Abstract
BACKGROUND
Among the diverse animal models proposed for schizophrenia, the neonatal ventral hippocampal lesion (NVHL) is one of the most widely used. However, its construct validity can be questioned because there is no evidence of a lesion present in schizophrenia. Other approaches that have tried to capture environmental influences on development include diverse models of maternal infection.
METHODS
As the early postnatal days in rodents are equivalent to the third trimester of human pregnancy in terms of brain development, we decided to test whether a neonatal immune challenge with an injection of the bacterial endotoxin lipopolysaccharide (LPS) into the ventral hippocampus caused deficits in interneuron function similar to those reported for the NVHL.
RESULTS
Neonatal LPS injection caused a persistent elevation in cytokines in several brain regions, deficits in prepulse inhibition of the acoustic startle response and a loss of the periadolescent maturation in the response of prefrontal cortical fast-spiking interneurons to dopamine.
CONCLUSIONS
The same phenotypes elicited by a NVHL can be obtained with an intra-hippocampal immune challenge, suggesting that perinatal environmental factors can affect adult prefrontal interneuron maturation during adolescence.
Keywords: schizophrenia, prefrontal cortex, animal model, electrophysiology, dopamine, interneuron
INTRODUCTION
As the genetic factors that may confer predisposition for schizophrenia are being unveiled, the case for a developmental nature in this disorder is strengthening. A variety of rodent models have been employed to test pathophysiological and behavioral changes derived from genetic, environmental and developmental alterations. Perhaps one of the most extensively studied models is the neonatal ventral hippocampal lesion (NVHL), which yields a variety of cellular, synaptic and behavioral deficits that emerge during or after adolescence (1, 2). A critical finding with this model is the absence of prefrontal cortical interneuron maturation during this late developmental stage (3), and this observation may relate to the consistent observation of parvalbumin interneuron deficits in schizophrenia patients (4, 5). A major drawback of the NVHL model, however, is the presence of a lesion, which is not typically observed in the disease. Although it has been argued that the construct validity of this model does not reside in the hippocampal lesion but in its downstream consequences on prefrontal cortical development at a critical stage (6), it remains to be determined whether interfering with ventral hippocampal function without causing a lesion yields abnormal prefrontal interneuron function in the adult.
Other models testing environmental factors include prenatal immune challenge with maternal injection of the bacterial endotoxin lipopolysaccharide (LPS), which yields abnormal behaviors in the offspring (7). LPS is a protein-free endotoxin derived from the cell wall of gram-negative bacteria following multiplication or lysis, and it causes the release of a variety of proinflammatory mediators, including interleukins, from immune cells (8). Systemic administration of synthetic double-strand RNA (poly I:C) to mimic maternal viral infection also causes deficits in latent inhibition and prepulse inhibition (PPI), as well as enhanced sensitivity to amphetamine, cognitive impairment and changes in dopamine (DA) turnover and DA receptor binding (9, 10), which are only evident in the adult offspring. Prenatal viral inoculation has also been shown to cause abnormal hippocampal morphology (11). Furthermore, neonatal intracerebral injection of lymphocytic choriomeningitis virus yields deficits in hippocampal interneurons and a hyper-excitable hippocampus (12), inoculation of Borna disease virus causes hippocampus-dependent deficits in memory functions (13), and systemic neonatal injection of LPS yields pathological changes in hippocampal parvalbumin interneurons (14). Thus, perinatal immune challenge can affect hippocampal structure and function, yielding schizophrenia-related phenomena in the adult animal.
Here, we combined both approaches to produce ventral hippocampal deficits without a lesion. We assessed whether injecting LPS into the ventral hippocampus (VH) at postnatal day (PD) 6–7 can cause (i) persistent activation of immune factors, (ii) PPI deficits, and (iii) alter PFC circuit maturation, in particular the DA modulation of fast-spiking interneurons that matures during adolescence (15). The neonatal time window of LPS injection was chosen because disruption of hippocampal network activity during this early period can exert marked deficits in PFC physiology with a periadolescent onset (2, 3, 16), and we used a dose known to activate the immune system within the brain (17).
