Abstract
Post hoc analyses of the Digitalis Investigation Group (DIG) trial indicate that digoxin at low (0.5–0.9 ng/ml) serum digoxin concentration (SDC) reduces mortality, which is eliminated at higher (≥1 ng/ml) SDC, and that low-dose (≤ 0.125 mg/day) digoxin predicts low SDC. In the DIG trial, ambulatory chronic systolic and diastolic heart failure (HF) patients (n=7788) in normal sinus rhythm receiving angiotensin converting enzyme inhibitors and diuretics were randomized to receive placebo (n=3899) or digoxin (n=3889). The median dose of digoxin (0.25 mg/day) and the target SDC (0.8–2.5 ng/ml) were higher than what are currently recommended, which may explain in part the lack of long-term mortality benefit of digoxin in the DIG trial. To test this hypothesis, we examined the effect of digoxin on short-term outcomes. One-year all-cause mortality occurred in 392 and 448 patients respectively in the digoxin and placebo groups (hazard ratio for digoxin, 0.87, 95% confidence interval {CI}, 0.76– 0.995; P=0.043). Respective hazard ratios (95% CI) for cardiovascular and HF deaths were 0.87 (0.76–1.01; P=0.072) and 0.66 (0.52–0.85; P=0.001). All-cause hospitalization occurred in 1411 and 1529 digoxin and placebo patients respectively (hazard ratio, 0.89, 95% CI, 0.83–0.96; P =0.002). Respective hazard ratios (95% CI) for cardiovascular and HF hospitalizations were 0.82 (0.75–0.89; P<0.0001) and 0.59 (0.52–0.66; P<0.0001). In conclusion, digoxin reduced one-year mortality and hospitalization in chronic HF patients receiving angiotensin converting enzyme inhibitors and diuretics. Randomized clinical trials are needed to determine the effect of digoxin in contemporary chronic heart failure patients.
Keywords: Digoxin, low dose, heart failure, one-year, morbidity, mortality
In the Digitalis Investigation Group (DIG) trial, digoxin reduced hospitalization due to worsening heart failure (HF), but had no long-term effect on mortality.1 However, data from post hoc analyses of the DIG trial suggest that digoxin reduces mortality at low (0.5–0.9 ng/ml) serum digoxin concentrations (SDC),2–7 but had not effect at higher (≥1 ng/ml) SDC.5,6 A close examination of the Kaplan-Meier survival plots in the DIG trial reveals an early mortality reduction in the digoxin group, followed by later virtual overlap of the plots, suggesting that while the early survival benefit was eliminated in later years, there was no increase in mortality. This lack of a long-term effect of digoxin on mortality may be due to use of open-labeled digoxin in the placebo group,8 and a cumulative effect of the use of high-dose digoxin. In the DIG trial, a SDC of 0.8–2.5 ng/ml was considered therapeutic and was used as a basis for dose adjustment.9 Accordingly, over 80% of the DIG participants were receiving ≥0.25 mg of digoxin or matching placebo, which was higher than the currently recommended daily dosage of digoxin.4,6,7,10,11 The continued use of high-dose digoxin in patients who grew older with deteriorating kidney function may have resulted in higher SDC during later years of the trial. The objective of this study was to examine the effect of digoxin on mortality and hospitalization during the first year of the follow up after randomization.
Methods
The design of the DIG trial has been previously described.1,12 Briefly, the DIG was a multicenter randomized placebo-controlled trial of digoxin in HF. Patients were recruited from the US (186 centers) and Canada (116 centers) during 1991–1993.9 Patients were randomized to 0.125, 0.25, 0.375, or 0.50 mg of digoxin or matching placebo.1,9 The dose recommended was aimed at achieving a SDC of 0.8–2.5 ng/ml.9 The median daily dose of digoxin in the DIG trial was 0.25 mg. DIG participants (N=7788) were ambulatory patients with chronic stable HF in normal sinus rhythm. Patients with left ventricular ejection fraction ≤45% (n=6800) and >45% (n=988) were respectively enrolled into the main and ancillary DIG trials. Most patients were receiving diuretics (>80%) and angiotensin converting enzyme (ACE) inhibitors (>90%). All 7788 patients were included in the current analysis.
