2 mmol) in methanol (25 mL). The resultant mixture was left at room temperature for 4 h. The excess diazomethane was quenched with drops of acetic acid. Removal of the solvent afforded a residue which was crystallized from methanol to give the methyl ester 17 (9.5 g, 95%).13 mp 248–250 °C; νmax(neat)/cm−1 3395, 2946 and 1725; 1H NMR (300 MHz; CDCl3):δ 5.28 (1H, br s), 3.71 (1H, br s), 3.61 (3H, s), 3.57 (1H, br s), 3.48 (2H, m), 2.84 (1H, d, J 13.2 Hz), 1.95–0.67 (41H, m); 13C NMR (75 MHz; CDCl3): δ 178.3, 144.0, 122.3, 80.1, 69.8, 69.7, 58.3, 51.7, 48.1, 47.7, 46.9, 46.0, 41.9, 41.5, 39.5, 38.3, 38.2, 34.0, 33.1, 32.7, 32.5, 30.7, 28.2, 27.8, 26.1, 23.6, 23.5, 23.2, 18.4, 17.1 and 12.9.
Preparation of the isopropylidene derivative 18
A mixture of methyl arjunolate 17 (500 mg, 1 mmol) in dry acetone (50 mL) containing a catalytic amount of conc. hydrochloric acid and activated 4Å molecular sieves was stirred for 6 h. The reaction mixture was filtered, concentrated and the residue was purified by column chromatography (hexane/ethyl acetate, 7/3) to give 18 (412 mg, 76%); mp 179–181°C (from ethyl acetate); (Found: C, 75.3; H, 10.1; C34H54O5 requires C, 75.2 and H, 10.0%); Rf (30% ethyl acetate/hexane 0.4); νmax(neat)/cm−1 3492, 2945 and 1725; 1H NMR (300 MHz; CDCl3): δ 5.28 (1H, br t), 3.78 (1H, dt, J 4.5 and 9.7 Hz), 3.62 (3H, s), 3.61 (1H, d, J 9.7 Hz), 3.48 (1H, d, J 3.3 Hz), 3.32 (1H, d, J 9.3 Hz), 2.87 (1H, dd, J 14.4 and 5.5 Hz), 2.23 (1H, s), 2.07–0.88 (44H, m); 13C NMR (75 MHz; CDCl3): δ 177.6, 143.4, 122.0, 99.4, 82.0, 72.5, 64.9, 51.4, 51.3, 47.6, 46.4, 46.2, 45.7, 41.5, 41.1, 39.3, 37.9, 36.8, 33.8, 33.1, 32.2, 32.0, 30.6, 29.6, 27.5, 25.9, 23.6, 23.1, 22.9, 19.3, 17.7, 17.4, 16.7 and 13.4.
Xanthate derivative 19
A solution of 18 (1.1 g, 2 mmol), sodium hydride (240 mg, 10 mmol), imidazole (50 mg, 0.74 mmol) in THF (10 mL) was stirred for 30 min. and freshly distilled carbon disulphide (1 mL, excessive amount) was added and the mixture was heated under reflux for 2 h. The reaction mixture was cooled to ambient conditions and methyl iodide (1.5 mL, excess) added. The mixture was further heated under reflux for 1 h. Water (15 mL) was added to the cooled mixture and extracted with ether (3 x 20 mL). The organic layer was washed with brine and dried. Removal of the solvent under reduced pressure afforded a residue which was purified by chromatography on silica gel (hexane/ethyl acetate, 10/1) to obtain 19 (1.16 g, 92%). mp 214–216°C; (Found: C, 68.55; H, 9.0; C36H56O5S2 requires C, 68.3 and H, 8.9%); νmax(neat)/cm−1 2946, 1725 and 1197; 1H NMR (300 MHz; CDCl3):δ 5.78 (1H, dd, J 10.3 and 4.2 Hz), 5.26 (1H, br s), 3.80 (1H, d, J 10.3 Hz), 3.62 (3H, s), 3.44 (1H, d, J 10.3 Hz), 2.86 (1H, dd, J 4.0 and 13.0 Hz), 2.51 (3H, s), 2.36 (1H, dd, J 4.8 and 12.3 Hz), 2.00–0.70 (44H, m); 13C NMR (75 MHz; CDCl3):δ 178.2, 143.7, 121.8, 99.4, 78.8, 78.6, 77.20, 72.6, 51.5, 51.1, 47.7, 46.6, 45.8, 43.4, 41.6, 41.2, 39.4, 38.8, 37.8, 33.8, 33.1, 32.3, 32.0, 30.7, 29.7, 27.6, 26.9, 26.0, 23.6, 23.3, 23.0, 19.2, 18.5, 17.4, 17.3, 16.6 and 13.5.
