Abstract
OBJECTIVE
To detect the burden of vancomycin-resistant Enterococcus (VRE) colonization among pediatric oncology patients and to determine risk factors for VRE acquisition.
DESIGN
Retrospective case-control study.
SETTING
The Children’s Hospital of Philadelphia.
PATIENTS
Pediatric oncology patients hospitalized from June 2006 through December 2007.
METHODS
Prevalence surveys revealed an increased VRE burden among pediatric oncology patients. For the case-control study, the 16 case patients were pediatric oncology patients who had 1 stool sample negative for VRE at screening before having a stool sample positive for VRE at screening; the 62 control patients had 2 consecutive screenings in which stool samples were negative for VRE. Case and control patients were matched on the duration of the interval between screens. Analyses were performed to determine the association between multiple exposures and VRE acquisition.
RESULTS
The prevalence survey revealed that 5 (9.6%) of 52 patients had unsuspected VRE colonization at the time of hospitalization. Multivariate analysis identified a lack of empirical contact precautions (odds ratio [OR], 17.16 [95% confidence interval {CI}, 1.49–198.21]; P = .02) and the presence of a gastrointestinal device (OR, 4.03 [95% CI, 1.04–15.56]; P = .04) as significant risk factors for acquisition of VRE. Observations in the interventional radiology department revealed that staff could not access the portions of the electronic medical record in which isolation precautions were documented. Simple interventions that granted access and that trained interventional radiology staff to review the need for precautions, coupled with enhanced infection control practices, interrupted ongoing transmission and reduced the proportion of VRE screens that were positive to 15 (1.2%) of 1,270.
CONCLUSIONS
Inadequate communication with regard to infection control precautions can increase the risk of transmission of epidemiologically important organisms, particularly when patients receive care at multiple clinic locations. Adherence to infection control practices across the spectrum of care may limit the spread of resistant organisms.
Enterococcus species are a common cause of bloodstream infection in hospitalized pediatric and adult patients.1 The emergence of vancomycin-resistant Enterococcus (VRE) species in the past 20 years has led to invasive infections that are more difficult to treat. Patients with hematologic malignancies have an increased risk of developing VRE blood-stream infection, likely associated with prolonged episodes of neutropenia and repeated antibiotic exposures.2–5 Additionally, hematologic malignancy has been shown to be an independent risk factor for death among patients with VRE bloodstream infection.6
Previous reports of outbreaks of VRE suggest that an increase in the colonization rate leads to an increase in the rate of clinical infection.7,8 We describe an outbreak of VRE colonization in pediatric oncology patients that was first detected by an increase in the number of cases of VRE bloodstream infection. We sought to detect the burden of vancomycin-resistant Enterococcus (VRE) colonization among pediatric oncology patients and to determine risk factors for VRE acquisition.
METHODS
Patients and Setting
The VRE outbreak occurred at the Children’s Hospital of Philadelphia, a tertiary care pediatric hospital with 430 beds, 42 of which are situated in the pediatric oncology ward. The study population were taken from pediatric oncology patients who were hospitalized at the Children’s Hospital of Philadelphia from June 2006 through December 2007 and who had clinical or surveillance cultures that were positive for VRE during this period. Patients who only visited an outpatient pediatric oncology clinic site and did not require hospitalization were not included in this study because only hospitalized patients had routine stool cultures for VRE performed during the study period.
Infection Control Practices for Patients With VRE
At the Children’s Hospital of Philadelphia, the use of contact precautions is standard for patients who have a culture positive for VRE. These precautions include the following: (1) the patient must remain in his or her room and must not visit common areas on the ward (eg, the playroom or the kitchen), (2) all staff must perform hand hygiene and don a gown and gloves before entering the room, (3) gown and glove procedures must be followed if the patient travels to another part of the hospital (eg, the operating room or the radiology department), and (4) when the patient is discharged, the room must undergo a thorough disinfection, known as a “terminal cleaning.”
Identification and Management of the Outbreak
Outbreak recognition
Active surveillance for hospital-associated bloodstream infection is conducted by the infection prevention and control department for all patients hospitalized at our institution. In June 2006, an increase in the number of cases of bloodstream infection caused by VRE was noted among patients in the pediatric oncology inpatient unit. A VRE colonization prevalence survey was conducted among these patients in July 2006.
Outbreak management
Upon recognition of the unexpectedly high prevalence of VRE colonization, multiple interventions were implemented to decrease the rate of acquisition of VRE. A multidisciplinary team from the infection control and oncology departments was developed, and the team initially met weekly to review VRE screening results, assess interventions, and discuss future plans.
Active surveillance for VRE colonization
Upon recognition of the outbreak, all pediatric oncology patients at admission had a stool sample obtained for VRE screening culture. If the initial screening culture was negative, hospitalized patients were screened weekly thereafter to detect newly acquired VRE; this took place from August 2006 through December 2007. After the initial prevalence survey, weekly data were combined to determine monthly prevalence rates from August 2006 through December 2007.
