Abstract
Influenza A viruses pose a substantial threat to the human population whether by purposeful manipulation and release or by the natural process of interspecies transmissions from animal reservoirs. The challenge with preparing for these events with vaccination strategies is that the best forms of protective immunity target the most variably of the viral proteins, hemagglutinin. Add to this even just the natural extent of variation in this protein and the challenges to vaccinologists become great. Progress must be made in the area of streamlining the conventional vaccine approaches, but also in further defining and testing other more conserved protective antigens. Within the context of biodefense, the issue will be to reach a balance where some of the diversity of influenza viruses can be encompassed within a vaccine while maintaining an acceptable level of efficacy.
Keywords: Influenza, Hemagglutinin, Immunogenicity
Introduction
The inherent virulence of select H5 and H7 influenza A strains and the advent of plasmid-based reverse genetics systems for these viruses has led to the inclusion of influenza as an organism of interest for biodefense [1]. The potential impact of a highly transmissible, highly pathogenic influenza in human or other animal populations is substantial as highlighted by the financial, societal, and political fallout from the H5N1 epizootic in recent years. The sporadic appearance of unexpected and novel pandemic influenza viruses into human populations over the centuries [2] has laid the foundation for development of influenza vaccines and although the challenges are large, considerable resources have been channeled into this area and some progress made.
The challenges of Influenza vaccination
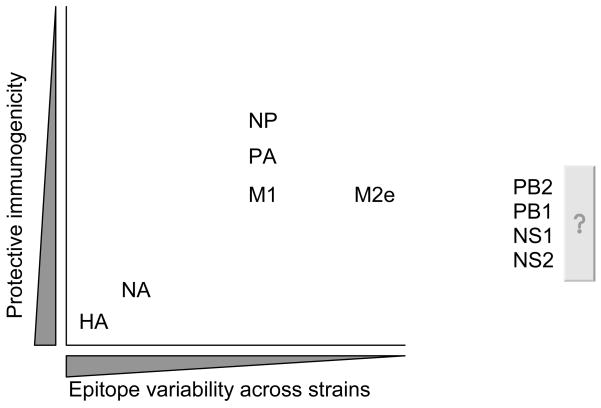
Because of the antigenic diversity encompassed by the avian reservoirs of influenza A viruses, the emergence of a novel strain into humans, whether by nature or by design, poses a number of problems in terms of vaccine design. The Achilles heel of preparing for an influenza outbreak is that the most effective vaccines against the virus promote an immune response against the most variable viral protein, hemagglutinin (HA) (Figure 1). The HA is a surface glycoprotein that attaches and promotes entry of the virus into a host cell. As such, antibodies that can bind to the hemagglutinin can mediate neutralization of the virus through a number of mechanisms including steric hindrance [3]. As discussed below, the HA is the mainstay of conventional influenza vaccine approaches.
Figure 1. The dilemma of influenza vaccination.
Generally speaking, the most protective epitopes, HA and NA, are also the most variable viral proteins. The key is finding the balance between immunogenicity and protein variability or enhancing methods of delivering HA antigen.
Conventional approaches to influenza vaccination
Inactivated virus vaccines
Most of the currently licensed influenza vaccines are in the form of inactivated antigen preparations. Although three major formulations are, or have been, available, all work primarily through the generation of antibodies to HA. As such, they are potent, but prone to antigenic changes such as would be expected with the emergence of a new strain in a population. Clinical studies have shown that inactivated vaccines have an efficacy, measured by reducing serologically confirmed influenza illnesses, of 70% in the age group between 14–60 years [4]. The efficacy is reduced with both infants and the elderly [5]. The first of the inactivated vaccine formulation is whole virion, the experimental use of which dates back to 1940s. The second is split virion which is derived by disrupting whole virus particles with disinfectants and finally, the subunit form, which is prepared by enriching for the viral surface glycoproteins HA and neuraminidase (NA) following disruption of viral particles. Although arguably more immunogenic [6, 7] the reactogenicity associated with inactivated whole virus vaccine preparations, particularly in infants and children drove the development of the split vaccine technology back in the 1960s [8, 9]. Split and subunit vaccines have subsequently proven to be safe and have been delivered to many countless of millions of people [10, 11]. Unfortunately the relatively poor immunogenicity of split vaccines means that at least two doses of vaccine must be provided to generate protective immune responses in naïve individuals [12, 13]. In the event of a release of a novel and virulent strain of influenza, a two dose schedule for vaccination poses logistical and temporal issues.
