Abstract
Partnered tango dance can improve balance and gait in individuals with Parkinson disease (PD). Partnered dance may allow individuals with PD to challenge balance more than non-partnered dance. Alternatively, partnered practice could reduce balance gains because the patient may rely on the partner as a balance aid when challenged. We compared effects of partnered to non-partnered dance on balance and mobility in 39 people (11 females) with mild-moderate PD (Hoehn & Yahr stages I-III). Participants were randomly assigned to partnered or non-partnered tango and attended 1-hour classes twice per week, completing 20 lessons within 10 weeks. Balance and gait were evaluated in the weeks immediately before, immediately after, and one month after the intervention. Both groups significantly improved on the Berg Balance scale, comfortable and fast as possible walking velocity and cadence. Improvements were maintained at the one-month follow-up. The non-partnered class improved as much as the partnered class; however, Partner participants expressed more enjoyment and interest in continuing.
Keywords: Parkinson disease, exercise, dance, retention, gait, balance
Introduction
Parkinson disease (PD), a progressive neurodegenerative movement disorder affecting more than 1 million Americans, can cause postural instability and impaired functional mobility, often leading to falls and decreased quality of life. Seventy percent of individuals with PD fell within a year, and half fell again the following year 1. Persons with PD have a 3.2 fold greater hip fracture risk than those without 2. Balance and gait impairments are not fully addressed by pharmacological agents, thus non-pharmacological approaches are necessary 3.
Rehabilitative programs for postural instability are most effective if they incorporate dynamic balance practice and continual adjustment to environmental demands 4, 5. Traditional exercise programs can meet these requirements, but often suffer from high attrition 6, 7, 8. Considered enjoyable, motivating and engaging 9, 10, 11, dance may be an excellent way to address motor impairments. Dance requires dynamic balance and continual adjustment to the environment while promoting enjoyment and fostering interest in continuing participation. Habitual social dancing over several years is associated with superior balance, gait function and leg reaction times compared to age-matched non-dancers 12, 13. Older adults who danced demonstrated improved balance and functional mobility and were more motivated to pursue healthy, exercise-related behaviors 14, 15. Greater balance and complex gait improvements in elders that participated in tango lessons were noted when compared to a walking group 16.
For individuals with PD, balance and functional mobility improvements after an Argentine tango program exceeded those of traditional exercise after a long duration, moderate dosage program 17, 18. Improvements were also demonstrated after a short duration, high dosage tango program 19. Composed of improvisational, small step elements, tango patterns may enhance motor abilities by targeting PD-related impairments, and possibly better than partner dances like Waltz and Foxtrot 20. Tango involves frequent movement initiation and cessation, a range of speeds, rhythmic variation and spontaneous multi-directional perturbations, features that might target difficulties with balance, movement initiation, and bradykinesia.
Although supporting evidence is building for partnered dance for those with PD, the partner's importance and influence remains equivocal. Ostensibly, the partner's physical contact may aid balance for those with PD, as even light touch facilitates postural stability 21. Because of this balancing aid, persons with PD might feel able to challenge their limits of stability more with a partner than without one. Alternatively, habitual partnered practice could reduce balance gains because when challenged, persons with PD might develop a dependence on the partner as a balance aid. Partnered dance may facilitate balance and permit an individual to learn motor skills more quickly, but gains might not be sustained when walking or doing un-partnered activities. On the other hand, dancing solo might be more difficult at first but could ultimately facilitate independent walking and un-partnered activities. Additionally, some individuals may lack a partner and/or prefer non-partnered dance. Non-partnered dance, such as line, folk and aerobic dancing, are popular among elderly persons. This study aimed to determine if individuals with PD would benefit more with respect to functional mobility if they participated in partnered or non-partnered tango lessons. We hypothesized that both groups would improve but that beneficial effects of partnered dance would exceed those of non-partnered dance.
Methods
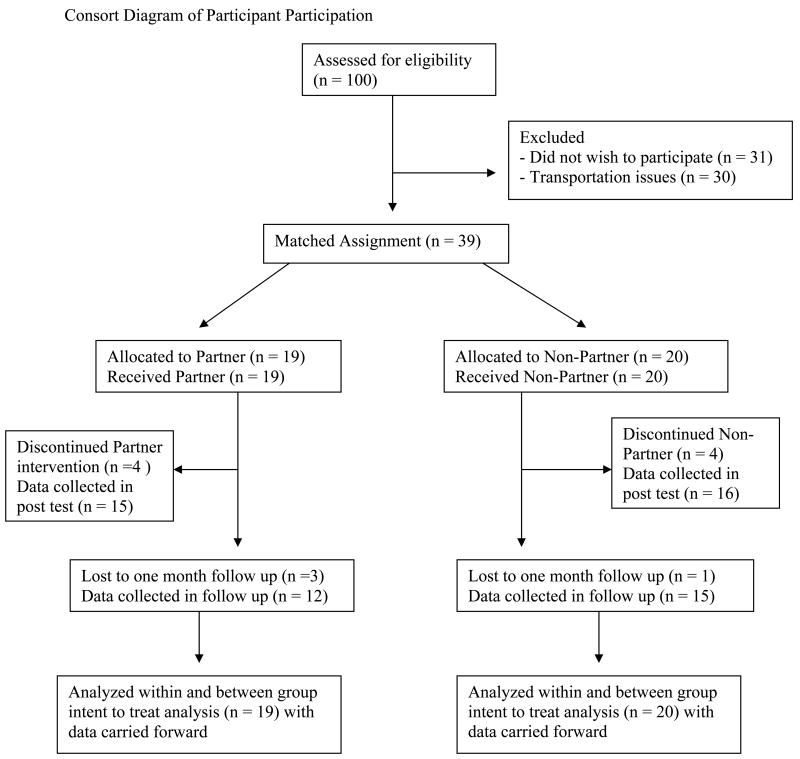
The Human Research Protection Office at Washington University in St. Louis approved this work. Participants provided written informed consent before participating. Figure 1 is a consort diagram describing participation throughout the trial.