Microbiological methods
Because of the decreased sensitivity of rectal swab specimens,9 stool specimens were used to screen patients for Enterococcus faecium and Enterococcus faecalis; rectal swab specimens were accepted if no stool specimen could be obtained after 24 hours of hospitalization. Bacterial isolation was performed using selective media of bile esculin agar plates supplemented with vancomycin at a concentration of 10 µg/mL (Hardy Diagnostics). All plates were incubated at 37°C, examined daily, and held for 48 hours before being considered negative. VRE isolates were identified using standard laboratory procedures. Species identification and vancomycin susceptibility were confirmed using the Vitek 2 fluorescent card assay (bioMérieux). Vancomycin-resistant Enterococcus gallinarum and Enterococcus casseliflavus species were excluded from the study. Antibiotic susceptibility results were categorized according to the recommendations of the Clinical and Laboratory Standards Institute.10 Sixteen VRE isolates (3 from blood and 13 from stool samples) were sent to a commercial laboratory for pulsed-field gel electrophoresis (PFGE) to determine the relatedness of the strains.
Case-Control Study
Study design
Because the transmission of VRE continued after the institution of infection control interventions, we conducted a retrospective, matched case-control study to determine potential factors associated with VRE transmission. Case patients were defined as pediatric oncology patients who had at least 1 surveillance culture negative for VRE before their first surveillance or clinical culture positive for VRE. Patients were eligible to be control patients if they had no previous surveillance or clinical cultures positive for VRE and they had 2 consecutive screening cultures negative for VRE. Case patients and control patients were selected from patients hospitalized at any time from July 1 through December 31, 2006. To adjust for time at risk, case patients were matched to control patients on the basis of the interval from their last negative screen to their first positive screen (for case patients) or the interval between 2 sequential negative screens (for control patients); this interval was defined as the exposure window.
Data collection
Data from the exposure window were extracted from the electronic medical record for case patients and control patients and were recorded on a structured data collection form. Information obtained included demographic data, dates of outpatient clinic visits, presence of indwelling medical devices, antibiotic use, results of radiologic studies, and performance of invasive procedures.
Statistical analysis
The primary outcome, VRE acquisition, was a dichotomous variable. Summary statistics were constructed using the frequency and proportion for categorical variables and the median and interquartile range for continuous variables. Univariate analyses were conducted to evaluate possible associations between VRE acquisition and exposures during the exposure window. All variables with a P value less than .2 on univariate analysis and all variables deemed to be clinically important were considered for inclusion in the multivariate model. The presence of confounding was defined by a difference of 15% in the odds ratio (OR) of the primary exposure when adjusting for a given covariate. Conditional multivariate analysis was conducted to identify independent risk factors for VRE colonization among the pediatric oncology population. The level of significance was set at .05. All statistical analyses were conducted using STATA software, version 8.0 (StataCorp).
RESULTS
Outbreak Recognition
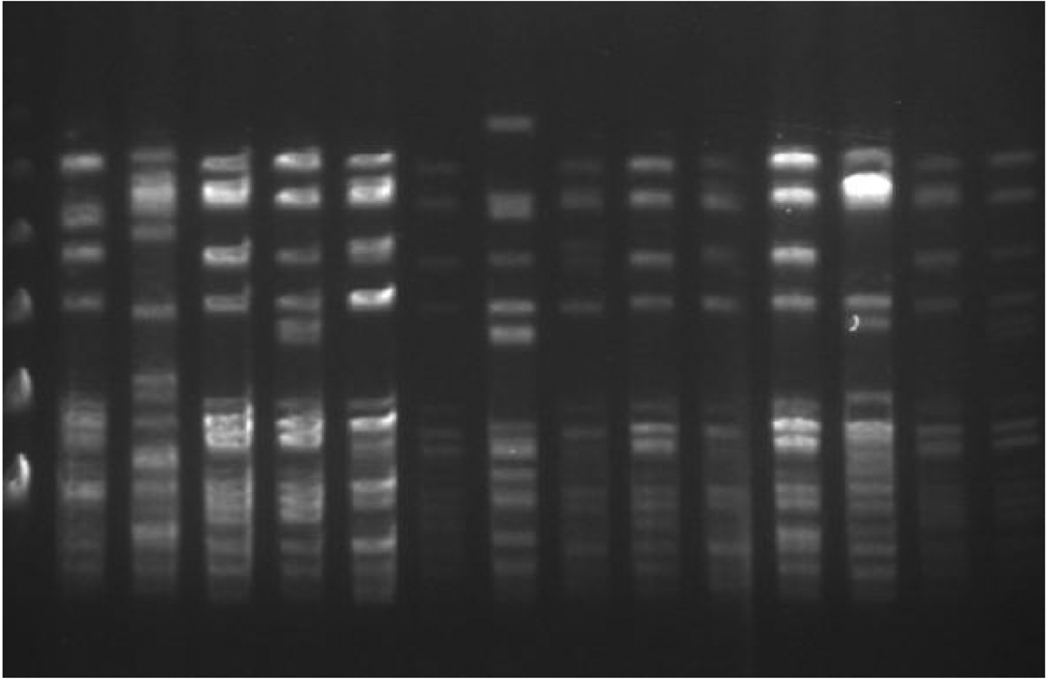
In June 2006, we recognized an increased incidence of bloodstream infection due to VRE among pediatric oncology patients not previously known to be colonized with VRE. The period prevalence survey of all newly hospitalized pediatric oncology patients, conducted in July 2006, revealed that 5 (9.6%) of 52 patients had unsuspected VRE colonization at the time of hospitalization. Patients previously known to be colonized or infected with VRE were not included in this survey. Sixteen isolates underwent analysis with PFGE, which revealed band patterns that indicated the VRE strains involved in the outbreak were not from a single clone (Figure 1). During this time, no other hospital unit or patient group was identified as having an increased prevalence of VRE in clinical cultures or in scheduled point prevalence surveys.