Other issues that limit the utility of inactivated vaccines for biodefense use include the reliance of the production system on embryonated chickens eggs and the length of time between selection of vaccine strains and the availability of the first doses of formulated vaccines [14–16]. As discussed, inactivated vaccines target HA and as such are relatively specific towards the strain in the vaccine, i.e., the immunity generated is not particularly broad and their efficacy is largely dependent on the degree of matching between vaccine and circulating strain. Therefore matching vaccines can only be produced after an outbreak has started. This fact has, nevertheless, not stopped the stockpiling of inactivated vaccines targeting animal strains considered of high zoonotic risk and a stockpiled library approach has been suggested as one tool in biodefense. Another key issue with the inactivated approach is that it is based on inactivation of live viruses that must be grown in bulk. Generation of bulk volumes of a novel virus, even reassortants with attenuating gene segments which are typically used, carries with it some safety concerns [15, 17].
Live attenuated virus vaccines
Unlike inactivated vaccines, live attenuated virus vaccines (LAIVs) are administered by intranasal inoculation of replication competent virus. In these vaccine viruses the HA and NA of the target strain is introduced into the backbone, for the licensed LAIV’s, of an attenuated, cold-adapted virus [18, 19]. The resulting virus has the antigenic phenotype of the target strain but the attenuated phenotype of the master strain. For completeness, it should also be noted that a number of other mechanisms, besides cold adaptation, of attenuating influenza vaccines are being developed for exploitation in LAIV’s. Such approaches target a number of specific gene segments such as M2, PB2, and NS1 [20–23].
The perceived advantages of the LAIV approach is that both a local immune neutralizing antibody and a cell mediated response can theoretically be generated. Cell mediated immunity is an attractive goal for influenza vaccines due to the fact that such immunity targets the more conserved viral proteins [24]. In an ideal world, such immunity would protect across a range of different virus strains. In practice, in the few side by side comparisons of LAIV and inactivated vaccines in a seasonal influenza setting, the theoretical advantage of a broadened immune response is only seen in the younger cohorts [25, 26]. This is hypothesized to be due to the over attenuation of the viruses in an immunologically primed population. Within the context of a release of a novel strain, however, the population will essentially be naïve and LAIV could have an important role to play in terms of corresponding vaccine strategies.
Despite some advantages, LAIV do suffer from some of the same issues as do their inactivated counterparts. LAIV will also likely require two doses to elicit optimal immune responses and their generation time is not substantially different from inactivated vaccines. LAIV also have some unique drawbacks in that it is possible not all HA and NA combinations will form viable viruses on the attenuated backbone, the vaccine virus must be able to infect the human upper respiratory tract (potentially an issue for avian strains [27, 28]), and potential safety risks of administering a live virus into a population before the target strain is widespread.
Novel approaches to influenza vaccination
As discussed, the major challenge with the development of vaccines against a novel influenza strain is the fact that generally the most potent forms of immunity target the most variable proteins. As such, and due to the enormous antigenic diversity possible within influenza viruses, most potent vaccines must be produced in response to an emergency rather than in advance of one. The time needed for such processes essentially means the first months of an emergent virus will not be met with any available vaccine. In very general terms, there are two major areas where newer generation influenza vaccines are poised to improve our ability to respond to or prepare for an influenza pandemic; increasing the speed at which matching vaccines can be produced or improving the breadth of immunity generated therefore extending the usefulness of stockpiled vaccines. Below we will briefly review, certainly not exhaustively, some of the more advances of these approaches.
Increasing the speed of vaccine preparation and delivery
Reverse Genetics
One of the more touted advances in influenza vaccine processes in recent times has been the utilization of plasmid based reverse genetics systems [29, 30]. These systems allow the generation of viruses of defined genetic composition. Although these processes do not speed up the actual time of manufacture for influenza vaccines, they can provide advantages in the development of seed strains which are typically produced within laboratories associated with the World Health Organization. It is these strains that are sent to manufacturers for bulk vaccine production. Although reverse genetics systems can potentially speed up the generation of the typical reassortant seed strains to seasonal and low pathogenic strains, their real value comes to the fore when the circulating virus is one of the highly pathogenic H5 or H7 strains [31, 32]. In these instances, the molecular features which primarily confer this high virulence, and coincidently reside within the HA protein, can be removed to produce a safe matching seed strain. Indeed, these technologies have been the backbone to the success of the production of inactivated and LAIV vaccines to H5N1 viruses.
