Abstract
Objective
To test the hypothesis that diffuse abnormalities precede axonal damage and atrophy in the MRI normal-appearing tissue of relapsing-remitting (RR) multiple sclerosis (MS) patients, and that these processes continue during clinical remission.
Methods
Twenty-one recently diagnosed mildly disabled (mean disease duration 2.3 years, mean Expanded Disability Status Scale score of 1.4) RR MS patients and 15 healthy matched controls were scanned with MRI and proton MR spectroscopic imaging (1H-MRSI) at 3 T. Metabolite concentrations: N-acetylaspartate (NAA) for neuronal integrity; choline (Cho) for membrane turnover rate; creatine (Cr) and myo-inositol (mI) for glial status were obtained in a 360 cm3 volume-of-interest (VOI) with 3D multivoxel 1H-MRSI. They were converted into absolute amounts using phantom replacement and normalized into absolute concentrations by dividing by the VOI tissue volume fraction obtained from MRI segmentation.
Results
The patients’ mean VOI tissue volume fraction, 0.92 and NAA concentration, 9.6 mM, were not different from controls’ 0.94 and 9.6 mM. In contrast, the patients’ mean Cr, Cho and mI levels 7.7, 1.9, 4.1 mM were 9%, 14% and 20%, higher than the controls’ 7.1, 1.6 and 3.4 mM (p=0.0097, 0.003 and 0.0023).
Conclusions
The absence of early tissue atrophy and apparent axonal dysfunction (NAA loss) in these RR MS patients suggests that both are preceded by diffuse glial proliferation (astrogliosis), as well as possible inflammation, de- and re-myelination reflected by elevated mI, Cho and Cr, even during clinical remission and despite immunomodulatory treatment.
Keywords: Multiple Sclerosis, normal-appearing tissue, MR Spectroscopy
INTRODUCTION
The sensitivity of T1- and T2-weighted MRI to white matter (WM) lesions and to regional as well as global atrophy has made this modality central to the diagnosis and treatment monitoring of multiple sclerosis (MS).[1-4] Paradoxically, these findings correlate only weakly with clinical disability,[5] an incongruity due MRI's insensitivity to microscopic pathology and lack of specificity to distinguish inflammation from demyelination, axonal loss, or gliosis.[1, 6] Since MS is now recognized to be a diffuse disease of both normal-appearing WM (NAWM) and gray matter (GM),[7, 8] these sensitivity and specificity issues limit the potential of clinical MRI to fully assess the total load of the disease and consequently, to monitor treatment response.[6]
Although diffuse pathology that leads to microscopic axonal damage is often MRI-occult, it is nevertheless detectable with quantitative MR methods.[6] For example, proton MR spectroscopy (1H-MRS) can probe tissue metabolism;[9] whereas magnetization transfer and diffusion tensor imaging can assess myelin and WM integrity.[1] These have shown that NAWM abnormalities may (a) be present in all MS subgroups, (b) correlate with disability and cognitive impairment, (c) occur prior to lesion development, all suggesting they play a role in lesion formation.[6, 7]
1H-MRS offers the ability to monitor several pathological processes via their specific metabolic markers: N-acetylaspartate (NAA) for neuronal integrity, whose decline correlated better with disability than MRI metrics;[10] myo-inositol (mI) for glial proliferation,[6] whose elevation has also correlated with impairment;[11] creatine (Cr) for energy status and cell density and choline (Cho) for inflammation, de- and re-myelination.[10] Unfortunately, most studies to date use single-voxel or 2D 1H-MRS that sample under 10% of the brain, subjecting them to the implicit assumption that such small volumes are globally representative. In addition, quantification with metabolite ratios may have confounded interpretation since Cr (the frequent denominator) levels are reported to also vary.[12, 13]
Only one study addressed both issues with absolute quantification in a large (~½ liter) WM volume of relapsing-remitting (RR) MS patients and controls.[12] It reported lower NAA and elevated Cho and Cr in the patients but could not ascribe the latter to either astrogliosis or myelin repair since Cr levels are higher in both astrocytes and oligodendrocytes;[14] and the long echo-time 1H-MRS used precluded detection of the astrocyte-specific marker – mI.[6] Furthermore, the NAA loss could not distinguish isolated event(s) from chronic inflammation due to the long, 6+ years, disease duration. Our goal, therefore, is to quantify the glial, de- and re-myelination markers (mI, Cho and Cr) in NAWM of recently diagnosed RR MS patients with short, TE=35 ms, 1H-MRS to test the hypothesis that these processes precede axonal damage (NAA).
MATERIALS AND METHODS
Human Subjects
Twenty-one (15 women) patients meeting the Poser criteria for RR MS were enrolled.[15] Their mean age was 33 (range 21 – 42) years; mean disease duration was 2.3 (range 0.3 – 5.2) years; and mean Expanded Disability Status Scale (EDSS[16]) score was 1.25 (range 0 – 4), as shown in table 1. All were on immunomodulatory medication and none experienced a relapse in the preceding 3 months. Fifteen age- and gender-matched controls (12 women, mean age 30, range 20 – 44 years) were also enrolled. Their “healthy” status was based on negative answers to a list of disqualifying neurological conditions before the study and unremarkable MRI afterwards. All participants gave written Institutional Review Board-approved informed consent.
Table 1.
