Abstract
Strigolactones (SLs) are newly discovered plant hormones that regulate plant growth and development including shoot branching. They also stimulate symbiosis with arbuscular mycorrhizal fungi. Rice has at least three genes that are involved in SL synthesis (D10, D17/HTD1 and D27) and at least two genes that are involved in SL signaling (D3) and SL signaling or downstream metabolism (D14/D88/HTD2). We observed that mesocotyl elongation in darkness was greater in rice mutants defective in these genes than in the wild type. Exogenous application of a synthetic SL analog, GR24, rescued the phenotype of mesocotyl elongation in the SL-deficient mutants, d10-1, d17-1 and d27-1, in a dose-dependent manner, but did not affect mesocotyl lengths of the SL-insensitive mutants, d3-1 and d14-1. No significant differences in cell length were found between the d mutants and the wild type, except for some cells on the lower half of the d3-1 mesocotyl that were shortened. On the other hand, the number of cells in the mesocotyls was 3- to 6-fold greater in the d mutants than in the wild type. Treatment with GR24 reduced the number of cells in the d10-1 mesocotyl to the wild-type level, but did not affect the number of cells in the d3-1 and d14-1 mesocotyls. These findings indicate that SLs negatively regulate cell division, but not cell elongation, in the mesocotyl during germination and growth of rice in darkness.
Keywords: Cell division, dwarf mutants, Mesocotyl, Rice, Strigolactones
Introduction
Strigolactones (SLs), a group of terpenoid lactones, were initially isolated from root exudates, that triggered germination of seeds of parasitic plants of the genus Striga and stimulated plant symbiosis with arbuscular mycorrhizal fungi (Cook et al. 1972, Akiyama et al. 2005, Besserer et al. 2008, Yoneyama et al. 2008). They have recently been identified as a new hormonal signal that regulates shoot branching in plants (Gomez-Roldan et al. 2008, Umehara et al. 2008). The involvement of SLs in the regulation of shoot branching, particularly in inhibiting the outgrowth of axillary buds, was assessed using a series of enhanced shoot branching mutants, which include more axillary growth (max) of Arabidopsis thaliana, ramosus (rms) of pea (Pisum sativum), decreased apical dominance (dad) of petunia (Petunia hybrida) and dwarf (d) or high-tillering dwarf (htd) of rice (Oryza sativa) (Dun et al. 2009, Leyser 2009, Beveridge and Kyozuka 2010). SLs also probably have other roles in growth and development, including seed germination, root growth, flower development and leaf senescence (Bradow et al. 1988, 1990, Woo et al. 2001, Snowden et al. 2005, Yan et al. 2007). Indeed, it was recently reported that SL biosynthesis/ signaling genes were expressed in mature seeds and young seedlings as well as adult plants, suggesting that their gene products play roles in germination and early development of Arabidopsis (Mashiguchi et al. 2009).
In rice, five DWARF (D) genes have been demonstrated to be responsible for SL biosynthesis (D10, D17/HTD1 and D27), SL signaling (D3) and SL signaling or downstream metabolism (D14/D88/HTD2) (Beveridge and Kyozuka 2010), and recessive mutants defective in these genes have reduced stature and increased numbers of tillers (Ishikawa et al. 2005). Among the SL biosynthetic genes, D10 encodes a protein that is a type of CAROTENOID CLEAVAGE DIOXYGENASE 8 (CCD8) (Arite et al. 2007), while D17/HTD1 encodes CCD7, another subclass of CCDs (Zou et al. 2006, Umehara et al. 2008). D10 and D17/HTD1 appear to catalyze sequential carotenoid cleavage reactions of precursors of SL synthesis (Umehara et al. 2008). D27 was recently shown to encode an iron-containing protein, which works downstream of steps mediated by CCD7 and CCD8 in plastids (Lin et al. 2009). On the other hand, D3 is involved in SL signaling and D14/D88/HTD2 is involved in SL signaling or downstream metabolism (Umehara et al. 2008). D3 encodes an F-box leucine-rich repeat (LRR) protein, which possibly acts in the ubiquitin-mediated degradation of a key factor of the SL signaling pathway (Ishikawa et al. 2005). D14/D88/HTD2 encodes a protein of the α/β-hydrolase superfamily, which is proposed to function downstream of SL synthesis, as a component of hormone signaling or as an enzyme that participates in the conversion of SLs to the bioactive form (Arite et al. 2009, Gao et al. 2009, Liu et al. 2009).