Use of adjuvants
The poor immunogenicity of conventional influenza vaccines is a major hurdle in the rapid response to an emerging virus. The impact is twofold, first higher doses of antigen are needed per dose meaning it takes longer to produce the required amounts, and second, is that two doses are needed in vaccine recipients, therefore the time to when they are protected is extended. The use of adjuvants to overcome poor immunogenicity and for antigen sparing is not new to vaccinology, indeed, adjuvants such as Alum salts for several human vaccines started several decades ago [33]. Addition of Alum adjuvant to split or subunit influenza vaccines has, however, induced only marginal improvements [10, 34]. Whole virus vaccines have feared slightly better, but are still less than optimal even in the presence of alum [35–37]. In contrast, oil-in-water based adjuvants (e.g., MF59 and AS03) have reduced the antigen requirements of H5N1 vaccines by at least 6 fold and have induced protective response after only single doses [38, 39]. MF59 has shown encouraging results in a number of influenza formulations and is licensed in seasonal influenza vaccine in Europe [40, 41]. These results strongly suggest that unless novel production and/or adjuvant systems are developed, oil-in-water adjuvants should go hand-in-hand with the development of influenza vaccines for biodefense.
DNA vaccination
Although some advances in improving the speed of conventional vaccines are being made, it is probable that the most advances in this area of flu vaccinology will require newer generation technologies. Although not new technology in itself, DNA vaccines are also being explored for their utility in influenza vaccines. DNA vaccines expressing various combinations of the viral HA or NA as well as other viral genes have been shown to be protective in animal models [42–44]. Production of these vaccines is relatively safe and economic and potentially rapid. The historical concern with DNA vaccines in humans is their poor immunogenicity, although clinical studies utilizing plasmids expressing H3 HA proved effective at a single 4 microgram dose [45]. More clinical studies are, however, required to fully understand the safety, immunogenicity, and effectiveness of DNA vaccines in humans. Of the newer approaches they do, nevertheless, possess many advantages while still targeting the preferred viral target, HA.
Increasing the breadth of immunity
Although increasing the speed of producing a matching vaccine is a key area, in the context of biodefense, perhaps of greater importance is the generation of more broadly reactive vaccines. The rational for this conclusion is that having a vaccine able to protect against a myriad of different strains would mean that the vaccine could be produced, manufactured, and stored prior to the emergence of a virus, potentially saving many lives. Although a long sought after goal for influenza vaccinologists, it is fair to say that such a vaccine does not currently exist and as discussed those vaccine approaches that are less strain specific tend to be less effective. Perhaps, however, we must lower our expectations of a biodefense influenza vaccine and strive for the target of reducing severe disease and death as opposed to reducing infection; at least while a matching vaccine is in development. In this scenario, some of the approaches described below become more attractive.
Use of adjuvants
In addition to their ability to enhance vaccine immunogenicity, adjuvants have also been suggested to expand the breadth of an immune response. It has been claimed that oil-in-water adjuvants induce higher serum antibody titers as well as more cross reactive responses when administered with split or subunit H5N1 vaccines [38, 46]. Although these vaccines are unlikely to protect across subtypes, they do appear to be more cross reactive across the variations within a subtype. Despite the claims, however, it is a little unclear if the cross reactivity noted is due to a qualitative difference in the specificity of the antibody response or if it is simply a reflection of the higher overall titers, i.e., the specificity of an adjuvanted vaccine induced antibody is the same but the minor populations have been elevated to above detectable levels. Recently, Malherbe and colleagues have shown that different adjuvants can promote the accumulation of different dominant clonotypes of CD4+ T-cells in the context of protein vaccination [47], perhaps through differential activation of antigen presenting cell populations, and it would certainly not be surprising to find that adjuvanted influenza vaccines do induce a qualitatively different response.
Universal target approach
Due to the inherent variation of the influenza HA, a number of attempts have been made to design influenza vaccines based on more conserved viral epitopes. In theory this sounds appealing, but in practice few of these approaches have come to fruition. One of the most studied and developed of these “universal target” antigen vaccines are those that target the extracellular portion of the M2 protein (M2e) [48]. The M2 protein is a transmembrane ion channel and studies have shown that in appropriate configurations and in certain models, antibodies to the M2e domain can be protective; virtually no M2e antibodies are produced after natural infection. Vaccination of mice with M2e has proved to be protective against a range of influenza strains, but the data is less convincing in other animal models and even in mice the potency of the immunity has been questioned [49–51]. Nevertheless, clinical trials have been initiated with M2e approaches, and many issues will become clearer in the near future [48].
