Abstract
Strigolactones are a group of terpenoid lactones that act as a host-derived signal in the rhizosphere communication of plants with arbuscular mycorrhizal (AM) fungi and root parasitic weeds as well as an endogenous plant hormone regulating shoot branching in plants. Strigolactones induce hyphal branching in AM fungi at very low concentrations, suggesting a highly sensitive perception system for strigolactones present in AM fungi. However, little is known about the structural requirements of strigolactones for hyphal branching in AM fungi. Here, we tested a series of natural and synthetically modified strigolactones as well as non-strigolactone-type germination stimulants for hyphal branching-inducing activity in germinating spores of the AM fungus Gigaspora margarita. All tested compounds with a tricyclic lactone coupled to a methylbutenolide via an enol ether bond showed activity, but differed in the active concentration and in the branching pattern of hyphae. Truncation of the A- and AB-rings in the tricyclic ABC lactone of strigolactones resulted in a drastic reduction in hyphal branching activity. Although the connection of the C-ring in the tricyclic lactone to the methylbutenolide D-ring was shown to be essential for hyphal branching, the bridge structure in the C–D part was found not necessarily to be enol ether, being replaceable with either alkoxy or imino ethers. These structural requirements in AM fungi are very similar but not identical to those observed in root parasitic weeds, especially with respect to the enol ether bridge in the C–D part.
Keywords: Arbuscular mycorrhizal fungi, Gigaspora margarita, Hyphal branching, Root parasitic weeds, Shoot branching, Strigolactone
Introduction
Arbuscular mycorrhiza (AM) is formed by >80% of land plants with fungi that belong to the ancient phylum Glomeromycota (Smith and Read 2008, Schüßler et al. 2001). AM fungi are obligate symbionts, relying on carbon provided by their hosts to complete their life cycle. The fungi colonize the cortex of roots, where they differentiate into highly branched tree-like structures called arbuscules, which are thought to be the main site of nutrient exchange between the fungal and plant symbiotic partners. Concomitant development of extraradical hyphae outside the roots allows the fungi to supply the host with the essential inorganic nutrients phosphate and nitrate, and other minerals from the soil. In return, AM fungi receive carbohydrates derived from photosynthesis in the host. Thus, AM symbioses support the growth and development of the host, and also confer resistance to the plant against pathogens and environmental stresses. The evolutionary origin of AM fungi dates back to about 460 million years ago, as shown by fossil records from the Ordovician and Devonian eras (Remy et al. 1994, Redecker et al. 2000), suggesting that AM symbioses have been instrumental in the successful colonization of land by plants. AM fungi are ancient symbionts that have co-evolved with land plants over 460 million years, and thus are of central importance in both agriculture and natural ecosystems (Parniske 2008).
In one of the first stages of host recognition, the hyphae of AM fungi show extensive branching in the vicinity of host roots, which helps them to ensure contact with the host root and the establishment of symbiosis (Giovannetti et al. 1993, Giovannetti et al. 1994). Host roots release signal molecules called ‘branching factors’ that act as early cues for differential hyphal branching in AM fungi (Giovannetti et al. 1996). A strigolactone, (+)-5-deoxystrigol, has been isolated and identified as an inducer of hyphal branching through bioassay-guided fractionation of the root exudates from the model legume Lotus japonicus (Akiyama et al. 2005). In addition to hyphal branching, strigolactones have also been found to stimulate spore germination and molecular and cellular responses involved in the energy metabolism of mitochondria in AM fungi (Besserer et al. 2006, Besserer et al. 2008). Strigolactones are a group of terpenoid lactones, previously isolated as seed germination stimulants for the parasitic weeds Striga and Orobanche (Bouwmeester et al. 2007). These terpenoid lactones have a unique structure, consisting of a tricyclic lactone and a methylbutenolide coupled with an enol ether bond. The tricyclic lactone of strigolactones was shown to be formed by oxidative cleavage of C40 carotenoids that originate from the plastidic non-mevalonate methylerythritol phosphate pathway (Matusova et al. 2005). Analysis of shoot branching mutants of rice and pea impaired in genes encoding carotenoid cleavage dioxygenases (CCDs), CCD7 and CCD8, showed that strigolactones act as a new hormone class or their biosynthetic precursors in regulating shoot branching (Gomez-Roldan et al. 2008, Umehara et al. 2008). The strigolactone-deficient ccd8 mutants of pea significantly lost the capacity to develop AM symbiosis, indicating an important role for strigolactones as a rhizosphere signal that mediates host location in AM fungi (Gomez-Roldan et al. 2008).
Strigolactones show potent activity at very low concentrations, suggesting a highly sensitive perception system for strigolactones present in AM fungi (Akiyama and Hayashi 2006). However, little is known about the structural requirements of strigolactones for the biological effects on AM fungi. Although >10 natural strigolactones have been isolated from a variety of plants and a number of structural analogs have been synthesized to date (Yoneyama et al. 2009, Zwanenburg et al. 2009), only a limited number of them have been examined for their effects on AM fungi. In contrast, extensive studies on the structure–activity relationships of strigolactones in germination stimulation of root parasitic weed seeds were conducted after the discovery of strigol in 1966 (Cook et al. 1966). The C- and D-rings connected with an enol ether bond in the strigolactone molecule were identified to be the essential structure for germination stimulation (Mangnus and Zwanenburg 1992b), and both constitution and configuration of the stimulants were also shown to influence the biological activity greatly (Yoneyama et al. 2009, Zwanenburg et al. 2009). The inherent instability of strigolactone is principally due to easy cleavage of the enol ether bond by nucleophilic agents, including water (Mangnus and Zwanenburg 1992b). Taken with our previous observations that all strigolactones tested were highly active on AM fungi, and that their activity drastically decreased after concentrating or storing a solution in nucleophilic solvents such as pure or aqueous methanol, it appears that the C–D part is also essential for hyphal branching in AM fungi (Akiyama et al. 2005).
To clarify the structural requirements of strigolactones for hyphal branching in AM fungi, we tested a series of natural and synthetically modified strigolactones as well as non-strigolactone-type terpene lactones for hyphal branching- inducing activity in germinating spores of the AM fungus Gigaspora margarita in this study. We found that the structural requirements of strigolactones for hyphal branching in AM fungi are very similar but not identical to those observed in root parasitic weeds, especially with respect to the enol ether bridge in the C–D part.
Results
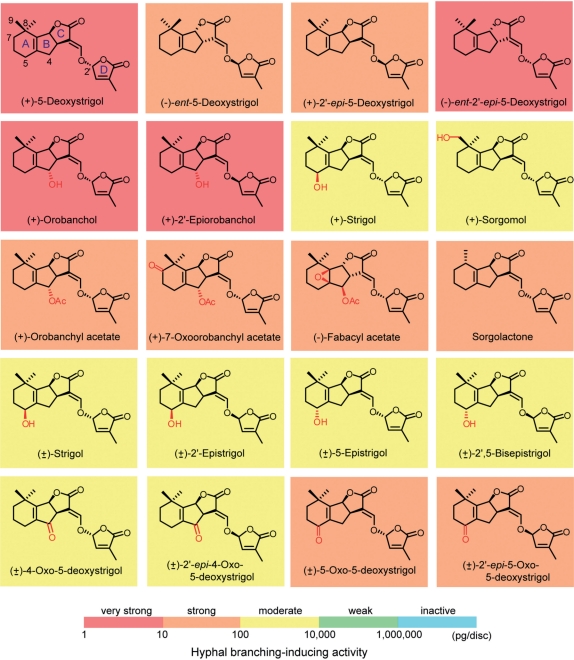
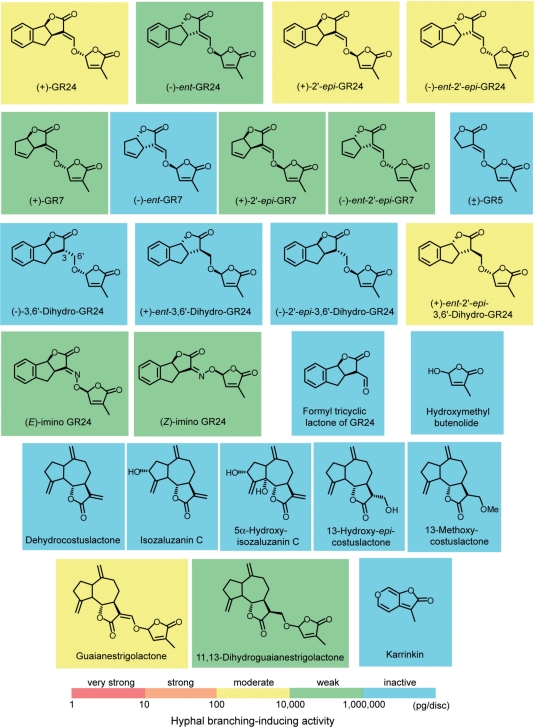
In total, 44 compounds including natural and chemically modified natural strigolactones (Fig. 1), synthetic GR (‘germination releaser’) analogs and non-strigolactone-type germination stimulants (Fig. 2) were tested for hyphal branching-inducing activity in germinating spores of an AM fungus G. margarita by paper disc assay. The activity was evaluated by determining the minimum effective concentration using serial dilutions of the test compounds (Table 1).
Fig. 1.
Chemical structures of natural and chemically modified natural strigolactones. Hyphal branching-inducing activities in Gigaspora margarita are indicated by a colored background to each chemical structure.
Fig. 2.
Chemical structures of synthetic GR (‘germination releaser’) analogs and non-strigolactone-type germination stimulants. Formyl tricyclic lactone and hydroxymethylbutenolide identified as inactive water degradation products of GR24 are also included. Hyphal branching-inducing activities in Gigaspora margarita are indicated by a colored background to each chemical structure.
Table 1.
