Abstract
Objective
This study examined perceptual and motor inhibition in a longitudinal sample of adolescents/young adults who were diagnosed with ADHD in childhood, and as a function of the relative persistence of ADHD.
Method
Ninety-eight participants diagnosed with ADHD in childhood were re-evaluated approximately 10 years later. Eighty-five never-ADHD controls similar in age, IQ, sociodemographic background, and gender distribution served as a comparison group. Participants were administered a psychiatric interview and the Stimulus and Response Conflict Tasks (Nassauer & Halperin, 2003).
Results
Participants with childhood ADHD demonstrated slower and less accurate responses to both control and conflict conditions relative to the comparison group, as well as more variable responses in both conditions of the motor inhibition task; there was no specific effect of childhood ADHD on perceptual or motor inhibition. ADHD persisters and partial remitters did not differ in overall accuracy, speed or variability in responding, but relative to partial remitters, persisters demonstrated greater slowing in response to perceptual conflict.
Conclusions
These findings are consistent with theories positing state regulation, but not inhibitory control deficits in the etiology of ADHD, and suggest that improved perceptual inhibition may be associated with better outcome for ADHD.
Keywords: Conflict (Psychology), Attention Deficit Hyperactivity Disorder (ADHD), Young Adults, Inhibitory Control, Longitudinal
INTRODUCTION
Inhibitory control is a multi-faceted cognitive process that facilitates resistance to interference from irrelevant environmental stimuli and the suppression of previously activated inappropriate motor responses (Bjorklund & Harnishfeger, 1995; Lampe, et al., 2007). In essence, inhibitory control is the ability to restrain behaviors that would disrupt the efficient completion of a goal. A prevailing perspective in the clinical and developmental literature on attention-deficit/hyperactivity disorder (ADHD) has been that the inattentive, impulsive, and hyperactive characteristics of individuals with ADHD closely relate to, and may result from, a core deficit in resistance to interference and capacity for motor and cognitive inhibition (Barkley, 1997; Castellanos & Tannock, 2002; Crosbie, Perusse, Barr, & Schachar, 2008; Nigg, 2001; Sergeant, Geurts, & Oosterlaan, 2002; Tannock, 1998). However, the specificity and centrality of this inhibitory control deficit in ADHD has been questioned (Rommelse, et al., 2007; Sergeant, et al., 2002).
Although problems with inhibition were once conceptualized as being universal among individuals with ADHD (Barkley, 1997; Pennington & Ozonoff, 1996), recent research suggests that only approximately half of children and adolescents with ADHD show deficits in inhibitory control (Bedard, et al., 2003; Biederman, et al., 2004; Nigg, Willcutt, Doyle, & Sonuga-Barke, 2005). In fact, a recent large-scale study reported that the frequency of deficits in response inhibition did not differ between adolescents with ADHD (9%) and controls (8%) (Loo, et al., 2007). Furthermore, data suggest that the poorer performance on many inhibitory control tasks in individuals with ADHD may result from deficiencies in lower-order cognitive processes and not from the higher-order cognitive process of inhibitory control (Rommelse, et al., 2007). Regardless, impaired inhibitory control is neither necessary nor sufficient to account for ADHD symptoms alone.
Although several meta-analytic reviews have found robust deficits in the ability to suppress a pre-potent response in children and adults with ADHD (Alderson, Rapport, & Kofler, 2007; Boonstra, Oosterlaan, Sergeant, & Buitelaar, 2005; Lijffijt, Kenemans, Verbaten, & van Engeland, 2005; Oosterlaan, Logan, & Sergeant, 1998; Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005), evidence pertaining to the specificity of this deficit remains equivocal. Some reviews have found decreased performance not only on tasks measuring aspects of motor inhibition, but also in skills that fall outside of the classical definitions of “executive functions” such as memory, information processing speed and basic motor functions (Hervey, Epstein, & Curry, 2004; Schoechlin & Engel, 2005). For example, hyperactive/inattentive preschool children (Berwid, et al., 2005), school-aged children (Rommelse, et al., 2007), and adults (Lampe, et al., 2007) with ADHD are not disproportionally slower or inaccurate relative to matched controls on motor inhibition tasks relative to performance on comparison conditions that require less inhibition (e.g. congruent trials of the Stroop, general RTs of the stop signal and go/no-go tasks).
Similarly, findings regarding perceptual inhibition, or what Barkley (1997) refers to as interference control, in ADHD also challenge the centrality of this cognitive function in the etiology of ADHD. Although some reviews have suggested that children with ADHD demonstrate impairments in perceptual inhibition as indexed by Stroop task performance (Barkley, 1997; Lansbergen, Kenemans, & van Engeland, 2007; Pennington & Ozonoff, 1996), other studies have reported equivalent Stroop effects in individuals with ADHD compared to controls (Halperin, Trampush, Miller, Marks, & Newcorn, 2008; Nigg, 2001; Pritchard, Neumann, & Rucklidge, 2007; Schwartz & Verhaeghen, 2008; van Mourik, Oosterlaan, & Sergeant, 2005; van Mourik, et al., 2009). Potential explanations for these discrepancies across studies include whether the method used to calculate the Stroop interference score accounted for basic naming speed, and the potential confound of color perception abilities and reading fluency on interference liability (Albrecht, et al., 2008; Lansbergen, et al., 2007; Rucklidge & Tannock, 2002; van Mourik, et al., 2005). The above-noted problems in using Stroop-like paradigms to evaluate perceptual inhibition/interference control in individuals with ADHD indicate that alternative methods are needed.
The Stimulus and Response Conflict Tasks (SRCT: Nassauer & Halperin, 2003) were developed to provide separate measures of early perceptual inhibition (i.e., susceptibility to interference) and late motor inhibition (i.e., inhibition of prepotent responding) using a series of simple reaction time tasks that use the same stimuli, responses, and paired control conditions that differ only in the context of the stimulus-response associations. The SRCT were designed to be independent of verbal ability and to minimize interference effects to due extraneous stimulus-response modality conflicts. A validation study of the SRCT in college students found significant increases in reaction time (RT) during both perceptual and motor inhibition. Performance on the perceptual inhibition component, but not the motor inhibition component, was associated with the Stroop interference effect (Nassauer & Halperin, 2003).
A modified version of the SRCT was used to measure perceptual and motor inhibition in hyperactive/inattentive preschoolers and matched control children (Marks, et al., 2005). These ‘at-risk’ preschoolers committed more errors and had longer reaction times (RTs) than a matched comparison group, suggestive of non-specific, perhaps state-regulation deficits. However, no specific impairments in perceptual or motor inhibition were found after controlling for nonexecutive abilities. Further, no significant associations were found between inhibition scores on the SRCT, and parent- and teacher-ratings of ADHD behaviors.
The SRCT has also been used in conjunction with functional magnetic resonance imaging (fMRI) to examine the neurobiological underpinnings of response inhibition. Adolescents previously diagnosed with ADHD during childhood exhibited differential neural responses to the perceptual, but not the motor inhibitory control tasks in several brain regions previous implicated in the pathophysiology of ADHD (e.g., ventrolateral prefrontal cortex) relative to age, gender, and IQ matched adolescents with no history of ADHD (Schulz, et al., 2005).
The current study examined perceptual and motor inhibition using the SRCT in a large, longitudinal sample of adolescents/young adults who were diagnosed with ADHD in childhood as compared to a never-ADHD comparison group. Based on findings using the SRCT in preschoolers at risk for ADHD (Marks, et al., 2005), we predicted that adolescents with childhood ADHD would show global impairments in task performance, as reflected by significant main effects for Group, but not specific deficits in perceptual and response inhibition compared to matched controls after controlling for baseline performance. We additionally examined among probands the degree to which performance varied as a function of relative persistence of ADHD diagnosis in adolescence/young adulthood. Here we predicted that SRCT performance would indicate greater inhibitory capacity in those who had ADHD in childhood, but some remission of symptoms over development, as compared to those with persisting ADHD symptoms, and that this trajectory-related difference in inhibitory control would be reflected by significant Group × Condition interactions. This latter prediction is based on the hypothesis of Halperin and Schulz (2006) that developmental changes in frontal lobe function which occur during adolescence are associated with diminution of ADHD symptoms and improved executive and inhibitory control.