FIGURE 1.
Banding patterns determined by pulsed-field gel electrophoresis of clinical isolates of vancomycin-resistant Enterococcus species indicate multiple clones.
Outbreak Management
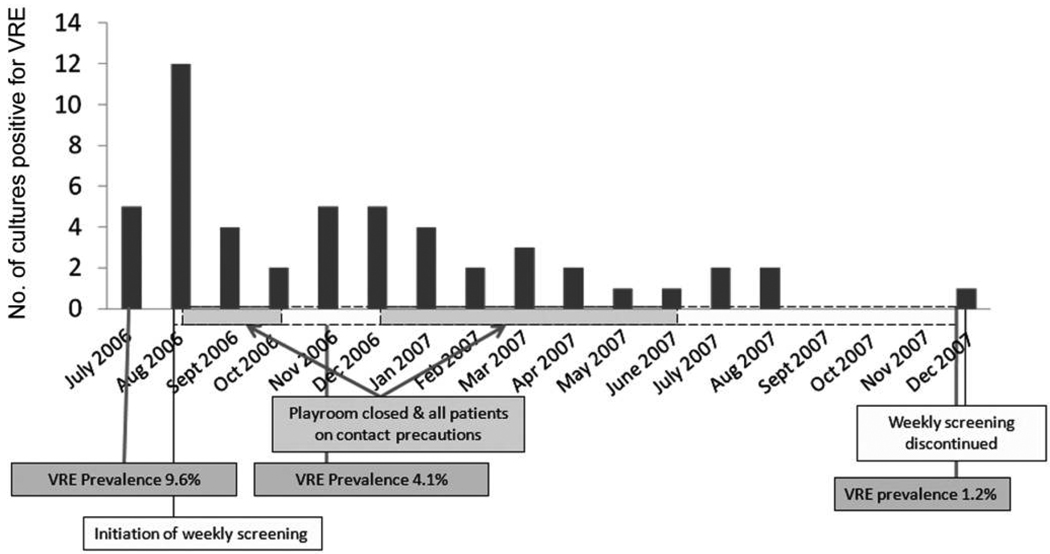
On confirmation of this multiclonal VRE outbreak, empirical contact precautions were applied to all currently hospitalized and all newly admitted pediatric oncology patients pending the results of a stool sample surveillance culture for VRE. Patients with negative culture results for VRE were removed from contact precautions, but repeat VRE surveillance cultures were performed on a weekly basis for the duration of their hospitalization and at readmission. Additional measures to interrupt transmission in inpatient areas included closure of the pediatric oncology playrooms and suspension of between-room visiting by patients and families (Figure 2).
FIGURE 2.
Graph shows number of positive results of screening cultures for vancomycin-resistant Enterococcus (VRE) species compared with timing of infection control interventions.
Multiple enhanced infection control measures were also introduced in the outpatient pediatric oncology clinic. These included (1) exclusion of VRE-positive patients from the waiting area of the outpatient clinic by immediate placement in an examination room, (2) removal of all small, shared play objects (eg, crayons, blocks, and cars), (3) re-education of staff in the environmental services department about disinfection practices, and (4) immediate disinfection of all examination rooms occupied by VRE-positive patients in the pediatric oncology clinic by environmental services department staff before use by the next patient.
For both inpatient and outpatient areas (including satellite pediatric oncology clinics), focused education through in-service meetings and written communication was provided to staff and families about the organism and the importance of hand hygiene, contact precautions, and environmental cleaning in the reduction of the transmission of VRE. The hospital-wide e-mail system was used to update staff throughout the institution of any changes in the infection control recommendations for pediatric oncology patients. Families were also kept abreast of the situation by memoranda written by the infection prevention and control department and the pediatric oncology clinical leadership and distributed by staff.