Our primary outcome of interest was all-cause mortality during the first year after randomization. Because HF secondary to valvular heart disease (VHD) is quite different from HF secondary to a poorly functioning left ventricle, we repeated our analysis after excluding 171 patients with VHD. Secondary outcomes included one-year cause-specific mortalities and hospitalizations. Data on vital status were 98.9% complete.13 The cause of death or the primary diagnosis leading to hospitalization was classified by DIG investigators who were blinded to the patient’s study-drug assignment. Kaplan–Meier analysis and log-rank statistic were used to construct and compare one-year mortality and hospitalization plots for patients receiving digoxin and placebo. Cox proportional-hazards models were used to compare the effects of digoxin versus placebo on various outcomes. Because, we had no data on renal function during follow up, to determine to what extent renal function may have declined during follow up (median, 38 months), we compared the median serum creatinine levels in patients 65 years and 68 years at baseline. All analyses were performed on an intention-to-treat basis with two-sided P values <0.05 considered significant using SPSS-15 for Windows.14
Results
Patients had a median age of 65 years, 25% were women, 14% were non-white, and 13% had ejection fraction >45%. There were no significant differences in baseline characteristics between the 3899 patients randomly assigned to placebo and 3889 patients assigned to placebo (Table 1). At baseline, median serum creatinine levels for patients 65 years (n=322) and 68 year (n=313) of age were, respectively, 1.2 milligram per deciliter and 1.8 milligram per deciliter (p=0.014).
Table 1.
Baseline patient characteristics by treatment group
| Variables | Placebo (N=3899) | Digoxin (N=3889) |
|---|---|---|
| Age (years), mean (±SD) | 64.0 (±10.8) | 63.9 (±11.0) |
| Ejection fraction (%), mean (±SD) | 31.9 (±12.6) | 32.0 (±12.5) |
| Serum creatinine (mg/dL), mean (±SD) | 1.28 (±0.37) | 1.28 (±0.37) |
| Estimated glomerular filtration rate (ml/min/1.73 sq. meter), mean (±SD) | 63.4 (±24.5) | 63.6 (±20.1) |
| Median duration of heart failure (months) | 17 | 17 |
| Age ≥ 65 years | 52.1 % | 51.5 % |
| Women | 24.7 % | 24.7 % |
| Non-whites | 14.6 % | 14.3 % |
| Estimated glomerular filtration rate <60 ml/min/1.73 sq. meter | 45.7 % | 44.9 % |
| Ejection fraction ≥ 0.45 | 12.7 % | 12.7 % |
| Cardiothoracic ratio >0.55 | 33.4 % | 33.6 % |
| New York Heart Association functional class | ||
| I | 14.0 % | 14.4 % |
| II | 54.8 % | 49.6 % |
| III | 29.3 % | 29.4 % |
| IV | 1.9 % | 2.1 % |
| Number of signs or symptoms of heart failure† | ||
| <4 | 19.0 % | 19.0 % |
| ≥4 | 81.0 % | 80.5 % |
| Medical history | ||
| Previous myocardial infarction | 63.2 % | 62.8 % |
| Current angina pectoris | 26.8 % | 27.5 % |
| Diabetes mellitus | 28.8 % | 28.2 % |
| Hypertension | 47.2 % | 47.1 % |
| Previous digoxin use | 43.6 % | 42.8 % |
| Primary cause of heart failure | ||
| Ischemic | 68.7 % | 69.0 % |
| Non-ischemic | 31.3 % | 31.0 % |
| Hypertensive | 10.6 % | 10.0 % |
| Idiopathic | 13.7 % | 14.8 % |
| Others‡ | 7.0 % | 6.2 % |
| Concomitant medications | ||
| Non-potassium-sparing diuretics | 78.3 % | 77.8 % |
| Potassium-sparing diuretics | 8.2 % | 7.1 % |
| Angiotensin-converting enzyme inhibitors | 93.7 % | 93.1 % |
| Nitrates | 42.9 % | 41.9 % |
| Other vasodilators§ | 1.5 % | 1.0 % |
| Daily dose of study medication (mg) | ||
| 0.125 | 18.4 % | 18.5 % |
| 0.250 | 69.8 % | 70.1 % |
| 0.375 | 10.8 % | 10.3 % |
| 0.500 | 1.0 % | 1.0 % |
The clinical signs or symptoms studied included râles, elevated jugular venous pressure, peripheral edema, dyspnea at rest or on exertion, orthopnea, limitation of activity, S3 gallop, and radiologic evidence of pulmonary congestion.
This category included valvular and alcohol-related causes of heart failure.