METHOD
Participants
Ninety-eight adolescents/young adults who were evaluated in a research protocol during childhood and diagnosed with ADHD (Halperin, et al., 1997) participated in a follow-up evaluation on average 9.29 (SD = 1.69, range 6.30–15.38) years later, with all but one occurring within 13.5 years of the original evaluation. They were drawn from a group of 169 youth who were recruited between 1990 and 1997. Of the original 169 participants in the childhood sample, 18 declined participation, two were deceased, five were incarcerated, and 46 were lost to follow-up. Nine participants did not complete the SRCT during the follow-up assessment; hence, 89 ADHD probands were included in the final analyses. Those who were and were not followed did not differ significantly in age at initial evaluation, race/ethnicity, sex, childhood comorbidity, socioeconomic status (SES), or ADHD behavior ratings at baseline (all p > 0.05) (see Halperin, et al., 2008), but did differ in that those followed had a slightly higher full-scale IQ (Followed: 93.96 ±14.27 vs. Not Followed 89.31 ± 14.95; F1, 159 = 3.98, p = 0.05).
In childhood (ages 7 – 11 years), participants were evaluated using parent report on the Diagnostic Interview Schedule for Children (DISC), version 2.1 or 2.3, depending upon when they were recruited (Fisher, et al., 1993; Shaffer, et al., 1996). Parent and teacher reports using the Child Behavior Checklist (CBCL: Achenbach, 1991a) and IOWA Conners Rating Scale (Loney & Milich, 1982), respectively, were also obtained. Childhood assessments determined using DSM-III-R, which only consisted of 14 as opposed to the 18 items included in DSM-IV, were systematically reviewed in relation to DSM-IV criteria for ADHD; it is likely that most, if not all, participants met criteria for ADHD-Combined Type as defined in DSM-IV.
In addition, 85 never-ADHD controls were recruited during adolescence/young adulthood via advertisements in neighborhoods that matched the ADHD sample by zip code. The Never-ADHD controls resembled the probands on most important demographic variables including age, race/ethnicity, SES, and IQ (P > 0.05; see Table 1). The ratio of males to females (M:F) did not differ statistically between the childhood ADHD (79:10) and Never-ADHD (74:11) groups (χ2 (1, 174) = 0.73, p = 0.82). Like the original ADHD sample, prospective Never-ADHD controls were excluded if they had any chronic medical/neurological condition or psychosis, or were non-English-speaking. Controls interested in participating were screened by telephone for study eligibility prior to scheduling a full evaluation.
Table 1.
Sample characteristics based on childhood diagnosis
| Measure | ADHD-in childhood (n=89) Mean (SD) | Never-ADHD (n=85) Mean (SD) | t | Cohen’s d |
|---|---|---|---|---|
| Age (years) | 18.34 (1.62) | 18.50 (1.68) | 0.51 | 0.10 |
| SES | 43.75 (17.32) | 40.89 (16.60) | 1.11 | 0.17 |
| WAIS-III Full-Scale IQ | 92.42 (14.50) | 96.79 (15.33) | 1.93 | 0.29 |
| Parent Report | ||||
| DSM-IV Inattention | 14.47 (7.39) | 3.81 (4.36) | 11.29* | 1.76 |
| DSM-IV Hyper.-Impuls. | 9.70 (7.82) | 1.89 (4.09) | 8.05* | 1.25 |
| CBCL Attention probs. | 61.10 (10.07) | 51.52 (2.89) | 8.28* | 1.29 |
| Adolescent Self Report | ||||
| DSM-IV Inattention | 9.71 (6.50) | 2.94 (3.33) | 8.54* | 1.31 |
| DSM-IV Hyper.-Impuls. | 7.93 (7.03) | 1.92 (2.47) | 7.44* | 1.14 |
| YSR Attention problems | 57.51 (9.85) | 50.53 (2.87) | 6.30* | 0.96 |
Note:
p < 0.001.
Severity of ADHD symptoms was determined dimensionally by self- and parent-ratings on a 4-point scale made-up of all 18 DSM-IV Inattentive and Hyperactive-Impulsive items.
The sample was predominately male (88%) and racially/ethnically diverse (26% African-American, 23% Caucasian, 36% Hispanic, and 15% mixed or other ancestry). Ages ranged from 16 to 21 years1. SES, estimated using the socioeconomic prestige scale (Nakao & Treas, 1994), was 42.35 (SD = 16.98). This scale was developed at the National Opinion Research Center and approaches the issue of measuring socioeconomic status by ranking the relative prestige of the individual’s occupation on a scale from 1 to 100. The rankings were derived from surveys that asked respondents to attach a ranking to the occupation. Thousands of occupations are classified and the rankings are updated periodically. Information about parental occupation was obtained from parents during the follow-up assessment. The sample comprised individuals with a broad range of scores on this measure (20 – 96), representing, on average, a low to lower-middle status group, with a fairly large number of participants (18.9%) at the poverty level.
All procedures were approved by the Institutional Review Boards of the participating institutions. Written informed consent was obtained from all participants above the age of 18 years and the parent(s) of those under age 18 years. Verbal assent was obtained from youth under the age of 18 years.
Clinical Assessment Measures at Follow-up
The Kiddie-SADS Present and Lifetime Version (Kaufman, et al., 1997) was administered at follow-up to each participant and his/her parent(s) to assess the presence of ADHD. Evaluators were Ph.D.-level Psychologists or trained Psychology graduate students blind to group membership. These evaluators derived a summary score from a combination of parent and participant responses, in accordance with published methods (Bird, Gould, & Staghezza, 1992). In this method, separate responses from interviews of mothers and from interviews of children are amalgamated by using data given by both informants. Based on the Kiddie-SADS interview, the ADHD group was subdivided into those who continued to meet diagnostic criteria for ADHD (‘persisters’ n = 38) and those who clearly did not (‘partial remitters’ n = 29). Persistence was defined as meeting DSM-IV criteria for ADHD with at least six symptoms of inattention and/or hyperactivity-impulsivity, plus evidence of impairment across multiple settings. Although most participants likely met criteria for ADHD-C in childhood, there was a distribution of subtypes at follow-up among the persisters: 28.9% ADHD-C, 50.0% ADHD-Inattentive Type, and 21.1% ADHD-Hyperactive-Impulsive Type. The partial remitter group was operationally-defined to exclude individuals with sub-threshold ADHD (i.e., those who missed qualifying for diagnosis based on one, two or three items on one or another item list), and thereby provide adequate separation from the persister group for analytic purposes. Participants classified as partial remitters were required to have no more than three symptoms in each domain of hyperactivity-impulsivity and inattention. The ratio of males to females (M:F) did not differ statistically between the ADHD-partial remitters (26:3) and the ADHD-persisters (35:3) groups (χ2 (1, 67) = 0.73, p = 0.53). Characteristics of the ADHD-persisters compared to the ADHD-partial remitters are summarized as Table 2. Of note, persisters and partial remitters differed significantly in SES (p < .05).
Table 2.
Childhood and Adolescent/Young Adult Characteristics of ADHD Persisters and Partial Remitters
| Measure | ADHD-persisters (n=38) Mean (SD) | ADHD-partial remitters (n=29) Mean (SD) | t | Cohen’s d |
|---|---|---|---|---|
| Childhood Assessment | ||||
| Age (years) | 8.75 (1.23) | 9.54 (1.07) | 2.77** | 0.69 |
| WISC-III Full-Scale IQ | 93.08 (14.41) | 96.11 (13.17) | 0.39 | 0.22 |
| IOWA Conners I/O Score | 11.30 (3.43) | 11.04 (3.01) | 0.32 | 0.08 |
| CBCL Attention Probs | 71.06 (10.80) | 71.44 (10.70) | 0.14 | 0.04 |
| Adolescent Assessment | ||||
| Age (years) | 18.12 (2.01) | 18.93(1.17) | 1.96 | 0.49 |
| Child to Adolescent Interval | 9.35 (1.84) | 9.41 (1.59) | 0.15 | 0.04 |
| SES | 39.24 (15.88) | 49.31 (17.65) | 2.45* | 0.60 |
| WAIS-III Full-Scale IQ | 90.61 (12.63) | 96.03 (15.02) | 1.61 | 0.39 |
| Parent Report | ||||
| DSM-IV Inattention | 17.89 (5.63) | 9.22 (6.40) | 5.75** | 1.44 |
| DSM-IV Hyper.-Impuls. | 13.41 (8.00) | 4.67 (5.93) | 4.79** | 1.24 |
| CBCL Attention Probs. | 65.30 (12.40) | 55.15 (5.91) | 3.94** | 1.05 |
| Adolescent Self Report | ||||
| DSM-IV Inattention | 12.44 (6.37) | 6.25 (4.63) | 4.33** | 1.11 |
| DSM-IV Hyper.-Impuls. | 11.06 (7.55) | 3.39 (3.99) | 4.87** | 1.27 |
| YSR Attention problems | 58.33 (9.83) | 53.50 (7.90) | 2.04* | 0.54 |
Note:
p < 0.05;
p < 0.001.
