Synopsis
Heart failure is common and is associated with poor prognosis. Chronic kidney disease is common in heart failure, and shares many risk factors with heart failure such as age, hypertension, diabetes, and coronary artery disease. Over half of all heart failure patients may have moderate to severe chronic kidney disease. The presence of chronic kidney disease is associated with increased morbidity and mortality, yet it is also associated with underutilization of evidence-based heart failure therapy that may reduce morbidity and mortality. Understanding the epidemiology and outcomes of chronic kidney disease in heart failure is essential to ensure proper management of these patients.
Keywords: Heart failure, chronic kidney disease, epidemiology, risk factors, management
Heart Failure: A Multidisciplinary Syndrome of Epidemic Proportion
Heart failure is a multi-disciplinary syndrome of epidemiologic importance. The vast majority of the estimated 5 million heart failure patients in the United States are older adults [1, 2]. Most of the over 1 million hospitalizations and over 300,000 heart failure-related deaths annually also occur in patients ≥65 years [1, 2]. The incidence of heart failure increases exponentially with age. Heart failure shares many features of major geriatric syndromes, and from an epidemiologic viewpoint heart failure can be considered a geriatric syndrome [3]. However, heart failure is also a complex cardiovascular syndrome with complicated pathophysiology, poor prognosis, and rapidly evolving therapy that demands continued basic and clinical research. Thus, mechanistically speaking, heart failure is thus very much a cardiac syndrome. As the number one reason for hospital admission for Medicare beneficiaries and as the primary cause of over 1 million hospitalizations, it is also a leading health services research syndrome. Currently, over half of the annual cost of heart failure care of an estimated 30 billion dollars is spent for inpatient care of heart failure [1]. With the aging of the baby boomers, the proportion of United States population ≥65 years is projected to increase from a current 13% to an estimated 20% in the next couple of decades [4]. With this increase in the population, the absolute number of heart failure hospitalization is projected to double to over 2 million per year posing a great challenge to the health care system of this nation.
Heart Failure: A Syndrome of Comorbidities
Because over 80% heart failure patients are ≥65 years, most of these patients suffer from one or more comorbidities. Further, because heart failure is a syndrome and not a disease per se, there are often one or more underlying causes that lead to the development of heart failure. Some of the most common causes of heart failure are hypertension, coronary artery disease, diabetes, and chronic kidney disease (CKD), which are often present as comorbidities in heart failure patients. A study of 105,388 heart failure patients in the Acute Decompensated Heart Failure National Registry (ADHERE) reported that 57% of these patients had coronary artery disease, 73% had hypertension, 44% had diabetes, and over 60% had kidney disease [5, 6]. These patients had a mean age of 72 years, 20% were African Americans and 52% were women. Age is an important risk factor for heart failure as it is for many of its risk factors including CKD [7, 8]. Therefore, the prevalence of CKD is expected to be higher in patients with heart failure than in the general population.
Chronic Kidney Disease in Heart Failure: Case Scenarios
Case 1
An 86-year-old white woman with a history of hypertension and atrial fibrillation developed progressive dyspnea about two years ago. Recently her dyspnea worsened. She could barely walk inside her home and often slept on a recliner to avoid orthopnea. She denied paroxysmal nocturnal dyspnea, cough, wheezing, or chest pain. She also reported right upper quadrant pain, nausea, loss of appetite, and severe leg swelling. She had no emergency room visits or hospitalizations due to dyspnea. Her current medications included a thiazide diuretic, digoxin, and a beta blocker. Her physical examination was remarkable for blood pressure of 156/88 mm of mercury, a heart rate of 85 beats/minute, an estimated jugular venous pressure of 16 cm of water, a positive hepatojugular reflux, a right-sided third heart sound, no pulmonary râles or wheezing, an enlarged soft tender liver, and severe bilateral lower extremity edema up to mid-thigh with brown pigmentation and induration of skin, and multiple blisters over lower legs. An accentuated second heart sound at left fifth intercostal space suggested pulmonary hypertension, with an estimated pulmonary artery systolic pressure of 50 millimeter of mercury. Her current serum creatinine level was 1.1 mg/dL (97 μmol/L) and her last serum creatinine from six months ago was 1 mg/dL (88 μmol/L). Her serum potassium was 3.6 mEq/L. She had a normal electrocardiogram and a chest radiograph revealed marked cardiomegaly and pulmonary congestion. An echocardiogram showed a left ventricular ejection fraction of >55%.
Case 2
A 59-year-old African American man with a history of hypertension and diabetes, developed dyspnea after an acute myocardial infarction. His left ventricular ejection fraction was 35%. He underwent coronary stenting, and his coronary symptoms have remained stable for the past five years. He currently had no dyspnea on usual exertion. He also denies dyspnea at rest, orthopnea, paroxysmal nocturnal dyspnea, cough, wheezing, or chest pain. He is currently receiving an aspirin, an angiotensin-converting enzyme (ACE) inhibitor, a beta blocker, and a statin drug. He has had no emergency room visits or hospitalizations due to dyspnea. His physical examination was unremarkable except for mild pitting edema around his ankles. He had normal jugular venous pressure, no hepatojugular reflux, no third heart sound, and no pulmonary râles. His most recent serum creatinine level was 1.4 mg/dL (124 μmol/L) and has remained stable around this level for the past one year. His serum potassium was 4.6 mEq/L. A spot urine sample revealed an albumin to creatinine ratio of 130 mg/gram suggesting microalbuminuria. A twelve-lead electrocardiogram showed old anteroseptal myocardial infarction, and findings from a chest x-ray were normal.
Relevance of Epidemiology to Patient Care
Do these patients have CKD? Is it possible to establish the diagnosis of CKD in these patients based on the information provided here? Serum creatinine levels in both these patients were <1.5 mg/dL, a cutoff often used by many clinical laboratories as the upper limit of normal serum creatinine levels. What are the prognostic and therapeutic implications of CKD in patients with heart failure? How heart failure patients with CKD should be treated? How common is CKD in heart failure? How common is heart failure in CKD?
Diagnosis of Chronic Kidney Disease in Heart Failure
According to the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (K/DOQI) guideline (http://www.kidney.org/professionals/kdoqi/guidelines_ckd/toc.htm) serum creatinine alone should not be used to make the diagnosis of CKD. Serum creatinine is not a sensitive marker of glomerular filtration rate (GFR) [9]. A basic evaluation for CKD includes an estimate of GFR, urinalysis and quantification of albuminuria [10]. An estimated GFR <60 ml/min/1.73 m2 body surface area (BSA) suggests CKD, even in the absence of evidence of kidney damage such as albuminuria [10]. In patients with GFR ≥60 ml/min/1.73 m2, evidence of kidney damage must be present, before a diagnosis of CKD can be established. A decreased GFR or kidney damage must be present for >3 months for the diagnosis of CKD. The K/DOQI classification for CKD is shown in Table 1.
Table 1.