These drugs included clonidine hydrochloride, doxazosin mesylate, flosequinan, labetalol hydrochloride, minoxidil, prazosin hydrochloride, and terazosin hydrochloride
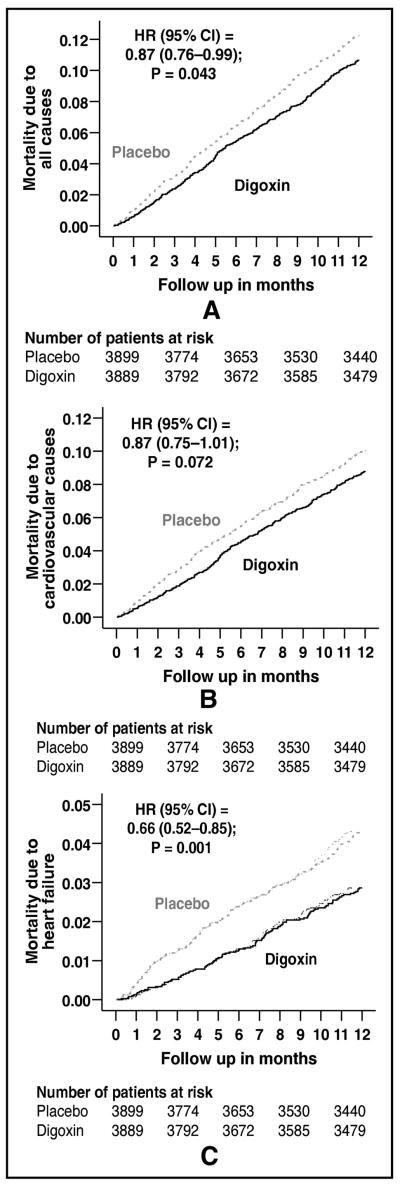
All-cause mortality occurred in 448 patients in the placebo group and 392 patients in the digoxin group during the first year of follow up (HR, when digoxin is compared with placebo, 0.87; 95% confidence interval {CI}, 0.76–0.99; p=0.043; Figure 1a and Table 2). When we repeated our analyses after excluding after excluding 171 patients VHD, we found a similar association between digoxin and all-cause mortality (HR, 0.88; 95% CI, 0.76–1.005; p=0.060). One-year cardiovascular mortality occurred in 368 patients in the placebo group and 323 patients in the digoxin group (HR, 0.87; 95% CI, 0.75–1.01; P=0.072; Figure 1b and Table 2). One-year mortality due to progressive HF occurred in 158 patients in the placebo group and 105 patients in the digoxin group (HR, 0.66; 95% CI, 0.52–0.85; P=0.001; Figure 1c and Table 2). Effects of digoxin on other cause-specific mortalities are displayed in Table 2. The effect of digoxin on one-year all-cause mortality was similar in a wide spectrum of chronic HF patients (Figure 2).
Figure 1.
Kaplan-Meier plots for one-year mortality due to (a) all causes, (b) cardiovascular causes, and (c) heart failure
Table 2.
Cause-specific mortalities during the first year after randomization in heart failure patients receiving placebo and digoxin
| Cause for death: Number of deaths during first year (% of all events) | Rate/10,000 person-years (Deaths/follow-up in years) |
Rate difference (per 10,000 person- years)* | Hazard ratio (95% confidence interval)† | P value | |
|---|---|---|---|---|---|
| Placebo (N=3899) | Digoxin (N=3889) | ||||
| All-cause | 1225 (448/3657) | 1065 (392/3682) | − 160 | 0.87 (0.76–0.995) | 0.043 |
| Cardiovascular | 1006 (368/3657) | 877 (323/3682) | − 129 | 0.87 (0.75–1.01) | 0.072 |
| Worsening heart failure‡ | 432 (158/3657) | 285 (105/3682) | − 147 | 0.66 (0.52–0.85) | 0.001 |
| Other cardio-vascular§ | 574 (210/3657) | 592 (218/3682) | + 17 | 1.03 (0.85–1.25) | 0.750 |
| Non-cardio-vascular | 164 (60/3657) | 130 (48/3682) | − 34 | 0.79 (0.54–1.16) | 0.234 |
| Unknown | 55 (20/3657) | 57 (21/3682) | + 2 | 1.04 (0.57–1.92) | 0.894 |
Absolute rate differences were calculated by subtracting the rates of death in the low-potassium group from the rates of death in the normal potassium group (before values were rounded).
Hazard ratios and confidence intervals (CI) were estimated from matched Cox proportional-hazards models.
This category includes patients who died from worsening heart failure, even if the final event was an arrhythmia.