Demographic, clinical, volumetric and metabolic data for patients (1-21, sorted by disease duration) and controls (22-36).
| Subject number | Age/gender | aDisease duration | EDSS | bLesion volume | cTf | NAA mM | Cr mM | Cho mM | mI mM |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 38/M | 0.3 | 1 | 3.7 | 0.95 | 10.3 | 7.4 | 1.7 | 4.0 |
| 2 | 39/M | 0.3 | 0 | 1.6 | 0.92 | 8.4 | 7.0 | 1.7 | 4.0 |
| 3 | 26/F | 0.9 | 3.5 | 10.7 | 0.97 | 8.7 | 7.3 | 1.7 | 3.8 |
| 4 | 27/F | 0.9 | 1 | 5.1 | 0.92 | 9.2 | 7.4 | 1.6 | 3.4 |
| 5 | 35/F | 1.5 | 1.5 | 2.4 | 0.89 | 9.3 | 8.1 | 1.9 | 4.2 |
| 6 | 38/F | 1.5 | 0 | 1.7 | 0.95 | 7.7 | 6.5 | 1.6 | 3.5 |
| 7 | 30/M | 1.6 | 0 | 8.3 | 0.93 | 8.8 | 7.2 | 1.9 | 3.4 |
| 8 | 35/F | 1.6 | 1 | 2.7 | 0.94 | 10.9 | 7.0 | 2.2 | 4.0 |
| 9 | 37/F | 1.9 | 0 | 11.4 | 0.88 | 8.4 | 7.6 | 1.9 | 4.6 |
| 10 | 34/M | 2.0 | 2 | 2.6 | 0.89 | 11.8 | 8.5 | 1.9 | 5.5 |
| 11 | 26/F | 2.1 | *4.0 | 1.6 | 0.84 | 11.8 | 8.2 | 2.4 | 3.1 |
| 12 | 42/F | 2.1 | 0 | 4.2 | 0.98 | 9.0 | 7.5 | 1.9 | 3.6 |
| 13 | 21/F | 2.3 | 1.5 | 23.2 | 0.94 | 9.0 | 8.3 | 2.0 | 5.5 |
| 14 | 28/M | 2.3 | 1 | 5.7 | 0.97 | 8.5 | 7.3 | 1.9 | 4.4 |
| 15 | 32/F | 2.7 | 2 | 7.3 | 0.90 | 8.8 | 7.9 | 1.8 | 4.8 |
| 16 | 29/M | 3.2 | 1 | 4.9 | 0.88 | 11.3 | 8.5 | 2.1 | 3.7 |
| 17 | 29/F | 3.4 | 2.5 | 16.5 | 0.85 | 8.8 | 6.8 | 1.7 | 4.0 |
| 18 | 27/F | 4.0 | 2 | 11.2 | 0.94 | 11.3 | 9.0 | 2.1 | 4.2 |
| 19 | 42/F | 4.5 | 1 | 1.2 | 0.90 | 9.9 | 8.2 | 1.8 | 4.3 |
| 20 | 38/F | 4.9 | 2 | 2.2 | 0.94 | 11.1 | 8.7 | 2.0 | 3.9 |
| 21 | 31/F | 5.2 | 2 | 2.8 | 0.94 | 8.9 | 7.1 | 1.6 | 3.7 |
| | |||||||||
| 22 | 20/F | - | - | - | 0.93 | 9.5 | 6.8 | 1.8 | 3.5 |
| 23 | 25/F | - | - | - | 0.97 | 11.1 | 7.8 | 2.0 | 2.2 |
| 24 | 26/F | - | - | - | 0.93 | 9.7 | 7.0 | 1.7 | 3.9 |
| 25 | 26/F | - | - | - | 0.90 | 11.3 | 8.2 | 1.8 | 4.2 |
| 26 | 27/M | - | - | - | 0.96 | 9.0 | 6.5 | 1.6 | 3.3 |
| 27 | 28/M | - | - | - | 0.94 | 7.9 | 5.7 | 1.4 | 3.1 |
| 28 | 28/F | - | - | - | 0.94 | 10.3 | 8.2 | 1.7 | 3.2 |
| 29 | 28/F | - | - | - | 0.87 | 9.0 | 7.9 | 1.7 | 3.9 |
| 30 | 29/F | - | - | - | 0.95 | 7.9 | 6.5 | 1.2 | 3.1 |
| 31 | 29/F | - | - | - | 0.94 | 9.8 | 6.9 | 1.8 | 3.1 |
| 32 | 31/F | - | - | - | 0.91 | 8.7 | 6.2 | 1.4 | 3.1 |
| 33 | 35/M | - | - | - | 0.94 | 8.3 | 6.6 | 1.5 | 3.8 |
| 34 | 36/F | - | - | - | 0.95 | 11.8 | 8.1 | 1.7 | 4.0 |
| 35 | 37/F | - | - | - | 0.99 | 8.8 | 6.7 | 1.6 | 3.8 |
| 36 | 44/F | - | - | - | 0.92 | 10.4 | 7.2 | 1.8 | 3.0 |
| | |||||||||
| Ctrl. avg. | 30 | - | - | - | 0.94±0.03 | 9.6±1.2 | 7.1±0.8 | 1.6±0.2 | 3.4±0.5 |
| | |||||||||
| Pat. avg. | 33 | 2.3 | 1.38 | 6.2 | 0.92±0.04 | 9.6±1.3 | 7.7±0.7 d | 1.9±0.2 d | 4.1±0.6 d |
Years
inside the VOI, in cm3
tissue fraction
p<0.01.
The horizontal line separates patients (above) from controls (below).
EDSS dominated by permanent visual impairment in one eye.
