Abstract
Ontogenetic studies using a social interaction paradigm have shown that adolescent rats are less sensitive to anxiolytic properties of acute ethanol than their adult counterparts. It is not known, however, whether adaptations to these anxiolytic effects upon repeated experiences with ethanol would be similar in adolescents and adults. The present study investigated sensitivity to the anxiolytic effects of ethanol in adolescent and adult male and female Sprague-Dawley rats following 7 days of exposure [postnatal day (P) 27–33 for adolescents and P62–68 for adults] to 1 g/kg ethanol or saline (i.p.), as well as in animals left non-manipulated during this time. Anxiolytic effects of ethanol (0, 0.75, 1.0, 1.25, and 1.5 g/kg for adolescents and 0, 0.25, 0.5, 0.75, 1.0, and 1.25 g/kg for adults in Experiments 1 and 2, respectively) were examined 48 hours after the last exposure using a modified social interaction test under unfamiliar test circumstances. At both ages, repeated ethanol exposure resulted in the development of apparent sensitization to anxiolytic effects of ethanol indexed via enhancement of social investigation and transformation of social avoidance into social indifference or preference, as well as expression of tolerance to the socially inhibiting effects induced by higher ethanol doses. Evidence for the emergence of sensitization in adults and tolerance at both ages was seen not only following chronic ethanol, but also after chronic saline exposure, suggesting that chronic manipulation per se may be sufficient to alter the sensitivity of both adolescents and adults to socially-relevant effects of ethanol.
Keywords: Adolescence, Anxiety, Ethanol, Rat, Social Interactions
Introduction
Adolescence is a time of acquiring new social skills for survival away from parents. This gradual transformation from immaturity and dependence to maturity and independence is a developmental phase that can be identified across different mammalian species, with adolescents sometimes differing dramatically from those younger or older in the ways they respond to and interact with stimuli in their environment (Spear, 2000). It has been suggested that human adolescents experience more stressors and negative life events than children and adults (Buchanan et al., 1992; Larson and Asmussen, 1991). The developmental transition from immaturity toward maturity itself can be stressful, especially if the capacity of adolescents to cope with different environmental and social challenges is overburdened by the magnitude and speed of adolescent-associated neural, behavioral, and hormonal changes (Collins, 2001; Davis, 2003; Jessor, 1993). Given the number of different stressors to which adolescents may be routinely exposed, it is not surprising that human adolescents consume alcohol in part for coping reasons (Cooper et al., 2000). Indeed, adolescents who expect alcohol to alleviate their anxiety and to relieve their personal problems are especially likely to engage in heavy and problem drinking (Bates and Labouvie, 1997; Kuntsche et al., 2005; Montgomery et al., 1993). Therefore, anxiolytic effects of ethanol may contribute to high levels of alcohol use during adolescence.
High levels of ethanol use are pervasive during adolescence, with approximately 11% of 8th graders, 22% of 10th graders and 25% of high school seniors reporting a binge pattern of drinking (i.e., consumption of 5 or more drinks per occasion) in the last 2 weeks (Johnston et al., 2007). Even more elevated rates of binge drinking are reported among adolescents in many European countries (Ahlström and Osterberg, 2005). High levels of ethanol consumption are not restricted to human adolescents but may be seen in other mammalian species as well. For instance, adolescent rats typically drink 2–3 times more ethanol relative to their body weights than do adults (Brunell and Spear, 2005; Doremus et al., 2005; Vetter et al., 2007). Adolescents may be able to sustain relatively large ethanol intakes due to their relative insensitivity to particular adverse and incapacitating effects that may serve as cues to terminate drinking, as well as to certain seemingly desirable consequences of ethanol (Spear, 2000; Spear and Varlinskaya, 2005). Adverse effects of ethanol to which adolescents are less sensitive than adults include ethanol-induced motor impairment (Hollstedt et al., 1980; Silveri and Spear, 2001; White et al., 2002), suppression of locomotor activity (Little et al., 1996), social impairment (Varlinskaya and Spear, 2002), and sedation (Draski et al., 2001; Moy et al., 1998; Silveri and Spear, 1998, 1999, 2002, 2004), whereas anxiolytic effects are among the desired effects of ethanol to which young adolescent rats appear insensitive relative to their more mature counterparts (Varlinskaya and Spear, 2002, 2006a).
Anxiolytic properties of ethanol can be experimentally studied in rats in a number of ways, including assessment of social interactions in an unfamiliar environment (File and Seth, 2003). An unfamiliar test situation is viewed as anxiety-provoking, and the suppression of social behavior seen under this condition (i.e., environment-induced social inhibition) has been applied as an animal model of anxiety (File and Hyde, 1978; File, 1980). Social inhibition observed under such unfamiliar test circumstances is reversed by a number of anxiolytic compounds, including ethanol (File and Seth, 2003). We have demonstrated recently that suppression of social behavior under unfamiliar test circumstances is accompanied by social avoidance (i.e., a tendency to move away from test partners rather than to follow them) in both adolescents and adults (Varlinskaya & Spear, 2008). Ontogenetic studies have shown that responsiveness to the anxiolytic effects of ethanol in the social interaction test differs as a function of age, with young adolescent rats being less sensitive to ethanol-induced anxiolysis than their older adolescent and adult counterparts, when indexed in terms of enhancement of social investigation and transformation of social avoidance into social preference (Varlinskaya and Spear, 2002, 2006a).
These previous ontogenetic studies assessed anxiolytic effects of only acute ethanol, and hence it is not known whether adaptations to its anxiolytic effects upon repeated experiences with ethanol would be similar in adolescents and adults. Indeed, few studies have assessed adaptations to the anxiolytic effects of ethanol following repeated ethanol exposure even in adults (Criswell and Breese, 1989; Koob et al., 1987; Sharma et al., 2007). Consequently, the present study investigated possible changes in sensitivity to the anxiolytic effects of ethanol in adolescent and adult Sprague-Dawley rats following 7 days of exposure to 1 g/kg ethanol or saline injected intraperitoneally (i.p.). This ethanol dose was chosen for investigation because it produces peak blood ethanol concentrations (BECs) around 110–120 mg/dl in both adolescent and adult rats (Varlinskaya & Spear, 2006b). These BECs are within the range currently accepted by NIAAA to define human binge drinking (i.e., a pattern of drinking that brings BEC to 80 mg/dl or above). An additional group of control animals left non-manipulated during the chronic exposure period was also included to assess whether the stress of repeated i.p. injections in the saline control group itself was sufficient to alter sensitivity to ethanol-induced anxiolysis, and perhaps differentially so across age.
General Methods
Subjects and Experimental Design
Adolescent (Experiment 1) and adult (Experiment 2) male and female Sprague-Dawley rats bred and reared in our colony at Binghamton University were used in these experiments. Although male/female comparisons were not a focus of this work, both male and female subjects were used in the present study, given our desire to maximally utilize generated litters and evidence that, in sufficiently powered experiments, we have not observed significant sex differences in responsiveness to the social anxiolytic effects of ethanol among adolescent and adult animals (e.g., Varlinskaya and Spear, 2002).