M2e is not the only conserved epitope between influenza viruses and a number of other proteins have been targeted. These include nucleoprotein and polymerase proteins through T cell mediated approaches and also more conserved domains of HA [24, 52, 53]. Recent studies with monoclonal antibodies have shown that a domain in the stalk region of HA is conserved across a number of subtypes, and these results have led to speculation about using the epitope as an immunizing antigen [54]. The immunogenicity of this part of the HA molecule and its protective potential when used as the immunizing antigen is yet to be determined.
Summary
Although in comparison to other organisms of biodefense concern, the influenza virus has minimal genetic material and much is known about the protective vaccine epitopes, we do not yet have a vaccine that could be considered optimal for emergency situations. Spurred to some degree by the influx of federal and private industry investment into H5N1 vaccine research there has, however, been a flurry of recent research effort targeted at addressing this deficiency. As such, the short to midterm should hopefully see a number of key steps completed in the development of faster, more cross reactive, and more effective vaccines.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Krug RM. The potential use of influenza virus as an agent for bioterrorism. Antiviral Res. 2003 Jan;57(1–2):147–50. doi: 10.1016/s0166-3542(02)00207-3. [DOI] [PubMed] [Google Scholar]
- 2.Potter CW. A history of influenza. J Appl Microbiol. 2001 Oct;91(4):572–9. doi: 10.1046/j.1365-2672.2001.01492.x. [DOI] [PubMed] [Google Scholar]
- 3.Skehel JJ, Wiley DC. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu Rev Biochem. 2000;69:531–69. doi: 10.1146/annurev.biochem.69.1.531. [DOI] [PubMed] [Google Scholar]
- 4.Demicheli V, Rivetti D, Deeks JJ, Jefferson TO. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2000;(2):CD001269. doi: 10.1002/14651858.CD001269. [DOI] [PubMed] [Google Scholar]
- 5.Jefferson T, Rivetti D, Rivetti A, Rudin M, Di Pietrantonj C, Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet. 2005 Oct 1;366(9492):1165–74. doi: 10.1016/S0140-6736(05)67339-4. [DOI] [PubMed] [Google Scholar]
- 6.Gross PA, Ennis FA, Gaerlan PF, Denson LJ, Denning CR, Schiffman D. A controlled double-blind comparison of reactogenicity, immunogenicity, and protective efficacy of whole-virus and split-product influenza vaccines in children. J Infect Dis. 1977 Nov;136(5):623–32. doi: 10.1093/infdis/136.5.623. [DOI] [PubMed] [Google Scholar]
- 7.Wright PF, Thompson J, Vaughn WK, Folland DS, Sell SH, Karzon DT. Trials of influenza A/New Jersey/76 virus vaccine in normal children: an overview of age-related antigenicity and reactogenicity. J Infect Dis. 1977 Dec;136 Suppl:S731–41. doi: 10.1093/infdis/136.supplement_3.s731. [DOI] [PubMed] [Google Scholar]
- 8.Mostow SR, Schoenbaum SC, Dowdle WR, Coleman MT, Kaye HS. Studies with inactivated influenza vaccines purified by zonal centrifugation. 1. Adverse reactions and serological responses. Bull World Health Organ. 1969;41(3):525–30. [PMC free article] [PubMed] [Google Scholar]
- 9.Peck FB., Jr Purified influenza virus vaccine. A study of viral reactivity and antigenicity. JAMA. 1968 Dec 2;206(10):2277–82. [PubMed] [Google Scholar]
- 10.Bresson JL, Perronne C, Launay O, Gerdil C, Saville M, Wood J, et al. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet. 2006 May 20;367(9523):1657–64. doi: 10.1016/S0140-6736(06)68656-X. [DOI] [PubMed] [Google Scholar]