The minimum effective concentrations (MECs) of tested compounds for hyphal branching-inducing activity in Gigaspora margarita
| Compound | MEC (pg per disc)a |
|---|---|
| (+)-5DS | 3 |
| (−)-ent-5DS | 30 |
| (+)-2′-epi-5DS | 30 |
| (−)-ent-2′-epi-5DS | 3 |
| (+)-Orobanchol | 1 |
| (+)-2′-Epiorobanchol | 1 |
| (+)-Strigol | 100 |
| (+)-Sorgomol | 100 |
| (±)-Strigol | 100 |
| (±)-2′-Epistrigol | 100 |
| (±)-5-Epistrigol | 100 |
| (±)-2′,5-Bisepistrigol | 100 |
| (+)-Orobanchyl acetate | 10 |
| (+)-7-Oxoorobanchyl acetate | 10 |
| (±)-4-Oxo-5DS | 100 |
| (±)-2′-epi-4-Oxo-5DS | 100 |
| (±)-5-Oxo-5DS | 10 |
| (±)-2′-epi-5-Oxo-5DS | 10 |
| (−)-Fabacyl acetate | 10 |
| (±)-Sorgolactoneb | <30 |
| (+)-GR24 | 100 |
| (−)-ent-GR24 | 10,000 |
| (+)-2′-epi-GR24 | 1,000 |
| (−)-ent-2′-epi-GR24 | 1,000 |
| (+)-GR7 | 10,000 |
| (−)-ent-GR7 | >1,000,000 |
| (+)-2′-epi-GR7 | 1,000,000 |
| (−)-ent-2′-epi-GR7 | 1,000,000 |
| (±)-GR5 | >1,000,000 |
| (−)-3,6′-Dihydro-GR24 | >1,000,000 |
| (+)-ent-3,6′-Dihydro-GR24 | >1,000,000 |
| (−)-2′-epi-3,6′-Dihydro-GR24 | >1,000,000 |
| (+)-ent-2′-epi-3,6′-Dihydro-GR24 | 3,000 |
| (±)-Imino GR24F | 10,000 |
| (±)-Imino GR24S | 10,000 |
| Formyl lactone of GR24 | >300,000 |
| Hydroxymethylbutenolide | >300,000 |
| Dehydrocostuslactone | >1,000,000 |
| Isozaluzanin C | >1,000,000 |
| 5α-Hydoxyisozaluzanin C | >1,000,000 |
| 13-Hydroxy-epi-costuslactone | >1,000,000 |
| 13-Methoxycostuslactone | >1,000,000 |
| Guaianestrigolactone | 1,000 |
| 11, 13-Dihydroguaianestrigolactone | 100,000 |
| Karrikin (KAR1) | >1,000,000 |
a Determined by serial 10-fold dilutions.
b Tested previously (Akiyama et al. 2005).
Hyphal branching activity of natural and chemically modified strigolactones
5-Deoxystrigol (5DS) and its 2′-epimer each synthesized as a racemate (Akiyama et al. 2005, Umehara et al. 2008) were separated into their enantiomers by chiral HPLC to give four stereoisomers, (+)-5DS, (−)-ent-5DS, (+)-2′-epi-5DS and (−)-ent-2′-epi-5DS. The absolute configurations at C-3a, 8b and 2′ of each isomer were determined by comparison of their circular dichroism (CD) spectra with those of the corresponding stereoisomers of strigol and sorgolactone (Frischmuth et al. 1991, Sugimoto et al. 1998, Reizelman et al. 2000; Supplementary Fig. S1). The two 2′(R)-isomers, (+)-5DS and (−)-2′-epi-5DS, were more active than their respective 2′(S)-enantiomers, (−)-ent-5DS and (+)-ent-2′-epi-5DS (Fig. 3B). The minimum effective concentrations of the (R)- and (S)-isomers in inducing hyphal branching of the AM fungi G. margarita were 3 and 30 pg per disc, respectively.
Fig. 3.
Hyphal branching of Gigaspora margarita induced by natural and synthetically modified strigolactones. (A) Control (70% ethanol in water). (B) (+)-5DS, (3 pg per disc). (C) (+)-Orobanchol (1 pg per disc). (D) (+)-2′-Epiorobancol (10 pg per disc). (E) (+)-7-Oxoorobanchyl acetate (100 pg per disc). (F) (−)-Fabacyl acetate (10 pg per disc). (G) (±)-2′,5-Bisepistrigol (100 pg per disc). (H) (±)-2′-epi-5-Oxo-5DS (10 pg per disc). (I) (+)-ent-2′-epi-3,6′-dihydro-GR24 (10 ng per disc). (J) Imino GR24F (1 μg per disc). Arrows indicate the direction of growth of primary hyphae. Control hyphae, which were treated with 70% ethanol–water, formed no hyphal branches, as shown in A. Scale bars, 1 mm.
Among four monohydroxylated derivatives of 5DS, (+)- orobanchol and (+)-2′-epiorobanchol (Yokota et al. 1998, Xie et al. 2007) were highly active, inducing hyphal branching at 1 pg per disc (Fig. 3C, D). The two 4α-hydroxyl-5DSs, having a (R) and (S) configuration at C-2′, respectively, were the most potent among all tested compounds in this study. (+)-Strigol and (+)-sorgomol (Cook et al. 1966, Xie et al. 2008b), possessing 5β- and 9-hydroxyl on (+)-5DS, respectively, were 100-fold less active than the two 4α-hydroxyl-5DSs, exhibiting activity at 100 pg per disc. All four racemic strigol diastereoisomers, (±)-strigol, (±)-2′-epistrigol, (±)-5-epistrigol and (±)-2′,5-bisepistrigol, prepared by organic synthesis (Reizelman et al. 2000) showed activity at 100 pg per disc (Fig. 3G). (+)-Orobanchyl acetate (Müller et al. 1992, Matsuura et al. 2008, Xie et al. 2008a), in which the 4α-hydroxyl group of (+)-orobanchol is acetylated, were 10-fold less active than (+)-orobanchol (10 pg per disc). (+)-7-Oxoorobanchyl acetate (Xie et al. 2009a) was as active as (+)-orobanchyl acetate (10 pg per disc) (Fig. 3E). Racemic 4- and 5-oxo-5DS and their respective 2′-epimers were prepared by allylic oxidation of racemic 5DS and 2′-epi-5DS with chromium trioxide-3,5-dimethylpyrazole complex (Hirayama and Mori 1999). 4- and 5-Oxo-strigolactones have not yet been isolated from natural sources. (±)-4-Oxo-5DS and its (±)-2′-epimer showed activity at 100 pg per disc, whereas (±)-5-oxo-5DS and its (±)-2′-epimer were 10-fold more active than the 4-oxo-strigolactones (10 pg per disc) (Fig. 3H). (−)-Fabacyl acetate (Xie et al. 2009b), the first natural ent-strigolactone containing an epoxide group, exhibited activity at 10 pg per disc (Fig. 3F). We previously reported that synthetically prepared racemic sorgolactone (Hauck et al. 1992, Sugimoto et al. 1998), a mono-8-demethyl derivative of 5-DS, exhibited activity at concentrations as low as 30 pg per disc, although its activity at <30 pg per disc has not yet been evaluated (Akiyama et al. 2005).
Hyphal branching activity of synthetic strigolactone analogs
The synthetic strigolactone analog, GR24, was prepared as a mixture of two racemic diastereomers (Mangnus et al. 1992b), and following chiral HPLC separation gave four stereoisomers, (+)-GR24, (−)-ent-GR24, (+)-2′-epi-GR24 and (−)-ent-2′-epi-GR24 (Thuring et al. 1997). (+)-GR24, which has the same configuration as (+)-5DS, was considerably more active than its enantiomer (−)-ent-GR24. The difference in activity amounts to a factor of 100 (100 pg vs. 10 ng per disc). The two enantiomeric isomers (+)-2′-epi-GR24 and (−)-ent-2′-epi-GR24 were less active than (+)-GR24 but more active than (−)-ent-GR24, exhibiting activity at 1 ng per disc.
Four stereoisomers of GR7, the A-ring-truncated analog, were prepared by starting from commercially available, pure enantiomers of ‘Corey’s lactone’ as reported (Mangnus and Zwanenburg 1992a). (+)-GR7, which has the same stereochemistry as (+)-GR24 at the corresponding chiral centers, was only weakly active (10 ng per disc). Its enantiomer (−)-ent-GR7 was totally inactive, eliciting no branching response even at the highest concentration (1 μg per disc) tested in our assays. The two enantiomeric isomers (+)-2′-epi-GR7 and (−)-ent-2′-epi-GR7 exhibited activity only at the highest concentration (1 μg per disc). A racemic mixture of the AB-ring-truncated synthetic analog, GR5 (Mangnus et al. 1992a), was inactive even at 1 μg per disc.
A reduced analog of GR24, 3,6′-dihydro-GR24 (Mangnus and Zwanenburg 1992b), in which the carbon double bond in the C–D-connecting enol ether is reduced to a single bond, is totally inactive in inducing germination of root parasitic weeds. To test the essentiality of the enol ether bond in the C–D part for hyphal branching-inducing activity in AM fungi, we synthesized reduced GR24 and tested it for hyphal branching. Hydroxymethylation of tricyclic lactone was conducted with base and formaldehyde vapors as previously described, which afforded 3α-hydroxymethyl lactone as a major product. Coupling of the hydroxymethyl lactone with 4-bromo-2-methyl-2-buten-4-olide gave reduced GR24 as a mixture of two racemic diastereomers. This mixture of four stereoisomers of reduced GR24 showed hyphal branching activity at concentrations as low as 100 ng per disc. The four stereoisomers were separated into optically pure forms by successive normal-phase and chiral HPLC. The absolute configuration of (−)-2′-epi-3,6′-dihydro-GR24, obtained from the (−)-3a(R),8b(S)-tricyclic lactone, was determined as 3(S), 3a(R), 8b(S) and 2′(S) by X-ray crystallographic analysis. Consequently, the configuration of the remaining stereoisomers could be assigned on the basis of the synthetic sequence and chiral HPLC analysis (Supplementary Fig. S2). Among the four isomers tested, only one diastereomer (+)-ent-2′-epi-3,6′-dihydro-GR24 with the same configurations as (−)-ent-2′-epi-GR24 at the corresponding chiral centers, showed hyphal branching activity in the AM fungus. The activity of this compound was nearly comparable with that of (−)-ent-2′-epi-GR24 (3 ng per disc) (Fig. 3I).