MR Acquisition
All measurements were done in a 3 T Trio MRI scanner (Siemens AG, Erlangen Germany) with a TEM3000 transmit-receive head coil (MRInstruments, Minneapolis, MN). For tissue segmentation, sagittal MP-RAGE (TE/TI/TR=2.6/1800/1360 ms) was acquired at 240×240 mm2 field-of-view (FOV), 256×256 matrix and reconstructed in 1 mm thick axial, and coronal slices. To guide the spectroscopic volume-of-interest (VOI), T2-weighted FLAIR (TE/TI/TR= 88/2500/9000 ms) images were obtained at the same FOV and matrix, but 3.7 mm slice thickness.
Following our chemical-shift imaging (CSI) based shim procedure, a 10 cm anterior-posterior (AP) ×8 cm left-right (LR) ×4.5 cm inferior-superior (IS) =360 cm3 1H-MRSI VOI was centered on the corpus callosum (fig. 1) and excited using TE/TR=35/1800 ms PRESS. The VOI was partitioned using Hadamard spectroscopic imaging[17] into six axial slices which were encoded with 16×16 2D-CSI over a 16×16 cm2 (LR×AP) FOV. The VOI was defined in the planes of these slices by the two PRESS 11.2 ms long numerically optimized 180° pulses yielding 8×10×6=480 voxels, 1.0×1.0×0.75 cm3 each. The signal was acquired for 256 ms at ±1 kHz bandwidth. At two averages the 1H-MRSI took 34 minutes and the entire protocol was an hour.
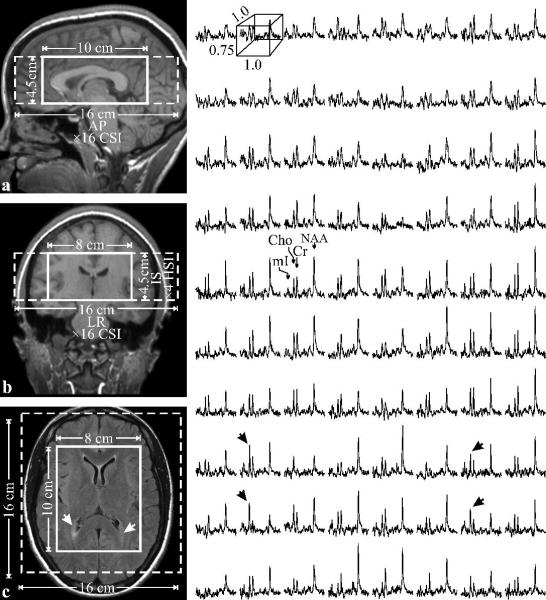
Fig. 1.
Left: Sagittal (a) and coronal (b) T1-weighted and axial T2-weighted FLAIR (c) MRI of patient #12 in table 1, with the VOI and FOV (solid and dashed white frames) superimposed. Note characteristic periventricular hyperintensities on c (arrows) and little or no atrophy.
Right: Real part of the 8×10 (LR×AP) 1H spectra matrix from the VOI on c, on common frequency (1.4 to 3.8 ppm) and intensity scales. Note the spectral resolution and SNR in these 0.75 cm3 voxels and elevated Cho and mI at the lesions (black arrows).
Tissue fraction (Tf) and lesion volume in the VOI
Since the VOI includes ventricles and sulci (cf. fig. 1) correction for their cerebro-spinal fluid (CSF) partial volume is needed. To this end the MP-RAGE images were segmented using our MIDAS package.[18] The process starts by automatic detection of the WM signal intensity, IWM, in a periventricular “seed” region. Following selection of all pixels at or above 55, but below 135% (to exclude the fat) of IWM, a tissue-mask is constructed per slice in three steps: morphological erosion, recursive region growth retaining pixels connected to the “seed”; and morphological inflation to reverse the effect of erosion. Pixels whose intensity was under 55% of IWM were defined as CSF. Tf for each subject was the product of the number of “tissue” pixels in the VOI and their volume divided by the nominal VOI-volume. Lesion volumes were obtained from the FLAIR images using the same procedure, but applying a threshold of 120% of IWM.
Absolute metabolite quantification
Processed offline using in-house software, the MRS data was voxel shifted to align the CSI grid with the NAA VOI, Fourier transformed in the time, AP and LR dimensions and Hadamard reconstructed along the IS direction. Each of the VOI's 480 spectra was frequency-aligned and zero-order phased in reference to the NAA peak and then summed. This retained the individual spectra (narrow) linewidth and improved the signal-to-noise-ratio (SNR) by 480½≈22.[12]
Relative levels of the ith (NAA, Cr, Cho, mI) metabolite in the jth subject were estimated from their peak area, Sij, using spectral modeling[19] Sij were scaled into concentrations, Cij, relative to signals from a 2 L sphere of Civitro=12.5, 10.0, 3.0 and 7.5 mM NAA, Cr, Cho and mI in water:
| [1] |
where SR is the sphere's metabolite signal, Vj180° and VR180° are the RF voltages needed for a non-selective 1 ms 180° inversion pulse on subject and reference sphere. The Cijs were corrected for relaxation time differences in vivo (T1vivo, T2vivo) and in the phantom (T1vitro, T2vitro) with a factor[12]:
| (2) |
using the T1vivo =1.4, 1.3 and 1.2 s, T2vivo= 343, 172 and 248 ms reported at 3 T for NAA, Cr and Cho[20, 21] and T1vitro=605, 336 and 235 ms, T2vitro=483, 288 and 200 ms in the phantom. Since J-coupling modulation and low voxel SNR for mI prevent obtaining its T1 and T2, we assumed f=1 for it in Eq. [2]. Finally, the Cijs were divided by Tf to convert into tissue concentrations.