This category includes cardiac deaths presumed to result from arrhythmia without evidence of worsening heart failure and deaths due to atherosclerotic coronary disease, bradyarrhythmias, low-output states, and cardiac surgery, and vascular deaths due to stroke, embolism, peripheral vascular disease, vascular surgery, and carotid endarterectomy.
Figure 2.
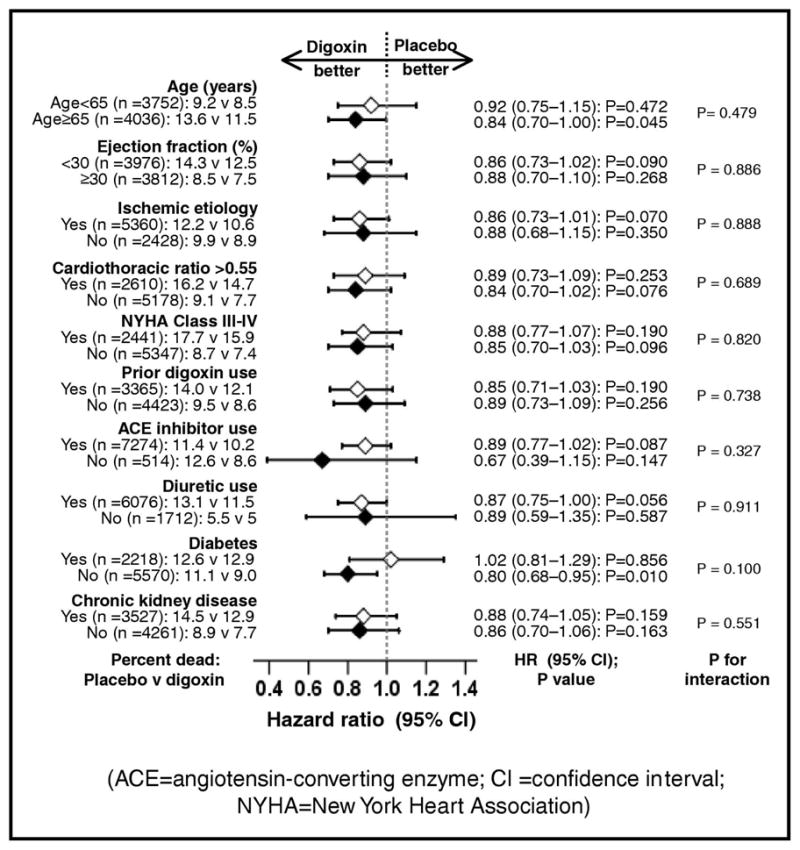
Effects of digoxin on one-year mortality in subgroups of patients (CI =confidence interval)
All-cause hospitalization occurred in 1529 patients in the placebo group and 1411 patients in the digoxin group during the first year of follow up (HR, 0.89; 95% CI, 0.83–0.96; p=0.002; Table 3). One-year cardiovascular hospitalization occurred in 1191 patients in the placebo group and 1016 patients in the digoxin group (HR, 0.82; 95% CI, 0.75–0.89; P<0.0001; Table 3). One-year hospitalization due to worsening HF occurred in 739 patients in the placebo group and 457 patients in the digoxin group (HR, 0.59; 95% CI, 0.52–0.66; P<0.0001; Table 3). Effects of digoxin on other cause-specific hospitalizations are displayed in Table 3.
Table 3.