To examine further the significance of the bridge structure of the C–D part in hyphal branching, we tested the GR24 imino analog for its biological effect on the AM fungus. The carbon–carbon double bond of the enol ether in GR24 is replaced by a carbon–nitrogen double bond in the imino analog (Kondo et al. 2007). Among the four possible racemic stereoisomers, we isolated two stereoisomers from the reaction product mixture, possibly being a pair of epimers at C-2′ having either (E) or (Z) configuration with respect to the C–N double bond as deduced from the close similarity in their 1H-NMR (nuclear magnetic resonance) spectra. We tentatively called the two epimers imino GR24F (fast-moving) and imino GR24S (slow-moving) according to their chromatographic behavior on HPLC. Both diastereomers of the GR24 imino analog, imino GR24F and imino GR24S, were active on the AM fungus, exhibiting activity at 10 ng per disc (Fig. 3J).
Hyphal branching activity of non-strigolactone-type germination stimulants
The non-strigolactone-type germination stimulants were tested for hyphal branching activity in G. margarita. Guaianolide sesquiterpene lactones induce seed germination of the sunflower parasite, Orobanche cumana (De Luque et al. 2000, Galindo et al. 2002). None of the four guaianolide sesquiterpene lactones showed activity on the fungus even at 1 μg per disc. However, a guaianestrigolactone (Macías et al. 2009), a synthetic analog of guaianolide sesquiterpene lactone having an enol ether-bridged methylbutenolide, showed activity comparable with GR24 (1 ng per disc). The reduced analog of guaianestrigolactone, 11,13-dihydroguaianestrigolactone (Macías et al. 2009), was also active at concentrations as low as 100 ng per disc. A karrikin, 3-methyl-2H-furo[2,3-c]pyran-2-one (KAR1), a butenolide isolated from smoke water as a primary seed germination stimulant in smoke (Nelson et al. 2009), was inactive on hyphal branching even at 1 μg per disc.
Hyphal branching patterns
Extensive evaluation of natural and synthetic strigolactones for hyphal branching in G. margarita enabled us to classify the branching inducers into three groups according to the branching pattern of hyphae. (+)-Orobanchol, (+)-7-oxoorobanchyl acetate and (+)-sorgomol induced highly branched short hyphae up to the sixth order from the secondary hyphae treated (Fig. 3C, E). The remaining natural strigolactones stimulated the formation of lower order branches up to the fourth order, mainly consisting of long tertiary hyphae, from the secondary hyphae of the AM fungus (Fig. 3B, D, F, G, H). Low order branches up to the fourth order, consisting mostly of short tertiary hyphae, were formed from the secondary hyphae upon treatment with synthetic strigolactone analogs, GR24, GR7, reduced GR24, imino GR24 and the two non-strigolactone-type guaianestrigolactones (Fig. 3I, J).
Identification of water degradation products of strigolactones
Strigolactones are unstable in water, where they are degraded into inactive products. A tentative reaction mechanism proposed for the water degradation of strigolactones involves a Michael addition of water to the enol ether bond, followed by elimination of the D-ring (Mangnus and Zwanenburg 1992b). However, the putative inactive degradation products have not yet been identified. To clarify the water degradation products of strigolactones, we incubated GR24 (as a mixture of two racemic diastereomers) in water containing 3% methanol at 32°C, and monitored the degradation reaction by HPLC. Along with a gradual decrease of GR24, two new peaks appeared at 26.8 and 11.0 min, and gradually increased. The compounds corresponding to these two peaks were identified to be formyl tricyclic ABC lactone and hydroxymethylbutenolide by the comparison of their retention times on HPLC, and electron ionization mass spectrometry (EI-MS) and 1H-NMR spectra with those of the synthetic standards (Fig. 4A, B). These two degradation products did not show any hyphal branching- inducing activities on G. margarita at 300 ng per disc. (±)-5DS was also degraded in water to the corresponding formyl tricyclic lactone and hydroxymethylbutenolide, the identity of which were confirmed by EI-MS analysis (Fig. 4C). The half-lives of 5DS and GR24 in water were about 1.5 and 10 d, respectively (Fig. 4D, E). The level of the formyl tricyclic lactone of 5DS rapidly increased within 24 h, and then gradually decreased over time, indicating further degradation of the formyl lactone possibly by oxidation and hydrolysis. In contrast, no appreciable decrease was observed in 5DS concentration when incubated in acetone at 32°C for 21 d (Fig. 4D).
Fig. 4.
Spectroscopic analysis of water degradation products of GR24 and 5DS. EI-MS spectra of formyl tricyclic lactone (A) and hydroxymethylbutenolide (B) isolated as degradation products of GR24 in water. (C) EI-MS spectrum of formyl tricyclic lactone isolated in the water degradation experiment of 5DS. (D) Time course of 5DS levels in water (filled circle) or acetone (open circle) incubated at 32°C. (E) Time course of GR24 levels in water incubated at 32°C.
Discussion
All tested natural and synthetic strigolactones with a tricyclic lactone coupled to a methylbutenolide via an enol ether bond showed hyphal branching activity in the AM fungus G. margarita. Truncation of the A- and AB-rings in the tricyclic ABC lactone of strigolactones resulted in a drastic reduction in hyphal branching activity. Although the A-ring truncated analog GR7 was weakly active, deletion of the AB-ring from the strigolactone skeleton as in GR5 resulted in the complete loss of the biological effect on the AM fungus. A drastic reduction in hyphal branching activity upon truncation of the A-ring was also observed for another AM fungus, Gigaspora rosea (Besserer et al. 2006). Formyl tricyclic ABC lactone and hydroxymethylbutenolide, identified as water degradation products of strigolactones for the first time in this study, were inactive, confirming that cleavage of the enol ether bond results in the loss of activity on the AM fungus. Interestingly, a moderate activity was observed for the guaianestrigolactone, in which the bicyclo[4.3.0]nonane ring system in the AB-ring of strigolactones is rearranged to bicyclo[5.3.0]decane. This suggests that a certain variation is allowed in the bicyclic AB-ring systems for the activity. Taking all this together, the C–D moiety fused to at least one ring system is necessary for the compounds to act as an inducer of hyphal branching in AM fungi.
Hyphal branching activity on the AM fungus varied significantly depending on the substitution on the AB-ring of strigolactones. The introduction of a hydroxyl group at either C-5 or C-9 in the A-ring of 5DS as in strigol isomers and (+)-sorgomol weakened the activity. Replacing dimethylcyclohexene with benzene also resulted in a significant decrease in hyphal branching activity as indicated by 5DS between GR24. Racemic sorgolactone showed activity comparable with that of racemic 5DS in a previous study (Akiyama et al. 2005), indicating that removal of a methyl group from the geminal dimethyl in the A-ring does not affect the activity. Oxidation of methylene to carbonyl at either C-5 or C-7 also did not affect the activity. Taken together, polar substitution and ring replacement in the A-ring reduce the activity of strigolactone in the AM fungus.
In contrast to those observed for the A-ring, the introduction of an α-oriented hydroxyl group at C-4 in the B-ring considerably strengthened the hyphal branching activity. The two 4α-hydroxyl-5DSs, (+)-orobanchol and (+)-2′-epiorobanchol, were the most potent inducers of hyphal branching among all the compounds tested in our assay. The acetyl and oxo derivatives, (+)-orobanchyl acetate and 5-oxo-5DSs, were weaker than the two 4α-hydroxyl-5DSs. This suggests that the hydrogen attached to oxygen is important for potent activity on the AM fungus, which could act as a hydrogen bond donor in binding interactions with the putative strigolactone receptor in the AM fungus.
The absolute configuration at C-2′ has been regarded as an important structural feature of strigolactones for their germination stimulation activities in root parasitic weeds (Yoneyama et al. 2009). There have been trends that (R)-epimers are more active than their respective (S)-isomers throughout the extensive evaluation of germination stimulation activity of natural and synthetic strigolactone stereoisomers since the isolation of (+)-strigol, the first natural strigolactone, in 1966 (Cook et al. 1966). However, 2′-epiorobanchol, the first natural strigolactone with the 2′(S)-configuration, is slightly more active than 2′(R)-orobanchol on seed germination of Orobanche minor and O. ramosa (Xie et al. 2007). Similar trends were observed for hyphal branching in AM fungi in this study. 2′(R)-Epimers of 5DS, 2′-epi-5DS, GR24 and GR7 were more active than their respective 2′(S)-isomers, and both (+)-orobanchol and (+)-2′-epiorobanchol were equally highly active on AM fungus. This indicates that the introduction of a 4α-hydroxyl moiety increases the activity of strigolactones, especially of 2′(S)-epimers, in both AM fungi and root parasitic weeds. It should be noted that the other chiral centers at C-3a and 8b common to all strigolactones also significantly affect the biological activity in AM fungus as exemplified by the finding that (+)-GR24 and (+)-2′-epi-GR24 were more active than their respective 3a,8b-bisepimers.
The enol ether bond connecting the C- and D-rings in strigolactone molecules was shown to be responsible for stimulating germination of root parasitic weeds (Mangnus and Zwanenburg 1992b). A proposed molecular mechanism for germination stimulation involves the addition of a nucleophilic species, present at a putative receptor site, to the enol ether carbon double bond in a Michael fashion, followed by elimination of the D-ring. Indeed, reduced GR24, in which the carbon double bond in the enol ether is reduced to a single bond, is totally inactive in inducing germination of root parasitic weeds (Mangnus and Zwanenburg 1992b). However, imino analogs of strigolactones, in which the carbon–carbon double bond in the enol ether is replaced by a carbon–nitrogen double bond, were recently found to show germination stimulation activity in root parasitic weeds (Kondo et al. 2007). Therefore, the essentiality of the enol ether structure in the C–D part for weed seed germination is still undetermined. In an AM fungus G. margarita, both reduced and imino GR24 analogs showed activity at slightly higher concentrations than GR24. Taken together, the requirement with respect to the enol ether bridge in the biological effects differs between AM fungi and root parasitic weeds, and the C–D part is not necessarily bridged by enol ether for hyphal branching in the case of the AM fungus.