- 11.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med. 2006 Mar 30;354(13):1343–51. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- 12.Atmar RL, Keitel WA, Patel SM, Katz JM, She D, El Sahly H, et al. Safety and immunogenicity of nonadjuvanted and MF59-adjuvanted influenza A/H9N2 vaccine preparations. Clin Infect Dis. 2006 Nov 1;43(9):1135–42. doi: 10.1086/508174. [DOI] [PubMed] [Google Scholar]
- 13.Stephenson I, Nicholson KG, Colegate A, Podda A, Wood J, Ypma E, et al. Boosting immunity to influenza H5N1 with MF59-adjuvanted H5N3 A/Duck/Singapore/97 vaccine in a primed human population. Vaccine. 2003 Apr 2;21(15):1687–93. doi: 10.1016/s0264-410x(02)00632-1. [DOI] [PubMed] [Google Scholar]
- 14.Fedson DS. Preparing for pandemic vaccination: an international policy agenda for vaccine development. J Public Health Policy. 2005 Apr;26(1):4–29. doi: 10.1057/palgrave.jphp.3200008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fedson DS. Vaccine development for an imminent pandemic: why we should worry, what we must do. Hum Vaccin. 2006 Jan-Feb;2(1):38–42. doi: 10.4161/hv.2.1.2554. [DOI] [PubMed] [Google Scholar]
- 16.Gerdil C. The annual production cycle for influenza vaccine. Vaccine. 2003 May 1;21(16):1776–9. doi: 10.1016/s0264-410x(03)00071-9. [DOI] [PubMed] [Google Scholar]
- 17.Wood JM, Robertson JS. From lethal virus to life-saving vaccine: developing inactivated vaccines for pandemic influenza. Nat Rev Microbiol. 2004 Oct;2(10):842–7. doi: 10.1038/nrmicro979. [DOI] [PubMed] [Google Scholar]
- 18.Maassab HF, Heilman CA, Herlocher ML. Cold-adapted influenza viruses for use as live vaccines for man. Adv Biotechnol Processes. 1990;14:203–42. [PubMed] [Google Scholar]
- 19.Wareing MD, Tannock GA. Live attenuated vaccines against influenza; an historical review. Vaccine. 2001 May 14;19(25–26):3320–30. doi: 10.1016/s0264-410x(01)00045-7. [DOI] [PubMed] [Google Scholar]
- 20.Murphy BR, Park EJ, Gottlieb P, Subbarao K. An influenza A live attenuated reassortant virus possessing three temperature-sensitive mutations in the PB2 polymerase gene rapidly loses temperature sensitivity following replication in hamsters. Vaccine. 1997 Aug-Sep;15(12–13):1372–8. doi: 10.1016/s0264-410x(97)00031-5. [DOI] [PubMed] [Google Scholar]
- 21.Parkin NT, Chiu P, Coelingh K. Genetically engineered live attenuated influenza A virus vaccine candidates. J Virol. 1997 Apr;71(4):2772–8. doi: 10.1128/jvi.71.4.2772-2778.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talon J, Salvatore M, O’Neill RE, Nakaya Y, Zheng H, Muster T, et al. Influenza A and B viruses expressing altered NS1 proteins: A vaccine approach. Proc Natl Acad Sci U S A. 2000 Apr 11;97(8):4309–14. doi: 10.1073/pnas.070525997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe T, Watanabe S, Ito H, Kida H, Kawaoka Y. Influenza A virus can undergo multiple cycles of replication without M2 ion channel activity. J Virol. 2001 Jun;75(12):5656–62. doi: 10.1128/JVI.75.12.5656-5662.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas PG, Keating R, Hulse-Post DJ, Doherty PC. Cell-mediated protection in influenza infection. Emerg Infect Dis. 2006 Jan;12(1):48–54. doi: 10.3201/eid1201.051237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edwards KM, Dupont WD, Westrich MK, Plummer WD, Jr, Palmer PS, Wright PF. A randomized controlled trial of cold-adapted and inactivated vaccines for the prevention of influenza A disease. J Infect Dis. 1994 Jan;169(1):68–76. doi: 10.1093/infdis/169.1.68. [DOI] [PubMed] [Google Scholar]
- 26.Ohmit SE, Victor JC, Rotthoff JR, Teich ER, Truscon RK, Baum LL, et al. Prevention of antigenically drifted influenza by inactivated and live attenuated vaccines. N Engl J Med. 2006 Dec 14;355(24):2513–22. doi: 10.1056/NEJMoa061850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shinya K, Ebina M, Yamada S, Ono M, Kasai N, Kawaoka Y. Avian flu: influenza virus receptors in the human airway. Nature. 2006 Mar 23;440(7083):435–6. doi: 10.1038/440435a. [DOI] [PubMed] [Google Scholar]