Methyl hederagenin 21 (from arjunolic acid)
A catalytic amount of AIBN was added to a mixture of 19 (1.0 g, 1.58 mmol) and TBTH (0.5 mL) in dry benzene (10 mL). The reaction mixture was heated under reflux for 2 h, the solvent was removed under reduced pressure and the residue refined by chromatography (hexane/ethyl acetate, 10/1) to obtain a white powder 20 (673 mg, 81%). This compound was sufficiently pure to be used for the subsequent operation. νmax(neat)/cm−1 2942 and 1724; 1H NMR (300 MHz; CDCl3): δ 5.28 (1H, t, J 3.6 Hz), 3.62 (3H, s), 3.47 (1H, m), 3.53 (1H, d, J 10.0 Hz), 3.44 (1H, d, J 10.0 Hz), 2.86 (1H, dd, J 14.0 and 4.8 Hz), 2.04–0.70 (46H, m). Amberlite-450 was added to 20 (600 mg, 1.14 mmol) in methanol (6 mL) and the resultant mixture allowed to stand at ambient temperature for 12 h with occasional shaking. The mixture was filtered through a pad of celite and the filtrate concentrated under reduced pressure. The resultant residue was purified by column chromatography (hexane/ethyl acetate, 3:2) to give methyl hederagenin as a colorless solid 21 (482 mg, 87%).14 mp 240–242°C; Rf (40% ethyl acetate/hexane 0.42); identical with an authentic sample prepared from hederagenin, νmax(neat)/cm−1 3507, 2942 and 1724; 1H NMR (300 MHz; CDCl3): δ 5.28 (1H, br t), 3.74 (1H, d, J 10.0 Hz), 3.63 (1H, br s), 3.62 (3H, s), 3.44 (1H, d, J 10.0 Hz), 2.86 (1H, dd, J 13.2 and 3.3 Hz), 2.32–0.70 (42H, m); 13C NMR (75 MHz; CDCl3): δ 178.5, 144.2, 122.4, 118.2, 80.2, 69.6, 69.5, 51.5, 48.0, 47.6, 46.5, 45.0, 41.7, 41.5, 39.6, 38.3, 38.2, 34.0, 33.4, 32.5, 32.3, 30.3, 28.4, 27.8, 26.2, 23.5, 23.4, 23.1, 17.1 and 13.1.
Isolation of hederagenin (3,23-dihydroxyolean-12-en-28-oic acid)
Dry pericap of soapnuts, Sapindus mukorossi, (S. emarginatus) abundantly available in southern states of India were collected. The pulverized, dried pericaps of soap nuts (125 g) were defatted by extraction with hexane (200 mL) in a soxhlet extractor. This was further extracted with ethyl acetate (300 mL) followed by methanol (300 mL). The methanol extract (16.2 g) was submitted for hydrolysis with 6N hydrochloric acid (60 mL) for 2 h. The acidic mass was carefully neutralized with 5% sodium hydroxide solution and extracted with ethyl acetate (5 x 60 mL). The combined ethyl acetate extract was evaporated and dried under vacuum. The crude sapogenin, hederagenin (6.5 g) was crystallized from methanol to afford pure hederagenin, mp 333–335 °C; νmax(neat)/cm−1 3447, 2930, 1698 and 756; 1H NMR (300 MHz; CDCl3): δ 5.46 (1H, br s), 4.22–4.24 (3H, m), 3.72 (1H, d, J 10.2 Hz), 3.32 (1H, d, J 10.2 Hz), 2.34 (1H, d, J 9.6 Hz), 2.16–0.91 (41H, m); m/z (DI) 248, 203 (100%), 191, 173, 133.