Despite the use of substantial infection control interventions designed to interrupt the spread of VRE, transmission continued. During the initial 4 months of enhanced infection control measures (July–October 2006), surveillance cultures of stool samples revealed that 26 (4.1%) of 629 patients screened were VRE positive. This proportion represented a significant decrease (P < .001) from that of the previous period prevalence survey, but it suggested continued nosocomial transmission. At this point, a case-control study was undertaken to identify risk factors associated with VRE transmission.
Case-Control Study
Patient characteristics
We identified 16 case patients and 62 matched control patients for the study. For 1 case patient, we were able to identify only 2 control patients because of the case patient’s prolonged exposure window (39 days). There were no statistically significant differences in the characteristics of case patients and control patients (Table 1).
TABLE 1.
Demographic and Clinical Characteristics of Pediatric Oncology Patients (Either Colonized With Vancomycin-Resistant Enterococcus Species [Case Patients] or Not Colonized [Control Patients])
| Characteristic | Case patients (n = 16) |
Control patients (n = 62) |
Odds ratio (95% CI)a | P valueb |
|---|---|---|---|---|
| Age, median (IQR), years | 4.15 (2.14–11.32) | 7.65 (3.12–13.86) | 0.94 (0.85–1.04) | .217 |
| Female sex | 5 (31) | 27 (44) | 0.56 (0.17–1.82) | .338 |
| Ethnicity | 1.98 (0.72–5.40) | .342 | ||
| Hispanic | 2 (12) | 4 (6) | ||
| Non-Hispanic | 10 (63) | 48 (78) | ||
| Unknown | 4 (25) | 10 (16) | ||
| Race | 1.32 (0.81–2.15) | .395 | ||
| Asian | 0 (0) | 2 (3) | ||
| Black | 4 (25) | 12 (19) | ||
| White | 9 (56) | 43 (69) | ||
| Other and/or unknown | 3 (19) | 5 (8) |
NOTE. Data are no. (%) of patients, unless otherwise specified, CI, confidence interval; IQR, interquartile range.
Calculated by univariate analysis.
Adjusted for matched case patients and control patients.
Risk factors for VRE acquisition
The occurrence of multiple exposures was analyzed as a potential risk factor for new acquisition of VRE colonization (Table 2). We hypothesized that a procedure or a visit to another clinical location within the hospital might have been associated with VRE acquisition. Neither visits to an outpatient clinic nor visits to the emergency department were associated with VRE acquisition. We found that a visit to the interventional radiology department for the placement of a gastrointestinal device was associated with VRE acquisition (P = .004).
TABLE 2.
Potential Risk Factors for VRE Acquisition Among Pediatric Oncology Patients
| Characteristic | Case patients (n = 16) |
Control patients (n = 62) |
Odds ratio (95% CI)a | P valueb |
|---|---|---|---|---|
| Outpatient visitc | ||||
| To clinic 1 | 8 (50) | 34 (55) | 0.75 (0.22–2.53) | .644 |
| To clinic 2 | 0 (0) | 3 (5) | … | >.99 |
| To clinic 3 | 3 (19) | 7 (11) | 1.73 (0.40–7.52) | .464 |
| To any emergency department | 5 (31) | 14 (23) | 1.58 (0.42–5.98) | .500 |
| Presence of indwelling medical deviced | ||||
| Any non-CVC device | 11 (69) | 19 (31) | 4.69 (1.38–15.97) | .013 |
| CVC | 15 (94) | 57 (92) | 1.27 (0.14–11.81) | .832 |
| Gastrointestinal device | 9 (56) | 17 (27) | 2.91 (0.97–8.72) | .056 |
| Procedure and/or study | ||||
| Underwent invasive proceduree | 6 (38) | 20 (32) | 1.13 (0.36–3.53) | .829 |
| Underwent radiologic study | 10 (63) | 44 (71) | 0.68 (0.22–2.12) | .508 |
| Underwent other procedure | 7 (44) | 25 (40) | 1.07 (0.36–3.13) | .905 |
| Travel away from oncology department | ||||
| To operating room | 4 (25) | 14 (23) | 1.11 (0.35–3.54) | .863 |
| To interventional radiology department | ||||
| For invasive or other procedure | 4 (25) | 7 (11) | 2.22 (0.61–8.04) | .223 |
| For placement of gastrointestinal devicef | 6 (38) | 1 (2) | 22.98 (2.75–192.17) | .004 |
| Environmental status during study period | ||||
| Playroom openg | 13 (82) | 48 (77) | 1.18 (0.28–5.03) | .820 |
| Lack of empirical contact precautions | 15 (94) | 39 (63) | 10.6 (1.22–91.81) | .032 |
| Receipt of antibiotics | 16 (100) | 52 (84) | … | >.99 |
NOTE. Data are no. (%) of patients, unless otherwise specified. CI, confidence interval; CVC, central venous catheter.