A total of 66 litters provided the 330 male and female offspring serving as experimental subjects and 330 like offspring serving as partners for these studies. All animals were housed in a temperature-controlled (22°C) vivarium maintained on a 14-/10-hr light/dark cycle (lights on at 07:00 hr) with ad libitum access to food (Purina Rat Chow, Lowell, MA) and water. Pups were housed until weaning with their mothers in standard maternity cages with pine shavings as bedding material. Litters were culled to 10 (5 males and 5 females) pups within 24 hr after birth on postnatal day (P) 0. Rats were weaned on P21 and placed into standard plastic cages with same-sex littermates (5 animals in a cage). In all respects, maintenance and treatment of the animals were in accord with guidelines for animal care established by the National Institutes of Health, using protocols approved by the Binghamton University Institutional Animal Care and Use Committee.
Experimental subjects were either repeatedly exposed to ethanol (chronic ethanol group) or saline (chronic saline control), or left undisturbed in their home cages (non-manipulated control). All animals from a given litter received the same chronic treatment and were kept together in groups of 5 same-sex littermates (Spear and File, 1996). On the test day, each animal was assigned randomly to one of the acute challenge groups, with order of testing counterbalanced across the experiment. To avoid the possible confounding of litter with treatment effects (Holson and Pearce, 1992; Zorrilla, 1997), animals were assigned semi-randomly to the experimental groups, with the constraint that no more than one subject of a given sex from a given litter was assigned to a particular acute challenge condition. Equal numbers of males and females were included into each experimental group to allow for maximal utilization of generated litters, with 5 experimental subjects per sex placed into each group. Assessment of sex differences was not a focus of these studies, and hence the studies were not so powered, given prior evidence that sex is not a significant variable in the anxiolysis induced by ethanol during mid adolescence and adulthood (see Varlinskaya and Spear, 2002).
Chronic Exposure
Experimental subjects received either 1 g/kg of ethanol or saline intraperitoneally (i.p.) for 7 consecutive days (P27–P33 for adolescents and P62–P68 for adults). Ethanol was administered as a 12.6% (v/v) solution, a relatively low concentration that induced little (if any) tissue irritation at the site of injection. Saline was given in a volume equal to the volume of ethanol administered (i.e., at 1% of the body weight of experimental animal). The solutions were administered at room temperature. A non-manipulated control group was left undisturbed in their home cages except for daily body weighing. Percentage body weight gain from day 1 (P27 for adolescents and P62 for adults) to day 7 (P33 for adolescents and P68 for adults) was calculated for males and females from each chronic exposure condition and the non-manipulated control group using litter as a unit of analysis (i.e., analyzing mean body weights for animals of each sex within each litter; see Holson and Pearce, 1992).
Testing Procedure
Behavioral testing occurred 48 hr after the last exposure to ethanol (P35 or P70). All animals were tested in an unfamiliar environment, since an unfamiliar test situation is traditionally viewed as an anxiety provoking condition (File and Hyde, 1978; File, 1980). The test apparatuses consisted of Plexiglas (Binghamton Plate Glass, Binghamton, NY) chambers (30 × 20 × 20 cm for adolescents and 45 × 30 × 20 cm for adults) containing clean pine shavings. Each test apparatus was divided into two equally sized compartments by a clear Plexiglas partition that contained an aperture (7 × 5 cm for adolescents and 9 × 7 cm for adults) to allow movement of the animals between compartments (Varlinskaya et al., 1999, 2001). On the test day, each subject was injected i.p. with ethanol or saline. The ethanol dose range used for testing differed for adolescents (Experiment 1) and adults (Experiment 2), given previously reported age-related differences in sensitivity to the anxiolytic effects of ethanol (Varlinskaya and Spear, 2002). Ethanol challenge dose was varied by altering the volume of the 12.6% ethanol solution to avoid concentration-induced differences in ethanol absorption rate (see Linakis and Cunningham, 1979). Control animals were injected with isotonic saline in a volume equal to that of the highest dose of ethanol administered.
Immediately after ethanol administration, each experimental animal was marked by a vertical line on the back and isolated in an opaque plastic holding cage (30 × 20 × 20 cm) for 30 min prior to testing (e.g., File, 1993). Thereafter, each animal was placed into the testing chamber simultaneously with a same age and sex test partner. Partners were always non-exposed animals that had not been socially isolated prior to testing and who were unfamiliar with both the test apparatus and the experimental animal with which they were paired for testing. Weight differences between test subjects and their partners were minimized as much as possible, with this weight difference not exceeding 10 g for each pair of animals at P35 and 20 g at P70, and test subjects always being heavier than their partners. The order of testing was counterbalanced for all treatment groups. During the 10-min test session, the behavior of the animals was recorded by a video camera (Panasonic model AF-X8, Secaucus, NJ), with real time being directly recorded onto the videotape for later scoring (Easy Reader II Recorder; Telcom Research TCG 550, Burlington, Ontario). After each test, the apparatus was wiped with 3% peroxide hydrochloride and the shavings were replaced with fresh ones. All testing procedures were conducted between 9:00 and 13:00 hr under dim light (15–20 lx). Trunk blood samples were collected immediately after the test.
Behavioral Measures
Anxiolytic effects of ethanol emerge reliably with two anxiety-sensitive measures of social behavior under unfamiliar test circumstances in both adolescent and adult rats. Under these test circumstances, social investigation is enhanced by acute ethanol challenge, whereas social avoidance is transformed into social preference (Varlinskaya and Spear, 2002, 2006a). Play fighting -- an adolescent-characteristic form of social interactions – is not sensitive to the anxiolytic effects of ethanol when animals are tested in an unfamiliar, anxiety-provoking environment. Therefore, the main focus of the present study was on social investigation and social preference/avoidance as two main indices of the anti-anxiety effects of ethanol. For investigation of whether adaptations to repeated ethanol exposure in the present study were specific to its anxiolytic effects, two anxiety-insensitive measures, namely play fighting and overall locomotor activity in the social context were also analyzed.
The frequency with which each test subject emitted these behaviors was analyzed from the video recordings (Thor and Holloway, 1984; Vanderschuren et al., 1997; Varlinskaya and Spear, 2002). Social investigation was defined as the sniffing of any part of the body of the partner. Play fighting was assessed by summing the frequencies of the following behavioral acts and postures: pouncing or playful nape attack (the experimental subject lunges at the partner with its forepaws extended outward); following and chasing (the experimental animal rapidly pursues the partner); pinning (the experimental subject stands over the exposed ventral area of the partner, pressing it against the floor). Play fighting differs from serious fighting in the laboratory rat by target of attack: during play fighting, snout or oral contact is directed toward the partner's nape, while during serious fighting the object of the attack is the partner's rump (Pellis and Pellis, 1987). In the present experiments, subjects did not demonstrate serious fighting and, hence, frequency of this behavior was not scored.
Social preference/avoidance was analyzed by scoring the number of crossovers (movements between compartments) demonstrated by the experimental subject toward the non-manipulated ethanol-naive peer and the number of crossovers away from the peer (Varlinskaya et al., 1999, 2001). Social motivation was assessed by means of a coefficient of preference/avoidance [Coefficient (%) = (crossovers to - crossovers from) / (crossovers to + crossovers from) × 100]. Social preference was defined by significantly positive values of the coefficient, while social avoidance was associated with negative values that differed significantly from 0. The values of the coefficient that did not differ from 0 reflected social indifference. Total number of crossovers was used as an index of general locomotor activity under these test circumstances.
The videotape records were scored by two observers without knowledge of the chronic exposure condition or acute challenge of any animal. Agreement between observers scoring the same videotape was in excess of 90% for each measure of social behavior and social preference.