METHODS AND MATERIALS
Neonatal ventral hippocampal LPS injection
All experimental procedures were performed according to the United States Public Health Service Guide for Care and Use of Laboratory Animals and approved by the Albany Medical College Institutional Animal Care and Use Committee and the University of Maryland School of Medicine Institutional Animal Care and Use Committee. Timed pregnant Sprague-Dawley females were obtained at embryonic day 15–18 from Charles River (MA), and individually housed with free access to food and water in a temperature and humidity controlled environment with a 12h:12h light/dark cycle (lights on at 7:00 am). Pups were left undisturbed until postnatal day (PD) 7, when they were weighed and their sex determined. Female pups and underdeveloped male pups were euthanized. Between PD7 and PD8, healthy male pups (15–20 g) received a bilateral injection of either vehicle (sham) or LPS in the VH. LPS (Salmonella enteritidis LPS B – batch #651628) was obtained from Difco Labs (Detroit, MI). Pups were anesthetized by hypothermia and secured to a platform placed in a stereotaxic apparatus (David Kopf, Tujunga, CA), and the scalp incised. Rats received bilateral infusions (0.3 μl side; 0.15 μl/min) of LPS (10 μg/μl in artificial CSF) into the ventral hippocampus, at 3 mm caudal to bregma, 3.5 mm lateral to midline, and 5 mm from the surface of the skull. Sham animals received bilateral infusions of artificial CSF into the ventral hippocampus at the same coordinates. The infusion needle was left in place for 3 min following each infusion to allow for diffusion away from the needle tip. The wound was closed with wound clips, and pups were placed on a warming pad until their respiration and locomotor activity levels returned to normal. Pups were returned to their mothers and remained undisturbed except for husbandry until the wound clips were removed at approximately PD23. All rats were maintained on a 12 h light/dark cycle with food and tap water available ad libitum until the time of the experiments.
Behavioral testing
We tested PPI in LPS and sham rats at PD>56 (late adolescence/young adult stage) (18). Rats were placed in a sound-attenuated startle chamber (San Diego Instruments) with a 70 dB background white noise. After a 5 min. period of adaptation, the PPI test was initiated with pseudorandom trials every 20–30 s. Either pulse (120 dB), prepulse (75, 80 or 85 dB), no pulse, or prepulse + pulse were delivered. Trials lasted 23 min. and 8–10 repetitions of pulse or prepulse + pulse trials were acquired, while null or prepulse only trials were repeated 5 times for each prepulse amplitude. The startle box is equipped with an acceleration-sensitive transducer that conveys the extent of motor reaction to the tones to a computer. Overall startle was measured with the deflections caused by pulse alone, and PPI was calculated as the ratio in startle between prepulse + pulse and pulse alone, and expressed as percent reduction. The initial five trials (all pulse alone) were used for habituation and not included in the analysis. Following behavioral testing, the rats were returned to their cages and used for either electrophysiological recordings or cytokine measurements a week later.
Electrophysiology
Whole-cell recordings from PFC interneurons were conducted in rats from both LPS and sham groups tested for PPI. Slices containing the medial PFC were prepared one week after the behavioral testing. Rats were deeply anesthetized with chloral hydrate (400 mg/kg, i.p.) before decapitation. Brains were quickly and gently removed and 4-mm-thick coronal tissue blocks were cut using rat brain matrices. The blocks were sectioned in ice-cold artificial CSF (aCSF) containing: 125 mM NaCl, 25 mM NaHCO3, 10 mM glucose, 3.5 mM KCl, 1.25 mM NaH2PO4, 0.5 mM CaCl2, 3 mM MgCl2; pH=7.45; osmolarity 295 mOsm. Coronal slices (350 μm) containing the medial PFC were cut on a Vibratome in ice-cold aCSF and transferred to a warm (35°C) aCSF solution constantly oxygenated with 95% O2-5% CO2 for at least 1 hour before recording. Slices were transferred to a submersion-type recording chamber maintained at 33–35°C and superfused with oxygenated recording aCSF at a flow rate of 2 ml/min delivered with a peristaltic pump. In the recording aCSF, CaCl2 was changed to 2 mM and MgCl2 was reduced to 1 mM. Patch-pipettes (6–9 MΩ) were obtained from 1.5 mm borosilicate glass capillaries (WPI) pulled with a horizontal Flaming-Brown puller (P97; Sutter Instruments; Novato, CA) and filled with 115 mM K-gluconate, 10 mM HEPES, 2 mM MgCl2, 20 mM KCl, 2 mM Mg-ATP, 2 mM Na2-ATP, 0.3 mM GTP; pH=7.3; 280–285 mOsm Neurobiotin (0.125%) was added to the internal solution for labeling of the recorded cells. Quinpirole and eticlopride were purchased from Sigma (St. Louis, MO), and were mixed into oxygenated recording aCSF in known concentrations. PFC interneurons were identified under visual guidance using infrared-differential interference contrast (IR-DIC) with an Olympus BX51-WI microscope and an infrared camera (Dage-MTI; Michigan City, IN). Whole-cell current-clamp recordings were performed with a Multiclamp 700A amplifier (Axon instruments; Sunnyvale, CA). Data collected were digitized at a sampling rate of 10 kHz and transferred to a computer via a data acquisition board (Digidata 1322A; Axon). At the end of the experiment, the slices were placed in 4% paraformaldehyde and a subsequent avidin-biotin reaction was used to label Neurobiotin-injected cells.