The hyphal branching inducers differed not only in the active concentration but also in the branching pattern of hyphae they induced. The phenotype of branching hyphae can be classified into three types: (I) highly branched short hyphae up to the sixth order; (II) low order branches up to the fourth order, mainly consisting of long tertiary hyphae; and (III) low order branches up to the fourth order, mainly consisting of short tertiary hyphae. The two epimeric isomers, (+)-orobanchol and (+)-2′-epiorobanchol, were equally highly active on G. margarita, inducing hyphal branching at 1 pg per disc, but the hyphal morphologies were quite different between the two; the former induces type I branches, but the latter induces type II branches. Taken with the previous finding that all the plant species examined so far exude mixtures of strigolactones, varying in the amounts and ratios depending on growth stages and nutrient conditions (Sato et al. 2003, Yoneyama et al. 2007a, Yoneyama et al. 2007b, López-Ráez and Bouwmeester 2008, Yoneyama et al. 2008, Yoneyama et al. 2009), it is tempting to speculate that hyphae of AM fungi are successfully attached to host roots through the successive formation of long coarse and short fine branching hyphae by detecting a blend of strigolactones emitted from the host. The lifetime of individual strigolactones in the rhizosphere would be another critical factor for host location in AM fungi, which must be considerably different among natural strigolactones as indicated by the water degradation experiments conducted for 5DS and GR24 in this study.
Our extensive structure–activity relationship study demonstrates that strigolactones show potent activity at very low concentrations in a structure-dependent manner, suggesting that the induction of hyphal branching in AM fungi proceeds via a receptor-mediated mechanism. Although detailed structure–activity relationship studies of strigolactones have not yet been accomplished, genetic approaches using strigolactone-insensitive shoot branching mutants have led to the isolation of the two genes, MAX2/RMS4/D3 and D14/D88/HTD2, encoding an F-box protein and an α/β-fold hydrolase, respectively (Ishikawa et al. 2005, Arite et al. 2009). They are expected to be a strong candidate of the strigolactone receptor since some of their families function as a component for the perception of plant hormones (Beveridge and Kyozuka 2010). In an effort to search for the strigolactone receptor in root parasitic weeds, a binding protein of strigolactone of 60 kDa was detected in the membrane fractions of pre-conditioned seeds of Striga hermonthica by applying the affinity labeling technique using biotinylated GR24 (Reizelman et al. 2003, Zwanenburg et al. 2009). Having established the structural requirements for strigolactones for hyphal branching in AM fungi, we are now designing and synthesizing molecular probes for affinity labeling studies, aiming at identification of the strigolactone receptor in AM fungi.
Materials and Methods
Hyphal branching assay
Hyphal branching assays for G. margarita Becker & Hall were conducted as reported previously (Akiyama et al. 2005, Yoneyama et al. 2008). Spores of G. margarita (CGC1411; Central Glass Co., Tokyo, Japan), surface-sterilized with 0.2% NaClO and 0.05% Triton X-100, were inserted into a 0.2% Phytagel gel (Sigma-Aldrich, Tokyo, Japan) containing 3 mM MgSO4 in 60 mm plastic Petri dishes. The dishes were incubated vertically for 5–7 d in a 2% CO2 incubator at 32°C. Test samples were first dissolved in acetone and then diluted with 70% ethanol in water. Paper discs (6 mm in diameter, ADVANTEC, Tokyo, Japan) loaded with test sample solution were placed in front of the tips of the secondary hyphae. The control was 70% ethanol in water. The hyphal branching patterns were observed 24 h after treatment. The sample was scored as positive if the clusters of hyphal branches of higher order are induced from the treated secondary hyphae located proximal to the paper discs. No hyphae or an occasional single branching hypha was induced in the 70% EtOH–H2O control. The assay was repeated at least three times, using three dishes for each concentration.
Spectroscopic methods
Infrared (IR) spectra were recorded with a JASCO FT/IR-460plus spectrometer, and ultraviolet spectra were measured with a Hitachi U-3210 instrument. Mass spectra were recorded on a JEOL JMS-700 instrument or a Shimadzu GCMS-QP2010 Plus instrument in the direct injection mode. 1H- and 13C-NMR spectra were obtained with a JEOL JNM-AL400 NMR spectrometer. CD spectra were measured with a JASCO J-820 spectropolarimeter.
Chemicals
(+)-Orobanchol, (+)-2′-epiorobanchol, (+)-strigol, (+)-sorgomol, (+)-orobanchyl acetate, (+)-7-oxoorobanchyl acetate and (−)-fabacyl acetate were a generous gift of Koichi Yoneyama (Utsunomiya University, Japan). Dehydrocostuslactone, isozaluzanin C, 5α-hydroxyisozaluzanin C, 13-hydroxy-epi-costuslactone, 13-methoxycostuslactone, guaianestrigolactone and 11,13-dihydroguaianestrigolactone were kindly provided by Juan C. G. Galindo (Universidad de Córdoba, Spain). 3-Methyl-2H-furo[2,3-c]pyran-2-one was provided by Gavin R. Flematti (The University of Western Australia, Australia). (±)-5-Deoxystrigol, (±)-2′-epi-5-deoxystrigol and the corresponding formyl tricyclic lactone were prepared as reported previously (Akiyama et al. 2005, Umehara et al. 2008). (±)-Strigol, (±)-2′-epistrigol, (±)-5-epistrigol and (±)-2′,5-bisepistrigol were synthesized from (±)-5-deoxystrigol and (±)-2′-epi-5-deoxystrigol as reported (Reizelman et al. 2000). (±)-GR24, (±)-2′-epi-GR24 and the corresponding formyl tricyclic lactone (Mangnus et al. 1992b), 3,6′-dihydro-GR24 (Mangnus and Zwanenburg. 1992b) and GR24 imino analog (Kondo et al. 2007) were synthesized according to previously reported procedures. (+)-GR7 and (+)-2′-epi-GR7, and (−)-ent-GR7 and (−)-ent-2′-epi-GR7 were prepared by starting, respectively, from (1R,5S)-(+)- and (1S,5R)-(−)-2-oxabicyclo[3.3.0]-oct-6-en-3-one (optical purity 99%, purchased from Sigma-Aldrich, Tokyo, Japan) as reported (Mangnus and Zwanenburg 1992a). (±)-GR5 was prepared by one pot synthetic procedures as reported previously (Mangnus at al. 1992a). All other chemicals were obtained from Wako Pure Chemical Industries (Osaka, Japan), Sigma-Aldrich (Tokyo, Japan) and Kanto Chemical (Tokyo, Japan).
Optical resolution of (+)-5-deoxystrigol, (−)-ent-5-deoxystrigol, (+)-2′-epi-5-deoxystrigol and (−)-ent-2′-epi-5-deoxystrigol
(±)-5-Deoxystrigol was chromatographed on a semi-preparative Chiralpak AD column HPLC column (φ 10 × 250 mm, Daicel, Osaka, Japan) employing isocratic elution with 10% isopropanol– n-hexane at a flow rate of 3.8 ml min−1. Compounds eluted from the column were monitored with a photodiode array detector. (+)-5-Deoxystrigol and (−)-ent-5-deoxystrigol, eluting as a single peak at 13.3 and 10.8 min, respectively, were collected. (±)-2′-epi-5-deoxystrigol was chromatographed as above to afford (+)-2′-epi-5-deoxystrigol and (−)-ent-2′-epi- 5-deoxystrigol that were eluted from the column at 11.9 and 11.2 min, respectively. The enantiomeric purities of four stereoisomers were estimated to be >99% e.e., respectively, by analytical HPLC using a Chiralpak AD-H column (φ 4.6 × 250 mm, 5 μm, Daicel, Osaka, Japan) employing isocratic elution with 20% isopropanol–n-hexane at a flow rate of 0.5 ml min−1 monitored with a photodiode array detector.
(+)-5-Deoxystrigol: 1H-NMR (400 MHz, CDCl3) δ 1.09 (3H, s, Me-10), 1.10 (3H, s, Me-9), 1.33–1.50 (2H, m, H-7), 1.63–1.71 (2H, m, H-6), 1.86–2.00 (2H, m, H-5), 2.03 (3H, t, J = 1.6 Hz, H-7′), 2.33 (1H, d, J = 18.0 Hz, H-4β), 2.71 (1H, dd, J = 9.0, 18.0 Hz, H-4α), 3.56–3.61 (1H, m, H-3a), 5.52 (1H, d, J = 8.0 Hz, H-8b), 6.15 (1H, m, H-2′), 6.92 (1H, m, H-3′), 7.41 (1H, m, H-6′); 13C-NMR (100 MHz, CDCl3) δ 10.8 (C-7′), 19.2 (C-6), 26.4 (C-5), 27.8 (C-9), 28.1 (C-10), 31.9 (C-8), 36.6 (C-3a), 39.0 (C-7), 41.3 (C-4), 88.5 (C-8b), 100.4 (C-2′), 114.5 (C-3), 136.0 (C-3′), 139.7 (C-4a), 140.9 (C-4′), 141.6 (C-8a), 149.7 (C-6′), 170.2 (C-5′), 171.7 (C-2); EI-MS m/z (relative intensity): 330 [M+] (7), 315 (3), 233 (28), 216 (16), 215 (34), 205 (8), 201 (12), 187 (25), 97 (100); UV (CH3CN) λmax 234 nm, (ε 17,000); [α]D32 +296° (c 0.0453, CH3CN); CD (acetonitrile) λmax (Δε) 262 (−1.7), 230 (25.7) nm.
(−)-ent-5-Deoxystrigol: 1H-NMR (400 MHz, CDCl3) δ 1.09 (3H, s, Me-10), 1.10 (3H, s, Me-9), 1.33–1.50 (2H, m, H-7), 1.63–1.71 (2H, m, H-6), 1.86–2.00 (2H, m, H-5), 2.03 (3H, t, J = 1.4 Hz, H-7′), 2.33 (1H, d, J = 17.2 Hz, H-4β), 2.71 (1H, dd, J = 9.4, 17.2 Hz, H-4α), 3.56–3.61 (1H, m, H-3a), 5.52 (1H, d, J = 8.0 Hz, H-8b), 6.15 (1H, m, H-2′), 6.92 (1H, m, H-3′), 7.41 (1H, m, H-6′); 13C NMR (100 MHz, CDCl3) δ 10.8 (C-7′), 19.2 (C-6), 26.4 (C-5), 27.8 (C-9), 28.1 (C-10), 31.9 (C-8), 36.6 (C-3a), 39.0 (C-7), 41.3 (C-4), 88.5 (C-8b), 100.4 (C-2′), 114.5 (C-3), 136.0 (C-3′), 139.7 (C-4a), 140.9 (C-4′), 141.6 (C-8a), 149.7 (C-6′), 170.2 (C-5′), 171.7 (C-2); EI-MS m/z (relative intensity): 330 [M+] (8), 315 (4), 233 (31), 216 (17), 215 (37), 205 (9), 201 (12), 187 (26), 97 (100); UV (CH3CN) λmax 233 nm, (ε 16,800); [α]D32 −294° (c 0.0462, CH3CN); CD (acetonitrile) λmax (Δε) 262 (1.7), 230 (−26.9) nm.