Blood Ethanol Determination
Trunk blood samples were collected immediately after behavioral testing in heparinized tubes, rapidly frozen, and maintained at −80°C until analysis of BECs. Samples were assessed for BEC via headspace gas chromatography using a Hewlett Packard (HP) 5890 series II Gas Chromatograph (Wilmington, DE). At the time of assay, blood samples were thawed and 25-µl aliquots were placed in airtight vials. Vials were placed in a HP 7694E Auto-Sampler, which heated each individual vial for 8 min, and then extracted and injected a 1.0 ml sample of the gas headspace into the gas chromatograph. Ethanol concentrations in each sample were determined using HP Chemstation software, which compares the peak area under the curve in each sample with those of standard curves derived from reference standard solutions.
Data Analysis
Dependent variables, including blood ethanol concentration, frequencies of social investigation and play fighting, coefficient of social preference/avoidance, and locomotor activity were examined using analyses of variance (ANOVAs), with pre-test condition and ethanol challenge dose used as between subject factors. The ANOVA of body weight gain included the factor of pre-test condition as well as sex, given well-known sex differences in body weight gain; this later variable was calculated separately using the mean body weights for animals of each sex within each litter. Where significant interactions involving pre-test condition and ethanol challenge dose were evident, planned ANOVAs within each pre-test condition were conducted to explore consequences of repeated ethanol or saline exposure on responsiveness to acute ethanol challenge. Ethanol-induced changes of social behavior and social motivation were assessed by post hoc comparisons (Fisher’s planned least significant difference test) between ethanol-challenged groups and the saline-challenged controls. T-tests were used to assess whether values of the coefficient differed significantly from 0.
Experiment 1. Ethanol anxiolysis following chronic adolescent ethanol exposure
A total of 150 adolescent animals served as experimental subjects, with 150 additional animals serving as their partners. On the test day (P35), animals were injected with one of the 5 doses of ethanol (0, 0.75, 1.0, 1.25, and 1.5 g/kg), and their social interactions with non-manipulated partners were assessed under unfamiliar circumstances. Therefore, the design of Experiment 1 was a 3 (pre-test condition: no manipulation, chronic saline, chronic ethanol) × 5 (ethanol challenge dose) factorial, with an equal number of males and females placed into each experimental group.
Body Weight Gain
A 3 (pre-test condition) × 2 (sex) ANOVA (see General Methods) for percent body weight gain from day 1 to day 7 of the chronic exposure period revealed significant main effects of pre-test condition, F(1,24) = 4.30, p < 0.05, and sex, F(1,24) = 28.44, p < 0.0001. Adolescent animals chronically exposed to saline (59.93% ± 1.97) gained significantly less weight than their non-manipulated counterparts (65.96% ± 1.62), whereas adolescents chronically exposed to ethanol (63.02% ± 2.07) did not differ significantly from either control group. Adolescent males gained significantly more weight across the exposure period than females (67.6% ± 1.2 vs. 58.3% ± 1.1, respectively).
Social Investigation and Social Preference/Avoidance
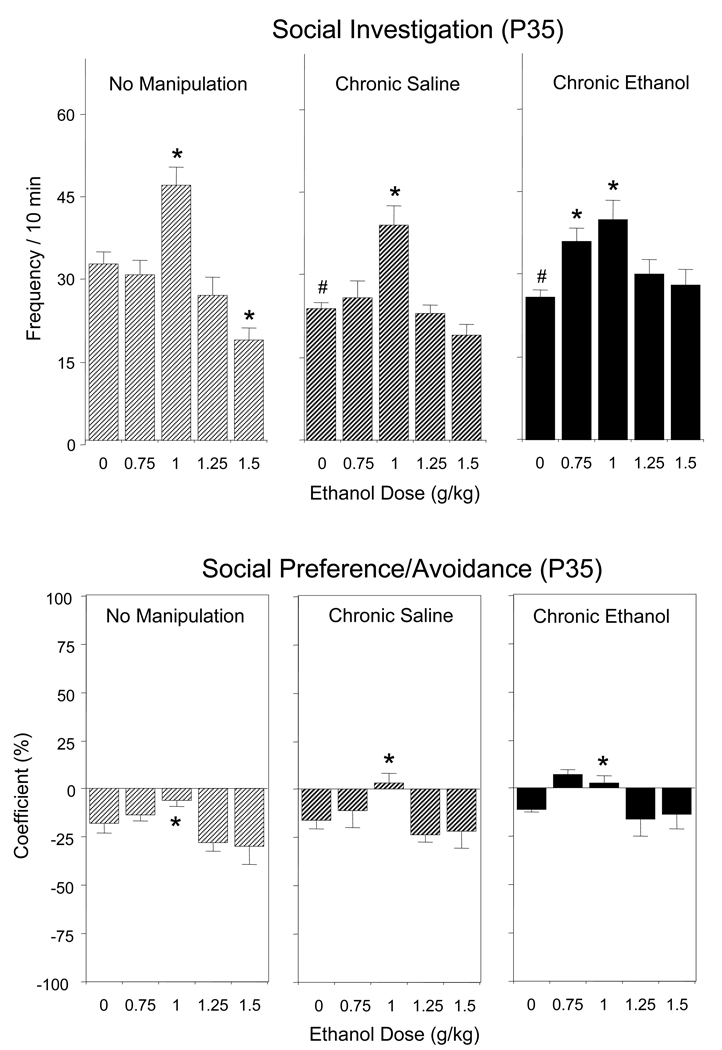
Ethanol-induced changes in social investigation of adolescent animals differed as a function of pre-test condition [pre-test condition × ethanol challenge dose interaction, F(8,120) = 2.08, p < 0.05]. Chronic exposure to saline or ethanol during adolescence significantly reduced baseline levels of social investigation; that is, chronically injected animals exhibited less social investigation than non-manipulated controls when challenged with saline (0 g/kg ethanol) on test day (see Fig.1, top). Ethanol-induced increases in social investigation were seen in all groups, although adolescents chronically exposed to ethanol demonstrated increases in social investigation at doses of 0.75 and 1 g/kg, whereas both non-manipulated and saline controls exhibited this anxiolytic effect only following the 1 g/kg of ethanol (see Figure 1, top). Non-manipulated controls demonstrated an inhibition of social investigation at 1.5 g/kg ethanol, whereas adolescents chronically exposed to ethanol or saline were insensitive to ethanol-induced social inhibition within this dose range.
Figure 1.
Effects of acute challenge with 0, 0.75, 1.0, 1.25, and 1.5 g/kg ethanol on social investigation (top) and social preference/avoidance (bottom) of adolescent rats left non-manipulated or repeatedly exposed to saline or 1 g/kg ethanol for 7 days prior to the social interaction test. Data are collapsed across sex (n=10 per group). Asterisks (*) indicate significant acute ethanol-induced changes relative to corresponding controls acutely challenged with saline (0 g/kg) within each pretest condition (p < 0.05) for social investigation and significant ethanol-induced changes relative to saline controls on data collapsed across pre-test condition for social preference/avoidance. # - significant changes in chronically saline- or ethanol-exposed animals relative to non-manipulated controls when challenged with saline on test day (p < 0.05).
The coefficient of social preference/avoidance differed as a function of pre-test condition, F(2,120) = 5.55, p < 0.01, and ethanol challenge dose, F(4,120) = 7.60, p < 0.001 (see Figure 1, bottom), with adolescent chronically exposed to ethanol demonstrating significantly less social avoidance than their non-manipulated counterparts and animals challenged with a dose of 1 g/kg ethanol showing transformation of social avoidance (t = −6.77, p < 0.001) into social indifference (t = −0.62, p > 0.05).