Whole-cell recordings from interneurons were used to test the response to the D2 agonist quinpirole. Current-clamp recordings were used to determine membrane properties (resting potential, input resistance) and cell excitability. The latter was assessed by measuring the latency to the first spike and the number of action potentials evoked by an intracellular 500 ms current pulse (up to 200 pA). Current pulses were adjusted so as to evoke 6–15 action potentials and their amplitude was left constant as the pulses were delivered every 20 sec. Pyramidal neurons and fast-spiking interneurons (FSI) could be identified by their morphology in the infrared field and by their distinct electrophysiological profile (15, 19). Once 10 min. of baseline excitability data had been recorded, the D2 family agonist quinpirole (1 μM) was applied for 5–10 minutes, and then washed out. This concentration was used based on previous determination of dose response curves in FSI (15).
Cytokine assays
Another set of adult rats that had a neonatal LPS injection or sham treatment were used to harvest brain tissue for determining cytokine levels. Rats were anesthetized by a brief exposure to ether, decapitated and the brains rapidly and gently removed. Pieces containing the areas of interest (i.e., the hippocampus, PFC, and nucleus accumbens) were dissected and frozen at −70°C until the time of the assay. These pieces were run for ELISA assays of IL-1, IL-2 and TNF-α. Each tissue piece was added to 0.5–1.0 ml of Iscove’s culture medium containing 5% fetal calf serum and a cocktail enzyme inhibitor (in mM: 100 amino-n-caproic acid, 10 EDTA, 5 benzamidine-HCl, and 0.2 phenylmethylsulfonyl fluoride). Tissue was mechanically dissociated using an ultrasonic cell disruptor (Heat Systems, Inc., Farmingdale, NY) for 10 s. Sonicated samples were centrifuged at 10,000 rpm at 4°C for 10 min., and supernatants were removed and stored at 4°C until an ELISA was performed. Bradford protein assays were also performed to determine total protein concentrations in brain sonication samples. IL-1β, IL-2, and TNF-α concentrations were measured in tissue homogenates using ELISAs specific for rat IL-1β, IL-2, and TNF-α (Assay Designs, Ann Harbor, MI) respectively, according to the manufacturer’s instructions. After samples and standards were added to wells, plates were incubated for 1 h at 37°C. Wells were washed seven times with wash solution, at which point, antibody was added to each well (except the blank) and incubated for 30 min at 4°C. After two additional wash procedures, substrate solution was added to each well, and plates were further incubated for 30 min at room temperature in the dark, at which point, stop solution was added to all wells. A UV spectrophotometer (model Ceres UV900 HDI; Bio-Tek Instruments, Inc., Winooski, VT) was used to read plates at 450 nm. Data are presented as mean ± SD and were analyzed by two-way ANOVA and then Tukey multiple comparison test using SigmaStat 3.0 (SPSS, Chicago, Ill). A two-sided P value of less than 0.05 was considered significant. Cytokine levels in homogenates are expressed as pg per 100 μg of total protein (detection limits: ~5 pg/ml for IL-1β, ~5 pg/ml for IL-2, and ~5 pg/ml for TNF-α).
Histological analysis
Rats were deeply anesthetized and transcardially perfused with cold saline followed by 4% paraformaldehyde. Brains were extracted and postfixed in 4% paraformaldehyde for 24 hrs before being transferred to a 30% sucrose solution. After 72 hrs in sucrose solution, the brains were cut into 50 μm sections using a freezing microtome, mounted on gelatin coated slides, and then Nissl stained to allow for the identification of any damage to the ventral hippocampus resulting from LPS or saline injection.
RESULTS
LPS and hippocampal Structure
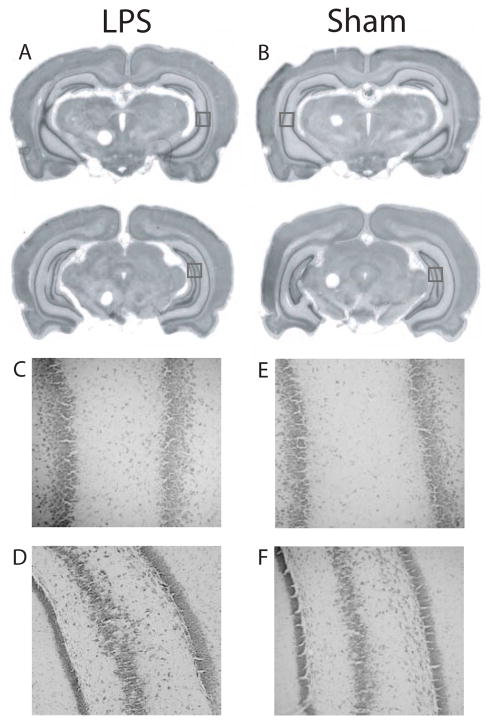
Histological analysis revealed no apparent alteration to the gross morphology of the hippocampus or surrounding brain regions following neonatal injection of LPS (Fig. 1). In some cases, the lateral ventricles appeared slightly enlarged, but this was observed in both LPS-treated and sham-operated rats.