The mesocotyl is a tissue located between the coleoptilar node and a basal part of the seminal root in young seedlings (Hoshikawa 1989). Its growth is controlled not only by the lengthwise elongation of each cell, but also by an increase in the number of cells by cell division. Cell elongation begins with swelling from water absorption, and then cell division is promoted and the elongation and growth of each cell occurs (Hoshikawa 1989). Previous studies have suggested that elongation of the mesocotyl is controlled by multiple genetic, developmental and environmental signals (Jones et al. 1991, Mori et al. 2002, Sawers et al. 2002, Choi et al. 2003). In general, light absorption by phytochrome in plants inhibits mesocotyl growth (Loercher 1966, Vanderhoef and Briggs 1978). Moreover, gibberellins (GAs) are known as facilitators of mesocotyl elongation, and auxin has recently been shown to down-regulate expression of a gene encoding polyamine oxidase, which was tightly correlated with inhibition of cell extension in the outer tissues of maize mesocotyl, thereby possibly enhancing elongation of the mesocotyl (Jones et al. 1991, Laurenzi et al. 1999, Cona et al. 2003). On the other hand, mesocotyl elongation seems to be inhibited by endogenous abscisic acid (ABA) in maize (Saab et al. 1992) and by endogenous jasmonate (JA) in rice (Riemann et al. 2003).
In this study, we found that mesocotyl elongation in dark-grown rice seedlings was higher in the SL-related d mutants than in the wild type. To understand how SLs affect mesocotyl elongation in dark-grown rice seedlings, we examined (i) whether the phenotype of the mesocotyl elongation in the d mutants was rescued by treatment with GR24, a synthetic SL analog, and (ii) whether cell division and/or cell elongation during mesocotyl growth contributed to the enhanced mesocotyl elongation of the d mutants. Possible roles of SLs in mesocotyl elongation in dark-grown rice seedling are discussed.
Results
Phenotype of rice SL mutants
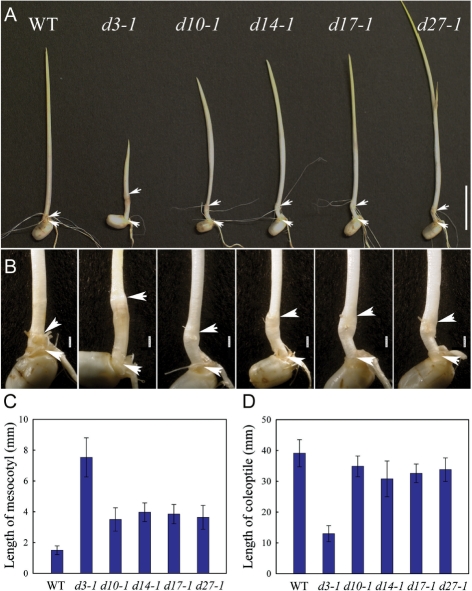
After growing in darkness for 8 d, mesocotyls of SL-deficient (d10-1, d17-1 and d27-1) and SL-insensitive (d3-1 and d14-1) mutants were at least 2- to 5-fold longer than those in the wild type (cv. Shiokari) (Fig. 1A–C). Interestingly, the d3-1 mesocotyls were ∼2-fold longer than the mesocotyls of the other mutants (Fig. 1C). On the other hand, the lengths of coleoptiles were not significantly different between the mutants and the wild type, except for the d3-1 coleoptile, which was only 40% as long as the other mutants and the wild type (Fig. 1D). When the seedlings were grown under light conditions, however, the mesocotyl and coleoptile lengths of the mutants were comparable to those of the wild type (data not shown), suggesting that the enhanced mesocotyl elongation in all the mutants and the reduced coleoptile elongation in the d3-1 mutant occur only under dark conditions.
Fig. 1.