Figure 1.
Consort diagram of participant participation.
Participants
Participants were recruited from the St. Louis community through advertisement at community support groups and events. While some participants self-identified, most were directly recruited via telephone from the Washington University Movement Disorders Center database.
Thirty-nine participants with PD, without history of other neurological deficit, were recruited. Participants were at least 40 years of age, could stand at least 30 minutes, and walk independently for three or more meters with or without an assistive device. All participants had a diagnosis of idiopathic PD (Hoehn and Yahr (H&Y) stages of I-III) using diagnostic criteria for clinically defined “definite PD” 22 and demonstrated clear benefit from levodopa. They were tested “ON” medications at a standardized time of day to reduce medication-related fluctuations in performance.
Intervention
The first author assigned individuals to the partnered (Partner) or non-partnered dance class (Non-partner) by randomly selecting a condition from a hat. Evaluations were videotaped for a rater, a specially trained physical therapy student, otherwise not involved in the study. Participants were told they were participating to further information about dance exercise effects in those with PD but all participants were blinded to study hypotheses. Participants were instructed not to change habitual exercise routines over the course of the study. The same instructor, both an experienced professional ballroom dance instructor and an American Council on Exercise-certified personal trainer, taught both progressive partnered and non-partnered tango lessons.
Both Partner and Non-Partner classes began with identical standing warm-ups to upbeat Latin music. After warm-up, both classes listened to and danced to identical commercial tango music selections, in the same order of presentation. In the Partner class, both sexes spent equal time leading and following dance steps, performed in a “closed practice” position, an adaptation of the traditional ballroom frame in which participants hold hands facing one another with bent elbows, maintaining forearms parallel to the floor. Non-partner learned the same Argentine “leading” and “following” tango-based steps as Partner, but performed them without a partner. The instructor advised participants to take breaks as needed.
In the partnered dance class, participants with PD always danced with individuals without PD. These individuals included caregivers and loved ones who elected to participate in classes as well as young adult volunteers. These volunteers were recruited from physical therapy, pre-physical therapy and pre-medical programs at Washington University. Caregivers, loved ones, and volunteers participated in the Non-partner class as well.
Testing Protocol
Videotaped assessments of participants were conducted the week before initiation of training (pre-testing), within the week following completion of 20 sessions (post-testing) and again four weeks after post-testing (follow-up). Participants self-determined an optimal performance time for pre-testing and were tested at the same time of day for post-testing and follow-up. Data files were coded for blinded ratings. Participants were evaluated with the Unified Parkinson's Disease Rating Scale Motor Subscale 3 (UPDRS) 23 at pre-testing. At all measurements, using a standardized script with specific instructions for each task, participants were assessed on the Berg Balance Scale (BBS) 24, tandem stance (TS), one leg stance (OLS) 25 the Timed Up and Go test (TUG) 26, and the six minute walk test (6MWT) 27. Comfortable and fast-as-possible gait were assessed along a 5m instrumented, computerized GAITRite walkway (CIR Systems, Inc., Havertown, PA). Variables of interest were gait velocity, cadence, stride length, swing percent and double support percent. We averaged the results from three trials of each condition. Post-intervention testing included an exit questionnaire completed by participants to assess program experience, asking them to rank items on a scale of 1-5 (1 = strongly agree, 2 = somewhat agree, 3 = neither agree nor disagree, 4 = somewhat disagree, 5 = strongly disagree.) Item 1 asked if participants enjoyed participating. Items 2 through 7 asked if participants noted improved aspects of physical well-being. Item 8 asked if participants would continue with classes if possible.
Statistical Analyses
The Berg Balance Scale, the primary variable of interest, was used for power calculations. A previous study noted an effect size of 0.90 on the BBS with partnered tango20. We powered our study to be able to detect differences between groups of just half that magnitude, i.e. an effect size of 0.45. With this effect size, 20 individuals per group would provide 81% power to test for differences between the Partner and Non-partner groups.
An intent-to-treat analysis was performed and the last observation was carried forward for participants who dropped out of the program before post-testing and/or follow-up testing. Data were analyzed using Sigmastat software (Systat, Richmond, VA). Participant baseline demographics and the exit questionnaire responses were compared for differences with one-way ANOVAs or Kruskall-Wallis one-way ANOVAs on ranks for non-parametric data. Two-way repeated measures ANOVAs (group [Partner, Non-partner] × time [pre, post, follow-up]), with Holms-Sidak post-hoc tests, determined statistical significance of changes from pre to post to follow-up. Level of significance was set at p = 0.05.