Severity of ADHD symptoms was determined dimensionally by self- and parent-ratings on a 4-point scale made-up of all 18 DSM-IV Inattentive and Hyperactive-Impulsive items. IOWA Conners I/O Score refers to the raw score on the Inattentive/Overactive subscale of the IOWA Conners Rating Scale.
Severity of ADHD symptoms encompassing the past six months was also determined dimensionally by self- and parent-ratings on a 4-point scale made-up of all 18 DSM-IV Inattentive and Hyperactive-Impulsive items. Possible scores for each item ranged from 0 (“not at all”) to 3 (“very much”). Parent- and self- reports of competence and problem items were also gathered using three-point response scales (CBCL: Achenbach, 1991a; YSR: Achenbach, 1991b). Not surprisingly, persisters and partial remitters differed significantly on all parent- and self-reports of ADHD-related behaviors obtained at follow-up.
Neuropsychological measures
Stimulus and Response Conflict Tasks (Nassauer & Halperin, 2003)
The SRCT consisted of five computerized tasks designed to evaluate the ability to inhibit inappropriate motor responses and/or ignore irrelevant stimulus characteristics. The SRCT was administered individually in the context of a comprehensive day-long neuropsychological assessment that included the WAIS, WIAT, CPT, Stroop (see Halperin, et al., 2008). The SRCT was always administered at the same point in this battery (i.e., in the morning), and the SCT task always preceded the RCT task. Evaluators were blind to group status.
Participants used the index and middle fingers of their dominant hand to respond. For right-handed individuals, the index finger indicated a left response and the middle finger indicated a right response; the opposite was the case for left-handed individuals. The response device was a standard two-button mouse for use with a desktop personal computer. Trials were randomized in terms of right/left responses so handedness should not have affected performance. Participants were encouraged to respond as quickly as possible without sacrificing accuracy. The primary dependent measures were the mean reaction time for correct responses (mean RT) and standard deviation of reaction time for correct responses (SDRT; both recorded in milliseconds) for each condition. In addition, the percent of correct responses out of the total number of valid trials was calculated for each condition as a measure of accuracy, although due to the intentional simplicity of the task, few errors were anticipated.
Perceptual Inhibition (Conditions 1–3)
The first three conditions of the SRCT were designed to assess perceptual inhibition/interference control. Condition 1 consisted of 40 randomized trials in which a left- (20 trials) or right- (20 trials) pointing arrow appeared in the middle of the screen. The participant was instructed to press either the left or right mouse button depending upon where the arrow was pointing; thus, the required response was in the direction of centrally displayed arrows (control condition). Condition 2 consisted of 40 trials in which a rectangular box appeared randomly either on the left (20 trials) or right (20 trials) side of the computer monitor. The participant was instructed to press the button that was located on the same side as the rectangle. Data from Condition 2 were not used in analyses; rather, the purpose was to establish stimulus location as the prepotent response (location condition). Condition 3 consisted of 80 randomized trials in which there was a left- (40 trials) or right- (40 trials) pointing arrow that appeared randomly on either the left (40 trials) or right (40 trials) side of the monitor. The participant was instructed to ignore the location of the arrow and to respond to the direction to which the arrow pointed (incompatible stimulus-response condition). Thus, this condition entailed the suppression of interference from task-irrelevant location information that previous data indicated were more salient than direction information (Marks, et al., 2005; Nassauer & Halperin, 2003; Schulz, et al., 2005).
Motor Inhibition (Conditions 4–5)
The ability to suppress a prepotent motor response was measured using a computerized two-choice, self-paced RT task that consisted of a total of 80 trials administered in two conditions of varying levels of competition between motor responses. The conditions were either no conflict (i.e., condition 4) or conflict (i.e., condition 5). In Condition 4, centrally located left- or right-pointing arrows appeared on the screen and participants responded with the mouse button compatible with the direction indicated by the arrow (compatible stimulus-response condition). In Condition 5, participants responded with the mouse button incompatible with the direction indicated by the arrow (e.g., if the arrow pointed to the right, the left button was pressed; incompatible stimulus-response condition). Thus, all trials in Condition 5 involved competition between the prepotent and correct responses. Each of these conditions consisted of 40 randomized trials with a 50% probability of either a left- or right-pointing stimulus.
Statistical Methods
Data Preparation
Due to equipment failure or data recovery difficulties, data from conditions 1 and 3 (perceptual inhibition) were unavailable for 8 participants (3 ADHD-in childhood; 5 Never-ADHD) and data from conditions 4 and 5 (motor inhibition) were unavailable for 2 participants (both ADHD-in childhood). The RT from the first trial for each condition was excluded from the analyses as this trial primarily involved adapting to the new condition and may have been influenced by a transient task adjustment process (as evidenced by the longer RTs for these first trials compared to the remaining trials within a condition)(see Geurts, et al., 2008 for similar methodology). For each participant, data from 39/40 trials was examined for Conditions 1, 4 and 5 and data from 79/80 trials was included for Condition 3. This resulted in excluding 676/33,680 (2.0%) trials, leaving 33,004 possible trials for analysis.
There was variability in performance across the different task components, with some individuals displaying extreme performance on individual tasks but performing within a normal range on others. Similar to the methods used by Krusch et al.(1996), the selection of valid trials for each of the seven conditions was constrained to control for the effects of extraneous variables on correct RTs (e.g., random, unintentional, or impulsive responding, lapses in attention or effort lasting several trials). First, RTs were only included if they were between 200 ms (i.e., below which our participants were empirically determined to perform at chance levels) and 2,000 ms (i.e., the RT representing the 90th percentile of all correct responses). This resulted in excluding 392/33,004 (1.2%) of total possible trials, leaving 32,612 trials. Although these outliers occurred in both the ADHD-in childhood and Never-ADHD groups, there was a greater proportion of invalid trials in the ADHD-in childhood group (307/16,934; 1.8%) compared to the Never-ADHD group (85/16,070; 0.50%) (χ2 (1, 33004) = 115.82, p < 0.001). This is consistent with a large literature suggesting that reaction time variability in individuals with ADHD is accounted for by outliers (Di Martino, et al., 2008; Geurts, et al., 2008; Williams, Strauss, Hultsch, Hunter, & Tannock, 2007).
In order to ensure that an adequate number of valid trials were being used in the calculation of dependent variables (mean RT and SDRT) for each condition, we excluded data from individual conditions in which the [remaining] number of valid trials, having RTs >200ms and <2,000ms, was less than 75% of the total number of possible trials (i.e., fewer than 30/39 trials for Conditions 1, 4 and 5; and fewer than 60/79 trials for Condition 3). This resulted in excluding data from 7/589 (1.2%) SRCT conditions across the whole sample, all of which were from the childhood ADHD group.
Accuracy of performance within each of the four conditions for each participant was then calculated by dividing the number of trials in which a correct response was executed by the total number of valid trials (i.e., the number of trials with RTs >200ms and <2000ms). Data were excluded from further analyses for situations in which accuracy within a condition was less than 75%. This resulted in excluding data from 10/582 (1.7%) of the conditions with an adequate number of valid trials, seven of which were in Condition 3 (2 Never-ADHD; 5 ADHD-in childhood) and three of which were in Condition 5 (all ADHD-in childhood).
Data Analyses
Mean RT within each condition served as the primary measure of response speed; variability in response performance was estimated by RTSD. Accuracy of performance was assessed in each condition using percent correct responses.