(+)-2′-epi-5-Deoxystrigol: 1H-NMR (400 MHz, CDCl3) δ 1.09 (3H, s, Me-10), 1.11 (3H, s, Me-9), 1.32–1.50 (2H, m, H-7), 1.63–1.71 (2H, m, H-6), 1.85–1.99 (2H, m, H-5), 2.03 (3H, t, J = 1.6 Hz, H-7′), 2.32 (1H, d, J = 17.6 Hz, H-4β), 2.68 (1H, dd, J = 9.2, 17.6 Hz, H-4α), 3.55–3.60 (1H, m, H-3a), 5.52 (1H, d, J = 8.0 Hz, H-8b), 6.13 (1H, m, H-2′), 6.93 (1H, m, H-3′), 7.42 (1H, m, H-6′); 13C-NMR (100 MHz, CDCl3) δ 11.0 (C-7′), 19.5 (C-6), 26.6 (C-5), 28.1 (C-9), 28.4 (C-10), 32.2 (C-8), 36.8 (C-3a), 39.3 (C-7), 41.6 (C-4), 88.8 (C-8b), 100.8 (C-2′), 114.8 (C-3), 136.1 (C-3′), 139.8 (C-4a), 141.3 (C-4′), 142.0 (C-8a), 150.3 (C-6′), 170.6 (C-5′), 172.0 (C-2); EI-MS m/z (relative intensity): 330 [M+] (9), 315 (4), 233 (31), 216 (17), 215 (35), 205 (9), 201 (12), 187 (26), 97 (100); UV (CH3CN) λmax 234 nm, (ε 15,500); [α]D32 +184° (c 0.0426, CH3CN); CD (acetonitrile) λmax (Δε) 240 (2.6), 218 (−8.9) nm.
(−)-ent-2′-epi-5-Deoxystrigol: 1H-NMR (400 MHz, CDCl3) δ 1.07 (3H, s, Me-10), 1.09 (3H, s, Me-9), 1.30–1.48 (2H, m, H-7), 1.61–1.69 (2H, m, H-6), 1.82–1.97 (2H, m, H-5), 2.01 (3H, t, J = 1.6 Hz, H-7′), 2.30 (1H, d, J = 16.8 Hz, H-4β), 2.66 (1H, dd, J = 8.8, 16.8 Hz, H-4α), 3.52–3.58 (1H, m, H-3a), 5.50 (1H, d, J = 8.0 Hz, H-8b), 6.11 (1H, m, H-2′), 6.91 (1H, m, H-3′), 7.40 (1H, m, H-6′); 13C NMR (100 MHz, CDCl3) δ 10.8 (C-7′), 19.2 (C-6), 26.3 (C-5), 27.8 (C-9), 28.2 (C-10), 31.9 (C-8), 36.5 (C-3a), 39.0 (C-7), 41.3 (C-4), 88.5 (C-8b), 100.6 (C-2′), 114.5 (C-3), 135.9 (C-3′), 139.5 (C-4a), 141.0 (C-4′), 141.8 (C-8a), 150.0 (C-6′), 170.3 (C-5′), 171.8 (C-2); EI-MS m/z (relative intensity): 330 [M+] (8), 315 (3), 233 (31), 216 (17), 215 (35), 205 (9), 201 (12), 187 (25), 97 (100); UV (CH3CN) λmax 234 nm, (ε 15,600); [α]D32 −168° (c 0.0520, CH3CN); CD (acetonitrile) λmax (Δε) 240 (−2.2), 218 (8.4) nm.
Synthesis of (±)-4-oxo-5-deoxystrigol, (±)-2′-epi-4-oxo-5-deoxystrigol, (±)-5-oxo-5-deoxystrigol and (±)-2′-epi-5-oxo-5-deoxystrigol
To a suspension of chromium trioxide (7.6 g, 76 mmol) in dry dichloromethane (175 ml) was added 3,5-dimethylpyrazole (7.3 g, 76 mmol) in one portion at −20°C under argon. The mixture was stirred for 30 min at −20°C and then a solution of a mixture of two racemic diastereomers of 5-deoxystrigol (250 mg, 0.76 mmol) in dry dichloromethane (10 ml) was added. After stirring for 4 h at −20°C, the reaction mixture was washed successively with 1 N HCl and saturated NaCl solution, and the organic layer was dried over MgSO4, and then concentrated under reduced pressure. The residue was chromatographed on silica gel (Wakogel C-200, Wako Pure Chemical Industries, Osaka, Japan) employing 10% stepwise elution with n-hexane and ethyl acetate. A portion of the 50 and 60% EtOAc eluates containing 5-oxo- and 4-oxo-5-deoxystrigol (123 mg, 0.36 mmol, 47%) was chromatographed on a semi-preparative Inertsil ODS-3 HPLC (φ 10 × 250 mm, 5 μm, GL Sciences, Tokyo, Japan) and eluted by a linear gradient from 60 to 100% methanol in water within 30 min at a flow rate of 3.8 ml min−1. Two epimeric isomers of 5-oxo-5-deoxystrigol (12 mg) and 4-oxo-5-deoxystrigol (3 mg) eluting as a single peak at 10.4 and 12.7 min, respectively, were collected. 5-Oxo-5-deoxystrigol was subjected to a semi-preparative Inertsil SIL-100A HPLC (φ 10 × 250 mm, 5 μm, GL Sciences, Tokyo, Japan), employing isocratic elution with 10% ethanol–n-hexane at a flow rate of 3.8 ml min−1. (±)-5-Oxo-5-deoxystrigol (3.8 mg) and (±)-2′-epi-5-oxo-5-deoxystrigol (7.1 mg) eluting as a single peak at 35.6 and 24.2 min, respectively, were collected. 4-Oxo-5-deoxystrigol was chromatographed as above to afford (±)-4-oxo-5- deoxystrigol (1.5 mg) and (±)-2′-epi-4-oxo-5-deoxystrigol (1.2 mg) that were eluted from the column at 29.9 and 45.9 min, respectively.
(±)-5-Oxo-5-deoxystrigol: 1H-NMR (400 MHz, CDCl3) δ 1.28 (3H, s, CH3), 1.31 (3H, s, CH3), 1.83–1.97 (2H, m, H-7), 2.04 (3H, t, J = 1.5 Hz, H-7′), 2.40–2.60 (2H, m, H-6), 2.60–2.70 (1H, m, H-4β), 2.80–3.00 (1H, dd, J = 9.2, 17.5 Hz, H-4α), 3.65–3.75 (1H, m, H-3a), 5.66–5.71 (1H, d, J = 8.3 Hz, H-8b), 6.15–6.17 (1H, m, H-2′), 6.92–6.94 (1H, m, H-3′), 7.47–7.85 (1H, d, J = 2.7 Hz, H-6′); EI-MS m/z 344 [M+], 247, 230, 215, 97.
(±)-2′-epi-5-Oxo-5-deoxystrigol: 1H-NMR (400 MHz, CDCl3) δ 1.28 (3H, s, CH3), 1.31 (3H, s, CH3), 1.83–1.97 (2H, m, H-7), 2.04 (3H, t, J = 1.5 Hz, H-7′), 2.40–2.60 (2H, m, H-6), 2.60–2.70 (1H, m, H-4β), 2.80–3.00 (1H, dd, J = 9.2, 17.5 Hz, H-4α), 3.65–3.75 (1H, m, H-3a), 5.66–5.71 (1H, d, J = 8.3 Hz, H-8b), 6.13–6.15 (1H, m, H-2′), 6.94–6.96 (1H, m, H-3′), 7.51–7.52 (1H, d, J = 2.7 Hz, H-6′); EI-MS m/z 344 [M+], 247, 230, 215, 97.
(±)-4-Oxo-5-deoxystrigol: 1H-NMR (400 MHz, CDCl3) δ 1.25 (3H, s, CH3), 1.28 (3H, s, CH3), 1.47–1.64 (2H, m, H-7), 1.68–1.75 (2H, m, H-6), 2.00–2.05 (3H, t, J = 1.7 Hz, H-7′), 2.06–2.14 (2H, m, H-5), 3.84–3.86 (1H, dd, J = 2.4, 6.1 Hz, H-3a), 5.50–5.54 (1H, d, J = 6.1 Hz, H-8b), 6.18–6.20 (1H, m, H-2′), 7.00–7.02 (1H, m, H-3′), 7.53–7.54 (1H, d, J = 2.7 Hz, H-6′); EI-MS m/z 344 [M+], 247, 219, 202, 97.
(±)-2′-epi-4-Oxo-5-deoxystrigol: 1H-NMR (400 MHz, CDCl3) δ 1.25 (3H, s, CH3), 1.28 (3H, s, CH3), 1.47–1.64 (2H, m, H-7), 1.68–1.75 (2H, m, H-6), 2.00–2.05 (3H, t, J = 1.7 Hz, H-7′), 2.06–2.14 (2H, m, H-5), 3.84–3.86 (1H, dd, J = 2.4, 6.1 Hz, 3a-H), 5.50–5.54 (1H, d, J = 6.1 Hz, 8b-H), 6.17–6.18 (1H, m, 2′-H), 7.05–7.07 (1H, m, H-3′), 7.65–7.67 (1H, d, J = 2.7 Hz, H-6′); EI-MS m/z 344 [M+], 247, 219, 202, 97.