Results
Nineteen individuals began Partner classes: one withdrew because of a progressive decline in mental status, two withdrew because of excessive traveling distance, and another felt the classes were too fatiguing. Twenty participants began Non-partner classes: one withdrew after two classes expressing lack of interest, one began a job that interfered with the class schedule, one withdrew after week 5 for unrelated medical problems, and another completed 17 lessons, but work commitments prevented him from attending the remaining required lessons. Four weeks after post-testing, three participants from Partner and one from Non-partner were unable to return for follow-up measures. Data points from all participants who did not complete post or follow-up testing were carried forward from the most recent testing point in an intent-to-treat analysis. Data from only 12 Partner and 15 Non-partner participants were collected in follow-up measures. Eighty percent of participants in Non-partner and 79% of the partnered participants completed post-testing and 75% and 63%, respectively completed the follow-up assessment 1 month after completing the intervention.
At baseline, the groups did not differ significantly in age, UPDRS, H&Y, disease duration or gender distribution (Table 1). In the Partner group, there were 9 individuals in Stage 2, 2 in Stage 2.5, 7 in Stage 3, and 1 in Stage 4 of the H&Y scale. In the Non-partner group, there was 1 individual in Stage 1, 10 in Stage 2, 4 in Stage 2.5, and 5 participants in Stage 3 of the H&Y scale.
Table 1.
Baseline Participant Demographics
| Variable | Partner (n = 19) |
Non-partner (n = 20) |
P values |
|---|---|---|---|
| Age (years) | 69.6 ± 8.5 | 69.6 ± 9.5 | 0.991 |
| Sex (male/female) | 13/6 | 15/5 | 0.652* |
| Time with PD (years) | 9.5 ± 5.3 | 7.9 ± 4.7 | 0.272* |
| UPDRS Motor Subscale III | 31.1 ± 7.8 | 29.2 ± 9.2 | 0.494 |
| Hoehn &Yahr Stage | 2.5 (2, 3) | 2 (2, 2.6) | 0.144 |
Values are means ± SDs except Hoehn & Yahr values which are medians (interquartiles). One-way ANOVAs or Kruskall-Wallis One-way ANOVAs on ranks (*) tested for differences between groups.
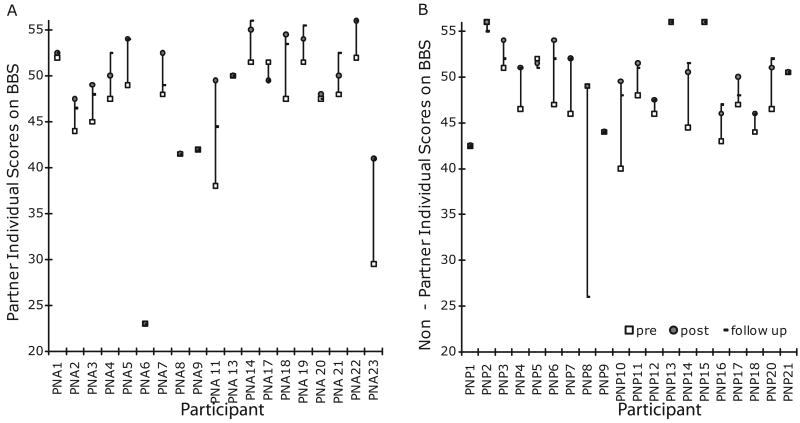
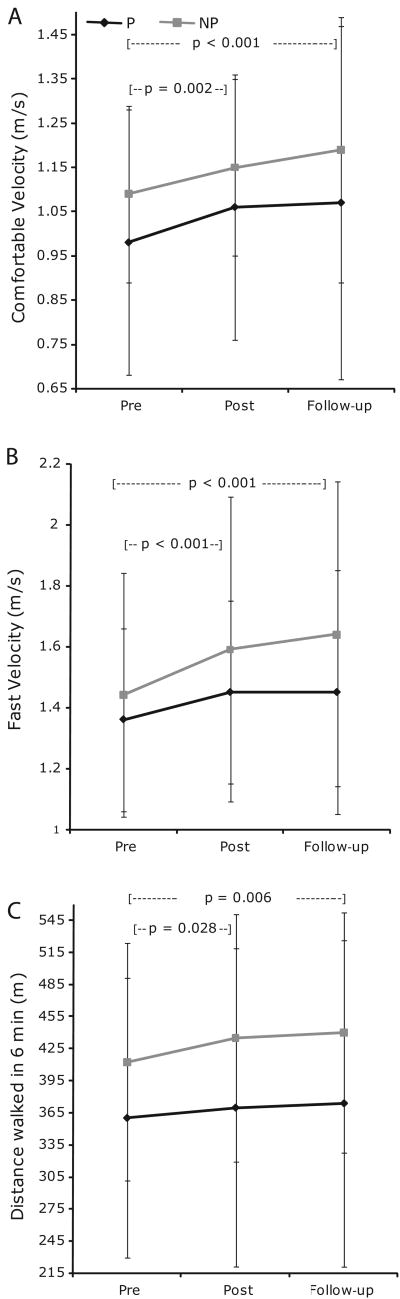
At post-testing, the majority of individuals in both groups demonstrated improved BBS scores (Figures 2AB). Twelve of the 15 Partner and 14 of the 16 Non-partner participants who completed post-testing demonstrated improved BBS scores. When considering group means, both groups had improved significantly on the BBS (Table 2), comfortable walking velocity (Figure 3A) and fast-as-possible walking velocity (Figure 3B) at post-testing. Both groups also improved significantly on one leg stance time, tandem stance time, cadence and double support percent (Table 2) at post-testing. With the exception of one leg stance time, all improvements that were significant at post-testing were maintained at follow-up. Two measures, the 6MWT (p = 0.028, critical level p = 0.025, Figure 3C) and fast-as possible walking swing percent (p = 0.041, critical level p = 0.025, Table 2) were close to significance at post-testing, and attained significance at follow-up. Comfortable and fast-as-possible stride length demonstrated nearly significant main effects of time (p = 0.051). Those in Non-partner had longer stride lengths than those in Partner. There were no group-by-time interactions. No participants took part in dance classes between post-testing and follow-up measures.