Figure 1.
Injection of LPS did not cause damage to the dorsal or ventral hippocampus. A, B. Rostral (top row) and caudal (bottom row) views of the ventral hippocampus in representative LPS injected (A) and sham operated (B) rats indicate that LPS did not alter the morphology of the hippocampus or surrounding brain regions. C, D. Higher magnification of the squares indicated in A for a representative LPS-treated rat. E, F. Higher magnification of the squares indicated in B. The pyramidal layer in these sections is intact, and the areas represented are those typically damaged in the NVHL model.
LPS and cytokine levels
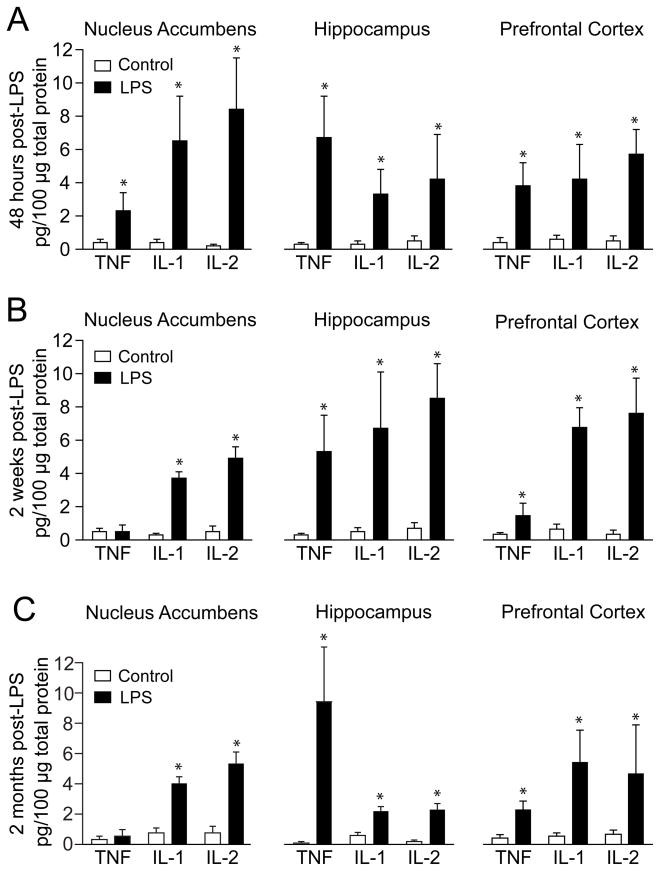
The effects of a bilateral neonatal VH LPS injection on cytokine levels were assessed in different brain regions in adult rats. LPS-treated rats exhibited higher levels of IL-1, IL-2 and TNF-α than controls in all three regions (Fig. 2A). In another set of rats, cytokine levels were assessed 2 weeks after the injection (n=6 LPS; n=6 sham). IL-1, IL-2 and TNF-α were increased in the PFC and hippocampus of LPS rats, while in the nucleus accumbens only IL-1 and IL-2 remained elevated (Fig. 2B). Cytokines were measured in the same brain regions in a third group of LPS (n=4) and sham (n=4) rat once they become adults (i.e., PD60 or older). These assays revealed a similar pattern of cytokine increases in LPS rats as the one observed 2 weeks following the injection (Fig. 2C). The data indicate that a neonatal intra-hippocampal LPS injection can alter cytokine levels in critical brain areas in the adult brain.
Figure 2.
Effect of LPS treatment on TNF, IL-1 and IL-2 protein levels in the PFC, NA and hippocampus, measured by ELISA. A. Interleukin levels 48 hours after the LPS injection. Open bars: vehicle injected rats; black bars: LPS-treated rats. B. Similar measures two weeks following the LPS injection. C. Cytokine levels in adult brains. All data in this and subsequent figures are mean ± SD. *p<0.05; Tukey multiple comparison test following a significant two-way ANOVA.