- 28.van Riel D, Munster VJ, de Wit E, Rimmelzwaan GF, Fouchier RA, Osterhaus AD, et al. H5N1 Virus Attachment to Lower Respiratory Tract. Science. 2006 Apr 21;312(5772):399. doi: 10.1126/science.1125548. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann E, Neumann G, Kawaoka Y, Hobom G, Webster RG. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc Natl Acad Sci U S A. 2000 May 23;97(11):6108–13. doi: 10.1073/pnas.100133697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neumann G, Watanabe T, Ito H, Watanabe S, Goto H, Gao P, et al. Generation of influenza A viruses entirely from cloned cDNAs. Proc Natl Acad Sci U S A. 1999 Aug 3;96(16):9345–50. doi: 10.1073/pnas.96.16.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subbarao K, Chen H, Swayne D, Mingay L, Fodor E, Brownlee G, et al. Evaluation of a genetically modified reassortant H5N1 influenza A virus vaccine candidate generated by plasmid-based reverse genetics. Virology. 2003 Jan 5;305(1):192–200. doi: 10.1006/viro.2002.1742. [DOI] [PubMed] [Google Scholar]
- 32.Webby RJ, Perez DR, Coleman JS, Guan Y, Knight JH, Govorkova EA, et al. Responsiveness to a pandemic alert: use of reverse genetics for rapid development of influenza vaccines. Lancet. 2004 Apr 3;363(9415):1099–103. doi: 10.1016/S0140-6736(04)15892-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindblad EB. Aluminium compounds for use in vaccines. Immunol Cell Biol. 2004 Oct;82(5):497–505. doi: 10.1111/j.0818-9641.2004.01286.x. [DOI] [PubMed] [Google Scholar]
- 34.Bernstein DI, Edwards KM, Dekker CL, Belshe R, Talbot HK, Graham IL, et al. Effects of adjuvants on the safety and immunogenicity of an avian influenza H5N1 vaccine in adults. J Infect Dis. 2008 Mar 1;197(5):667–75. doi: 10.1086/527489. [DOI] [PubMed] [Google Scholar]
- 35.Govorkova EA, Webby RJ, Humberd J, Seiler JP, Webster RG. Immunization with reverse-genetics-produced H5N1 influenza vaccine protects ferrets against homologous and heterologous challenge. J Infect Dis. 2006 Jul 15;194(2):159–67. doi: 10.1086/505225. [DOI] [PubMed] [Google Scholar]
- 36.Lipatov AS, Hoffmann E, Salomon R, Yen HL, Webster RG. Cross-protectiveness and immunogenicity of influenza A/Duck/Singapore/3/97(H5) vaccines against infection with A/Vietnam/1203/04(H5N1) virus in ferrets. J Infect Dis. 2006 Oct 15;194(8):1040–3. doi: 10.1086/507709. [DOI] [PubMed] [Google Scholar]
- 37.Stephenson I, Nicholson KG, Gluck R, Mischler R, Newman RW, Palache AM, et al. Safety and antigenicity of whole virus and subunit influenza A/Hong Kong/1073/99 (H9N2) vaccine in healthy adults: phase I randomised trial. Lancet. 2003 Dec 13;362(9400):1959–66. doi: 10.1016/S0140-6736(03)15014-3. [DOI] [PubMed] [Google Scholar]
- 38.Baras B, Stittelaar KJ, Simon JH, Thoolen RJ, Mossman SP, Pistoor FH, et al. Cross-protection against lethal H5N1 challenge in ferrets with an adjuvanted pandemic influenza vaccine. PLoS One. 2008;3(1):e1401. doi: 10.1371/journal.pone.0001401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicholson KG, Colegate AE, Podda A, Stephenson I, Wood J, Ypma E, et al. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet. 2001 Jun 16;357(9272):1937–43. doi: 10.1016/S0140-6736(00)05066-2. [DOI] [PubMed] [Google Scholar]
- 40.Frey S, Poland G, Percell S, Podda A. Comparison of the safety, tolerability, and immunogenicity of a MF59-adjuvanted influenza vaccine and a non-adjuvanted influenza vaccine in non-elderly adults. Vaccine. 2003 Oct 1;21(27–30):4234–7. doi: 10.1016/s0264-410x(03)00456-0. [DOI] [PubMed] [Google Scholar]