Group differences in perceptual and motor inhibition were tested using two-way mixed analyses of variance (ANOVA) with perceptual inhibition (incompatible-condition 3 vs. compatible-condition 1) and motor inhibition for direction (incompatible-condition 5 vs. compatible-condition 4) entered separately as the within-subjects factor, and group (Never-ADHD, ADHD-partial remitters, ADHD-persisters) as a between-subjects factor for mean RT, RTSD, and accuracy. Post hoc analyses were carried out to examine specific relationships. Partial eta-squared (partial η2) was used to estimate effect sizes: 0.01 is considered a small effect, 0.06 is considered a medium effect, and 0.14 is considered a large effect (Stevens, 2002). For these analyses, a main effect for Condition, with poorer performance in the incompatible conditions, would provide support for the validity of the task manipulation; and a main effect for Group, would suggest overall, potentially non-specific, differences between the groups that would be consistent with a global, perhaps state regulation deficit. Significant Group × Condition interactions would provide support for the hypothesis that the dependent measure is being specifically affected by a deficit in inhibitory control.
In addition, Pearson’s product-moment correlations were used to dimensionally examine the association between inhibitory control indices and current ADHD clinical symptom severity for participants with childhood ADHD. Current ADHD severity was indexed by calculating a single factor score for ADHD symptoms endorsed at the time of the SRCT task administration based on the self and parent reported scores on the DSM-IV 18 item ADHD checklist. For each participant, the total number of ADHD symptoms was computed by adding the number of ADHD symptoms endorsed by either parent or self report. Difference scores to index perceptual inhibition and motor inhibition were computed by subtracting performance on the corresponding control (compatible stimuli) task from performance on the conflict (incompatible stimuli) task for mean RT and for RTSD. For accuracy, difference scores were taken by subtracting conflict from control conditions. This was done for consistency: for all three indices of task performance, the larger the positive difference score, the greater the negative impact of conflict. Statistical significance was determined by using a significant p-value of 0.0083 adjusted for False Discovery Rate (Benjamini & Hochberg, 1995) and all probabilities were based on two-tailed tests.
RESULTS
Perceptual Inhibition
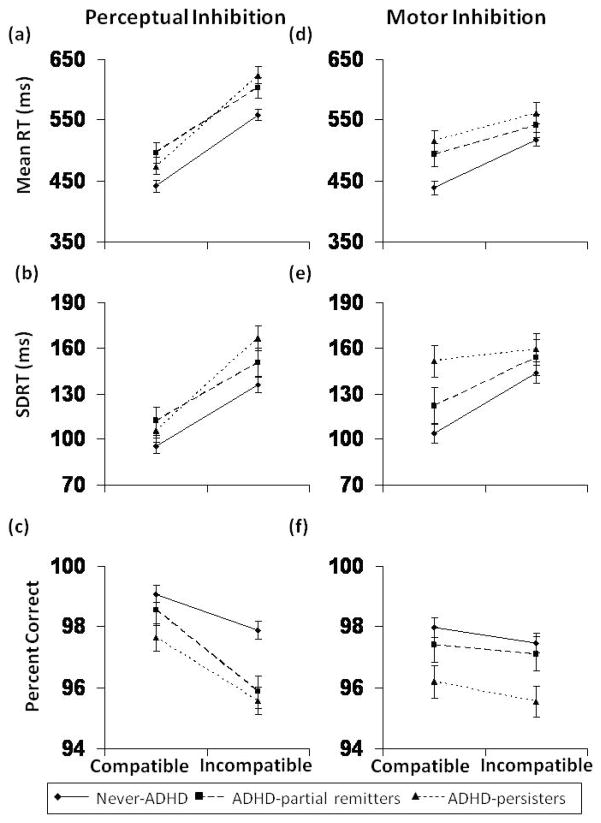
For perceptual inhibition, significant main effects of Condition were identified, such that participants were less accurate (F(1, 134) = 24.88, p < 0.001, partial η2 = 0.16), more variable (F(1, 134) = 59.07, p < 0.001, partial η2 = 0.31), and slower (F(1, 134) = 335.14, p < 0.001, partial η2 = 0.71) on the incompatible task (condition 3) relative to the compatible task (condition 1), regardless of group (see Figure 1a–c). In addition, significant main effects for Group status were also observed for two of the three dependent measures (Mean RT: F(2, 134) = 5.69, p = 0.004, partial η2 = 0.08; SDRT: F(2, 134) = 2.54, p = 0.08, partial η2 = 0.04; Accuracy: F(2, 134) = 6.86, p = 0.001, partial η2 = 0.09). The lack of a statistically significant difference in SDRT may be due to the fact that those with childhood ADHD had more “outlier” responses removed (i.e., < 200 msec or > 2000 msec) relative to the never-ADHD comparison group, thus, artificially deflating their measured SDRT. Post-hoc analyses revealed that, compared to the Never-ADHD comparison group, the ADHD-persisters were slower (F(1, 110) = 8.17, p = 0.005, partial η2 = 0.07), more variable (F(1, 110) = 4.95, p = 0.03, partial η2 = 0.04), and less accurate (F(1, 110) = 14.20, p < 0.001, partial η2 = 0.11). Further, compared to the Never-ADHD group, the ADHD-partial remitters were significantly slower (F(1, 101) = 6.92, p = 0.01, partial η2 = 0.06) and less accurate (F(1, 110) = 5.54, p = 0.02, partial η2 = 0.05). There were no significant overall Group differences between the ADHD-partial remitters and persisters. In addition, there was a significant Group × Condition interaction for reaction time (F(2, 134) = 3.24, p = 0.04, partial η2 = 0.05). Posthoc analyses showed that this was accounted for by increased slowing during the incompatible condition for the ADHD-persisters relative to both the ADHD-partial remitters (F(1, 57) = 4.74, p = 0.03, partial η2 = 0.08) and the never-ADHD group (F(1, 110) = 4.45, p = 0.04, partial η2 = 0.04) (see Figure 1a). However, there were not significant Group × Condition interactions for variability or accuracy (SDRT: F(2, 134) = 1.42, p = 0.25, partial η2 = 0.02; Accuracy: F(2, 134) = 1.44, p = 0.24, partial η2 = 0.02).
Fig. 1.
Performance on the SRCT task by ADHD group (Never-ADHD, ADHD-partial remitters, ADHD-persisters): Perceptual inhibition performance for (a) response speed (RT), (b) response variability (SDRT), and (c) accuracy (% correct). Motor inhibition performance for (d) response speed, (e) response variability, and (f) accuracy.
Motor Inhibition
As indicated by main effects for Condition, performance on the incompatible direction task (condition 5) was more variable (F(1, 144) = 14.72, p < 0.001, partial η2 = 0.09) and slower (F(1, 144) = 83.51, p < 0.001, partial η2 = 0.37) than performance on the compatible direction task (condition 4) across the whole sample, whereas accuracy did not differ between compatible and incompatible conditions (F(1, 144) = 2.00, p = 0.16, partial η2 = 0.01) (Figure 1d–f). There were main effects of group for accuracy (F(2, 144) = 4.64, p = 0.01, partial η2 = 0.06), variability (F(2, 144) = 3.13, p =0.05, partial η2 = 0.04), and speed (F(2, 144) = 4.97, p = 0.008, partial η2 = 0.07). Post-hoc analyses showed that, compared to the Never-ADHD group, the ADHD-persisters were less accurate (F(1, 117) = 8.88, p = 0.004, partial η2 = 0.07), more variable (F(1, 117) = 6.86, p = 0.01, partial η2 = 0.06), and slower (F(1, 117) = 8.61, p = 0.004, partial η2 = 0.07); the ADHD-partial remitters only had slower response speed (F(1, 111) = 4.03, p = 0.05, partial η2 = 0.04). In addition, there was a Group × Condition interaction for mean RT (F(2, 144) = 4.09, p = 0.02, partial η2 = 0.05). As evidenced by the different slopes, the never-ADHD group showed greater slowing in response to incompatible direction compared to both the ADHD-partial remitters (F(1, 111) = 5.00, p = 0.03, partial η2 = 0.04) and the ADHD-persisters (F(1, 117) = 5.56, p = 0.02, partial η2 = 0.05) (Figure 1d). There was no significant interaction of Condition × Group on variability (F(2, 144) = 2.24, p = 0.11, partial η2 = 0.03) or accuracy (F(2, 144) = 0.07, p = 0.93, partial η2 = 0.001) of performance (Figure 1e–f).