Comparison of the morphology of d mutant and wild-type seedlings grown in the dark. (A) Phenotypes of seedlings of d mutants and the wild type (WT) when germinated and grown for 8 d under darkness. Upper arrows and lower arrows indicate the positions of the coleoptilar node and a basal part of the seminal root, respectively. The mesocotyl is the tissues between the two arrows. Bars = 1 cm. (B) Higher magnification of the mesocotyl regions in (A). Some roots were removed to clearly see the mesocotyls. Bars = 1 mm. (C) Length of mesocotyls of 8-day-old seedlings. Values are mean ± SD, n = 15. (D) Length of coleoptiles of 8-day-old seedlings. Values are mean ± SD, n = 15.
Effect of SL on mesocotyl length of rice d mutants
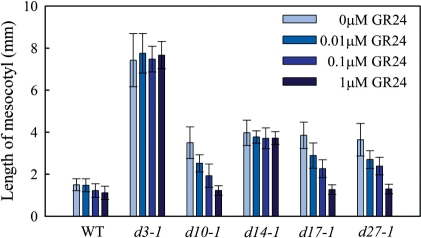
To examine the role of SLs in mesocotyl elongation, seedlings were germinated and grown on agar plates containing different concentrations (0, 0.01, 0.1 and 1 μM) of GR24, a synthetic SL analog, for 8 d in darkness. GR24 did not affect mesocotyl elongation of the wild type but decreased the lengths of mesocotyls of SL-deficient mutants (d10-1, d17-1 and d27-1) in a dose-dependent manner (Fig. 2). At a GR24 concentration of 1 μM, the lengths of mesocotyls were almost the same between the SL-deficient mutants and the wild type. On the other hand, the lengths of mesocotyls of SL-insensitive mutants (d3-1 and d14-1) were not changed by treatment with GR24 (Fig. 2).
Fig. 2.
Effect of GR24 on mesocotyl elongation of 8-day-old dark-grown d mutant and wild-type (WT) seedlings. Each value represents mean ± SD, n = 10–15.
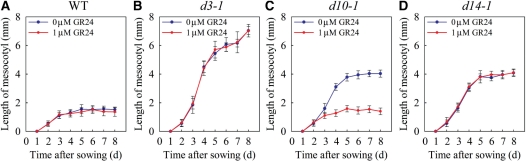
When growth was observed over time, the mesocotyls of mutant and wild-type seedlings began to elongate after 1 d and continued to elongate to 2 d at the same rate (Fig. 3, blue curves). Subsequently, mesocotyl elongation slowed and almost stopped in the wild type but continued in d3-1, d10-1 and d14-1 (Fig. 3, blue curves). GR24 (1 μM) did not affect mesocotyl elongation of the wild type or the SL-insensitive mutants (d3-1 and d14-1) but it decreased the mesocotyl length of SL-deficient d10-1, making it similar to the wild type (Fig. 3, red curves).
Fig. 3.
Time-course of mesocotyl elongation of dark-grown d mutant and wild-type (WT) seedlings. Blue and red curves indicate untreated and GR24-treated seedlings, respectively. Each value represents mean ± SD, n = 10.
Enhancement of cell division, but not cell elongation, in the mesocotyls of rice d mutants
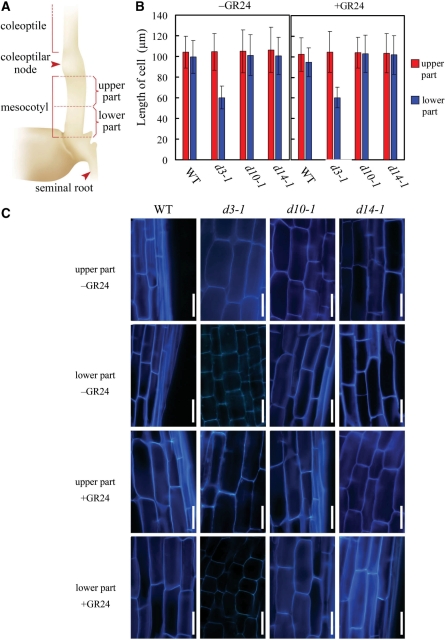
Is the enhanced mesocotyl elongation of the d mutants the result of increased cell division or increased cell elongation? We measured cell length in vertical sections of the mesocotyls in d3-1, d10-1, d14-1 and the wild type. During this investigation, we noticed that cell length differed between the upper half and the lower half of the d3-1 mesocotyl, and thus we decided to separate the mesocotyls into two parts (upper part and lower part; Fig. 4A). Cell lengths in both the upper and lower parts of all the mesocotyls were ∼100 μm, except for d3-1, in which the cell lengths in the lower half of the mesocotyl were only ∼60 μm (Fig. 4B–C). Treatment with 1 μM GR24 had no visible effect on cell length (Fig. 4B–C).