Statistical Analysis
Analysis of variance (ANOVA) based on ranks was used to compare patients’ and controls’ NAA, Cho, Cr, mI and Tf. A separate analysis was conducted for each measure. In each case, the observed values were converted to ranks (to better satisfy underlying distributional assumptions) that were then used as the dependent variable. Each model included age and gender as subject level covariates and subject group (patient versus control) as the between subjects factor. Pearson product moment and Spearman rank correlation coefficients were used to evaluate association of disease duration with each measure among patients. When a significant correlation was detected, least squares regression was used to characterize the change in that measure as function of disease duration. SAS version 9.0 (SAS Institute, Cary, NC) was used for statistical calculations.
RESULTS
Our shim procedure adjusted the whole-head water linewidth to 27±4 Hz full-width at half maximum (FWHM) among the 36 subjects, which improved to 21±3 Hz in the VOI. Examples of the size and placement of the VOI and 1H spectra are shown in figs. 1 and 2. The SNR, estimated as the peak-height divided by the root-mean-square of the noise in 16,800 voxels (35 subjects ×480 spectra each) were: NAA=24.0±10.0, Cr=13.8±4.8, Cho=13.3±4.8 and mI=3.8±1.0 (mean±standard deviation). The metabolites’ FWHM linewidth, Δω, was 5.5±1.2 Hz across the 16,800 voxels reflecting a T2* (=1/πΔω assuming Lorentzian lines) of 57.8±13.2 ms.
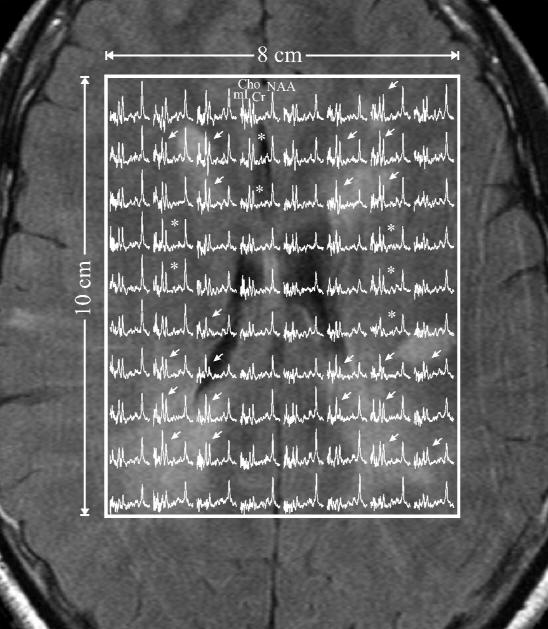
Fig. 2.
Axial T2-weighted FLAIR image of the patient (#13) with the heaviest lesion load superimposed with the VOI (white frame) and 8×10 (LR×AP) 1H spectra on common frequency (1.4 to 3.8 ppm) and intensity scales. Note extensive hyperintensities and visible Cho and mI elevation in voxels within these lesions (arrows) and in the NAWM (asterisks), i.e., their overall elevation is truly diffuse not merely multifocal.
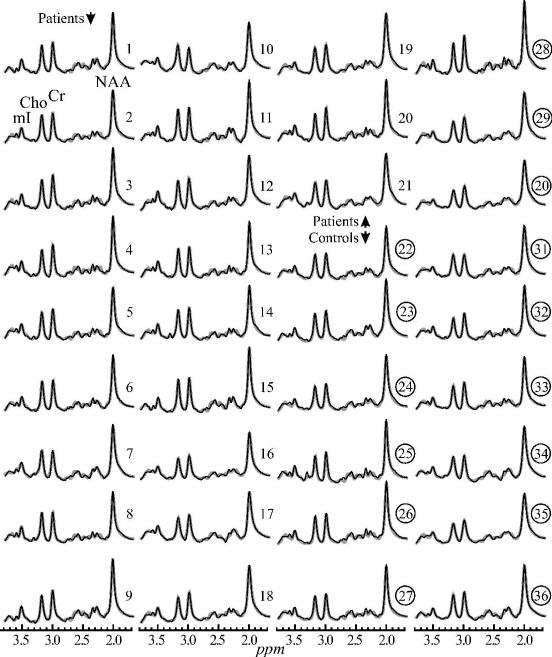
The 480 summed VOI spectra from each of the 21 patients and 15 controls superimposed with their model functions are shown in fig. 3. Their SNRs range from a low of ~75 for mI to over 500 for the NAA. The average linewidth in the sums increased only slightly to 6.8±0.6 Hz versus the individual voxels (vide supra). These demonstrate the advantages of the aligned summation strategy: preservation of individual voxel linewidth in the sum and substantial SNR boost.[12]
Fig. 3.
Real part of the 1H 480 summed spectra from the VOI (black lines) for each of the 36 subjects in table 1: patients (1–21) and controls (22–36 - circled), superimposed with the fitted model functions (gray lines) on common scales. Note: (i) excellent 75 (mI) – 500 (NAA) SNRs and linewidths of the sums compared with the individual voxels in Fig. 1 (benefit of summation and alignment); (ii) the quality of the fit; and (iii) the visual similarity in NAA levels between patients and controls versus elevated Cr, Cho and mI.