Figure 2.
BBS scores for each participant in the Partner (A) and Non-partner (B) groups at pre-test, post-test and follow-up.
Table 2.
Balance, Mobility, & Gait Measures
| Partner n = 19 |
Non-partner n = 20 |
||
|---|---|---|---|
| Berg Balance Scale (out of 56) | Pre: | 45.2 ± 7.8 | 47.8 ± 4.6 |
| Post: | 48.4 ± 7.6* | 50.4 ± 3.8* | |
| Follow-up: | 48.2 ± 7.8* | 49.0 ± 6.5* | |
| Tandem stance (s) | 15.7 ± 21 | 15.8 ± 19 | |
| 25.0 ± 26* | 24.1 ± 23* | ||
| 26.4 ± 25* | 25.0 ± 22* | ||
| OLS (s) | 7.1 ± 7 | 9.7 ± 15 | |
| 17.6 ± 25* | 11.5 ± 13* | ||
| 11.3 ± 15 | 10.6 ± 13 | ||
| TUG (s) | 13.6 ± 9 | 9.7 ± 3 | |
| 13.2 ± 9 | 9.8 ± 3 | ||
| 13.4 ± 11 | 9.6 ± 4 | ||
| Comfortable cadence (steps/min) | 109 ± 12 | 106 ± 14 | |
| 114 ± 13* | 110 ± 10* | ||
| 115 ± 13* | 110 ± 13* | ||
| Comfortable stride length (m) ˆ‡ | 1.08± 0.3 | 1.24 ± 0.2 | |
| 1.09 ± 0.2 | 1.26 ± 0.2 | ||
| 1.12 ± 0.3 | 1.30 ± 0.2 | ||
| Comfortable swing percent | 34.0 ± 3.6 | 35.1 ± 2.0 | |
| 34.3 ± 3.8 | 35.4 ± 2.0 | ||
| 34.3 ± 3.9 | 35.4 ± 2.0 | ||
| Comfortable double support percent | 32.0 ± 7.7 | 30.0 ± 3.8 | |
| 31.4 ± 7.8 | 29.3 ± 3.8 | ||
| 31.5 ± 8.0 | 29.2 ± 3.7 | ||
| Fast cadence (steps/min) | 127 ± 16 | 121 ± 18 | |
| 133 ± 23* | 130 ± 19* | ||
| 133 ± 21* | 131 ± 19* | ||
| Fast stride length (m) ‡ | 1.28 ± 0.3 | 1.42 ± 0.2 | |
| 1.29 ± 0.3 | 1.48 ± 0.3 | ||
| 1.29 ± 0.3 | 1.51 ± 0.3 | ||
| Fast swing percent | 36.1 ± 0.8 | 36.9 ± 0.6 | |
| 36.6 ± 0.9* | 37.6 ± 0.6* | ||
| 36.4 ± 1.1* | 38.3 ± 0.6* | ||
| Fast double support percent | 26.5 ± 8.6 | 26.3 ± 35.2 | |
| 26.5 ± 7.9* | 24.7 ± 5.6* | ||
| 26.4 ± 8.4* | 23.8 ± 6.1* | ||
Values are pre-test, post-test, and one month follow-up means +/- SDs. Unless otherwise noted, all main effects were of time. P values presented are for significant pair-wise time comparisons. Two-way repeated measures ANOVAs with Holms-Sidak post-hoc tests determined statistical significance between groups (p ≤ 0.05).
significantly different from pre-test,
main effect of group,
main effect of time
Figure 3.
Six minute walk (A), comfortable walking velocity (B) and fast-as-possible walking velocity (C) for the Partner (black) and Non-partner (gray) groups at pre-test, post-test, and follow-up measures. Values plotted are means ± SDs. Both groups demonstrated improvements at post-test that were retained at follow-up.