Methyl hederagenin 21 (from hederagenin)
Ethereal diazomethane was added to an ice-cold suspension of hederagenin (600 mg, 1.20 mmol) in methanol (4 mL). The resultant mixture was left at ambient temperature for 4 h. The excess diazomethane was quenched using drops of acetic acid. The solvent was removed by evaporation under reduced pressure and the residue purified by column chromatography on silica gel (hexane/ethyl acetate, 3:2) to give the methyl ester (570 mg, 95%). This compound was identical with compound 21.14 mp 240–242°C.
23-O-t-butyldimethylsilyl derivative of methyl hederagenin 22
tert-Butyldimethylsilyl chloride (159 mg, 1.06 mmol) in dichloromethane (1 mL) was added to a mixture of the diol 21 (432 mg, 0.88 mmol) and imidazole (108 mg, 1.59 mmol) in dry dichloromethane (1.5 mL) at 0°C over a period of 30 min. The reaction mixture was stirred for 6 h and worked up to give a residue which was purified by chromatography. Elution with hexane/ethyl acetate (9/1) afforded a viscous oil 22 (480 mg, 91%), (Found: C, 73.94; H, 10.73; C37H64O4Si, requires C, 74.0 and H, 10.33%); Rf (5% ethyl acetate/hexane 0.3); νmax(neat)/cm−1 3420, 2949 and 1726; 1H NMR (300 MHz; CDCl3): δ 5.27 (1H, br t), 3.67 (1H, d, J 9.0 Hz), 3.61 (3H, s), 3.60 (1H, d, J 5.4 Hz), 3.35 (1H, d, J 9.0 Hz), 2.85 (1H, dd, J 14.0 and 4.0 Hz), 2.16 (1H, s), 1.95–0.70 (55H, m).
Methyl 23-O-t-butyldimethylsilyl-3-oxoolean-12-en-28-oate 23
A solution of alcohol 22 (376 mg, 0.67 mmol) in dry dichloromethane was added to mixture of pyridinium chlorochromate (731 mg, 3.40 mmol) and sodium acetate (730 mg, 8.9 mmol) in dry dichloromethane. The reaction mixture was stirred for 4 h before filtering through a pad of celite. The concentrated residue was refined by column chromatography on silica gel (hexane/ethyl acetate, 95/5) to give viscous oil 23 (292 mg, 78%). (Found: C, 73.65; H, 10.75; C37H62O4Si requires C, 74.25 and H, 10.36%); Rf (5% ethyl acetate/hexane 0.5); νmax(neat)/cm−1 2927, 2856, 1731 and 1703; 1H NMR (300 MHz; CDCl3) 5.33 (1H, br t), 3.62 (3H, s), 3.61 (1H, d, J 9.0 Hz), 3.27 (1H, d, J 9.0 Hz), 2.86 (1H, m), 2.38 (2H, m), 2.04–0.8 (53H, m).