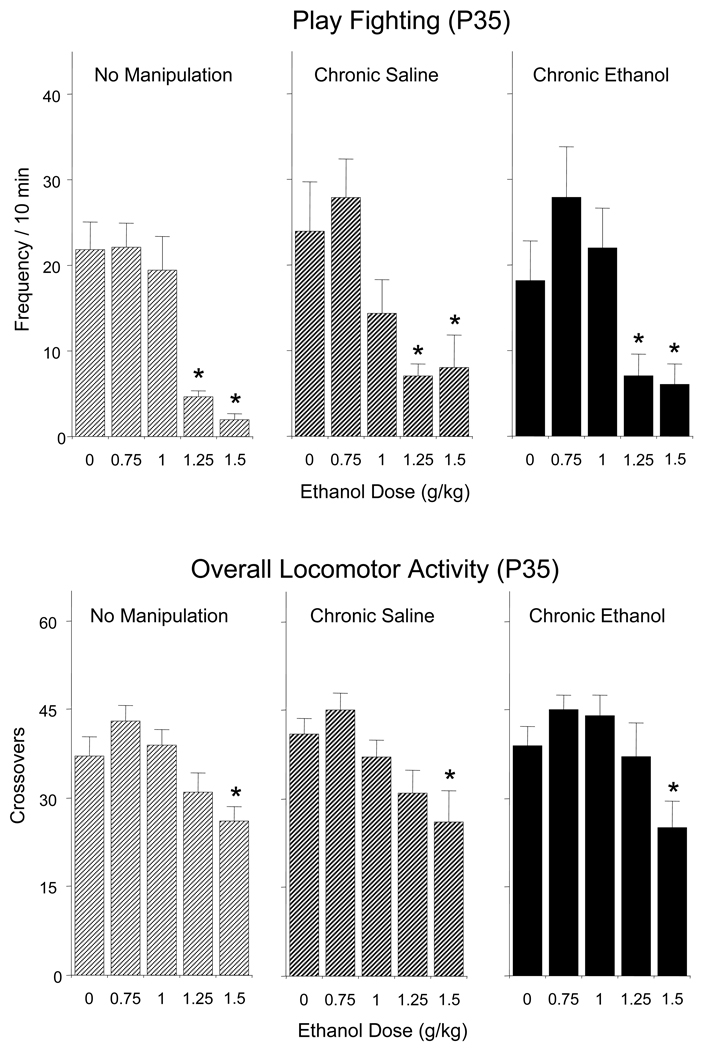
Play Fighting and Overall Locomotor Activity
As presented in Figure 2 (top), chronic exposure had no effects on ethanol-induced social inhibition indexed by decreases in play fighting, with adolescents from all chronic conditions demonstrating significant declines in play fighting after doses of 1.25 and 1.5 g/kg ethanol [a significant main effect of ethanol challenge dose, F(4,120) = 18.89, p < 0.001]. Similarly, no effects of chronic exposure were evident in terms of ethanol-induced changes in locomotor activity in the social context (Figure 2, bottom), with all groups of adolescent animals showing significant decreases in total number of crossovers when challenged with 1.5 g/kg ethanol [a significant main effect of ethanol challenge dose, F(4,120) = 11.88, p < 0.0001].
Figure 2.
Effects of acute challenge with 0, 0.75, 1.0, 1.25, and 1.5 g/kg ethanol on play fighting (top) and locomotor activity in the social context, indexed via overall number of crossovers (bottom) of adolescent rats left non-manipulated or repeatedly exposed to saline or 1 g/kg ethanol for 7 days prior to the social interaction test. Asterisks (*) indicate significant acute ethanol-induced changes relative to controls acutely challenged with saline (0 g/kg) (p < 0.05), with data collapsed across sex.
Blood Ethanol Concentration
BECs increased in a dose-dependent fashion, F(3,96) = 101.20, p < 0.0001, but did not differ as a function of chronic treatment in adolescent animals (see Table 1).
Table 1.
Blood Ethanol Concentrations (mean ± SEM) in Adolescent Rats.
| Ethanol Dose (g/kg) |
No Manipulation (mg/dl) |
Chronic Saline (mg/dl) |
Chronic Ethanol (mg/dl) |
|---|---|---|---|
| 0.75 | 41.9 ± 1.5 | 46.5 ± 3.9 | 43.9 ± 2.1 |
| 1.0 | 64.5 ± 4.3 | 69.5 ± 1.7 | 68.2 ± 3.4 |
| 1.25 | 85.8 ± 4.0 | 91.0 ± 3.0 | 82.4 ± 7.4 |
| 1.5 | 107.3 ± 6.7 | 108.9 ± 7.8 | 106.7 ± 4.7 |
Experiment 2. Ethanol anxiolysis following chronic adult ethanol exposure
A total of 180 animals served as experimental subjects, and 180 served as partners in this experiment. On test day (P70), adult rats from all 3 chronic conditions were injected i.p. with one of the 6 doses of ethanol (0, 0.25, 0.5, 0.75, 1.0, 1.25), with their social interactions with non-manipulated partners being assessed 30 min post-injection under unfamiliar circumstances. Adult animals were challenged with lower doses of ethanol than their adolescent counterparts (see Experiment 1), since they are more sensitive to the anxiolytic effects of ethanol in the social interaction test (Varlinskaya and Spear, 2002). Therefore, the design of Experiment 2 was a 3 (pre-test condition: no manipulation, chronic saline, chronic ethanol) × 6 (ethanol challenge dose) factorial, with an equal number of animals of each sex placed into each experimental condition.
Body Weight Gain
A 3 (pre-test condition) × 2 (sex) ANOVA for percent body weight gain from day 1 to day 7 of chronic adult exposure showed significant main effects of pre-test condition, F(1,30) = 5.81, p < 0.01, and sex, F(1,30) = 15.72, p < 0.001. In contrast to their adolescent counterparts, adults chronically exposed to saline (8.34% ± 0.57) did not differ from non-manipulated adults (9.44% ± 0.59), whereas animals chronically exposed to ethanol (7.38% ± 0.39) gained significantly less weight than non-manipulated controls, but not than chronic saline control animals. Adult males gained significantly more weight across the exposure period than adult females (9.37% ± 0.47 vs. 7.40% ± 0.33, respectively).