Dimensional Analyses
Using a more dimensional approach in those with childhood ADHD, high levels of ADHD symptoms at follow-up were positively correlated with the effects of perceptual inhibition on mean RT (r = 0.21, p = 0.008) such that a greater number of ADHD symptoms endorsed was associated with more slowing on the stimulus incompatible task relative to the stimulus compatible task. There were no significant associations between ADHD symptom count and changes in RTSD (r = 0.07, p = 0.37) or in accuracy (r= 0.04, p = 0.59) associated with greater conflict. For the motor inhibition task, ADHD symptom count was not significantly associated with changes in any aspect of performance as a function of increasing conflict (mean RT: r = −0.03, p = 0.68; RTSD: r = 0.003, p = 0.96; Accuracy: r = −0.11, p = 0.15).
DISCUSSION
Results from this study demonstrate that the SRCT worked as intended, providing separate measures of early perceptual (i.e., susceptibility to interference) and late motor inhibition (i.e., inhibition of prepotent responding) using a series of reaction time tasks that use the same stimuli, responses, and paired control conditions that differ only in the context of the stimulus-response associations. Significant main effects for condition were identified for both the perceptual and motor conflict tasks in the SRCT, such that participants, irrespective of ADHD status, performed worse on the incompatible conflict conditions compared with the compatible control conditions. Such patterns support the validity of the task manipulations in that the paired conditions were designed to be identical except for the greater inhibitory control requirements of the stimulus-response incompatible conditions. These findings replicate previous data generated using the SRCT (Marks, et al., 2005; Nassauer & Halperin, 2003).
The adolescents who were diagnosed with ADHD in childhood responded slower, more variably and less accurately on virtually all measures relative to the participants who were never diagnosed with ADHD. However, the weaker performance by the ADHD-in childhood group was consistently evident across control (i.e., low inhibitory control demands) and conflict (i.e., high demand for inhibitory control) conditions. Thus, the overall weaker performance of the ADHD-in childhood adolescents on the SRCT cannot be specifically attributed to impairments in perceptual or motor inhibition, per se. These results are in line with those from previous studies demonstrating a lack of impairment in interference control and response competition in individuals with ADHD, when baseline sensory and motor performance is taken into account (e.g., Berwid, et al., 2005; Engelhardt, Nigg, Carr, & Ferreira, 2008; Lampe, et al., 2007; Marks, et al., 2005; Rommelse, et al., 2007). Rather, the global deficits seen in individuals with childhood-ADHD may reflect difficulties in the cooperative work of multiple low-level, state-regulation systems. According to the non-optimal activation state regulation hypothesis in ADHD (Sergeant, Oosterlaan, & van der Meere, 1999; van der Meere, 1996) an inhibition deficit might reflect just one aspect of a broader cognitive deficit. Children with ADHD might not have response inhibition deficits per se, but may be unable to modulate their energetic state (i.e., levels of arousal and activation) according to task and situational demands, and be easily under- or overactivated resulting in poor response inhibition and impaired motor processing. Extensive research supports this hypothesis (Banaschewski, et al., 2004; Scheres, Oosterlaan, & Sergeant, 2001; Sergeant, et al., 1999; Sergeant & Van der Meere, 1994; van der Meere, 1996). While a state regulation model would predict a pattern of diffuse, nonspecific impairments and as such the findings are consistent with that model, these data certainly do not prove it. A similar pattern of diffuse, non-specific impairments could potentially result from an array of brain defects that affect neurotransmission such as white matter anomalies (e.g., Hamilton, et al., 2008; Silk, Vance, Rinehart, Bradshaw, & Cunnington, 2009), or from impairments more closely linked to impaired reward-related motivational systems (Sonuga-Barke, De Houwer, De Ruiter, Ajzenstzen, & Holland, 2004). Nevertheless, difficulties in these basic regulatory abilities have consistently been shown in individuals with ADHD (e.g., Kuntsi, Oosterlaan, & Stevenson, 2001; Sergeant, 2000; Sikstrom & Soderlund, 2007; van der Meere, Gunning, & Stemerdink, 1996; Zentall & Meyer, 1987).
Many studies have examined inhibitory control and other aspects of neuropsychological functioning in adolescents and adults diagnosed with ADHD (e.g., Boonstra, et al., 2005; Engelhardt, et al., 2008; Hervey, et al., 2004; King, Colla, Brass, Heuser, & von Cramon, 2007; Marchetta, Hurks, De Sonneville, Krabbendam, & Jolles, 2008; Murphy, Barkley, & Bush, 2001; Nigg, Butler, Huang-Pollock, & Henderson, 2002; Schoechlin & Engel, 2005; Schwartz & Verhaeghen, 2008; Wodushek & Neumann, 2003). These studies have provided important information regarding the neurocognitive correlates of the disorder as they manifest during these later developmental phases. Fewer have examined neuropsychological functioning specifically in longitudinal samples of adolescents and adults diagnosed with ADHD in childhood (e.g., Biederman, et al., 2008; Biederman, et al., 2007; Fischer, Barkley, Edelbrock, & Smallish, 1990; Fischer, Barkley, Smallish, & Fletcher, 2005; Seidman, Biederman, Faraone, Weber, & Ouellette, 1997), and even fewer (e.g., Carr, Nigg, & Henderson, 2006; Drechsler, Brandeis, Foldenyi, Imhof, & Steinhausen, 2005; Halperin, et al., 2008) have examined the degree to which inhibitory control and other neurocognitive deficits are specifically associated with symptom diminution over the lifespan. Most of these studies have in fact suggested that developmental factors such as age seem to be important in moderating the relationship between neurocognitive functioning and symptom stability in ADHD (e.g., Balint, et al., 2008). It is likely that such developmentally-focused data will help elucidate mechanisms associated with the highly diverse outcomes characteristic of ADHD.
A particular strength of this study is that the participants were not self-referred during adolescence/young adulthood; rather, they were comprehensively diagnosed with ADHD in childhood and prospectively followed. This is advantageous for several reasons. For one, retrospective report of childhood ADHD has been shown to be problematic (Mannuzza, Klein, Klein, Bessler, & Shrout, 2002). Another advantage is that we had an ADHD cohort that was relatively phenotypically homogeneous in childhood, but over time, became much more heterogeneous. This allowed us to study a group with variable degrees of ADHD-related symptomatology at follow-up as well as the natural trajectory of ADHD symptomatology. If we would have recruited a new 16–21 year-old ADHD cohort, we would have seen a much more restricted range of psychopathology. For example, those classified as ‘partial remitters’ in this study would not meet inclusion criteria for most studies of ADHD in adolescents or adults. As such, we were able to investigate the association of SRCT performance with a wide range of current ADHD symptom severity.
Notably, there were no differences in overall performance between persisters and partial remitters of ADHD, suggesting that overall regulatory ability was comparable across the groups despite differences in their ADHD-related functioning. Also, although there was little evidence to suggest that, beyond state regulation deficiencies, there are specific deficits in perceptual and motor inhibition between those with and without ADHD in childhood, there was some evidence from the perceptual inhibition task to suggest that ADHD-persisters had poorer interference control than both ADHD-partial remitters and Never-ADHD controls. Based on the reasoning of Carr, Nigg and Henderson (2006), perceptual inhibition deficits may be an epiphenomenon, and not a core characteristic of ADHD that parallels symptom recovery. Alternatively, as hypothesized by Halperin and Schulz (2006), it may be that improved top-down control, as evidenced by better perceptual inhibition, serves to moderate ADHD severity over development. It is important to note that these data cannot clarify whether this improved perceptual inhibition caused the diminution of ADHD symptom severity or was the result of it.