Optical resolution of (+)-GR24, (−)-ent-GR24, (+)-2′-epi-GR24 and (−)-ent-2′-epi-GR24
(±)-GR24 was chromatographed on a semi-preparative Chiralpak AD-H column HPLC column (φ 10 × 250 mm, Daicel, Osaka, Japan) employing isocratic elution with 40% isopropanol– n-hexane at a flow rate of 2.4 ml min−1. Compounds eluted from the column were monitored with a photodiode array detector. (+)-GR24 and (−)-ent-GR24 eluting as a single peak at 20.7 and 14.4 min, respectively, were collected. (±)-2′-epi-GR24 was chromatographed as above to afford (+)-2′-epi-GR24 and (−)-ent-2′-epi-GR24 that were eluted from the column at 16.6 and 14.8 min, respectively. The enantiomeric purities of four stereoisomers were estimated to be >99% e.e. by analytical HPLC using a Chiralpak AD-H column (φ 4.6 × 250 mm, 5 μm, Daicel, Osaka, Japan) employing isocratic elution with 40% isopropanol–n-hexane at a flow rate of 0.5 ml min−1 monitored with a photodiode array detector.
(+)-GR24, [α]D15 +463° (c 0.12, CHCl3) {[α]D +436° (c 0.25, CHCl3), Thuring et al. 1997}; (−)-ent-GR24, [α]D15 −473° (c 0.12, CHCl3) {[α]D −446° (c 0.25, CHCl3), Thuring et al. 1997}; (+)-2′-epi-GR24, [α]D15 +267° (c 0.079, CHCl3) {[α]D +273° (c 0.2, CHCl3), Thuring et al. 1997}; (−)-ent-2′-epi-GR24, [α]D15 −243° (c 0.084, CHCl3) {[α]D −272° (c 0.2, CHCl3, Thuring et al. 1997}.
Synthesis, optical resolution and absolute configuration determination of reduced GR24 (3,6′-dihydro-GR24) stereoisomers
Hydroxymethyl lactone, 3-(hydroxymethyl)-3,3a,4,8b-tetrahydroindeno [1,2-b]furan- 2-one, was synthesized from the corresponding tricyclic lactone by the procedures previously described (Mangnus and Zwanenburg 1992b). The mixture of 3α- and 3β-hydroxymethyl lactones was subjected to a semi-preparative ODS HPLC column (φ 10 × 250 mm, 5 μm, GL Sciences, Tokyo, Japan) employing isocratic elution with 40% acetonitrile–H2O at a flow rate of 3.8 ml min−1. Compounds eluted from the column were monitored with a photodiode array detector. 3α- and 3β-Hydroxymethyl lactones eluting as a single peak at 9.2 and 8.5 min respectively, were collected.
3α-Hydroxymethyl lactone: 1H-NMR (400 MHz, CDCl3) δ 2.44–2.49 (1H, m, H-3), 2.90–2.96 (1H, m, H-3a), 3.24–3.31 (2H, m, H-4), 3.88 (1H, dd, J = 5.5, 11.2 Hz, CH2OH), 4.02 (1H, dd, J = 5.5, 11.2 Hz, CH2OH), 5.91 (1H, d, J = 6.8 Hz, H-8b), 7.20–7.48 (4H, m, H-5–8); 13C-NMR (100 MHz, CDCl3) δ 36.4, 40.2, 48.9, 61.1, 86.1, 125.4, 126.0, 127.6, 129.7, 138.6, 141.4, 178.2; IR (KBr) 3,440 cm−1 (OH), 1,641 cm−1 (C = O, lactone); EI-MS m/z 204 [M+], 186, 174, 160, 142, 129, 115, 91, 77.
3β-Hydroxymethyl lactone: 1H-NMR (400 MHz, CDCl3) δ 3.03–3.15 (2H, m, H-4), 3.16 (1H, d, J = 6.7, 8.8 Hz, H-3), 3.32–3.40 (1H, m, H-3a), 3.87 (1H, dd, J = 6.8, 11.2 Hz, CH2OH), 3.98 (1H, dd, J = 6.8, 11.2 Hz, CH2OH), 5.68 (1H, d, J = 6.0 Hz, H-8b), 7.20–7.48 (4H, m, H-5–8); 13C-NMR (CDCl3, 100 MHz) δ 31.6, 41.1, 45.9, 60.2, 86.8, 124.9, 126.3, 127.2, 130.2, 137.8, 144.3, 177.7; IR (KBr) 3,433 cm−1 (OH), 1,646 cm−1 (C = O, lactone); EI-MS m/z 204 [M+], 186, 174, 142, 129, 115, 91, 77.
Alkylation of 3α-hydroxymethyl lactone with 4-bromo-2-methyl-2-buten-4-olide afforded 3,6′-dihydro-GR24 as a mixture of two epimers as reported previously (Mangnus and Zwanenburg 1992b). This mixture was subjected to a semi- preparative Inertsil SIL-100A HPLC (φ 10 × 250 mm, 5 μm, GL Sciences, Tokyo, Japan), employing isocratic elution with 15% ethanol–n-hexane at a flow rate of 3.8 ml min−1 to afford 3,6′-dihydro-GR24 (tR 9.6 min) and 2′-epi-3,6′-dihydro-GR24 (tR 10.9 min). Racemic 3,6′-dihydro-GR24 was chromatographed on a semi-preparative Chiralpak AD-H column HPLC column (φ 10 × 250 mm, Daicel, Osaka, Japan) employing isocratic elution with 100% ethanol at a flow rate of 1 ml min−1 to give (+)-ent-3,6′-dihydro-GR24 (tR 57.2 min) and (−)-3,6′-dihydro-GR24 (tR 84.7 min). Racemic 2′-epi-3,6′-dihydro-GR24 was chromatographed as above to afford (+)-ent-2′-epi-3,6′-dihydro-GR24 (tR 35.4 min) and (−)-2′-epi-3,6′-dihydro-GR24 (tR 47.2 min). The enantiomeric purities of four stereoisomers were estimated to be >99% e.e., respectively, by analytical HPLC using a Chiralpak AD-H column (φ 4.6 × 250 mm, 5 μm, Daicel, Osaka, Japan) employing isocratic elution with 65% ethanol–n-hexane at a flow rate of 0.5 ml min−1 monitored with a photodiode array detector.
(−)-2′-epi-3,6′-Dihydro-GR24: 1H-NMR (400 MHz, CDCl3) δ 1.91 (3H, t, J = 1.5 Hz, H-7′), 2.50 (1H, dt, J = 3.6, 7.2 Hz, H-3), 2.88 (1H, d, J = 16.0 Hz, H-4), 3.25 (1H, dd, J = 7.6, 16.0 Hz, H-4), 3.33 (1H, ddd, J = 2.0, 8.0, 15.6 Hz, H-3a), 4.06 (1H, dd, J = 4.0, 9.6 Hz, H-6′), 4.13 (1H, dd, J = 3.2, 9.6 Hz, H-6′), 5.79 (1H, dq, J = 1.5, 1.6 Hz, H-2′), 5.81 (1H, d, J = 7.6 Hz, H-8b), 6.80 (1H, dq, J = 1.5, 1.6 Hz, H-3′) 7.24–7.45 (4H, benzene, H-5–8); 13C-NMR (100 MHz, CDCl3) δ 10.6 (C-7′), 36.7 (C-4), 40.4 (C-3a), 47.7 (C-3), 68.0 (C-6′), 86.0 (C-8b), 101.6 (C-2′), 125.5 (C-7), 126.1 (C-8), 127.6 (C-5), 129.8 (C-6), 134.1 (C-4′), 141.6 (C-4a), 142.6 (C-3′), 138.7 (C-8a), 171.7 (C-5′), 176.5 (C-2); EI-MS m/z 300 [M+], 282, 270, 203, 186, 173, 142, 129, 116, 97, 91. [α]D −80° (c 0.1, CHCl3).
(+)-ent-2′-epi-3,6′-Dihydro-GR24: 1H-NMR, 13C-NMR and mass data were the same as for (−)-2′-epi-3,6′-dihydro-GR24. [α]D +103° (c 0.1, CHCl3).
(−)-3,6′-Dihydro-GR24: 1H-NMR (400 MHz, CDCl3) δ 1.95 (3H, t, J = 1.4 Hz, H-7′), 2.54 (1H, m, H-3), 2.90 (1H, d, J = 15.6 Hz, H-4), 3.28 (1H, dd, J = 7.6, 16.0 Hz, H-4), 3.34 (1H, ddd, J = 1.8, 7.8, 15.6 Hz, H-3a), 3.93 (1H, dd, J = 4.4, 9.6 Hz, H-6′), 4.03 (1H, dd, J = 5.8, 9.8 Hz, H-6′), 5.89 (1H, m, H-2′), 5.89 (1H, d, J = 7.4 Hz, H-8b), 6.84 (1H, dq, J = 1.4, 1.5 Hz, H-3′), 7.25–7.46 (4H, benzene, H-5–8); 13C-NMR (100 MHz, CDCl3) δ 10.6 (C-7′), 36.8 (C-4), 41.2 (C-3a), 47.4 (C-3), 66.4 (C-6′), 86.0 (C-8b), 101.0 (C-2′), 125.5 (C-5), 126.2 (C-8), 127.6 (C-7), 129.9 (C-6), 134.8 (C-4′), 138.7 (C-8a), 141.6 (C-4a), 142.6 (C-3′), 171.4 (C-5′), 176.3 (C-2); EI-MS m/z 300 [M+], 282, 270, 203, 186, 173, 142, 129, 116, 97, 91; [α]D −94° (c 0.1, CHCl3).
(+)-ent-3,6′-Dihydro-GR24: 1H-NMR, 13C-NMR and mass data were the same as for (−)-3,6′-dihydro-GR24. [α]D +101° (c 0.1, CHCl3).
For the determination of the absolute configuration of 3,6′-dihydro-GR24 stereoisomers, the four isomers were synthesized as a pair of epimers at C-2′ from (+)-3a(S),8b(R)- and (−)-3a(R),8b(S)-tricyclic lactones ([α]D +82° (c 0.1, CHCl3), lit. [α]D +102.5° (c 0.4, CHCl3); [α]D −86° (c 0.09, CHCl3), lit. [α]D −107.0° (c 0.4, CHCl3), (Thuring et al. 1997). (−)-3a(R),8b(S)-tricyclic lactone gave (−)-ent-2′-epi-3,6′-dihydro-GR24, the relative configuration of which was established by an X-ray crystallographic analysis using colorless needles from dichloromethane/diisopropyl ether. Thus, the absolute configuration of (−)-ent-2′-epi-3,6′-dihydro-GR24 was determined as 3(S), 3a(R), 8b(S) and 2′(S). Consequently, the absolute configuration of the remaining stereoisomers could be assigned on the basis of the synthetic sequence and chiral HPLC analysis.