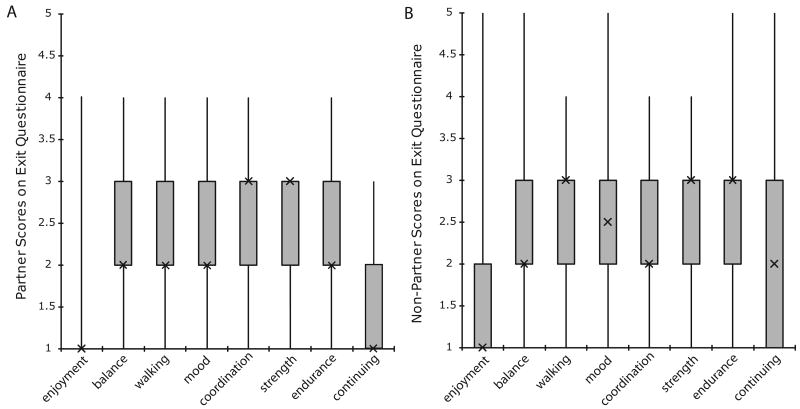
Both groups reported enjoying classes and noted improvements in physical well-being as evidenced by their answers to the exit questionnaire (Figures 4A, 4B). Partner participants expressed stronger agreement with the statements, “I enjoyed participating” and “I would continue participating if possible”. However, there were no significant differences between groups on any exit questionnaire item.
Figure 4.
Exit Questionnaire values for Partner (n = 15) (A) and Non-Partner (n = 16) (B). Values plotted are medians, interquartiles, maxima and minima. These values include measures from only the participants who completed post-testing.
Discussion
After ten weeks of one-hour partnered or non-partnered dance lessons twice per week, two cohorts of people with PD improved in measures of gait, balance and functional mobility. Participants reported enjoying classes, and 80% of those originally recruited completed the intervention. This is the first study to demonstrate maintenance of gait and balance gains in persons with PD beyond the week immediately after completing a rehabilitative dance program. Evidence of maintained improvements one month after completing dance interventions is encouraging and important.
We hypothesized that Partner would experience more gains than Non-partner would, but treatments appeared similarly effective. The lack of difference between groups cannot be attributed to a lack of statistical power, as the sample size was sufficient to detect meaningful differences between groups. Thus, a partner may not be essential to rehabilitative dance interventions, but those with more severe PD who habitually use walking aids, and/or experience freezing may need a partner. As partners maintain physical connection, potentially aiding balance, more severely affected participants might be able to challenge their movement boundaries safely with the assistance of a partner 28. Dancing with a partner does not appear to reduce balance gains or to create dependence on the partner as a balance aid. Teamwork that fosters social support might critically affect perceptions of partnered dance by individuals with PD.
Patient self-perception of improvement is greatly important to the effectiveness of a therapeutic physical activity. The self-completed questionnaire showed both groups enjoyed their program (100% in Partner) and noticed improved balance. Non-partner appeared to note greater improvements in coordination, while Partner noted greater improvements in walking and endurance. Individuals from Partner expressed greater interest in continuing. While none of these differences were significant, this might speak to the useful aid in attaining mobility that a partner might represent for those with PD, particularly those in more severe stages of the disease 28.
Functional Relevance of Improvements
The minimal detectable change (MDC) on the Berg Balance Score in parkinsonism is 2.84 points 29, exceeded by our study's significant 3 point balance improvement. Both groups achieved effect sizes above 0.40 on the BBS. Increases in six-minute walk distance may reflect increased endurance and increased gait speed. There is discrepancy in the literature about the amount of change in gait speed that is meaningful. The suggested MDC for those with PD for comfortable gait speed is 0.18 m/s 30, while a change of 0.1 m/s, demonstrated by both intervention groups in the present study, has been deemed functionally significant for seniors at risk of falling 31. The groups also significantly increased fast-as-possible gait speed, which could positively affect daily activities such as crossing the street. Many gains noted, nearly all of which were retained one month after treatment cessation, may have clinical and/or functional significance. More than half of participants who completed the intervention in both groups improved on the BBS at post-test, and maintained these improvements in the follow up testing procedures (Figures 2A, 2B).
Potential Roles of Attentional Focus and External Cues
Perhaps attention influenced these gains noted with dance, given that attending to critical movement aspects allows individuals with PD to achieve nearly normal speed and amplitude 32-34. After a tango lesson involving engaged and enhanced focus on walking and movement, healthy subjects have exhibited increased activity of supplementary motor and premotor cortices during imagined walking 35, In addition, external cues, ever present in dance, may allow for bypassing of the dysfunctional basal ganglia 36, 37. For example, pathways from the visual cortex may reach the motor cortex via pontine and cerebellar relays 38 while auditory cues may access cortical circuitry via the thalamus or the cerebellum 39, 40 Synchronizing movement to rhythm may facilitate movement 41.
The results raise questions about optimal cue usage in rehabilitative movement strategies. Do individuals with PD achieve more mobility and postural stability gains from practicing internal generation of movement (without cues), or while extensively exploiting cues? People with PD have faster reaction times when externally cued than for self-initiated movement 42,43. Partner participants both generated movement internally and effected motor responses to externally cued movement as they all both led and followed step patterns. ‘Leaders’ generated movement internally to determine step length, single support time, velocity, timing, and partner unit trajectories. Conversely, ‘followers’ reflexively responded to the leader's external cuing. Proper following involves focus on simpler concepts of direction, rotation, distance, and speed, which allows the follower to respond to the smallest movements of the leader by reacting to multidirectional perturbations from moment to moment. This strategy of breaking down complex movements into simpler elements may facilitate motor performance 44, 45. To understand which role provides greater benefit, future research should investigate the effects of dancing leading or following roles exclusively.