Calculated with univariate analysis.
Adjusted for matched case patients and control patients.
Outpatient visits were recorded at the Children’s Hospital of Philadelphia main hospital oncology clinic (clinic 1), 2 satellite clinics (clinics 2 and 3), and any emergency department (the Children’s Hospital of Philadelphia or other).
Indwelling medical devices included nasogastric tubes, endotracheal tubes, gastrostomy tubes, Foley catheters, tracheostomy cannulas, continuous positive airway pressure and/or bilevel positive airway pressure machines, and central lines.
Invasive procedures included surgery, endoscopy, colonoscopy, bronchoscopy, skin biopsy, and any procedure performed in the interventional radiology department. Other procedures included lumbar puncture, bone marrow aspiration, intrathecal chemotherapy, audiology examination, and echocardiography.
Before or during the study window.
For approximately 12 weeks during the outbreak investigation from luly to December 2006, the playroom on the oncology floor was dosed. During the first open period, all children with no history of VRE were allowed in the playroom; during subsequent periods, only children with a negative VRE screen from the current admission were permitted in the playroom.
Nearly all patients in the study received antimicrobial agents during the exposure window: 16 (100%) of 16 case patients and 52 (84%) of 62 control patients (P > .99); however, if antibiotic use was grouped by spectrum of activity, disparities in use by group were seen (Table 3). Case patients were more likely than control patients to receive fluoroquinolones (31% vs 8%; P = .023). Case patients also tended to receive vancomycin and third-generation cephalosporins more frequently, although this difference was not statistically significant.
TABLE 3.
Select Antibiotic Utilization By Pediatric Oncology Patients Colonized With Vancomycin-Resistant Enterococcus (Case Patients) and Not Colonized (Control Patients)
| Antibiotic group | Case patients (n = 16) |
Control patients (n = 62) |
Odds ratio (95% CI)a | P valueb |
|---|---|---|---|---|
| Fluoroquinolonesc | 5 (31) | 5 (8) | 4.65 (1.23–17.56) | .023 |
| Glycopep tidesd | 7 (44) | 15 (24) | 2.34 (0.76–7.21) | .139 |
| 3G cephalosporinse | 10 (63) | 27 (44) | 2.51 (0.73–8.57) | .143 |
| Aminoglycosidesf | 9 (56) | 28 (45) | 1.54 (0.47–5.08) | .475 |
| Antianaerobesg | 3 (19) | 13 (21) | 0.85 (0.22–3.31) | .815 |
| Carbapenemsh | 0 (0) | 3 (5) | … | >.99 |
NOTE. Data are no. (%) of patients, unless otherwise specified CI, confidence interval; 3G, third-generation.
Calculated by univariate analysis.
Adjusted for matched case patients and control patients.
Includes ciprofloxacin and levofloxacin.
Includes vancomycin.
Includes ceftazidime and ceftriaxone.
Includes amikacin, gentamicin, and tobramycin.
Includes amoxicillin/davulanate, ampicillin/sulbactam, clindamycin, metronidazole, piperacillin/tazobactam, and ticarcillin/clavulanate.
Includes imipenem and meropenem.
We examined environmental factors possibly associated with VRE acquisition and found that new VRE colonization was more likely during a period when empirical contact precautions were not used (P = .032); 15 (94%) of the 16 case patients who became colonized with VRE were hospitalized during a period when empirical contact precautions were not in place. In contrast, only 39 (63%) of 62 control patients were hospitalized during a period when empirical contact precautions were not in place (P = .032).
Factors independently associated with VRE colonization at multivariate analysis included presence of a gastrointestinal device (OR, 4.03 [95% confidence interval {CI}, 1.04–15.56]) and lack of use of empirical contact precautions (OR, 17.16 [95% CI, 1.49–198.21]). Because of concordance between “visit to the interventional radiology department for placement of a gastrointestinal device” and “presence of a gastrointestinal device,” only “presence of a gastrointestinal device” was included in the multivariate model.
Application of risk factor analysis
On completion of this analysis, observations by a certified infection control practitioner were performed in the interventional radiology suite. We identified several factors that might have contributed to VRE transmission in this setting. First, 2 different computer systems were used to register patients in the interventional radiology department, but only 1 of the systems recorded the patients’ isolation status. Second, the interventional radiology staff lacked easy access to the clinical portion of the electronic medical record, which contained information about patients’ isolation status. To identify patients who required isolation precautions, interventional radiology staff asked patients and/or caregivers if precautions were necessary. We observed patients and caregivers who did not appropriately identify the need for isolation precautions.
Once this issue was recognized, the interventional radiology department instituted a policy that assigned a staff member to verify each patient’s isolation status before the patient’s arrival in the department. During the next 8 months, the proportion of VRE screens that were positive decreased to 15 (1.2%) of 1,270 (Figure 2).