LPS and PPI
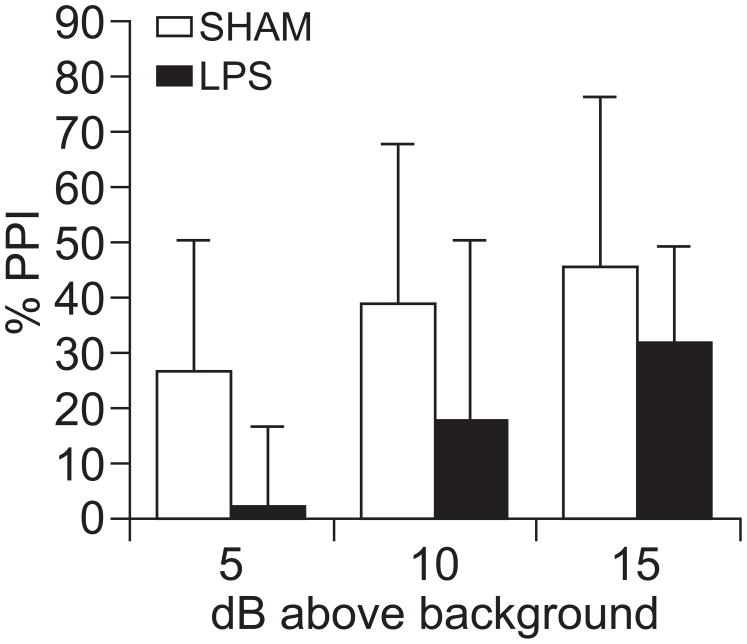
Next, we assessed PPI as a behavioral measure for sensorimotor gating deficits in another set of LPS (n=7) or vehicle (n=6) treated rats once the rats reached late adolescence or a young adult age (PD>56). Startle amplitude was not affected by the neonatal treatment (data not shown). PPI was disrupted in LPS-treated rats, when compared to the sham group (Fig. 3). Thus, a neonatal immune challenge localized to the VH can affect adult sensorimotor gating performance.
Figure 3.
PPI was disrupted in LPS-treated adult rats. Bar graph illustrating percent PPI with three prepulse intensities in sham (light) and LPS (dark) rats. Treatment status: F(1,82)=5.09; p=0.03. PPI level: F(2,82) = 3.202; p=0.055. No interaction: F(2,82) = 0.131; p=0.878.
LPS and PFC interneuron responses to DA
One week following PPI testing, PFC slices were prepared for electrophysiology experiments. Whole cell recordings were conducted from FSI located in layer V of the prelimbic and infralimbic regions of the medial PFC, as verified with Neurobiotin staining. As in other cortical regions, FSI in the adult PFC responded with constant firing to depolarizing current pulses, exhibited larger afterhyperpolarization (>15 mV) and faster spike kinetics (<0.6 ms) than pyramidal neurons. FSI recorded from sham (n=10 cells from 6 rats) and LPS-treated rats (n=9 cells from 7 rats) exhibited similar resting membrane potential (−63.0 ± 2.7 vs. −62.0 ± 2.6 mV, Sham vs. LPS) and input resistance (238 ± 44 vs. 246 ± 58 MΩ, Sham vs. LPS), within the ranges obtained from naïve rats (n=25; −61.6 ± 2.7 mV; 234 ± 66 MΩ). Furthermore, no differences in FSI afterhyperpolarization amplitude (naïve: 19.1 ± 3.4 mV; sham: 18.4 ± 2.4 mV; LPS: 17.1 ± 1.9 mV) or half-width duration (naïve: 5.5 ± 1.9 ms; sham: 5.7 ± 2.3 ms; LPS: 5.2 ± 1.8 ms), or in action potential half-width (naïve: 0.5 ± 0.1 ms; sham: 0.5 ± 0.1 ms; LPS: 0.6 ± 0.2 ms), spike amplitude (naïve: 68.6 ± 6.5 mV; sham: 68.9 ± 5.8 mV; LPS: 67.7 ± 8.8 mV) and threshold (sham: −43.8 ± 2.7 mV; LPS: −44.4 ± 3.4 mV) were observed among naïve, sham and LPS-treated rats. These results indicate that membrane properties and action potential firing of FSI in the medial PFC were not affected by neonatal LPS injection into the VH.
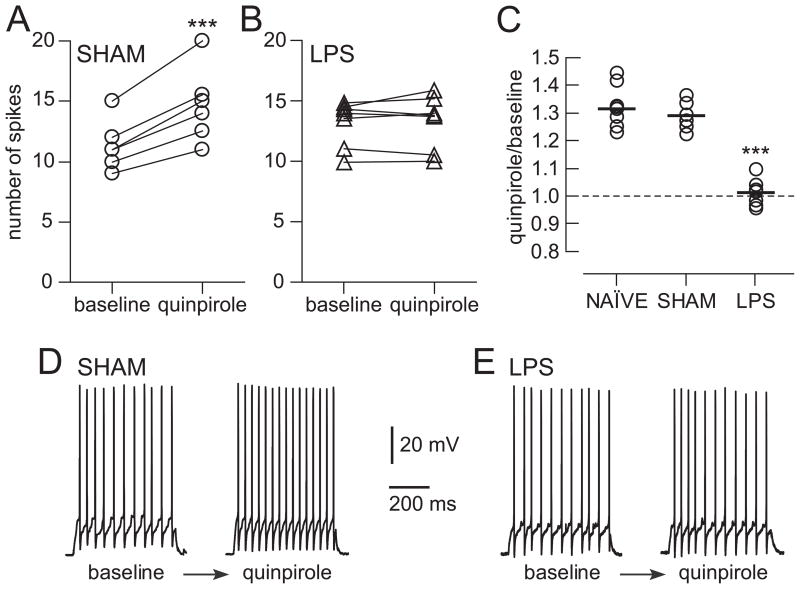
The DA modulation of PFC interneuron function acquires an adult profile during adolescence (15), and this maturation is not present in the PFC following a NVHL (3). We tested whether this is also the case in rats with a neonatal intra-hippocampal LPS challenge by examining the effects of the D2 agonist quinpirole on FSI excitability. Cell excitability was assessed by determining the latency to the first spike and number of evoked spikes evoked with constant-amplitude intracellular current pulses and, as previously reported (15, 19, 20). After determining baseline excitability, quinpirole (1 μM) was added to the bath while continuing to deliver the pulses. Within 5 minutes, the number of spikes evoked rose from 11.3 ± 2.1 to 14.7 ± 3.1 in adult sham rats (n=6 cells from 6 rats; Fig. 4A, D) and from 10.1 ± 1.8 to 13.3 ± 2.5 in naïve rats (n=9; Fig 4C). In LPS-injected rats, quinpirole did not change interneuron excitability; evoked spikes were 13.1 ± 1.9 before and 13.3 ± 2.2 after quinpirole (n=7 cells from 7 rats; Fig. 4B, E). Thus, in sham animals, quinpirole increased excitability by nearly 30%, resembling the response pattern observed in slices from naïve rats. However, the majority of interneurons recorded in LPS-treated animals were not affected by quinpirole (Fig. 4C), a pattern similar to what was observed in slices from pre-adolescent rats (15) and from adult NVHL rats (21). The data therefore suggest that a neonatal immune challenge can alter the periadolescent maturation of PFC interneurons by virtue of its impact on the hippocampus.
Figure 4.