Stages of chronic kidney disease [10]
| Stage | Description | GFR*, mL/min/1.73m2 of BSA |
|---|---|---|
| 1 | Kidney damage with normal or increased GFR | >90 |
| 2 | Kidney damage with mildly decreased GFR | 60–89 |
| 3 | Moderately decreased GFR | 30–59 |
| 4 | Severely decreased GFR | 15–29 |
| 5 | Kidney failure | <15 or dialysis |
GFR estimated from serum creatinine by abbreviated Modification of Diet in Renal Disease Study equation based on age, sex, race, and calibration for serum creatinine.
In most situations, GFR estimating equations are preferred over 24-hour urine creatinine clearance studies because they are less cumbersome and more accurate. Both the MDRD and Cockcroft-Gault equations are widely used (Box 1). The abbreviated (4-variable: age, gender, race, serum creatinine) MDRD equation is frequently used in epidemiology studies. An advantage of the MDRD equation over the Cockcroft-Gault equation is that it does not require a body weight. The MDRD-estimated GFR is expressed per 1.73 m2 BSA which is the normal mean value for young adults. Adjustment for BSA allows comparison of a patient’s estimated GFR to normal values. The MDRD has been validated in whites, African Americans and patients with diabetes [11]. Both equations are based on serum creatinine and assume stable kidney function. These methods underestimate GFR when GFR is ≥60 ml/min/1.73 m2. They may also be less accurate when serum creatinine production is abnormal, such as in severe malnutrition, muscle wasting, limb amputation or cirrhosis. Cystatin C, a cysteine proteinase inhibitor that is freely filtered at the glomerulus, has been proposed as an alternative to creatinine based estimates of GFR [12–14]. However, cystatin C is also influenced by factors such as hypothyroidism, tobacco use and inflammation, and is not widely available [15, 16].
Box 1. Formulas for estimating glomerular filtration rate and creatinine clearance.
Abbreviated (4-variable) Modification of Diet in Renal Disease (MDRD) study equation
Cockcroft-Gault equation
Renal damage is assessed by either a dipstick for albuminuria and hematuria, or a more sensitive dipstick for smaller amounts of albumin (microalbuminuria). Albuminuria should be quantified by using a random urine sample for an albumin to creatinine ratio. Albuminuria is defined as a urinary albumin to creatinine ratio of ≥30 mg/gm (≥3.5 mg/mmol), with a ratio of 30–299 mg/gm (3.5–35 mg/mmol) suggesting microalbuminuria and ≥300 mg/gm (≥35 mg/mmol) indicating macroalbuminuria [17].
Using an online GFR calculator (http://www.nephron.com/MDRD_GFR.cgi) based on the four-variable MDRD equation, case 1 had an estimated GFR of 50 ml/min/1.73 m2. She had stage-3 CKD and according to the K/DOQI guideline she was at moderate risk of complications, for which she should receive nephrology consultation. The estimated GFR of case 2 was 67 ml/min/1.73 m2. His GFR alone was not sufficient for the diagnosis of CKD. However, considering his risk of kidney damage from his hypertension, diabetes, and heart failure, a spot urine sample was tested for albumin to creatinine ratio, and a diagnosis of microalbuminuria was made. According to the K/DOQI guideline, he had stage-2 CKD and was at mild risk of complications, and should also receive nephrology consultation. Using four readily available variables, a diagnosis of CKD was made in both these patients with apparently normal serum creatinine levels. So, what is the significance of the presence of CKD in heart failure patients?
Prognostic Implications of Chronic Kidney Disease in Heart Failure
The presence of CKD is associated with poor prognosis in heart failure and can be used to risk stratify patients for targeted intervention [6, 18–24]. According to one study, the risk of death in heart failure may be more strongly associated with a decline in the GFR than with a decline in the left ventricular ejection fraction [22]. Among patients with chronic heart failure in the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) trial, compared to patients without CKD, those with CKD (MDRD-based GFR <60 ml/min/1.73 m2 BSA) had increased risk of cardiovascular death or heart failure hospitalization [23]. This risk increased progressively with decreasing GFR.
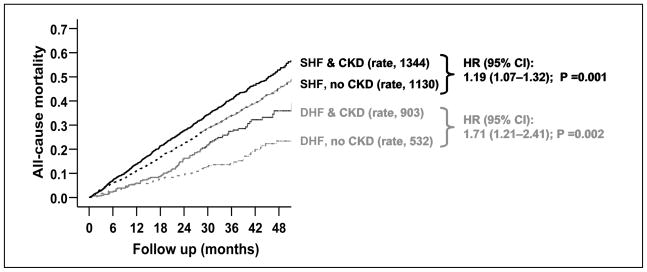
In a propensity-matched study of the effect of CKD on total mortality in patients with systolic (ejection fraction <45%; n=6800) and diastolic (ejection fraction ≥45%; n=988) heart failure in the Digitalis Investigation Group trial, there were 757 and 882 deaths, respectively, in patients without and with CKD (hazard ratio when CKD was compared with no CKD, 1.22, 95% confidence interval {CI}, 1.09–1.36; p <0.0001) [18]. CKD was defined by MDRD-based GFR of <60 ml/min/1.73 m2 of BSA. In that study, CKD-associated mortality was higher in patients with diastolic heart failure (hazard ratio, 1.71, 95% CI, 1.21–2.41; p =0.002) than in systolic heart failure (hazard ratio, 1.19, 95% CI, 1.07–1.32, p =0.001, Figure 1). This difference in CKD-associated death between patients with systolic and diastolic heart failure was statistically significant (adjusted p for interaction =0.034). Further, the risk of CKD-associated death progressively increased with increasing ejection fraction. The hazard ratios for CKD-associated mortality for patients with ejection fraction <35%, 35–55%, and >55% were respectively 1.15 (95% CI, 1.02–1.29), 1.35 (95% CI, 1.11–1.64), and 2.33 (95% CI, 1.34–4.06) [18]. CKD has a similar association with cause-specific mortality and hospitalization in these patients [25].
Figure 1.
Kaplan-Meier plots for cumulative risk of chronic kidney disease (CKD)-associated all-cause death systolic (SHF) and diastolic (DHF) heart failure patients, with rates of death (expressed per 10,000 person-years of follow up), and hazard ratio (HR) and 95% confidence interval (CI), when CKD is compared with no CKD (Adopted form Ahmed et al. Am J Cardiol. 2007. Ref. 18)
Data from 118,465 patients with acute heart failure in the ADHERE registry demonstrate a similar effect of CKD [6]. In that study, 2%, 2%, 4%, 8% and 7% patients respectively with stages I, 2, 3, 4 and 5 CKD died in the hospital. CKD was also associated with increased morbidity. An estimated 17%, 17%, 18%, 22% and 26% patients respectively with stages 1, 2, 3, 4 and 5 CKD were admitted to intensive care units [6]. CKD was also a risk factor for worsening kidney function during hospitalization in acute heart failure, which in turn was associated with increased total mortality and heart failure hospitalization [19, 20].