Fig. 4.
Cell lengths in mesocotyls of dark-grown seedlings. (A) Definition of the upper part and the lower part of the mesocotyl. (B) Vertical length of cells in the upper and lower parts of mesocotyls in 8-day-old seedlings treated with (+GR24) or without (−GR24) 1 μM GR24. Each value represents mean ± SD, n = 400–450. (C) Representative tissue sections of mesocotyls from the wild type (WT), d3-1, d10-1 and d14-1. Bars = 50 μm.
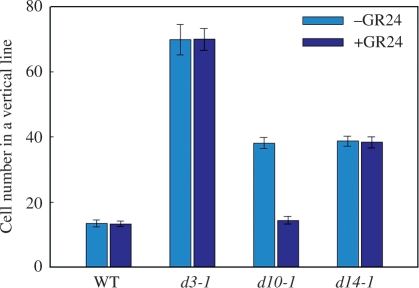
The cells in the mesocotyl are rectangular and elongated in the vertical direction as shown in Fig. 4C. The average number of cells from the bottom to the top of the mesocotyl was ∼13 in the wild type, ∼70 in d3-1 and ∼38 in d10-1 and d14-1 (Fig. 5, −GR24). When the seedlings were grown in the presence of 1 μM GR24, the cell number in the d10-1 mesocotyl was reduced to almost that of the wild type, whereas the cell numbers in d3-1, d14-1 and wild-type mesocotyls were not changed (Fig. 5, +GR24). Taken together, these results indicate that SLs negatively regulate cell division, but not cell elongation, in the mesocotyl during germination and growth of rice in darkness.
Fig. 5.
Cell numbers and effect of GR24 on cell numbers in a vertical line in mesocotyls of dark-grown seedlings. Values indicate the numbers of cells between the coleoptilar node and the basal part of the seminal root in dark-grown 8-day-old seedlings of d mutants and the wild type (WT). GR24 concentration was 1 μM. Each value represents mean ± SD, n = 30.
Discussion
As mentioned above, SLs stimulate the growth of mycorrhizal fungi and control shoot branching, and probably affect seed germination, root growth, flower development and leaf senescence. Here we show that SLs or their downstream metabolites also negatively regulate mesocotyl elongation in rice when germinated and grown in darkness. This new role is demonstrated by our findings that rice mesocotyl elongation was enhanced in mutants deficient in or insensitive to SL (Fig. 1), and that a synthetic SL, GR24, rescued the phenotype of mesocotyl elongation in the SL-deficient mutants but not in the SL-insensitive mutants (Figs. 2, 3).
The results shown in Figs. 4, 5 indicate that the enhancement of mesocotyl elongation in the d mutants is due to enhanced cell division, but not to enhanced cell elongation, in the mesocotyls, and that SLs or their downstream metabolites have an inhibitory effect on cell division in the dark-grown mesocotyls. In preliminary analyses, mRNA levels of some cell division-related genes were higher in the d3-1 mesocotyl than in the wild-type mesocotyl a few days after sowing (data not shown). Together, these results raise the possibility that SLs also negatively regulate cell division in the axillary buds, which might explain the observations that shoot branching of the d mutants is enhanced (Ishikawa et al. 2005). To understand the general roles of SLs in plant growth and development, it would be of interest to examine this possibility.
The d3-1 seedlings, which are defective in SL signaling, have more severe phenotypes [longer mesocotyl and shorter coleoptile (Fig. 1)] than the other d mutants. Why? It may be that in the SL-related mutants other than d3-1, either (i) SLs could be produced by some other pathway or (ii) even in the absence of SLs, SCFD3 (an SCF-type ubiquitin E3 ligase) might still interact with its substrate, both of which would result in more moderate phenotypes. In the d3-1 mutant, however, SL signaling would be severely affected, leading to more severe phenotypes. Another explanation for the more severe phenotypes of the d3-1 mutants is that D3 may have roles in SL-independent processes.