Although conflict during the motor inhibition component of the SRCT appeared to increase required effort, as indicated by increased reaction time, this measure yielded an unexpected result. Specifically, as compared to both ADHD-persisters and ADHD-partial remitters, the never-ADHD comparison group had greater slowing during the conflict condition. From an additive factors perspective (Sergeant, 1996; Sergeant, Geurts, Huijbregts, Scheres, & Oosterlaan, 2003; Sternberg, 1969), this would imply that the comparison group had more difficulty than the ADHD group with the effortful process of conflict resolution. While post hoc explanations of this unanticipated result must be considered tentative, we offer three possible explanations. First, it is possible that those in the comparison group demonstrated better self-regulation since they slowed down when the task became more challenging. However, the groups did not differ in error rates and those with childhood ADHD did not have a greater decrease in accuracy during the motor conflict condition. Another, potentially more important possibility is that the motor conflict task does not adequately challenge top-down executive control despite the overall slowing in RT. Not only were main effects for condition substantially smaller for motor inhibition as compared to perceptual inhibition, but the manipulation did not significantly alter error rates. This possibility is consistent with fMRI data which indicate that perceptual inhibition as measured using the SRCT resulted in activation of the ventrolateral prefrontal cortex in both probands and controls, however, motor inhibition did not increase prefrontal activation in either group (Schulz, et al., 2005). Thus, it is possible that the motor conflict portion of the SRCT is not challenging enough to engage effortful top-down executive processes. Finally, it is possible that the unexpected results were due to the fact that the ADHD-persisters and ADHD-partial remitters started out slower, and thus experienced some ceiling effect in terms of slow RT. Perhaps the comparison group simply had farther to fall in the incompatible condition because they were faster in the compatible condition than those with ADHD-in childhood. The seemingly inverse pattern of ADHD persisters showing increased slowing in the perceptual task and faster responses in the motor task in response to interference compared to the Never ADHD group may be related to the fact that the motor task was too easy, and does not really tap prefrontal processes as clearly as the perceptual task does (Schulz, et al., 2005).
The SRCT used in this study differs in many important ways from the go/no-go and stop tasks that have been more commonly used to study inhibition in individuals with ADHD. The SRCT measures the ability to resolve perceptual conflict among competing stimulus characteristics and motoric competition between prepotent incorrect and more effortful correct responses. The go/no-go and stop tasks measure the inhibition of prepotent incorrect and appropriately initiated ongoing responses, respectively. The motor inhibition component of the SRCT involves the inhibition of the prepotent (but incorrect) response, but unlike the go/no-go and stop tasks, it also requires the selection and execution of a competing response. Further, and perhaps more importantly, unlike the go/no-go and stop tasks, the SRCT includes a direct experimental manipulation which allows for greater isolation of the construct of interest. Had that manipulation not been employed, and only the conflict conditions administered to the participants, it would appear from our data that those with childhood ADHD have inhibitory control deficits rather than more generalized impairments.
These findings and conclusions must be viewed within the context of several limitations. First, despite considerable effort, the attrition rate of this sample from the childhood study is high, although available data suggest that our subsample was representative of the original group. Second, we do not have childhood data on the Never-ADHD control group since they were not recruited until the follow-up phase of the study. Third, the task order was not counterbalanced in that the perceptual inhibition (conditions 1–3) was always measured first, followed by motor inhibition for location (conditions 4–5). Accordingly, we cannot rule out potential practice/fatigue effects. In fact, this may account for the surprising impact of the incompatible motor inhibition condition of slowing responding speed in the Never-ADHD controls compared to those with ADHD in childhood, which was not in the anticipated direction. Lastly, the methods used are not a direct test of most variants of ADHD state regulation models and thus cannot be used to prove state regulation deficits in this study.
In spite of these shortcomings, these results have significant clinical and theoretical implications. Specific impairments in perceptual or motor inhibition were not observed in adolescents/young adults with childhood ADHD as evidenced by performance on the SRCT, a task that includes direct experimental manipulations which allow for greater isolation of the inhibition constructs. Had we only administered the conflict conditions to the participants without this manipulation, our data would have incorrectly suggested that those with childhood ADHD have inhibitory control deficits rather than more generalized impairments. Further, we would not have been able to demonstrate that these more generalized impairments persist into adulthood and are unrelated to symptom recovery. Although several studies have demonstrated similar, presumably state-regulation deficits in children and adults with ADHD, these data indicate that such deficits persist even when ADHD symptoms do not. Finally, there was some evidence to suggest that partial remitters had better interference control than persisters in adolescence/young adulthood. Further studies will be important in clarifying the dynamic relationship between ADHD symptom severity and this aspect of inhibitory control over development.
Acknowledgments
This research was supported by grants # RO1 MH046448 and RO1 MH060698 from NIMH to Jeffrey Halperin, and by a Canadian Institutes of Health Research Fellowship Award to Anne-Claude Bedard.
Footnotes
Three participants in the ADHD group were 15.55, 25.50, and 26.29 years-old at follow-up.
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/neu
References
- Achenbach TM. Manual for the Child Behavior Checklist 4/18 and 1991 Profile. Burlington: University of Vermont; 1991a. [Google Scholar]
- Achenbach TM. Manual for the Youth Self-Report and 1991 Profile. Burlington, VT: University of Vermont, Department of Psychiatry; 1991b. [Google Scholar]
- Albrecht B, Rothenberger A, Sergeant J, Tannock R, Uebel H, Banaschewski T. Interference control in attention-deficit/hyperactivity disorder: differential Stroop effects for colour-naming versus counting. J Neural Transm. 2008;115(2):241–247. doi: 10.1007/s00702-007-0818-1. [DOI] [PubMed] [Google Scholar]
- Alderson RM, Rapport MD, Kofler MJ. Attention-deficit/hyperactivity disorder and behavioral inhibition: a meta-analytic review of the stop-signal paradigm. J Abnorm Child Psychol. 2007;35(5):745–758. doi: 10.1007/s10802-007-9131-6. [DOI] [PubMed] [Google Scholar]
- Balint S, Czobor P, Komlosi S, Meszaros A, Simon V, Bitter I. Attention deficit hyperactivity disorder (ADHD): gender- and age-related differences in neurocognition. Psychol Med. 2008:1–9. doi: 10.1017/S0033291708004236. [DOI] [PubMed] [Google Scholar]
- Banaschewski T, Brandeis D, Heinrich H, Albrecht B, Brunner E, Rothenberger A. Questioning inhibitory control as the specific deficit of ADHD--evidence from brain electrical activity. J Neural Transm. 2004;111(7):841–864. doi: 10.1007/s00702-003-0040-8. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Bedard AC, Ickowicz A, Logan GD, Hogg-Johnson S, Schachar R, Tannock R. Selective inhibition in children with attention-deficit hyperactivity disorder off and on stimulant medication. J Abnorm Child Psychol. 2003;31(3):315–327. doi: 10.1023/a:1023285614844. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B. 1995;57(1):289–300. [Google Scholar]
- Berwid OG, Curko Kera EA, Marks DJ, Santra A, Bender HA, Halperin JM. Sustained attention and response inhibition in young children at risk for Attention Deficit/Hyperactivity Disorder. J Child Psychol Psychiatry. 2005;46(11):1219–1229. doi: 10.1111/j.1469-7610.2005.00417.x. [DOI] [PubMed] [Google Scholar]