Anemia and secondary hyperparathyroidism are common complications of CKD and may further complicate prognosis and management of HF patients with CKD. Anemia of CKD is associated with poor prognosis [26–28]. Even though data from one small study suggest that aggressive treatment of anemia may improve outcomes in heart failure [29], data from large prospective trials of CKD patients (with and without heart failure) suggest that such treatment not improve outcomes [30–32]. Secondary hyperparathyroidism and abnormalities in calcium and phosphorus metabolism also common in CKD, and may play a role in abnormal vascular calcifications in heart failure patients with CKD [33–36]
Therapeutic Implications of Chronic Kidney Disease in Heart Failure
There are two aspects in the therapeutic implications of CKD in heart failure: the impact of the presence of CKD on the receipt of evidence-based therapy, and the importance of administration of such therapy to heart failure patients with CKD. ACE inhibitors and angiotensin receptor blockers have been shown to reduce mortality in patients with systolic heart failure [37]. However, these drugs are often underused in heart failure and one of the reasons for this underuse is the perception that the increase in serum creatinine associated with the use of these drugs may be an indication of worsening kidney function [38, 39]. This is despite the fact that a rise in serum creatinine associated with the use of ACE inhibitors and angiotensin receptor blockers is often mild and reversible, and often considered a marker of their effectiveness as is a decrease is heart rate in patients receiving beta blockers [40]. ACE inhibitors and angiotensin receptor blockers have been shown to reduce the progression of kidney damage in wide spectrum of patients with kidney disease [41]. Therefore, these drugs may be both cardio- and reno-protective in systolic heart failure patients with CKD.
This is especially important considering the increased risk of death associated with CKD in heart failure. Effects of drugs are often more pronounced in subgroups of patients with more severe disease or higher comorbidity burden [42]. Even though patients with CKD were excluded from most randomized clinical trials of ACE inhibitors and angiotensin receptors in heart failure, post hoc and subgroup analyses and other observational data suggest that these drugs are equally effective in heart failure patients with CKD, and the absolute benefit may even be greater in patients with CKD than in those without CKD [21, 43–46]. The cardio-protective benefit of ACE inhibitors and angiotensin receptors is not well-established in diastolic heart failure. However, these patients benefit from the reno-protective effects of these drugs, which may indirectly improve prognosis. However, this has not been demonstrated in large-scale randomized clinical trials. Data from patients with stable coronary artery disease and normal left ventricular ejection fraction demonstrated that randomized use of trandolapril, an ACE inhibitor, was associated with a reduction in total mortality in patients with CKD, but not in those with normal kidney function [47]. These data suggest that kidney function may be used to risk stratify patients with stable coronary artery disease and normal left ventricular ejection fraction and identify those that are most likely to benefit from therapy with ACE inhibitors or angiotensin receptors for cardiovascular protection.
Thus, initial and subsequent assessment of heart failure patients should include an assessment for the presence of CKD. The presence of CKD is a marker of poor prognosis, regardless of ejection fraction, and these patients should be appropriately treated with inhibitors of the renin-angiotensin system. Any associated hypertension, diabetes, or other shared risk factors should also be appropriately treated. Therefore, to appreciate the correlates and impact of CKD in heart failure better, it is important to understand the epidemiology of CKD in the population, in general, and in patients with heart failure, in particular.
Epidemiology of Chronic Kidney Disease in the Population in the United States
Like heart failure, CKD is also common. According to a recent study based on the 1999–2004 National Health and Nutrition Examination Surveys (NHANES), an estimated 13% of the population ≥20 years or nearly 26 million Americans in that age group have CKD [48]. The prevalence of stage 1, 2, 3 and 4 CKD was respectively 1.8% (3.5 million), 3.2% (6.3 million), 7.7% (15.2 million) and 0.35% (0.7 million) of the population. Participants in that study had a mean age of 46 years, 51% were women and 11% were African Americans. CKD was diagnosed by both persistent albuminuria (albumin to creatinine ration of ≥30 mg/gm based on a random spot urine sample) and decreased MDRD-estimated GFR [48]. CKD was categorized into 4 stages based on the K/DOQI classification system (Table 1).
The results of that study also demonstrated that the prevalence of CKD has increased from 10% of the population ≥20 years (an estimated 18 million) in the NHANES (1988–1994) to 13% of the population ≥20 years (an estimated 26 million) in the NHANES (1999–2004) [48]. This increase in the prevalence of CKD has been attributed to the increased prevalence of diabetes, hypertension, and obesity during that period. An earlier study by the Center for Disease Control and Prevention using the same NHANES (1988–1994) and NHANES (1999–2004) surveys suggested somewhat higher prevalence [49]. According to that study the prevalence of CKD in the NHANES (1988–1994) and NHANES (1999–2004) were respectively 14.5% and 16.8% of the population ≥20 years. These differences are likely due to the more conservative methodology used in the former study.
Age is an important risk factor for CKD. Of the13233 participants in NHANES (1999–2004) study, more than three-fourth were younger than 60 years [48]. The estimated prevalence of CKD was <5% for participants 20–39 years, <10% for those 40–59 years, >20% for those 60–69 years, and about 50% for those 70 years and older. This is an important observation as the vast majority of heart failure patients are 65 years and older.
Age and the Epidemiology of Chronic Kidney Disease
The importance of age as a risk factor of CKD is further highlighted by a recent study of the prevalence of CKD in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study [50]. REGARDS is a nationally representative, population-based cohort study of 20,667 participants ≥45 years. REGARDS has been designed to identify risk factors for excess stroke mortality among African Americans and those living in the Southeastern United States. The goal of REGARDS is to recruit 30,000 participants free of stroke at baseline, and this report was based on the first 20,667 participants enrolled. The results of this study demonstrated a 43% prevalence of CKD among REGARDS participants [50]. In that study CKD was defined by an estimated GFR <60 ml/min/1.73 m2 of BSA and the GFR was estimated using the abbreviated MDRD Study equation. However, serum creatinine values were calibrated to values reported by the Cleveland Clinic Foundation, and REGARDS values were found to be lower than the Cleveland Clinic Foundation values.
REGARDS participants in that study had a mean age of 66 years, 42% were African Americans, and 51% were women [50]. The prevalence of CKD was 19.3%, 31.6%, 51.5%, 62.7% and 71.0% respectively for participants 45–54 years, 55–64 years, 65–74 years, 75 to 84 years, and ≥85 years [50]. The prevalence of CKD was higher among whites (50% versus 34% among African Americans) and women (48% versus 39% in men). The lower prevalence of CKD among African Americans was mostly observed at lower stages of CKD and reversed at higher stages suggesting a higher rate of disease progression among African Americans with CKD. The prevalence of CKD was significantly higher among those with hypertension (48% versus 37% among those without hypertension), diabetes (45% versus 43% for those without diabetes), prior myocardial infarction (54% versus 42% for those without myocardial infarction) and prior stroke (54% versus 43% for those without stroke) [50].