These hypotheses may also explain differences in phenotypes that are observed in SL-related mutants of Arabidopsis. Arabidopsis has several SL-related genes (MAX1–MAX4) and mutants of these genes (max1–max4) show enhanced shoot branching. One of the MAX genes (MAX2) is very similar to D3 in sequence, and like D3 is involved in SL signaling. Also like the d3-1 mutant, the max2 mutant has more severe phenotypes (elongated hypocotyls when grown in the light and hyposensitivity of germination to red and far-red light) (Stirnberg et al. 2002, Shen et al. 2007) than do the other max mutants.
Recently, some progress has been made in elucidating the regulatory mechanisms of mesocotyl elongation through studies of some mutants affecting mesocotyl growth in rice. For instance, rice phytochrome A (phyA) and coleoptile photomorphogenesis 1 (cpm1) impair the phytochrome-mediated inhibition of mesocotyl and coleoptile elongation (Takano et al. 2001, Biswas et al. 2003). The rice phyA mutant is insensitive to far-red light, which negatively affects the elongation of the mesocotyl and coleoptile (Takano et al. 2001). In the cpm1 mutant, the phytochrome signaling that inhibits growth is impaired (Biswas et al. 2003). Interestingly, the mesocotyl and coleoptile elongate more in cpm1 than in the wild type in darkness (Biswas et al. 2003). On the other hand, mesocotyl elongation in the rice JA-deficient mutant hebiba was enhanced when germinated and grown under dark conditions as well as under illumination with red light (Riemann et al. 2003), suggesting that JA is also involved in negative regulation of mesocotyl elongation in rice. Rice genes (e.g. OsAOS1 and OsAOS4) encoding allene oxide synthase, which is a key enzyme in JA synthesis, are up-regulated by red and far-red light in shoots (Haga and Iino 2004), and thus JA levels dramatically increase in rice seedlings in response to red light (Riemann et al. 2003). The induction of JA synthesis in rice seedlings under light conditions may explain why the phenotype of elongated mesocotyls in the d mutants was not observed when seedlings were germinated and grown under light conditions. In other words, the increased JA levels may mask the effect of a deficiency of SLs or impairment of SL signaling on mesocotyl elongation in light-grown seedlings of the d mutants. Indeed, in preliminary experiments, we found that exogenous application of JA to the d mutants during germination in darkness suppressed their elongated mesocotyl phenotype (data not shown). Further studies are needed to understand the relationship between SLs and JA and how it affects mesocotyl elongation in rice seedlings.
Materials and Methods
Plant materials and growth conditions
Rice (Oryza sativa L.) tillering dwarf mutants, d3-1, d10-1, d14-1, d17-1 and d27-1, and their wild type (cv. Shiokari) were used in this study (Kinoshita and Takahashi 1991, Ishikawa et al. 2005).
Dehusked rice caryopses were sterilized in a 10% (v/v) sodium hypochlorite solution for 1 h. After washing with deionized water, seeds were kept in the water for 10 h at 4°C in darkness, and then ∼15 seeds were sown on each 0.7% (w/v) agar plate. The agar plate was placed in a dark box made of black cardboard (Haga and Iino 2004) and then the dark box was covered by black cloths. The boxes were put in the growth chamber, and the seeds were germinated and grown at 28°C under completely dark conditions. For the time-course experiments, independent dark boxes were used for each time point.
SL treatment and analysis
GR24 was synthesized as described previously (Umehara et al. 2008). For SL treatment, 0.7% (w/v) agar was melted by autoclaving, and then GR24 (100 mM; dissolved in 100% acetone) was added to the melted agar (∼50°C) to prepare agar plates with 0.01, 0.1 or 1 μM GR24 (final concentration) (each plate contained 0.001% acetone). The desterilized seeds were placed on the agar plates with 0.01, 0.1 or 1 μM GR24 and were germinated and grown at 28°C under complete darkness. For negative controls, agar plates with 0.001% acetone were used.