Chromatographic separation of epimers of the GR24 imino analog
The GR24 imino analog was obtained probably as an (EZ) mixture with respect to the C–N double bond through previously described procedures (Kondo et al. 2007). This mixture was first subjected to a semi-preparative ODS HPLC column (φ 10 × 250 mm, 5 μm, GL Sciences, Tokyo, Japan) employing isocratic elution with 60% methanol–water at a flow rate of 3.8 ml min−1. Compounds eluted from the column were monitored with a photodiode array detector. One of the two epimeric isomers having either (E) or (Z) configuration with respect to the CN double bond eluting as a single peak at 11.3 min was collected. These epimeric isomers were chromatographed on a semi-preparative Inertsil SIL-100A HPLC (φ 10 × 250 mm, 5 μm, GL Sciences, Tokyo, Japan), employing isocratic elution with 20% ethanol–n-hexane at a flow rate of 2.4 ml min−1. Fast-moving and slow-moving epimers, tentatively called imino GR24F and imino GR24S, eluting as a single peak at 16.0 and 18.7 min, respectively, were collected.
Imino GR24F: 1H-NMR (400 MHz, CDCl3) δ 2.04 (3H, t, J = 1.5 Hz, H-7′), 3.21 (1H, dd, J = 4.3, 17.3 Hz, H-4), 3.58 (1H, dd, J = 10.5, 17.3 Hz, H-4), 4.07 (1H, ddd, J = 4.3, 7.3, 10.5 Hz, H-3a), 6.08 (1H, d, J = 7.3 Hz, H-8b), 6.63 (1H, d, J = 1.5 Hz, H-2′), 6.98 (1H, d, J = 1.5 Hz, H-3′), 7.25–7.58 (4H, m, H-5,6,7,8); EI-MS m/z 299 [M+], 203, 202, 116, 115, 97.
Imino GR24S: 1H-NMR (400 MHz, CDCl3) δ 2.05 (3H, t, J = 1.5 Hz, H-7′), 3.22 (1H, dd, J = 4.4, 17.4 Hz, H-4), 3.56 (1H, dd, J = 10.4, 17.4 Hz, H-4), 4.04 (1H, ddd, J = 4.4, 7.6, 10.4 Hz, H-3a), 6.08 (1H, d, J = 7.6 Hz, H-8b), 6.63 (1H, d, J = 1.3 Hz, H-2′), 6.98 (1H, d, J = 1.6 Hz, H-3′), 7.25–7.58 (4H, m, H-5,6,7,8); EI-MS m/z 299 [M+], 203, 202, 116, 115, 97.
Water degradation experiments of GR24 and 5DS
Aqueous solutions of GR24 [as an equimolar mixture of (±)-GR24 and (±)-2′-epi-GR24, 200 μg ml−1] and (±)-5-deoxystrigol (50 μg ml−1) containing 3% methanol were incubated at 32°C in the HPLC vials. The compounds were first dissolved in methanol, and then were diluted to the final concentrations with water. The time course of degradation was monitored by HPLC analysis using an Inertsil ODS-3 column (φ 4.6 × 250 mm, 5 μm, GL Sciences, Tokyo, Japan), eluted by a linear gradient from 5 to 100% acetonitrile in water containing 0.1% acetic acid within 40 min at a flow rate of 0.8 ml min−1. Compounds eluted from the column were detected with a photodiode array detector. A semi-preparative Inertsil ODS-3 column (φ 10 × 250 mm, 5 μm, GL Sciences, Tokyo, Japan) eluting at a flow rate of 3.8 ml min−1 with the same gradient program as for analytical HPLC was used for large-scale preparation of degradation products.
4-Bromo-2-methyl-2-buten-4-olide (50 μl, 0.5 mmol) was dissolved in 1 N KOH aqueous solution (500 μl). After stirring for 3 h at room temperature, the reaction mixture was poured into 1 N HCl, and then extracted with ethyl acetate. The organic layer was washed, dried over Na2SO4 and then concentrated under reduced pressure. The residue was chromatographed on Wakogel C-200 (Wako Pure Chemical Industries, Osaka, Japan) silica gel employing 10% stepwise elution with n-hexane and ethyl acetate containing a few drops of acetic acid to give 4-hydroxy-2-methylbut-2-en-4-olide (43 mg, 0.37 mmol, 75%) in 40–50% ethyl acetate eluates.
4-Hydroxy-2-methylbut-2-en-4-olide: 1H-NMR (400 MHz, CDCl3) δ 1.97 (3H, t, J = 1.6 Hz, CH3), 6.08 (1H, m, H-4), 6.88 (1H, m, H-3); 13C-NMR (100 MHz, CDCl3) δ 10.5, 96.4, 134.0, 144.0, 171.9; EI-MS m/z 113 [M+]-H, 97, 86, 69, 68, 55, 53, 51.
Supplementary data
Supplementary data are available at PCP online.
Funding
This work was supported by a the Japan Society of the Promotion of Science [Grant-in-Aid for Scientific Research (No. 18208010 (A)]; the Program for Promotion of Basic and Applied Researches for Innovations in Bio-oriented Industry (BRAIN).
Supplementary Material
Acknowledgements
We thank Koichi Yoneyama (Utsunomiya University), Juan C. G. Galindo (Universidad de Córdoba, Spain) and Gavin R. Flematti (The University of Western Australia, Australia) for providing the compounds described in the Materials and Methods, and Toshiji Tada (Osaka Prefecture University) for the X-ray analysis.
Glossary
Abbreviations
- AM
arbuscular mycorrhiza
- CCD
carotenoid cleavage dioxygenase
- CD
circular dichroism
- 5DS
5-deoxystrigol
- EI-MS
electon ionization mass spectrometry
- GR
germination releaser
- IR
infrared
- MEC
minimum effective concentration
- NMR
nuclear magnetic resonance
- tR
retention time.
References
- Akiyama K., Hayashi H. Strigolactones: chemical signals for fungal symbionts and parasitic weeds in plant roots. Ann. Bot. 2006;97:925–931. doi: 10.1093/aob/mcl063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama K., Matsuzaki K., Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 2005;435:824–827. doi: 10.1038/nature03608. [DOI] [PubMed] [Google Scholar]
- Arite T., Umehara M., Ishikawa S., Hanada A., Maekawa M., Yamaguchi S., et al. D14, a strigolactone-insensitive mutant of rice, shows an accelerated outgrowth of tillers. Plant Cell Physiol. 2009;50:1416–1424. doi: 10.1093/pcp/pcp091. [DOI] [PubMed] [Google Scholar]
- Besserer A., Puech-Pagès V., Kiefer P., Gomez-Roldan V., Jauneau A., Roy S., et al. Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol.y. 2006;4:1239–1247. doi: 10.1371/journal.pbio.0040226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besserer A., Bécard G., Jauneau A., Roux C., Séjalon-Delmas N. GR24, a synthetic analog of strigolactones, stimulates the mitosis and growth of the arbuscular mycorrhizal fungus Gigaspora rosea by boosting its energy metabolism. Plant Physiol. 2008;148:402–413. doi: 10.1104/pp.108.121400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge C.A., Kyozuka J. New genes in the strigolactone-related shoot branching pathway. Curr. Opin. Plant Biol. 2010;13:34–39. doi: 10.1016/j.pbi.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Bouwmeester H.J., Roux C., Lopez-Raez J.A., Bécard G. Rhizosphere communication of plants, parasitic plants and AM fungi. Trends Plant Sci. 2007;12:224–230. doi: 10.1016/j.tplants.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Cook C.E., Whichard L.P., Turner B., Wall M.E., Egley G.H. Germination of witchweed (Striga lutea Lour.): isolation and properties of a potent stimulant. Science. 1966;154:1189–1190. doi: 10.1126/science.154.3753.1189. [DOI] [PubMed] [Google Scholar]
- De Luque A.P., Galindo J.C.G., Macías F.A., Jorrín J. Sunflower sesquiterpene lactone models induce Orobanche cumana seed germination. Phytochemistry. 2000;53:45–50. doi: 10.1016/s0031-9422(99)00485-9. [DOI] [PubMed] [Google Scholar]
- Frischmuth K., Samson E., Kranz A., Welzel P., Meuer H., Sheldrick W.S. Routes to derivatives of strigol (the witchweed germination factor) modified in the 5-position. Tetrahedron. 1991;47:9793–9806. [Google Scholar]
- Galindo J.C.G., De Luque A.P., Jorrín J., Macías F.A. SAR studies of sesquiterpene lactones as Orobanche cumana seed germination stimulants. J. Agric. Food Chem. 2002;50:1911–1917. doi: 10.1021/jf0110809. [DOI] [PubMed] [Google Scholar]
- Giovannetti M., Sbrana C., Avio L., Citernesi A.S., Logi C. Differential hyphal morphogenesis in arbuscular mycorrhizal fungi during preinfection stages. New Phytol. 1993;125:587–593. doi: 10.1111/j.1469-8137.1993.tb03907.x. [DOI] [PubMed] [Google Scholar]
- Giovannetti M., Sbrana C., Logi C. Early process involved in host recognition by arbuscular mycorrhizal fungi. New Phytol. 1994;127:703–709. doi: 10.1111/j.1469-8137.1994.tb02973.x. [DOI] [PubMed] [Google Scholar]
- Giovannetti M., Sbrana C., Silvia A., Avio L. Analysis of factors involved in fungal recognition response to host-derived signals by arbuscular mycorrhizal fungi. New Phytol. 1996;133:65–71. [Google Scholar]
- Gomez-Roldan V., Fermas S., Brewer P.B., Puech-Pagès V., Dun E.A., Pillot J.-P., et al. Strigolactone inhibition of shoot branching. Nature. 2008;455:189–194. doi: 10.1038/nature07271. [DOI] [PubMed] [Google Scholar]
- Hauck C., Müller S., Schildknecht H. A germination stimulant for parasitic flowering plants from Sorghum bicolor, a genuine host plant. J. Plant Physiol. 1992;139:474–478. [Google Scholar]