Social Investigation and Social Preference/Avoidance
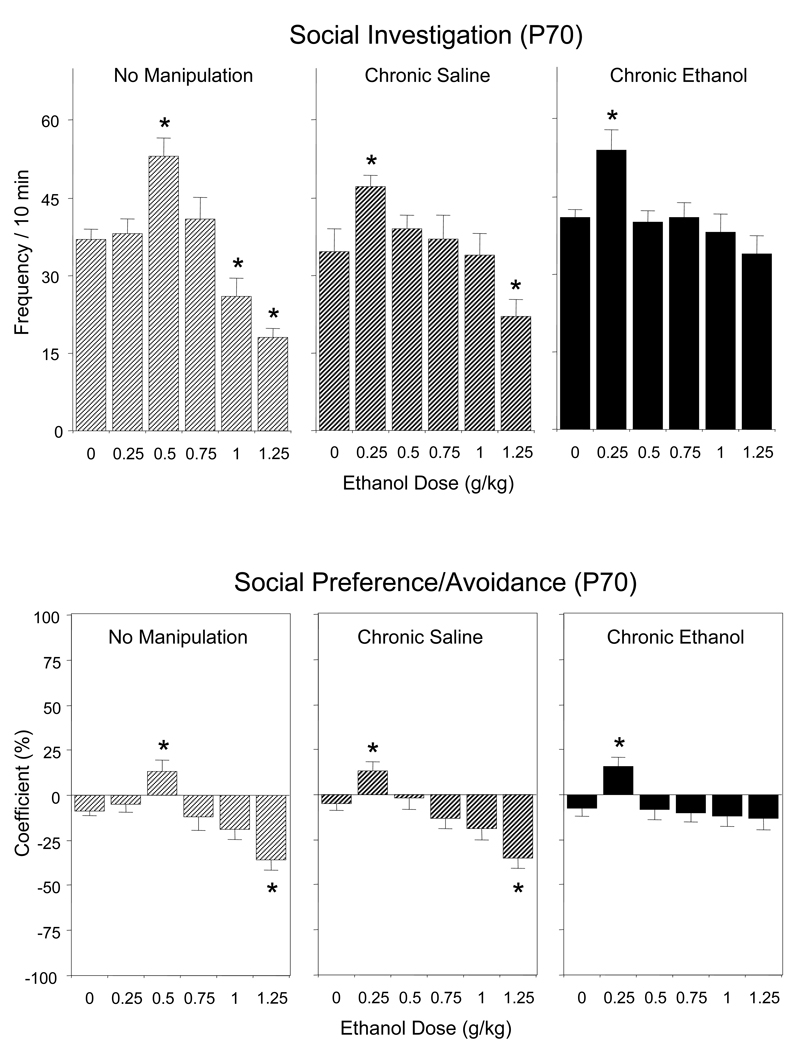
As seen in Figure 3 (top), effects of acute ethanol challenge on social investigation of adult animals varied with pre-test condition [pre-test condition × ethanol challenge dose interaction, F(10,144) = 13.37, p < 0.001]. Ethanol-induced anxiolysis, as indexed by significant increases in social investigation, was seen at a dose of 0.5 g/kg ethanol in non-manipulated animals and at a lower dose of 0.25 g/kg in animals chronically exposed to saline or ethanol. Ethanol-induced suppression of social investigation was observed following administration of 1.0 and 1.25 g/kg ethanol in non-manipulated control animals, whereas this suppression was seen only at a dose of 1.25 g/kg in animals chronically exposed to saline and was not evident at any dose in the range tested following chronic ethanol.
Figure 3.
Effects of acute challenge with 0, 0.75, 1.0, 1.25, and 1.5 g/kg ethanol on social investigation (top) and social preference/avoidance (bottom) of adult rats left non-manipulated or repeatedly exposed to saline or 1 g/kg ethanol for 7 days prior to the social interaction test. Asterisks (*) indicate significant acute ethanol-induced changes relative to corresponding controls acutely challenged with saline (0 g/kg) within each pre-test condition (p < 0.05), with data collapsed across sex.
Acute effects of ethanol on the coefficient of social preference/avoidance were likewise affected by pre-test condition [pre-test condition × ethanol challenge dose interaction, F(10,144) = 2.45, p < 0.01]. Social avoidance (t = − 3.65, p < 0.05) was transformed into social preference (t = 2.28, p < 0.05) by a dose of 0.5 g/kg ethanol in non-manipulated adults, whereas adults chronically treated either with saline or ethanol became more sensitive to this ethanol effect, demonstrating transformation of social indifference (i.e., values of the coefficient not different from 0) to social preference (t = 2.62, p < 0.05 for repeated saline and t = 3.30, p < 0.05 for repeated ethanol) following administration of 0.25 g/kg ethanol (Figure 3, bottom). An ethanol-induced enhancement of social avoidance (or transformation of social indifference into social avoidance), indexed by significant lowering of the preference/avoidance coefficient, was evident at the highest dose of ethanol tested (1.25 g/kg) in both control groups but was not seen in the animals chronically exposed to ethanol.
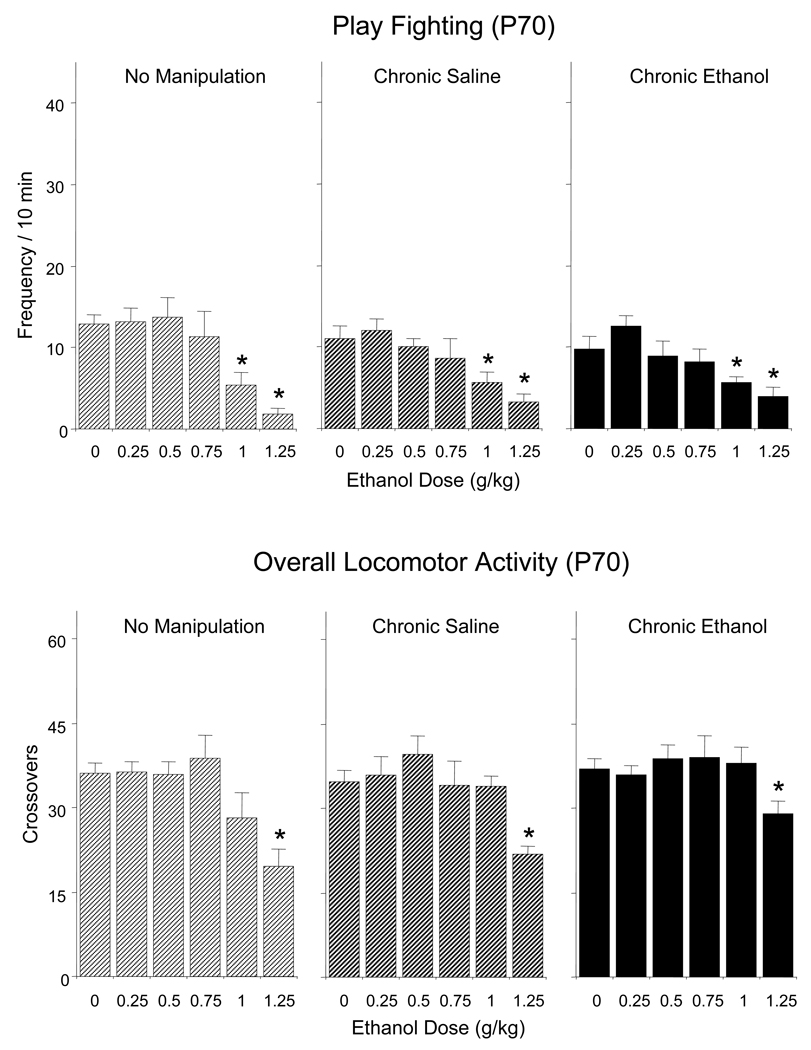
Play Fighting and Overall Locomotor Activity
As presented in Figure 4 (top), ethanol-induced social suppression in terms of play fighting were seen in adults at doses of 1.0 and 1.25 g/kg regardless of chronic exposure condition [ethanol challenge dose effect, F(5,144) = 14.65, p < 0.0001]. Likewise, all groups of adult animals showed significant decreases in total number of crossovers following a dose of 1.25 g/kg ethanol [a significant main effect of ethanol challenge dose, F(5,144) = 12.42, p < 0.0001], with no effect of pre-test condition evident for this measure of locomotor activity in the social context (Figure 4, bottom).
Figure 4.