Study Limitations and Conclusions
In conclusion, after twenty lessons of tango individuals with mild-moderate PD experienced gains in gait, mobility and balance, which were retained one month after completing lessons. Participants expressed enjoyment, satisfaction with improved physical well-being and interest in continuing dance classes, particularly those in Partner. Limitations include small training dosage and sample, participant attrition, the possibility of practice effects on the tasks representing a portion of the benefits noted, and lack of information about transference of effects to activities of daily living. There were some non-significant differences between groups at baseline on certain measures, which may have affected the results and may limit conclusions that can be reached from this work. Nevertheless, the effect of the treatment appeared to have been equally effective in incurring improvement in both groups, and in similar magnitudes. Finally, the BBS is known to have ceiling effects that may have affected the data. However, plots of individual data show that this was not a factor for most subjects (Figures 2A, 2B), and that the groups were similar in the number of participants who topped out on the BBS (3 in Non-partner and 2 in Partner).
This is the first study to provide evidence for retention at a follow-up visit of mobility gains obtained through dancing in persons with PD. Prior studies in dance have only examined participants with PD immediately following dance intervention completion. Because dance interests and engages older individuals, it could be lastingly effective and enjoyable for individuals with PD, which is critical; as 60% of American seniors do not engage in the recommended daily amount of physical activity 46 Activity levels in individuals with PD are a further 15% lower than those of age-matched controls 47. Future work should include larger samples and longer-term studies to determine: 1) the best blending of dance's characteristics and its external cues to provide optimal rehabilitation, 2) the long-term effectiveness of such programs, and 3) the cost effectiveness of such programs and whether they reduce the need for utilization of more expensive health services, as has been suggested for dance programs for the healthy elderly 15.
Acknowledgments
We would like to acknowledge Jeff Becket, Laurie Bonkowski, Ashley Brosius, Brooke Cheatham, Megan Chochol, Stephanie Brosius, Callie Chen, Mike Falvo, Alex Fisher, Josh Funk, Vanessa Heil-Chapdelaine, Stephanie Higgins, Callie Mosiman, Heidi Schmidt, John Scott, Jennifer Sylvester, and Anne Thompson for their assistance with this project. A grant from the American Parkinson Disease Association and NIH grant K01-HD048437-05 supported this work. The study sponsors played no role in the study design, collection, analysis and interpretation of data, the writing of the manuscript, the conclusions drawn or in the decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Statement
The authors have no personal or financial conflicts of interest associated with this work.
Author Roles: Drs. Madeleine Hackney and Gammon Earhart equally contributed to the study design, data collection, analysis and interpretation, the writing of the manuscript, the conclusions drawn and in the decision to submit the manuscript for publication.
References
- 1.Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and Freezing of Gait in Parkinson's Disease: A Review of Two Interconnected, Episodic Phenomena. Mov Disord. 2004;19(8):871–884. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- 2.Melton LJ, III, Leibson CL, Achenbach SJ, Bower JH, Maraganore DM, Ober AL, Rocca WA. Fracture risk after the diagnosis of Parkinson's disease: influence of concomitant dementia. Mov Disord. 2006;21(9):1361–1367. doi: 10.1002/mds.20946. [DOI] [PubMed] [Google Scholar]
- 3.Gage H, Storey L. Rehabilitation for Parkinson's disease: a systematic review of available evidence. Clin Rehab. 2004;18:463–482. doi: 10.1191/0269215504cr764oa. [DOI] [PubMed] [Google Scholar]
- 4.Hu MH, Woollacott MH. Multisensory training of standing balance in older adults: I. Postural stability and one leg stance balance. J Gerontol. 1994;49:M52–61. doi: 10.1093/geronj/49.2.m52. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch MA, Toole T, Maitland CG, Rider RA. The effects of balance training and high-intensity resistance training on person with idiopathic Parkinson's diseasel. Arch Phys Med Rehabil. 2003;84:1109–1117. doi: 10.1016/s0003-9993(03)00046-7. [DOI] [PubMed] [Google Scholar]
- 6.Baker MK, Kennedy DJ, Bohle PL, Campbell DS, Knapman L, Fiatarone MA, et al. Efficacy and Feasibility of a Novel Tri-Modal Robust Exercise Prescription in a Retirement Community: A Randomized, Controlled Trial. J Am Geriatr Soc. 2007;55:1–10. doi: 10.1111/j.1532-5415.2006.01031.x. [DOI] [PubMed] [Google Scholar]