Isoxazole of methyl hederagenin 25
A mixture of the ketone 23 (250 mg, 0.42 mmol), ethyl formate (0.2 mL, excess) and sodium tertiary amylate {prepared from sodium (48 mg, 2.09 mmol) and t-amyl alcohol (1.5 mL)}in benzene (2 mL) was stirred at room temperature for 8 h. The reaction mixture was acidified with 2N hydrochloric acid and extracted with ether (3 x 15 mL), washed with brine and dried over anhydrous sodium sulfate. The organic layer was concentrated to afford a glassy solid 24 which was directly used in the next step. νmax(neat)/cm−1 2948, 2856 and 1728; 1H NMR (300 MHz; CDCl3): δ 8.31 (1H, d, J 3.3 Hz), 5.30 (1H, br s), 3.70 (1H, d, J 9.0 Hz), 3.63 (3H, s), 3.62 (1H, br s), 3.34 (1H, d, J 9.0 Hz), 2.95-2.85 (1H, m), 2.21–0.80 (55H, m). The crude formyl derivative 24 in methanol (3 mL), hydroxylamine hydrochloride (58 mg, 0.84 mmol) and sodium acetate (68 mg, 0.84 mmol) were heated under refluxed for 8 h. The reaction mixture was cooled and the solvent removed under reduced pressure. The residue in water (6 mL) was extracted with ethyl acetate (15 x 3 mL). The combined organic layers were washed with water, brine and dried over anhydrous sodium sulfate. The residue was purified by column chromatography. Elution with (hexane/ethyl acetate, 4/1) afforded the pure isoxazole 25 (146 mg, 68% for two steps). mp 218–220°C; (Found: C, 74.95; H. 9.3; N, 2.45; C32H47NO4 requires C, 75.4; H. 9.3 and N. 2.75%); Rf (20% ethyl acetate/hexane 0.5); ν-max(neat)/cm−1 2974, 1732, 1656, 1620, 1460, 1385 and 870; 1H NMR (300 MHz; CDCl3): δ 8.02 (1H, s), 5.34 (1H, br t), 3.80 (1H, d, J 11.0 Hz), 3.63 (3H, s), 3.55 (1H, d, J 11.0 Hz), 2.9 (1H, dd, J 4.0 and 14.4 Hz), 2.5–0.8 (39H, m); m/z(DI) 507 (M+), 310, 276, 135 (100).
Methyl 2-cyano-23-hydroxy-3-oxooleana-1,12-dien-28-oate 6
The cyano-ketone (179 mg, 96%), Rf (20% ethyl acetate in hexane 0.3) was prepared from the isoxazole 25 (186 mg, 0.36 mmol) by hydrolysis using sodium methoxide in methanol. Since this compound could not be purified, it was directly used in the next step. The crude cyano-ketone (155 mg, 0.30 mmol) was oxidized using DDQ to give the cyano-enone 6 (98 mg, 65%). mp 221–223 °C; (Found: C, 75.5; H, 8.7; N, 2.6; C32H45NO4 requires C, 75.7; H, 8.9 and N, 2.8%); Rf (20% ethyl acetate in hexane 0.25) νmax(neat)/cm−1 2951, 2232, 1727, 1685, 1655, 1610 and 802; 1H NMR (300 MHz; CDCl3): δ 7.74 (1H, s), 5.34 (1H, t, J 3.3 Hz), 3.76 (1H, d, J 10.8 Hz), 3.63 (3H, s), 3.62 (1H, br s), 3.43 (1H, d, J 10.8 Hz), 2.89 (1H, dd, J 5.7 and 14.7 Hz), 2.15–2.12 (2H, m), 2.00–1.92 (2H, m), 1.71–0.83 (32H, m); 13C NMR (75 MHz; CDCl3): δ 198.0, 177.6, 169.5, 144.8, 121.0, 115.0, 114.3, 67.4, 51.6, 50.2, 46.7, 45.7, 45.3, 42.2, 41.6, 41.3, 40.5, 40.4, 34.0, 33.4, 32.3, 32.0, 30.9, 27.8, 26.0, 23.8, 23.4, 23.0, 19.1, 18.5, 17.6 and 17.2.
Isolation of β-boswellic acid from the gum of Boswellia serrata:15
The commercially available gum (100 g) of Boswellia serrata was extracted with ethyl acetate (3 x 100 mL) and the ethyl acetate extract was filtered and concentrated under vacuum. The syrupy mass (45 g) was treated with 5% sodium hydroxide solution and stirred till a uniform emulsion is formed. The emulsion was extracted with hexane (3 x 100 mL). The aqueous extract was acidified with dilute hydrochloric acid and the precipitate was extracted with ethyl acetate (3 x 100 mL). The ethyl acetate extract was thoroughly washed with water, dried over anhydrous sodium sulfate and concentrated to afford a creamy powder (22 g) consisting a mixture of boswellic acids. This mixture (5 g) was chromatographed on a silica gel column. Elution with hexane-ethyl acetate (4:1) afforded a mixture of β-, and α-boswellic acids (2.2 g) in the ratio of 9:1, Rf (20% ethyl acetate/hexane 0.3).