A study of the 5808 non-institutionalized individuals ≥65 years in the Cardiovascular Health Study demonstrated that 1249 (22%) had CKD at baseline and the presence of CKD was associated with older age, male sex, white race, and a higher prevalence of cardiovascular disease [51]. The Cardiovascular Health Study is a prospective cohort study of risk factors for cardiovascular disease in elderly men and women. This currently ongoing study recruited eligible participants and their spouses from Forsyth County, North Carolina, Sacramento County, California, Washington County, Maryland, and Pittsburgh, Pennsylvania. CKD was defined as abbreviated MDRD-estimated GFR <60 ml/min/1.73 m2 of BSA. Serum creatinine values were also calibrated to values reported by the Cleveland Clinic Foundation. However, it is not clear why the prevalence of CKD was much lower in this elderly cohort compared to the relatively younger REGARDS population, despite similar methodology used in both the studies.
Chronic Kidney Disease: A Global Epidemic
A study of the prevalence of CKD using a primary care database in the United Kingdom reported an overall prevalence of 8.5% among population ≥18 years, with women having a higher prevalence (10.6% versus 5.8% in men) [52]. In that study, 68% of the patients were excluded due to lack of valid data on serum creatinine. Patients in that study had a mean age of 58 years, and 56% were women. As expected the prevalence of CKD increased with age: from 0.01% in the age group 18–24 years to 45% in those ≥85 years. CKD was defined by GFR <60 ml/min/1.73 m2 of BSA, based on the four-variable MDRD formula.
Another study, also from the United Kingdom, among older adults in residential care homes reported 82% prevalence of CKD [53]. The prevalence of stage 3 and 4 CKD was respectively 72% and 10% in that study. Patients in that study had a mean age of 86 years and 79% were women. CKD was defined as MDRD-estimated GFR <60 ml/min/1.73 m2 of BSA. When Cockcroft–Gault equation was used to estimate GFR, the prevalence of CKD was 97% with 66% and 31% respectively with stage 3 and 4 CKD. The finding from this study further highlights the importance of age as a risk factor for CKD in the general population.
A study of 11,247 non-institutionalized adult population ≥25 years in Australia reported an 11% prevalence of CKD, which was defined as an estimated GFR <60 ml/min/1.73 m2 based on the Cockcroft-Gault method [54]. In that study the prevalence of CKD increased with age: 2.5% for those 45–64 years and 55% for those ≥65 years. A study of a population of 527,594 participants, age ≥20 years, in Japan reported a 20% prevalence of CKD [55]. The prevalence increased significantly with age: 1%, 4%, 11%, 16%, 32%, 44%, and 59%, respectively for population 20–29, 30–39, 40–49, 50–59, 60–69, 70–79, and 80–89 years.
A study of 2,353 participants in Beijing, China reported an 11% prevalence of CKD, defined as a GFR of <60 ml/min/1.73 m2 of BSA [56]. Men and women had similar prevalence in that study. The GFR in that study was calculated by a new GFR-estimating equation which was developed by modifying the MDRD equation based on data from Chinese patients with CKD. Little is know about the prevalence of CKD in India [57]. It has been suggested that the prevalence of CKD may be lower in India as the GFR may be lower in the predominantly vegetarian Indian subjects with different creatinine generation rates.
Epidemiology of Chronic Kidney Disease in Cardiovascular Disease
Because CKD and cardiovascular disease share many risk factors, the prevalence of CKD is expected to be higher in these patients than in the general population. However, studies on the prevalence of CKD in patients with cardiovascular disease are not as well conducted as those for the general population. In the Cooperative Cardiovascular Project, an estimated 13% of patients had blood urea nitrogen >40 mg/dL or serum creatinine >2.5 mg/dL [58]. In a study of 1,609 patients with established cardiovascular disease in the West of Ireland had 1,272 (79%) patients with one or more serum creatinine measurements during the study period and 36% had GFR <60 ml/min/1.73 m2 of BSA based on MDRD formula [59]. In another study of all patients undergoing cardiac catheterization at Duke University Medical Center between 1995 and 2000 with at least one coronary artery stenosis of ≥75%, 24% had CKD based on the Cockcroft-Gault formula based estimated creatinine clearance of <60 ml/min [60]. In the China Heart Survey, among the 3,513 participants with coronary heart disease, 25% had CKD [61]. However, data on the prevalence of CKD in patients in randomized clinical trials of cardiovascular disease is limited as these patients are often excluded from these trials [62, 63].
Epidemiology of Chronic Kidney Disease in Heart Failure
Unlike in the general population, the epidemiology of CKD in heart failure is not very well studied. Patients with high serum creatinine levels have often been excluded form randomized clinical trials in heart failure [64]. Data on the prevalence of CKD in heart failure are, therefore, best derived from large heart failure registries involving hospitalized acute heart failure patients (Table 2).
Table 2.
Prevalence of chronic kidney disease in heart failure (HF)
| Study | Data source | Number of HF patients | Mean age | Women (%) | African American (%) | Method to define CKD | Advanced CKD excluded | Prevalence of CKD |
|---|---|---|---|---|---|---|---|---|
| Heywood JT et al., 2007 [6] | ADHERE | 118465 | 72 | 52 | 21 | MDRD | No | 64% |
| Fonarow GC, et al., 2007 [66] | OPTIMIZE | 48612 | 73 | 52 | 18 | ? CRI | No | 20% |
| Masoudi FA et al., 2005 [67, 68] | NHCP | 62376 | 79 | 58 | 10 | MDRD | No | 67% |
| Ahmed A* | AHFP, 1994 [39] | 1091 | 79 | 60 | 18 | MDRD | No | 68% |
| Ahmed A* | AHFP, 2001 [69] | 8555 | 76 | 58 | 24 | MDRD | No | 65% |
| Hillege HL et al., 2006 [23, 65] | CHARM | 2680 | 65 | 33 | 4 | MDRD | Yes | 36% |
| Khan NA et al., 2006 [70] | SOLVD | 6440 | 60 | 14 | 12 | MDRD | Yes | 33% |
| Ahmed A et al., 2007 [18] | DIG | 7788 | 64 | 25 | 15 | MDRD | Yes | 45% |
| Ahmed A* | BEST [71] | 2707 | 60 | 22 | 23 | MDRD | Yes | 37% |
| Ahmed A* | EPHESUS [72] | 6632 | 64 | 29 | 1 | MDRD | Yes | 34% |
| Ahmed A* | CHS [73] | 262 | 75 | 52 | 20 | MDRD | No | 53% |
| Ahmed A* | FHS [74] | 70 | 74 | 70 | 0 | MDRD | No | 74% |
| Ahmed A et al. 2008 [75] | REGARDS | 357 | 65 | 65 | 59 | MDRD | No | 55% |
| McAlister FA et al., 2004 [44] | University Clinic | 754 | 67 | 34 | - | No | 56% | |
| Ezekowitz J et al., 2004 [21] | APPROACH | 6427 | 69 | 35 | - | C-G equation | No | 39% |
| Bibbins-Domingo K et al., 2004 [76] | HERS | 702 | 68 | 100 | 12 | C-G equation | No | 58% |
| Berger AK et al., 2007 [46] | Minnesota Heart Survey | 2169 | 69 | 47 | - | C-G equation | No | 53% |
| Cowie MR et al., 2006 [19] | POSH | 299 | 68 | 26 | - | ** | No | 33% |
| Jose P et al., 2006 [77] | SAVE | 1854 | 59 | 17 | - | ** | Yes | 12% |
AHFP=Alabama Heart Failure Project, C-G=Cockcroft-Gault, CKD=chronic kidney disease, CRI=chronic renal insufficiency (not defined), FHS=Framingham Heart Study, MDRD=Modification of Diet in Renal Disease, NHCP=National Heart Care Project,
Unpublished data, analysis performed by author (AA), using original data;
Change in serum creatinine >0.3 mg/dL
A study of about 120,000 contemporary acute hospitalized heart failure patients from the ADHERE registry indicates that CKD (defined as MDRD GFR <60 ml/min/1.73m2 of BSA) was present in 75,382 (64%) patients, of whom 51,553 (44%), 15,553 (13%) and 8,276 patients (7%) respectively had stage 3, 4 and 5 CKD [6]. These patients had a median age of 75 years, 52% were women, and 25% were African Americans, with mean (standard deviation) serum creatinine, GFR, blood urea nitrogen levels of 1.8 (±1.6) mg/dL, 55 (±30) ml/min/1.73 m2 of BSA, and 32 (±21) mg/dL. Data from other similar registries are presented in Table 2.