DISCUSSION
The successful management and investigation of this VRE outbreak demonstrate the need for a multifaceted intervention strategy to interrupt transmission, systematic investigation to identify locations of possible transmission, and assessment to define breaches in practices. Our case-control study revealed that lack of empirical contact precautions and visits to the interventional radiology department for placement of a gastrointestinal device significantly increased the risk for VRE acquisition. Observations in the interventional radiology department revealed that inaccurate communication, both between families and staff and between computer systems, caused a breakdown in the proper institution of contact precautions. This failure likely contributed to the spread of the organism throughout the pediatric oncology patient population.
The use of contact precautions has frequently been shown to decrease transmission of resistant organisms.11–17 Patients in our study who became colonized with VRE were much more likely than control patients to have been hospitalized during periods of the study when empirical contact precautions were not in use (15 [94%] of 16 case patients vs 39 [63%] of 62 control patients). The initial response to the outbreak was to impose strict contact precautions on all pediatric oncology patients in both the inpatient and outpatient settings. As cumulative prevalence rates decreased, use of these precautions was relaxed so that they were applied only to patients with a stool sample positive for VRE on screening or with a history of VRE carriage. It was during these periods of relaxed use that a higher percentage of patients acquired VRE. These observations support recommendations to conduct active surveillance cultures with empirical contact precautions until culture results become available, as mentioned in the Society for Healthcare Epidemiology of America guidelines.18
Because our case-control study demonstrated that case patients were more likely than control patients to have visited the interventional radiology department for the placement of a gastrointestinal device, this location was identified as a potential source of the ongoing transmission. The failure of a computer system commonly used in the interventional radiology department to report isolation status demonstrates the need for a single, well-integrated electronic medical record system throughout a hospital system. As practitioners become more reliant on electronic medical records, it is imperative that the information contained in them be accurate and consistent to ensure proper patient care and adherence to hospital policy. Also, despite educational efforts by the pediatric oncology department in teaching patients’ families and caretakers about VRE and the importance of controlling the outbreak, families did not consistently volunteer patients’ VRE status and their need for contact precautions. Thus, without a unified electronic medical record or a system with proper checks and balances, breakdowns in communication among practitioners, as well as between families and medical personnel, can cause errors with substantial consequences.
Our study identified other risk factors for VRE transmission. The presence of an indwelling medical device, in particular a gastrostomy tube or nasogastric tube, was associated with VRE acquisition. Previous studies have shown that patients with central venous catheters, urinary catheters, and enteral tubes are all associated with colonization by drug-resistant organisms.19–21 A recent cross-sectional study in several nursing homes also found enteral feeding tubes to be a risk factor for colonization with resistant bacteria; however, methicillin-resistant Staphylococcus aureus was the colonizing pathogen.22 Because VRE is known to contaminate the environment, live for extended periods on surfaces, and colonize the gastrointestinal tract, it is unclear whether these enteric devices were contaminated from an endogenous or an exogenous source. Our patients were more likely to be identified as VRE carriers after the placement of enteral tubes; therefore, we hypothesize that VRE might have been transferred from an external source to the patient at the time of placement or manipulation of these devices. Cultures of neither environmental surfaces nor healthcare worker hands were performed; therefore, we were unable to assess this possible mechanism of transmission.
Antibiotic use has been implicated as an important risk factor in previously reported VRE outbreaks. Several classes of antibiotics have been shown to be associated with VRE acquisition, including glycopeptides (vancomycin), third-generation cephalosporins, antianaerobic medications, fluoroquinolones, aminoglycosides, and carbapenems.20,23–29 In our study, fluoroquinolones were the only antibiotics found to be significantly associated with VRE acquisition, although both vancomycin and third-generation cephalosporins were also more frequently used by case patients than by control patients. The high rates of antibiotic use and the small number of subjects in both groups make conclusions with regard to the role of antibiotic usage in VRE acquisition difficult to determine.
Several factors limited this case-control study. During the study period, we identified only 16 cases of new VRE acquisition, which limited our power to detect differences between the groups. The study was retrospective, and data were gathered via chart review, which could affect the completeness and accuracy of the information collected. Steps were taken to minimize the incompleteness of data by reviewing pharmacy records as well as orders and written documentation. The accuracy of data extraction was enhanced by having 2 reviewers independently examine each chart. To minimize bias associated with different durations of exposure, we matched case patients and control patients on the interval between VRE surveillance cultures and limited our data collection to potential exposures during this period.
As with any case-control study, selection and misclassification biases can be important issues. Selection bias in our study was decreased by identifying case patients and control patients from the same group of hospitalized pediatric oncology patients. By sampling subjects from this limited population, we reduced selection bias at the cost of the generalizability of our findings to other patient populations. Misclassification bias was diminished by identifying case patients and control patients by means of microbiological methods. The likelihood of error in recovering VRE arises primarily from sampling issues. To minimize the possibility of error, the large majority of screens used stool specimens rather than rectal swab specimens.