Methyl boswellates 27
The methyl esters of α- and β-boswellic acids 27 (606 mg, 98%) were obtained from boswellic acids 26 (602 mg, 1.32 mmol) by esterification with ethereal diazomethane.15 m.p 195°C; (Found: C, 78.83; H, 10.50; C31H50O3 requires C, 79.10; H, 10.78%); Rf (20% ethyl acetate/hexane 0.6); νmax(neat)/cm−1 3511, 2921 and 1722; 1H NMR (300 MHz; CDCl3): δ 5.13 (1H, s), 4.10 (1H, br s), 3.61 (3H, s), 2.23–0.78 (45H, m); m/z (DI) 470 (M+), 394, 161.
Methyl 3-ketoboswellates 28
A solution of the alcohol 27 (658 mg, 1.40 mmol) and PDC (602 mg, 1.60 mmol) were taken in dry dichloromethane (6 mL). The reaction mixture was stirred for 4 h before filtering through a pad of celite. The concentrated residue was purified by column chromatography on silica gel (20% ethyl acetate in hexane) to give a colorless solid 28 (467 mg, 71%).15 m.p. 160°C; (Found: C, 79.35; H, 10.25; C31H48O3 requires C, 79.40; H, 10.30%); Rf (20% ethyl acetate/hexane 0.8); νmax(neat)/cm−1 2949, 1742, 1714 and 1662; 1H NMR (300 MHz; CDCl3): δ 5.54 and 5.30 (1H, br s), 3.68 (3H, s), 2.92 (1H, dt, J 6.0 and 14.7 Hz), 2.34 (1H, d, J 14.7 Hz Hz), 2.16–0.76 (42H, m); 13C NMR (75 MHz; CDCl3): δ 207.9, 173.9, 139.9, 124.0, 59.2, 58.2, 57.4, 52.0, 46.6, 42.3, 41.5, 41.1, 39.9, 39.7, 37.2, 36.5, 33.9, 33.0, 31.3, 28.9, 28.1, 26.6, 23.7, 23.2, 21.5, 21.0, 20.6, 17.6, 16.9 and 13.4; m/z (DI) 468 (M+), 218 (100%).
Preparation of the isoxazole 30
Employing a protocol similar to the synthesis of 25 from 23, the crude of the 2-hydroxy-methylene derivative 29 was obtained from 28 (456 mg, 0.98 mmol) which was directly used for the synthesis of isoxazole 30 (358 mg, 74%). (Found: C, 77.8; H, 9.6; N, 2.85; C32H47NO3 requires C, 77.9; H, 9.53; N, 2.84%); Rf (20% ethyl acetate/hexane 0.57); νmax(neat)/cm−1 2918, 1732 and 1646; 1H NMR (300 MHz; CDCl3): δ 8.05 (1H, s), 5.24 and 5.19 (1H, br t), 3.63 (3H, s), 2.53 (1H, m), 2.15–0.77 (41H, m); 13C NMR (75 MHz; CDCl3): δ 172.9, 166.9, 150.1, 139.7, 123.9, 121.27, 111.2, 77.2, 59.1, 55.3, 52.1, 47.3, 46.8, 46.3, 45.1, 42.2, 41.8, 41.4, 39.8, 39.7, 39.5, 38.2, 38.2, 37.0, 35.9, 35.6, 34.6, 33.8, 33.3, 32.5, 32.4, 32.0, 31.2, 31.1, 28.8, 28.4, 28.1, 26.9, 26.6, 26.1, 25.6, 24.4, 23.6, 23.0, 21.3, 19.8, 17.4, 16.4, 16.3, 14.5 and 14.3; m/z (DI) 493 (M+), 218 (100%).