Cause-specific hospitalizations during the first year after randomization in heart failure patients receiving placebo and digoxin
| Cause for hospitalization*: Number of events during first year (% of all events) | Rate/10,000 person-years follow up (Hospitalizations/follow-up in years) |
Rate difference (/10,000 person-years)† | Hazard ratio (95% confidence interval)‡ | P value | |
|---|---|---|---|---|---|
| Placebo (N=3899) | Digoxin (N=3889) | ||||
| All-cause | 5409 (1529/2827) | 4803 (1411/2938) | − 606 | 0.89 (0.83–0.96) | 0.002 |
| Cardiovascular | 3941 (1191/3022) | 3211 (1016/3164) | − 730 | 0.82 (0.75–0.89) | <0.0001 |
| Worsening heart failure | 2258 (739/3273) | 1315 (457/3475) | − 943 | 0.59 (0.52–0.66) | <0.0001 |
| Ventricular arrhythmia/cardiac arrest: 122 (41%) | 157 (57/3635) | 178 (65/3654) | + 21 | 1.14 (0.80–1.62) | 0.485 |
| Supraventricular arrhythmias§ | 213 (77/3613) | 175 (64/3649) | − 38 | 0.82 (0.59–1.15) | 0.254 |
| Atrioventricular block, bradyarrhythmia | 5 (2/3656) | 30 (11/3676) | + 24 | 5.47 (1.21–24.69) | 0.027 |
| Suspected digoxin Toxicity | 36 (13/3652) | 120 (44/3658) | + 85 | 3.38 (1.82–6.28) | <0.0001 |
| Myocardial infarction | 240 (87/3622) | 233 (85/3652) | − 7 | 0.97 (0.72–1.31) | 0.838 |
| Unstable angina | 580 (206/3554) | 649 (231/3558) | + 69 | 1.12 (0.93–1.35) | 0.237 |
| Stroke | 179 (65/3630) | 183 (67/3654) | + 4 | 1.02 (0.73–1.44) | 0.893 |
| Coronary revascularization¶ | 96 (35/3640) | 96 (35/362) | 0 | 1.00 (0.62–1.59) | 0.983 |
| Heart transplantation | 11 (4/3656) | 8 (3/3680) | − 3 | 0.74 (0.17–3.32) | 0.697 |
| Other cardiovascular causes|| | 505 (180/3562) | 628 (224/3566) | + 123 | 1.24 (1.02–1.51) | 0.030 |
| Respiratory infection | 311 (112/3607) | 291 (106/3640) | − 20 | 0.94 (0.72–1.22) | 0.634 |
| Other non-cardiovascular causes | 1570 (534/3402) | 1712 (581/3393) | + 142 | 1.09 (0.97–1.23) | 0.147 |
| Unspecified | 25 (9/3653) | 24 (9/3678) | − 1 | 0.99 (0.39–2.50) | 0.987 |
Data shown include the first hospitalization of each patient due to each cause.
Absolute differences were calculated by subtracting the percentage of patients hospitalized in the placebo group from the percentage of patients hospitalized in the digoxin group (before values were rounded).
Hazard ratios and confidence intervals (CI) were estimated from a Cox proportional-hazards models that used the first hospitalization of each patient for each reason.
Supraventricular (SV) arrhythmias include atrioventricular (AV) block and bradyarrhythmias
This category includes coronary-artery bypass grafting and percutaneous transluminal coronary angioplasty
This category includes embolism, venous thrombosis, peripheral vascular disease, hypertension, other vascular surgery, cardiac catheterization, other types of catheterization, pacemaker implantation, installation of automatic implantable cardiac defibrillator, electrophysiologic testing, transplant-related evaluation, nonspecific chest pain, atherosclerotic heart disease, hypotension, orthostatic hypotension, and valve operation
At the end of first 12 months of follow up, 85% of the patients were taking the study drug and 84% of patients were taking >80% of the study drug prescribed: more patients in digoxin group were taking the study drug (86% versus 84% placebo patients; Chi square P=0.005) and were taking >80% of the prescribed dosage (85% versus 82% placebo patients; Chi square P=0.001) at the end of 12 months of follow up. Study drug was discontinued in 1010 patients during the first 12 months after randomization, of which 41% were due to use of open-label digoxin to treat worsening HF (30%) and atrial fibrillation (11%); discontinuation of the study drug for open label digoxin use occurred in 31% of patients in the digoxin group and 49% of the patients in the placebo group (Chi square test P <0.0001). The median daily doses of the study drug both at randomization and at 12 months were 0.25 milligram. At baseline, 18%, 70%, 11% and 1% patients were respectively receiving ≤0.125 milligram, 0.250 milligram, 0.375 milligram and 0.50 milligram per day of the study drug. Twelve months after randomization, 21%, 68%, 10% and 1% patients were respectively receiving ≤0.125 milligram, 0.25 milligram, 0.375 milligram and ≥0.50 milligram per day of the study drug.
Overall, 110 (1.4%) patients were hospitalized for suspected or confirmed digoxin toxicity during the first 12 months after randomization. Hospitalization due to digoxin toxicity occurred in 13 patients in the placebo group and 44 patients in the digoxin group during the first year of follow up (HR, 3.38; 95% CI, 1.82–6.28; P <0.0001; Table 3). Hospitalization due to atrioventricular block or bradyarrhythmia toxicity occurred in 2 patients in the placebo group and 11 patients in the digoxin group during the first year of follow up (HR, 5.47; 95% CI, 1.21–24.69; P=0.027; Table 3).