D2 effects on interneurons. A. Increase in excitability by the D2 agonist quinpirole in fast-spiking interneurons recorded in PFC slices from sham rats (***p<0.001). B. Lack of effect in slices from LPS treated rats. C. Graph showing normalized responses to quinpirole in both groups, revealing a 30% increase in excitability in sham rats but no change in LPS treated rats. A third group from naïve rats showing a 30% increase in excitability is also shown. D, E. Representative traces illustrating the effect of quinpirole in a slice from a sham rat (D) and in a slice from an LPS treated rat (E).
DISCUSSION
Our data revealed that bilateral LPS injection in the ventral hippocampus at PD6–7 elicits persistent increases in IL-1 and IL-2 in the hippocampus, PFC and nucleus accumbens that extend into adulthood. Adult rats with a neonatal immune challenge also presented disrupted PPI, and whole-cell recordings in slices from the same rats revealed that the D2 modulation of FSI was absent.
Although our experiments have not addressed the time of onset of the electrophysiological deficits, the ontogeny of DA effects on interneurons suggests they may occur during the peri-adolescent period. FSI excitability is not affected by D2 agonists in juvenile naïve rats, but D2 agonists become excitatory on FSI of rats older than P55 (15). The absence of the excitatory D2 effect in our results suggests that FSI failed to acquire this modulation in the transition to adulthood in LPS rats. This failure of interneuron activation would contribute to abnormally enhanced firing in PFC pyramidal neurons when DA systems are strongly active, and this could be interpreted as a disinhibited state in PFC circuits. As PFC disinhibition is considered a central pathophysiological feature of schizophrenia, our data suggest this state can be achieved by abnormal FSI maturation.
An intriguing finding is the persistent cytokine elevation in different brain regions. We expected changes to be short lasting, but only TNF-α goes down to control levels in the nucleus accumbens two weeks after LPS injection. The elevation of IL-1 and IL-2 in the PFC and nucleus accumbens, on the other hand, outlasts the time course of LPS and extends into the adult stage. Such a persistent increase of cytokines suggests that neonatal LPS injection in the hippocampus can trigger and sustain long-lasting immune/inflammatory responses in brain regions implicated in schizophrenia. The persistent increase of IL-1 and IL-2 outlasts the time course of LPS and suggests this agent can induce a persistent immune response in the brain. As cytokines in the brain are signaling molecules, it is conceivable that several neural processes may become affected by their persistent elevation.
PPI deficits and electrophysiological alterations in LPS-treated rats were similar to what has been reported in the NVHL model (3, 22), although the present study does not characterize whether and how the magnitude of these effects may differ between these experimental interventions. This suggests that the lesion is not a requirement for the outcome in NVHL rats. Indeed, transient inactivation of the VH with tetrodotoxin at the same postnatal age caused behavioral deficits similar to those elicited by the lesion (23). Thus, it is possible that disturbing hippocampal activity has downstream consequences on areas targeted by the ventral hippocampus such as the medial PFC. The time of the immune challenge coincides with the onset of parvalbumin expression in local circuits interneurons in the PFC (24). Interfering with hippocampal afferents may therefore impair the normal postnatal maturation of this critical neural population. Furthermore, it is unlikely that the deficits observed in LPS-treated rats are due to non-specific factors as pups recover well from this procedure, and behavioral, neurochemical and electrophysiological deficits are observed in adult rats. Together, these deficits indicate that a neonatal VH immune challenge can reproduce aspects of the NVHL model, with altered developmental trajectory of circuits involved in the regulation of PFC inhibition.