- 41.Vesikari T, Pellegrini M, Karvonen A, Groth N, Borkowski A, O’Hagan DT, et al. Enhanced Immunogenicity of Seasonal Influenza Vaccines in Young Children Using MF59 Adjuvant. Pediatr Infect Dis J. 2009 Jul;28(7):563–71. doi: 10.1097/INF.0b013e31819d6394. [DOI] [PubMed] [Google Scholar]
- 42.Epstein SL, Tumpey TM, Misplon JA, Lo CY, Cooper LA, Subbarao K, et al. DNA vaccine expressing conserved influenza virus proteins protective against H5N1 challenge infection in mice. Emerg Infect Dis. 2002 Aug;8(8):796–801. doi: 10.3201/eid0808.010476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kodihalli S, Kobasa DL, Webster RG. Strategies for inducing protection against avian influenza A virus subtypes with DNA vaccines. Vaccine. 2000 May 22;18(23):2592–9. doi: 10.1016/s0264-410x(99)00485-5. [DOI] [PubMed] [Google Scholar]
- 44.Qiu M, Fang F, Chen Y, Wang H, Chen Q, Chang H, et al. Protection against avian influenza H9N2 virus challenge by immunization with hemagglutinin- or neuraminidase-expressing DNA in BALB/c mice. Biochem Biophys Res Commun. 2006 May 19;343(4):1124–31. doi: 10.1016/j.bbrc.2006.03.088. [DOI] [PubMed] [Google Scholar]
- 45.Drape RJ, Macklin MD, Barr LJ, Jones S, Haynes JR, Dean HJ. Epidermal DNA vaccine for influenza is immunogenic in humans. Vaccine. 2006 May 22;24(21):4475–81. doi: 10.1016/j.vaccine.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 46.Banzhoff A, Gasparini R, Laghi-Pasini F, Staniscia T, Durando P, Montomoli E, et al. MF59-adjuvanted H5N1 vaccine induces immunologic memory and heterotypic antibody responses in non-elderly and elderly adults. PLoS One. 2009;4(2):e4384. doi: 10.1371/journal.pone.0004384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malherbe L, Mark L, Fazilleau N, McHeyzer-Williams LJ, McHeyzer-Williams MG. Vaccine adjuvants alter TCR-based selection thresholds. Immunity. 2008 May;28(5):698–709. doi: 10.1016/j.immuni.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schotsaert M, De Filette M, Fiers W, Saelens X. Universal M2 ectodomain-based influenza A vaccines: preclinical and clinical developments. Expert Rev Vaccines. 2009 Apr;8(4):499–508. doi: 10.1586/erv.09.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fan J, Liang X, Horton MS, Perry HC, Citron MP, Heidecker GJ, et al. Preclinical study of influenza virus A M2 peptide conjugate vaccines in mice, ferrets, and rhesus monkeys. Vaccine. 2004 Aug 13;22(23–24):2993–3003. doi: 10.1016/j.vaccine.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 50.Jegerlehner A, Schmitz N, Storni T, Bachmann MF. Influenza A vaccine based on the extracellular domain of M2: weak protection mediated via antibody-dependent NK cell activity. J Immunol. 2004 May 1;172(9):5598–605. doi: 10.4049/jimmunol.172.9.5598. [DOI] [PubMed] [Google Scholar]
- 51.Slepushkin VA, Katz JM, Black RA, Gamble WC, Rota PA, Cox NJ. Protection of mice against influenza A virus challenge by vaccination with baculovirus-expressed M2 protein. Vaccine. 1995;13(15):1399–402. doi: 10.1016/0264-410x(95)92777-y. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y, Qin K, Wu WL, Li G, Zhang J, Du H, et al. Broad cross-protection against H5N1 avian influenza virus infection by means of monoclonal antibodies that map to conserved viral epitopes. J Infect Dis. 2009 Jan 1;199(1):49–58. doi: 10.1086/594374. [DOI] [PubMed] [Google Scholar]
- 53.Epstein SL, Kong WP, Misplon JA, Lo CY, Tumpey TM, Xu L, et al. Protection against multiple influenza A subtypes by vaccination with highly conserved nucleoprotein. Vaccine. 2005 Nov 16;23(46–47):5404–10. doi: 10.1016/j.vaccine.2005.04.047. [DOI] [PubMed] [Google Scholar]
- 54.Ekiert DC, Bhabha G, Elsliger MA, Friesen RH, Jongeneelen M, Throsby M, et al. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009 Apr 10;324(5924):246–51. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]