- Hirayama K., Mori K. Plant bioregulators, 5. Synthesis of (+)-strigol and (+)-orobanchol, the germination stimulants and their stereoisomers by employing lipase-catalyzed asymmetric acetylation as the key step. Eur. J. Org. Chem. 1999;1999:2211–2217. [Google Scholar]
- Ishikawa S., Maekawa M., Arite T., Onishi K., Takamure I., Kyozuka J. Suppression of tiller bud activity in tillering dwarf mutants of rice. Plant Cell Physiol. 2005;46:79–86. doi: 10.1093/pcp/pci022. [DOI] [PubMed] [Google Scholar]
- Kondo Y., Tadokoro E., Matsuura M., Iwasaki K., Sugimoto Y., Miyake H., et al. Synthesis and seed germination stimulating activity of some imino analogs of strigolactones. Biosci. Biotechnol. Biochem. 2007;71:2781–2786. doi: 10.1271/bbb.70398. [DOI] [PubMed] [Google Scholar]
- López-Ráez J.A., Bouwmeester H. Fine-tuning regulation of strigolactone biosynthesis under phosphate starvation. Plant Signal. Behav. 2008;3:963–965. doi: 10.4161/psb.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macías F.A., García-Díaz M.D., Pérez-De-Luque A., Rubiales D., Galindo J.C.G. New chemical clues for broomrape-sunflower host–parasite interactions: synthesis of guaianestrigolactones. J. Agric. Food Chem. 2009;57:5853–5864. doi: 10.1021/jf900870j. [DOI] [PubMed] [Google Scholar]
- Mangnus E.M., Zwanenburg B. Synthesis, structural characterization and biological evaluation of all four enantiomers of strigol analogue GR7. J. Agric. Food Chem. 1992a;40:697–700. [Google Scholar]
- Mangnus E.M., Zwanenburg B. Tentative molecular mechanisms for germination stimulation of Striga and Orobanche seeds by strigol and its synthetic analogues. J. Agric. Food Chem. 1992b;40:1066–1070. [Google Scholar]
- Mangnus E.M., Dommerholt F.J., Jong R.L.P.D., Zwanenburg B. Improved synthesis of strigol analog GR24 and evaluation of the biological activity of its diastereomers. J. Agric. Food Chem. 1992b;40:1230–1235. [Google Scholar]
- Mangnus E.M., Vliet L.A., Vandenput D.A. L., Zwanenburg B. Structural modifications of strigol analogues. Influence of the B and C rings on the bioactivity of the germination stimulant GR24. J. Agric. Food Chem. 1992a;40:1222–1229. [Google Scholar]
- Matsuura H., Ohashi K., Sasako H., Tagawa N., Takano Y., Ioka Y., et al. Germination stimulant from root exudates of Vigna unguiculata. Plant Growth Regul. 2008;54:31–36. [Google Scholar]
- Matusova R., Rani K., Verstappen F.W.A., Franssen M.C.R., Beale M.H., Bouwmeester H. The strigolactone germination stimulants of the plant parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant Physiol. 2005;139:920–934. doi: 10.1104/pp.105.061382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller S., Hauck C., Schildknecht H. Germination stimulants produced by Vigna unguiculata Walp cv Saunders Upright. J. Plant Growth Regul. 1992;11:77–84. [Google Scholar]
- Nelson D.C., Riseborough J.-A., Flematti G.R., Stevens J., Ghisalberti E.L., Dixon K.W., et al. Karrikins discovered in smoke trigger arabidopsis seed germination by a mechanism requiring gibberellic acid synthesis and light. Plant Physiol. 2009;149:863–873. doi: 10.1104/pp.108.131516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parniske M. Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nat. Rev. Microbiol. 2008;6:763–775. doi: 10.1038/nrmicro1987. [DOI] [PubMed] [Google Scholar]
- Redecker D., Kodner R., Graham L.E. Glomalean fungi from the Ordovician. Science. 2000;289:1920–1921. doi: 10.1126/science.289.5486.1920. [DOI] [PubMed] [Google Scholar]
- Reizelman A., Scheren M., Nefkens G.H.L., Zwanenburg B. Synthesis of all eight stereoisomers of the germination stimulant strigol. Synthesis. 2000;13:1944–1951. [Google Scholar]
- Reizelman A., Wigchert S.C.M., del-Bianco C., Zwanenburg B. Synthesis and bioactivity of labelled germination stimulants for the isolation and identification of the strigolactone receptor. Org. Biomol. Chem. 2003;1:950–959. doi: 10.1039/b210678g. [DOI] [PubMed] [Google Scholar]
- Remy W., Taylor T.N., Hass H., Kerp H. Four hundred-million-year-old vesicular arbuscular mycorrhizae. Proc. Natl Acad. Sci. USA. 1994;91:11841–11843. doi: 10.1073/pnas.91.25.11841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato D., Awad A.A., Chae S.H., Yokota T., Sugimoto Y., Takeuchi Y., et al. Analysis of strigolactones, germination stimulants for Striga and Orobanche, by high-performance liquid chromatography/tandem mass spectrometry. J. Agric. Food Chem. 2003;51:1162–1168. doi: 10.1021/jf025997z. [DOI] [PubMed] [Google Scholar]
- Schüßler A., Schwarzott D., Walker C. A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycol. Res. 2001;105:1413–1421. [Google Scholar]
- Smith S.E., Read D.J. Mycorrhizal Symbiosis. San Diego: Academic Press; 2008. [Google Scholar]
- Sugimoto Y., Wigchert S.C.M., Thuring J.W.J.F., Zwanenburg B. Synthesis of all eight stereoisomers of the germination stimulant sorgolactone. J. Org. Chem. 1998;63:1259–1267. [Google Scholar]
- Thuring J.W.J.F., Nefkens G.H.L., Zwanenburg B. Asymmetric synthesis of all stereoisomers of the strigol analogue GR24. Dependence of absolute configuration on stimulatory activity of Striga hermonthica and Orobanche crenata seed germination. J. Agric. Food Chem. 1997;45:2278–2283. [Google Scholar]
- Umehara M., Hanada A., Yoshida S., Akiyama K., Arite T., Takeda-Kamiya N., et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature. 2008;455:195–200. doi: 10.1038/nature07272. [DOI] [PubMed] [Google Scholar]
- Xie X., Kusumoto D., Takeuchi Y., Yoneyama K., Yamada Y., Yoneyama K. 2′-epi-orobanchol and solanacol, two unique strigolactones, germination stimulants for root parasitic weeds, produced by tobacco. J Agric. Food Chem. 2007;55:8067–8072. doi: 10.1021/jf0715121. [DOI] [PubMed] [Google Scholar]
- Xie X., Yoneyama K., Harada Y., Fusegi N., Yamada Y., Ito S., et al. Fabacyl acetate, a germination stimulant for root parasitic plants from Pisum sativum. Phytochemistry. 2009b;70:211–215. doi: 10.1016/j.phytochem.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Xie X., Yoneyama K., Kurita J.-Y., Harada Y., Yamada Y., Takeuchi Y., et al. 7-Oxoorobanchyl acetate and 7-oxoorobanchol as germination stimulants for root parasitic plants from flax (Linum usitatissimum) Biosci. Biotechnol. Biochem. 2009a;73:1367–1370. doi: 10.1271/bbb.90021. [DOI] [PubMed] [Google Scholar]
- Xie X., Yoneyama K., Kusumoto D., Yamada Y., Takeuchi Y., Sugimoto Y., et al. Sorgomol, germination stimulant for root parasitic plants, produced by Sorghum bicolor. Tetrahedron Lett. 2008b;49:2066–2068. [Google Scholar]
- Xie X., Yoneyama K., Kusumoto D., Yamada Y., Yokota T., Takeuchi Y., et al. Isolation and identification of alectrol as (+)-orobanchyl acetate, a germination stimulant for root parasitic plants. Phytochemistry. 2008a;69:427–431. doi: 10.1016/j.phytochem.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Yokota T., Sakai H., Okuno K., Yoneyama K., Takeuchi Y. Alectrol and orobanchol, germination stimulants for Orobanche minor, from its host red clover. Phytochemistry. 1998;49:1967–1973. [Google Scholar]
- Yoneyama K., Xie X., Kusumoto D., Sekimoto H., Sugimoto Y., Takeuchi Y., et al. Nitrogen deficiency as well as phosphorus deficiency in sorghum promotes the production and exudation of 5-deoxystrigol, the host recognition signal for arbuscular mycorrhizal fungi and root parasites. Planta. 2007b;227:125–132. doi: 10.1007/s00425-007-0600-5. [DOI] [PubMed] [Google Scholar]
- Yoneyama K., Xie X., Sekimoto H., Takeuchi Y., Ogasawara S., Akiyama K., et al. Strigolactones, host recognition signals for root parasitic plants and arbuscular mycorrhizal fungi, from Fabaceae plants. New Phytol. 2008;179:484–494. doi: 10.1111/j.1469-8137.2008.02462.x. [DOI] [PubMed] [Google Scholar]
- Yoneyama K., Xie X., Yoneyama K., Takeuchi Y. Strigolactones: structures and biological activities. Pest Manag. Sci. 2009;65:467–470. doi: 10.1002/ps.1726. [DOI] [PubMed] [Google Scholar]
- Yoneyama K., Yoneyama K., Takeuchi Y., Sekimoto H. Phosphorus deficiency in red clover promotes exudation of orobanchol, the signal for mycorrhizal symbionts and germination stimulant for root parasites. Planta. 2007a;225:1031–1038. doi: 10.1007/s00425-006-0410-1. [DOI] [PubMed] [Google Scholar]
- Zwanenburg B., Mwakaboko A.S., Reizelman A., Anilkumar G., Sethumadhavan D. Structure and function of natural and synthetic signalling molecules in parasitic weed germination. Pest Manag. Sci. 2009;65:478–491. doi: 10.1002/ps.1706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.