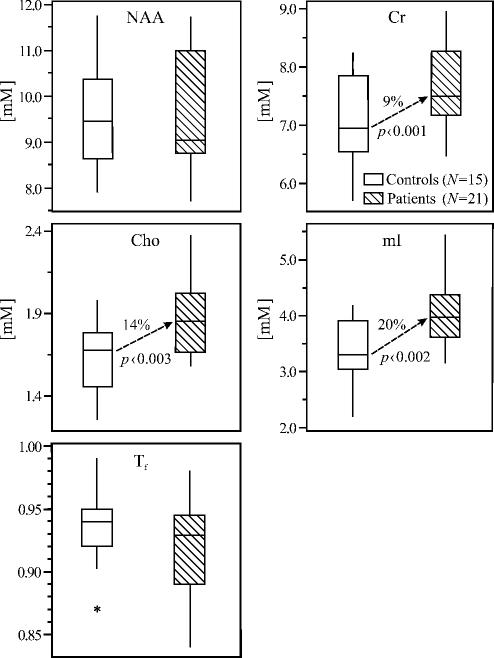
Average absolute metabolic concentrations obtained from the summed spectra of fig. 3 using Eqs. [ 1 ] and [ 2 ], Tf-s and lesion volumes are given in table 1. Their box plots (fig. 4) show that the patients’ medians: Tf=0.92 and NAA=9.6 mM, are not significantly different from the controls’ 0.94 and 9.6 mM. (Based on their standard deviations the smallest difference detectable with 80% power at the 5% significance level is 0.04 for Tf and 1.25 mM for NAA; alternatively, to establish the significance of these observed differences with 80% power would require larger cohorts: 56 subjects in each for Tf and 1100 for NAA). In contrast, the patients’ Cr, Cho and mI levels: 7.7, 1.9, and 4.1 mM were significantly (9%, 14% and 20%, p=0.0097, 0.003 and 0.0023) higher than the controls’ 7.1, 1.6 and 3.4 mM even after Bonferroni correction. No correlations were found between any metric and time from diagnosis except a trend (p=0.06) for Cr increase.
Fig. 4.
Box plots displaying the 25%, median and 75% (box), 95% (whiskers) and outliers (*) of the NAA, Cr, Cho and mI concentrations as well as tissue-fraction, Tf, distributions in the VOIs of patients and controls. Note the absence of atrophy and NAA loss versus significant 9, 14 and 20% elevation of Cr, Cho and mI in the recently diagnosed patients.
DISCUSSION
Lack of convincing correlation between disability and T2 lesion load in MS is often attributed to the inability of MRI to account for microscopic NAWM damage.[5] Since lesions comprise on average less than 3% of the brain volume[5] it is possible that milder, but widespread pathology influences disability. Indeed, quantitative metrics that assess all the tissue in a given volume, e.g., the NAA level, correlate better with clinical impairment.[22] Yet, despite its specificity, 1H-MRS sensitivity is limited by SNR and WM/GM/CSF partial volume. Furthermore, placement of a single VOI over a lesion or NAMW may miss distal damage propagated by Wallerian and retrograde degeneration.[23-25]
To address both SNR and limited coverage we assessed the metabolite levels in a large (360 cm3) VOI of mostly NAWM. Summing all 480 frequency-aligned spectra in the VOI yielded excellent spectral resolution and sufficient SNR to detect subtle metabolic changes. This approach has previously shown decreased Tf and NAA reflecting axonal loss and dysfunction, as well as elevated Cr and Cho in 11 RR MS patients of 6+ years disease duration.[12] Based on those findings our goals were to: a) test for the presence of astrogliosis (via its mI marker[26]); b) ascertain the temporal relationship between demyelination, gliosis and NAA decline; in order to c) test the hypothesis that widespread pathogenesis precedes axonal damage and atrophy.
The results support the hypothesis and suggest the following sequence of disease evolution: (i) astrogliosis, possibly accompanied by inflammation, de- and re-myelination, preceding (ii) axonal damage, that is perhaps followed by (iii) atrophy.
Diffuse abnormalities
Since our cohort's average VOI lesion load was under 2% (cf. table 1), differences of 9%, 14% and 20% for Cr, Cho and mI from controls must reflect widespread NAWM pathology, (fig. 2). Moreover, the metabolic pattern, seen in the global VOI spectra of fig. 3, is similar to that observed in the lesions (cf. figs. 1 and 2), known foci of ongoing activity.[9, 27] This similarity may suggest that the same processes may also be widespread in the NAWM, early in the disease course, even during remission and despite immunomodulatory treatment. This conjecture is supported by strong correlations of MRS observed elevated mI and Cho with biopsy findings (astrogliosis and inflammation) in lesions,[28] and the well-documented NAWM abnormalities - astrogliosis and inflammation in autopsied tissue.[7]
Glial pathology
While Cr and Cho are found in all brain cells, their levels are highest in mature and progenitor oligodendrocytes and astrocytes.[14] Their elevation, therefore, may represent glial activity: combinations of inflammation, de- and re-myelination as well as gliosis. Corroboration and specificity is provided by the mI, an osmolyte and constituent of phosphate- and lipid-containing compounds involved in signal transduction in the brain.[29] Its 1H-MRS peak is thought to originate from intracellular stores in astrocytes[30] and its increase, therefore, indicate astrogliosis,[6] i.e., cell hypertrophy and hyperplasia.[31]
Evidence of glial abnormalities at the earliest stages is offered by the largest (96 patients) 1H-MRS study of clinically isolated syndrome suggestive of MS (CIS).[32] It reported elevated mI, Cr and Cho (but no NAA decline) most pronounced in patients who also satisfied the McDonald criteria. Indeed, elevated mI suggestive of astrogliosis is a consistent finding in all disease durations.[11, 33, 34] While astroglial scars are known to form on severed axons, the cause of astrogliosis in the context of (MRS inferred) healthy neurons is unclear. It is known that astrocytes directly contact the endothelial cells comprising the blood-brain barrier (BBB) and enhance its impermeability.[35] Both the subtle BBB breakdown from microscopic inflammation[36] and hypoperfusion[37] that were proposed as possible pathogenic mechanisms may conceivably cause astrogliosis: the former in response to increased leakage through the BBB and (or) micro plaque formation; the latter due to oxygen deprivation, as shown in a rat model of chronic ischemia.[38]
Axonal sparing
NAA's almost exclusive confinement to neurons renders its 1H-MRS signal a marker of their integrity.[6] Since axonal loss is thought to proceed mainly by Wallerian or retrograde degeneration from lesions,[24, 39] conflicting reports of normal[33, 34] and low[11-13, 40] NAA may reflect different disease course, duration and subjective VOI placement. The last issue was addressed with whole-brain NAA quantification showing average 22% decline already in CIS.[41] However, since over 60% of that NAA signal originates from cortical GM (known to be affected early in MS[11, 40, 42]), that finding is not necessarily in conflict with the NAA levels reported here which reflect mainly WM.