An interdepartmental response with strict adherence to contact precautions and a concerted effort to improve communication with regard to patients’ isolation status controlled the VRE outbreak at our institution and decreased the colonization prevalence of VRE from 9.6% to 1.2%. Before identification of this VRE outbreak, active surveillance for VRE was not performed. However, point prevalence surveys are now conducted at regularly scheduled intervals to ensure early identification of rising rates of VRE colonization.
ACKNOWLEDGMENTS
We acknowledge the efforts of the nurses, physicians, and staff who care for the oncology patients and the cooperation of the oncology patients and their families in controlling the VRE outbreak.
Footnotes
Potential conflicts of interest. All authors report no conflicts of interest relevant to this article.
REFERENCES
- 1.Raad I, Hachem R, Hanna H, et al. Sources and outcome of bloodstream infections in cancer patients: the role of central venous catheters. Eur J Clin Microbiol Infect Dis. 2007;26:549–556. doi: 10.1007/s10096-007-0320-6. [DOI] [PubMed] [Google Scholar]
- 2.Shay DK, Maloney SA, Montecalvo M, et al. Epidemiology and mortality risk of vancomycin-resistant enterococcal bloodstream infections. J Infect Dis. 1995;172:993–1000. doi: 10.1093/infdis/172.4.993. [DOI] [PubMed] [Google Scholar]
- 3.Ostrowsky BE, Trick WE, Sohn AH, et al. Control of vancomycin-resistant Enterococcus in healthcare facilities in a region. N Engl J Med. 2001;344:1427–1433. doi: 10.1056/NEJM200105103441903. [DOI] [PubMed] [Google Scholar]
- 4.Husni R, Hachem R, Hanna H, Raad I. Risk factors for vancomycin-resistant Enterococcus (VRE) infection in colonized patients with cancer. Infect Control Hosp Epidemiol. 2002;23:102–103. doi: 10.1086/502016. [DOI] [PubMed] [Google Scholar]
- 5.Henning KJ, Delencastre H, Eagan J, et al. Vancomycin-resistant Enterococcus faecium on a pediatric oncology ward: duration of stool shedding and incidence of clinical infection. Pediatr Infect Dis J. 1996;15:848–854. doi: 10.1097/00006454-199610000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Shay DK, Maloney SA, Montecalvo M, et al. Epidemiology and mortality risk of vancomycin-resistant enterococcal bloodstream infections. J Infect Dis. 1995;172:993–1000. doi: 10.1093/infdis/172.4.993. [DOI] [PubMed] [Google Scholar]
- 7.Bonten MJ, Slaughter S, Ambergen AW, et al. The role of “colonization pressure” in the spread of vancomycin-resistant enterococci: an important infection control variable. Arch Intern Med. 1998;158:1127–1132. doi: 10.1001/archinte.158.10.1127. [DOI] [PubMed] [Google Scholar]
- 8.Ofner-Agostini ME, Conly J, Paton S, et al. Vancomycin-resistant enterococci (VRE) in Canada—results of the Canadian Nosocomial Infection Surveillance Program 1996: VRE point prevalence surveillance project. Can J Infect Dis. 1997;8:73–78. doi: 10.1155/1997/297038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Agata EM, Gautam S, Green WK, Tang YW. High rate of false-negative results of the rectal swab culture method in detection of gastrointestinal colonization with vancomycin-resistant enterococci. Clin Infect Dis. 2002;34:167–172. doi: 10.1086/338234. [DOI] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute . Performance standards for antimicrobial susceptibility testing: 15th informational supplement. CLSI document M100-S15. Wayne, PA: Clinical and Laboratory Standards Institute; 2005. [Google Scholar]
- 11.Sakka V, Tsiodras S, Galani L, et al. Risk factors and predictors of mortality in patients colonised with vancomycin-resistant enterococci. Clin Microbiol Infect. 2008;14:14–21. doi: 10.1111/j.1469-0691.2007.01840.x. [DOI] [PubMed] [Google Scholar]
- 12.Mody L, Maheshwari S, Galecki A, Kauffman CA, Bradley SF. Indwelling device use and antibiotic resistance in nursing homes: identifying a high-risk group. J Am Geriatr Soc. 2007;55:1921–1926. doi: 10.1111/j.1532-5415.2007.01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanna H, Umphrey J, Tarrand J, Mendoza M, Raad I. Management of an outbreak of vancomycin-resistant enterococci in the medical intensive care unit of a cancer center. Infect Control Hosp Epidemiol. 2001;22:217–219. doi: 10.1086/501892. [DOI] [PubMed] [Google Scholar]