Of the 7,599 contemporary ambulatory chronic heart failure patients in the CHARM trial, baseline serum creatinine levels (<3 mg/dL) were available from 2,680 patients enrolled from North America [65]. Of these 931 were from the CHARM-Added trial (37% of 2,548 systolic heart failure patients, all receiving an ACE inhibitors), 1,087 were from the CHARM-Preserved trial (36% of 3,023 patients with diastolic heart failure, <20% receiving ACE inhibitors) and 662 were from the CHARM-Alternative trial (33% of 2,028 patients with systolic heart failure who were intolerant to ACE inhibitors). The overall prevalence of CKD (GFR <60 ml/min/1.73 m2 of BSA) was 36% [23]. Prevalence of CKD in the CHARM-Added, CHARM-Preserved and CHARM- Alternative trials was respectively 33%, 35% and 43%. The prevalence of CKD was similar regardless of ejection fraction. The high prevalence of CKD in the CHARM-Alternative trial was likely due to the fact these patients were intolerant of ACE inhibitors. This is not surprising as a high baseline serum creatinine or a worsening kidney function is often perceived as a contraindication to the use of ACE inhibitors [40].
Risk Factors for Chronic Kidney Disease in Heart Failure
Prospective epidemiological data on the risk factor of CKD in patients with heart failure are scarce, and most associations are derived from cross-sectional studies. Data from the Framingham Heart Study suggest that age, baseline GFR, body mass index, smoking, and diabetes are risk factors for new-onset CKD in the general population [78]. Cross-sectional data from the NHANES survey suggest that in the general population age, ethnicity, education, diabetes, hypertension, cardiovascular disease, and body mass index are associated with CKD [49]. Similar associations are observed in heart failure patients, and because of the high prevalence of many of these risk factors in heart failure patients, the risk of CKD in heart failure is also likely high. Data from the ADHERE registry indicate that age, sex and ethnicity are associated with CKD [6]. However, these associations were somewhat unique among stage 5 CKD patients, of whom about 68% were receiving chronic dialysis. The mean age of patients increased progressively with worsening kidney function, from 62 years in stage 1, to 70 years in stage 2 and 76 years in stage 3 and 4, but decreased to 67 years for those in stage 5 CKD. Most heart failure patients with CKD were women (54%, 58% and 54% respectively for stage 3, 4 and 5), while most with stage 1 and 2 kidney function were men (57% and 53, respectively). The proportion of African Americans among heart failure patients with stage 1 to 5 kidney function were respectively 39%, 24%, 15%, 15% and 33% [6], suggesting that these patients were less likely to develop CKD, but once developed, they were more likely to progress into stage 5 CKD, often requiring chronic dialysis. A similar association between race and CKD was also observed in the general population. In REGARDS, the prevalence of CKD (MDRD-estimated GFR <60 ml/min/1.73 m2) was higher among whites (50% versus 34% for African Americans); however, the prevalence of stage 4 and 5 CKD (GFR <30 ml/min/1.73 m2) was higher among African Americans (0.3% versus 0.1% for whites) [50].
Among heart failure patients in the ADHERE registry, the prevalence of hypertension, a risk factor for CKD, progressively increased with the worsening of kidney function: 68%, 70%, 73%, 77% and 85% respectively for patients with stage 1 through 5 CKD [6]. The prevalence of diabetes, also a risk factor for CKD, was the lowest in patients with stage 1 and 2 patients (38% and 37% respectively), intermediate in stage 3 (45%), and the highest in those with stage 4 and 5 CKD (respectively 54% and 55%). The prevalence of coronary artery disease, another risk factor, also increased with worsening kidney function (41%, 51%, 61% and 67% respectively for patients with stage 1 through 4), but dropped in those with stage 5 disease (56%) [6]. This may in part be due to the higher mortality of heart failure patients with coronary artery disease and stage 5 CKD [79, 80]. The prevalence of systolic heart failure (ejection fraction <40%), on the other hand, was the lowest among patients with stage 5 CKD (53%, 52%, 52%, 52% and 45% for those with stage 1 to 5 disease) [6], suggesting that either fewer patients with systolic heart failure progressed to stage 5 CKD, or heart failure patients with low ejection fraction and stage 5 CKD receiving dialysis had disproportionately high mortality rates. Similar associations with these risk factors are also observed in ambulatory chronic heart failure patients [18, 23].
Chronic Kidney Disease as a Risk Factor for Heart Failure
Because heart failure and CKD share common risk factors that often co-exist, it may be at times difficult to determine if CKD in heart failure is a case of prevalent or incident CKD, or a manifestation of cardiorenal syndrome [81–84]. Data from the Cardiovascular Health Study indicate that among older adults, increasing baseline serum creatinine levels were associated with a graded increase in the risk for incident heart failure [7, 85]. A further analysis of the Cardiovascular Health Study suggests that among older adults with no baseline CKD, a pre-clinical CKD identified by cystatin C was associated with increased risk of heart failure [12]. CKD has a poor prognosis among African Americans [6, 50]. However, little is known about racial variations in the CKD-associated risk of heart failure, which will be studied prospectively in REGARDS, in which half of the estimated 30,000 participants are African Americans [75].