Despite contradictory reports, the concept of eventual NAA decreases,[9] axonal loss[7] and atrophy[2] in NAWM is now generally accepted. We report on glial abnormalities early in the disease course, in the absence of, i.e., prior to overt axonal injury, reflected in patients’ NAA level and Tf not significantly different from the controls’. These findings support the notion that glial processes including astrogliosis precede axonal damage, which in turn precedes tissue atrophy – the final non-specific end point of the various pathologies of this disease.
Based on these findings we conjecture that some axonal damage may arise from astrogliosis and/or inflammation. Indeed, disability's modest correlation with lesion load[7] and histological evidence of axonal loss in the absence of apparent demyelination suggest that not all damage is due to lesions.[43] Indeed, both biopsy and autopsy show that axonal damage correlates better with inflammatory infiltrates than with demyelination.[44, 45] The concept of astrogliosis driven damage is supported by the EAE animal model of MS that shows astrocytic hypertrophy that is spatially and temporally related to axonal damage already in the earliest pre-inflammatory stage.[46]
Caveats
The sensitivity to metabolic changes reported here comes at a price - regional differences, especially between lesions and NAWM, are averaged out. Although the original localized information is still available (see figs. 1 and 2) the proposed strategy reflects a post-processing choice of maximum sensitivity at the expense of localization. Monitoring the global effects of diffuse pathogenesis may make this tradeoff attractive, especially for the much weaker mI signal.
A cautionary note raised by this study underscores the importance of absolute quantification of 1H-MRS in MS versus use of metabolite ratios to assumed stable Cr levels. This and other reports[34] that show increased Cr and unchanged NAA suggest that lower NAA/Cr (interpreted in the past as “axonal dysfunction”) may in fact be due primarily to elevated Cr. Similarly, the 15% Cho increase observed in patients here and in other studies will attenuate to ~5% in a Cho/Cr due to the concomitant Cr increase, reducing the ratio's discriminatory power.
CONCLUSION
Diffuse glial proliferation (elevated mI and Cr) and membrane turnover (high Cho) in the absence of atrophy or axonal injury (normal NAA levels) in RR MS patients suggest ongoing diffuse inflammation, de- and (or) re-myelination, as well as astrogliosis early in the disease course, even during clinical remission and despite immunomodulatory treatment. Consequently, mI, Cho and Cr may serve as earlier MRS markers of disease activity than the traditional NAA decline and atrophy that represent in order a later and the final stage of MS pathogenesis.
AKNOWLEDGEMENTS
We thank Drs. Andrew A. Maudsley and Brian J. Soher for the use of their SITools software.
Footnotes
COMPETING INTERESTS
None
FUNDING
This work was supported by NIH Grants NS050520, NS29029 and EB01015.