Cyano-enone of methyl boswellates 7
Following the bromination-dehydrobromination protocol employed for the synthesis of 4 from 13, the cyano-enone of α- and β-methyl boswellates 7 (90 mg, 54% yield) was obtained from 30 (174 mg, 0.34 mmol) as an amorphous solid. The isomers were observed in a (1:9) ratio, based on the integration of the 1H proton signals. (Found: C, 78.4; H, 9.0; N, 2.5. C32H45NO3 requires C, 78.2; H, 9.2; N, 2.85%); Rf (20% ethyl acetate/hexane 0.4); νmax(neat)/cm−1 2922, 2234, 1729, 1698 and 1612; 1H NMR (300 MHz; CDCl3): δ 7.62 and 7.60 (1H, s), 5.17 and 5.12 (1H, br t), 3.59 (3H, s), 2.17–0.79 (40H, m); 13C NMR (75 MHz; CDCl3): δ 190.3, 172.5, 168.0, 146.0, 140.7, 122.5, 120.0, 114.7, 113.6, 59.0, 54.5, 54.4, 54.3, 52.5, 47.3, 46.5, 42.5, 41.4, 41.3, 41.0, 40.8, 40.7, 39.54, 39.5, 36.9, 34.6, 33.7, 33.3, 32.6, 32.5, 32.3, 31.6, 31.1, 31.0, 28.3, 27.8, 26.9, 26.6, 26.4, 25.9, 25.7, 23.6, 23.5, 23.3, 23.1, 22.6, 21.3, 20.9, 19.3, 17.5, 17.4, 14.1, 14.0 and 13.9; m/z (DI) 491 (M+), 476, 432, 218 (100%), 203.
BIOASSAYS
Nitric Oxide assay
Thioglycolate (4%) elicited peritoneal macrophages were isolated from two months old C57black6 male mice after sacrificing by cervical dislocation. The cells were cultured for 72h in presence of mouse interferon gamma (10U/ml) either alone or in presence of test compounds. Induced nitric oxide rapidly oxidized into nitrite in culture medium (RPMI+10%FBS) and the nitrite concentration in supernatants was taken as a measure of nitric oxide production. Griess assay10 was done to measure the nitrite in the medium.
MTT assay for cytotoxicity
Cancer cell lines were plated in 96-well plates (Nunc, Denmark) at a density of 5 x 103 – 8 x 103 cells/well depending on the cell line. The plates were incubated at 37°C in a humidified 5% CO2 atmosphere. Cells were allowed to adhere to the surface for 3–4 h and different concentrations of the compounds were added and incubated for 72 h. Later, 20μL of MTT solution (5mg/mL) was added to each well and the incubation continued for an additional 3h. The dark blue formazan crystals formed within the healthy cells were solubilized with DMSO and the absorbance was measured in an ELISA plate reader at 550 nm. The EC50 values of the compounds were calculated by Graphpad Prism.