- 14.Timmers GJ, van der Zwet WC, Simoons-Smit IM, et al. Outbreak of vancomycin-resistant Enterococcus faecium in a haematology unit: risk factor assessment and successful control of the epidemic. Br J Haematol. 2002;116:826–833. doi: 10.1046/j.0007-1048.2002.03339.x. [DOI] [PubMed] [Google Scholar]
- 15.Christiansen KJ, Tibbett PA, Beresford W, et al. Eradication of a large outbreak of a single strain of vanB vancomycin-resistant Enterococcus faecium at a major Australian teaching hospital. Infect Control Hosp Epidemiol. 2004;25:384–390. doi: 10.1086/502410. [DOI] [PubMed] [Google Scholar]
- 16.Peta M, Carretto E, Barbarini D, et al. Outbreak of vancomycin-resistant Enterococcus spp. in an Italian general intensive care unit. Clin Microbiol Infect. 2006;12:163–169. doi: 10.1111/j.1469-0691.2005.01331.x. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt-Hieber M, Blau IW, Schwartz S, et al. Intensified strategies to control vancomycin-resistant enterococci in immunocompromised patients. Int J Hematol. 2007;86:158–162. doi: 10.1532/IJH97.E0632. [DOI] [PubMed] [Google Scholar]
- 18.Muto CA, Jernigan JA, Ostrowsky BE, et al. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and Enterococcus. Infect Control Hosp Epidemiol. 2003;24:362–386. doi: 10.1086/502213. [DOI] [PubMed] [Google Scholar]
- 19.NNIS System National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470–485. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 20.Hwang YS, Brinton BG, Leonard RB, Blue SR, Woods ML, Carroll KC. Investigation of an outbreak of vancomycin-resistant Enterococcus faecium in a low prevalence university hospital. J Investig Med. 1998;46:435–443. [PubMed] [Google Scholar]
- 21.Martínez JA, Ruthazer R, Hansjosten K, Barefoot L, Snydman DR. Role of environmental contamination as a risk factor for acquisition of vancomycin-resistant enterococci in patients treated in a medical intensive care unit. Arch Intern Med. 2003;163:1905–1912. doi: 10.1001/archinte.163.16.1905. [DOI] [PubMed] [Google Scholar]
- 22.Mascini EM, Troelstra A, Beitsma M, et al. Genotyping and preemptive isolation to control an outbreak of vancomycin-resistant Enterococcus faecium. Clin Infect Dis. 2006;42:739–746. doi: 10.1086/500322. [DOI] [PubMed] [Google Scholar]
- 23.Furtado GH, Mendes RE, Pignatari AC, Wey SB, Medeiros EA. Risk factors for vancomycin-resistant Enterococcus faecalis bacteremia in hospitalized patients: an analysis of two case-control studies. Am J Infect Control. 2006;34:447–451. doi: 10.1016/j.ajic.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Byers KE, Anglim AM, Anneski CJ, et al. A hospital epidemic of vancomycin-resistant Enterococcus: risk factors and control. Infect Control Hosp Epidemiol. 2001;22:140–147. doi: 10.1086/501880. [DOI] [PubMed] [Google Scholar]
- 25.Chavers LS, Moser SA, Funkhouser E, et al. Association between antecedent intravenous antimicrobial exposure and isolation of vancomycin-resistant enterococci. Microb Drug Resist. 2003;9(Suppl 1):S69–S77. doi: 10.1089/107662903322541928. [DOI] [PubMed] [Google Scholar]
- 26.Bhavnani SM, Drake JA, Forrest A, et al. A nationwide, multicenter, case-control study comparing risk factors, treatment, and outcome for vancomycin-resistant and -susceptible enterococcal bacteremia. Diagn Microbiol Infect Dis. 2000;36:145–158. doi: 10.1016/s0732-8893(99)00136-4. [DOI] [PubMed] [Google Scholar]
- 27.Lautenbach E, LaRosa LA, Marr AM, Nachamkin I, Bilker WB, Fishman NO. Changes in the prevalence of vancomycin-resistant enterococci in response to antimicrobial formulary interventions: impact of progressive restrictions on use of vancomycin and third-generation cephalosporins. Clin Infect Dis. 2003;36:440–446. doi: 10.1086/346153. [DOI] [PubMed] [Google Scholar]
- 28.Carmeli Y, Eliopoulos GM, Samore MH. Antecedent treatment with different antibiotic agents as a risk factor for vancomycin-resistant Enterococcus. Emerg Infect Dis. 2002;8:802–807. doi: 10.3201/eid0808.010418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fridkin SK, Edwards JR, Courval JM, et al. Intensive Care Antimicrobial Resistance Epidemiology (ICARE) Project and the National Nosocomial Infections Surveillance (NNIS) System Hospitals The effect of vancomycin and third-generation cephalosporins on prevalence of vancomycin-resistant enterococci in 126 US adult intensive care units. Ann Intern Med. 2001;135:175–183. doi: 10.7326/0003-4819-135-3-200108070-00009. [DOI] [PubMed] [Google Scholar]