Summary
Heart failure is common and is associated with poor prognosis. Heart failure and CKD share many common risk factors and they often coexist. About one-third of trial-eligible chronic heart failure patients and about two-thirds of real-life hospitalized acute heart failure patients are estimated to have CKD defined as MDRD estimated GFR of <60 ml/min/1.73 m2 of BSA. CKD is associated with increased morbidity and mortality in heart failure. CKD is also associated with underuse of ACE inhibitors or angiotensin-receptor blockers. Despite concerns for rising serum creatinine, use of these drugs is associated with improved outcomes. Clinicians should routinely risk stratify heart failure patients by the presence of CKD based on MDRD estimated GFR and take preventive and therapeutic measures based on current guidelines and appropriate nephrology consultation.
Acknowledgments
Grant Supports: Dr. Ahmed is supported by the National Institutes of Health through grants from the National Heart, Lung, and Blood Institute (5-R01-HL085561-02 and P50-HL077100). Dr. Campbell is supported by the National Institutes of Health through a grant from the National Institute of Diabetes and Digestive and Kidney Diseases, 1-K23-DK-64649-1A2 and by the National Kidney Foundation Young Investigator Program
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ali Ahmed, Division of Gerontology, Geriatrics and Palliative Care, Department of Medicine, School of Medicine, and Department of Epidemiology, School of Public Health, Scientist, Center for Aging, and Center for Heart Failure Research, and Director, Geriatric Heart Failure Clinics, University of Alabama at Birmingham, and Veterans Affairs Medical Center, Birmingham, Alabama.
Ruth C. Campbell, Division of Nephrology, Department of Medicine, School of Medicine, University of Alabama at Birmingham, UAB Hospital, 1802 6th Avenue South, Birmingham, AL 35249 and Veterans Affairs Medical Center, Birmingham, Alabama.
References
- 1.Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.National Heart L, and Blood Institute, National Institutes of Health. Congestive Heart Failure in the United States: A New Epidemic. 1996. [Google Scholar]
- 3.Ahmed A. DEFEAT heart failure: clinical manifestations, diagnostic assessment, and etiology of geriatric heart failure. Heart Fail Clin. 2007;3:389–402. doi: 10.1016/j.hfc.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Redfield MM. Heart failure--an epidemic of uncertain proportions. N Engl J Med. 2002;347:1442–1444. doi: 10.1056/NEJMe020115. [DOI] [PubMed] [Google Scholar]
- 5.Adams KF, Jr, Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007;13:422–430. doi: 10.1016/j.cardfail.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Gottdiener JS, Arnold AM, Aurigemma GP, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 8.Yamagata K, Ishida K, Sairenchi T, et al. Risk factors for chronic kidney disease in a community-based population: a 10-year follow-up study. Kidney Int. 2007;71:159–166. doi: 10.1038/sj.ki.5002017. [DOI] [PubMed] [Google Scholar]
- 9.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 10.The National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 11.Stevens LA, Coresh J, Feldman HI, et al. Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol. 2007;18:2749–2757. doi: 10.1681/ASN.2007020199. [DOI] [PubMed] [Google Scholar]
- 12.Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145:237–246. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 13.Randers E, Erlandsen EJ, Pedersen OL, Hasling C, Danielsen H. Serum cystatin C as an endogenous parameter of the renal function in patients with normal to moderately impaired kidney function. Clin Nephrol. 2000;54:203–209. [PubMed] [Google Scholar]
- 14.Hojs R, Bevc S, Ekart R, Gorenjak M, Puklavec L. Serum cystatin C as an endogenous marker of renal function in patients with mild to moderate impairment of kidney function. Nephrol Dial Transplant. 2006;21:1855–1862. doi: 10.1093/ndt/gfl073. [DOI] [PubMed] [Google Scholar]
- 15.Knight EL, Verhave JC, Spiegelman D, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65:1416–1421. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 16.Herget-Rosenthal S, Bokenkamp A, Hofmann W. How to estimate GFR-serum creatinine, serum cystatin C or equations? Clin Biochem. 2007;40:153–161. doi: 10.1016/j.clinbiochem.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Justesen TI, Petersen JL, Ekbom P, Damm P, Mathiesen ER. Albumin-to-creatinine ratio in random urine samples might replace 24-h urine collections in screening for micro- and macroalbuminuria in pregnant woman with type 1 diabetes. Diabetes Care. 2006;29:924–925. doi: 10.2337/diacare.29.04.06.dc06-1555. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed A, Rich MW, Sanders PW, et al. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol. 2007;99:393–398. doi: 10.1016/j.amjcard.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cowie MR, Komajda M, Murray-Thomas T, Underwood J, Ticho B. Prevalence and impact of worsening renal function in patients hospitalized with decompensated heart failure: results of the prospective outcomes study in heart failure (POSH) Eur Heart J. 2006;27:1216–1222. doi: 10.1093/eurheartj/ehi859. [DOI] [PubMed] [Google Scholar]
- 20.Damman K, Navis G, Voors AA, et al. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J Card Fail. 2007;13:599–608. doi: 10.1016/j.cardfail.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Ezekowitz J, McAlister FA, Humphries KH, et al. The association among renal insufficiency, pharmacotherapy, and outcomes in 6,427 patients with heart failure and coronary artery disease. J Am Coll Cardiol. 2004;44:1587–1592. doi: 10.1016/j.jacc.2004.06.072. [DOI] [PubMed] [Google Scholar]
- 22.Hillege HL, Girbes AR, de Kam PJ, et al. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation. 2000;102:203–210. doi: 10.1161/01.cir.102.2.203. [DOI] [PubMed] [Google Scholar]
- 23.Hillege HL, Nitsch D, Pfeffer MA, et al. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113:671–678. doi: 10.1161/CIRCULATIONAHA.105.580506. [DOI] [PubMed] [Google Scholar]
- 24.Go AS, Yang J, Ackerson LM, et al. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation. 2006;113:2713–2723. doi: 10.1161/CIRCULATIONAHA.105.577577. [DOI] [PubMed] [Google Scholar]
- 25.Campbell RC, Sui X, Filippatos G, et al. Association of chronic kidney disease and outcomes in chronic heart failure: a propensity-matched study. 2008. (Revision re-submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levin A, Thompson CR, Ethier J, et al. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis. 1999;34:125–134. doi: 10.1016/s0272-6386(99)70118-6. [DOI] [PubMed] [Google Scholar]