Discussion
The key findings of the current post hoc analysis of the DIG trial are that digoxin reduced deaths and hospitalizations due to all causes, cardiovascular causes, and HF during the first year after randomization in a wide spectrum of ambulatory chronic systolic and diastolic HF patients receiving ACE inhibitors and diuretics. These benefits of digoxin were observed despite the use of relatively high doses of digoxin that are now considered high and regardless of SDC. There is evidence that digoxin may be underused in HF patients,15–17 and the findings form the current analysis may help rehabilitate digoxin in HF care.18
Digoxin, long known for its positive inotropic effects,19,20 is now also known to suppress sympathetic and renin-angiotensin-aldosterone systems.21–26 It has been suggested that the neurohormonal properties of digitalis are more pronounced at low SDC.27,28 This is consistent with the findings from post hoc analyses of the DIG trial that suggested that digoxin may reduce mortality at low (<1 ng/ml) SDC.4–7 Data from these studies also suggest that low-dose digoxin is one the strongest predictors of low SDC.4–7 However, in the current analysis patients were receiving digoxin in higher doses and the analysis was not restricted to patients with low SDC. So, how can one explain an early mortality reduction from a drug that showed no long-term mortality reduction?
In the DIG trial, patients were receiving the same daily dose of 0.25 mg of digoxin both at the time of randomization and at the end of one year of follow up. A daily dose of 0.25 mg of digoxin may have been proper at baseline when patients were relatively younger (median age, 65 years). However, continued use of digoxin at the same dose in patients with increasing age and declining renal function may have resulted in higher SDC. This is important as both age and renal function are important predictors of SDC.6,7,10 During the median follow up of over three years, with the increase in the median age of patients from 65 to 68 years, the mean serum creatinine levels increased from 1.2 mg/dL to 1.8 mg/dL (a 50% increase).
Dose of digoxin is a strong predictor of SDC and is particularly stronger in older that younger HF patients.6,7,10 Thus, the dose of digoxin that was normal at the trial onset may have been high during later years of follow up, leading to higher SDC. Results of post hoc analysis of DIG trial suggest that digoxin at higher SDC (≥1 ng/ml) had no effect on mortality.5,6 In the DIG trial, the digoxin-associated early mortality reduction was eliminated in later years, but there was no increase in mortality 1, suggesting that an early low SDC may have turned high in later years. The lack of a long-term mortality benefit of digoxin may also be due to an increased cross-over of during later years of follow up. At 12 months, 86% of digoxin patients were receiving digoxin and 83% placebo patients were receiving placebo. At the study end, 71% of surviving digoxin patients were receiving digoxin and another 10% were receiving open-labeled digoxin; on the other hand, 68% of surviving placebo patients were receiving placebo but 16% were receiving open-labeled digoxin.1
Because the median age of real-life HF patients is generally more than a decade older than those in the DIG trial,16 a daily dose of 0.125 mg of digoxin may be a more appropriate starting dose for most HF patients. There is no need for routine SDC monitoring if digoxin is used in lower dosages. The dose of digoxin may be increased to 0.25 mg/day for patients who remain symptomatic at lower doses. However, for patients who are elderly, women and have renal insufficiency, any increase in dose should be guided by SDC and tailored to achieve a SDC (0.5–0.9 ng/ml) associated with mortality reduction. Since the DIG trial, beta-blockers and aldosterone antagonists have been added to the list of drugs that can favorably alter the natural history end points in chronic systolic HF and the role of digoxin in these patients remain uncertain. Data from post hoc and subgroup analyses suggest safety and efficacy of digoxin in patients receiving beta-blockers and aldosterone antagonists.29,30 However, digoxin and beta-blockers may have complex interactions. The inotropic effect of digoxin may improve the tolerability for beta-blockers, while beta blockers may reduce the risk of digoxin-induced serious arrhythmias in sensitive pro-arrhythmic patients. However, digoxin may also potentially increase some of the adverse effects of beta-blockers such as atrioventricular blocks. Therefore, the effect of digoxin in contemporary HF patients receiving beta-blockers needs to be tested in a well-designed randomized clinical trial that would recruit equal number of men and women with systolic and diastolic HF.
Digoxin is one of the most inexpensive HF drugs. For HF patients in developing nations who cannot afford ACE inhibitors, angiotensin receptor blockers or beta-blockers, digoxin may be useful in reducing mortality and morbidity. In the developed nations, HF patients receiving ACE inhibitors who cannot afford or tolerate beta-blockers should be treated with low-dose digoxin. Over 90% of all patients in the DIG trial were receiving ACE inhibitors.