Measurement of cell length and cell numbers in vertical sections of the mesocotyls
Six dark-grown 8-day-old seedlings from each mutant line and the wild type were selected for measuring and counting mesocotyl cells. The mesocotyls were embedded in 5% (w/v) agar and cut in 8- to 10-μm-thick vertical sections with a microtome (MicroRom HM 650 V; Nikon, Tokyo, Japan). The sections were observed with a fluorescence microscope (ECLIPSE E600; Nikon) and photographed with a Micro Color camera (DS-Ri1; Nikon). The cells could be clearly seen by the autofluorescence of their cell walls. The lengths of 50–100 cells in both the upper and lower parts of the mesocotyl (Fig. 4A) (a total of 400–450 cells for each part) were measured with NIS-Elements D (Nikon).
The numbers of cells in the outer layers (including the epidermis) of the mesocotyls from the coleoptilar node to the basal part of the seminal root in vertical sections were counted under a microscope. This number was counted for 30 columns of cells in each line of rice.
Acknowledgments
We thank Drs Moritoshi Iino and Ken Haga for instructions on how to grow rice seedlings under completely dark conditions and for stimulating discussions. We thank Drs Eiji Nambara, Yusuke Jikumaru, Mikihisa Umehara, Kiyoaki Kato, Masao Watanabe, Yuhua Li, Shin-ichi Arimura for stimulating discussions. This work was partly supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan, a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan, and a grant from the Bio-oriented Technology Research Advancement Institution (Promotion of Basic Research Activities for Innovative Biosciences).
Glossary
Abbreviations
- ABA
abscisic acid
- CCD
carotenoid cleavage dioxygenase
- GA
giberellin
- JA
jasmonate
- SL
strigolactone.
References
- Akiyama K., Matsuzaki K., Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 2005;435:824–827. doi: 10.1038/nature03608. [DOI] [PubMed] [Google Scholar]
- Arite T., Iwata H., Ohshima K., Maekawa M., Nakajima M., Kojima M., et al. DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J. 2007;51:1019–1029. doi: 10.1111/j.1365-313X.2007.03210.x. [DOI] [PubMed] [Google Scholar]
- Arite T., Umehara M., Ishikawa S., Hanada A., Maekawa M., Yamaguchi S., et al. d14, a strigolactone-insensitive mutant of rice, shows an accelerated outgrowth of tillers. Plant Cell Physiol. 2009;50:1416–1424. doi: 10.1093/pcp/pcp091. [DOI] [PubMed] [Google Scholar]
- Besserer A., Bécard G., Jauneau A., Roux C., Séjalon-Delmas N. GR24, a synthetic analog of strigolactones, stimulates the mitosis and growth of the arbuscular mycorrhizal fungus Gigaspora rosea by boosting its energy metabolism. Plant Physiol. 2008;148:402–413. doi: 10.1104/pp.108.121400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge C.A., Kyozuka J. New genes in the strigolactone-related shoot branching pathway. Curr. Opin. Plant Biol. 2010;13:34–39. doi: 10.1016/j.pbi.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Biswas K.K., Neumann R., Haga K., Yatoh O., Iino M. Photomorphogenesis of rice seedlings: a mutant impaired in phtyochrome-mediated inhibition of coleoptile growth. Plant Cell Physiol. 2003;44:242–254. doi: 10.1093/pcp/pcg040. [DOI] [PubMed] [Google Scholar]
- Bradow J.M., Connick W.J., Pepperman A.B. Comparison of the seed germination effects of synthetic analogs of strigol, gibberellic acid, cytokinins and other plant growth regulators. J. Plant Growth Regul. 1988;7:227–239. [Google Scholar]
- Bradow J.M., Connick W.J., Pepperman A.B., Wartelle L.H. Germination stimulation in wild oats (Avena fatua L.) by synthetic strigol analogues and gibberellic acid. J. Plant Growth Regul. 1990;9:35–41. [Google Scholar]