Effects of acute challenge with 0, 0.75, 1.0, 1.25, and 1.5 g/kg ethanol on play fighting (top) and locomotor activity in the social context, indexed via overall number of crossovers (bottom) of adult rats left non-manipulated or repeatedly exposed to saline or 1 g/kg ethanol for 7 days prior to the social interaction test. Asterisks (*) indicate significant acute ethanol-induced changes relative to controls acutely challenged with saline (0 g/kg (p < 0.05), with data collapsed across sex.
Blood Ethanol Concentration
BECs for adult animals increased with ethanol challenge dose, F(4,120) = 214.51, p < 0.0001, but were not affected by pre-test condition (see Table 2).
Table 2.
Blood Ethanol Concentrations (mean ± SEM) in Adult Rats.
| Ethanol Dose (g/kg) |
No Manipulation (mg/dl) |
Chronic Saline (mg/dl) |
Chronic Ethanol (mg/dl) |
|---|---|---|---|
| 0.25 | 7.4 ± 1.0 | 7.7 ± 2.1 | 8.1 ± 0.6 |
| 0.5 | 25.0 ± 2.3 | 28.4 ± 2.8 | 26.3 ± 1.7 |
| 0.75 | 55.1 ± 3.1 | 47.3 ± 3.8 | 59.8 ± 3.7 |
| 1.0 | 79.6 ± 6.5 | 75.0 ± 5.4 | 85.1 ± 3.1 |
| 1.25 | 96.6 ± 5.0 | 101.0 ± 9.5 | 102.0 ± 6.2 |
Discussion
Anxiolytic effects of ethanol emerged reliably with the two anxiety-sensitive measures in both adolescent (Experiment 1) and adult (Experiment 2) animals: social investigation in an unfamiliar context was enhanced by challenge with ethanol, whereas social avoidance was transformed into social indifference/preference. These observations are in agreement with our previous findings that have demonstrated the selective sensitivity of these two measures for detection of ethanol-induced anxiolysis under unfamiliar test circumstances (Varlinskaya and Spear, 2002, 2006a). During adolescence, chronic ethanol exposure resulted in the development of apparent sensitization to ethanol anxiolysis: adolescents chronically treated with ethanol showed significant increases in social investigation at a lower dose than non-manipulated adolescents as well as those chronically injected with saline. Among adults, apparent sensitization to the anxiolytic effects of ethanol was seen not only following repeated ethanol exposure, but also after chronic saline injections, and hence would not have been detected if a non-manipulated control group had not been included. These results suggest that the chronic manipulations per se were sufficient for sensitization of adult animals to ethanol-induced anxiolysis (i.e., possible stress-induced sensitization).
Very few studies have assessed adaptations to the anxiolytic effects of ethanol following repeated ethanol exposure, with only adult rodents used as experimental subjects. The development of tolerance to the anxiolytic effects of ethanol in conflict paradigms (Criswell and Breese, 1989; Koob et al., 1987) and in an elevated plus maze test of anxiety (Sharma et al., 2007) has been reported following repeated exposure of adult rats to ethanol – findings that are notably in contrast to the sensitization to ethanol anxiolysis observed here. The discrepancy between the present findings and those earlier studies may be related to procedural differences, including levels and duration of chronic ethanol exposure, housing conditions of the experimental animals, amount of stress associated with the experimental manipulations (food deprivation, electric shock, etc.), and model used to test anxiety-like behavior. For instance, in the Criswell and Breese (1989) and Sharma et al. (2007) studies, adult rats were housed individually and received liquid ethanol diets, with average daily ethanol intakes of around 10 – 12 g/kg, whereas Koob et al. (1987) exposed group-housed, food deprived animals to 0.75 g/kg ethanol per day, although the duration of chronic ethanol exposure in all of these studies was comparable to that employed here (7–12 days). A particularly substantial contributor may be the context in which anxiety is tested: in the studies by Criswell and Breese (1989), Koob et al. (1987) and Sharma et al. (2007), experimental subjects were tested individually, whereas we tested adolescent and adult animals in a social context. Taken together, these findings lead to the tentative suggestion that whereas sensitization develops to the social anxiety-reducing effects of ethanol, repeated ethanol exposure more readily produces tolerance to ethanol’s anticonflict and/or antipunishment properties.
In contrast to the sensitization to ethanol-induced anxiolysis seen following chronic ethanol administration, tolerance emerged to the social inhibitory effects of ethanol at doses above the anxiolytic range and was indexed by attenuation in the inhibitory effects of these higher doses on social investigation in both adolescents and adults. This decreased sensitivity to ethanol-induced suppression of social investigation among adolescents and adults was seen not only following repeated ethanol, but also after chronic saline exposure, suggesting some degree of possible stress-induced tolerance. In adults, chronic ethanol, but not saline exposure also resulted in the development of tolerance to the ethanol-induced increase in social avoidance seen at the highest ethanol challenge dose. Tolerance was not evident with other measures, with ethanol-induced suppression of play fighting and locomotor activity seen at these high doses of ethanol in the social context uninfluenced by chronic treatment at either age. These findings suggest that ethanol-induced social inhibition indexed by decreases in social investigation at both ages and social avoidance in adults do not simply mirror changes in overall locomotor activity, given that these measures are differentially affected by chronic ethanol and saline exposure. That is, the attenuations in social investigation and social motivation at the highest dose were eliminated or reduced by the chronic exposure regimen, whereas ethanol-induced decreases in overall number of crossovers were unaffected, data that support the suggestion that initial declines in social investigation and social motivation in ethanol-challenged animals likely reflect ethanol-induced social inhibition and/or anxiogenesis, rather than general locomotor suppressing effects of ethanol evident at higher exposure levels.
A number of studies have compared propensities for the emergence of chronic tolerance following repeated ethanol exposure during adolescence and adulthood, although the results obtained have varied across studies. For instance, adolescent, but not adult rats were found to develop chronic tolerance to the sedative effects of ethanol following 7 days of exposure to 4 g/kg given intragastrically twice daily (Swartzwelder et al, 1998). Likewise, in a study where ethanol was administered via vapor inhalation for 64 hr to adult and adolescent mice, and animals were tested 6 weeks later for a conditioned taste aversion to ethanol, only adolescent ethanol exposure resulted in tolerance development (Diaz-Granados and Graham, 2007). In contrast to these studies reporting greater tolerance development among adolescents, Ristuccia and Spear (2005) found that tolerance to hypothermic effects of ethanol developed faster in adult rats than in their younger counterparts following exposure to ethanol vapor (Ristuccia and Spear, 2005). In a study equating initial functional motor impairment across age by dose adjustments, equivalent levels of tolerance to ethanol-induced motor impairment were observed across the two ages (Silveri and Spear, 2001). Similarly, exposure to a relatively modest dose of ethanol (1 g/kg ethanol i.p. daily for 7 days) during adolescence or early adulthood resulted in expression of chronic tolerance at both ages to the social consequences of ethanol under familiar test circumstances (Varlinskaya and Spear, 2007). Among the variables that may contribute to such differing age-related patterns in the relative rate of ethanol adaptation is the response measure under investigation, as well as dose administered.
The adaptations to repeated ethanol observed in the present study appear to be functional and not metabolic in nature, given that BECs determined from trunk blood did not differ as a function of pre-test chronic treatment at either age. These findings differ from our previously reported observations of decreased ethanol levels in tail blood on test day following similar repeated ethanol exposure in adult but not adolescent rats (Varlinskaya and Spear, 2007). Trunk blood samples were used in the present study, given that they are thought to be a more accurate reflection of brain ethanol concentrations after i.p. administration in laboratory rodents than tail blood samples (Gehle and Erwin, 2000; Ponomarev and Crabbe, 2002; Sunahara et al., 1978; Varlinskaya and Spear, 2006b).