The results of this post hoc analysis of DIG trial should be interpreted with caution. The DIG protocol pre-specified post hoc analyses of two-year outcomes. However, based on the Kaplan-Meier plots, the higher target SDC and the higher doses of digoxin used in the DIG trial, we restricted our analysis to one-year outcomes. Patients in the DIG trial were younger than real-life HF patients and were predominantly male and whites, and were in normal sinus rhythm, thus limiting generalizability to other patients. In conclusion, digoxin reduced one-year mortality and hospitalization in ambulatory patients with chronic mild to moderate systolic and diastolic HF and normal sinus rhythm, receiving ACE inhibitors and diuretics. Randomized clinical trials are needed to determine the effect of digoxin in contemporary chronic HF patients.
Acknowledgments
Funding Support
Dr. Ahmed is supported by the National Institutes of Health through grants from the National Heart, Lung, and Blood Institute (5-R01-HL085561-02 and P50-HL077100), and a generous gift from Ms. Jean B. Morris of Birmingham, Alabama.
“The Digitalis Investigation Group (DIG) study was conducted and supported by the NHLBI in collaboration with the DIG Investigators. This Manuscript was prepared using a limited access dataset obtained from the NHLBI and does not necessarily reflect the opinions or views of the DIG Study or the NHLBI.”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–533. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 2.Smith TW, Butler VP, Jr, Haber E. Determination of therapeutic and toxic serum digoxin concentrations by radioimmunoassay. N Engl J Med. 1969;281:1212–1216. doi: 10.1056/NEJM196911272812203. [DOI] [PubMed] [Google Scholar]
- 3.Eraker SA, Sasse L. The serum digoxin test and digoxin toxicity: a Bayesian approach to decision making. Circulation. 1981;64:409–420. doi: 10.1161/01.cir.64.2.409. [DOI] [PubMed] [Google Scholar]
- 4.Adams KF, Jr, Gheorghiade M, Uretsky BF, Patterson JH, Schwartz TA, Young JB. Clinical benefits of low serum digoxin concentrations in heart failure. J Am Coll Cardiol. 2002;39:946–953. doi: 10.1016/s0735-1097(02)01708-4. [DOI] [PubMed] [Google Scholar]
- 5.Rathore SS, Curtis JP, Wang Y, Bristow MR, Krumholz HM. Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA. 2003;289:871–878. doi: 10.1001/jama.289.7.871. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed A, Rich MW, Love TE, Lloyd-Jones DM, Aban IB, Colucci WS, Adams KF, Gheorghiade M. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur Heart J. 2006;27:178–186. doi: 10.1093/eurheartj/ehi687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed A, Pitt B, Rahimtoola SH, Waagstein F, White M, Love TE, Braunwald E. Effects of digoxin at low serum concentrations on mortality and hospitalization in heart failure: a propensity-matched study of the DIG trial. Int J Cardiol. 2008;123:138–146. doi: 10.1016/j.ijcard.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruelaz RA, Rahimtoola SH. Was it digoxin toxicity? …very likely. J Card Fail. 2005;11:87–90. doi: 10.1016/j.cardfail.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 9.The Digitalis Investigation Group. Final protocol. Bethesda, Maryland: National Heart, Lung, and Blood Institute; 1992. Protocol: Trial to evaluate the effect of digitalis on mortality in heart failure. [Google Scholar]
- 10.Ahmed A. Digoxin and reduction in mortality and hospitalization in geriatric heart failure: importance of low doses and low serum concentrations. J Gerontol A Biol Sci Med Sci. 2007;62:323–329. doi: 10.1093/gerona/62.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Veldhuisen DJ. Low-dose digoxin in patients with heart failure. Less toxic and at least as effective? J Am Coll Cardiol. 2002;39:954–956. doi: 10.1016/s0735-1097(02)01710-2. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed A, Rich MW, Fleg JL, Zile MR, Young JB, Kitzman DW, Love TE, Aronow WS, Adams KF, Jr, Gheorghiade M. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary digitalis investigation group trial. Circulation. 2006;114:397–403. doi: 10.1161/CIRCULATIONAHA.106.628347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins JF, Howell CL, Horney RA. Determination of vital status at the end of the DIG trial. Control Clin Trials. 2003;24:726–730. doi: 10.1016/j.cct.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 14.SPSS. SPSS for Windows, Rel. 14. Chicago, IL: SPSS Inc., Chicago, IL; 2007. [Google Scholar]