REFERENCES
- 1.Bakshi R. Magnetic resonance imaging advances in multiple sclerosis. J Neuroimaging. 2005;15(4 Suppl):5S–9S. doi: 10.1177/1051228405283362. [DOI] [PubMed] [Google Scholar]
- 2.Arnold DL, Matthews PM. MRI in the diagnosis and management of multiple sclerosis. Neurology. 2002;58(8 Suppl 4):S23–31. doi: 10.1212/wnl.58.8_suppl_4.s23. [DOI] [PubMed] [Google Scholar]
- 3.McFarland HF, Barkhof F, Antel J, et al. The role of MRI as a surrogate outcome measure in multiple sclerosis. Mult Scler. 2002;8(1):40–51. doi: 10.1191/1352458502ms767xx. [DOI] [PubMed] [Google Scholar]
- 4.Miller DH, Barkhof F, Frank JA, et al. Measurement of atrophy in multiple sclerosis: pathological basis, methodological aspects and clinical relevance. Brain. 2002;125(Pt 8):1676–95. doi: 10.1093/brain/awf177. [DOI] [PubMed] [Google Scholar]
- 5.Li DK, Held U, Petkau J, et al. MRI T2 lesion burden in multiple sclerosis: a plateauing relationship with clinical disability. Neurology. 2006;66(9):1384–9. doi: 10.1212/01.wnl.0000210506.00078.5c. [DOI] [PubMed] [Google Scholar]
- 6.Bakshi R, Thompson AJ, Rocca MA, et al. MRI in multiple sclerosis: current status and future prospects. Lancet Neurol. 2008;7(7):615–25. doi: 10.1016/S1474-4422(08)70137-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller DH, Thompson AJ, Filippi M. Magnetic resonance studies of abnormalities in the normal appearing white matter and grey matter in multiple sclerosis. J Neurol. 2003;250(12):1407–19. doi: 10.1007/s00415-003-0243-9. [DOI] [PubMed] [Google Scholar]
- 8.Allen IV, McKeown SR. A histological, histochemical and biochemical study of the macroscopically normal white matter in multiple sclerosis. J Neurol Sci. 1979;41(1):81–91. doi: 10.1016/0022-510x(79)90142-4. [DOI] [PubMed] [Google Scholar]
- 9.De Stefano N, Filippi M. MR spectroscopy in multiple sclerosis. J Neuroimaging. 2007;17(Suppl 1):31S–35S. doi: 10.1111/j.1552-6569.2007.00134.x. [DOI] [PubMed] [Google Scholar]
- 10.Narayana PA. Magnetic resonance spectroscopy in the monitoring of multiple sclerosis. J Neuroimaging. 2005;15(4 Suppl):46S–57S. doi: 10.1177/1051228405284200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chard DT, Griffin CM, McLean MA, et al. Brain metabolite changes in cortical grey and normal-appearing white matter in clinically early relapsing-remitting multiple sclerosis. Brain. 2002;125(Pt 10):2342–52. doi: 10.1093/brain/awf240. [DOI] [PubMed] [Google Scholar]
- 12.Inglese M, Li BS, Rusinek H, et al. Diffusely elevated cerebral choline and creatine in relapsing-remitting multiple sclerosis. Magn Reson Med. 2003;50(1):190–5. doi: 10.1002/mrm.10481. [DOI] [PubMed] [Google Scholar]
- 13.He J, Inglese M, Li BS, et al. Relapsing-remitting multiple sclerosis: metabolic abnormality in nonenhancing lesions and normal-appearing white matter at MR imaging: initial experience. Radiology. 2005;234(1):211–7. doi: 10.1148/radiol.2341031895. Epub 2004 Nov 04. [DOI] [PubMed] [Google Scholar]
- 14.Urenjak J, Williams SR, Gadian DG, et al. Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J Neurosci. 1993;13(3):981–9. doi: 10.1523/JNEUROSCI.13-03-00981.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: Guidelines for research protocols. Annals of Neurology. 1983;13(3):227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 16.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 17.Goelman G, Liu S, Hess D, et al. Optimizing the efficiency of high-field multivoxel spectroscopic imaging by multiplexing in space and time. Magn Reson Med. 2006;56(1):34–40. doi: 10.1002/mrm.20942. [DOI] [PubMed] [Google Scholar]
- 18.De Santi S, de Leon MJ, Rusinek H, et al. Hippocampal formation glucose metabolism and volume losses in MCI and AD. Neurobiol Aging. 2001;22(4):529–39. doi: 10.1016/s0197-4580(01)00230-5. [DOI] [PubMed] [Google Scholar]
- 19.Soher BJ, Young K, Govindaraju V, et al. Automated spectral analysis III: application to in vivo proton MR spectroscopy and spectroscopic imaging. Magn Reson Med. 1998;40(6):822–31. doi: 10.1002/mrm.1910400607. [DOI] [PubMed] [Google Scholar]
- 20.Traber F, Block W, Lamerichs R, et al. 1H metabolite relaxation times at 3.0 tesla: Measurements of T1 and T2 values in normal brain and determination of regional differences in transverse relaxation. J Magn Reson Imaging. 2004;19(5):537–45. doi: 10.1002/jmri.20053. [DOI] [PubMed] [Google Scholar]
- 21.Kirov I, Fleysher L, Fleysher R, et al. Age dependence of regional proton metabolites T2 relaxation times in the human brain at 3 T. Magn Reson Med. 2008;60(4):790–5. doi: 10.1002/mrm.21715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Stefano N, Matthews PM, Fu L, et al. Axonal damage correlates with disability in patients with relapsing-remitting multiple sclerosis. Results of a longitudinal magnetic resonance spectroscopy study. Brain. 1998;121(Pt 8):1469–77. doi: 10.1093/brain/121.8.1469. [DOI] [PubMed] [Google Scholar]
- 23.Trapp BD, Peterson J, Ransohoff RM, et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338(5):278–85. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 24.Vrenken H, Geurts JJ, Knol DL, et al. Normal-appearing white matter changes vary with distance to lesions in multiple sclerosis. AJNR Am J Neuroradiol. 2006;27(9):2005–11. [PMC free article] [PubMed] [Google Scholar]