Social investigation of adolescent and adult rats is extremely sensitive to anxiogenic and/or anxiolytic manipulations (Doremus-Fitzwater et al., 2009; Varlinskaya and Spear, 2002, 2006a, 2007), with repeated exposure to restraint stress decreasing this form of social behavior and anxiolytic compounds (including ethanol) increasing social investigation. The results of the current study demonstrate an age-related difference in the impact of chronic manipulation on baseline levels of social investigation. That is, adolescents from both chronic exposure conditions given saline on test day exhibited significantly less social investigation than non-manipulated adolescents challenged with saline – an effect not seen in adults. Thus, adolescents may be more sensitive to the anxiety-provoking effects of chronic manipulations than their adult counterparts, and as a consequence may perceive saline injections as a mild stressor, robust enough to produce anxiogenic action.
The unusual sensitivity of adolescent animals to the mild stress associated with repeated saline injections was also evident in terms of body weight gain during the exposure period. That is, adolescent animals were more sensitive to chronic saline than to chronic ethanol exposure, showing a significant reduction of body weight gain relative to non-manipulated controls only following repeated saline. In contrast, adults gained less weight than non-manipulated controls only when they were chronically exposed to ethanol. These effects were slight, however, with no significant differences in body weight gain observed between the two chronic conditions at either age. Nevertheless, these findings are reminiscent of work by Stone and Quartermain (1997), showing reduction in weight gain following restraint stress only in 4-week-old but not 8-week-old male Swiss Webster mice.
To some extent, the enhanced sensitivity to chronic injection stress seen in adolescents in terms of angiogenic and body weight effects in the present study is reminiscent of a recent study by Silvers et al. (2006), who have reported more pronounced increases in allopregnanolone levels following saline challenge in animals exposed to chronic saline injections during adolescence than in those exposed to ethanol injections (Silvers et al., 2006). Such stress-enhanced anxiety of adolescents animals could potentially serve to encourage ethanol intake for its anxiolytic properties during adolescence. Yet, the results of studies investigating the relationship between anxiety levels and ethanol intake in adult animals are often controversial, with some researchers demonstrating positive correlations between anxiety and ethanol intake (Izidio & Ramos, 2007; Stewart et al., 1993) and others not finding this relationship (e.g., Langen & Fink, 2004). Unquestionably, more ontogenetic studies are needed for our better understanding of the relationship between anxiety levels, sensitivity to the anxiolytic effects of ethanol, and drinking.
In conclusion, the results of the present study suggest that adaptations to both chronic ethanol exposure and the repeated stress of chronic injection per se may possibly serve as permissive factors fostering high ethanol intake in adolescents and adults, although the nature of these adaptations seemingly varies across age. Repeated exposure to ethanol or to the mild stress of the repeated injection procedure alone seemingly increases baseline anxiety in adolescent animals, anxiety that may promote ethanol consumption during adolescence for its anxiolytic properties. In adults, however, chronic stress and, especially, repeated ethanol exposure reduce sensitivity to adverse effects of ethanol that may normally serve to limit drinking, thereby permitting higher levels of use.
Acknowledgments
The research presented in this paper was supported by NIH grants R37 AA012525, R01 AA018026, R01 AA016887 to Linda P. Spear and R01 AA012453 to Elena I. Varlinskaya.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlström SK, Österberg EL. International perspectives on adolescent and young adult drinking. Alcohol Res. Health. 2005;28:258–268. [Google Scholar]
- Bates ME, Labouvie EW. Adolescent risk factors and the prediction of persistent alcohol and drug use into adulthood. Alcohol. Clin. Exp. Res. 1997;21:944–950. [PubMed] [Google Scholar]
- Brunell SC, Spear LP. Effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcohol. Clin. Exp. Res. 2005;29:1641–1653. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- Buchanan CM, Eccles JS, Becker JB. Are adolescents the victims of raging hormones: evidence for activational effects of hormones on moods and behavior at adolescence. Psychological Bulletin. 1992;111:62–107. doi: 10.1037/0033-2909.111.1.62. [DOI] [PubMed] [Google Scholar]
- Collins ME. Transition to adulthood for vulnerable youths: A review of research and implications for policy. Social Service Rev. 2001;75:271–291. [Google Scholar]
- Cooper ML, Agocha VB, Sheldon MS. A motivational perspective on risky behaviors: the role of personality and affect regulatory processes. J. Pers. 2000;68:1059–1088. doi: 10.1111/1467-6494.00126. [DOI] [PubMed] [Google Scholar]
- Criswell HE, Breese GR. A conflict procedure not requiring deprivation: evidence that chronic ethanol treatment induces tolerance to the anticonflict action of ethanol and chlordiazepoxide. Alcohol. Clin. Exper. Res. 1989;13:680–685. doi: 10.1111/j.1530-0277.1989.tb00404.x. [DOI] [PubMed] [Google Scholar]
- Davis M. Addressing the needs of youth in transition to adulthood. Admin. Policy Ment. Health. 2003;30:495–509. doi: 10.1023/a:1025027117827. [DOI] [PubMed] [Google Scholar]
- Diaz-Granados JL, Graham DL. The effects of continuous and intermittent ethanol exposure in adolesence on the aversive properties of ethanol during adulthood. Alcohol. Clin. Exper. Res. 2007;31:2020–2027. doi: 10.1111/j.1530-0277.2007.00534.x. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol. Clin. Exper. Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiol Behav. 2009;97:484–494. doi: 10.1016/j.physbeh.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draski LJ, Bice PJ, Deitrich RA. Developmental alterations of ethanol sensitivity in selectively bred high and low alcohol sensitive rats. Pharmacol. Biochem. Behav. 2001;70:387–396. doi: 10.1016/s0091-3057(01)00621-9. [DOI] [PubMed] [Google Scholar]
- File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J. Neurosci. Methods. 1980;2:219–238. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- File SE. The social interaction test of anxiety. Neurosci. Protocol. 1993;10:1–7. [Google Scholar]
- File SE, Hyde JR. Can social interaction be used to measure anxiety? Br. J. Pharmacol. 1978;62:19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur. J. Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Gehle VM, Erwin VG. The genetics of acute functional tolerance and initial sensitivity to ethanol for an ataxia test in the LSxSS RI strains. Alcohol. Clin. Exper. Res. 2000;24:579–587. [PubMed] [Google Scholar]
- Hollstedt C, Olsson O, Rydberg U. Effects of ethanol on the developing rat. II. Coordination as measured by the tilting-plane test. Med. Biol. 1980;58:164–168. [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol. Teratol. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Izidio GS, Ramos A. Positive association between ethanol consumption and anxiety-related behaviors in two selected rat lines. Alcohol. 2007;41:517–524. doi: 10.1016/j.alcohol.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Jessor R. Successful adolescent development among youth in high risk settings. Amer. Psychol. 1993;48:117–126. doi: 10.1037//0003-066x.48.2.117. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the future national survey results on drug use, 1975–2006: Volume 1, Secondary school students. Bethesda, MD: National Institute on Drug Abuse; 2007. (NIH Publication No. 07-6205) [Google Scholar]