The mechanism by which LPS causes a deficit similar to a ventral hippocampal lesion in the PFC remains to be elucidated. Cytokines are signaling molecules extensively used for intercellular communication, not unlike hormones and neurotransmitters, playing a role as signaling compounds in the CNS (25). A cytokine of important translational value is IL-2, which has been reported to be elevated in schizophrenia patients (26) and to cause psychotic episodes when administered (27). Furthermore, bacterial endotoxins and viruses can activate TNF release in the CNS, and this activation can lead to profound changes in synaptic efficacy and plasticity. IL-1 and TNF have been shown to suppress normal expression of BDNF (28) and prenatal poly I:C injection alters TNF and BDNF levels in the neonatal brain (29), suggesting that immune activation could affect developmental trajectories of neural populations. A potential role of immune activation on the inhibitory circuit dysfunction proposed for schizophrenia is highlighted by recent observations that IL-6 mediates the deleterious effects of non-competing NMDA antagonists (a well-studied pharmacological model of schizophrenia) on cortical interneurons (30). Thus, it can be speculated that both the neonatal lesion and immune challenge can alter the developmental trajectory of interneuron maturation in the PFC. As the responses of these neurons to DA matures during adolescence, their deficits will only become evident after that period.
In summary, we interpret these findings as evidence that hippocampal inputs are critical during early developmental stages for a proper assembly of PFC circuits. As some aspects of PFC maturation occur during adolescence, electrophysiological and behavioral anomalies may become evident in adult animals. The reduced PPI and altered DA modulation of interneuron function observed in adult NVHL rats can also be produced by intra-hippocampal immune challenge, indicating that at least for some of the NVHL model deficits, the lesion is not necessary.
Acknowledgments
This work was supported by NIH grant MH57683 (PO’D)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lipska BK, Jaskiw GE, Weinberger DR. Postpuberal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology. 1993;90:67–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- 2.Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav Brain Res. 2008 doi: 10.1016/j.bbr.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tseng KY, Lewis BL, Hashimoto T, Sesack SR, Kloc M, Lewis DA, et al. A neonatal ventral hippocampal lesion causes functional deficits in adult prefrontal cortical interneurons. J Neurosci. 2008;28:12691–12699. doi: 10.1523/JNEUROSCI.4166-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volk DW, Lewis DA. Impaired prefrontal inhibition in schizophrenia: relevance for cognitive dysfunction. Physiol Behav. 2002;77:501–505. doi: 10.1016/s0031-9384(02)00936-8. [DOI] [PubMed] [Google Scholar]
- 5.Maldonado-Aviles JG, Curley AA, Hashimoto T, Morrow AL, Ramsey AJ, O’Donnell P, et al. Altered markers of tonic inhibition in the dorsolateral prefrontal cortex of subjects with schizophrenia. Am J Psychiatry. 2009;166:450–459. doi: 10.1176/appi.ajp.2008.08101484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Donnell P. Increased cortical excitability as a critical element in schizophrenia pathophysiology. In: O’Donnell P, editor. Cortical deficits in schizophrenia: from genes to function. New York, NY: Springer; 2008. pp. 219–236. [Google Scholar]
- 7.Borrell J, Vela JM, Arevalo-Martin A, Molina-Holgado E, Guaza C. Prenatal immune challenge disrupts sensorimotor gating in adult rats. Implications for the etiopathogenesis of schizophrenia. Neuropsychopharmacology. 2002;26:204–215. doi: 10.1016/S0893-133X(01)00360-8. [DOI] [PubMed] [Google Scholar]
- 8.Van Amersfoort ES, Van Berkel TJ, Kuiper J. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin Microbiol Rev. 2003;16:379–414. doi: 10.1128/CMR.16.3.379-414.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol Psychiatry. 2006;59:546–554. doi: 10.1016/j.biopsych.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 10.Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28:1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]
- 11.Fatemi SH, Emamian ES, Kist D, Sidwell RW, Nakajima K, Akhter P, et al. Defective corticogenesis and reduction in Reelin immunoreactivity in cortex and hippocampus of prenatally infected neonatal mice. Mol Psychiatry. 1999;4:145–154. doi: 10.1038/sj.mp.4000520. [DOI] [PubMed] [Google Scholar]