- 27.Al-Ahmad A, Rand WM, Manjunath G, et al. Reduced kidney function and anemia as risk factors for mortality in patients with left ventricular dysfunction. J Am Coll Cardiol. 2001;38:955–962. doi: 10.1016/s0735-1097(01)01470-x. [DOI] [PubMed] [Google Scholar]
- 28.McClellan WM, Flanders WD, Langston RD, Jurkovitz C, Presley R. Anemia and renal insufficiency are independent risk factors for death among patients with congestive heart failure admitted to community hospitals: a population-based study. J Am Soc Nephrol. 2002;13:1928–1936. doi: 10.1097/01.asn.0000018409.45834.fa. [DOI] [PubMed] [Google Scholar]
- 29.Silverberg DS, Wexler D, Blum M, et al. The use of subcutaneous erythropoietin and intravenous iron for the treatment of the anemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalizations. J Am Coll Cardiol. 2000;35:1737–1744. doi: 10.1016/s0735-1097(00)00613-6. [DOI] [PubMed] [Google Scholar]
- 30.Levin A, Djurdjev O, Thompson C, et al. Canadian randomized trial of hemoglobin maintenance to prevent or delay left ventricular mass growth in patients with CKD. Am J Kidney Dis. 2005;46:799–811. doi: 10.1053/j.ajkd.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 32.Drueke TB, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 33.Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- 34.Russo D, Palmiero G, De Blasio AP, Balletta MM, Andreucci VE. Coronary artery calcification in patients with CRF not undergoing dialysis. Am J Kidney Dis. 2004;44:1024–1030. doi: 10.1053/j.ajkd.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 35.Ketteler M, Schlieper G, Floege J. Calcification and cardiovascular health: new insights into an old phenomenon. Hypertension. 2006;47:1027–1034. doi: 10.1161/01.HYP.0000219635.51844.da. [DOI] [PubMed] [Google Scholar]
- 36.Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116:85–97. doi: 10.1161/CIRCULATIONAHA.106.678342. [DOI] [PubMed] [Google Scholar]
- 37.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). Developed in Collaboration With the American College of Chest Physicians and the International Society for Heart and Lung Transplantation. Endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 38.Masoudi FA, Rathore SS, Wang Y, et al. National patterns of use and effectiveness of angiotensin-converting enzyme inhibitors in older patients with heart failure and left ventricular systolic dysfunction. Circulation. 2004;110:724–731. doi: 10.1161/01.CIR.0000138934.28340.ED. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed A, Allman RM, DeLong JF, Bodner EV, Howard G. Age-related underutilization of angiotensin-converting enzyme inhibitors in older hospitalized heart failure patients. South Med J. 2002;95:703–710. [PubMed] [Google Scholar]
- 40.Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160:685–693. doi: 10.1001/archinte.160.5.685. [DOI] [PubMed] [Google Scholar]
- 41.Maschio G, Alberti D, Janin G, et al. Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group. N Engl J Med. 1996;334:939–945. doi: 10.1056/NEJM199604113341502. [DOI] [PubMed] [Google Scholar]
- 42.Rothwell PM. Treating individuals 2. Subgroup analysis in randomised controlled trials: importance, indications, and interpretation. Lancet. 2005;365:176–186. doi: 10.1016/S0140-6736(05)17709-5. [DOI] [PubMed] [Google Scholar]
- 43.Ahmed A, Kiefe CI, Allman RM, Sims RV, DeLong JF. Survival benefits of angiotensin-converting enzyme inhibitors in older heart failure patients with perceived contraindications. J Am Geriatr Soc. 2002;50:1659–1666. doi: 10.1046/j.1532-5415.2002.50457.x. [DOI] [PubMed] [Google Scholar]
- 44.McAlister FA, Ezekowitz J, Tonelli M, Armstrong PW. Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation. 2004;109:1004–1009. doi: 10.1161/01.CIR.0000116764.53225.A9. [DOI] [PubMed] [Google Scholar]
- 45.Ahmed A, Love TE, Sui X, Rich MW. Effects of angiotensin-converting enzyme inhibitors in systolic heart failure patients with chronic kidney disease: a propensity score analysis. J Card Fail. 2006;12:499–506. doi: 10.1016/j.cardfail.2006.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berger AK, Duval S, Manske C, et al. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in patients with congestive heart failure and chronic kidney disease. Am Heart J. 2007;153:1064–1073. doi: 10.1016/j.ahj.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 47.Solomon SD, Rice MM, KAJ, et al. Renal function and effectiveness of angiotensin-converting enzyme inhibitor therapy in patients with chronic stable coronary disease in the Prevention of Events with ACE inhibition (PEACE) trial. Circulation. 2006;114:26–31. doi: 10.1161/CIRCULATIONAHA.105.592733. [DOI] [PubMed] [Google Scholar]
- 48.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. Jama. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 49.Centers for Disease Control and Prevention. Prevalence of chronic kidney disease and associated risk factors--United States, 1999–2004. MMWR Morb Mortal Wkly Rep. 2007;56:161–165. [PubMed] [Google Scholar]
- 50.McClellan W, Warnock DG, McClure L, et al. Racial differences in the prevalence of chronic kidney disease among participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Cohort Study. J Am Soc Nephrol. 2006;17:1710–1715. doi: 10.1681/ASN.2005111200. [DOI] [PubMed] [Google Scholar]
- 51.Shlipak MG, Fried LF, Cushman M, et al. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 2005;293:1737–1745. doi: 10.1001/jama.293.14.1737. [DOI] [PubMed] [Google Scholar]
- 52.Stevens PE, O’Donoghue DJ, de Lusignan S, et al. Chronic kidney disease management in the United Kingdom: NEOERICA project results. Kidney Int. 2007;72:92–99. doi: 10.1038/sj.ki.5002273. [DOI] [PubMed] [Google Scholar]
- 53.Carter JL, O’Riordan SE, Eaglestone GL, Delaney MP, Lamb EJ. Chronic Kidney Disease Prevalence in a UK Residential Care Home Population. Nephrol Dial Transplant. 2007 doi: 10.1093/ndt/gfm792. [DOI] [PubMed] [Google Scholar]
- 54.Chadban SJ, Briganti EM, Kerr PG, et al. Prevalence of kidney damage in Australian adults: The AusDiab kidney study. J Am Soc Nephrol. 2003;14:S131–138. doi: 10.1097/01.asn.0000070152.11927.4a. [DOI] [PubMed] [Google Scholar]
- 55.Imai E, Horio M, Iseki K, et al. Prevalence of chronic kidney disease (CKD) in the Japanese general population predicted by the MDRD equation modified by a Japanese coefficient. Clin Exp Nephrol. 2007;11:156–163. doi: 10.1007/s10157-007-0463-x. [DOI] [PubMed] [Google Scholar]