- Koob GF, Wall TL, Schafer J. Rapid induction of tolerance to the antipunishment effects of ethanol. Alcohol (Fayetteville, NY) 1987;4:481–484. doi: 10.1016/0741-8329(87)90090-5. [DOI] [PubMed] [Google Scholar]
- Kuntsche E, Knibbe R, Gmel G, Engels R. Why do young people drink? A review of drinking motives. Clin. Psychol. Rev. 2005;25:841–861. doi: 10.1016/j.cpr.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Langen B, Fink H. Anxiety as a predictor of alcohol preference in rats? Prog Neuropsychopharmacol & Biological Psychiatry. 2004;28:961–968. doi: 10.1016/j.pnpbp.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Larson R, Asmussen L. Anger, worry, and hurt in early adolescence: an enlarging world of negative emotions. In: Colten ME, Gore S, editors. Adolescent Stress: Causes and Consequences. New York: Aldine de Gruyter; 1991. pp. 21–41. [Google Scholar]
- Linakis JG, Cunningham CL. Effects of concentration of ethanol injected intraperitoneally on taste aversion, body temperature, and activity. Psychopharmacology. 1979;64:61–65. doi: 10.1007/BF00427346. [DOI] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcohol. Clin. Exper. Res. 1996;20:1346–1351. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Montgomery RL, Benedicto JA, Haemmerlie FM. Personal vs social motivations of undergraduates for using alcohol. Psychol. Rep. 1993;73:960–962. doi: 10.2466/pr0.1993.73.3.960. [DOI] [PubMed] [Google Scholar]
- Moy SS, Duncan GE, Knapp DJ, Breese GR. Sensitivity to ethanol across development in rats: comparison to [3H]zolpidem binding. Alcohol. Clin. Exper. Res. 1998;22:1485–1492. [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. Play-fighting differs from serious fighting in both target of attack and tactics of fighting in the laboratory rat Rattus norvegicus. Aggress. Behav. 1987;13:227–242. [Google Scholar]
- Ponomarev I, Crabbe JC. A novel method to assess initial sensitivity and acute functional tolerance to hypnotic effects of ethanol. J. Pharmacol. Exp. Ther. 2002;302:257–263. doi: 10.1124/jpet.302.1.257. [DOI] [PubMed] [Google Scholar]
- Ristuccia RC, Spear LP. Sensitivity and tolerance to autonomic effects of ethanol in adolescent and adult rats during repeated vapor inhalation sessions. Alcohol. Clin. Exper. Res. 2005;29:1809–1820. doi: 10.1097/01.alc.0000183010.72764.cd. [DOI] [PubMed] [Google Scholar]
- Sharma AN, Chopde CT, Hirani K, Kokare DM, Ugale RR. Chronic progesterone treatment augments while dehydroepiandrosterone sulphate prevents tolerance to ethanol anxiolysis and withdrawal anxiety in rats. Eur. J. Pharmacol. 2007;567:211–222. doi: 10.1016/j.ejphar.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol. Clin. Exper. Res. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Ontogeny of rapid tolerance to the hypnotic effects of ethanol. Alcohol. Clin. Exper. Res. 1999;23:1180–1184. [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Acute, rapid, and chronic tolerance during ontogeny: observations when equating ethanol perturbation across age. Alcohol. Clin. Exper. Res. 2001;25:1301–1308. [PubMed] [Google Scholar]
- Silveri MM, Spear LP. The effects of NMDA and GABAA pharmacological manipulations on ethanol sensitivity in immature and mature animals. Alcohol. Clin. Exper. Res. 2002;26:449–456. [PubMed] [Google Scholar]
- Silveri MM, Spear LP. The effects of NMDA and GABAA pharmacological manipulations on acute and rapid tolerance to ethanol during ontogeny. Alcohol. Clin. Exper. Res. 2004;28:884–894. doi: 10.1097/01.alc.0000128221.68382.ba. [DOI] [PubMed] [Google Scholar]
- Silvers JM, Tokunaga S, Mittleman G, O'Buckley T, Morrow AL, Matthews DB. Chronic intermittent ethanol exposure during adolescence reduces the effect of ethanol challenge on hippocampal allopregnanolone levels and Morris water maze task performance. Alcohol. 2006;39:151–158. doi: 10.1016/j.alcohol.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, File SE. Methodological considerations in neurobehavioral teratology. Pharmacol. Biochem. Behav. 1996;55:455–457. doi: 10.1016/s0091-3057(96)00272-9. [DOI] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Dev. Alcohol. 2005;17:143–159. [PubMed] [Google Scholar]
- Stewart RB, Gatto GJ, Lumeng L, Li TK, Murphy JM. Comparison of alcohol-preferring (P) and nonpreferring (NP) rats on tests of anxiety and for the anxiolytic effects of ethanol. Alcohol. 1993;10:1–10. doi: 10.1016/0741-8329(93)90046-q. [DOI] [PubMed] [Google Scholar]
- Stone EA, Quartermain D. Greater behavioral effects of stress in immature as compared to mature male mice. Physiol. Behav. 1997;63:143–145. doi: 10.1016/s0031-9384(97)00366-1. [DOI] [PubMed] [Google Scholar]
- Sunahara GI, Kalant H, Schofield M, Grupp L. Regional distribution of ethanol in the rat brain. Can. J. Physiol. Pharmacol. 1978;56:988–992. doi: 10.1139/y78-157. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Richardson RC, Markwiese-Foerch B, Wilson WA, Little PJ. Developmental differences in the acquisition of tolerance to ethanol. Alcohol. 1998;15:311–314. doi: 10.1016/s0741-8329(97)00135-3. [DOI] [PubMed] [Google Scholar]
- Thor DH, Holloway WR., Jr Social play in juvenile rats: a decade of methodological and experimental research. Neurosci. Biobehav. Rev. 1984;8:455–464. doi: 10.1016/0149-7634(84)90004-6. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Niesink RJ, Van Ree JM. The neurobiology of social play behavior in rats. Neurosci. Biobehav. Rev. 1997;21:309–326. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol. Clin. Exp. Res. 2002;26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Differences in the social consequences of ethanol emerge during the course of adolescence in rats: social facilitation, social inhibition, and anxiolysis. Develop. Psychobiol. 2006a;48:146–161. doi: 10.1002/dev.20124. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Ontogeny of acute tolerance to ethanol-induced social inhibition in Sprague-Dawley rats. Alcohol. Clin. Exp. Res. 2006b;30:1833–1844. doi: 10.1111/j.1530-0277.2006.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Chronic tolerance to the social consequences of ethanol in adolescent and adult Sprague-Dawley rats. Neurotoxicol. Teratol. 2007;29:23–30. doi: 10.1016/j.ntt.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Social interactions in adolescent and adult Sprague-Dawley rats: impact of social deprivation and test context familiarity. Behav. Brain Res. 2008;188:398–405. doi: 10.1016/j.bbr.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP, Spear NE. Social behavior and social motivation in adolescent rats: role of housing conditions and partner's activity. Physiol. Behav. 1999;67:475–482. doi: 10.1016/s0031-9384(98)00285-6. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP, Spear NE. Acute effects of ethanol on behavior of adolescent rats: role of social context. Alcohol. Clin. Exp. Res. 2001;25:377–385. [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol. Clin. Exp. Res. 2007;31:1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder HS. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol. Biochem. Behav. 2002;73:673–677. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Dev. Psychobiol. 1997;30:141–150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]