- 25.De Stefano N, Narayanan S, Matthews PM, et al. In vivo evidence for axonal dysfunction remote from focal cerebral demyelination of the type seen in multiple sclerosis. Brain. 1999;122:1933–1939. doi: 10.1093/brain/122.10.1933. [DOI] [PubMed] [Google Scholar]
- 26.Danielsen EA, Ross B. Magnetic Resonance Spectroscopy Diagnosis of Neurological Diseases. Marcel Dekker, Inc.; New York: 1999. p. 327. [Google Scholar]
- 27.Bitsch A, Kuhlmann T, Stadelmann C, et al. A longitudinal MRI study of histopathologically defined hypointense multiple sclerosis lesions. Ann Neurol. 2001;49(6):793–6. doi: 10.1002/ana.1053. [DOI] [PubMed] [Google Scholar]
- 28.Bitsch A, Bruhn H, Vougioukas V, et al. Inflammatory CNS demyelination: histopathologic correlation with in vivo quantitative proton MR spectroscopy. AJNR Am J Neuroradiol. 1999;20(9):1619–27. [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher SK, Novak JE, Agranoff BW. Inositol and higher inositol phosphates in neural tissues: homeostasis, metabolism and functional significance. J Neurochem. 2002;82(4):736–54. doi: 10.1046/j.1471-4159.2002.01041.x. [DOI] [PubMed] [Google Scholar]
- 30.Frahm J, Hanefeld F. Localized proton magnetic spectroscopy of brain disorders in childhood. In: Bachelard HS, editor. Magnetic Resonance Spectroscopy and Imaging in Neurochemistry. Plenum Press; New York: 1997. pp. 329–402. [Google Scholar]
- 31.Williams A, Piaton G, Lubetzki C. Astrocytes--friends or foes in multiple sclerosis? Glia. 2007;55(13):1300–12. doi: 10.1002/glia.20546. [DOI] [PubMed] [Google Scholar]
- 32.Fernando KT, McLean MA, Chard DT, et al. Elevated white matter myo-inositol in clinically isolated syndromes suggestive of multiple sclerosis. Brain. 2004;127(Pt 6):1361–9. doi: 10.1093/brain/awh153. [DOI] [PubMed] [Google Scholar]
- 33.Kapeller P, Brex PA, Chard D, et al. Quantitative 1H MRS imaging 14 years after presenting with a clinically isolated syndrome suggestive of multiple sclerosis. Mult Scler. 2002;8(3):207–10. doi: 10.1191/1352458502ms822oa. [DOI] [PubMed] [Google Scholar]
- 34.Vrenken H, Barkhof F, Uitdehaag BM, et al. MR spectroscopic evidence for glial increase but not for neuro-axonal damage in MS normal-appearing white matter. Magn Reson Med. 2005;53(2):256–66. doi: 10.1002/mrm.20366. [DOI] [PubMed] [Google Scholar]
- 35.Abbott N. Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat. 2002;200(5):527. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silver NC, Tofts PS, Symms MR, et al. Quantitative contrast-enhanced magnetic resonance imaging to evaluate blood-brain barrier integrity in multiple sclerosis: a preliminary study. Mult Scler. 2001;7(2):75–82. doi: 10.1177/135245850100700201. [DOI] [PubMed] [Google Scholar]
- 37.Law M, Saindane AM, Ge Y, et al. Microvascular abnormality in relapsing-remitting multiple sclerosis: perfusion MR imaging findings in normal-appearing white matter. Radiology. 2004;231(3):645–52. doi: 10.1148/radiol.2313030996. [DOI] [PubMed] [Google Scholar]
- 38.Wakita H, Tomimoto H, Akiguchi I, et al. Glial activation and white matter changes in the rat brain induced by chronic cerebral hypoperfusion: an immunohistochemical study. Acta Neuropathol. 1994;87(5):484–92. doi: 10.1007/BF00294175. [DOI] [PubMed] [Google Scholar]
- 39.Dutta R, Trapp BD. Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology. 2007;68(22 Suppl 3):S22–31. doi: 10.1212/01.wnl.0000275229.13012.32. discussion S43-54. [DOI] [PubMed] [Google Scholar]
- 40.Kapeller P, McLean MA, Griffin CM, et al. Preliminary evidence for neuronal damage in cortical grey matter and normal appearing white matter in short duration relapsing-remitting multiple sclerosis: a quantitative MR spectroscopic imaging study. J Neurol. 2001;248(2):131–8. doi: 10.1007/s004150170248. [DOI] [PubMed] [Google Scholar]
- 41.Filippi M, Bozzali M, Rovaris M, et al. Evidence for widespread axonal damage at the earliest clinical stage of multiple sclerosis. Brain. 2003;126(Pt 2):433–7. doi: 10.1093/brain/awg038. [DOI] [PubMed] [Google Scholar]
- 42.Inglese M, Ge Y, Filippi M, et al. Indirect evidence for early widespread gray matter involvement in relapsing-remitting multiple sclerosis. Neuroimage. 2004;21(4):1825–9. doi: 10.1016/j.neuroimage.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Bjartmar C, Kinkel RP, Kidd G, et al. Axonal loss in normal-appearing white matter in a patient with acute MS. Neurology. 2001;57(7):1248–52. doi: 10.1212/wnl.57.7.1248. [DOI] [PubMed] [Google Scholar]
- 44.Kutzelnigg A, Lucchinetti CF, Stadelmann C, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128(Pt 11):2705–12. doi: 10.1093/brain/awh641. [DOI] [PubMed] [Google Scholar]
- 45.Bitsch A, Schuchardt J, Bunkowski S, et al. Acute axonal injury in multiple sclerosis. Correlation with demyelination and inflammation. Brain. 2000;123(Pt 6):1174–83. doi: 10.1093/brain/123.6.1174. [DOI] [PubMed] [Google Scholar]
- 46.Wang D, Ayers MM, Catmull DV, et al. Astrocyte-associated axonal damage in pre-onset stages of experimental autoimmune encephalomyelitis. Glia. 2005;51(3):235–40. doi: 10.1002/glia.20199. [DOI] [PubMed] [Google Scholar]