- 12.Pearce BD, Valadi NM, Po CL, Miller AH. Viral infection of developing GABAergic neurons in a model of hippocampal disinhibition. Neuroreport. 2000;11:2433–2438. doi: 10.1097/00001756-200008030-00019. [DOI] [PubMed] [Google Scholar]
- 13.Rubin SA, Sylves P, Vogel M, Pletnikov M, Moran TH, Schwartz GJ, et al. Borna disease virus-induced hippocampal dentate gyrus damage is associated with spatial learning and memory deficits. Brain Res Bull. 1999;48:23–30. doi: 10.1016/s0361-9230(98)00133-6. [DOI] [PubMed] [Google Scholar]
- 14.Jenkins TA, Harte MK, Stenson G, Reynolds GP. Neonatal lipopolysaccharide induces pathological changes in parvalbumin immunoreactivity in the hippocampus of the rat. Behav Brain Res. 2009 doi: 10.1016/j.bbr.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Tseng KY, O’Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb Cortex. 2007;17:1235–1240. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Donnell P, Lewis BL, Weinberger DR, Lipska BK. Neonatal hippocampal damage alters electrophysiological properties of prefrontal cortical neurons in adult rats. Cereb Cortex. 2002;12:975–982. doi: 10.1093/cercor/12.9.975. [DOI] [PubMed] [Google Scholar]
- 17.Boje KM, Jaworowicz D, Jr, Raybon JJ. Neuroinflammatory role of prostaglandins during experimental meningitis: evidence suggestive of an in vivo relationship between nitric oxide and prostaglandins. J Pharmacol Exp Ther. 2003;304:319–325. doi: 10.1124/jpet.102.041533. [DOI] [PubMed] [Google Scholar]
- 18.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 19.Tseng KY, O’Donnell P. Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci. 2004;24:5131–5139. doi: 10.1523/JNEUROSCI.1021-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, O’Donnell P. D1 dopamine receptors potentiate NMDA-mediated excitability increase in rat prefrontal cortical pyramidal neurons. Cerebral Cortex. 2001;11:452–462. doi: 10.1093/cercor/11.5.452. [DOI] [PubMed] [Google Scholar]
- 21.Tseng KY, Lewis BL, Lipska BK, O’Donnell P. Post-Pubertal Disruption of Medial Prefrontal Cortical Dopamine-Glutamate Interactions in a Developmental Animal Model of Schizophrenia. Biol Psychiatry. 2007;62:730–738. doi: 10.1016/j.biopsych.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipska BK, Swerdlow NR, Geyer MA, Jaskiw GE, Braff DL, Weinberger DR. Neonatal excitotoxic hippocampal damage in rats cause post-pubertal changes in prepulse inhibition of startle and its disruption by apomorphine. Psychopharmacol. 1995;132:303–310. doi: 10.1007/BF02246439. [DOI] [PubMed] [Google Scholar]
- 23.Lipska BK, Halim ND, Segal PN, Weinberger DR. Effects of reversible inactivation of the neonatal ventral hippocampus on behavior in the adult rat. J Neurosci. 2002;22:2835–2842. doi: 10.1523/JNEUROSCI.22-07-02835.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levitt P, Eagleson KL, Powell EM. Regulation of neocortical interneuron development and the implications for neurodevelopmental disorders. Trends Neurosci. 2004;27:400–406. doi: 10.1016/j.tins.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Rothwell NJ, Hopkins SJ. Cytokines and the nervous system II: Actions and mechanisms of action. Trends Neurosci. 1995;18:130–136. doi: 10.1016/0166-2236(95)93890-a. [DOI] [PubMed] [Google Scholar]
- 26.Gaughran F, O’Neill E, Cole M, Collins K, Daly RJ, Shanahan F. Increased soluble interleukin 2 receptor levels in schizophrenia. Schizophr Res. 1998;29:263–267. doi: 10.1016/s0920-9964(97)00099-6. [DOI] [PubMed] [Google Scholar]
- 27.Walker LG, Wesnes KP, Heys SD, Walker MB, Lolley J, Eremin O. The cognitive effects of recombinant interleukin-2 (rIL-2) therapy: a controlled clinical trial using computerised assessments. Eur J Cancer. 1996;32A:2275–2283. doi: 10.1016/s0959-8049(96)00300-0. [DOI] [PubMed] [Google Scholar]
- 28.Schulte-Herbruggen O, Nassenstein C, Lommatzsch M, Quarcoo D, Renz H, Braun A. Tumor necrosis factor-alpha and interleukin-6 regulate secretion of brain-derived neurotrophic factor in human monocytes. J Neuroimmunol. 2005;160:204–209. doi: 10.1016/j.jneuroim.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 29.Gilmore JH, Jarskog LF, Vadlamudi S. Maternal poly I:C exposure during pregnancy regulates TNF alpha, BDNF, and NGF expression in neonatal brain and the maternal-fetal unit of the rat. J Neuroimmunol. 2005;159:106–112. doi: 10.1016/j.jneuroim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Behrens MM, Ali SS, Dugan LL. Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. J Neurosci. 2008;28:13957–13966. doi: 10.1523/JNEUROSCI.4457-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]