- 7.Qutubuddin AA, Cifu DX, Armistead-Jehle P, Carne W, McGuirk TE, Baron MS. A comparison of computerized dynamic posturography therapy to standard balance physical therapy in individuals with Parkinson's disease: A pilot study. NeuroRehabilitation. 2007;22:261–265. [PubMed] [Google Scholar]
- 8.Jancey J, Lee A, Howat P, Clarke A, Wang K, Shilton T. Reducing attrition in physical activity programs for older adults. J Aging Phys Act. 2007;15(2):152–65. doi: 10.1123/japa.15.2.152. [DOI] [PubMed] [Google Scholar]
- 9.Palo-Bengtsson L, Winblad B, Ekman SL. Social dancing: a way to support the intellectual, emotional and motor function in persons with dementia. J Psychiatr Ment Health Nurs. 1998;5(6):545–54. doi: 10.1046/j.1365-2850.1998.560545.x. [DOI] [PubMed] [Google Scholar]
- 10.Belardinelli R, et al. Waltz Dancing in Patients with Chronic Heart Failure: A New Form of Exercise Training. Circulation: Heart Failure. 2008;1:107–114. doi: 10.1161/CIRCHEARTFAILURE.108.765727. Available from: http://circheartfailure.ahajournals.org/cgi/content/short/1/2/107. [DOI] [PubMed]
- 11.Federici A, Bellagamba S, Rocchi MB. Does Dance based training improve balance in adult and young old subjects? A Pilot randomized controlled trial. Aging Clin Exp Res. 2005;17(5):385–9. doi: 10.1007/BF03324627. [DOI] [PubMed] [Google Scholar]
- 12.Verghese J. Cognitive and Mobility Profile of Older Social dancers. J Am Geriatr Soc. 2006;54:1241–1244. doi: 10.1111/j.1532-5415.2006.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang JG, Ishikawa-Takata K, Yamazaki H, Morita T, Ohta T. Postural Stability and physical performance in social dancers. Gait Posture. 2008;27:697–701. doi: 10.1016/j.gaitpost.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Eyigor S, Karapolat H, Durmaz B, Ibisoglu U, Cakir S. A randomized controlled trial of Turkish folklore dance on the physical performance, balance, depression and quality of life in older women. Arch Gerontol Geriatr. 2007 doi: 10.1016/j.archger.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Song R, June KJ, Kim CG, Jeon MY. Comparisons of Motivation, Health Behaviors, and Functional Status among Elders in Residential Homes in Korea. Public Health Nursing. 2004;21(4):361–371. doi: 10.1111/j.0737-1209.2004.21410.x. [DOI] [PubMed] [Google Scholar]
- 16.McKinley P, Jacobson A, Leroux A, Bednarczyk V, Rossignol M, Fung J. Effect of a community-based Argentine tango dance program on functional balance and confidence in older adults. J Aging Phys Act. 2008;16(4):435–53. doi: 10.1123/japa.16.4.435. [DOI] [PubMed] [Google Scholar]
- 17.Hackney ME, Kantorovich S, Earhart GM. A study on the effects of Argentine tango as a form of partnered dance for those with Parkinson disease and healthy elderly. Am J Dance Ther. 2007;29(2):109–127. [Google Scholar]
- 18.Hackney ME, Kantorovich S, Levin R, Earhart GM. Effects of tango on functional mobility in Parkinson disease: A preliminary study. J Neurol Phys Ther. 2007;31:173–179. doi: 10.1097/NPT.0b013e31815ce78b. [DOI] [PubMed] [Google Scholar]
- 19.Hackney ME, Earhart GM. Short Duration, Intensive Tango Dancing for Parkinson Disease: An Uncontrolled Pilot Study. Complement Ther Med. 2008 doi: 10.1016/j.ctim.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hackney ME, Earhart GM. Effects of Dance on Movement Control in Parkinson's Disease: A Comparison of Argentine Tango and American Ballroom. J Rehabil Med. 2009;41:475–481. doi: 10.2340/16501977-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeka JJ. Light touch contact as a balance aid. Phys Ther. 1997;77(5):476–87. doi: 10.1093/ptj/77.5.476. [DOI] [PubMed] [Google Scholar]
- 22.Racette BA, Rundle M, Parsian A, Perlmutter JS. Evaluation of a screening questionnaire for genetic studies of Parkinson's disease. Am J Med Genet. 1999;88(5):539–43. [PubMed] [Google Scholar]
- 23.Movement Disorder Society Task force on Rating Scales for Parkinson's Disease. The Unified Parkinson's disease rating scale (UPDRS): status and recommendations. Mov Disord. 2003;18(7):738–50. doi: 10.1002/mds.10473. [DOI] [PubMed] [Google Scholar]
- 24.Berg K, Wood-Dauphinee S, Williams JI. The Balance Scale: reliability assessment with elderly residents and patients with acute stroke. Scand J Rehabil Med. 1995;27:27–36. [PubMed] [Google Scholar]
- 25.Vellas BJ, Wayne SJ, Romero L, Baumgartner RN, Rubenstein LZ, Garry PJ. One-leg balance is an important predictor of injurious falls in older persons. J Am Geriatr Soc. 1997;45:735–738. doi: 10.1111/j.1532-5415.1997.tb01479.x. [DOI] [PubMed] [Google Scholar]
- 26.Morris S, Morris ME, Iansek R. Reliability of measurements obtained with the Timed “Up & Go” test in people with Parkinson disease. Phys Ther. 2001;81:810–818. doi: 10.1093/ptj/81.2.810. [DOI] [PubMed] [Google Scholar]
- 27.Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, Berman LB. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132(8):919–923. [PMC free article] [PubMed] [Google Scholar]