- Choi D., Lee Y., Cho H., Kende H. Regulation of expansin gene expression affects growth and development in transgenic rice plants. Plant Cell. 2003;15:1386–1398. doi: 10.1105/tpc.011965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cona A., Cenci F., Cervelli M., Federico R., Mariottini P., Moreno S., et al. Polyamine oxidase, a hydrogen peroxide-producing enzyme, is up-regulated by light and down-regulated by auxin in the outer tissues of the maize mesocotyl. Plant Physiol. 2003;131:803–813. doi: 10.1104/pp.011379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook C.E., Whichard L.P., Monroe W.E., Egley G.H., Coggon P., Luhan P.A., et al. Germination stimulants II. Structure of strigol—a potent seed germination stimulant for witchweed (Striga lutea Lour) J. Am. Chem. Soc. 1972;94:6198–6199. [Google Scholar]
- Dun E.A., Hanan J., Beveridge C.A. Computational modeling and molecular physiology experiments reveal new insights into shoot branching in pea. Plant Cell. 2009;21:3459–3472. doi: 10.1105/tpc.109.069013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z., Qian Q., Liu X., Yan M., Feng Q., Dong G., et al. Dwarf 88, a novel putative esterase gene affecting architecture of rice plant. Plant Mol. Biol. 2009;71:265–276. doi: 10.1007/s11103-009-9522-x. [DOI] [PubMed] [Google Scholar]
- Gomez-Roldan V., Fermas S., Brewer P.B., Puech-Pagès V., Dun E.A., Pillot J.P., et al. Strigolactone inhibition of shoot branching. Nature. 2008;455:189–194. doi: 10.1038/nature07271. [DOI] [PubMed] [Google Scholar]
- Haga K., Iino M. Phytochrome-mediated transcriptional up-regulation of ALLENE OXIDE SYNTHASE in rice seedlings. Plant Cell Physiol. 2004;45:119–128. doi: 10.1093/pcp/pch025. [DOI] [PubMed] [Google Scholar]
- Hoshikawa K. The growing rice plant: an anatomical monograph. Tokyo: Nobunkyou; 1989. pp. 39–50. [Google Scholar]
- Ishikawa S., Maekawa M., Arite T., Onishi K., Takamure I., Kyozuka J. Suppression of tiller bud activity in tillering dwarf mutants of rice. Plant Cell Physiol. 2005;46:79–86. doi: 10.1093/pcp/pci022. [DOI] [PubMed] [Google Scholar]
- Jones A.M., Cochran D.S., Lamerson P.M., Evans M.L., Cohen J.D. Red light-regulated growth: 1. Changes in the abundance of indoleacetic acid and a 22-kilodalton auxin-binding protein in the maize mesocotyl. Plant Physiol. 1991;97:352–358. doi: 10.1104/pp.97.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T., Takahashi M. The one hundredth report of genetic studies on rice plant. J. Fac. Agr. Hokkaido Univ. 1991;65:1–61. [Google Scholar]
- Laurenzi M., Rea G., Federico R., Tavladoraki P., Angelini R. De-etiolation causes a phytochrome-mediated increase of polyamine oxidase expression in outer tissues of the maize mesocotyl: a role in the photomodulation of growth and cell wall differentiation. Planta. 1999;208:146–154. [Google Scholar]
- Leyser O. The control of shoot branching: an example of plant information processing. Plant Cell Environ. 2009;32:694–703. doi: 10.1111/j.1365-3040.2009.01930.x. [DOI] [PubMed] [Google Scholar]
- Lin H., Wang R., Qian Q., Yan M., Meng X., Fu Z., et al. DWARF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell. 2009;21:1512–1525. doi: 10.1105/tpc.109.065987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Wu C., Fu Y., Hu G., Si H., Zhu L., et al. Identification and characterization of HTD2: a novel gene negatively regulating tiller bud outgrowth in rice. Planta. 2009;230:649–658. doi: 10.1007/s00425-009-0975-6. [DOI] [PubMed] [Google Scholar]
- Loercher L. Phytochrome changes correlated to mesocotyl inhibition in etiolated Avena seedlings. Plant Physiol. 1966;41:932–936. doi: 10.1104/pp.41.6.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashiguchi K., Sasaki E., Shimada Y., Nagae M., Ueno K., Nakano T., et al. Feedback-regulation of strigolactone biosynthetic genes and strigolactone-regulated genes in Arabidopsis. Biosci. Biotechnol. Biochem. 2009;73:2460–2465. doi: 10.1271/bbb.90443. [DOI] [PubMed] [Google Scholar]