- 56.Zhang L, Zuo L, Xu G, et al. Community-based screening for chronic kidney disease among populations older than 40 years in Beijing. Nephrol Dial Transplant. 2007;22:1093–1099. doi: 10.1093/ndt/gfl763. [DOI] [PubMed] [Google Scholar]
- 57.Rao M, Pereira BJ. Chronic kidney disease in India--a hidden epidemic. Indian J Med Res. 2007;126:6–9. [PubMed] [Google Scholar]
- 58.Krumholz HM, Chen J, Chen YT, Wang Y, Radford MJ. Predicting one-year mortality among elderly survivors of hospitalization for an acute myocardial infarction: results from the Cooperative Cardiovascular Project. J Am Coll Cardiol. 2001;38:453–459. doi: 10.1016/s0735-1097(01)01395-x. [DOI] [PubMed] [Google Scholar]
- 59.Glynn LG, Reddan D, Newell J, Hinde J, Buckley B, Murphy AW. Chronic kidney disease and mortality and morbidity among patients with established cardiovascular disease: a West of Ireland community-based cohort study. Nephrol Dial Transplant. 2007;22:2586–2594. doi: 10.1093/ndt/gfm222. [DOI] [PubMed] [Google Scholar]
- 60.Reddan DN, Szczech LA, Tuttle RH, et al. Chronic kidney disease, mortality, and treatment strategies among patients with clinically significant coronary artery disease. J Am Soc Nephrol. 2003;14:2373–2380. doi: 10.1097/01.asn.0000083900.92829.f5. [DOI] [PubMed] [Google Scholar]
- 61.Liu H, Yu J, Chen F, Li J, Hu D. Inpatients with coronary heart disease have a high prevalence of chronic kidney disease based on estimated glomerular filtration rate (eGFR) in China. Heart Vessels. 2007;22:223–228. doi: 10.1007/s00380-006-0964-7. [DOI] [PubMed] [Google Scholar]
- 62.Coca SG, Krumholz HM, Garg AX, Parikh CR. Underrepresentation of renal disease in randomized controlled trials of cardiovascular disease. JAMA. 2006;296:1377–1384. doi: 10.1001/jama.296.11.1377. [DOI] [PubMed] [Google Scholar]
- 63.Charytan D, Kuntz RE. The exclusion of patients with chronic kidney disease from clinical trials in coronary artery disease. Kidney Int. 2006;70:2021–2030. doi: 10.1038/sj.ki.5001934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Masoudi FA, Havranek EP, Wolfe P, et al. Most hospitalized older persons do not meet the enrollment criteria for clinical trials in heart failure. Am Heart J. 2003;146:250–257. doi: 10.1016/S0002-8703(03)00189-3. [DOI] [PubMed] [Google Scholar]
- 65.Pfeffer MA, Swedberg K, Granger CB, et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759–766. doi: 10.1016/s0140-6736(03)14282-1. [DOI] [PubMed] [Google Scholar]
- 66.Fonarow GC, Abraham WT, Albert NM, et al. Influence of a performance-improvement initiative on quality of care for patients hospitalized with heart failure: results of the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF) Arch Intern Med. 2007;167:1493–1502. doi: 10.1001/archinte.167.14.1493. [DOI] [PubMed] [Google Scholar]
- 67.Masoudi FA, Baillie CA, Wang Y, et al. The complexity and cost of drug regimens of older patients hospitalized with heart failure in the United States, 1998–2001. Arch Intern Med. 2005;165:2069–2076. doi: 10.1001/archinte.165.18.2069. [DOI] [PubMed] [Google Scholar]
- 68.Rathore SS, Foody JM, Wang Y, et al. Race, quality of care, and outcomes of elderly patients hospitalized with heart failure. JAMA. 2003;289:2517–2524. doi: 10.1001/jama.289.19.2517. [DOI] [PubMed] [Google Scholar]
- 69.Ahmed A, DeShazo WR, Dell’Italia LJ, Allman RM. Discharge prescription of beta blockers reduces mortality in hospitalized older adults with systolic heart failure: A propensity score analysis. Circulation. 2005;112:II–353. [Google Scholar]
- 70.Khan NA, Ma I, Thompson CR, et al. Kidney function and mortality among patients with left ventricular systolic dysfunction. J Am Soc Nephrol. 2006;17:244–253. doi: 10.1681/ASN.2005030270. [DOI] [PubMed] [Google Scholar]
- 71.The Beta-Blocker Evaluation of Survival Trial Investigators. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med. 2001;344:1659–1667. doi: 10.1056/NEJM200105313442202. [DOI] [PubMed] [Google Scholar]
- 72.Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 73.Gottdiener JS, McClelland RL, Marshall R, et al. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med. 2002;137:631–639. doi: 10.7326/0003-4819-137-8-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 74.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 75.Ahmed A, Howard VJ, Zhang R, et al. Prevalence of heart failure in REGARDS: A unique opportunity to study racial and geographical variations in heart failure epidemiology. To be presented at the 48th Cardiovascular Disease Epidemiology and Prevention Annual Conference; March 11–15, 2008; Colorado: Broadmoor Hotel in Colorado Springs; 2008. [Google Scholar]
- 76.Bibbins-Domingo K, Lin F, Vittinghoff E, Barrett-Connor E, Grady D, Shlipak MG. Renal insufficiency as an independent predictor of mortality among women with heart failure. J Am Coll Cardiol. 2004;44:1593–1600. doi: 10.1016/j.jacc.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 77.Jose P, Skali H, Anavekar N, et al. Increase in creatinine and cardiovascular risk in patients with systolic dysfunction after myocardial infarction. J Am Soc Nephrol. 2006;17:2886–2891. doi: 10.1681/ASN.2006010063. [DOI] [PubMed] [Google Scholar]
- 78.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. Jama. 2004;291:844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 79.Herzog CA, Ma JZ, Collins AJ. Comparative survival of dialysis patients in the United States after coronary angioplasty, coronary artery stenting, and coronary artery bypass surgery and impact of diabetes. Circulation. 2002;106:2207–2211. doi: 10.1161/01.cir.0000035248.71165.eb. [DOI] [PubMed] [Google Scholar]
- 80.Goodman WG, Goldin J, Kuizon BD, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 81.Bongartz LG, Cramer MJ, Doevendans PA, Joles JA, Braam B. The severe cardiorenal syndrome: ‘Guyton revisited’. Eur Heart J. 2005;26:11–17. doi: 10.1093/eurheartj/ehi020. [DOI] [PubMed] [Google Scholar]
- 82.Heywood JT. The cardiorenal syndrome: lessons from the ADHERE database and treatment options. Heart Fail Rev. 2004;9:195–201. doi: 10.1007/s10741-005-6129-4. [DOI] [PubMed] [Google Scholar]
- 83.Schrier RW. Cardiorenal versus renocardiac syndrome: is there a difference? Nat Clin Pract Nephrol. 2007;3:637. doi: 10.1038/ncpneph0673. [DOI] [PubMed] [Google Scholar]
- 84.Shlipak MG, Massie BM. The clinical challenge of cardiorenal syndrome. Circulation. 2004;110:1514–1517. doi: 10.1161/01.CIR.0000143547.55093.17. [DOI] [PubMed] [Google Scholar]
- 85.Fried LF, Shlipak MG, Crump C, et al. Renal insufficiency as a predictor of cardiovascular outcomes and mortality in elderly individuals. J Am Coll Cardiol. 2003;41:1364–1372. doi: 10.1016/s0735-1097(03)00163-3. [DOI] [PubMed] [Google Scholar]