- 28.Hackney ME, Earhart GM. Effects of dance on balance and gait in stage V Parkinson disease: A case study. Disabil Rehabil. doi: 10.3109/09638280903247905. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim LIIK, van Wegen EEH, de Goede CJT, Jones D, Rochester L, Hetherington V, Nieuwboer A, Willems AM, Kwakkel G. Measuring gait and gait-related activities in Parkinson's patients own home environment: a reliability, responsiveness and feasibility study Parkinsonism. Relat Disord. 2005;11:19–24. doi: 10.1016/j.parkreldis.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Steffen T, Seney M. Test-Tetest Reliability and Minimal Detectable change on Balance and Ambulation Tests, the 36-Item Short From health Survey, and the Unified Parkinson Disease Rating Scale in People with Parkinsonism. Phys Ther. 2008;88(6):1–14. doi: 10.2522/ptj.20070214. [DOI] [PubMed] [Google Scholar]
- 31.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful Change and Responsiveness in Common Physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 32.Baker K, Rochester L, Nieuwboer A. The immediate effect of attentional, auditory, and a combined cue strategy on gait during single and dual tasks in Parkinson's disease. Arch Phys Med Rehabil. 2007;88(12):1593–600. doi: 10.1016/j.apmr.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 33.Morris ME, Iansek R, Matyas TA, Summers JJ. Stride length regulation in Parkinson's disease: normalization strategies and underlying mechanisms. Brain. 1996;119:551–568. doi: 10.1093/brain/119.2.551. [DOI] [PubMed] [Google Scholar]
- 34.Morris ME, Huxham FE, McGinley J, Iansek R. Gait disorders and gait rehabilitation in Parkinson's disease. Adv Neurol. 2001;87:347–361. [PubMed] [Google Scholar]
- 35.Sacco K, Cauda F, Cerliani L, Mate D, Duca S, Geminiani GC. Motor imagery of walking following training in locomotor attention. The effect of ‘the tango lesson’. NeuroImage. 2006;32:1441–1449. doi: 10.1016/j.neuroimage.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 36.Nieuwboer A, Kwakkel G, Rochester L, Jones D, van Wegen E, Willems AM, Chavret F, Hetherington V, Baker K, Lim I. Cueing training in the home improves gait-related mobility in Parkinson's disease: The RESCUE trial. J Neurol Neurosurg Psychiatry. 2007;78:134–140. doi: 10.1136/jnnp.200X.097923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freedland RL, Festa C, Sealy M, McBean A, Elghazaly P, Capan A, Brozycki L, Nelson AJ, Rothman J. The effects of pulsed auditory stimulation on various gait measurements in persons with Parkinson's Disease. NeuroRehabilitation. 2002;17:81–87. [PubMed] [Google Scholar]
- 38.Cerasa A, Hagberg GE, Peppe A, et al. Functional changes in the activity of cerebellum and frontostriatal regions during externally and internally timed movement in Parkinson's disease. Brain Res Bull. 2006;71(1-3):259–269. doi: 10.1016/j.brainresbull.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 39.Nieuwboer A, Feys P, De Weerdt W, Dom R. Is using a cue the clue to the treatment of freezing in Parkinson's disease? Physiother Res Int. 1997;2(3):125–134. doi: 10.1002/pri.94. [DOI] [PubMed] [Google Scholar]
- 40.Chuma T, Faruque Reza M, Ikoma K, Mano Y. Motor learning of hands with auditory cue in patients with Parkinson's disease. J Neural Transm. 2006;113:175–185. doi: 10.1007/s00702-005-0314-4. [DOI] [PubMed] [Google Scholar]
- 41.Howe TE, Lovgreen B, Cody FW, Ashton VJ, Oldham JA. Auditory cues can modify the gait of persons with early-stage Parkinson's disease: a method for enhancing parkinsonian walking performance? Clin Rehabil. 2003;17(4):363–367. doi: 10.1191/0269215503cr621oa. [DOI] [PubMed] [Google Scholar]
- 42.Siegert RJ, Harper DN, Cameron FB, Abernethy D. Self-initiated versus externally cued reaction times in Parkinson's disease. J Clin Exp Neuropsychol. 2002;24(2):146–153. doi: 10.1076/jcen.24.2.146.991. [DOI] [PubMed] [Google Scholar]
- 43.Ballanger B, Thobois S, Baraduc P, Turner RS, Broussolle E, Desmurget M. “Paradoxical kinesis” is not a hallmark of Parkinson's disease but a general property of the motor system. Mov Disord. 2006;21(9):1490–1495. doi: 10.1002/mds.20987. [DOI] [PubMed] [Google Scholar]
- 44.Tamir R, Dickstein R, Huberman M. Integration of motor imagery and physical practice in group treatment applied to subjects with Parkinson's disease. Neurorehabil Neural Repair. 2007;21(1):68–75. doi: 10.1177/1545968306292608. [DOI] [PubMed] [Google Scholar]
- 45.Morris ME. Movement Disorders in People with Parkinson Disease: A Model for Physical Therapy. Phys Ther. 2000;80(6):578–597. [PubMed] [Google Scholar]
- 46.Macera CA, Ham SA, Yore MM, Jones DA, Ainsworth BE, Kimsey CD, Kohl HW., 3rd Prevalance of physical activity in the United States: behavioral risk factor surveillance system, 2001. Prev Chronic Dis. 2005;2(2):A17. [PMC free article] [PubMed] [Google Scholar]
- 47.Toth MJ, Fishman PS, Pehlman ET. Free-living daily energy expenditure in patients with Parkinson's disease. Neurology. 1997;48:88–91. doi: 10.1212/wnl.48.1.88. [DOI] [PubMed] [Google Scholar]