- Biederman J, Monuteaux MC, Doyle AE, Seidman LJ, Wilens TE, Ferrero F, et al. Impact of executive function deficits and attention-deficit/hyperactivity disorder (ADHD) on academic outcomes in children. J Consult Clin Psychol. 2004;72(5):757–766. doi: 10.1037/0022-006X.72.5.757. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Doyle AE, Spencer T, Henderson CS, Marion B, et al. Stability of executive function deficits in girls with ADHD: a prospective longitudinal followup study into adolescence. Dev Neuropsychol. 2008;33(1):44–61. doi: 10.1080/87565640701729755. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Fried R, Doyle AE, Spencer T, Seidman LJ, et al. Stability of executive function deficits into young adult years: a prospective longitudinal follow-up study of grown up males with ADHD. Acta Psychiatr Scand. 2007;116(2):129–136. doi: 10.1111/j.1600-0447.2007.01008.x. [DOI] [PubMed] [Google Scholar]
- Bird HR, Gould MS, Staghezza B. Aggregating data from multiple informants in child psychiatry epidemiological research. J Am Acad Child Adolesc Psychiatry. 1992;31(1):78–85. doi: 10.1097/00004583-199201000-00012. [DOI] [PubMed] [Google Scholar]
- Bjorklund DF, Harnishfeger KK. The evolution of inhibition mechanisms and their role in human cognition and behavior. In: Dempster FN, Brainerd CJ, editors. Interference and inhibition in cognition. San Diego: Academic Press; 1995. pp. 142–169. [Google Scholar]
- Boonstra AM, Oosterlaan J, Sergeant JA, Buitelaar JK. Executive functioning in adult ADHD: a meta-analytic review. Psychol Med. 2005;35(8):1097–1108. doi: 10.1017/s003329170500499x. [DOI] [PubMed] [Google Scholar]
- Carr LA, Nigg JT, Henderson JM. Attentional versus motor inhibition in adults with attention-deficit/hyperactivity disorder. Neuropsychology. 2006;20(4):430–441. doi: 10.1037/0894-4105.20.4.430. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci. 2002;3(8):617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Crosbie J, Perusse D, Barr CL, Schachar RJ. Validating psychiatric endophenotypes: inhibitory control and attention deficit hyperactivity disorder. Neurosci Biobehav Rev. 2008;32(1):40–55. doi: 10.1016/j.neubiorev.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Ghaffari M, Curchack J, Reiss P, Hyde C, Vannucci M, et al. Decomposing intra-subject variability in children with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;64(7):607–614. doi: 10.1016/j.biopsych.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsler R, Brandeis D, Foldenyi M, Imhof K, Steinhausen HC. The course of neuropsychological functions in children with attention deficit hyperactivity disorder from late childhood to early adolescence. J Child Psychol Psychiatry. 2005;46(8):824–836. doi: 10.1111/j.1469-7610.2004.00384.x. [DOI] [PubMed] [Google Scholar]
- Engelhardt PE, Nigg JT, Carr LA, Ferreira F. Cognitive inhibition and working memory in attention-deficit/hyperactivity disorder. J Abnorm Psychol. 2008;117(3):591–605. doi: 10.1037/a0012593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Barkley RA, Edelbrock CS, Smallish L. The adolescent outcome of hyperactive children diagnosed by research criteria: II. Academic, attentional, and neuropsychological status. J Consult Clin Psychol. 1990;58(5):580–588. doi: 10.1037//0022-006x.58.5.580. [DOI] [PubMed] [Google Scholar]
- Fischer M, Barkley RA, Smallish L, Fletcher K. Executive functioning in hyperactive children as young adults: attention, inhibition, response perseveration, and the impact of comorbidity. Dev Neuropsychol. 2005;27(1):107–133. doi: 10.1207/s15326942dn2701_5. [DOI] [PubMed] [Google Scholar]
- Fisher PW, Shaffer D, Piacentini JC, Lapkin J, Kafantaris V, Leonard H, et al. Sensitivity of the Diagnostic Interview Schedule for Children, 2nd edition (DISC-2.1) for specific diagnoses of children and adolescents. J Am Acad Child Adolesc Psychiatry. 1993;32(3):666–673. doi: 10.1097/00004583-199305000-00026. [DOI] [PubMed] [Google Scholar]
- Geurts HM, Grasman RP, Verte S, Oosterlaan J, Roeyers H, van Kammen SM, et al. Intra-individual variability in ADHD, autism spectrum disorders and Tourette’s syndrome. Neuropsychologia. 2008;46(13):3030–3041. doi: 10.1016/j.neuropsychologia.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Newcorn JH, Schwartz ST, Sharma V, Siever LJ, Koda VH, et al. Age-related changes in the association between serotonergic function and aggression in boys with ADHD. Biol Psychiatry. 1997;41(6):682–689. doi: 10.1016/S0006-3223(96)00168-0. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Schulz KP. Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychol Bull. 2006;132(4):560–581. doi: 10.1037/0033-2909.132.4.560. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Trampush JW, Miller CJ, Marks DJ, Newcorn JH. Neuropsychological outcome in adolescents/young adults with childhood ADHD: profiles of persisters, remitters and controls. J Child Psychol Psychiatry. 2008;49(9):958–966. doi: 10.1111/j.1469-7610.2008.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton LS, Levitt JG, O’Neill J, Alger JR, Luders E, Phillips OR, et al. Reduced white matter integrity in attention-deficit hyperactivity disorder. Neuroreport. 2008;19(17):1705–1708. doi: 10.1097/WNR.0b013e3283174415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervey AS, Epstein JN, Curry JF. Neuropsychology of adults with attention-deficit/hyperactivity disorder: a meta-analytic review. Neuropsychology. 2004;18(3):485–503. doi: 10.1037/0894-4105.18.3.485. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- King JA, Colla M, Brass M, Heuser I, von Cramon D. Inefficient cognitive control in adult ADHD: evidence from trial-by-trial Stroop test and cued task switching performance. Behav Brain Funct. 2007;3:42. doi: 10.1186/1744-9081-3-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusch DA, Klorman R, Brumaghim JT, Fitzpatrick PA, Borgstedt AD, Strauss J. Methylphenidate slows reactions of children with attention deficit disorder during and after an error. J Abnorm Child Psychol. 1996;24(5):633–650. doi: 10.1007/BF01670104. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Oosterlaan J, Stevenson J. Psychological mechanisms in hyperactivity: I. Response inhibition deficit, working memory impairment, delay aversion, or something else? J Child Psychol Psychiatry. 2001;42(2):199–210. [PubMed] [Google Scholar]
- Lampe K, Konrad K, Kroener S, Fast K, Kunert HJ, Herpertz SC. Neuropsychological and behavioural disinhibition in adult ADHD compared to borderline personality disorder. Psychol Med. 2007;37(12):1717–1729. doi: 10.1017/S0033291707000517. [DOI] [PubMed] [Google Scholar]
- Lansbergen MM, Kenemans JL, van Engeland H. Stroop interference and attention-deficit/hyperactivity disorder: a review and meta-analysis. Neuropsychology. 2007;21(2):251–262. doi: 10.1037/0894-4105.21.2.251. [DOI] [PubMed] [Google Scholar]
- Lijffijt M, Kenemans JL, Verbaten MN, van Engeland H. A meta-analytic review of stopping performance in attention-deficit/hyperactivity disorder: deficient inhibitory motor control? J Abnorm Psychol. 2005;114(2):216–222. doi: 10.1037/0021-843X.114.2.216. [DOI] [PubMed] [Google Scholar]
- Loney J, Milich R. Hyperactivity, inattention, and aggression in clinical practice. Advances in Developmental and Behavioral Pediatrics. 1982;3:113–147. [Google Scholar]
- Loo SK, Humphrey LA, Tapio T, Moilanen IK, McGough JJ, McCracken JT, et al. Executive functioning among Finnish adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1594–1604. doi: 10.1097/chi.0b013e3181575014. [DOI] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Klein DF, Bessler A, Shrout P. Accuracy of adult recall of childhood attention deficit hyperactivity disorder. Am J Psychiatry. 2002;159(11):1882–1888. doi: 10.1176/appi.ajp.159.11.1882. [DOI] [PubMed] [Google Scholar]
- Marchetta ND, Hurks PP, De Sonneville LM, Krabbendam L, Jolles J. Sustained and focused attention deficits in adult ADHD. J Atten Disord. 2008;11(6):664–676. doi: 10.1177/1087054707305108. [DOI] [PubMed] [Google Scholar]
- Marks DJ, Berwid OG, Santra A, Kera EC, Cyrulnik SE, Halperin JM. Neuropsychological correlates of ADHD symptoms in preschoolers. Neuropsychology. 2005;19(4):446–455. doi: 10.1037/0894-4105.19.4.446. [DOI] [PubMed] [Google Scholar]
- Murphy KR, Barkley RA, Bush T. Executive functioning and olfactory identification in young adults with attention deficit-hyperactivity disorder. Neuropsychology. 2001;15(2):211–220. doi: 10.1037//0894-4105.15.2.211. [DOI] [PubMed] [Google Scholar]