- Mori M., Nomura T., Ooka H., Ishizaka M., Yokota T., Sugimoto K., et al. Isolation and characterization of a rice dwarf mutant with a defect in brassinosteroid biosynthesis. Plant Physiol. 2002;130:1152–1161. doi: 10.1104/pp.007179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann M., Müller A., Korte A., Furuya M., Weiler E.W., Nick P. Impaired induction of the jasmonate pathway in the rice mutant hebiba. Plant Physiol. 2003;133:1820–1830. doi: 10.1104/pp.103.027490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab I.N., Sharp R.E., Pritchard J. Effect of inhibition of abscisic acid accumulation on the spatial distribution of elongation in the primary root and mesocotyl of maize at low water potentials. Plant Physiol. 1992;99:26–33. doi: 10.1104/pp.99.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawers R.J., Linley P.J., Farmer P.R., Hanley N.P., Costich D.E., Terry M.J., et al. elongated mesocotyl1, a phytochrome-deficient mutant of maize. Plant Physiol. 2002;130:155–163. doi: 10.1104/pp.006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H., Luong P., Huq E. The F-box protein MAX2 functions as a positive regulator of photomorphogenesis in Arabidopsis. Plant Physiol. 2007;145:1471–1483. doi: 10.1104/pp.107.107227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden K.C., Simkin A.J., Janssen B.J., Templeton K.R., Loucas H.M., Simons J.L., et al. The Decreased apical dominance1/Petunia hybrida CAROTENOID CLEAVAGE DIOXYGENASE8 gene affects branch production and plays a role in leaf senescence, root growth, and flower development. Plant Cell. 2005;17:746–759. doi: 10.1105/tpc.104.027714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirnberg P., van de Sande K., Leyser H.M. MAX1 and MAX2 control shoot lateral branching in Arabidopsis. Development. 2002;129:1131–1141. doi: 10.1242/dev.129.5.1131. [DOI] [PubMed] [Google Scholar]
- Takano M., Kanegae H., Shinomura T., Miyao A., Hirochika H., Furuya M. Isolation and characterization of rice phytochrome A mutants. Plant Cell. 2001;13:521–534. doi: 10.1105/tpc.13.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umehara M., Hanada A., Yoshida S., Akiyama K., Arite T., Takeda-Kamiya N., et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature. 2008;455:195–200. doi: 10.1038/nature07272. [DOI] [PubMed] [Google Scholar]
- Vanderhoef L.N., Briggs W.R. Red light-inhibited mesocotyl elongation in maize seedlings. Plant Physiol. 1978;61:534–537. doi: 10.1104/pp.61.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo H.R., Chung K.M., Park J.H., Oh S.A., Ahn T., Hong S.H., et al. ORE9, an F-Box protein that regulates leaf senescence in Arabidopsis. Plant Cell. 2001;13:1779–1790. doi: 10.1105/TPC.010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H., Saika H., Maekawa M., Takemure I., Tsutsumi N., Kyozuka J., et al. Rice tillering dwarf mutant dwarf3 has increased leaf longevity during darkness-induced senescence or hydrogen peroxide-induced cell death. Genes Genet. Syst. 2007;82:361–366. doi: 10.1266/ggs.82.361. [DOI] [PubMed] [Google Scholar]
- Yoneyama K., Xie X., Sekimoto H., Takeuchi Y., Ogasawara S., Akiyama K., et al. Strigolactones, host recognition signals for root parasitic plants and arbuscular mycorrhizal fungi, from Fabaceae plants. New Phytol. 2008;179:484–494. doi: 10.1111/j.1469-8137.2008.02462.x. [DOI] [PubMed] [Google Scholar]
- Zou J., Zhang S., Zhang W., Li G., Chen Z., Zhai W., et al. The rice HIGH-TILLERING DWARF1 encoding an ortholog of Arabidopsis MAX3 is required for negative regulation of the outgrowth of axillary buds. Plant J. 2006;48:687–696. doi: 10.1111/j.1365-313X.2006.02916.x. [DOI] [PubMed] [Google Scholar]