Acknowledgments
This work is supported by a grant from NIH-Fogarty International Program to M.B.S. Our thanks are due to CSIR and INSA for financial assistance to GSRSR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Honda T, Rounds BV, Gribble GW, Suh N, Wang Y, Sporn MB. Bioorg Med Chem Lett. 1998;8:2711. doi: 10.1016/s0960-894x(98)00479-x. [DOI] [PubMed] [Google Scholar]
- 2.Chauhan D, Li G, Poddar K, Hideshima T, Shringarpure R, Catley L, Mitsiades C, Munshi N, Tai YT, Suh N, Gribble GW, Honda T, Schlossman R, Richardson P, Sporn MB, Anderson K, Lipper J. Blood. 2004;103:3158. doi: 10.1182/blood-2003-08-2873. [DOI] [PubMed] [Google Scholar]
- 3.Place AE, Suh N, Williams CR, Risingsong R, Honda T, Honda Y, Gribble GW, Leesnitzer LM, Stimmel JB, Wilson TM, Rosen E, Sporn MB. Clin Cancer Res. 2003;9:2798. [PubMed] [Google Scholar]
- 4.Honda T, Rounds BV, Bore L, Favaloro FG, Jr, Gribble GW, Suh N, Wang Y, Sporn MB. Bioorg Med Chem Lett. 1999;9:3429. doi: 10.1016/s0960-894x(99)00623-x. [DOI] [PubMed] [Google Scholar]
- 5.Honda T, Gribble GW, Suh N, Finlay HJ, Rounds BV, Bore L, Favaloro FG, Jr, Wang Y, Sporn MB. J Med Chem. 2000;43:1866. doi: 10.1021/jm000008j. [DOI] [PubMed] [Google Scholar]
- 6.Honda T, Rounds BV, Bore L, Finlay HJ, Favalaro FG, Jr, Suh N, Wang Y, Sporn MB, Gribble GW. J Med Chem. 2000;43:4233. doi: 10.1021/jm0002230. [DOI] [PubMed] [Google Scholar]
- 7.Suh N, Wang Y, Honda T, Gribble GW, Dmitrovsky E, Hickey WF, Maue RA, Place AE, Porter DM, Spinella MJ, Williams CR, Wu C, Dannenberg AJ, Flanders KC, Letterio JJ, Mangelsdorf DJ, Nathan CF, Nguyen L, Porter WW, Ren RF, Roberts AB, Roche NS, Subbaramaiah K, Sporn MB. Cancer Res. 1999;59:336. [PubMed] [Google Scholar]
- 8.Wang Y, Porter WW, Suh N, Honda T, Gribble GW, Leesnitzer LM, Plunket KD, Mangelsdorf DJ, Blanchard SG, Wilson TM, Sporn MB. Mol Endocrinol. 2000;14:1550. doi: 10.1210/mend.14.10.0545. [DOI] [PubMed] [Google Scholar]
- 9.Beaton JM, Spring FS. J Chem Soc. 1955:3126. [Google Scholar]
- 10.Johnson WS, Shelberg WE. J Am Chem Soc. 1945;67:1745. [Google Scholar]
- 11.Doorenbos NJ, Milewich L. J Org Chem. 1966;31:3193. doi: 10.1021/jo01348a024. [DOI] [PubMed] [Google Scholar]
- 12.Campsteyn H, Dupont L, Lamotte J, Dideberg O, Vermeire M. Acta Cryst B. 1977;33:3443. [Google Scholar]
- 13.Row LR, Subba Rao GSR. Tetrahedron. 1962;18:827. [Google Scholar]
- 14.Jacobs WA. J Biol Chem. 1925;63:621. [Google Scholar]
- 15.Taneja SC, Sethi VK, Dhar KL, Kapil RS. European Patent 755940. 1997; Simpson JCE, Williams NE. J hem Soc. 1938:686. [Google Scholar]
- 16.Konopleva M, Tsao T, Ruvolo P, Stiouf I, Estroy Z, Leysath CE, Zhao S, Harris D, Chang S, Jackson CE, Munsell M, Suh N, Gribble GW, Honda T, May WS, Sporn MB. Blood. 2002;99:326. doi: 10.1182/blood.v99.1.326. [DOI] [PubMed] [Google Scholar]
- 17.Ji Y, Lee HJ, Goodman C, Uskokovic M, Liby K, Sporn MB, Suh N. Mol Can Ther. 2006;5:1452. doi: 10.1158/1535-7163.MCT-06-0136. [DOI] [PubMed] [Google Scholar]
- 18.Honda T, Liby KL, Su X, Sundararajan C, Honda Y, Suh N, Risingsong R, Williams CR, Royce DB, Sporn MB, Gribble GW. Bioorg Med Chem Lett. 2006;16:6306. doi: 10.1016/j.bmcl.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding A, Nathan C, Graycar J, Derynck R, Stuehr DJ, Srimal S. J Immunol. 1990;145:940. [PubMed] [Google Scholar]