- 15.Ahmed A, Young JB, Gheorghiade M. The underuse of digoxin in heart failure, and approaches to appropriate use. CMAJ. 2007;176:641–643. doi: 10.1503/cmaj.061239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gheorghiade M, Zannad F, Sopko G, Klein L, Pina IL, Konstam MA, Massie BM, Roland E, Targum S, Collins SP, Filippatos G, Tavazzi L. Acute heart failure syndromes: current state and framework for future research. Circulation. 2005;112:3958–3968. doi: 10.1161/CIRCULATIONAHA.105.590091. [DOI] [PubMed] [Google Scholar]
- 17.Wehrmacher WH. Digitalis treatment decreases mortality and morbidity in heart failure patients. Reanalysis of digitalis treatment. Cardiology. 2006;108:157–158. doi: 10.1159/000096567. [DOI] [PubMed] [Google Scholar]
- 18.Brophy JM. Rehabilitating digoxin. Eur Heart J. 2006;27:127–129. doi: 10.1093/eurheartj/ehi686. [DOI] [PubMed] [Google Scholar]
- 19.Braunwald E, Pool PE. Mechanism of action of digitalis glycosides (II) Mod Concepts Cardiovasc Dis. 1968;37:135–139. [PubMed] [Google Scholar]
- 20.Goldman RH, Coltart DJ, Friedman JP, Nola GT, Berke DK, Schweizer E, Harrison DC. The inotropic effects of digoxin in hyperkalemia. Relation to (Na+,K+)-ATPase inhibition in the intact animal. Circulation. 1973;48:830–838. doi: 10.1161/01.cir.48.4.830. [DOI] [PubMed] [Google Scholar]
- 21.Torretti J, Hendler E, Weinstein E, Longnecker RE, Epstein FH. Functional significance of Na− K-ATPase in the kidney: effects of ouabain inhibition. Am J Physiol. 1972;222:1398–1405. doi: 10.1152/ajplegacy.1972.222.6.1398. [DOI] [PubMed] [Google Scholar]
- 22.Covit AB, Schaer GL, Sealey JE, Laragh JH, Cody RJ. Suppression of the renin-angiotensin system by intravenous digoxin in chronic congestive heart failure. Am J Med. 1983;75:445–447. doi: 10.1016/0002-9343(83)90346-7. [DOI] [PubMed] [Google Scholar]
- 23.Ferguson DW, Berg WJ, Sanders JS, Roach PJ, Kempf JS, Kienzle MG. Sympathoinhibitory responses to digitalis glycosides in heart failure patients. Direct evidence from sympathetic neural recordings. Circulation. 1989;80:65–77. doi: 10.1161/01.cir.80.1.65. [DOI] [PubMed] [Google Scholar]
- 24.Gheorghiade M, Ferguson D. Digoxin. A neurohormonal modulator in heart failure? Circulation. 1991;84:2181–2186. doi: 10.1161/01.cir.84.5.2181. [DOI] [PubMed] [Google Scholar]
- 25.Bolognesi R, Tsialtas D, Manca C. Digitalis and heart failure: does digitalis really produce beneficial effects through a positive inotropic action? Cardiovasc Drugs Ther. 1992;6:459–464. doi: 10.1007/BF00055601. [DOI] [PubMed] [Google Scholar]
- 26.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup ML, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. American College of Cardiology; [Accessed on August 17, 2005, 2005]. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) Web Site. Available at: http://www.acc.org/clinical/guidelines/failure//index.pdf. [DOI] [PubMed] [Google Scholar]
- 27.Gheorghiade M, van Veldhuisen DJ, Colucci WS. Contemporary use of digoxin in the management of cardiovascular disorders. Circulation. 2006;113:2556–2564. doi: 10.1161/CIRCULATIONAHA.105.560110. [DOI] [PubMed] [Google Scholar]
- 28.Rahimtoola SH. Digitalis therapy for patients in clinical heart failure. Circulation. 2004;109:2942–2946. doi: 10.1161/01.CIR.0000132477.32438.03. [DOI] [PubMed] [Google Scholar]
- 29.Eichhorn EJ, Lukas MA, Wu B, Shusterman N. Effect of concomitant digoxin and carvedilol therapy on mortality and morbidity in patients with chronic heart failure. Am J Cardiol. 2000;86:1032–1035. A1010–1031. doi: 10.1016/s0002-9149(00)01146-2. [DOI] [PubMed] [Google Scholar]
- 30.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]