- Nakao K, Treas J. Updating occupational prestige and socioeconomic scores: how the new measures measure up. Sociological Methods & Research. 1994;24:1–72. [Google Scholar]
- Nassauer KW, Halperin JM. Dissociation of perceptual and motor inhibition processes through the use of novel computerized conflict tasks. J Int Neuropsychol Soc. 2003;9(1):25–30. doi: 10.1017/s1355617703910034. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Is ADHD a disinhibitory disorder? Psychol Bull. 2001;127(5):571–598. doi: 10.1037/0033-2909.127.5.571. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Butler KM, Huang-Pollock CL, Henderson JM. Inhibitory processes in adults with persistent childhood onset ADHD. J Consult Clin Psychol. 2002;70(1):153–157. doi: 10.1037//0022-006x.70.1.153. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJ. Causal heterogeneity in attention-deficit/hyperactivity disorder: do we need neuropsychologically impaired subtypes? Biol Psychiatry. 2005;57(11):1224–1230. doi: 10.1016/j.biopsych.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Oosterlaan J, Logan GD, Sergeant JA. Response inhibition in AD/HD, CD, comorbid AD/HD + CD, anxious, and control children: a meta-analysis of studies with the stop task. J Child Psychol Psychiatry. 1998;39(3):411–425. [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. J Child Psychol Psychiatry. 1996;37(1):51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Pritchard VE, Neumann E, Rucklidge JJ. Interference and negative priming effects in adolescents with attention deficit hyperactivity disorder. Am J Psychol. 2007;120(1):91–122. [PubMed] [Google Scholar]
- Rommelse NN, Altink ME, de Sonneville LM, Buschgens CJ, Buitelaar J, Oosterlaan J, et al. Are motor inhibition and cognitive flexibility dead ends in ADHD? J Abnorm Child Psychol. 2007;35(6):957–967. doi: 10.1007/s10802-007-9146-z. [DOI] [PubMed] [Google Scholar]
- Rucklidge JJ, Tannock R. Neuropsychological profiles of adolescents with ADHD: effects of reading difficulties and gender. J Child Psychol Psychiatry. 2002;43(8):988–1003. doi: 10.1111/1469-7610.00227. [DOI] [PubMed] [Google Scholar]
- Scheres A, Oosterlaan J, Sergeant JA. Response execution and inhibition in children with AD/HD and other disruptive disorders: the role of behavioural activation. J Child Psychol Psychiatry. 2001;42(3):347–357. [PubMed] [Google Scholar]
- Schoechlin C, Engel RR. Neuropsychological performance in adult attention-deficit hyperactivity disorder: meta-analysis of empirical data. Arch Clin Neuropsychol. 2005;20(6):727–744. doi: 10.1016/j.acn.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Schulz KP, Tang CY, Fan J, Marks DJ, Newcorn JH, Cheung AM, et al. Differential prefrontal cortex activation during inhibitory control in adolescents with and without childhood attention-deficit/hyperactivity disorder. Neuropsychology. 2005;19(3):390–402. doi: 10.1037/0894-4105.19.3.390. [DOI] [PubMed] [Google Scholar]
- Schwartz K, Verhaeghen P. ADHD and Stroop interference from age 9 to age 41 years: a meta-analysis of developmental effects. Psychol Med. 2008;38(11):1607–1616. doi: 10.1017/S003329170700267X. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Biederman J, Faraone SV, Weber W, Ouellette C. Toward defining a neuropsychology of attention deficit-hyperactivity disorder: performance of children and adolescents from a large clinically referred sample. J Consult Clin Psychol. 1997;65(1):150–160. doi: 10.1037/0022-006X.65.1.150. [DOI] [PubMed] [Google Scholar]
- Sergeant J. A theory of attention: An information processing perspective. In: Lyon GR, Krasnegor NA, editors. Attention, memory, and executive function. Baltimore, MD: Paul H. Brookes Publishing Co; 1996. pp. 57–69. [Google Scholar]
- Sergeant J. The cognitive-energetic model: an empirical approach to attention-deficit hyperactivity disorder. Neurosci Biobehav Rev. 2000;24(1):7–12. doi: 10.1016/s0149-7634(99)00060-3. [DOI] [PubMed] [Google Scholar]
- Sergeant J, Geurts H, Huijbregts S, Scheres A, Oosterlaan J. The top and the bottom of ADHD: a neuropsychological perspective. Neurosci Biobehav Rev. 2003;27(7):583–592. doi: 10.1016/j.neubiorev.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Sergeant J, Geurts H, Oosterlaan J. How specific is a deficit of executive functioning for attention-deficit/hyperactivity disorder? Behav Brain Res. 2002;130(1–2):3–28. doi: 10.1016/s0166-4328(01)00430-2. [DOI] [PubMed] [Google Scholar]
- Sergeant JA, Oosterlaan J, van der Meere JJ. Information processing and energetic factors in attention-deficit/hyperactivity disorder. In: Quay HC, Hogan A, editors. Handbook of Disruptive Behavior Disorders. New York: Plenum Press; 1999. pp. 75–104. [Google Scholar]
- Sergeant JA, Van der Meere J. Toward an empirical child psychopathology. In: Routh DK, editor. Disruptive behavior disorders in children. New York: Plenum Press; 1994. pp. 59–85. [Google Scholar]
- Shaffer D, Fisher P, Dulcan MK, Davies M, Piacentini J, Schwab-Stone ME, et al. The NIMH Diagnostic Interview Schedule for Children Version 2.3 (DISC-2.3): description, acceptability, prevalence rates, and performance in the MECA Study. Methods for the Epidemiology of Child and Adolescent Mental Disorders Study. J Am Acad Child Adolesc Psychiatry. 1996;35(7):865–877. doi: 10.1097/00004583-199607000-00012. [DOI] [PubMed] [Google Scholar]
- Sikstrom S, Soderlund G. Stimulus-dependent dopamine release in attention-deficit/hyperactivity disorder. Psychol Rev. 2007;114(4):1047–1075. doi: 10.1037/0033-295X.114.4.1047. [DOI] [PubMed] [Google Scholar]
- Silk TJ, Vance A, Rinehart N, Bradshaw JL, Cunnington R. White-matter abnormalities in attention deficit hyperactivity disorder: a diffusion tensor imaging study. Hum Brain Mapp. 2009;30(9):2757–2765. doi: 10.1002/hbm.20703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, De Houwer J, De Ruiter K, Ajzenstzen M, Holland S. AD/HD and the capture of attention by briefly exposed delay-related cues: evidence from a conditioning paradigm. J Child Psychol Psychiatry. 2004;45(2):274–283. doi: 10.1111/j.1469-7610.2004.00219.x. [DOI] [PubMed] [Google Scholar]
- Sternberg S. Discovery of processing stages: Extensions of Donders’ method. In: Koster WG, editor. Attention and performance II. Amsterdam: 1969. pp. 276–315. [Google Scholar]
- Stevens JP. Applied multivariate statistics for the social sciences. Mahwah, New Jersey: Lawrence Erlbaum Associates; 2002. [Google Scholar]
- Tannock R. Attention deficit hyperactivity disorder: advances in cognitive, neurobiological, and genetic research. J Child Psychol Psychiatry. 1998;39(1):65–99. [PubMed] [Google Scholar]
- van der Meere J, Gunning WB, Stemerdink N. Changing a response set in normal development and in ADHD children with and without tics. J Abnorm Child Psychol. 1996;24(6):767–786. doi: 10.1007/BF01664739. [DOI] [PubMed] [Google Scholar]
- van der Meere JJ. The role of inattention in hyperactivity disorders. In: Sandberg S, editor. Monographs on child and adolescent psychiatry: Hyperactivity disorders. Cambridge, England: Cambridge University Press; 1996. pp. 109–146. [Google Scholar]
- van Mourik R, Oosterlaan J, Sergeant JA. The Stroop revisited: a meta-analysis of interference control in AD/HD. J Child Psychol Psychiatry. 2005;46(2):150–165. doi: 10.1111/j.1469-7610.2004.00345.x. [DOI] [PubMed] [Google Scholar]
- van Mourik R, Papanikolau A, van Gellicum-Bijlhout J, van Oostenbruggen J, Veugelers D, Post-Uiterweer A, et al. Interference control in children with attention deficit/hyperactivity disorder. J Abnorm Child Psychol. 2009;37(2):293–303. doi: 10.1007/s10802-008-9277-x. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57(11):1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Williams BR, Strauss EH, Hultsch DF, Hunter MA, Tannock R. Reaction time performance in adolescents with attention deficit/hyperactivity disorder: evidence of inconsistency in the fast and slow portions of the RT distribution. J Clin Exp Neuropsychol. 2007;29(3):277–289. doi: 10.1080/13803390600678020. [DOI] [PubMed] [Google Scholar]
- Wodushek TR, Neumann CS. Inhibitory capacity in adults with symptoms of Attention Deficit/Hyperactivity Disorder (ADHD) Arch Clin Neuropsychol. 2003;18(3):317–330. [PubMed] [Google Scholar]
- Zentall SS, Meyer MJ. Self-regulation of stimulation for ADD-H children during reading and vigilance task performance. J Abnorm Child Psychol. 1987;15(4):519–536. doi: 10.1007/BF00917238. [DOI] [PubMed] [Google Scholar]